209 PRODUCTION OF IN VITRO-FERTILIZED INTERSPECIES BLASTOCYSTS BETWEEN SHEEP OOCYTES AND GOAT SPERMATOZOA
D. Malakar A , A. K. De A and Y. S. Akshey AAnimal Biotechnology Center, NDRI, Karnal-132001, India
Reproduction, Fertility and Development 20(1) 184-184 https://doi.org/10.1071/RDv20n1Ab209
Published: 12 December 2007
Abstract
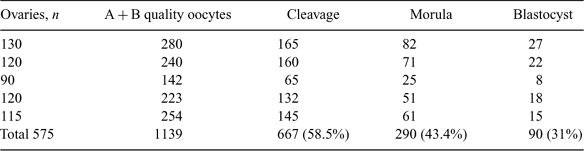
In rodents, chimeric blastocysts produced by combining embryonic cells of 2 different species have been used to investigate cell lineage and cell interaction during development. Interspecific chimerism offers new approaches to the study of reproductive incompatibilities between species. The aim of the present study was to produce interspecies embryos between sheep oocytes and goat spermatozoa through in vitro fertilization. Sheep ovaries were collected from a nearby abattoir and transported to the laboratory in 0.9% normal sterile saline containing antibiotics (50 μg mL–1 of gentamicin sulfate) at 30 to 35°C. Oocytes were aspirated by the puncturing method in a medium consisting of TCM-199 and 3 mg mL–1 of BSA. Only A and B grade COC with 3 or more layers of cumulus cells with homogeneous ooplasm were taken for maturation. The oocytes were washed 4 to 5 times in maturation medium containing TCM-199 (HEPES modified), 10 μg mL–1 of LH, 5 μg mL–1 of FSH, 1 μg mL–1 of estradiol-17β, 50 μg mL–1 of sodium pyruvate, 5.5 mg mL–1 of glucose, 3.5 μg mL–1 of L-glutamine, 50 μg mL–1 of gentamicin, 3 mg mL–1 of BSA, and 10% EGS (heat-inactivated goat serum). The COC (15 to 20 oocytes) were placed in 100-μL droplets of maturation medium, covered with paraffin oil in a 35-mm Petri dish, and incubated in a CO2 incubator (5% CO2 in air) with maximum humidity at 38.5°C for 24 h. Fresh semen was collected from a proven buck. The semen was washed at 300g 2 times in sperm-TALP (Parrish et al. 1986) medium to remove the seminal plasma and incubated with fert-TALP medium containing sperm-TALP supplemented with 50 μg mL–1 of heparin and 3 mg mL–1 of BSA for 1.5 h for capacitation. The matured sheep oocytes with expanded cumulus cells were coincubated with capacitated buck spermatozoa at a concentration of 2 × 106 sperm mL–1 for 10 h in 5% CO2 in air with maximum humidity at 38.5°C. The presumptive zygotes were then cultured in embryo development medium containing TCM-199 (HEPES modified), 0.03 mg mL–1 of sodium pyruvate, 0.1 mg mL–1 of L-glutamine, 0.05 mg mL–1 of gentamicin, 10 μL mL–1 of essential amino acids, 5 μL mL–1 of nonessential amino acids, 10 mg mL–1 of BSA (fraction V), 10% fetal calf serum, and 50 mm cysteamine along with sheep oviductal cells for further development. The cleavage was recorded at 36 to 48 h postinsemination, and morula- and blastocyst-stage embryos were obtained on Day 5 and Day 7, respectively. The cleavage percentage was found to be 58.6%. Among the cleaved embryos, 43% reached the morula stage, and among morula, 31% reached the blastocyst stage. We concluded that interspecies embryos between sheep and goat can be produced successfully in vitro up to the blastocyst stage.
|


