168 POST-THAW VIABILITY OF EPIDIDYMAL SPERM FROM WHITE-TAIL DEER THAT WAS FROZEN USING GLYCEROL OR DMSO AS THE CRYOPROTECTANT
M. A. Stout A B , J. S. Saenz A B , G. T. Gentry A B , S. P. Leibo C , K. R. Bondioli A B and R. A. Godke A BA Louisiana State University Agricultural Center, Baton Rouge, LA;
B Embryo Biotechnology Laboratory, Saint Gabriel, LA;
C Audubon Center for Research of Endangered Species, New Orleans, LA
Reproduction, Fertility and Development 21(1) 183-183 https://doi.org/10.1071/RDv21n1Ab168
Published: 9 December 2008
Abstract
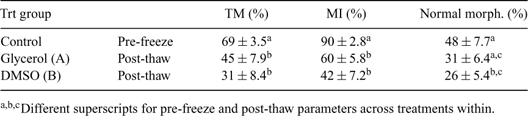
Cryoprotectants are compounds that protect and maintain the viability of cells when being subjected to cold environments. In addition to glycerol, DMSO has been evaluated for post-thaw epididymal sperm viability in various endangered species, but has not yet been assessed in White-tail deer. Testicles (in the scrotum) were collected from hunter harvested sexually mature White-tail bucks (n = 7) during the peak rutting season. Testicles within the scrotum, once removed from the postmortem buck, were placed in a plastic Ziploc bag and then into a Styrofoam ice chest (at ambient temperature) and transported to the Embryo Biotechnology Laboratory (EBL). Upon arrival at the EBL, sperm were flushed in a retrograde flow out of a small incision at the medial section of the cauda epididymides. Epididymal sperm were flushed from the epididymis using a non glycerol extender. Sperm from both epididymides of each buck were pooled and allowed a slow cool to 4°C. Upon reaching 4°C, the pooled epididymal sperm were equally divided and either 6% glycerol (A) or 6% DMSO (B) was added to each sperm sample. After the addition of the cryoprotectant sperm were loaded into 0.5-mL plastic straws and cryopreserved using liquid nitrogen vapor. Total motility (TM) was determined subjectively using an inverted Nikon Diaphot microscope. Membrane integrity (MI) was determined using SYBR 14 and propidium iodide staining under a microscope equipped with epiflurescence. Morphology was determined using an eosin-nigrosin staining. A paired t-test was used for statistical analyses. There were no differences between the glycerol and DMSO treatments for any of the three parameters measured (Table 1). However, post-thaw total motility and membrane integrity were significantly lower for both treatment groups (A and B) when compared with pre-freeze values. Furthermore, the DMSO treatment group had significantly lower normal morphology when compared with pre-freeze values, but was not different than the glycerol treatment group. Our results indicate that cryopreservation of epididymal sperm from White-tail deer with either 6% glycerol or 6% DMSO yield similar results for total motility, membrane integrity, and normal sperm morphology.
|
Tony Vidrine of the Louisiana Department of Wildlife and Fisheries.


