54 IN VITRO AND IN VIVO DEVELOPMENT OF FLAT-HEADED CAT (PRIONAILURUS PLANICEPS) CLONED EMBRYOS
A. Thongphakdee A , S. Manee-in A , N. Klincumhom A , B. Siriaroonrat B , S. Kamolnorranarth B , K. Chatdarong A and M. Techakumphu AA Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand;
B Zoological Park Organization under the Royal Patronage of H.M. the King, Bangkok, Thailand
Reproduction, Fertility and Development 21(1) 127-127 https://doi.org/10.1071/RDv21n1Ab54
Published: 9 December 2008
Abstract
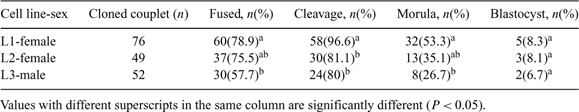
The objectives of the study were to investigate (1) the effect of individual cell line and gender of donor cells on flat-headed cat (FC) cloned embryo production (Study I) and (2) pregnancy establishment of recipients receiving cloned FC embryos with or without domestic cat (DC) IVF embryo co-transfer. The DC IVF embryos were used as a control (Study II). Study I Three cell lines of FC fibroblasts (passage 3–5) collected from 2 females (L1 and L2; biopsied from muscle and skin, respectively) and a male (L3; biopsied from skin) were used as donor cells for nuclear transfer. Donor cells were fused with enucleated in vitro matured DC oocytes. Fused couplets were induced by electrical pulses and subsequently incubated in activation medium containing 10 μg mL–1 cycloheximide and 5 μg mL–1 cytochalasin B for 4 h. Reconstructed embryos were cultured in SOFaa medium supplemented with 5% fetal bovine serum (FBS) at 38.5°C in air, and monitored for 7 days. Differences in the percentages of fusion and embryo development to a particular stage between cell lines and genders of donor cells were determined by chi-square analysis. Variations of fusion efficiency and embryo developmental success were observed between the cell lines. Greater cleavage number (P < 0.05) was observed when L1 was used as donor cells than that of L2 and L3. Developmental success to morula stage of embryo reconstructed from L1 was greater (P < 0.05) than that of L3 but not L2 (P > 0.05). However, there was no difference in the blastocyst formation success among cell lines. The development of the embryos derived from female and male donor cells at subsequent stages was not different. Study II Estrus and ovulation were induced in 15 DC recipients using 100 to 150 IU of pregnant mare serum gonadotropin (PMSG) and 100 IU of hCG (subcutaneous injection). Recipients were divided into 3 groups; (1) cloned group (n = 5) receiving FC cloned embryos (mean 41.4 ± 13), (2) co-transferred group (n = 4) receiving FC cloned and DC IVF embryos (mean 55 ± 15; 43.3 ± 15 of FC cloned and 10.8 ± 1.5 of DC IVF embryos), and (3) IVF/control group (n = 6) receiving only DC IVF embryos (mean 25 ± 9). Control DC IVF embryos were produced by co-incubation of DC oocytes with fresh DC semen for 18 h. Day 1 embryos were transferred into oviducts of recipients. Pregnancy evaluation using ultrasonography at Day 30 post-transfer demonstrated that pregnancy was not observed in any recipients in cloned group. One recipient from co-transferred group became pregnant and delivered DC IVF stillbirths (n = 2) and live kittens (n = 6). All recipients in IVF group became pregnant and 3 recipients delivered 5 DC kittens. These results indicate that (1) the individual cell line but not the gender of donor cells influences the development of FC cloned embryos and (2) with or without co-transfer of FC cloned and DC IVF embryos, FC cloned offspring was not able to be produced in the study.
|
This study was supported by the Zoological Park Organization under the Royal Patronage of H.M. the King, and the Reproductive Biotechnology Research Unit, Chulalongkorn University. A. Thongphakdee is supported by the Royal Golden Jubilee Ph.D. Program, and the Thailand Research Fund.


