271 NUCLEAR MATURATION OF WOOD BISON (BISON BISON ATHABASCAE) CUMULUS–OOCYTE COMPLEXES
M. P. Cervantes A , M. Anzar A , R. J. Mapletoft A , J. M. Palomino A and G. P. Adams AUniversity of Saskatchewan, Saskatoon, Saskatchewan, Canada
Reproduction, Fertility and Development 25(1) 283-283 https://doi.org/10.1071/RDv25n1Ab271
Published: 4 December 2012
Abstract
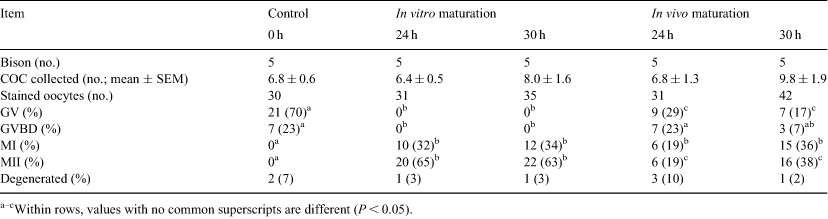
Methods of producing wood bison embryos in vivo and in vitro are being developed in an effort to preserve the genetic diversity of this threatened species. Previous data from our laboratory suggest that oocytes collected 24 h after LH treatment had not yet achieved nuclear maturation. The objectives of this study were (1) to determine the optimal interval of time after hCG treatment required for in vivo maturation of cumulus–oocyte complexes (COC) in wood bison, and (2) to compare the maturational characteristics of COC after in vitro v. in vivo maturation. Follicular wave emergence was synchronized among bison cows (n = 25) by follicular ablation (Day –1) from May to June. Ovarian superstimulation was induced with FSH IM diluted in 5 mg mL–1 of hyaluronan (MAP-5, Bioniche, Belleville, Ontario, Canada) given on Day 0 (300 mg) and Day 2 (100 mg). Superstimulated cows were assigned randomly to 5 groups (n = 5/group): COC collected on Day 4 with no maturation (control), or matured in vitro for 24 or 30 h, or collected 24 or 30 h after treatment with 2000 IU of hCG IM on Day 4. The COC were collected by transvaginal ultrasound-guided follicle aspiration. In vitro maturation was done in TCM-199 with 5% calf serum, 5 µg mL–1 of LH, 0.5 µg mL–1 of FSH, and 0.05 µg mL–1 of gentamicin, at 38.5°C and in 5% CO2. To assess nuclear maturation, oocytes were stained with anti-lamin AC/DAPI (4′,6-diamidino-2-phenylindole). Nuclear stages were classified as germinal vesicle (GV), GV breakdown (GVBD), metaphase I (MI), or metaphase II (MII). Comparisons among groups were made by ANOVA and Fisher’s exact test (Table 1). A mean (± SEM) of 7.6 ± 0.6 COC was collected per bison; no differences were observed among groups (P = 0.37). Cumulus cell expansion was more extensive after in vivo than in vitro maturation, and the percentage of fully expanded COC was highest in the in vivo 30-h group (97%; P < 0.05). No COC were expanded in the control (0 h) group, and none reached MI. Maximal nuclear maturation was achieved in vitro by 24 h; that is, there was no difference in the proportion of MII-stage COC at 24 versus 30 h. However, between 24 and 30 h of in vivo maturation, the percentage of nuclear stages GV + GVBD decreased from 54 to 24% (P < 0.05), whereas nuclear stages MI + MII increased from 39 to 74% (P < 0.05). In conclusion, nuclear maturation occurred earlier in vitro versus in vivo, but the consequences of this difference are unknown. Although more than one-third of oocytes matured in vivo for 30 h were mature enough to permit immediate IVF, whether additional in vivo maturation time would be beneficial to fertilization rates remains to be tested.
|
Thanks to Bioniche Canada.


