Invasive species and their impacts on agri-ecosystems: issues and solutions for restoring ecosystem processes
Peter J. S. Fleming A B D , Guy Ballard B C , Nick C. H. Reid B and John P. Tracey AA Vertebrate Pest Research Unit, New South Wales Department of Primary Industries, Orange Agricultural Institute, 1447 Forest Road, Orange, NSW 2800, Australia.
B Ecosystem Management, School of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia.
C Vertebrate Pest Research Unit, New South Wales Department of Primary Industries, Allingham Street, Armidale, NSW 2350, Australia.
D Corresponding author. Email: peter.fleming@dpi.nsw.gov.au
The Rangeland Journal 39(6) 523-535 https://doi.org/10.1071/RJ17046
Submitted: 17 May 2017 Accepted: 3 October 2017 Published: 28 November 2017
Journal compilation © Australian Rangeland Society 2017 Open Access CC BY-NC-ND
Abstract
Humans are the most invasive of vertebrates and they have taken many plants and animals with them to colonise new environments. This has been particularly so in Australasia, where Laurasian and domesticated taxa have collided with ancient Gondwanan ecosystems isolated since the Eocene Epoch. Many plants and animals that humans introduced benefited from their pre-adaptation to their new environments and some became invasive, damaging the biodiversity and agricultural value of the invaded ecosystems. The invasion of non-native organisms is accelerating with human population growth and globalisation. Expansion of trade has seen increases in purposeful and accidental introductions, and their negative impacts are regarded as second only to activities associated with human population growth. Here, the theoretical processes, economic and environmental costs of invasive alien species (i.e. weeds and vertebrate pests) are outlined. However, defining the problem is only one side of the coin. We review some theoretical underpinnings of invasive species science and management, and discuss hypotheses to explain successful biological invasions. We consider desired restoration states and outline a practical working framework for managing invasive plants and animals to restore, regenerate and revegetate invaded Australasian ecosystems.
Additional keywords: adaptive management, biological invasions, removal, rate of increase.
Introduction
Biological invasions of native ecosystems are pervasive and often degrading, requiring effort for restoration through removal, revegetation and regeneration (Elton 1958). Humans are the most invasive of vertebrates and they have taken many plants and animals with them to colonise new environments (Vitousek et al. 1997; Rotherham and Lambert 2011). This is particularly so in Australasia, where Old World Laurasian and domesticated taxa have collided with ancient and geographically isolated ancient Gondwanan ecosystems, the fauna and flora of which Darwin (1859) noted, were utterly dissimilar in form but analogous in function and trophic position. Plants and animals have also been introduced to New Zealand from Australia, for example, red necked wallabies (Notamacropus rufogriseus) and brush-tailed possums (Trichosurus vulpecula), the latter having devastating impacts on native biota, cattle production and the economy (Nugent et al. 2001).
Many plants and animals that humans introduced benefited from pre-adaptation to their new environments and some became invasive, damaging the biodiversity and agricultural value of the invaded ecosystems. The risk of invasion of non-native organisms is accelerating with human population growth and globalisation (McNeely 2011). Despite excellent quarantine services in both Australia and New Zealand, expansion of trade has seen increases in purposeful and accidental introductions, and their negative impacts are regarded as second only to activities associated with human population growth.
In this paper, we define invasive species and outline and discuss some theoretical underpinnings of invasive species science and management in agri-ecosystems, which we define as all anthropogenically modified ecosystems used for agriculture, both intensive and extensive. We do this to differentiate from the term, ‘agro-ecosystems’, which is often interpreted as highly modified ecosystems affected by agronomic practices. We also discuss existing and new hypotheses to explain successful biological invasions and review some conceptual issues affecting regeneration, revegetation and the restoration of invaded agri-ecosystems. A practical working framework for managing invasive plants and animals is outlined.
What are invasive species?
There are two types of invasive plant and animal species. Most readily understood to be invasive are those alien species that, when introduced, become established and harm human and environmental values (Pimentel 2002; Prins and Gordon 2014a). However, there are also native species that, when conditions are anthropogenically changed, harm those same values, for example, the large macropodids, red kangaroos, Osphranter rufus, and eastern grey kangaroos, Macropus giganteus. Even the iconic koala, Phascolarctos cinereus, has invasive impacts on vegetation in southern Victoria and on Kangaroo Island (Whisson et al. 2012). The impact component is important in this definition; some species have invasive or colonising qualities but are regarded as beneficial, for instance those endemic plants that recolonise after perturbations such as fire, flood, soil disturbance and erosion (Bazzaz 1979). Other examples of invasive species that are usually considered to be beneficial are exotic pasture plants that effectively persist as a productive part of naturalised swards (e.g. Phalaris aquatica) and non-endemic plants used to stabilise soils (e.g. Dactyloctenium australe).
Some theory and hypotheses about biological invasions: three fundamental curves, a rate & 13 hypotheses
Generalised invasion curve
The most frequently presented conceptual function describing the procession of activity classes and returns on investment for managing biological invasions is a sigmoidal curve (Fig. 1, Department of Environment and Primary Industries Victoria 2017), often termed the generalised invasion curve. There are four phases from ‘prevention’ through ‘quarantine’ and other biosecurity measures to ‘asset-based protection’ for established and widespread biological invaders (Braysher 2017).

|
Perusal of the generalised invasion curve reiterates the importance of prevention and eliminating small invasions early before establishment. This is when such actions are more likely to be logistically feasible. If the pest or weed, having escaped biosecurity measures, becomes established, focus can be shifted to containment in regions of establishment to limit the impacts to only those areas. The curve also implies that once an invasive species has become established, investment should be wound back to target the protection of high-value assets. Often, the impacts of the established pest are such that investment must be continuous to protect the assets (Williams et al. 1995; Fleming et al. 2001; Braysher 2017). Although appropriate for established pests and weeds with well-defined ranges, focal distributions and static or slow-moving invasion fronts, this simple concept may be unsuitable for wide-ranging (e.g. wild dogs, Thomson 1992; Claridge et al. 2009; Robley et al. 2010), migratory or widely dispersing species such as plants with seeds that are dispersed by wind (Higgins and Richardson 1999; Nathan and Muller-Landau 2000) or by wide-ranging animals (Cheal and Coman 2003). This is because control programs for these three groups of invasive species require community effort and cannot be adequately addressed by protecting assets at a smaller scale than the home ranges of the pest or the dispersion of their propagules. Solely protecting focal assets in these cases will likely result in broader distribution and greater cost to individuals and the community.
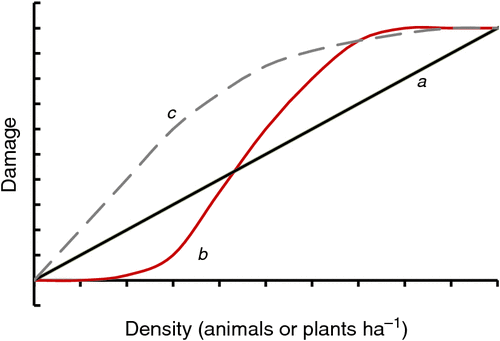
Density : damage functions
Before the economics of managing invasive species can be determined, the shape of the underlying response function to the density of the weed or pest is required (Hone 1994, 2007). These density : damage and density : yield curves describe the incremental increase in impacts or decrease in yield for each incremental increase in pest or weed density (Fig. 2). Multiple hypothetical density : damage functions exist. The simplest, a monotonic linear function, is often implicitly assumed in economic analyses (Hone 1994). More common underlying relationships are curvilinear functions that reach a plateau level over which no further damage is inflicted (e.g. 100% or unharvestable levels), a similar curvilinear function but with a threshold density below which no damage is evident, and sigmoidal curves similar to the generalised invasion curve. To establish these response curves requires measurement of invasive species’ density and the associated response in yield loss. Achieved yields are measured along a gradient of replicated invasive species densities, where those densities are obtained by adding or removing individuals (Caughley 1980), and the data are subsequently analysed by regression. Environmental impacts can also be fitted to density : damage functions where the damage, represented on the y-axis, is the population density of an affected species or another community or ecosystem measurement. The main benefit of these functions is to enable managers to identify break-even points for investment: when to invest, how much effort or expenditure is required to achieve a desired response, and when to stop (Hone 1994).
|
Rate of population increase: 
Practical management of invasive plants and animals involves manipulating the population dynamics through the rate of increase of the invader/s to minimise their adverse impacts (Sibly and Hone 2002). This is often manifest as reducing populations to below the break-even points of density : damage functions.
Rate of population increase is a fundamental concept required to understand population dynamics, and management of invasive species. In the absence of predation or harvesting, the rate at which a population increases is determined by the interaction of its life-history strategy and the quality of environmental conditions (Caughley and Birch 1971). This is the intrinsic rate of increase (rm), the exponential rate at which a demographically stable population grows without resource limits.
There are several other measures that track losses from a population (annual mortality and emigration) and additions to a population (annual births and immigration), including the observed rate of increase (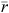 ), which is the exponential rate at which a population grows over a given period of time (Caughley 1980). The observed rate of increase has greater utility for recording the change in density of invasive species when subject to management. In general when:
), which is the exponential rate at which a population grows over a given period of time (Caughley 1980). The observed rate of increase has greater utility for recording the change in density of invasive species when subject to management. In general when:
-
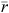 = 0, the population is stable;
= 0, the population is stable; -
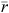 > 0, the population is growing and, for invasive species, potentially expanding, and
> 0, the population is growing and, for invasive species, potentially expanding, and -
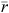 < 0, the population is declining. That is, if the sum of births and immigration are less than the sum of mortality and emigration, the population declines.
< 0, the population is declining. That is, if the sum of births and immigration are less than the sum of mortality and emigration, the population declines.
Control of invasive species is about achieving and maintaining 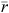 ≤ 0 until a density proportional to an acceptable level of damage is reached.
≤ 0 until a density proportional to an acceptable level of damage is reached.
Invasion population dynamics curves
Although the generalised invasion curve (Fig. 1) conceptualises invasion progression and the relative return for effort of management strategies at different phases, it has several implicit assumptions that may not prevail. Many restoration projects require that the invasive population size as well as the area invaded be reduced below the break-even point of investment for most efficient management and ecosystem recovery (Hone 2007). Another implicit assumption is that investment in a maintenance phase during asset protection from an established invasive species will be cheaper than suppressing invasive populations to much lower levels. Also implicitly, the shape of the density : damage curve is assumed to be linear (a in Fig. 2), which for invasive predators is unlikely (e.g. there is no simple relationship between wild dog density and livestock predation costs: Fleming et al. 2014), and for many invasive plants and animals it is unknown (Hone 1994; Braysher 2017).
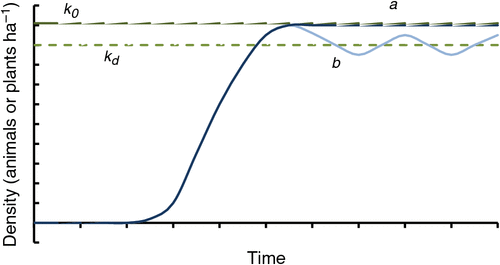
If the y-axis in Fig. 1 is replaced with population size or density, then an invasion population dynamics curve results (Fig. 3, after Caughley 1980).
|
The traditional generalised invasion curve (Fig. 1), which emphasises the investment returns for management effort as invasive species move from incursive to established pests, should be viewed with reference to density : damage curves (Fig. 2) and population dynamics curves (Fig. 3). Where density : damage functions establish break-even points for investment or thresholds above which investment fails to achieve productivity responses, these can also be considered as the desired stocking rate of the pest or weed. The marginal benefit or return on investment for an established pest may be greatest after the regional population is suppressed and returns to levels where containment is possible (Fig. 4).
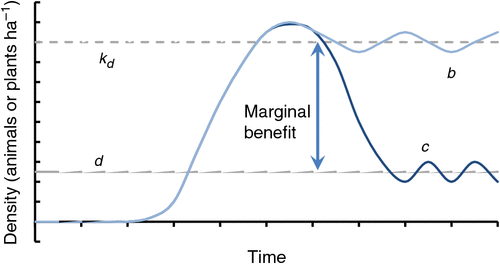
|
Suppression to a new, lower population dynamic is a possible objective when control tools and strategies are efficacious, but requires buy-in from the human community and often landscape-level application. Community buy-in may be the limiting factor and will usually require considerable investment in time and expertise to achieve a level of coverage to align with the size of effective management units (Braysher 2017). This alternative economic model of invasion requires further investigation.
Explanatory hypotheses
Prins and Gordon (2014a) summarised hypotheses that have been proffered to explain successful biological invasions. These hypotheses have been usually expressed as conditions where invasive species can invade, for example ‘Hypothesis 1: a species will not be able to invade an area that has abiotic conditions that are outside its physiological tolerances’ (Prins and Gordon 2014a). Importantly, some of these hypotheses are alternatives whereas others only need one falsification to be legitimate explanations of successful invasion and establishment. To permit these hypotheses to be tested, in order to establish their validity against alternatives, we have rewritten them in a null format (Table 1).

|
The first hypothesis (H1, Table 1) is tautological, and more a limiting statement than a predictor of invasiveness. Surprisingly, five of the articles summarised in Prins and Gordon (2014b) rejected the hypothesis that a species can only invade within its physiological tolerances. If an organism can survive and reproduce outside its physiological tolerances in a new environment, then its presumed tolerances were ipso facto incorrectly delimited.
The conclusion of those that rejected the hypothesis or found no support for it (4/16, Prins and Gordon 2014b) implies a confusion by some authors of physiological experiences with physiological tolerances. A species must be physiologically pre-adapted to the novel environment to survive, let alone reproduce and successfully invade. This does not mean that the source and destination environments have to be similar as implied by bio-climatic matching (e.g. Bomford and O’Brien 1995). For example, physiological features that enable plants to survive in salty air at sea level can decrease frost susceptibility at higher elevations (Seki et al. 2003). Human interferences are also important drivers of vegetation change (Whalley et al. 2011) and such anthropogenic preparation of novel environments can be critical for weed invasions (Ruttledge et al. 2015). Australasia has varied environments and the preadaptation of exotic animals to the abiotic conditions experienced when they were introduced could be expected. For example, rabbits (Oryctolagus cuniculus) are derived from Mediterranean stock and the original Australian and New Zealand propagules were sourced from the United Kingdom, so the spread of rabbits across Australasia would be expected.
The animals that have been introduced have in some instances fitted niches that were not filled when they arrived. For example, cane toads (Rhinella marina) have no Australian amphibian competitors of even approximate equivalence. Coupled with the anthropogenically changed environments into which they were introduced, their success as an invader was almost certain, particularly given that the neotropical abiotic conditions into which they were introduced matched their primary (South America) and secondary (Hawaii) sources (H1, Table 1). New Zealand had no arboreal mammals before the introductions and invasion of brush-tailed possums (Trichosurus vulpecula) from Tasmania, Victoria and possibly New South Wales (Sarre et al. 2014), that is, these were vacant niches that had not been occupied until the invasive species was anthropogenically introduced. These examples seem to support H2 and H3 (Table 1).
The inverse of H0,3 is the competitive exclusion principle (Gause 1934), which suggests that invasive species that occupy a native niche dissimilar to any occupied niche in the new environment are likely to be successful. For examples, feral cats (Felis catus) and red foxes (Vulpes vulpes) were larger than any quoll (Dasyurus) species at the time of their introductions, implying that they occupy niches that were between those of the largest extant quoll (D. maculatus) and dingoes.
The preponderance of marsupials in Australia, the host-specificity of many pathogens and the fact that anthropogenically introduced mammals have all been eutherian, is counter to hypothesis H4 (Table 1). Indeed, the novelty of the introduced hosts to extant pathogens could equally result in the novel ecosystem (i.e. the novel host) being unsuitable for the pathogens. For Australasia, the vast difference in extant parasites, pathogens and predators to those found in the places of origin of the invasive species could mean that it is less likely that the invasive species will be affected by them. Alternatively, the invasive microbes might be more harmful to the extant biota. This is supported by evidence that microbes and pathogens that have entered as passengers of domestic animals can have major impacts on native wildlife (e.g. toxoplasmosis – causal agent Toxoplasma gondii – on marsupial reproduction, Canfield et al. 1990; hydatidosis – causal agent Echinococcus granulosus – on brush-tailed rock-wallabies, Petrogale penicillata, Barnes et al. 2007; and bovine tuberculosis – causal agent Mycobacterium tuberculosis – in New Zealand brush-tailed possums, Nugent et al. 2001).
The fifth hypothesis (Table 1) is an unlikely scenario for Australasia. Most of the Australasian flora and fauna evolved separately in isolation from eutherian carnivores and ungulates, so Australia and New Zealand had no co-evolved prey or forage species for the invaders when they were introduced. Any co-evolved life-cycle-essential species likely immigrated with them, for instance within their guts or on their backs. Co-evolved plants were not necessary for the ungulate livestock and pests or lagomorphs to flourish; they just needed to be similar structurally and nutritionally to the co-evolved species, which supports the null hypothesis H0,5.
The applicability of the sixth hypothesis depends upon H2, H3 and H4. If there are no pathogens or predators, and no niche equivalents in the novel ecosystem, there is no reason to expect rarity in the native environment to preclude invasiveness in the novel environment. For example, koalas are usually uncommon or rare, and are endangered in some native environments. However, koalas can become invasive and destructive when introduced into ecosystems that are naive to them (e.g. Kangaroo Island, Masters et al. 2004). An example from the plant world is Cenchrus ciliaris L. which is becoming endangered in its native Tunisia, but is an environmental weed in parts of Australia and in Texas, USA (Kharrat-Souissi et al. 2014).
Although Hypothesis 7 (Table 1) is intuitively satisfying, its inverse is also likely. That is, when competing for a limiting resource, an introduced species is more likely to prevail because it may have competitive advantages aligned with other hypotheses, such as resistance to pathogens and absence of predators that affect the native species, advantageous life-history characteristics, or temporal niche separation.
Given that anthropogenic activities, such as urbanisation, forestry, agriculture and commercial grazing, usually disturb environments at a similar time to the introduction of new plants and animals, it is sometimes difficult to test whether disturbed habitats are easier to invade than undisturbed habitats (H8 and see H13, Table 1). There are many examples in the weed literature where disturbance favours invasive plant species, for example, altered disturbance situations in wetlands affected the invasion of native water couch communities (Paspalum distichum L.) by the introduced forb lippia (Phyla canescens (Kunth) Greene, Price et al. 2011).
Seedling establishment of many species of plants depends on disturbance of the existing vegetation to allow the germination of seed and the establishment of the resultant seedlings. This principle is well established (Scott 2000; Sheppard 2000) and the elimination of establishment niches is an important principle of weed management in pastures and crops throughout the world. It is also clear that the rate of invasion of weeds will be more rapid in disturbed than in undisturbed areas (H0,8) (Scott 2000; Sheppard 2000). Also, many weeds of pastures invade more rapidly under disturbance by grazing because their low acceptability by livestock increases their competitive advantage over native or sown acceptable grassland species (Medd et al. 1987). However, this may be due to the life-history characteristics of the invaders or coincidence of the introduction and the preparation for its introduction, particularly when considering the invasiveness of pasture plants and the ungulates that eat them.
Australia, being ancient and isolated for extended geological time, provides a site to test the ninth hypothesis that species of older lineage are more likely to be displaced than newer species (Table 1). However, separating the lineage factor from other likely coincident factors, such as those associated with niche overlap and separation, would be difficult.
A more r-selected species is only able to invade where extant niche occupants are less r-selected (H10, Table 1). This hypothesis may explain why bilby species (Macrotis spp.) were usurped by expanding populations of rabbits, which have a greater reproductive capacity. Additionally, we suggest that a species with a flexible life-history, that is, one that can be r-selected or K-selected (Krebs 2014) depending on circumstances, is more likely to be invasive. Among plants, those with back-up reproductive strategies, such as cleistogenes in Chilean needle grass, Nassella neesiana, (Trin. & Rupr.) Barkworth, a pasture invader in Australasia (BourdôT and Hurrell 1989; Gardener et al. 2003), or high fecundity, such as giant Parramatta grass, Sporobolus fertilis (Stued.) Clayton (Andrews et al. 1996), are likely to have a competitive advantage after disturbances.
The likelihood of a species being invasive is unpredictable, happening by chance (H11, Table 1). Prins and Gordon (2014a) suggested this as a possible null hypothesis for all the others, and, hence, formulating a null H0,11 is problematic. Any falsification of this hypothesis indicates that one or more of the previous hypotheses must explain the likelihood of invasion by a species.
In addition to the hypotheses of Prins and Gordon (2014a), we suggest the final two hypotheses, but acknowledge that they require testing for confirmation. We propose these hypotheses in the light of our observations while studying vertebrate pests and weeds: for animals, species with generalist tendencies are more likely to invade than specialists (H12, Table 1), and for plants, early successional plants are more likely to be successful invaders than later ones (H13, Table 1). The latter hypothesis is likely whether successional vegetation change is assumed to be a linear process or a state and transition model (e.g. Westoby et al. 1989) applies, as is the case for Australian grassland systems (Lodge and Whalley 1989; Whalley 1994).
Biological invasions of Australasia
Most ecosystems in Australasia have been affected by human activities, including the facilitation, whether purposeful or accidental, of biological invasions. Many of these are agri-ecosystems (i.e. those that are currently used for or influenced by agricultural activities or previously used for agriculture). Although only 6% of Australia’s land mass is arable, 53% of the total area is currently used for extensive livestock production (Australian Bureau of Statistics 2012) and much of the remainder has, at one time, had sheep or cattle grazed there. Some conservation reserves, even world heritage areas such as Kakadu National Park, have long-established populations of feral ungulates (e.g. feral goats, Capra hircus, Parkes 1993; Russell et al. 2011; feral water buffalo, Bubalus bubalis; feral horses, Equus caballus; banteng Bos javanicus, Edwards et al. 2004) and feral camels, Camelus dromedarius (McGregor and Edwards 2010; Hart and Edwards 2016). Aboriginal and Maori people also managed the land to increase productivity of desirable flora and fauna, and, in consequence, most of the Australia and New Zealand landscapes have been and are affected by human activities and the ecosystem dynamics changed (Morton et al. 1995; Gamage 2011).
Australasian invasive animals
Four groups of eutherian mammals successfully invaded Australia before 1788. First of these were the bats, which could fly across Wallace’s Line (Hand et al. 1994). These were followed by rodent members of the Muridae during the late Miocene, leading to the evolution of 66 native species present in 1788. Humans arrived at least 65 000 years ago (Clarkson et al. 2017) and what invasive passengers and chattels they brought with them is unknown. People were also responsible for the introduction of dingoes, an ancient breed of Canis familiaris introduced from South-East Asia ~4500 ybp (Jackson and Groves 2015; Jackson et al. 2017), that subsequently became feral (Fleming et al. 2014) and invaded most Australian environments (Johnson and Letnic 2014). The Maori people brought the now extinct Polynesian dog and Polynesian rats (Rattus exulans) to New Zealand ~650–700 ybp (Matisoo-Smith and Robins 2004).
In Australia, from 1788 until 1998, established invasions included 27 bird species, four reptiles, seven fish and one amphibian, the cane toad (Bomford and Hart 2002). Jackson and Groves (2015) list 33 established species of mammals and Bomford and Hart (2002), 28. Europeans brought 28 mammals, nine fish and six birds that have become established pests in New Zealand over the past 200 years (Clout 2002).
Australasian naturalised invasive plants
The number of invasive native and alien flora species of Australia (Groves 2002) and New Zealand (Webb et al. 1988) are close to parity in both countries. Some 2681 plant species are recognised as naturalised in Australia and ~2200 in New Zealand (Norton 2009). Not all naturalised species are aggressively invasive but all affect Australasian ecosystems to some degree. Most alien plants were deliberately introduced: grasses and forbs for livestock production (Cook and Dias 2006) and many ornamental plants. In addition, others were introduced accidentally as passengers of immigrant people and visitors.
Introduced plant species may remain in low abundance in their new host country before suddenly increasing in abundance and becoming a problem to manage. These have been given the name ‘sleeper weeds’ (Groves 2006) and it is often difficult to predict whether a particular species will become a weed in the future (Groves 2006).
So what can we do about invasive species?
A strategic approach: passive adaptive management
The most practical approach that has been brought to bear on the seemingly intractable issues of managing invasive species is the implementation of adaptive management (Holling 1978; Walters and Hilborn 1978; Walters and Holling 1990). Adaptive management permits the managers of pests and weeds to start with a working model then iteratively improve it by systematically acquiring reliable information to assist in decision making.
There are three types of adaptive management (Walters and Hilborn 1978; Walters and Holling 1990). In order of increasing information, inference and efficiency these are evolutionary, passive adaptive and active adaptive management. The latter involves a formal experiment that is imposed on the managed system. For example, a weed control strategy for an aquatic system might involve a multifactorial experimental design where treatments and their combinations are applied to replicate dams or waterways and contrasted with untreated invaded ecosystems. The relative efficacies and cost-efficiencies of each tool and strategy are measured to provide better information about how to cost-effectively manage the problem. Importantly, the required budget that is necessary to make a substantial reduction in negative impacts, such as oxygen depletion and subsequent change in aquatic faunal communities, can be determined. Without such information the risk is that, in an effort to be ‘doing something’, managers spend inadequate money to achieve real improvements (e.g. water hyacinth, Eichhornia crassipes: Villamagna and Murphy 2010).
In Australasia, the passive form of adaptive management (Walters and Hilborn 1978; Walters and Holling 1990) is more common than other types (Braysher 2017). It typically involves the use of historical data to form a single working model of management, which is modified as better information about system ecology, control tool efficacy and application comes to hand from a quasi-experimental framework. The system is considered passive because a formal, controlled randomised experimental intervention is often logistically or socially impossible (e.g. wild canid management, Fleming et al. 2014). A major advantage of the process is increased ownership of the invasive species issue and its solutions by stakeholders such that their on-going involvement in the process is more likely (Chapple et al. 2011; Braysher 2017). A further development of adaptive management principles for weeds is Integrated Weed Management (Sindel 2000).
Fleming et al. (2014) and Braysher (2017) presented a simple explanatory flow chart to lead managers through the principles of strategic management in a passive adaptive management framework (Fig. 5). Fleming et al. (2014) also provided detail that is pertinent to practical management of invasive species, including the crucial human dimension (McNeely 2001; Pejchar and Mooney 2009).
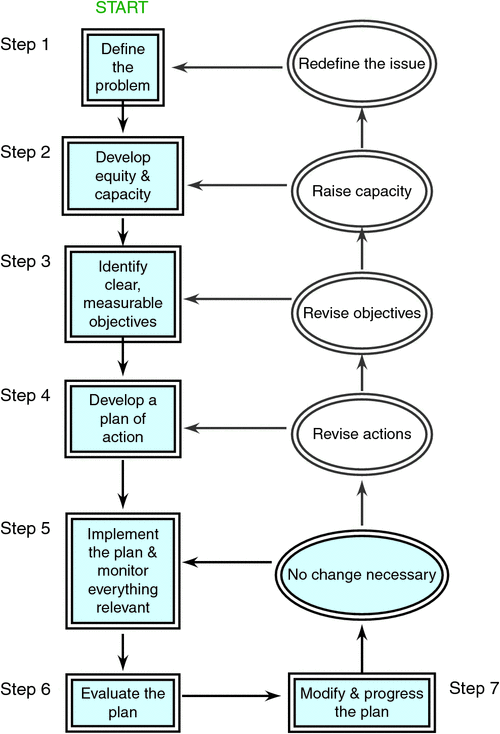
|
The critical components of passive adaptive management are problem definition, the development of equity and capacity, and monitoring. The problem definition step is most important because it identifies what the problem is, where it is, where it comes from, who has the problem, when it occurs and how critical it is. A useful way of defining the issue of invasiveness to be managed is to regard the positive, neutral and negative impacts of a species and the situation in which it occurs (Jarman 1990; Table 2 after Allison 2011).

|
A major difficulty in defining the issue for invasive plants is that some introduced species only become invasive after many years, i.e. sleeper weeds (Groves 2006). For example, Coolatai grass (Hyparrhenia hirta (L.) Stapf) was introduced into Northern NSW in the 1890s, was planted along roadsides for erosion control in the 1940s and only became recognised as an invasive weed in the late 1980s (Chejara et al. 2015).
Determining the human capacity of stakeholders is an important part of the issue definition stage. Human capacity to act effectively is multi-faceted but three common determinants are: knowledge of the problem and what to do to solve it, sufficient financial resources to do something useful about the problem, and the time to enact the practical aspects of management. If any of these three elements is missing, management will be suboptimal and the invasive species will continue to prosper. As an example, a comprehensive survey of landholders revealed that the majority of residential professional farmers in the Northern Tablelands were well aware of the problem of serrated tussock (Nasella trichotoma (Nees) Hack. Ex Arechav.) invasion and could recognise the species (Ruttledge et al. 2015). However, the majority of non-professional or absentee farmers generally did not have this knowledge or ability. In addition, the latter group almost universally did not adopt biosecurity precautions to control the spread of seeds by livestock, vehicles and machinery. The prognosis, therefore, was that without effectively engaging these people and increasing the knowledge component of their capacity, the continued spread of this species on the Northern Tablelands of NSW is inevitable (Ruttledge et al. 2015).
Monitoring is the final essential ingredient in effective management of invasive species. Without monitoring of management actions and the responses to them, no evidence of regeneration, revegetation or restoration of ecological processes and ecosystems can be demonstrated.
Invasive species research and management progress
The strategic approach outlined in Fig. 5 encompasses iteratively increasing knowledge about the invasive species issue and its solutions. The study and management of invasive species, and, indeed, the study of their management, broach many disciplines (Fig. 6).
Knowledge development, in the many fields identified (Fig. 6), is critical to defining the issue (Step 1, Fig. 5), building capacity (Step 2, Fig. 5), as well as determining the appropriate technologies and their application for restoring ecological processes where invasive species have been detrimental (Steps 3 and 4, Fig. 5). Ecology, invasion biology, agricultural sciences, conservation science, and human dimensions are central to this. However, all the other disciplines are important, which indicates that collaboration is essential both for useful research and effective management of invasive species.
Managing invasive species to restore ecological processes
Conceptual undercurrents
There are conceptual undercurrents implied in the three themes of the RRR conference when addressing invasive species impacts on ecosystem processes. First let us define the three themes: restoration is the repairing of an ecosystem by moving it to a prior desired state; regeneration means to generate again by re-establishment of the desired state by generating it from propagules within, and to revegetate is to reinstate vegetation with propagules from outside the system (i.e. an active process).
Issues: restoration
The primary questions here are to which state is the agri-ecosystem to be restored, and is restoration possible? Australasian ecosystems before Aboriginal, Maori and European invasions were dynamic (Johnson 2006). They now have new dynamics, with abiotic drivers that are essentially similar to those that have prevailed since the conclusion of the last Ice Age. However, climate change is affecting temperatures, rainfall totals, intensity and patterns, and will have consequences for Australasian ecosystems.
Some changes to biota have been and are desirable for human wellbeing since European settlement. Agricultural production primarily uses animal and plant species that are novel to Australasian ecosystems. There were no ungulates in either Australia or New Zealand until Europeans brought them, although it is possible that Asian wild boar (Sus scrofa) were introduced from South East Asia via Cape York before European introduction of Asian and European domestic pigs (Gongora et al. 2004). Ungulates were behaviourally, reproductively and, most importantly, morphologically different from any animals in Australasia. This led to impacts on soils and vegetation (e.g. piospheres of adverse impacts around permanent waters, Landsberg and Stol 1996) that were not evident from the largest extant marsupial herbivores. The interaction of ungulate domesticates with purposely introduced and naturalised forage species, as well as preferred native forage plants, is an essential component of profitable livestock production. Do we include these components of the new agri-ecosystem in the desired state for restoration?
Eradication of invasive species is rare and mainly demonstrated on islands (e.g. rodents on islands, Howald et al. 2007; feral cats and rabbits on Macquarie Island, (Robinson and Copson 2014; Sindel et al. 2017) where the scale is logistically tractable. No established invasive vertebrate or plant has been eradicated from a continent because efforts usually fail to meet three essential criteria (Bomford and O’Brien 1995). These are:
-
The rate of removal must be greater than
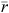 at all population densities;
at all population densities; -
Immigration must be zero; and
-
All reproductive animals (or plants and their seeds) must be at risk of control tool/s and strategies.
The finding and removal of the last few plants of a target invasive plant species (i.e. a weed) in complex vegetation is often impossible or prohibitively expensive (Gardener et al. 2010). The eradication of weeds can be more difficult than eradication of invasive animals because of long-lived seed banks that are impossible to find and remove or render unviable. In addition, the impact of the weed species on ecological processes within the region of occurrence must be determined, and if these effects are not an apocalyptic threat to biodiversity, then the cost of eradication is hard to justify (Davis et al. 2011).
Bomford and O’Brien (1995) listed a further three preferred criteria:
-
The animals (or plants and their propagules) can be monitored at low densities;
-
The discounted benefit : cost analysis favours eradication over ongoing suppression; and
-
The socio-political environment is suitable.
The final criterion is also critical for gaining acceptance of weed and pest animal management technologies and strategies. Apart from occasional lay and scientist contrarians (so described by Simberloff 2011), it is likely that the prevailing societal attitudes towards and perceptions of invasive species are negative. If true, this attitude will aid acceptance of the need for population reductions and eradications of invasive species for ecological restorations.
If eradication is essential for restoration of ecological processes, then in such cases restoration may be impossible. For example, the regeneration of drooping she-oak, Allocasuarina verticillata, and sweet bursaria, Bursaria spinosa, in arid ecosystems in South Australia cannot occur while rabbits occur at densities of ≥0.5 ha–1 (Bird et al. 2012). Without recruitment of the structural backbones of the ecosystem, it is destined for degradation and local extinction of dependent native fauna. Even livestock, as arguably desirable components of the new ecosystem, might be negatively impacted upon because of loss of shade and browse forage.
Issues: regeneration
Regeneration is a simpler concept; it is the encouragement of generation to increase populations within the degraded ecosystem. Invasive incursions by animals degrade systems and often reduce seedbanks or reduce recruitment by preying on juveniles. Invasive plants can outcompete and increase the relative abundance of their propagules at the expense of the extant natives or desirable introduced pasture species. In such cases, removal or reduction of the population of the invader is required to halt further degradation and permit regeneration.
Exclusion plots (e.g. Lunt et al. 2007) and exclusion fencing (e.g. Doupé et al. 2010) also allow regeneration of both plant and animal communities from remnant propagules, but have received some criticism (Hayward and Kerley 2009). These exclosures often demonstrate that invasion must be prevented for regeneration to occur and that contemporaneous removal of the invader is an essential component of regeneration. However, it could be pragmatic to accept the new, altered state and less regeneration of desired ecosystem components (i.e. containment and asset protection in Fig. 1).
Issues: revegetation
This is also a deceptively simple concept; that is, the reinstatement of desirable vegetation where it has been removed or outcompeted. Revegetation can be prevented by invasive native and alien animals, and invasive plants. In New Zealand, for example, the floristic assemblage of native forests is altered by selective browsing on preferred species by brush-tailed possums and regeneration of preferred species is only possible when considerable and regular control effort is exerted on the possums (Gormley et al. 2012). In those systems, continuous control effort will be required to suppress possum populations to maintain the forest species mix and dynamics (Gormley et al. 2012): such investment may not be possible in the long term and the new forest structure, assemblage and dynamics after selective browsing by possums might be inevitable.
The residual negative impacts of invasions, i.e. ecosystem degradation, can also affect the likelihood of successful regeneration. The re-establishment of Australian native plant species in revegetation programs, whether trees, shrubs or herbaceous species following landscape degradation, is often very difficult, particularly on shrink-swell vertosols common in parts of the continent (Watt and Whalley 1982a, 1982b; Waters et al. 2000; Chivers and Raulings 2009; Mitchell et al. 2015; Talonia et al. 2017). The seeds of many native Australian grasses and other herbaceous species germinate readily but the seedlings are initially slow growing and are very susceptible to weed competition (Barrett-Lennard et al. 1991; Waters et al. 2000; Chivers and Raulings 2009). In addition, these soils provide an inhospitable environment for seedling establishment as do the soils in many other degraded landscapes (Watt and Whalley 1982a, 1982b; Barrett-Lennard et al. 1991; Talonia et al. 2017).
Additionally, introduced plants can provide the same ecosystem services as extant natives: trees can provide shade (Bird et al. 1993), forage (e.g. nut crops for endangered regent parrots; Tracey and Fleming 2008), nest sites and nutrient recycling; shrubs can provide forage and cover; and grasses and forbs provide forage eaten by native animals, insects and livestock. Effectively, we have created new agri-ecosystems consisting of native and naturalised species with neutral effects, or that are beneficial for agricultural production, and of invasive native and introduced plants that are detrimental for production and environmental values. The determination of what states are most desirable requires value judgements: how much regeneration is to be encouraged, is revegetation to be active or indirect through suppression of invasive species, and what ecosystem is to be restored?
Conclusions
Restoring ecological processes through regeneration and revegetation encapsulates conservation activities and conservation science is based on principles of population and community ecology. The conservation of a population, community or ecosystem that is potentially threatened by invasive species requires first that its persistence be evaluated, either by measuring and monitoring changes, or by making observations of like systems previously exposed to the biological invader.
Because the requirements for eradication are rarely met except for plants and animals detected early on during an incursion (Fig. 1, Bomford and O’Brien 1995), most invasive species are here to stay. Therefore, management to remove or reduce impacts of invasive species will be ongoing. An adaptive management approach provides a workable framework that is readily adopted by stakeholders because it will involve them in applying the best practice and lessons about how to better deal with the problem animal or plant through the process.
We must decide whether the impacts of an invasive species require rectification or acceptance as a new part of the biota, naturalised and adding the ecological equivalent of multiculturalism. These are new agri-ecosystems with naturalised plants and animals that have a range of impacts from positive through neutral to negative. As a rule of thumb, positive values and impacts should be encouraged, negative impacts should be discouraged (usually through population suppression and exclusion) and no action is needed for neutral effects.
The human dimensions must be measured, evaluated and encapsulated in any invasive species management program or we will always fail (Gunderson 1999; Chapple et al. 2011). If the impacts are net detrimental (i.e. the sum of the deficits outweighs the sum of the benefits, if any), Australasians must decide if the best benefit : cost ratio is achieved by protecting environment, cultural and agricultural assets, as is suggested in the oft-cited generalised invasion curve (Fig. 1), or by applying the substantial investment required to push the state of the invasion from asset protection back to eradication or containment as we suggest (Fig. 4). We suspect that the marginal gains of this latter strategy, which changes the investment from costly suppression to manageable maintenance, will result in the best long-term benefit : cost ratio, more effective and sustainable restoration of ecosystem processes, and better environmental, agricultural and societal wellbeing.
Conflicts of interest
The authors declare no conflicts of interests.
Acknowledgements
This presentation and paper benefited from discussions with many people over the years, including but not exclusive to: Jim Hone, Mike Braysher, Glen Saunders, Andrew McConnachie, Kerinne Harvey, Mary Bomford, Bob Harden, Quentin Hart, Ben Russell, Guy Robinson, Wal Whalley, Paul Meek, Peter Caley, Tony Pople, John Fleming and the late David Choquenot. The comments of the editor and two anonymous reviewers improved the manuscript. The authors receive funding from the Invasive Animals Cooperative Research Centre.
References
Allison, S. K. (2011). The paradox of invasive species: do restorationists worry about them too much or too little? In: ‘Invasive and Introduced Plants and Animals: Human Perceptions, Attitudes, and Approaches to Management’. (Eds I. D. Rotherham and R. Lambert.) pp. 265–275. (Earthscan Press: London, UK.)Andrews, T. S., Whalley, R. D. B., and Jones, C. E. (1996). Seed production and seedling emergence of giant Parramatta grass on the North Coast of New South Wales. Australian Journal of Experimental Agriculture 36, 299–308.
| Seed production and seedling emergence of giant Parramatta grass on the North Coast of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Australian Bureau of Statistics (2012). 7121.0 – Agricultural Commodities, Australia, 2010–11. Available at: www.abs.gov.au/ausstats/abs@.nsf/Products/7121.0~2010-11~Main+Features~Land+Use?OpenDocument (accessed 15 March 2017).
Barnes, T. S., Hinds, L. A., Jenkins, D. J., and Coleman, G. T. (2007). Precocious development of hydatid cysts in a macropodid host. International Journal for Parasitology 37, 1379–1389.
| Precocious development of hydatid cysts in a macropodid host.Crossref | GoogleScholarGoogle Scholar |
Barrett-Lennard, E. G., Frost, F., Vlahos, S., and Richards, N. (1991). Revegetating salt-affected lands with shrubs. Journal of Agriculture Western Australia 32, 124–129.
Bazzaz, F. A. (1979). The physiological ecology of plant succession. Annual Review of Ecology and Systematics 10, 351–371.
| The physiological ecology of plant succession.Crossref | GoogleScholarGoogle Scholar |
Bird, P. R., Bicknell, D., Bulman, P. A., Burke, S. J. A., Leys, J. F., Parker, J. N., van der Sommen, F. J., and Voller, P. (1993). The role of shelter in Australia for protecting soils, plants and livestock. In: ‘The Role of Trees in Sustainable Agriculture: Review papers presented at the Australian Conference, The Role of Trees in Sustainable Agriculture’. Albury, Victoria, Australia, October 1991. (Ed. R. T. Prinsley.) pp. 59–86. (Springer: Dordrecht, The Netherlands.)
Bird, P., Mutze, G., Peacock, D., and Jennings, S. (2012). Damage caused by low-density exotic herbivore populations: the impact of introduced European rabbits on marsupial herbivores and Allocasuarina and Bursaria seedling survival in Australian coastal shrubland. Biological Invasions 14, 743–755.
| Damage caused by low-density exotic herbivore populations: the impact of introduced European rabbits on marsupial herbivores and Allocasuarina and Bursaria seedling survival in Australian coastal shrubland.Crossref | GoogleScholarGoogle Scholar |
Bomford, M., and Hart, Q. (2002). Non-indigenous vertebrates in Australia. In: ‘Biological Invasions: Economic and Environmental Costs of Alien Plant, Animal and Microbe species’. (Ed. D. Pimentel.) pp. 25–44. (CRC Press: New York.)
Bomford, M., and O’Brien, P. (1995). Eradication or control for vertebrate pests? Wildlife Society Bulletin 23, 249.
BourdôT, G. W., and Hurrell, G. A. (1989). Cover of Stipa neesiana Trin. & Rupr. (Chilean needle grass) on agricultural and pastoral land near Lake Grassmere, Marlborough. New Zealand Journal of Botany 27, 415–420.
| Cover of Stipa neesiana Trin. & Rupr. (Chilean needle grass) on agricultural and pastoral land near Lake Grassmere, Marlborough.Crossref | GoogleScholarGoogle Scholar |
Braysher, M. (2017). ‘Managing Australia’s Pest Animals: A Guide to Strategic Planning and Effective Management.’ (CSIRO Publishing: Melbourne.)
Canfield, P. J., Hartley, W. J., and Dubey, J. P. (1990). Lesions of toxoplasmosis in Australian marsupials. Journal of Comparative Pathology 103, 159–167.
| Lesions of toxoplasmosis in Australian marsupials.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M%2FmtVOktA%3D%3D&md5=c23abb1083c7e88725d15a8b700828a0CAS |
Caughley, G. (1980). ‘Analysis of Vertebrate Populations.’ Reprinted with corrections. (John Wiley and Sons: London, UK.)
Caughley, G., and Birch, L. C. (1971). Rate of increase. The Journal of Wildlife Management 35, 658–663.
| Rate of increase.Crossref | GoogleScholarGoogle Scholar |
Chapple, R. S., Ramp, D., Bradstock, R. A., Kingsford, R. T., Merson, J. A., Auld, T. D., Fleming, P. J. S., and Mulley, R. C. (2011). Integrating science into management of ecosystems in the Greater Blue Mountains. Environmental Management 48, 659–674.
| Integrating science into management of ecosystems in the Greater Blue Mountains.Crossref | GoogleScholarGoogle Scholar |
Cheal, D., and Coman, B. (2003). Pest plants and animals. In: ‘Ecology: an Australian Perspective’. (Eds P. Attiwill and B. Wilson.) pp. 457–473. (Oxford University Press: South Melbourne.)
Chejara, V. K., Kristiansen, P., Sindel, B. M., Johnson, S. B., Whalley, R. D. B., and Nadolny, C. (2015). The biology of Australian weeds 64. Hyparrhenia hirta (L.) Stapf. Plant Protection Quarterly 30, 2–11.
Chivers, I. H., and Raulings, K. A. (2009). ‘Australian Native Grasses: A Manual for Sowing, Growing and Using Them.’ (Native Seeds P/L: Melbourne.)
Claridge, A. W., Mills, D. J., Hunt, R., Jenkins, D. J., and Bean, J. (2009). Satellite tracking of wild dogs in south-eastern mainland Australian forests: Implications for management of a problematic top-order carnivore. Forest Ecology and Management 258, 814–822.
| Satellite tracking of wild dogs in south-eastern mainland Australian forests: Implications for management of a problematic top-order carnivore.Crossref | GoogleScholarGoogle Scholar |
Clarkson, C., Jacobs, Z., Marwick, B., Fullagar, R., Wallis, L., Smith, M., Roberts, R. G., Hayes, E., Lowe, K., Carah, X., Florin, S. A., McNeil, J., Cox, D., Arnold, L. J., Hua, Q., Huntley, J., Brand, H. E. A., Manne, T., Fairbairn, A., Shulmeister, J., Lyle, L., Salinas, M., Page, M., Connell, K., Park, G., Norman, K., Murphy, T., and Pardoe, C. (2017). Human occupation of northern Australia by 65,000 years ago. Nature 547, 306–310.
| Human occupation of northern Australia by 65,000 years ago.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXhtF2qurrI&md5=a919b32cc1c2533d0c15f47b28a71219CAS |
Clout, M. N. (2002). Ecological and economic costs of alien vertebrates in New Zealand. In: ‘Biological Invasions: Economic and Environmental Costs of Alien Plant, Animal and Microbe Species’. (Ed. D. Simberloff.) pp. 185–193. (CRC Press: New York.)
Cook, G. D., and Dias, L. (2006). It was no accident: deliberate plant introductions by Australian government agencies during the 20th century. Australian Journal of Botany 54, 601–625.
| It was no accident: deliberate plant introductions by Australian government agencies during the 20th century.Crossref | GoogleScholarGoogle Scholar |
Darwin, C. (1859) (republished 1998). ‘On the Origin of Species by Means of Natural Selection or, the Preservation of Favoured Races in the Struggle for Life.’ (Wordsworth Editions: Ware, Hertfordshire, UK.)
Davis, M. A., Chew, M. K., Hobbs, R. J., Lugo, A. E., Ewel, J. J., Vermeij, G. J., Brown, J. H., Rosenzweig, M. L., Suding, K. N., Ehrenfeld, J. G., Grime, J. P., Mascaro, J., and Briggs, J. C. (2011). Don’t judge species on their origins. Nature 474, 153–154.
| Don’t judge species on their origins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntFKltrs%3D&md5=9bd28bd7176e9eb56e328282b78fc0d3CAS |
Department of Environment and Primary Industries Victoria (2017). ‘Protecting Victoria from Pest Animals.’ (The State of Victoria: Melbourne, Vic.)
Doupé, R. G., Mitchell, J., Knott, M. J., Davis, A. M., and Lymbery, A. J. (2010). Efficacy of exclusion fencing to protect ephemeral floodplain lagoon habitats from feral pigs (Sus scrofa). Wetlands Ecology and Management 18, 69–78.
| Efficacy of exclusion fencing to protect ephemeral floodplain lagoon habitats from feral pigs (Sus scrofa).Crossref | GoogleScholarGoogle Scholar |
Edwards, G. P., Pople, A. R., Saalfeld, K., and Caley, P. (2004). Introduced mammals in Australian rangelands: future threats and the role of monitoring programmes in management strategies. Austral Ecology 29, 40–50.
| Introduced mammals in Australian rangelands: future threats and the role of monitoring programmes in management strategies.Crossref | GoogleScholarGoogle Scholar |
Elton, C. S. (1958). ‘The Ecology of Invasion by Plants and Animals.’ (Methguen: London, UK.)
Fleming, P., Corbett, L., Harden, B., and Thomson, P. (2001). ‘Managing the Impacts of Dingoes and Other Wild Dogs.’ (Bureau of Rural Sciences: Canberra, ACT.)
Fleming, P. J. S., Allen, B. L., Allen, L. R., Ballard, G., Bengsen, A. J., Gentle, M. N., McLeod, L. J., Meek, P. D., and Saunders, G. R. (2014). Management of wild canids in Australia: free-ranging dogs and red foxes. In: ‘Carnivores of Australia: Past, Present and Future’. (Eds A. S. Glen and C. R. Dickman.) pp. 105–149. (CSIRO Publishing: Melbourne.)
Gamage, B. (2011). ‘The Biggest Estate on Earth: How Aborigines made Australia.’ (Allen & Unwin: Sydney, NSW.)
Gardener, M. R., Whalley, R. D. B., and Sindel, B. M. (2003). Ecology of Nassella neesiana, Chilean needle grass, in pastures on the Northern Tablelands of New South Wales. I. Seed production and dispersal. Crop & Pasture Science 54, 613–619.
| Ecology of Nassella neesiana, Chilean needle grass, in pastures on the Northern Tablelands of New South Wales. I. Seed production and dispersal.Crossref | GoogleScholarGoogle Scholar |
Gardener, M. R., Cordell, S., Anderson, M., and Tunnicliffe, R. D. (2010). Evaluating the long-term project to eradicate the rangeland weed Martynia annua L.: linking community with conservation. The Rangeland Journal 32, 407–416.
| Evaluating the long-term project to eradicate the rangeland weed Martynia annua L.: linking community with conservation.Crossref | GoogleScholarGoogle Scholar |
Gause, G. F. (1934). ‘The Struggle for Existence.’ (Williams and Wilkins: Baltimore, MD.)
Gongora, J., Fleming, P., Spencer, P. B. S., Mason, R., Garkavenko, O., Meyer, J. N., Droegemueller, C., Lee, J. H., and Moran, C. (2004). Phylogenetic relationships of Australian and New Zealand feral pigs assessed by mitochondrial control region sequence and nuclear GPIP genotype. Molecular Phylogenetics and Evolution 33, 339–348.
| Phylogenetic relationships of Australian and New Zealand feral pigs assessed by mitochondrial control region sequence and nuclear GPIP genotype.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntVKqurg%3D&md5=ae9e6d273decefd0481700253c10586dCAS |
Gormley, A. M., Holland, E. P., Pech, R. P., Thomson, C., and Reddiex, B. (2012). Impacts of an invasive herbivore on indigenous forests. Journal of Applied Ecology 49, 1296–1305.
| Impacts of an invasive herbivore on indigenous forests.Crossref | GoogleScholarGoogle Scholar |
Groves, R. H. (2002). The impacts of alien plants in Australia. In: ‘Biological Invasions: Economic and Environmental Costs of Alien Plant, Animal and Microbe Species’. (Ed. D. Simberloff.) pp. 11–23. (CRC Press: New York.)
Groves, R. H. (2006). Are some weeds sleeping? Some concepts and reasons. Euphytica 148, 111–120.
| Are some weeds sleeping? Some concepts and reasons.Crossref | GoogleScholarGoogle Scholar |
Gunderson, L. (1999). Resilience, flexibility and adaptive management- antidotes for spurious certitude? Conservation Ecology 3, 7.
Hand, S. J., Novacek, M., Godthelp, H., and Archer, M. (1994). First Eocene bat from Australia. Journal of Vertebrate Paleontology 14, 375–381.
| First Eocene bat from Australia.Crossref | GoogleScholarGoogle Scholar |
Hart, Q., and Edwards, G. (2016). Guest Editorial: outcomes of the Australian feral camel management project. The Rangeland Journal 38, i–iii.
| Guest Editorial: outcomes of the Australian feral camel management project.Crossref | GoogleScholarGoogle Scholar |
Hayward, M. W., and Kerley, G. I. H. (2009). Fencing for conservation: Restriction of evolutionary potential or a riposte to threatening processes? Biological Conservation 142, 1–13.
| Fencing for conservation: Restriction of evolutionary potential or a riposte to threatening processes?Crossref | GoogleScholarGoogle Scholar |
Higgins, S. I., and Richardson, D. M. (1999). Predicting plant migration rates in a changing world: The role of long-distance dispersal. American Naturalist 153, 464–475.
Holling, C. S. (1978). ‘Adaptive Environmental Assessment and Management.’ Wiley International Series on Applied Systems Analysis, 3. (John Wiley & Sons: London, UK.)
Hone, J. (1994). ‘Analysis of Vertebrate Pest Control.’ (Cambridge University Press: Cambridge, UK.)
Hone, J. (2007). ‘Wildlife Damage Control.’ (CSIRO Publishing: Melbourne.)
Howald, G., Donlan, C., Galván, J. P., Russell, J. C., Parkes, J., Samaniego, A., Wang, Y., Veitch, D., Genovesi, P., Pascal, M., and Saunders, A. (2007). Invasive rodent eradication on islands. Conservation Biology 21, 1258–1268.
| Invasive rodent eradication on islands.Crossref | GoogleScholarGoogle Scholar |
Jackson, S. M., and Groves, C. P. (2015). Dingo. In: ‘Taxonomy of Australian Mammals’. (Eds S. M. Jackson and C. P. Groves.) pp. 287–289. (CSIRO Publishing: Melbourne.)
Jackson, S. M., Groves, C. P., Fleming, P. J. S., Aplin, K. P., Eldridge, M. D. B., Gonzalez, A., and Helgen, K. M. (2017). The wayward dog: Is the Australian native dog or Dingo a distinct species? Zootaxa 4317, 201–224.
| The wayward dog: Is the Australian native dog or Dingo a distinct species?Crossref | GoogleScholarGoogle Scholar |
Jarman, P. (1990). Bird pest research: the gap between research and application. In: ‘National Bird Post Workshop Proceedings’. University of New England, Armidale, 8–9 February 1990. (Eds P. Fleming, I. Temby and J. Thompson.) pp. 7–12. (NSW Agriculture & Fisheries: Glen Innes, NSW.)
Johnson, C. (2006). ‘Australia’s Mammal Extinctions: a 50000-year History.’ (Cambridge University Press: Cambridge, UK.)
Johnson, C. N., and Letnic, M. (2014). Introducing a new top predator, the dingo. In: ‘Invasion Biology and Ecological Theory: Insights from a Continent in Transformation’. (Eds H. H. T. Prins and I. J. Gordon.) pp. 414–428. (Cambridge University Press: Cambridge, UK.)
Kharrat-Souissi, A., Siljak-Yakovlev, S., Brown, S. C., Baumel, A., Torre, F., and Chaieb, M. (2014). The polyploid nature of Cenchrus ciliaris L. (Poaceae) has been overlooked: new insights for the conservation and invasion biology of this species – a review. The Rangeland Journal 36, 11–23.
| The polyploid nature of Cenchrus ciliaris L. (Poaceae) has been overlooked: new insights for the conservation and invasion biology of this species – a review.Crossref | GoogleScholarGoogle Scholar |
Krebs, C. J. (2014). ‘Ecology: The Experimental Analysis of Distribution and Abundance.’ 6th edn. (Pearson Education Limited: Harlow, UK.)
Landsberg, J., and Stol, J. (1996). Spatial distribution of sheep, feral goats and kangaroos in woody rangeland paddocks. The Rangeland Journal 18, 270–291.
| Spatial distribution of sheep, feral goats and kangaroos in woody rangeland paddocks.Crossref | GoogleScholarGoogle Scholar |
Lodge, G. M., and Whalley, R. D. B. (1989). ‘Native and natural pastures on the Northern Slopes and Tablelands of New South Wales – a review and annotated bibliography.’ Technical Bulletin No. 35. (NSW Agriculture and Fisheries: Orange, NSW.)
Lunt, I. D., Jansen, A. M. Y., Binns, D. L., and Kenny, S. A. (2007). Long-term effects of exclusion of grazing stock on degraded herbaceous plant communities in a riparian Eucalyptus camaldulensis forest in south-eastern Australia. Austral Ecology 32, 937–949.
| Long-term effects of exclusion of grazing stock on degraded herbaceous plant communities in a riparian Eucalyptus camaldulensis forest in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Masters, P., Duka, T., Berris, S., and Moss, G. (2004). Koalas on Kangaroo Island: from introduction to pest status in less than a century. Wildlife Research 31, 267–272.
| Koalas on Kangaroo Island: from introduction to pest status in less than a century.Crossref | GoogleScholarGoogle Scholar |
Matisoo-Smith, E., and Robins, J. H. (2004). Origins and dispersals of Pacific peoples: evidence from mtDNA phylogenies of the Pacific rat. Proceedings of the National Academy of Sciences of the United States of America 101, 9167–9172.
| Origins and dispersals of Pacific peoples: evidence from mtDNA phylogenies of the Pacific rat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltlWqtrY%3D&md5=27acbbad43a8cc5649700f7c88fd0500CAS |
McGregor, M., and Edwards, G. (2010). Guest Editorial: managing the impacts of feral camels. The Rangeland Journal 32, i–iii.
| Guest Editorial: managing the impacts of feral camels.Crossref | GoogleScholarGoogle Scholar |
McNeely, J. A. (2001). ‘The great reshuffling: human dimensions of invasive alien species.’ (IUCN, Biodiversity Policy Coordination Division: Gland, Switzerland.) Available at: https://portals.iucn.org/library/node/7850 (accessed 15 March 2017).
McNeely, J. A. (2011). Xenophobia or conservation: some human dimensions of invasive alien species. In: ‘Invasive and Introduced Plants and Animals: Human Perceptions, Attitudes and Approaches to Management’. (Eds I. D. Rotherham and R. A. Lambert.) pp. 19–36. (Earthscan: London, UK.)
Medd, R. W., Kemp, D. R., and Auld, B. A. (1987). Management of weeds in perennial pastures. In: ‘Temperate Pastures, their Production, Use and Management’. (Eds J. L. Wheeler, C. J. Pearson and G. E. Robards.) pp. 253–261. (CSIRO Publishing: Melbourne.)
Mitchell, M. L., Norman, H. C., and Whalley, R. D. B. (2015). Use of functional traits to identify Australian forage grasses, legumes and shrubs for domestication and use in pastoral areas under a changing climate. Crop & Pasture Science 66, 71–89.
| Use of functional traits to identify Australian forage grasses, legumes and shrubs for domestication and use in pastoral areas under a changing climate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXisFemt74%3D&md5=8068ad805b30515c8dba57e08a32e3dbCAS |
Morton, S. R., Stafford-Smith, D. M., Friedel, M. H., Griffin, G. F., and Pickup, G. (1995). The stewardship of arid Australia: Ecology and landscape management. Journal of Environmental Management 43, 195–217.
| The stewardship of arid Australia: Ecology and landscape management.Crossref | GoogleScholarGoogle Scholar |
Nathan, R., and Muller-Landau, H. C. (2000). Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends in Ecology & Evolution 15, 278–285.
| Spatial patterns of seed dispersal, their determinants and consequences for recruitment.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2sbitlOqtA%3D%3D&md5=5ac1decb69e9718a521925af40ecee48CAS |
Norton, D. A. (2009). Species invasions and the limits to restoration: learning from the New Zealand experience. Science 325, 569–571.
| Species invasions and the limits to restoration: learning from the New Zealand experience.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXptFOjsb8%3D&md5=119d69321574e3f55770ae02f795d565CAS |
Nugent, G., Fraser, W., and Sweetapple, P. (2001). Top down or bottom up? Comparing the impacts of introduced arboreal possums and ‘terrestrial’ ruminants on native forests in New Zealand. Biological Conservation 99, 65–79.
| Top down or bottom up? Comparing the impacts of introduced arboreal possums and ‘terrestrial’ ruminants on native forests in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Parkes, J. P. (1993). Feral goats: designing solutions for a designer pest. New Zealand Journal of Ecology 17, 71–83.
Pejchar, L., and Mooney, H. A. (2009). Invasive species, ecosystem services and human well-being. Trends in Ecology & Evolution 24, 497–504.
| Invasive species, ecosystem services and human well-being.Crossref | GoogleScholarGoogle Scholar |
Pimentel, D. (2002). Introduction: non-native species in the world. In: ‘Biological Invasions: Economic and Environmental Costs of Alien Plant, Animal and Microbe Species’. (Ed. D. Pimentel.) pp. 3–8. (CRC Press: Boca Raton, FL.)
Price, J. N., Berney, P. J., Ryder, D., Whalley, R. D. B., and Gross, C. L. (2011). Disturbance governs dominance of an invasive forb in a temporary wetland. Oecologia 167, 759–769.
| Disturbance governs dominance of an invasive forb in a temporary wetland.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbht1ertg%3D%3D&md5=2ca1f22678a52e6d2ad05e30aa96c87cCAS |
Prins, H. H. T., and Gordon, I. J. (2014a). Testing hypotheses about biological invasions and Charles Darwin’s two-creators rumination. In: ‘Invasion Biology and Ecological Theory: Insights from a Continent in Transformation’. (Eds H. H. T. Prins and I. J. Gordon.) pp. 1–19. (Cambridge University Press: Cambridge, UK.)
Prins, H. H. T., and Gordon, I. J. (2014b). A critique of ecological theory and a salute to natural history. In: ‘Invasion Biology and Ecological Theory: Insights from a Continent in Transformation’. (Eds H. H. T. Prins and I. J. Gordon.) pp. 497–516. (Cambridge University Press: Cambridge, UK.)
Robinson, S. A., and Copson, G. R. (2014). Eradication of cats (Felis catus) from subantarctic Macquarie Island. Ecological Management & Restoration 15, 34–40.
| Eradication of cats (Felis catus) from subantarctic Macquarie Island.Crossref | GoogleScholarGoogle Scholar |
Robley, A., Gormley, A., Forsyth, D. M., Wilton, A. N., and Stephens, D. (2010). Movements and habitat selection by wild dogs in eastern Victoria. Australian Mammalogy 32, 23–32.
| Movements and habitat selection by wild dogs in eastern Victoria.Crossref | GoogleScholarGoogle Scholar |
Rotherham, I. D., and Lambert, R. A. (2011). Balancing species history, human culture and scientific insight: introduction and overview. In: ‘Invasive and Introduced Plants and Animals: Human Perceptions, Attitudes and Approaches to Management’. (Eds I. D. Rotherham and R. A. Lambert.) pp. 3–18. (Earthscan: London, UK.)
Russell, B. G., Letnic, M., and Fleming, P. J. S. (2011). Managing feral goat impacts by manipulating their access to water in the rangelands. The Rangeland Journal 33, 143–152.
| Managing feral goat impacts by manipulating their access to water in the rangelands.Crossref | GoogleScholarGoogle Scholar |
Ruttledge, A., Whalley, R. D. B., Reeve, I., Backhouse, D. A., and Sindel, B. M. (2015). Preventing weed spread: a survey of lifestyle and commercial landholders about Nassella trichotoma in the Northern Tablelands of New South Wales, Australia. The Rangeland Journal 37, 409–423.
| Preventing weed spread: a survey of lifestyle and commercial landholders about Nassella trichotoma in the Northern Tablelands of New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Sarre, S. D., Aitken, N., Adamack, A. T., MacDonald, A. J., Gruber, B., and Cowan, P. (2014). Creating new evolutionary pathways through bioinvasion: the population genetics of brushtail possums in New Zealand. Molecular Ecology 23, 3419–3433.
| Creating new evolutionary pathways through bioinvasion: the population genetics of brushtail possums in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Scott, J. K. (2000). Weed invasion, distribution and succession. In: ‘Australian Weed Management Systems’. (Ed. B. M. Sindel.) pp. 19–38. (R.G. and F.J. Richardson: Melbourne.)
Seki, M., Kamei, A., Yamaguchi-Shinozaki, K., and Shinozaki, K. (2003). Molecular responses to drought, salinity and frost: common and different paths for plant protection. Current Opinion in Biotechnology 14, 194–199.
| Molecular responses to drought, salinity and frost: common and different paths for plant protection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjsVGlu7w%3D&md5=b8ebb23eb81d4394dd841815029dd287CAS |
Sheppard, A. W. (2000). Weed ecology and population dynamics. In: ‘Australian Weed Management Systems’. (Ed. B. M. Sindel.) pp. 39–60. (R.G. and F.J. Richardson: Melbourne.)
Sibly, R. M., and Hone, J. (2002). Population growth rate and its determinants: an overview. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 357, 1153–1170.
| Population growth rate and its determinants: an overview.Crossref | GoogleScholarGoogle Scholar |
Simberloff, D. (2011). The rise of modern invasion biology and American attitudes towards introduced species. In: ‘Invasive and Introduced Plants and Animals: Human Perceptions, Attitudes and Approaches to Management’. (Eds I. D. Rotherham and R. A. Lambert.) pp. 121–135. (Earthscan: London, UK.)
Sindel, B. M. (2000). The history of integrated weed management. In: ‘Australian Weed Management Systems’. (Ed. B. M. Sindel.) pp. 253–265. (R.G. and F.J. Richardson: Melbourne.)
Sindel, B. M., Kristiansen, P. E., Wilson, S. C., Shaw, J. D., and Williams, L. K. (2017). Managing native plants on sub-Antarctic Macquarie Island. The Rangeland Journal 39, .
Talonia, L. R., Reid, N., Gross, C. L., and Whalley, R. D. B. (2017). Germination ecology of six species of Eucalyptus in shrink–swell vertosols: moisture, seed depth and seed size limit seedling emergence. Australian Journal of Botany 65, 22–30.
| Germination ecology of six species of Eucalyptus in shrink–swell vertosols: moisture, seed depth and seed size limit seedling emergence.Crossref | GoogleScholarGoogle Scholar |
Thomson, P. C. (1992). The behavioural ecology of dingoes in north-western Australia: IV. Social and spatial organisation, and movements. Wildlife Research 19, 543–563.
| The behavioural ecology of dingoes in north-western Australia: IV. Social and spatial organisation, and movements.Crossref | GoogleScholarGoogle Scholar |
Tracey, J., and Fleming, P. (2008). Regent parrots and almonds: balancing conservation and production issues. Australian Nutgrower 22, 29–30.
Villamagna, A. M., and Murphy, B. R. (2010). Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): a review. Freshwater Biology 55, 282–298.
| Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): a review.Crossref | GoogleScholarGoogle Scholar |
Vitousek, P. M., D’Antonio, C. M., Loope, L. L., Rejm, X. C., Nek, M., and Westbrooks, R. (1997). Introduced species: A significant component of human-caused global change. New Zealand Journal of Ecology 21, 1–16.
Walters, C. J., and Hilborn, R. (1978). Ecological optimization and adaptive management. Annual Review of Ecology and Systematics 9, 157–188.
| Ecological optimization and adaptive management.Crossref | GoogleScholarGoogle Scholar |
Walters, C. J., and Holling, C. S. (1990). Large-scale management experiments and learning by doing. Ecology 71, 2060–2068.
| Large-scale management experiments and learning by doing.Crossref | GoogleScholarGoogle Scholar |
Waters, C. M., Whalley, R. D. B., and Huxtable, C. H. A. (2000). ‘Grassed Up (A guideline for revegetating with Australian native grasses).’ (NSW Agriculture: Dubbo, NSW.)
Watt, L. A., and Whalley, R. D. B. (1982a). Establishment of small-seeded perennial grasses on black clay soils in North-western N.S.W. Australian Journal of Botany 30, 611–623.
| Establishment of small-seeded perennial grasses on black clay soils in North-western N.S.W.Crossref | GoogleScholarGoogle Scholar |
Watt, L. A., and Whalley, R. D. B. (1982b). Effect of sowing depth and seedling morphology on establishment of grasses seedlings on cracking black earths. Australian Rangeland Journal 4, 52–60.
| Effect of sowing depth and seedling morphology on establishment of grasses seedlings on cracking black earths.Crossref | GoogleScholarGoogle Scholar |
Webb, C. J., Sykes, W. R., and Garnock-Jones, P. J. (1988). ‘Flora of New Zealand Vol. 4. Naturalized Pteridophytes, Gymnosperms, Dicotyledons.’ (Landcare New Zealand: Christchurch.) Available at: http://mfkp.org/INRMM/article/13524154, http://floraseries.landcareresearch.co.nz (accessed 15 March 2017).
Westoby, M., Walker, B., and Noy-Meir, I. (1989). Opportunistic management for rangelands not at equilibrium. Journal of Range Management 42, 266–274.
| Opportunistic management for rangelands not at equilibrium.Crossref | GoogleScholarGoogle Scholar |
Whalley, R. D. B. (1994). State and transitions models for rangelands. 1. Successional theory and vegetation change. Tropical Grasslands 28, 195–205.
Whalley, R. D. B., Price, J. N., Macdonald, M. J., and Berney, P. J. (2011). Drivers of change in the Social-Ecological Systems of the Gwydir Wetlands and Macquarie Marshes in northern New South Wales, Australia. The Rangeland Journal 33, 109–119.
| Drivers of change in the Social-Ecological Systems of the Gwydir Wetlands and Macquarie Marshes in northern New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Whisson, D. A., Holland, G. J., and Carlyon, K. (2012). Translocation of overabundant species: implications for translocated individuals. The Journal of Wildlife Management 76, 1661–1669.
| Translocation of overabundant species: implications for translocated individuals.Crossref | GoogleScholarGoogle Scholar |
Williams, K., Parer, I., Coman, B., Burley, J., and Braysher, M. (1995). ‘Managing Vertebrate Pests: Rabbits.’ (Bureau of Resource Sciences and CSIRO Division of Wildlife and Ecology, Australian Government Publishing Service: Canberra, ACT.)



