Asymptomatic non-chlamydial, non-gonococcal urethritis — an iatrogenic disease?
Basil DonovanSchool of Public Health, University of Sydney; and Sydney Sexual Health Centre, Sydney Hospital; address for correspondence: PO Box 1614, Sydney, NSW 2001, Australia; email: donovanb@sesahs.nsw.gov.au
Sexual Health 1(2) 65-67 https://doi.org/10.1071/SH03016
Submitted: 11 November 2003 Accepted: 5 March 2004 Published: 24 June 2004
Around the middle of the twentieth century, specialist services began routinely screening asymptomatic men with a urethral smear for gram staining. The practice persists in over 90% of United Kingdom services in line with national guidelines.1 If the smear reveals ≥ 5 polymorphs in five or more oil-immersion fields then a diagnosis of non-gonococcal urethritis (NGU) is made, despite the absence of symptoms or a specific pathogen. Some will even fish out threads or debris from an asymptomatic man’s first-void urine and stain these, in this case looking for ≥ 10 polymorphs/oil-immersion field. An alternative is a leucocyte esterase test on the urine. Thus (arbitrarily) a new disease was created; necessitating counselling, antibiotic treatment, contact tracing, and frequent psychosocial dilemmas.
The Americans generally go easy on the tracing of contacts of cases of asymptomatic NGU in the absence of a specific organism (C. trachomatis) being documented.2 While agreeing on the microscopic criteria for asymptomatic urethritis, in contrast to the UK neither the American nor the Australian guidelines advocate urethral smears for microscopy for asymptomatic men, though neither guidelines actually advise against the practice.3,4 Perhaps technology has advanced to the point where this time-consuming and often problematic diagnosis of asymptomatic non-chlamydial, non-gonococcal urethritis (NCNGU) (and its active pursuit in the clinic) needs to be reappraised.
First, we need to ask why did the practice start in the first place? After glancing over their shoulders, a few senior UK genitourinary physicians have intimated their sceptical view that the process was devised ‘to keep the statistics up in the clinics’. By the 1950s, the introduction of penicillin had made large inroads into the major concerns of the time — gonorrhoea and syphilis. The tactic worked. NGU filled the gap and the comprehensive network of UK clinics was preserved. In the meantime, in the USA, the practice did not become as widespread and much of their clinical network was de-funded. But these days the UK clinics are straining under a massive and growing clinical burden — yet more reason to question a traditional practice with a fading rationale.
Second, there was a strong hunch (confirmed by the 1970s) that NGU often indicated an important infection (namely C. trachomatis) with serious consequences for sexual partners, and chlamydial urethritis is commonly asymptomatic. At the time (in the absence of any other test for C. trachomatis infection) this provided a compelling argument for screening for asymptomatic NGU. However, these days, a specific test for C. trachomatis is part of the routine work-up in specialist services,1 so all the urethral smear now contributes is the diagnosis of asymptomatic NCNGU. Additionally, as control programmes take effect, C. trachomatis tends to contribute shrinking proportions (typically < 20%) of acute NGU cases and even smaller proportions of asymptomatic NGU cases.2
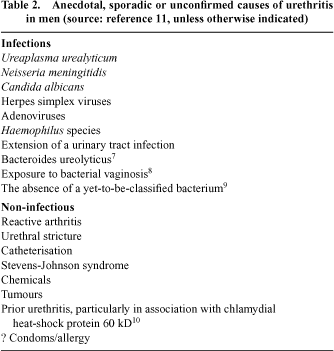
The only consistently documented causes of NCNGU are Mycoplasma genitalium 5 and Trichomonas vaginalis 6 [the latter largely confined to areas of high prevalence (Table 1)] but we don’t routinely test for these organisms. The reasons that we don’t screen for these infections are because the pathogenicity of M. genitalium has been unclear until recently5 while T. vaginalis tends to be uncommon in those services that can afford to test for it. There may be a scattering of other specific causes of NCNGU (Table 2)7–11 but there is no debate that we don’t know the cause of most cases. Thus we find ourselves in the odd predicament of having constructed and been required to manage an asymptomatic condition with generally no known cause and no known consequences.

|
|
Some may chose to continue the practice of urethral smears with the justification that a sinister (as yet undiscovered) pathogen is lurking in the urethrae of these men and that they wouldn’t otherwise get treated. But the treatments most commonly used for NGU or NCNGU in the public sectors in the UK and the USA (tetracyclines) have no effect on T. vaginalis and, on preliminary experience, a high failure rate for M. genitalium. 12 We have no way of knowing if this yet-to-be-discovered pathogen is susceptible to our undirected antibiotics — this hardly qualifies as evidence-based medicine.
Another proffered justification for urethral microscopy of asymptomatic men relates to clinic dynamics. It is claimed that more men (some of whom harbour C. trachomatis) get treated sooner so that they have less opportunity to infect others and are at less risk of being lost to follow-up without treatment. There is scant recent evidence for this supposition.13 A nucleic acid amplification test for C. trachomatis takes 1–4 h (equivalent to getting most patients treated in 1 or 2 days in real terms) which is hardly a major public health hazard. What about the resources and distress involved in counselling men diagnosed with asymptomatic NGU (C. trachomatis test pending) who have to be told that they ‘may or may not’ have a sexually transmissible infection, when we know in advance that they usually don’t? Compounding the poor positive predictive value of microscopy for the presence of major NGU pathogens is its poor sensitivity.14 What about the other potentially infected patients who have to wait for extended periods (often several working days) for appointments, in part because of the inherent inefficiency of the fractured double-consultation needed to deliver the gram-stain result? What about the physical discomfort due to the collection of the urethral smear and its effect on future health-care seeking behaviour? Cost effectiveness and patient acceptability studies are needed.
Nevertheless, the art of the gram-stained smear need not be lost. The smear remains relevant for urethrally symptomatic men, for the assessment of male contacts of women with pelvic inflammatory disease and of men suspected of having epididymitis or reactive arthritis, as well as men participating in research into urethritis.
Given the current limitations in our knowledge, if we want to do the best thing by our patients we should be lobbying our laboratories and diagnostic companies for multiplex nucleic acid amplification tests for the substantiated causes of urethritis (Table 1) and consider relegating to history the routine gram-stained urethral smear of the asymptomatic man.
[1] Carne CA, Foley E, Rowen D, Kell P. May R on behalf of the British Co-operative Clinical Group of the Medical Society for the Study of Venereal Diseases. Variation in clinical practice in genitourinary medicine clinics in the United Kingdom. Sex Transm Infect 2003; 79 240–2.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[2] Burnstein GR, Zenilman JM. Non-gonococcal urethritis — a new paradigm. Clin Infect Dis 1999; 28 S66–73.
| PubMed |

[3]
[4]
[5] Taylor-Robinson D. Mycoplasma genitalium — an update. Int J STD AIDS 2002; 13 145–51.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[6] Schwebke JR, Hook EW. High rates of Trichomonas vaginalis among men attending a sexually transmitted diseases clinic: implications for screening and urethritis management. J Infect Dis 2003; 188 465–8.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[7] Hawkins DA, Fontaine EAR, Thomas BJ, Bonstouller YL, Taylor-Robinson D. The enigma of non-gonococcal urethritis: role for Bacteroides ureolyticus. Genitourin Med 1988; 64 10–3.
| PubMed |

[8] Keane FE, Thomas BJ, Whitaker L, Renton A, Taylor-Robinson D. An association between non-gonococcal urethritis and bacterial vaginosis and the implications for patients and their sexual partners. Genitourin Med 1997; 73 373–7.
| PubMed |

[9] Riemersma WA, van der Schee CJ, van der Meijden WI, Verbrugh HA, van Belkum A. Microbial population diversity in the urethras of healthy males and males suffering from non-chlamydial, non-gonococcal urethritis. J Clin Microbiol 2003; 41 1977–86.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[10] Horner PJ, Cain D, McClure M,, et al. Association of antibodies to Chlamydia trachomatis heat-shock protein 60kD with chronic non-gonococcal urethritis. J Infect Dis 1997; 24 653–60.

[11]
[12] Falk L, Fredlund H, Jensen JS. Tetracycline does not eradicate Mycoplasma genitalium. Sex Transm Infect 2003; 79 318–9.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[13] Chen MY, Ryder N, Donovan B. Completeness and timeliness of treatment for chlamydia within a sexual health service. Int J STD AIDS 2004;

[14] Janier M, Lassau F, Casin I, et al. Male urethritis with and without discharge: a clinical and microbiological study. Sex Transm Dis 1994; 22 244–52.



