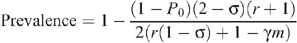Minimal impact of circumcision on HIV acquisition in men who have sex with men
Gregory J. Londish A , David J. Templeton B C , David G. Regan B , John M. Kaldor B and John M. Murray A B DA School of Mathematics and Statistics, University of New South Wales, Sydney, NSW 2052, Australia.
B National Centre in HIV Epidemiology and Clinical Research, University of New South Wales, Sydney, NSW 2052, Australia.
C Royal Prince Alfred Sexual Health, Royal Prince Alfred Hospital, Sydney, NSW 2050, Australia.
D Corresponding author. Email: J.Murray@unsw.edu.au
Sexual Health 7(4) 463-470 https://doi.org/10.1071/SH09080
Submitted: 11 August 2009 Accepted: 20 April 2010 Published: 10 November 2010
Abstract
Background: Men who have sex with men (MSM) are disproportionately affected by HIV. The proven efficacy of circumcision in reducing the risk of HIV acquisition among African heterosexual males has raised the question of whether this protective effect may extend to MSM populations. We examined the potential impact of circumcision on an HIV epidemic within a population of MSM. Methods: A mathematical model was developed to simulate HIV transmission in an MSM population. The model incorporated both circumcision and seropositioning, and was used to predict the reduction in HIV prevalence and incidence as a result of the two interventions. Estimates for the time required to achieve these gains were also calculated. Results: We derive simple formulae for the decrease in HIV prevalence with increased circumcision. Our model predicts that if an initially uncircumcised MSM population in a developed country with a baseline HIV prevalence of 10% underwent universal circumcision, HIV incidence would only be reduced to 95% of pre-intervention levels and HIV prevalence to 9.6% after 20 years. In the longer term, our model predicts that prevalence would only decrease from 10% to 6%, but this would take several generations to achieve. The effectiveness of circumcision increases marginally with higher degrees of seropositioning. Conclusions: The results of these calculations suggest that circumcision as a public health intervention will not produce a substantial decrease in HIV prevalence or incidence among MSM in the near future, and only modest reductions are achievable in the long-term.
Additional keywords: mathematical model, MSM, seropositioning.
Introduction
Men who have sex with men (MSM) remain disproportionately affected by HIV epidemic in resource-rich countries. 1 , 2 Despite widespread health promotion and education campaigns targeting gay men, increases in HIV incidence among MSM have been observed in several jurisdictions over the past decade. 2 – 5
Much research in recent years has focussed on potential biomedical interventions to prevent HIV transmission. Strategies, including candidate microbicides and vaccines, have thus far failed to provide protection against HIV infection. 6 , 7 Clinical trials have provided strong evidence that circumcision can reduce the risk of HIV acquisition by around 50% in heterosexual African men. 8 – 11 However, the evidence that circumcision provides protection against HIV among MSM is limited and conflicting. 9 Most MSM acquire HIV via receptive unprotected anal intercourse (UAI) 12 and the potential reduction in HIV risk afforded by circumcision would most likely be limited to the insertive UAI partner.
Two key factors could account for an increasingly important role of circumcision, should it afford MSM a degree of protection against HIV. First, the prevalence of circumcision is declining in many resource-rich countries. 13 , 14 Second, ‘seropositioning’, where HIV-negative MSM adopt the insertive role in UAI to reduce their HIV risk, is being commonly reported throughout the world. 5 , 15 Should increasing proportions of uncircumcised HIV-negative MSM take the insertive role in UAI with HIV-positive partners, then the proportion of new HIV infections attributable to insertive UAI is likely to increase.
At a population level, the effect of increased levels of male circumcision, either alone or in combination with seropositioning, on the MSM HIV epidemic is unknown. 16 , 17 We therefore developed a mathematical model to explore likely effects of male circumcision on HIV prevalence and incidence among MSM.
Methods
We used a mathematical model to investigate the effect of circumcision on HIV under different levels of circumcision coverage and seropositioning. The intention of this modelling is to determine a representation of the problem, containing the most important features of how circumcision may impact on HIV transmission among MSM, while still enabling the derivation of explicit formulae of how HIV prevalence in the MSM community will change with levels of circumcision. These formulae provide estimates of the expected reduction in HIV prevalence for the MSM community as well as establishing its relative benefits compared with practices such as seropositioning.
We assumed that the only mode of HIV transmission was UAI. We also assumed that circumcision reduced the risk of infection via insertive UAI by the same amount as shown for heterosexual men (i.e. 50%) but the risk of acquisition for receptive UAI was unchanged.
The compartmental mathematical model is described by the following system of differential equations where X and Y represent the number of uninfected and HIV-infected MSM (both diagnosed and undiagnosed) respectively:
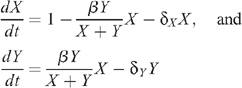
where population sizes have been scaled so that the rate of entry of new MSM to the uninfected population is 1 per year. The probability of HIV transmission per time unit through UAI between an infected and uninfected MSM is given by:
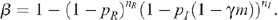
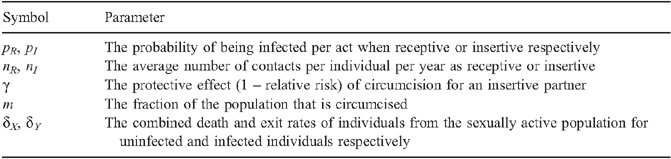
The parameters p R and p I denote the probabilities of HIV infection per act of UAI when receptive or insertive respectively, n R and n I represent the number of receptive and insertive UAI acts per unit of time, m is the fraction of MSM who are circumcised, γ is the reduced risk of HIV infection for insertive UAI due to circumcision, and δ X and δ Y are the rates at which MSM no longer practise UAI due to death or change in sexual activity for each of the uninfected and infected groups respectively. Since the probability of HIV transmission per act of UAI is small, 12 β simplifies to:
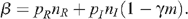
The value of β will vary from country to country. In the calculations below, which are relevant to a country such as Australia, the value of β is determined using current levels of HIV prevalence and mortality rates commensurate with a population where antiretroviral therapy (ART) is used to the extent typical of a resource-rich country; i.e. ART is widely available but not used by everyone. As such, it implicitly includes the effect of reduced probability of transmission due to lower viral loads with ART. A full description of the model is given in the Appendix.
Relative to HIV prevalence P 0, when there is no circumcision, an increase of circumcision within a fraction m of the MSM population results in an HIV prevalence that is determined from the steady-state level in the mathematical model, given by:

where r = p R /p I expresses the relative risk of infection of receptive to insertive acts (see the Appendix for the derivation of this and the following equations). So the decrease in prevalence is given by:

Seropositioning was modelled by increasing the proportion of total insertive UAI acts by uninfected men while not changing the total number of UAI acts. The degree to which seropositioning is practised was assumed to be the effective level in the population in the simulations. However, this is likely to be lower than the perceived level as a result of non-disclosure of HIV status to sexual partners or HIV infections remaining undiagnosed. The level of seropositioning is described by the variable σ = 1−n R /n I , such that σ = 0 represents no seropositioning so that the number of receptive and insertive acts are equal between HIV-infected and uninfected individuals, and σ = 1 represents full seropositioning so that there are no receptive acts by an uninfected MSM with an infected MSM. Relative to HIV prevalence P 0 when there is no circumcision and no seropositioning, an increase in both of these results in the steady-state level of:

The parameters used in the numerical simulation of the model can be found in Table 1. With no seropositioning, we assumed n R = n I = 15 UAI acts per year, which produced an HIV prevalence of 10%, a value consistent with the estimated 8% prevalence among MSM in Australia, 18 and with the average 12.8% prevalence among MSM in low and middle-income countries throughout the world (Table 1). 19 Estimates of change in HIV prevalence with increasing circumcision and seropositioning were determined from these steady-state values. Calculations of incidence were determined numerically from the model differential equations. Coverage by circumcision was varied between 0% and 100%, and seropositioning was varied such that the proportion of UAI acts by uninfected men that were insertive ranged from 50% to 100%.

|
Results
Circumcision in a MSM population without seropositioning
In this scenario, we investigated changes in HIV incidence and prevalence resulting from a circumcision intervention. We considered a population with no circumcised men initially and modelled the effect of circumcising a given percentage of men. The proportion of UAI acts by uninfected men that are insertive was kept constant at 50%. Studies of the per-contact risk of MSM acquiring HIV infection in the USA indicate that the risk of acquiring HIV from receptive UAI is 0.82% when the partner was known to be infected with HIV and 0.27% for someone with unknown serostatus. 12 However, the per-contact risk for insertive UAI was ~10-fold lower at 0.06% and 0.04% respectively. 12 Hence we assume that the relative risk of HIV infection from receptive UAI is much more likely than through insertive UAI.
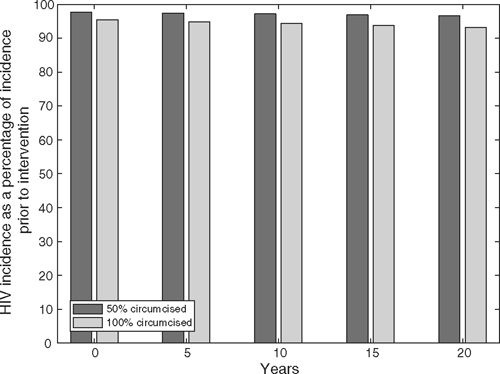
The change in HIV incidence after the intervention is shown in Fig. 1 for a typical resource-rich country where the availability of combination ART results in a mortality rate of HIV-infected individuals that is similar to that of uninfected individuals. 21 By circumcising 50% or 100% of the MSM population, incidence quickly drops to 97% or 95% of its value before the intervention respectively, as a consequence of the immediate reduction in risk due to circumcision. However, over the next 20 years, the additional reduction in HIV incidence is only minimal.
|
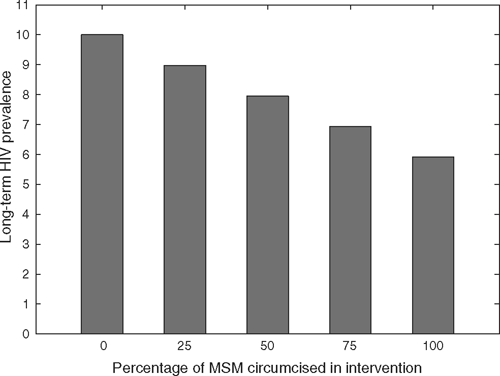
Long-term trends in HIV prevalence are shown in Fig. 2 and the decrease due to the intervention is given by the following expression, which was derived from our model (Eqn 2):
|

For example, in Australia ~10% of MSM are HIV-positive, 20 so the fraction uninfected is 0.90. Given this value and the values from Table 1, we obtain:
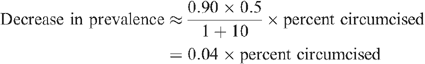
If the entire population was originally uncircumcised, complete circumcision coverage could eventually decrease HIV prevalence from 10% to just under 6%. However, after 20 years, HIV prevalence would only fall to 9.6% in a resource-rich country, indicating that the length of time required to achieve the majority of the long-term decrease in prevalence is on the order of generations. This slow rate of decrease is a consequence of the low mortality rate of individuals living with HIV infection in resource-rich countries where ART is widely available.
As would be expected, if the relative risk of infection from receptive v. insertive UAI was half the above value, so r = 5, then the benefits of circumcision are approximately twice that of the above calculations, so complete circumcision would reduce HIV prevalence from 10% to ~2%.
Circumcision in a MSM population practicing seropositioning
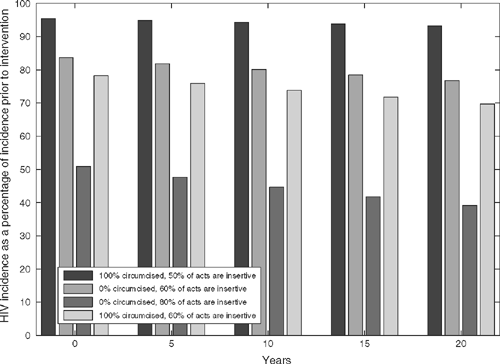
We assumed the same parameter values as above, but in addition, we considered the impact on HIV prevalence of increasing the level of seropositioning at the start of the intervention, holding it constant thereafter. HIV incidence for different levels of achieved seropositioning and circumcision is shown in Fig. 3. The graph shows that circumcision is slightly more effective as the proportion of acts which are insertive increases, but it also shows that the greatest reductions in incidence are due to seropositioning. If there is a slight increase in seropositioning such that 60% of acts by uninfected men are insertive then incidence falls to 76% of its previous level after 20 years for a resource-rich country. Circumcision of all MSM in addition to the increased level of seropositioning leads to a further decrease in incidence to 69% of the baseline. In addition, achieving a level of seropositioning such that 80% of acts were insertive causes the incidence to drop to 40% of its pre-intervention level after 20 years.
|
The combinations of seropositioning and circumcision that are necessary for a given reduction in HIV prevalence can be determined through a formula similar to that solely through circumcision (Eqn 3). With no increased circumcision, then seropositioning, to the extent that only 44% instead of 50% of UAI acts with HIV-infected MSM are receptive, will eventually reduce HIV prevalence to zero.
Discussion
The results from this model indicate that circumcision is unlikely to have a substantial impact on HIV incidence or prevalence in a MSM population. Given a baseline HIV prevalence of 10%, as in Australia, circumcising all MSM in an uncircumcised population in a resource-rich country where seropositioning is not practised would only reduce incidence to 95% of pre-intervention levels after 20 years (Fig. 1). For countries where HIV-associated mortality is significantly higher than mortality rates for those not living with HIV, reductions in HIV incidence will be proportionately faster, as the HIV-infected group is lost more quickly. However, the extent of reduction in HIV prevalence will not be affected.
If seropositioning was practised and 60% of UAI acts by uninfected men were insertive, HIV incidence after 20 years would be reduced to 69% of the baseline with complete circumcision of an initially uncircumcised MSM population. However, this level of seropositioning without a circumcision intervention would itself reduce incidence to 76% of the baseline (Fig. 3). HIV prevalence follows similar trends with a circumcision-only intervention, reducing prevalence from 10% to 9.6% after 20 years and to 6% in the long term in a resource-rich country. The low mortality rates of those living with HIV in this setting due to the availability of combination ART results in a slow change in HIV prevalence (Fig. 2). Greater reductions in prevalence occur with increasing practice of seropositioning.
Although the rate at which HIV prevalence is reduced is slow, as described above for the continued high levels of HIV incidence at 20 years, increased seropositioning can eventually eliminate HIV prevalence on its own. With no increased circumcision, a reduction of receptive UAI from 50% to 44% is sufficient to eventually remove HIV infection from the MSM community, provided there is no compensatory change in risk. Given the model predictions of the sensitivity of prevalence to seropositioning and the relative insensitivity to circumcision alone, it is therefore understandable that circumcision will have little additional benefit when combined with seropositioning.
In our model, the predicted benefits of circumcision among MSM are less than those observed among African heterosexuals in the three randomised circumcision trials. 8 , 10 , 11 Simulations of a circumcision intervention applied to a sub-Saharan population with heterosexual intercourse as the only mode of HIV transmission showed that HIV prevalence could decline to 50% of pre-intervention levels after 13 years; 22 however, the simulations presented here indicate that the prevalence among MSM in resource-rich countries would only fall to 96% of its pre-intervention level after 20 years (Fig. 1). For MSM, the great majority of new infections occur through receptive UAI, for which circumcision has no plausible effect. 9 This contrasts with insertive vaginal sex as the key risk factor for sexual acquisition among African heterosexual males in the randomised trials. Additionally, any changes in HIV prevalence in developed countries, where HIV infection among MSM contributes a much greater proportion of new infections than heterosexual transmission, will be much slower than in Africa, given lower AIDS-associated mortality due to access to ART.
A substantial number of MSM in developed countries are already circumcised. 13 , 14 Therefore, it is unlikely that such a circumcision intervention would provide the maximum estimated benefit, since the decrease in HIV prevalence is proportional to the size of the uncircumcised MSM population at the baseline. For example, the estimated reduction in HIV prevalence achieved by complete circumcision would only be 2% in a population of MSM among whom 50% are already circumcised.
A circumcision intervention among MSM who also practise seropositioning could result in a greater reduction in HIV prevalence compared with the decrease achieved with circumcision alone. However, our model suggests that similar benefits in terms of reductions in HIV prevalence could be achieved by minimal increases in the practice of seropositioning alone. Observational data on the benefits of circumcision are few and conflicting on HIV acquisition among MSM who predominantly practice the insertive role in UAI. 23 , 24 Nonetheless, our model predicts that if effective seropositioning is practised to a sufficient degree, HIV acquisition could be significantly reduced to a much greater extent than through circumcision.
Several limitations in our model should be noted. For simplicity and clarity, we assumed homogeneity in sexual behaviour for MSM who were not infected with HIV, and our model did not include other factors that have an impact on HIV transmission among MSM, such as different levels of HIV viraemia during the acute, chronic and AIDS stages of infection; concurrent sexually transmissible infections; the structure of sexual contacts and other risk behaviours such as injecting drug use. 25 , 26 We also assumed that following the intervention, there were no substantial changes in behaviour e.g. increases in the number of UAI acts per year.
There is evidence that in some settings MSM wholly or predominantly adopt a specific role (insertive or receptive) in UAI 27 and the benefit of circumcision to these groups may be somewhat different than the results presented here for the whole population. An analysis of circumcision and HIV incidence for MSM in Australia determined that for men where virtually all UAI with serodiscordant partners was in the insertive position, being circumcised provided a ~five-fold reduction in HIV incidence. 28 Our model did not include separate insertive or receptive only individuals, as this would have then required a numerical rather than analytical solution and the introduction of many more parameters, with an increasing level of uncertainty due to model complexity. Neither did the model determine benefit to subpopulations but to the community as a whole. However, even under these simplifications, we determined that seropositioning can substantially reduce HIV incidence for the entire community irrespective of circumcision. In our calculations, the additional benefit of circumcision was minor to the community as a whole but would be expected to directly benefit those who are preferentially insertive. A more detailed analysis could be informative regarding the benefits of circumcision to different groups within the MSM community. Nevertheless, our calculations should be useful for public health officials, HIV clinicians and researchers to estimate the impact that circumcision could have in their own MSM community without requiring the solution of complex systems of differential equations with a large number of parameters.
In contrast to the substantial protective effect circumcision affords African heterosexual men, 8 , 10 , 11 our model suggests that circumcision would have a minimal impact on the HIV epidemic among MSM in resource-rich countries. Due to the improved survival with effective ART for those living with HIV infection, any substantial impact would take many generations to achieve. Other more effective interventions to reduce HIV acquisition in MSM are required.
Conflicts of interest
None declared.
[1]
[2]
[3] Fisher M, Pao D, Murphy G, Dean G, McElborough D, Homer G, et al. Serological testing algorithm shows rising HIV incidence in a UK cohort of men who have sex with men: 10 years application. AIDS 2007; 21 2309–14.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[4] Katz MH, Schwarcz SK, Kellogg TA, Klausner JD, Dilley JW, Gibson S, et al. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am J Public Health 2002; 92 388–94.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[5] Marcus U, Voss L, Kollan C, Hamouda O. HIV incidence increasing in MSM in Germany: factors influencing infection dynamics. Euro Surveill 2006; 11 157–60.
| CAS | PubMed |

[6] van de Wijgert JH, Shattock RJ. Vaginal microbicides: moving ahead after an unexpected setback. AIDS 2007; 21 2369–76.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[7] Yang OO. Aiming for successful vaccine-induced HIV-1-specific cytotoxic T lymphocytes. AIDS 2008; 22 325–31.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[8] Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 2007; 369 643–56.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[9] Weiss HA, Halperin D, Bailey RC, Hayes RJ, Schmid G, Hankins CA. Male circumcision for HIV prevention: from evidence to action? AIDS 2008; 22 567–74.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[10] Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 2007; 369 657–66.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[11] Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med 2005; 2 e298.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[12] Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol 1999; 150 306–11.
| CAS | PubMed |

[13] Templeton DJ, Mao L, Prestage G, Kaldor JM, Kippax S, Grulich AE. Demographic predictors of circumcision status in a community-based sample of homosexual men in Sydney, Australia. Sex Health 2006; 3 191–3.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[14] Mor Z, Kent CK, Kohn RP, Klausner JD. Declining rates in male circumcision amidst increasing evidence of its public health benefit. PLoS One 2007; 2 e861.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[15] Jin F, Prestage GP, Ellard J, Kippax SC, Kaldor JM, Grulich AE. How homosexual men believe they became infected with HIV: the role of risk-reduction behaviors. J Acquir Immune Defic Syndr 2007; 46 245–7.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[16] MacDonald A, Humphreys J, Jaffe HW. Prevention of HIV transmission in the UK: what is the role of male circumcision? Sex Transm Infect 2008; 84 158–60.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[17] Rice BD, Delpech VC, Evans BG. Could male circumcision reduce HIV incidence in the UK? HIV Med 2008; 9 329–31.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |

[18] Prestage G, Jin F, Zablotska I, Imrie J, Kaldor JM, Grulich AE. Trends in HIV prevalence among homosexual and bisexual men in eastern Australian states. Sex Health 2008; 5 103–7.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[19] Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: a systematic review. PLoS Med 2007; 4 e339.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[20] Prestage G, Ferris J, Grierson J, Thorpe R, Zablotska I, Imrie J, et al. Homosexual men in Australia: population, distribution and HIV prevalence. Sex Health 2008; 5 97–102.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[21] Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med 2007; 146 87–95.
| PubMed |

[22] Londish GJ, Murray JM. Significant reduction in HIV prevalence according to male circumcision intervention in sub-Saharan Africa. Int J Epidemiol 2008; 37 1246–53.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[23] Millett GA, Ding H, Lauby J, Flores S, Stueve A, Bingham T, et al. Circumcision status and HIV infection among Black and Latino men who have sex with men in 3 US cities. J Acquir Immune Defic Syndr 2007; 46 643–50.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[24]
[25]
[26] Kral AH, Lorvick J, Ciccarone D, Wenger L, Gee L, Martinez A, et al. HIV prevalence and risk behaviors among men who have sex with men and inject drugs in San Francisco. J Urban Health 2005; 82(Suppl 1): i43–50.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[27] Trichopoulos D, Sparos L, Petridou E. Homosexual role separation and spread of AIDS. Lancet 1988; 332 965–6.
| Crossref | GoogleScholarGoogle Scholar |

[28]
Appendix
|

|
Derivation of equations
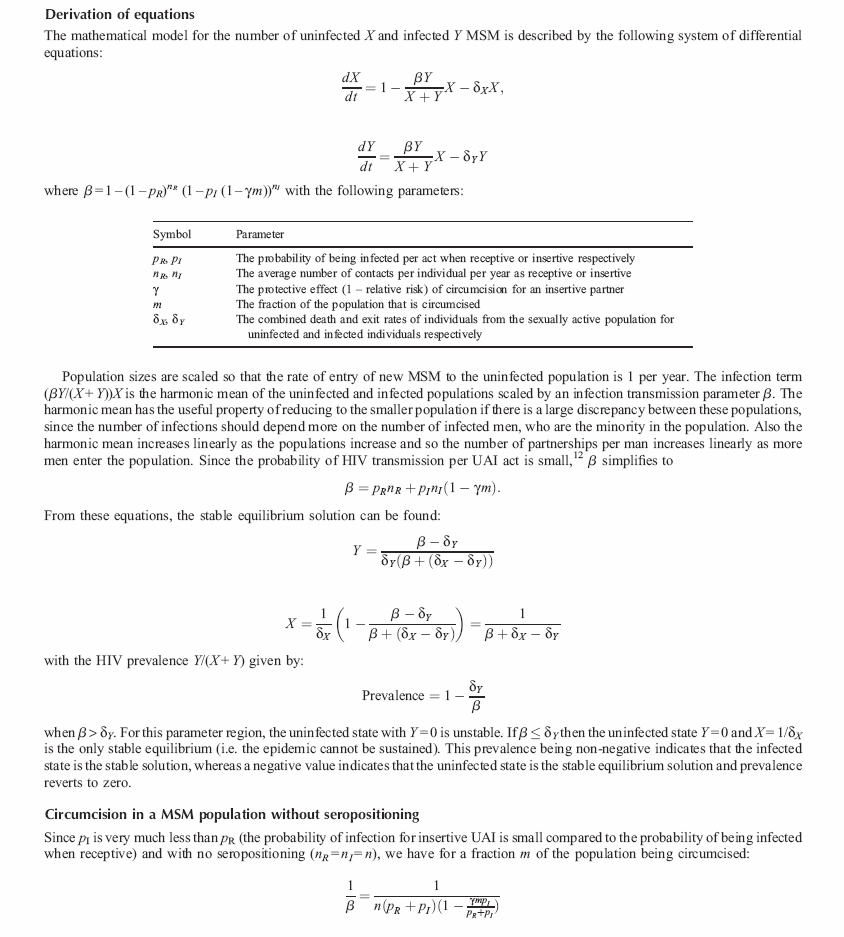
The mathematical model for the number of uninfected X and infected Y MSM is described by the following system of differential equations:
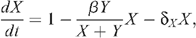
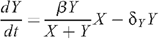
where β = 1 – (1 – p R ) n R (1 – p I (1 – γm)) n I with the following parameters:
|
Population sizes are scaled so that the rate of entry of new MSM to the uninfected population is 1 per year. The infection term (β Y/(X + Y))X is the harmonic mean of the uninfected and infected populations scaled by an infection transmission parameter β. The harmonic mean has the useful property of reducing to the smaller population if there is a large discrepancy between these populations, since the number of infections should depend more on the number of infected men, who are the minority in the population. Also the harmonic mean increases linearly as the populations increase and so the number of partnerships per man increases linearly as more men enter the population. Since the probability of HIV transmission per UAI act is small,12 β simplifies to
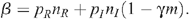
From these equations, the stable equilibrium solution can be found:
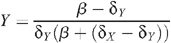
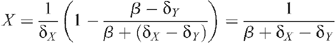
with the HIV prevalence Y/(X + Y) given by:
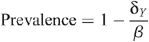
when β > δ Y . For this parameter region, the uninfected state with Y = 0 is unstable. If β ≤ δ Y then the uninfected state Y = 0 and X = 1/δ X is the only stable equilibrium (i.e. the epidemic cannot be sustained). This prevalence being non-negative indicates that the infected state is the stable solution, whereas a negative value indicates that the uninfected state is the stable equilibrium solution and prevalence reverts to zero.
Circumcision in a MSM population without seropositioning
Since p I is very much less than p R (the probability of infection for insertive UAI is small compared to the probability of being infected when receptive) and with no seropositioning (n R = n I = n), we have for a fraction m of the population being circumcised:
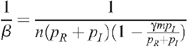
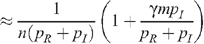
so that:
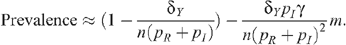
The HIV prevalence when there is no circumcision (m = 0) is given by
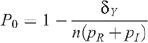
so that this last equation can be rewritten as:
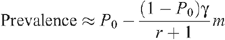
where r = p R /p I expresses the relative risk of receptive to insertive acts. This is the approximation used in estimates of the protective effect of circumcision in an MSM population.
Circumcision in a MSM population practicing seropositioning
We now consider how prevalence changes with circumcision where seropositioning is practised. With seropositioning, the number of acts between infected and uninfected individuals is assumed to remain constant, but the ratio of receptive to insertive acts between these individuals changes. We assume here that the number of UAI acts, N, is constant regardless of the level of seropositioning.
The level of seropositioning is described by the variable σ = 1 – n R /n I such that σ = 0 represents no seropositioning so that the number of receptive and insertive acts are equal between HIV-infected and uninfected individuals, and σ = 1 represents full seropositioning so that there are no receptive acts by an uninfected MSM with an infected MSM. HIV prevalence with circumcision under different levels of seropositioning is given by:
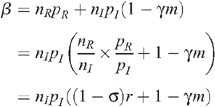
Since we assume that the total number of UAI is unchanged by seropositioning,
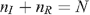
then:
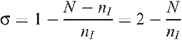
and the number of insertive UAI acts is given by n I = N/(2 – σ). Therefore:
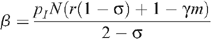
and:
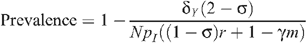
Prevalence in the absence of circumcision and seropositioning is given by
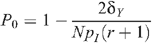
so that: