Australian sexually transmissible infection and HIV testing guidelines for asymptomatic men who have sex with men 2014: a review of the evidence
David J. Templeton A B C H , Phillip Read B D , Rajesh Varma E and Christopher Bourne F GA RPA Sexual Health, Royal Prince Alfred Hospital, Camperdown, NSW 2050, Australia.
B The Kirby Institute for Infection and Immunity in Society, The University of New South Wales, Kensington, NSW 2052, Australia.
C Central Clinical School, The University of Sydney, Sydney, NSW 2006, Australia.
D Kirketon Road Centre, PO Box 22, Kings Cross, Sydney, NSW 1340, Australia.
E Western Sydney Sexual Health Centre, Jeffery House, Level 1, 162 Marsden Street, Parramatta, NSW 2150, Australia.
F Sydney Sexual Health Centre, Sydney Hospital, PO Box 1614 Sydney, NSW 2001, Australia.
G School of Public Health and Community Medicine, The University of New South Wales, Kensington, NSW 2052, Australia.
H Corresponding author. Email: dtempleton@kirby.unsw.edu.au
Sexual Health 11(3) 217-229 https://doi.org/10.1071/SH14003
Submitted: 3 January 2014 Accepted: 9 February 2014 Published: 2 April 2014
Abstract
Men who have sex with men (MSM) in Australia and overseas are disproportionately affected by sexually transmissible infections (STIs), including HIV. Many STIs are asymptomatic, so regular testing and management of asymptomatic MSM remains an important component of effective control. We reviewed articles from January 2009–May 2013 to inform the 2014 update of the 2010 Australian testing guidelines for asymptomatic MSM. Key changes include: a recommendation for pharyngeal chlamydia (Chlamydia trachomatis) testing, use of nucleic acid amplification tests alone for gonorrhoea (Neisseria gonorrhoeae) testing (without gonococcal culture), more frequent (up to four times a year) gonorrhoea and chlamydia testing in sexually active HIV-positive MSM, time required since last void for chlamydia first-void urine collection specified at 20 min, urethral meatal swab as an alternative to first-void urine for urethral chlamydia testing, and the use of electronic reminders to increase STI and HIV retesting rates among MSM.
Additional keywords: chlamydia, first-void urine, gonorrhoea, nucleic acid amplification test, reminders, swabs.
Introduction
In Australia, men who have sex with men (MSM) continue to be disproportionately affected by sexually transmissible infections (STIs) including HIV.1–3 STI and HIV rates have been increasing in recent years among MSM,3,4 and have been attributed to several factors, including changes in sexual behaviour such as reductions in condom use for anal intercourse, and HIV risk reduction strategies.5,6 Many STIs do not lead to symptomatic presentations; therefore, regular STI testing will identify a large number of infections that would otherwise remain undiagnosed and untreated.7
Last year in Australia, there was a 10% increase in new HIV diagnoses,4 which mostly occurred among MSM. As other STIs facilitate HIV transmission,8 regular testing and management of identified STIs among MSM is also likely to be an important strategy in reducing new HIV infections in Australia.
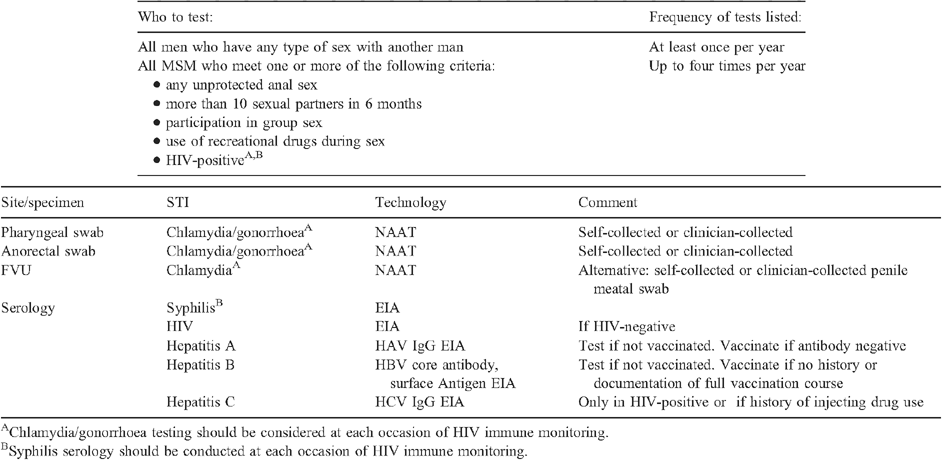
The Sexually Transmitted Infections in Gay Men Action group (STIGMA) was established in 2000 in response to the increasing prevalence of STIs among inner Sydney MSM. STIGMA is a collaborative body comprising representation from sexual health and HIV clinical services, public health units, health promotion, community-based organisations, research centres and general practice. The aim of the group is to reduce the community prevalence of STIs through a multifaceted approach including health promotion and education, research and guideline development. A STIGMA working group was formed in early 2013 to review and update the 2010 STI and HIV testing recommendations.9 The methods and findings of the comprehensive literature review informing this update are outlined in this paper, including a summary of the new 2014 guidelines in Table 1. Key changes from the 2010 guidelines are outlined in Table 2.
Methods
A PubMed search was performed for articles published between 1 January 2009 and 30 May 2013 using the MESH term ‘homosexual, male’, and terms specific for each infection as outlined below. For STIs not previously considered in the guidelines (Mycoplasma genitalium and Trichomonas vaginalis), all available articles, irrespective of publication date, were considered. All potentially relevant abstracts were reviewed and the reference lists of reviewed full-text articles checked. For each STI, the number of articles retrieved, abstracts reviewed and full-text articles reviewed were as follows: gonorrhoea (Neisseria gonorrhoeae) and chlamydia (Chlamydia trachomatis): 758, 216, 84; lymphogranuloma venereum (LGV): 168, 74, 32; M. genitalium: 17, 12, 9; T. vaginalis:19, 7, 5; hepatitis A virus (HAV): 17, 17, 4; hepatitis B virus (HBV): 57, 57, 17; hepatitis C virus: 236, 22, 22; syphilis: 288, 43, 28; HIV: 534, 86, 16; HIV coinfected: 454, 76, 15; herpes simplex virus (HSV): 55, 29, 19; human papillomavirus (HPV): 142, 45, 26. References from the 2010 guidelines, relevant national surveillance and public health reports, and international STI or HIV testing guidelines for MSM were also accessed.
Questions addressed by the review panel were:
-
Should we be testing asymptomatic MSM for each specific STI?
-
If so, should we be testing all MSM or a particular subgroup of MSM?
-
Which anatomical sites should be tested (if relevant)?
-
Overall frequency of testing and indications for more frequent testing
-
What tests or technology should be used for diagnostic testing?
-
Should testing recommendations differ depending on HIV status?
Chlamydia and gonorrhoea
Chlamydia and gonorrhoea are among the most common STIs affecting Australian MSM.10,11 Testing at nongenital as well as urethral sites is an important component of chlamydia and gonorrhoea control.10–14 However, such ‘comprehensive’ STI testing is occurring in less than half of Australian MSM.15
Recommendations for chlamydia and gonorrhoea testing among MSM in the 2010 STIGMA guidelines were largely based on the community-based Health in Men (HIM) cohort, which identified a variety of receptive nonpenile intercourse practices that were risk factors for anal chlamydia and gonorrhoea infections, including fingering, fisting, toys and rimming.10 In contrast to syphilis and HIV infection, there have been no published mathematical models assessing the likely impact of more frequent chlamydia and gonorrhoea testing in Australian MSM.
For pharyngeal infections, there was no association of either chlamydia or gonorrhoea with sore throat symptoms in the HIM cohort,11,16 while all pharyngeal chlamydia or gonorrhoea identified in a recent Dutch clinic-based study was asymptomatic.17 Most pharyngeal chlamydia or gonorrhoea infections are isolated to the pharynx11,16–18 and there is strong epidemiological evidence that supports transmission of chlamydia and gonorrhoea between pharyngeal and anogenital sites among MSM. The HIM study found an independent association of both urethral and anal chlamydia with receptive oral sexual practices among MSM, while the univariate association of anogenital gonorrhoea with oral sex was no longer significant after adjustment for demographic and behavioural risk factors.10 Likewise, independent associations were identified in the HIM study between pharyngeal chlamydia and receptive penile–oral sex,16 and between pharyngeal gonorrhoea and insertive rimming.11 Overseas, the prevalence of both urethral chlamydia and gonorrhoea infections was found to be over 4% among MSM whose only recent exposure was receptive penile–oral sex, and this was similar to urethral chlamydia and gonorrhoea prevalence among MSM who reported recent unprotected insertive anal intercourse.19 For pharyngeal chlamydia, a high prevalence-to-incidence ratio16 suggests that chlamydia infection may be long-lasting in the pharynx, in contrast to gonorrhoea, where a high incidence-to-prevalence ratio11 supports frequent spontaneous resolution.20,21 More recently, a pharyngeal chlamydia prevalence of up to 2.3% has been reported among MSM overseas14,18,22 and these studies have identified a higher prevalence of pharyngeal chlamydia among MSM than previously reported. Thus chlamydia as well as gonorrhoea testing is now recommended in the pharynx of MSM.
There is strong evidence in Australian MSM that nucleic acid amplification testing (NAAT) for gonorrhoea in those without urethral symptoms yields very few positive results. In a large Sydney clinical cohort, the prevalence was only 0.04% among almost 4500 asymptomatic MSM,23 and prevalence (0.33%) and incidence (0.26 per 100 person-years (PY)) were both low in the predominantly asymptomatic community-based HIM study.10 Thus, the previous recommendation not to test asymptomatic MSM for urethral gonorrhoea remains.
Commercially available NAAT is substantially more sensitive for anal and pharyngeal chlamydia and gonorrhoea detection among MSM than culture.24–27 NAAT is now recommended as the preferred method of chlamydia and gonorrhoea testing at all sites, provided that supplementary laboratory testing for nongenital gonorrhoea occurs28,29 and that gonococcal culture for antibiotic resistance surveillance is performed before treatment.29 Among MSM, gonococcal NAAT is particularly more sensitive than culture at extragenital sites and also among those with asymptomatic infections.30–32
Self-collected anorectal and pharyngeal swabs for chlamydia and gonorrhoea detection using commercially available assays perform equally well as clinician-collected swabs and are acceptable to MSM.14,33–37 In addition, self-collected samples remove the need for routine examination among asymptomatic HIV-negative MSM,38 with self-collected penile meatal swabs also performing well if adequate sampling of the urethral meatus occurs.39,40 If first-void urine (FVU) is to be used, studies from Australia41 and overseas42 have suggested the sensitivity for chlamydia detection in the male urethra is similar if urine specimens are collected 20 min after the last void, compared with 1 h after the last void.
Anal chlamydia and gonorrhoea infections have been identified as important risk factors for HIV acquisition among MSM in Australia43 and overseas.44–46 Irrespective of antiretroviral therapy, urethral chlamydia or gonorrhoea can increase seminal HIV viral load and thus infectiousness among HIV-positive MSM.47 Therefore, regular STI testing among HIV-positive MSM is likely to have public as well as individual health benefits.
Given that higher rates of gonorrhoea48 and chlamydia24,49 have been reported in Australia and overseas among HIV-positive MSM compared with HIV-negative MSM, more frequent chlamydia and gonorrhoea testing should be considered among sexually active HIV-positive MSM.
Lymphogranuloma venereum
Lymphogranuloma venereum (LGV) appears to have been endemic among MSM in the United States (US) since the early 1980s50 but, in the last decade, it has re-established itself as an important STI affecting MSM worldwide.51 The majority of LGV identified among MSM has been anorectal and symptomatic, and has predominantly affected highly sexually active and sexually adventurous core groups of HIV-positive MSM.52–54
A recent systematic review and meta-analysis55 found a significant association of LGV with HIV infection in MSM. LGV has also been described in association with incident HCV infections among MSM.52 However, it remains unclear if such associations are due to behavioural factors, biological factors or a combination of both.
Previous investigations in North America56,57 and the United Kingdom (UK)58 have failed to identify a substantial reservoir of asymptomatic LGV infection among MSM. This contrasts with other European data, where a large proportion of asymptomatic anorectal chlamydia has been identified as LGV.59,60 Anorectal LGV has been identified among Australian MSM in both clinic61,62 and community-based63,64 settings. In common with most overseas reports, Australian cases predominantly affect HIV-positive MSM, with symptoms of proctitis.
LGV was rarely identified on systematic typing of positive pharyngeal and anogenital chlamydia samples in Australian clinic- and community-based studies.61,63,65 Although LGV has been identified in the pharynx of MSM overseas,60,66 a single Australian study failed to identify any cases.63
Urethral LGV was recently identified in over 2% of MSM with anorectal LGV and almost 7% of their contacts at an Amsterdam clinic,67 with around half of these infections being asymptomatic. This report, from a relatively high-LGV prevalence setting contrasts sharply with other European58,60 and Australian63 data, where no reservoir of urethral LGV has been found among unselected MSM populations, irrespective of symptoms.
LGV remains comparatively rare among MSM in Australia. Given the low prevalence of LGV, especially in asymptomatic Australian MSM, routine LGV typing of chlamydia infections in asymptomatic MSM is not currently justified.
Mycoplasma genitalium
Anogenital M. genitalium appears to be uncommon in community-based Australian MSM. Prevalence at urethral and anorectal sites was <1% and <2%, respectively, in a cross-sectional study of over 500 Melbourne attendees at sex-on-premises venues (SOPV).68 There were no demographic or behavioural correlates of infection and site-specific symptoms were rare. The overall prevalence of anogenital M. genitalium was substantially lower than chlamydia or gonorrhoea, a pattern consistent with the prevalence of rectal infections among MSM clinic attendees in the US.69 This contrasts with findings from a UK clinic-based study,70 where the overall prevalence of all three infections was similar (5–8%), but among a subgroup of HIV-positive men, the prevalence of M. genitalium was substantially higher (21%) than that of either gonorrhoea (8%) or chlamydia (13%).
There is strong and consistent epidemiological and clinical evidence that M. genitalium can cause both acute and chronic nongonococcal urethritis (NGU).69 Findings from clinic-based studies suggest that M. genitalium is detected significantly more often in men with NGU than in asymptomatic patients.70–73 Nonetheless, M. genitalium-related NGU appears to be significantly less common among MSM compared with heterosexual men.72,74 Evidence for a role of M. genitalium in ascending male genital infections is lacking75 and there is currently no evidence that M. genitalium colonises or infects the pharynx of MSM.68
Although anorectal M. genitalium was identified in under 2% of Australian SOPV attendees,68 a higher anorectal M. genitalium prevalence (4–6%) has been reported among sexual health clinic attendees overseas.70,76 Urethral-to-anal transmission has been reported in MSM,77 although no significant association of M. genitalium with anorectal symptoms or clinical proctitis has been described.68,70,76
Multiple studies report an association between M. genitalium and HIV infection. M. genitalium prevalence appears to be significantly higher at both anal and genital sites among HIV-positive MSM compared with HIV-negative MSM in UK and US clinical settings.70,76 A 2009 systematic review and meta-analysis78 reported a statistically significant association of M. genitalium and HIV in 12 of 19 included studies, although only two included MSM. Most importantly, very few included studies were of prospective design and the critical question of temporal relationship remains unanswered. Longitudinal studies are required to address whether M. genitalium is a risk factor for HIV in MSM.
Before advocating routine M. genitalium testing, further studies to understand the contribution of M. genitalium to anogenital disease and its impact on HIV acquisition among MSM are required.
Trichomonas vaginalis
There are few data on T. vaginalis among MSM and none have reported its association with site-specific symptoms or clinical syndromes. To date, no studies have been performed among Australian MSM.
A T. vaginalis prevalence of 1.1% was found on urine NAAT testing of over 600 community-based MSM in El Salvador.79 However, over one-third of these MSM reported sex with a female partner in the past year.79 In contrast, a prospective primary care-based US study involving over 350 HIV-positive MSM failed to identify any prevalent or incident genital T. vaginalis infections.80 In a longitudinal US study comparing STI and HIV incidence between 600 Black and White MSM, no baseline genital T. vaginalis infections were identified.81 These studies, importantly, suggest that the notable racial disparities in T. vaginalis prevalence between Black and White heterosexual Americans are not present among MSM.
In two US studies involving a total of 725 MSM clinic attendees reporting recent receptive anal sex, only three NAAT-detected anal T. vaginalis infections were identified, with a prevalence of 0.9%82 and 0.2%.76 Anal T. vaginalis prevalence among MSM was almost tenfold lower than among women reporting anal sex82 and was substantially lower than the prevalence of all other anal STIs in MSM.82
Pharyngeal T. vaginalis has been identified among predominantly asymptomatic STI clinic attendees in the US, although most (>90%) infections were identified among heterosexual men.83
Taken together, these studies suggest that T. vaginalis rarely colonises the pharynx or anogenital mucosa of MSM, even in settings where the heterosexual community prevalence of T. vaginalis is substantial. Testing for T. vaginalis among asymptomatic Australian MSM is therefore not recommended.
Hepatitis A virus
Several sexually transmissible HAV outbreaks among MSM have been reported worldwide.84 These outbreaks are usually confined to subgroups of MSM such as SOPV attendees. Fortunately, most Australian jurisdictions have noted steady reductions in HAV notifications over the last decade, including in New South Wales, where notifications have declined by over 50%.85
A retrospective study comparing state HAV notifications with HAV antibody prevalence in MSM tested at Melbourne Sexual Health Centre found that the proportion found to be immune remained unchanged over a 20-year period until 2010, despite more MSM being tested for antibodies. Notably, the number of HAV notifications declined by 85% over this period, and the male-to-female ratio dropped from 4.2 to 0.9. The authors concluded that vaccination rates of 40–50% among MSM are required prevent further outbreaks of HAV86.
HAV vaccination rates among MSM remain low internationally,86,87 despite being recommended in Australia88 and overseas.89,90 Once an individual has been vaccinated, however, persistent HAV antibody responses remain in most individuals a decade of more after vaccination, irrespective of HIV status,.91
Hepatitis B virus
Each year in Australia ~7000 people are newly diagnosed with HBV, ~200 of whom have a newly acquired infection.4 MSM are at particular risk of HBV, although diagnoses among Australian men under 30 years old have declined since 2002.4
The most contemporary data on HBV incidence among Australian MSM comes from a retrospective 10-year Melbourne study.92 Encouragingly, despite a fall in immunity via vaccination or past resolved infection from 67% to 50%, this was not accompanied by a rise in HBV surface antigen detection.92
Most overseas studies among MSM are from high-prevalence settings such as Vietnam or China, where HBV prevalence is over 10%.4 Chronic HBV prevalence in Australians born overseas largely reflects their country of origin; 10% of newly-acquired HBV cases in Australia occur among people born in Asia.4 Therefore, Asian MSM are likely to be at particular risk of HBV infection.
Australia implemented a universal infant HBV vaccination in 2000 with a school-based catch-up program, meaning that many MSM born in Australia after 1986 are likely to have been vaccinated. Australian immunisation guidelines88 continue to recommend HBV vaccination for all MSM, as do other international agencies.89,90 However, ≤50% of MSM in some other countries have been vaccinated against HBV,93,94 with testing for HBV and vaccination of nonimmune HIV-positive individuals occurring in only 52% and 25%, respectively.95
Studies in immunocompetent adults continue to support the persistence of immune memory following HBV vaccination despite a fall in hepatitis B surface antibody (anti-HBs) titres, meaning further antibody testing and booster doses of HBV vaccine are unnecessary after completion of a vaccination course.96 This contrasts with the situation among those infected with HIV,97 in whom a potentially poorer and less longstanding anti-HBs response means that four double-dose vaccinations at 0, 1, 2 and 6 months are recommended, followed by double-dose boosters if annual anti-HBs titres fall below 10 IU mL–1.88
Hepatitis C virus
Over 10 000 diagnoses of HCV were made in Australia in 2011, of which ~400 were new infections. Over 80% of these new infections reported a history of injecting drug use (IDU).4 Non-IDU HCV acquisition is rarely identified among HIV-negative MSM in Australia and overseas,98–100 with an HCV prevalence of under 1% among HIV-negative non-IDU MSM overseas, arguing against routine HCV testing among HIV-negative MSM.101–104
Recently, the US Multicenter AIDS Cohort Study reported an association of incident HCV with higher numbers of receptive unprotected anal intercourse partners, as well as IDU and HIV-positive status. Nonetheless, HCV incidence was only 0.5 per 1000 PY in HIV-negative MSM compared with 4.2 per 1000 PY in HIV-positive MSM.105 Among HIV-positive MSM in Australia and overseas, there are recent data supporting sexual or permucosal HCV transmission.101,106,107 In a recent Australian study, 60% of HIV-positive Australian MSM with acute HCV did not report an IDU history.106 The exact mechanism of transmission and the reasons for an increased risk of HCV acquisition among HIV-positive MSM remain unclear, but may relate to traumatic sex practices such as fisting or shared sex toys, or to per-muscosal drug use.108,109
The most cost-effective strategy for HCV testing among asymptomatic HIV-positive MSM appears to be annual HCV antibody tests and 3–6 monthly testing of liver function,110,111 as would occur at most HIV monitoring visits.
Herpes simplex virus
Epidemiological data continue to show a variable but generally high prevalence of HSV-1 and HSV-2 among different populations of MSM. Several overseas studies involving MSM from sexual health clinics,112 community-based settings113 and MSM who recently seroconverted to HIV114 have identified a high seroprevalence of both HSV-1 and HSV-2, which is significantly higher among HIV-positive MSM compared with HIV-negative MSM.112,113
The association between HSV-1 and -2 infections and HIV-1 acquisition has been examined in several studies, both in Australia and overseas. In the STEP HIV-1 vaccine trial, which recruited over 1800 MSM from several sites internationally, including Australia, prevalent HSV-2 infection significantly increased the risk of HIV-1 acquisition.115 Longitudinal data from the Australian HIM cohort provided conflicting data, with no association being demonstrated between prevalent or incident HSV-2 and subsequent HIV infection. Prevalent HSV-1 infection was associated with risk of HIV acquisition; however, this association was no longer statistically significant when adjusted for behaviour.43 Although a transmission synergy between HSV and HIV is generally thought to exist,116 interventions to suppress HSV-2 viral replication with aciclovir have not been shown to reduce the risk of HIV acquisition in two randomised controlled trials.117,118
HSV western blot remains the gold standard for serological diagnosis of HSV.119 Type-specific HSV-2 serology that uses glycoprotein G2 antigen are commercially available, although there are concerns regarding test performance in different populations with varying HSV-2 prevalence.90,120 Some experts have advocated HSV-2 serology testing among those at high risk of acquiring HIV.119 However, the benefits of HSV-2 serological testing remain unclear. Further evaluation of testing asymptomatic MSM in terms of reducing HSV and HIV transmission, impact on sexual behaviour and psychological effects are required before any recommendations can be made for Australian MSM.
Human papillomavirus
The majority of adult MSM are infected with HPV often with multiple HPV types.121 HPV-related disease such as anogenital warts and malignancies cause substantial morbidity and mortality in these men,121–123 although the national introduction of a school-age male HPV vaccination program last year can be expected to have a major impact on HPV-related disease among future generations of Australian MSM.124
The majority of research on HPV-related disease in MSM has involved DNA detection of prevalent anal HPV infection. There are fewer prospective data on the frequency of acquisition and duration of anal HPV infection in MSM,125,126 and studies of penile and pharyngeal HPV infection in MSM are rare.123,127 A small number of studies have assessed HPV seroprevalence in MSM125 but this is unlikely to be a useful clinical diagnostic tool due to the low sensitivity of HPV serology as a marker of HPV infection.125
Estimated anal HPV prevalence in a recent large meta-analysis of 31 studies was 64% and 93% among HIV-negative and HIV-positive MSM, respectively, whereas the prevalence of high-risk (oncogenic) anal HPV types was 37% and 74%, respectively.128 Although the natural history of anogenital HPV in MSM has not been clearly defined, MSM appear to be at risk of both frequent reinfection and persistent infection, which, for high-risk anal HPV types, may explain the substantially elevated risk of anal cancer in this population.121 Studies that include assessing the utility of anal HPV testing to predict the risk of anal precancerous lesions are ongoing and are expected to guide future recommendations.129
There are few data regarding the impact of HPV infection and HIV acquisition.130 In a recent meta-analysis of the association of HIV acquisition with HPV infection at a variety of anogenital sites, a significant association was identified.131 Anal HPV infection was independently associated with HIV acquisition in the single included study that assessed anal infection among MSM.132
Considerable uncertainty surrounds the natural history of HPV infection and clinical utility of testing among MSM at present. Thus regular testing for HPV in MSM is not recommended.
HIV
Globally, HIV incidence, prevalence and the number of undiagnosed infections among MSM remain high.133–138 In Australia, an estimated 12–33% of HIV among MSM remains undiagnosed.139,140 Despite around two-thirds of gay-community-attached MSM consistently reporting testing,141 less than 40% of high-risk MSM return for HIV testing within 1 year of a previous test.142
HIV risk factors identified in Australia and overseas may indicate the need for more frequent testing in MSM and include: receptive unprotected anal intercourse, higher numbers of male sexual partners, IDU and noninjecting drug use during sex (including the use of amphetamines with oral erectile dysfunction medications), engaging in group sex, other (especially anal) STIs, sex with HIV-positive partners and high viral load in those partners.133,143–145 However, although identifying risk factors for HIV is important for targeted testing, one in eight HIV diagnoses in Australia may be missed by offering risk-based HIV testing only.146
Annual HIV testing is recommended for MSM by the US Centers for Disease Control,90 although only 60% of US MSM report being tested for HIV in the previous 12 months.147 British guidelines also recommend yearly HIV testing for MSM and more frequent testing if there are symptoms suggestive of seroconversion or ongoing high-risk exposures.148 In the US, HIV testing of MSM every 3 months when combined with high engagement in care, immediate initiation of antiretroviral therapy and inexpensive self-testing has recently been shown to be cost-effective compared with annual testing.149 However, differences in US health care systems and the availability of HIV testing means these modelling data are unlikely to be generalisable to Australian MSM populations.
Australian researchers have assessed the impact and acceptability of increased coverage and frequency of HIV testing on the number of HIV infections averted in MSM.150 Modelling indicated the single most effective scenario (increasing the frequency of testing among the 70–80% of MSM who already test annually to four times per year) resulted in a relatively modest 14% reduction in HIV incidence over 10 years, provided that other factors such as sexual behaviour and antiretroviral treatment initiation at a CD4 count of 350 cells µL–1 remain unchanged. If annual HIV testing coverage was increased to 100% of MSM, there would be a similar (11%) reduction in HIV infections over 10 years. Only one-third of Australian MSM in an online survey150 reported being ‘very likely’ to increase their testing frequency from current levels, although men reporting recent unprotected anal intercourse and who were thus at the highest risk of HIV, were more willing to increase HIV testing frequency. Current inflexible and inconvenient clinic-based testing was cited as a barrier to achieving this goal. The availability of clinic-, community- or home-based rapid HIV testing may address some of these concerns, although there are few controlled studies on the impact of such strategies on the coverage or frequency of HIV testing.151
Given that a large proportion of Australian MSM are not willing to test more frequently150 and that many do not even have annual HIV testing,142 testing for HIV at least once per year is recommended for all MSM. This recommendation is largely based on models suggesting little difference in the proportion of HIV infections averted between annual HIV testing among all MSM and current HIV testers being tested more often.150 However, those at highest HIV risk, including those reporting unprotected anal intercourse, more partners, drug use during sex or group sex (Table 1), should be targeted for more frequent HIV testing. This subgroup of the men at highest risk also appear to be the most willing to increase their frequency of HIV testing.150
Syphilis
The rate of new syphilis infections continue to rise among MSM overseas,152–156 whereas rates have plateaued in Australia since 2010.4 Syphilis is frequently diagnosed concurrently with new HIV infection.114 Globally, ~50% of infectious syphilis is diagnosed in HIV-positive MSM.157
Identified risk factors for syphilis in MSM with HIV are fisting, rimming, the use of anal sex toys, unprotected oral sex, multiple sexual partners and group sex.158–160 Mathematical modelling in Australia indicates testing high-risk MSM who report more than 10 partners per year and/or group sex (or both) every 3 months will substantially reduce the incidence and, subsequently, the prevalence of syphilis; in fact, more so than increasing the coverage of annual syphilis testing.160,161 MSM at are high risk of reinfection following a syphilis diagnosis.162 However, such men, as well as those having regular asymptomatic syphilis testing, are being tested less frequently than recommended,142,163 which is likely to jeopardise syphilis control efforts.
STI testing among HIV-positive MSM
The prevalence and incidence of STIs in MSM with HIV infection remains high in Australia164 and elsewhere in the world.137,158,165–169 Although HCV has emerged as a STI that is largely confined to HIV-positive MSM, and gonorrhoea and chlamydia are frequently identified,166 syphilis appears to be the most commonly reported STI coinfection among those with HIV.159,170 Nonetheless, STI testing rates for nongenital gonorrhoea, chlamydia171,172 and syphilis173 in MSM with HIV infection remain below recommended levels.
Overseas, among MSM attending clinics for HIV care, a 13–16% prevalence of STI coinfection has been identified,158,166,167 with an STI incidence of up to 20 per 100 PY166,167 and the majority of chlamydia and gonorrhoea being detected at nongenital sites. Independent risk factors for other STIs in these clinic-based studies included younger age, higher numbers of sexual partners, polysubstance use, sharing of sex toys with a sexual partner and enema use. In addition, syphilis was independently associated with fisting and rimming, whereas chlamydia was independently associated with drug use during sex.
Due to the high prevalence and incidence of STIs among HIV-positive MSM, consideration should be given to testing for gonorrhoea and chlamydia more than once per year, whereas syphilis testing up to four times a year should ideally occur at the same time as HIV monitoring blood tests.
Use of electronic reminders for STI testing
Electronic medical record alerts for clinicians,174 and short message service and email alerts to MSM themselves175,176 have been shown to increase detection of gonorrhoea, chlamydia and syphilis, as well as increasing retesting rates among MSM. Clinician alerts were also found to increase the yield of asymptomatic early syphilis, especially among HIV-positive MSM.167,177 Use of such technology, where available, is recommended to increase the frequency, regularity and yield of STI and HIV testing among MSM attending clinical services.
Conclusion
Efforts to improve the rates of comprehensive STI and HIV testing for MSM should focus on STI education, promotion of regular testing and building the sexual health capacity of general practice.178,179 To improve access to STI and HIV testing for Australian MSM, strategies such as rapid HIV testing, information technology and the use of social media180–182 should be fully utilised. The testing guidelines outlined in Table 1 aim to guide clinicians in their STI and HIV testing practice for MSM and provide an additional clinical tool to assist in reducing the community prevalence of STIs and HIV among Australian MSM.
Conflicts of interest
None declared.
Acknowledgements
We thank Dr Shi-Chi Kao for his assistance with the review process, Dr Mary Poynten for her review of the HPV section, A/Prof Catriona Bradshaw for her review of the M. genitalium section and A/Prof Rebecca Guy for her advice on the HIV section.
References
[1] Pedrana A, Hellard M, Guy R, El-Hayek C, Gouillou M, Asselin J, et al Stop the Drama Downunder: a social marketing campaign increases HIV/sexually transmitted infection knowledge and testing in Australian gay men. Sex Transm Dis 2012; 39 651–8.| Stop the Drama Downunder: a social marketing campaign increases HIV/sexually transmitted infection knowledge and testing in Australian gay men.Crossref | GoogleScholarGoogle Scholar | 22801349PubMed |
[2] Wilkinson A, El-Hayek C, Fairley CK, Leslie D, Roth N, Tee BK, et al Incidence and risk factors associated with chlamydia in men who have sex with men: a cohort analysis of Victorian Primary Care Network for Sentinel Surveillance data. Sex Transm Infect 2012; 88 319–24.
| Incidence and risk factors associated with chlamydia in men who have sex with men: a cohort analysis of Victorian Primary Care Network for Sentinel Surveillance data.Crossref | GoogleScholarGoogle Scholar | 22344714PubMed |
[3] Mallitt KA, Wilson DP, McDonald A, Wand H. HIV incidence trends vary between jurisdictions in Australia: an extended back-projection analysis of men who have sex with men. Sex Health 2012; 9 138–43.
| 22498157PubMed |
[4] The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia, annual surveillance report, 2013. Sydney: University of New South Wales; 2013.
[5] Zablotska IB, Kippax S, Grulich A, Holt M, Prestage G. Behavioural surveillance among gay men in Australia: methods, findings and policy implications for the prevention of HIV and other sexually transmissible infections. Sex Health 2011; 8 272–9.
| Behavioural surveillance among gay men in Australia: methods, findings and policy implications for the prevention of HIV and other sexually transmissible infections.Crossref | GoogleScholarGoogle Scholar | 21851766PubMed |
[6] Jin F, Prestage GP, Templeton DJ, Poynten IM, Donovan B, Zablotska I, et al The impact of HIV seroadaptive behaviors on sexually transmissible infections in HIV-negative homosexual men in Sydney, Australia. Sex Transm Dis 2012; 39 191–4.
| The impact of HIV seroadaptive behaviors on sexually transmissible infections in HIV-negative homosexual men in Sydney, Australia.Crossref | GoogleScholarGoogle Scholar | 22337105PubMed |
[7] Brill JR. Sexually transmitted infections in men. Prim Care 2010; 37 509–25.
| Sexually transmitted infections in men.Crossref | GoogleScholarGoogle Scholar | 20705196PubMed |
[8] Cohen MS. HIV and sexually transmitted diseases: lethal synergy. Top HIV Med 2004; 12 104–7.
| 15516707PubMed |
[9] New South Wales STI Programs Unit (STIPU). Sexually transmitted infection testing guidelines for men who have sex with men. Sydney: STIPU; 2010. Available online at: http://www.stipu.nsw.gov.au/icms_docs/163932_STI_testing_guidelines_for_MSM_2010.pdf [verified 2 December 2013].
[10] Jin F, Prestage GP, Mao L, Kippax SC, Pell CM, Donovan B, et al Incidence and risk factors for urethral and anal gonorrhoea and chlamydia in a cohort of HIV-negative homosexual men: the Health in Men Study. Sex Transm Infect 2007; 83 113–9.
| Incidence and risk factors for urethral and anal gonorrhoea and chlamydia in a cohort of HIV-negative homosexual men: the Health in Men Study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2s3it12ltQ%3D%3D&md5=c30f0c0de795622416e469a959f27e8cCAS | 17005541PubMed |
[11] Templeton DJ, Jin F, McNally LP, Imrie JC, Prestage GP, Donovan B, et al Prevalence, incidence and risk factors for pharyngeal gonorrhoea in a community-based HIV-negative cohort of homosexual men in Sydney, Australia. Sex Transm Infect 2010; 86 90–6.
| Prevalence, incidence and risk factors for pharyngeal gonorrhoea in a community-based HIV-negative cohort of homosexual men in Sydney, Australia.Crossref | GoogleScholarGoogle Scholar | 19841003PubMed |
[12] Ota KV, Fisman DN, Tamari IE, Smieja M, Ng LK, Jones KE, et al Incidence and treatment outcomes of pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections in men who have sex with men: a 13-year retrospective cohort study. Clin Infect Dis 2009; 48 1237–43.
| Incidence and treatment outcomes of pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections in men who have sex with men: a 13-year retrospective cohort study.Crossref | GoogleScholarGoogle Scholar | 19323630PubMed |
[13] Annan NT, Sullivan AK, Nori A, Naydenova P, Alexander S, McKenna A, et al Rectal chlamydia – a reservoir of undiagnosed infection in men who have sex with men. Sex Transm Infect 2009; 85 176–9.
| Rectal chlamydia – a reservoir of undiagnosed infection in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MznsFGntA%3D%3D&md5=b14ff58e066cdb5a5592b9c6a419b9afCAS | 19176570PubMed |
[14] Soni S, White JA. Self-screening for Neisseria gonorrhoeae and Chlamydia trachomatis in the human immunodeficiency virus clinic – high yields and high acceptability. Sex Transm Dis 2011; 38 1107–9.
| Self-screening for Neisseria gonorrhoeae and Chlamydia trachomatis in the human immunodeficiency virus clinic – high yields and high acceptability.Crossref | GoogleScholarGoogle Scholar | 22082720PubMed |
[15] Holt M, Hull P, Lea T, Guy R, Bourne C, Prestage G, et al Comprehensive testing for, and diagnosis of, sexually transmissible infections among Australian gay and bisexual men: findings from repeated, cross-sectional behavioural surveillance, 2003–2012. Sex Transm Infect 2013; •••
| Comprehensive testing for, and diagnosis of, sexually transmissible infections among Australian gay and bisexual men: findings from repeated, cross-sectional behavioural surveillance, 2003–2012.Crossref | GoogleScholarGoogle Scholar | 24234070PubMed |
[16] Templeton DJ, Jin F, Imrie J, Prestage GP, Donovan B, Cunningham PH, et al Prevalence, incidence and risk factors for pharyngeal chlamydia in the community based Health in Men (HIM) cohort of homosexual men in Sydney, Australia. Sex Transm Infect 2008; 84 361–3.
| Prevalence, incidence and risk factors for pharyngeal chlamydia in the community based Health in Men (HIM) cohort of homosexual men in Sydney, Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cnhslyqsg%3D%3D&md5=4b0a47f7720add7f8da650f2052b1f56CAS | 18596068PubMed |
[17] Peters RP, Verweij SP, Nijsten N, Ouburg S, Mutsaers J, Jansen CL, et al Evaluation of sexual history-based screening of anatomic sites for Chlamydia trachomatis and Neisseria gonorrhoeae infection in men having sex with men in routine practice. BMC Infect Dis 2011; 11 203
| Evaluation of sexual history-based screening of anatomic sites for Chlamydia trachomatis and Neisseria gonorrhoeae infection in men having sex with men in routine practice.Crossref | GoogleScholarGoogle Scholar | 21791061PubMed |
[18] Park J, Marcus JL, Pandori M, Snell A, Philip SS, Bernstein KT. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men – San Francisco, 2010. Sex Transm Dis 2012; 39 482–4.
| Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men – San Francisco, 2010.Crossref | GoogleScholarGoogle Scholar | 22592836PubMed |
[19] Bernstein KT, Stephens SC, Barry PM, Kohn R, Philip SS, Liska S, et al Chlamydia trachomatis and Neisseria gonorrhoeae transmission from the oropharynx to the urethra among men who have sex with men. Clin Infect Dis 2009; 49 1793–7.
| Chlamydia trachomatis and Neisseria gonorrhoeae transmission from the oropharynx to the urethra among men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 19911970PubMed |
[20] Apewokin SK, Geisler WM, Bachmann LH. Spontaneous resolution of extragenital chlamydial and gonococcal infections prior to therapy. Sex Transm Dis 2010; 37 343–4.
| 20393382PubMed |
[21] Hutt DM, Judson FN. Epidemiology and treatment of oropharyngeal gonorrhea. Ann Intern Med 1986; 104 655–8.
| Epidemiology and treatment of oropharyngeal gonorrhea.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL287osFSgtw%3D%3D&md5=e269f3ed8eca9fe1b1b2a09d89accbe2CAS | 2938529PubMed |
[22] Centers for Disease Control and Prevention Clinic-based testing for rectal and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections by community-based organizations –five cities, United States, 2007. MMWR Morb Mortal Wkly Rep 2009; 58 716–9.
| 19590491PubMed |
[23] Ryder N, Lockart IG, Bourne C. Is screening asymptomatic men who have sex with men for urethral gonorrhoea worthwhile? Sex Health 2010; 7 90–1.
| Is screening asymptomatic men who have sex with men for urethral gonorrhoea worthwhile?Crossref | GoogleScholarGoogle Scholar | 20152104PubMed |
[24] Turner AN, Reese PC, Ervin M, Davis JA, Fields KS, Bazan JA. HIV, rectal chlamydia, and rectal gonorrhea in men who have sex with men attending a sexually transmitted disease clinic in a midwestern US city. Sex Transm Dis 2013; 40 433–8.
| HIV, rectal chlamydia, and rectal gonorrhea in men who have sex with men attending a sexually transmitted disease clinic in a midwestern US city.Crossref | GoogleScholarGoogle Scholar | 23677015PubMed |
[25] Pinsky L, Chiarilli DB, Klausner JD, Kull RM, O’Keefe R, Heffer C, et al Rates of asymptomatic nonurethral gonorrhea and chlamydia in a population of university men who have sex with men. J Am Coll Health 2012; 60 481–4.
| Rates of asymptomatic nonurethral gonorrhea and chlamydia in a population of university men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 22857141PubMed |
[26] Bachmann LH, Johnson RE, Cheng H, Markowitz L, Papp JR, Palella FJ, et al Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol 2010; 48 1827–32.
| Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections.Crossref | GoogleScholarGoogle Scholar | 20335410PubMed |
[27] Ota KV, Tamari IE, Smieja M, Jamieson F, Jones KE, Towns L, et al Detection of Neisseria gonorrhoeae and Chlamydia trachomatis in pharyngeal and rectal specimens using the BD Probetec ET system, the Gen-Probe Aptima Combo 2 assay and culture. Sex Transm Infect 2009; 85 182–6.
| Detection of Neisseria gonorrhoeae and Chlamydia trachomatis in pharyngeal and rectal specimens using the BD Probetec ET system, the Gen-Probe Aptima Combo 2 assay and culture.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MznsFCqsA%3D%3D&md5=2a167ccc1b31446bef6f716de7a19078CAS | 19126571PubMed |
[28] McNally LP, Templeton DJ, Jin F, Grulich AE, Donovan B, Whiley DM, et al Low positive predictive value of a nucleic acid amplification test for nongenital Neisseria gonorrhoeae infection in homosexual men. Clin Infect Dis 2008; 47 e25–7.
| Low positive predictive value of a nucleic acid amplification test for nongenital Neisseria gonorrhoeae infection in homosexual men.Crossref | GoogleScholarGoogle Scholar | 18549310PubMed |
[29] Fairley CK, Chen MY, Bradshaw CS, Tabrizi SN. Is it time to move to nucleic acid amplification tests screening for pharyngeal and rectal gonorrhoea in men who have sex with men to improve gonorrhoea control? Sex Health 2011; 8 9–11.
| Is it time to move to nucleic acid amplification tests screening for pharyngeal and rectal gonorrhoea in men who have sex with men to improve gonorrhoea control?Crossref | GoogleScholarGoogle Scholar | 21371376PubMed |
[30] Sherley M, Kennedy KJ, Martin SJ. Screening with nucleic acid amplification tests for gonorrhoea in men who have sex with men. Med J Aust 2012; 197 332
| Screening with nucleic acid amplification tests for gonorrhoea in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 22994826PubMed |
[31] Harryman L, Scofield S, Macleod J, Carrington D, Williams OM, Fernandes A, et al Comparative performance of culture using swabs transported in Amies medium and the Aptima Combo 2 nucleic acid amplification test in detection of Neisseria gonorrhoeae from genital and extra-genital sites: a retrospective study. Sex Transm Infect 2012; 88 27–31.
| Comparative performance of culture using swabs transported in Amies medium and the Aptima Combo 2 nucleic acid amplification test in detection of Neisseria gonorrhoeae from genital and extra-genital sites: a retrospective study.Crossref | GoogleScholarGoogle Scholar | 22034496PubMed |
[32] Bissessor M, Tabrizi SN, Fairley CK, Danielewski J, Whitton B, Bird S, et al Differing Neisseria gonorrhoeae bacterial loads in the pharynx and rectum in men who have sex with men: implications for gonococcal detection, transmission, and control. J Clin Microbiol 2011; 49 4304–6.
| Differing Neisseria gonorrhoeae bacterial loads in the pharynx and rectum in men who have sex with men: implications for gonococcal detection, transmission, and control.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38%2FjtVantw%3D%3D&md5=38d6ac7a9be0333f26fea9252892f96bCAS | 21956992PubMed |
[33] Dodge B, Van Der Pol B, Reece M, Malebranche D, Martinez O, Goncalves G, et al Rectal self-sampling in non-clinical venues for detection of sexually transmissible infections among behaviourally bisexual men. Sex Health 2012; 9 190–1.
| 22498165PubMed |
[34] Sexton ME, Baker JJ, Nakagawa K, Li Y, Perkins R, Slack RS, et al How reliable is self-testing for gonorrhea and chlamydia among men who have sex with men? J Fam Pract 2013; 62 70–8.
| 23405376PubMed |
[35] Dodge B, Van Der Pol B, Rosenberger JG, Reece M, Roth AM, Herbenick D, et al Field collection of rectal samples for sexually transmitted infection diagnostics among men who have sex with men. Int J STD AIDS 2010; 21 260–4.
| Field collection of rectal samples for sexually transmitted infection diagnostics among men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c3kvFSqug%3D%3D&md5=65401876c6769b017a7e0e9afde68353CAS | 20378897PubMed |
[36] van der Helm JJ, Hoebe CJ, van Rooijen MS, Brouwers EE, Fennema HS, Thiesbrummel HF, et al High performance and acceptability of self-collected rectal swabs for diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men who have sex with men and women. Sex Transm Dis 2009; 36 493–7.
| High performance and acceptability of self-collected rectal swabs for diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men who have sex with men and women.Crossref | GoogleScholarGoogle Scholar | 19617869PubMed |
[37] Moncada J, Schachter J, Liska S, Shayevich C, Klausner JD. Evaluation of self-collected glans and rectal swabs from men who have sex with men for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of nucleic acid amplification tests. J Clin Microbiol 2009; 47 1657–62.
| Evaluation of self-collected glans and rectal swabs from men who have sex with men for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of nucleic acid amplification tests.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXosVejtbc%3D&md5=dc4029bc9e28062a345c69e1dbb56f20CAS | 19369445PubMed |
[38] Templeton DJ, Wang Y, Higgins AN, Manokaran N. Self-collected anal swabs in men who have sex with men: minimal benefit of routine peri-anal examination. Sex Transm Infect 2011; 87 204
| Self-collected anal swabs in men who have sex with men: minimal benefit of routine peri-anal examination.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M3ot1SqtA%3D%3D&md5=c89bf6710f0a0080aec1c8366ff9326eCAS | 21292689PubMed |
[39] Dize L, Agreda P, Quinn N, Barnes MR, Hsieh YH, Gaydos CA. Comparison of self-obtained penile-meatal swabs to urine for the detection of C. trachomatis, N. gonorrhoeae and T. vaginalis. Sex Transm Infect 2013; 89 305–7.
| Comparison of self-obtained penile-meatal swabs to urine for the detection of C. trachomatis, N. gonorrhoeae and T. vaginalis.Crossref | GoogleScholarGoogle Scholar | 23093735PubMed |
[40] Chernesky MA, Jang D, Portillo E, Smieja M, Gilchrist J, Ewert R, et al Self-collected swabs of the urinary meatus diagnose more Chlamydia trachomatis and Neisseria gonorrhoeae infections than first catch urine from men. Sex Transm Infect 2013; 89 102–4.
| Self-collected swabs of the urinary meatus diagnose more Chlamydia trachomatis and Neisseria gonorrhoeae infections than first catch urine from men.Crossref | GoogleScholarGoogle Scholar | 23024224PubMed |
[41] Kwan B, Ryder N, Knight V, Kenigsberg A, McNulty A, Read P, et al Sensitivity of 20-minute voiding intervals in men testing for Chlamydia trachomatis. Sex Transm Dis 2012; 39 405–6.
| Sensitivity of 20-minute voiding intervals in men testing for Chlamydia trachomatis.Crossref | GoogleScholarGoogle Scholar | 22504609PubMed |
[42] Mathew T, O’Mahony C, Mallinson H. Shortening the voiding interval for men having chlamydia nucleic acid amplification tests. Int J STD AIDS 2009; 20 752–3.
| Shortening the voiding interval for men having chlamydia nucleic acid amplification tests.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1Mjis1Whsw%3D%3D&md5=584bd8108a0d97646d2991940dd8ecdeCAS | 19875829PubMed |
[43] Jin F, Prestage GP, Imrie J, Kippax SC, Donovan B, Templeton DJ, et al Anal sexually transmitted infections and risk of HIV infection in homosexual men. J Acquir Immune Defic Syndr 2010; 53 144–9.
| Anal sexually transmitted infections and risk of HIV infection in homosexual men.Crossref | GoogleScholarGoogle Scholar | 19734801PubMed |
[44] Pathela P, Braunstein SL, Blank S, Schillinger JA. HIV incidence among men with and those without sexually transmitted rectal infections: estimates from matching against an HIV case registry. Clin Infect Dis 2013; 57 1203–9.
| HIV incidence among men with and those without sexually transmitted rectal infections: estimates from matching against an HIV case registry.Crossref | GoogleScholarGoogle Scholar | 23800942PubMed |
[45] Bernstein KT, Marcus JL, Nieri G, Philip SS, Klausner JD. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr 2010; 53 537–43.
| Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion.Crossref | GoogleScholarGoogle Scholar | 19935075PubMed |
[46] Sanders EJ, Okuku HS, Smith AD, Mwangome M, Wahome E, Fegan G, et al High HIV-1 incidence, correlates of HIV-1 acquisition, and high viral loads following seroconversion among MSM. AIDS 2013; 27 437–46.
| High HIV-1 incidence, correlates of HIV-1 acquisition, and high viral loads following seroconversion among MSM.Crossref | GoogleScholarGoogle Scholar | 23079811PubMed |
[47] Kalichman SC, Pellowski J, Turner C. Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect 2011; 87 183–90.
| Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention.Crossref | GoogleScholarGoogle Scholar | 21330572PubMed |
[48] Jin F, Prestage GP, Zablotska I, Rawstorne P, Kippax SC, Donovan B, et al High rates of sexually transmitted infections in HIV positive homosexual men: data from two community based cohorts. Sex Transm Infect 2007; 83 397–9.
| High rates of sexually transmitted infections in HIV positive homosexual men: data from two community based cohorts.Crossref | GoogleScholarGoogle Scholar | 17556503PubMed |
[49] Lim MS, Goller JL, Guy R, Gold J, Stoove M, Hocking JS, et al Correlates of Chlamydia trachomatis infection in a primary care sentinel surveillance network. Sex Health 2012; 9 247–53.
| Correlates of Chlamydia trachomatis infection in a primary care sentinel surveillance network.Crossref | GoogleScholarGoogle Scholar | 22697142PubMed |
[50] Christerson L, de Vries HJ, de Barbeyrac B, Gaydos CA, Henrich B, Hoffmann S, et al Typing of lymphogranuloma venereum Chlamydia trachomatis strains. Emerg Infect Dis 2010; 16 1777–9.
| Typing of lymphogranuloma venereum Chlamydia trachomatis strains.Crossref | GoogleScholarGoogle Scholar | 21029543PubMed |
[51] Ward H, Ronn M. Lymphogranuloma venereum in men who have sex in men: are we missing a reservoir of infection? Sex Transm Dis 2013; 40 609–10.
| Lymphogranuloma venereum in men who have sex in men: are we missing a reservoir of infection?Crossref | GoogleScholarGoogle Scholar | 23859906PubMed |
[52] White JA. Manifestations and management of lymphogranuloma venereum. Curr Opin Infect Dis 2009; 22 57–66.
| Manifestations and management of lymphogranuloma venereum.Crossref | GoogleScholarGoogle Scholar | 19532081PubMed |
[53] de Vries HJ, van der Bij AK, Fennema JS, Smit C, de Wolf F, Prins M, et al Lymphogranuloma venereum proctitis in men who have sex with men is associated with anal enema use and high-risk behavior. Sex Transm Dis 2008; 35 203–8.
| Lymphogranuloma venereum proctitis in men who have sex with men is associated with anal enema use and high-risk behavior.Crossref | GoogleScholarGoogle Scholar | 18091565PubMed |
[54] Hughes G, Alexander S, Simms I, Conti S, Ward H, Powers C, et al Lymphogranuloma venereum diagnoses among men who have sex with men in the UK: interpreting a cross-sectional study using an epidemic phase-specific framework. Sex Transm Infect 2013; 895 42–7.
[55] Rönn MM, Ward H. The association between lymphogranuloma venereum and HIV among men who have sex with men: systematic review and meta-analysis. BMC Infect Dis 2011; 11 70
| The association between lymphogranuloma venereum and HIV among men who have sex with men: systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 21418569PubMed |
[56] Tinmouth J, Gilmour MW, Kovacs C, Kropp R, Mitterni L, Rachlis A, et al Is there a reservoir of sub-clinical lymphogranuloma venereum and non-LGV Chlamydia trachomatis infection in men who have sex with men? Int J STD AIDS 2008; 19 805–9.
| Is there a reservoir of sub-clinical lymphogranuloma venereum and non-LGV Chlamydia trachomatis infection in men who have sex with men?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cjot1SqtA%3D%3D&md5=fa329fddfa5691818da646b78b37e14cCAS | 19050208PubMed |
[57] Geisler WM, Morrison SG, Bachmann LH. Absence of lymphogranuloma venereum strains among rectal Chlamydia trachomatis outer membrane protein A genotypes infecting women and men who have sex with men in Birmingham, Alabama. Sex Transm Dis 2008; 35 856–8.
| Absence of lymphogranuloma venereum strains among rectal Chlamydia trachomatis outer membrane protein A genotypes infecting women and men who have sex with men in Birmingham, Alabama.Crossref | GoogleScholarGoogle Scholar | 18580820PubMed |
[58] Ward H, Alexander S, Carder C, Dean G, French P, Ivens D, et al The prevalence of lymphogranuloma venereum infection in men who have sex with men: results of a multicentre case finding study. Sex Transm Infect 2009; 85 173–5.
| The prevalence of lymphogranuloma venereum infection in men who have sex with men: results of a multicentre case finding study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MznsFKjsA%3D%3D&md5=2e8a4480bdf91fd88463d17a7fc4e81eCAS | 19221105PubMed |
[59] Van der Bij AK, Spaargaren J, Morré SA, Fennema HSA, Mindel A, Coutinho RA, et al Diagnostic and clinical implications of anorectal lymphogranuloma venereum in men who have sex with men: a retrospective case–control study. Clin Infect Dis 2006; 42 186–94.
| Diagnostic and clinical implications of anorectal lymphogranuloma venereum in men who have sex with men: a retrospective case–control study.Crossref | GoogleScholarGoogle Scholar | 16355328PubMed |
[60] Haar K, Dudareva-Vizule S, Wisplinghoff H, Wisplinghoff F, Sailer A, Jansen K, et al Lymphogranuloma venereum in men screened for pharyngeal and rectal infection, Germany. Emerg Infect Dis 2013; 19 488–92.
| Lymphogranuloma venereum in men screened for pharyngeal and rectal infection, Germany.Crossref | GoogleScholarGoogle Scholar | 23621949PubMed |
[61] Templeton DJ, Sharp N, Gryllis S, O’Connor CC, Dubedat SM. Prevalence and predictors of lymphogranuloma venereum among men who have sex with men at a Sydney metropolitan sexual health clinic. Sex Health 2013; 10 190–1.
| Prevalence and predictors of lymphogranuloma venereum among men who have sex with men at a Sydney metropolitan sexual health clinic.Crossref | GoogleScholarGoogle Scholar | 23449004PubMed |
[62] Lee DM, Fairley CK, Owen L, Horvath L, Chen MY. Lymphogranuloma venereum becomes an established infection among men who have sex with men in Melbourne. Aust N Z J Public Health 2009; 33 94
| Lymphogranuloma venereum becomes an established infection among men who have sex with men in Melbourne.Crossref | GoogleScholarGoogle Scholar | 19236368PubMed |
[63] Templeton DJ, Grulich AE, Yew J, Twin J, Jin F, Prestage GP, et al Lymphogranuloma venereum is rare in Australian community-based samples of men who have sex with men. Sex Transm Dis 2011; 38 48–9.
| Lymphogranuloma venereum is rare in Australian community-based samples of men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 20625348PubMed |
[64] Templeton DJ, Twin J, Jin F, Grulich AE, Garland SM, Tabrizi SN. Chlamydia trachomatis serovars in community-based HIV-positive and HIV-negative men who have sex with men in Sydney, Australia. Sex Transm Infect 2011; 87 501–2.
| Chlamydia trachomatis serovars in community-based HIV-positive and HIV-negative men who have sex with men in Sydney, Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mfjs1aisQ%3D%3D&md5=56987422b0a1821d0621edd16b766f68CAS | 21813568PubMed |
[65] Twin J, Moore EE, Garland SM, Stevens MP, Fairley CK, Donovan B, et al Chlamydia trachomatis genotypes among men who have sex with men in Australia. Sex Transm Dis 2011; 38 279–85.
| 21085058PubMed |
[66] Korhonen S, Hiltunen-Back E, Puolakkainen M. Genotyping of Chlamydia trachomatis in rectal and pharyngeal specimens: identification of LGV genotypes in Finland. Sex Transm Infect 2012; 88 465–9.
| Genotyping of Chlamydia trachomatis in rectal and pharyngeal specimens: identification of LGV genotypes in Finland.Crossref | GoogleScholarGoogle Scholar | 22517888PubMed |
[67] de Vrieze NH, van Rooijen M, Speksnijder AG, de Vries HJ. Urethral lymphogranuloma venereum infections in men with anorectal lymphogranuloma venereum and their partners: the missing link in the current epidemic? Sex Transm Dis 2013; 40 607–8.
| Urethral lymphogranuloma venereum infections in men with anorectal lymphogranuloma venereum and their partners: the missing link in the current epidemic?Crossref | GoogleScholarGoogle Scholar | 23859905PubMed |
[68] Bradshaw CS, Fairley CK, Lister NA, Chen SJ, Garland SM, Tabrizi SN. Mycoplasma genitalium in men who have sex with men at male-only saunas. Sex Transm Infect 2009; 85 432–5.
| Mycoplasma genitalium in men who have sex with men at male-only saunas.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1Mnos1OqsQ%3D%3D&md5=30051c51464a70cefea1f9510b734eabCAS | 19254902PubMed |
[69] Cazanave C, Manhart LE, Bebear C. Mycoplasma genitalium, an emerging sexually transmitted pathogen. Med Mal Infect 2012; 42 381–92.
| Mycoplasma genitalium, an emerging sexually transmitted pathogen.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38bms1ygtw%3D%3D&md5=8f6731bbfb89aa3070f4aeeae2956877CAS | 22975074PubMed |
[70] Soni S, Alexander S, Verlander N, Saunders P, Richardson D, Fisher M, et al The prevalence of urethral and rectal Mycoplasma genitalium and its associations in men who have sex with men attending a genitourinary medicine clinic. Sex Transm Infect 2010; 86 21–4.
| The prevalence of urethral and rectal Mycoplasma genitalium and its associations in men who have sex with men attending a genitourinary medicine clinic.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c7gs1CrtA%3D%3D&md5=7ccf2d575a831144316b52d6716e3f4cCAS | 19843536PubMed |
[71] Weinstein SA, Stiles BG. A review of the epidemiology, diagnosis and evidence-based management of Mycoplasma genitalium. Sex Health 2011; 8 143–58.
| A review of the epidemiology, diagnosis and evidence-based management of Mycoplasma genitalium.Crossref | GoogleScholarGoogle Scholar | 21592428PubMed |
[72] Moi H, Reinton N, Moghaddam A. Mycoplasma genitalium is associated with symptomatic and asymptomatic non-gonococcal urethritis in men. Sex Transm Infect 2009; 85 15–8.
| Mycoplasma genitalium is associated with symptomatic and asymptomatic non-gonococcal urethritis in men.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M%2Fos12hsw%3D%3D&md5=01f35c1d943dca410b4550e483160126CAS | 18842690PubMed |
[73] Couldwell DL, Gidding HF, Freedman EV, McKechnie ML, Biggs K, Sintchenko V, et al Ureaplasma urealyticum is significantly associated with non-gonococcal urethritis in heterosexual Sydney men. Int J STD AIDS 2010; 21 337–41.
| Ureaplasma urealyticum is significantly associated with non-gonococcal urethritis in heterosexual Sydney men.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3czlvFWksg%3D%3D&md5=5945dbdb662c274089bbbe5986854085CAS | 20498103PubMed |
[74] Mezzini TM, Waddell RG, Douglas RJ, Sadlon TA. Mycoplasma genitalium: prevalence in men presenting with urethritis to a South Australian public sexual health clinic. Intern Med J 2013; 43 494–500.
| Mycoplasma genitalium: prevalence in men presenting with urethritis to a South Australian public sexual health clinic.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3szpvFShsQ%3D%3D&md5=3aef7b31751051ab9d8b3600436160ceCAS | 23425506PubMed |
[75] Street E, Joyce A, Wilson J. BASHH UK guideline for the management of epididymo-orchitis, 2010. Int J STD AIDS 2011; 22 361–5.
| BASHH UK guideline for the management of epididymo-orchitis, 2010.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MnlvVSiug%3D%3D&md5=9a1c9827fdaad218946fc925d5ad64beCAS | 21729951PubMed |
[76] Francis SC, Kent CK, Klausner JD, Rauch L, Kohn R, Hardick A, et al Prevalence of rectal Trichomonas vaginalis and Mycoplasma genitalium in male patients at the San Francisco STD clinic, 2005–2006. Sex Transm Dis 2008; 35 797–800.
| Prevalence of rectal Trichomonas vaginalis and Mycoplasma genitalium in male patients at the San Francisco STD clinic, 2005–2006.Crossref | GoogleScholarGoogle Scholar | 18607317PubMed |
[77] Edlund M, Blaxhult A, Bratt G. The spread of Mycoplasma genitalium among men who have sex with men. Int J STD AIDS 2012; 23 455–6.
| The spread of Mycoplasma genitalium among men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38fgvFGgtQ%3D%3D&md5=ff538a6b958a6cd524508a0e0a49fd5aCAS | 22807547PubMed |
[78] Napierala Mavedzenge S, Weiss HA. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS 2009; 23 611–20.
| Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 19194271PubMed |
[79] Creswell J, Guardado ME, Lee J, Nieto AI, Kim AA, Monterroso E, et al HIV and STI control in El Salvador: results from an integrated behavioural survey among men who have sex with men. Sex Transm Infect 2012; 88 633–8.
| HIV and STI control in El Salvador: results from an integrated behavioural survey among men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 22917694PubMed |
[80] Mayer KH, Bush T, Henry K, Overton ET, Hammer J, Richardson J, et al Ongoing sexually transmitted disease acquisition and risk-taking behavior among US HIV-infected patients in primary care: implications for prevention interventions. Sex Transm Dis 2012; 39 1–7.
| Ongoing sexually transmitted disease acquisition and risk-taking behavior among US HIV-infected patients in primary care: implications for prevention interventions.Crossref | GoogleScholarGoogle Scholar | 22183836PubMed |
[81] Kelley CF, Rosenberg ES, O’Hara BM, Sanchez T, del Rio C, Sullivan PS. Prevalence of urethral Trichomonas vaginalis in Black and White men who have sex with men. Sex Transm Dis 2012; 39 739
| Prevalence of urethral Trichomonas vaginalis in Black and White men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 22902674PubMed |
[82] Cosentino LA, Campbell T, Jett A, Macio I, Zamborsky T, Cranston RD, et al Use of nucleic acid amplification testing for diagnosis of anorectal sexually transmitted infections. J Clin Microbiol 2012; 50 2005–8.
| Use of nucleic acid amplification testing for diagnosis of anorectal sexually transmitted infections.Crossref | GoogleScholarGoogle Scholar | 22493338PubMed |
[83] Munson E, Wenten D, Phipps P, Gremminger R, Schuknecht MK, Napierala M, et al Retrospective assessment of transcription-mediated amplification-based screening for Trichomonas vaginalis in male sexually transmitted infection clinic patients. J Clin Microbiol 2013; 51 1855–60.
| Retrospective assessment of transcription-mediated amplification-based screening for Trichomonas vaginalis in male sexually transmitted infection clinic patients.Crossref | GoogleScholarGoogle Scholar | 23554208PubMed |
[84] Cotter SM, Sansom S, Long T, Koch E, Kellerman S, Smith F, et al Outbreak of hepatitis A among men who have sex with men: implications for hepatitis A vaccination strategies. J Infect Dis 2003; 187 1235–40.
| Outbreak of hepatitis A among men who have sex with men: implications for hepatitis A vaccination strategies.Crossref | GoogleScholarGoogle Scholar | 12696002PubMed |
[85] NSW Public Health Unit. Hepatitis A notifications by month and sex. Sydney: New South Wales Ministry of Health; 2013. Available online at: http://www.healthstats.nsw.gov.au/Indicator/com_hepanot/com_hepanot [verified July 2013].
[86] Weerakoon AP, Chen MY, Read TRH, Bradshaw C, Fairley CK. Immunity to hepatitis A when outbreaks of infection in men who have sex with men (MSM) are rare. Vaccine 2012; 30 3430–4.
| Immunity to hepatitis A when outbreaks of infection in men who have sex with men (MSM) are rare.Crossref | GoogleScholarGoogle Scholar | 22449421PubMed |
[87] Bialek SR, Barry V, Bell BP, Valleroy LA, Behel S, MacKellar DA, et al Seroprevalence and correlates of hepatitis A among HIV-negative American men who have sex with men. Sex Health 2011; 8 343–8.
| Seroprevalence and correlates of hepatitis A among HIV-negative American men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 21851774PubMed |
[88] Australian Government Department of Health and Ageing (DoHA). The Australian immunisation handbook, 10th edn. Canberra: DoHA; 2013.
[89] British Association of Sexual Health and HIV (BASHH). United Kingdom national guideline on the management of the viral hepatitides A, B & C., Macclesfield, UK: BASHH; 2008. Available online at: http://www.bashh.org/documents/1927.pdf [verified December 2013].
[90] Workowski KA, Berman S, Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 2010; 59 1–110.
| 21160459PubMed |
[91] Crum-Cianflone NF, Wilkins K, Lee AW, Grosso A, Landrum ML, Weintrob A, et al Long-term durability of immune responses after hepatitis A vaccination among HIV-infected adults. J Infect Dis 2011; 203 1815–23.
| Long-term durability of immune responses after hepatitis A vaccination among HIV-infected adults.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmvFChtrw%3D&md5=846771eb8e55b1a754bf8b1ab391ce99CAS | 21606540PubMed |
[92] Gamagedara N, Weerakoon AP, Zou H, Fehler G, Chen MY, Read TR, et al Cross-sectional study of hepatitis B immunity in MSM between 2002 and 2012. Sex Transm Infect 2014; 90 41–45.
| Cross-sectional study of hepatitis B immunity in MSM between 2002 and 2012.Crossref | GoogleScholarGoogle Scholar | 23920399PubMed |
[93] Baars JE, Boon BJ, Garretsen HF, van de Mheen D. The reach of a hepatitis B vaccination programme among men who have sex with men. Eur J Public Health 2011; 21 333–7.
| The reach of a hepatitis B vaccination programme among men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 20813894PubMed |
[94] Reiter PL, Brewer NT. Hepatitis B vaccination among a national sample of gay and bisexual men. Sex Transm Dis 2011; 38 235–38
| Hepatitis B vaccination among a national sample of gay and bisexual men.Crossref | GoogleScholarGoogle Scholar |
[95] Hoover KW, Butler M, Workowski KA, Follansbee S, Gratzer B, Hare CB, et al Low rates of hepatitis screening and vaccination of HIV-infected MSM in HIV clinics. Sex Transm Dis 2012; 39 349–53.
| Low rates of hepatitis screening and vaccination of HIV-infected MSM in HIV clinics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xls1Siurc%3D&md5=45f81a3d8dc1ad3be1998b096ac3ebe3CAS | 22504597PubMed |
[96] Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Crasta PD, Hardt K. Persistence and immune memory to hepatitis B vaccine 20 years after primary vaccination of Thai infants, born to HBsAg and HBeAg positive mothers. Hum Vaccin Immunother 2012; 8 896–904.
| Persistence and immune memory to hepatitis B vaccine 20 years after primary vaccination of Thai infants, born to HBsAg and HBeAg positive mothers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsF2nsbvE&md5=07644a0993c1270de655b4dbd41b14b8CAS | 22777097PubMed |
[97] Powis JE, Raboud J, Ostrowski M, Loutfy MR, Kovacs C, Walmsley SL. The Recombinant Hepatitis B Surface Antigen Vaccine in Persons With HIV: Is Seroconversion Sufficient for Long-term Protection? J Infect Dis 2012; 205 1534–8.
| The Recombinant Hepatitis B Surface Antigen Vaccine in Persons With HIV: Is Seroconversion Sufficient for Long-term Protection?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmsFarsbk%3D&md5=4ab9ebda9c2d9bf1ba8d9e607290a88fCAS | 22448009PubMed |
[98] Jin F, Prestage GP, Matthews G, Zablotska I, Rawstorne P, Kippax SC, et al Prevalence, incidence and risk factors for hepatitis C in homosexual men: data from two cohorts of HIV-negative and HIV-positive men in Sydney, Australia. Sex Transm Infect 2010; 86 25–8.
| Prevalence, incidence and risk factors for hepatitis C in homosexual men: data from two cohorts of HIV-negative and HIV-positive men in Sydney, Australia.Crossref | GoogleScholarGoogle Scholar | 19841001PubMed |
[99] van de Laar TJ, Paxton WA, Zorgdrager F, Cornelissen M, de Vries HJ. Sexual transmission of hepatitis C virus in human immunodeficiency virus-negative men who have sex with men: a series of case reports. Sex Transm Dis 2011; 38 102–4.
| Sexual transmission of hepatitis C virus in human immunodeficiency virus-negative men who have sex with men: a series of case reports.Crossref | GoogleScholarGoogle Scholar | 20706177PubMed |
[100] Jin F, Prestage GP, Kippax SC, Kaldor JM, Dore GJ, Grulich AE. Prevalence and risk factors of hepatitis C in HIV‐negative homosexual men in Sydney, Australia. Aust N Z J Public Health 2005; 29 536–9.
| Prevalence and risk factors of hepatitis C in HIV‐negative homosexual men in Sydney, Australia.Crossref | GoogleScholarGoogle Scholar | 16370051PubMed |
[101] Raymond HF, Chu P, Nieves-Rivera I, Louie B, McFarland W, Pandori M, et al Infection among men who have sex with men, San Francisco, 2011. Sex Transm Dis 2012; 39 985–6.
| Infection among men who have sex with men, San Francisco, 2011.Crossref | GoogleScholarGoogle Scholar | 23191955PubMed |
[102] Tseng Y-T, Sun H-Y, Chang S-Y, Wu C-H, Liu W-C, Wu P-Y, et al Seroprevalence of hepatitis virus infection in men who have sex with men aged 18–40 years in Taiwan. J Formos Med Assoc 2012; 111 431–8.
| Seroprevalence of hepatitis virus infection in men who have sex with men aged 18–40 years in Taiwan.Crossref | GoogleScholarGoogle Scholar | 22939661PubMed |
[103] Scott C, Day S, Low E, Sullivan A, Atkins M, Asboe D. Unselected hepatitis C screening of men who have sex with men attending sexual health clinics. J Infect 2010; 60 351–3.
| Unselected hepatitis C screening of men who have sex with men attending sexual health clinics.Crossref | GoogleScholarGoogle Scholar | 20153770PubMed |
[104] Yaphe S, Bozinoff N, Kyle R, Shivkumar S, Pai NP, Klein M. Incidence of acute hepatitis C virus infection among men who have sex with men with and without HIV infection: a systematic review. Sex Transm Infect 2012; 88 558–64.
| Incidence of acute hepatitis C virus infection among men who have sex with men with and without HIV infection: a systematic review.Crossref | GoogleScholarGoogle Scholar | 22859499PubMed |
[105] Witt MD, Seaberg EC, Darilay A, Young S, Badri S, Rinaldo CR, et al Incident hepatitis C virus infection in men who have sex with men: a prospective cohort analysis, 1984–2011. Clin Infect Dis 2013; 57 77–84.
| Incident hepatitis C virus infection in men who have sex with men: a prospective cohort analysis, 1984–2011.Crossref | GoogleScholarGoogle Scholar | 23532480PubMed |
[106] Gamage DG, Read TR, Bradshaw CS, Hocking JS, Howley K, Chen MY, et al Incidence of Hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis 2011; 11 39
| Incidence of Hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study.Crossref | GoogleScholarGoogle Scholar | 21291565PubMed |
[107] van der Helm JJ, Prins M, del Amo J, Bucher HC, Chêne G, Dorrucci M, et al The hepatitis C epidemic among HIV-positive MSM: incidence estimates from 1990 to 2007. AIDS 2011; 25 1083–91.
| The hepatitis C epidemic among HIV-positive MSM: incidence estimates from 1990 to 2007.Crossref | GoogleScholarGoogle Scholar | 21537114PubMed |
[108] Danta M, Brown D, Bhagani S, Pybus OG, Sabin CA, Nelson M, et al Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS 2007; 21 983–91.
| Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours.Crossref | GoogleScholarGoogle Scholar | 17457092PubMed |
[109] Bradshaw D, Matthews G, Danta M. Sexually transmitted hepatitis C infection: the new epidemic in MSM? Curr Opin Infect Dis 2013; 26 66–72.
| 1:CAS:528:DC%2BC38XhvVCru7jK&md5=4ba6ecdb7fff13fdd0b04c68bc533f5cCAS | 23242342PubMed |
[110] Linas BP, Wong AY, Schackman BR, Kim AY, Freedberg KA. Cost-effective screening for acute hepatitis C virus infection in HIV-infected men who have sex with men. Clin Infect Dis 2012; 55 279–90.
| Cost-effective screening for acute hepatitis C virus infection in HIV-infected men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 22491339PubMed |
[111] Albert M, Gilson R, Guiguet M, Hoepelman A, Horban A, Katlama C, et al Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference The European AIDS Treatment Network (NEAT) Acute Hepatitis C Infection Consensus Panel. AIDS 2011; 25 399–409.
[112] Hill C, McKinney E, Lowndes CM, Munro H, Murphy G, Parry JV, et al Epidemiology of herpes simplex virus types 2 and 1 amongst men who have sex with men attending sexual health clinics in England and Wales: implications for HIV prevention and management. Euro Surveill 2009; 14 19418
| 19941804PubMed |
[113] Bohl DD, Katz KA, Bernstein K, Wong E, Raymond HF, Klausner JD, et al Prevalence and correlates of herpes simplex virus type-2 infection among men who have sex with men, San Francisco, 2008. Sex Transm Dis 2011; 38 617–21.
| Prevalence and correlates of herpes simplex virus type-2 infection among men who have sex with men, San Francisco, 2008.Crossref | GoogleScholarGoogle Scholar | 21278625PubMed |
[114] Spielmann N, Munstermann D, Hagedorn HJ, an der Heiden M, Houareau C, Gunsenheimer-Bartmeyer B, et al Time trends of syphilis and HSV-2 co-infection among men who have sex with men in the German HIV-1 seroconverter cohort from 1996–2007. Sex Transm Infect 2010; 86 331–6.
| Time trends of syphilis and HSV-2 co-infection among men who have sex with men in the German HIV-1 seroconverter cohort from 1996–2007.Crossref | GoogleScholarGoogle Scholar | 20876750PubMed |
[115] Barnabas RV, Wasserheit JN, Huang Y, Janes H, Morrow R, Fuchs J, et al Impact of herpes simplex virus type 2 on HIV-1 acquisition and progression in an HIV vaccine trial (the Step study). J Acquir Immune Defic Syndr 2011; 57 238–44.
| Impact of herpes simplex virus type 2 on HIV-1 acquisition and progression in an HIV vaccine trial (the Step study).Crossref | GoogleScholarGoogle Scholar | 21860356PubMed |
[116] Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006; 20 73–83.
| Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies.Crossref | GoogleScholarGoogle Scholar | 16327322PubMed |
[117] Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany-Moretlwe S, et al Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371 2109–19.
| Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnsFGjurY%3D&md5=555c025d31f069c3327bb50ddad067c0CAS | 18572080PubMed |
[118] Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010; 362 427–39.
| Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhvVams70%3D&md5=21da836616a8dfc65b3e64ddd9b27dfeCAS | 20089951PubMed |
[119] Douglas JM, Berman SM. Screening for HSV-2 infection in STD clinics and beyond: a few answers but more questions. Sex Transm Dis 2009; 36 729–31.
| Screening for HSV-2 infection in STD clinics and beyond: a few answers but more questions.Crossref | GoogleScholarGoogle Scholar | 19809383PubMed |
[120] Patel R, Alderson S, Geretti A, Nilsen A, Foley E, Lautenschlager S, et al European guideline for the management of genital herpes, 2010. Int J STD AIDS 2011; 22 1–10.
| European guideline for the management of genital herpes, 2010.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M3jtVemsw%3D%3D&md5=af956b714c7c7f88c48de2d1071cd69aCAS | 21364059PubMed |
[121] Machalek DA, Grulich AE, Jin F, Templeton DJ, Poynten IM. The epidemiology and natural history of anal human papillomavirus infection in men who have sex with men. Sex Health 2012; 9 527–37.
| The epidemiology and natural history of anal human papillomavirus infection in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 23380235PubMed |
[122] Garland SM. Prevention strategies against human papillomavirus in males. Gynecol Oncol 2010; 117 S20–5.
| Prevention strategies against human papillomavirus in males.Crossref | GoogleScholarGoogle Scholar | 20138347PubMed |
[123] Read TR, Hocking JS, Vodstrcil LA, Tabrizi SN, McCullough MJ, Grulich AE, et al Oral human papillomavirus in men having sex with men: risk-factors and sampling. PLoS ONE 2012; 7 e49324
| Oral human papillomavirus in men having sex with men: risk-factors and sampling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVShs7nP&md5=74907b69d6c0bb8fbfce1e21b21ec247CAS | 23173054PubMed |
[124] Grulich AE, Hillman R, Brotherton JM, Fairley CK. Time for a strategic research response to anal cancer. Sex Health 2012; 9 628–31.
| Time for a strategic research response to anal cancer.Crossref | GoogleScholarGoogle Scholar | 23380237PubMed |
[125] Poynten IM, Jin F, Templeton DJ, Prestage GP, Donovan B, Pawlita M, et al Prevalence, incidence, and risk factors for human papillomavirus 16 seropositivity in Australian homosexual men. Sex Transm Dis 2012; 39 726–32.
| Prevalence, incidence, and risk factors for human papillomavirus 16 seropositivity in Australian homosexual men.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1alsbfL&md5=3ef3d5245b50c1152c51eaaecd4d3f60CAS | 22902671PubMed |
[126] Videla S, Darwich L, Canadas MP, Coll J, Pinol M, Garcia-Cuyas F, et al Natural history of human papillomavirus infections involving anal, penile, and oral sites among HIV-positive men. Sex Transm Dis 2013; 40 3–10.
| Natural history of human papillomavirus infections involving anal, penile, and oral sites among HIV-positive men.Crossref | GoogleScholarGoogle Scholar | 23250297PubMed |
[127] Nyitray AG, da Silva RJ, Baggio ML, Lu B, Smith D, Abrahamsen M, et al The prevalence of genital HPV and factors associated with oncogenic HPV among men having sex with men and men having sex with women and men: the HIM study. Sex Transm Dis 2011; 38 932–40.
| The prevalence of genital HPV and factors associated with oncogenic HPV among men having sex with men and men having sex with women and men: the HIM study.Crossref | GoogleScholarGoogle Scholar | 21934568PubMed |
[128] Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, et al Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 2012; 13 487–500.
| Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 22445259PubMed |
[129] Machalek DA, Grulich AE, Hillman RJ, Jin F, Templeton DJ, Tabrizi SN, et al The Study of the Prevention of Anal Cancer (SPANC): design and methods of a three-year prospective cohort study. BMC Public Health 2013; 13 946
| The Study of the Prevention of Anal Cancer (SPANC): design and methods of a three-year prospective cohort study.Crossref | GoogleScholarGoogle Scholar | 24107134PubMed |
[130] Brown B, Davtyan M, Galea J, Chow E, Leon S, Klausner JD. The role of human papillomavirus in human immunodeficiency virus acquisition in men who have sex with men: a review of the literature. Viruses 2012; 4 3851–8.
| The role of human papillomavirus in human immunodeficiency virus acquisition in men who have sex with men: a review of the literature.Crossref | GoogleScholarGoogle Scholar | 23250451PubMed |
[131] Lissouba P, Van de Perre P, Auvert B. Association of genital human papillomavirus infection with HIV acquisition: a systematic review and meta-analysis. Sex Transm Infect 2013; 89 350–6.
| Association of genital human papillomavirus infection with HIV acquisition: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 23761216PubMed |
[132] Chin-Hong PV, Husnik M, Cranston RD, Colfax G, Buchbinder S, Da Costa M, et al Anal human papillomavirus infection is associated with HIV acquisition in men who have sex with men. AIDS 2009; 23 1135–42.
| Anal human papillomavirus infection is associated with HIV acquisition in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 19390418PubMed |
[133] Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al Global epidemiology of HIV infection in men who have sex with men. Lancet 2012; 380 367–77.
| Global epidemiology of HIV infection in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 22819660PubMed |
[134] Birrell PJ, Gill ON, Delpech VC, Brown AE, Desai S, Chadborn TR, et al HIV incidence in men who have sex with men in England and Wales 2001–10: a nationwide population study. Lancet Infect Dis 2013; 13 313–8.
| HIV incidence in men who have sex with men in England and Wales 2001–10: a nationwide population study.Crossref | GoogleScholarGoogle Scholar | 23375420PubMed |
[135] German D, Sifakis F, Maulsby C, Towe VL, Flynn CP, Latkin CA, et al Persistently high prevalence and unrecognized HIV infection among men who have sex with men in Baltimore: the BESURE study. J Acquir Immune Defic Syndr 2011; 57 77–87.
| Persistently high prevalence and unrecognized HIV infection among men who have sex with men in Baltimore: the BESURE study.Crossref | GoogleScholarGoogle Scholar | 21297479PubMed |
[136] Frieden TR, Jaffe HW, Stephens JW, Thacker SB, Zaza S. HIV surveillance – United States, 1981–2008. MMWR Morb Mortal Wkly Rep 2011; 60 689–93.
[137] Jakopanec I, Grjibovski AM, Nilsen O, Blystad H, Aavitsland P. Trends in HIV infection surveillance data among men who have sex with men in Norway, 1995–2011. BMC Public Health 2013; 13 144
| Trends in HIV infection surveillance data among men who have sex with men in Norway, 1995–2011.Crossref | GoogleScholarGoogle Scholar | 23414557PubMed |
[138] Feng Y, Wu Z, Detels R, Qin G, Liu L, Wang X, et al HIV/STD prevalence among men who have sex with men in Chengdu, China and associated risk factors for HIV infection. J Acquir Immune Defic Syndr 2010; 53 S74–80.
| HIV/STD prevalence among men who have sex with men in Chengdu, China and associated risk factors for HIV infection.Crossref | GoogleScholarGoogle Scholar | 20104114PubMed |
[139] Mallitt K-A, Wilson DP, McDonald A, Wand H. HIV incidence trends vary between jurisdictions in Australia: an extended back-projection analysis of men who have sex with men. Sex Health 2012; 9 138–43.
| 22498157PubMed |
[140] Pedrana AE, Hellard ME, Wilson K, Guy R, Stoove M. High rates of undiagnosed HIV infections in a community sample of gay men in Melbourne, Australia. J Acquir Immune Defic Syndr 2012; 59 94–9.
| High rates of undiagnosed HIV infections in a community sample of gay men in Melbourne, Australia.Crossref | GoogleScholarGoogle Scholar | 21992925PubMed |
[141] Holt M, Rawstorne P, Wilkinson J, Worth H, Bittman M, Kippax S. HIV testing, gay community involvement and internet use: social and behavioural correlates of HIV testing among Australian men who have sex with men. AIDS Behav 2012; 16 13–22.
| HIV testing, gay community involvement and internet use: social and behavioural correlates of HIV testing among Australian men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC387jt1GjtQ%3D%3D&md5=f75496040664995a9ecfcbe0d9f4bad4CAS | 21213035PubMed |
[142] Guy R, Goller JL, Spelman T, El-Hayek C, Gold J, Lim M, et al Does the frequency of HIV and STI testing among men who have sex with men in primary care adhere with Australian guidelines? Sex Transm Infect 2010; 86 371–6.
| Does the frequency of HIV and STI testing among men who have sex with men in primary care adhere with Australian guidelines?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cflsVyjug%3D%3D&md5=4225b874ddc4566fe81494f7d34339a1CAS | 20460263PubMed |
[143] Guy RJ, Spelman T, Stoove M, El-Hayek C, Goller J, Fairley CK, et al Risk factors for HIV seroconversion in men who have sex with men in Victoria, Australia: results from a sentinel surveillance system. Sex Health 2011; 8 319–29.
| Risk factors for HIV seroconversion in men who have sex with men in Victoria, Australia: results from a sentinel surveillance system.Crossref | GoogleScholarGoogle Scholar | 21851771PubMed |
[144] Poynten IM, Jin F, Prestage GP, Kaldor JM, Kippax S, Grulich AE. Defining high HIV incidence subgroups of Australian homosexual men: implications for conducting HIV prevention trials in low HIV prevalence settings. HIV Med 2010; 11 635–41.
| Defining high HIV incidence subgroups of Australian homosexual men: implications for conducting HIV prevention trials in low HIV prevalence settings.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cbgslKisQ%3D%3D&md5=ff5974c3ee7e9847af4f2f9c766d422bCAS | 20456511PubMed |
[145] Prestage GP, Hudson J, Down I, Bradley J, Corrigan N, Hurley M, et al Gay men who engage in group sex are at increased risk of HIV infection and onward transmission. AIDS Behav 2009; 13 724–30.
| Gay men who engage in group sex are at increased risk of HIV infection and onward transmission.Crossref | GoogleScholarGoogle Scholar | 18818998PubMed |
[146] Vodstrcil LA, Fairley CK, Chen MY, Denham I. Risk-based HIV testing of men who have sex with men would result in missed HIV diagnoses. Sex Transm Dis 2012; 39 492
| Risk-based HIV testing of men who have sex with men would result in missed HIV diagnoses.Crossref | GoogleScholarGoogle Scholar | 22592839PubMed |
[147] Centers for Disease Control and Prevention Prevalence and awareness of HIV infection among men who have sex with men –21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep 2010; 59 1201–7.
| 20864920PubMed |
[148] National Institute for Health and Clinical Excellence. Increasing the uptake of HIV testing among men who have sex with men: guidance. London:National Health Service; 2011.
[149] Lucas A, Armbruster B. The cost-effectiveness of expanded HIV screening in the United States. AIDS 2013; 27 795–801.
| The cost-effectiveness of expanded HIV screening in the United States.Crossref | GoogleScholarGoogle Scholar | 23169333PubMed |
[150] Gray RT, Prestage GP, Down I, Ghaus MH, Hoare A, Bradley J, et al Increased HIV testing will modestly reduce HIV incidence among gay men in NSW and would be acceptable if HIV testing becomes convenient. PLoS ONE 2013; 8 e55449
| Increased HIV testing will modestly reduce HIV incidence among gay men in NSW and would be acceptable if HIV testing becomes convenient.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjsVarsLw%3D&md5=aa8ae1d44349c4eb179549844298230bCAS | 23457470PubMed |
[151] Napierala Mavedzenge S, Baggaley R, Corbett EL. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clin Infect Dis 2013; 57 126–38.
| A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXptFelt7c%3D&md5=d061638e0e6fe6aae95729900b58236eCAS | 23487385PubMed |
[152] Savage EJ, Marsh K, Duffell S, Ison CA, Zaman A, Hughes G. Rapid increase in gonorrhoea and syphilis diagnoses in England in 2011. Euro Surveill 2012; 17 20224
| 22835469PubMed |
[153] Van de Laar MSG. Increasing trends of gonorrhoea and syphilis and the threat of drug-resistant gonorrhoea in Europe. Euro Surveill 2012; 17 1–3.
[154] Centers for Disease Control and Prevention Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: o cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep 2012; 61 590–4.
| 22874837PubMed |
[155] Cohen SE, Chew Ng RA, Katz KA, Bernstein KT, Samuel MC, Kerndt PR, et al Repeat syphilis among men who have sex with men in California, 2002–2006: implications for syphilis elimination efforts. Am J Public Health 2012; 102 e1–8.
| Repeat syphilis among men who have sex with men in California, 2002–2006: implications for syphilis elimination efforts.Crossref | GoogleScholarGoogle Scholar | 22095364PubMed |
[156] Hao C, Yan H, Yang H, Huan X, Guan W, Xu X, et al The incidence of syphilis, HIV and HCV and associated factors in a cohort of men who have sex with men in Nanjing, China. Sex Transm Infect 2011; 87 199–201.
| The incidence of syphilis, HIV and HCV and associated factors in a cohort of men who have sex with men in Nanjing, China.Crossref | GoogleScholarGoogle Scholar | 21262785PubMed |
[157] Chow EPF, Wilson DP, Zhang L. HIV and syphilis co-infection increasing among men who have sex with men in China: a systematic review and meta-analysis. PLoS ONE 2011; 6 e22768
| HIV and syphilis co-infection increasing among men who have sex with men in China: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFGhtLjO&md5=00869cce73fe347fa8a4ac214c6e0620CAS |
[158] Heiligenberg M, Rijnders B, Schim van der Loeff MF, de Vries HJ, van der Meijden WI, Geerlings SE, et al High prevalence of sexually transmitted infections in HIV-infected men during routine outpatient visits in the Netherlands. Sex Transm Dis 2012; 39 8–15.
| High prevalence of sexually transmitted infections in HIV-infected men during routine outpatient visits in the Netherlands.Crossref | GoogleScholarGoogle Scholar | 22183837PubMed |
[159] Champenois K, Cousien A, Ndiaye B, Soukouna Y, Baclet V, Alcaraz I, et al Risk factors for syphilis infection in men who have sex with men: results of a case-control study in Lille, France. Sex Transm Infect 2013; 89 128–32.
| Risk factors for syphilis infection in men who have sex with men: results of a case-control study in Lille, France.Crossref | GoogleScholarGoogle Scholar | 22679099PubMed |
[160] Gray RT, Hoare A, Prestage GP, Donovan B, Kaldor JM, Wilson DP. Frequent testing of highly sexually active gay men is required to control syphilis. Sex Transm Dis 2010; 37 298–305.
| 20393383PubMed |
[161] Tuite AR, Fisman DN, Mishra S. Screen more or screen more often? Using mathematical models to inform syphilis control strategies. BMC Public Health 2013; 13 606
| Screen more or screen more often? Using mathematical models to inform syphilis control strategies.Crossref | GoogleScholarGoogle Scholar | 23800206PubMed |
[162] Marcus JL, Katz KA, Bernstein KT, Nieri G, Philip SS. Syphilis testing behavior following diagnosis with early syphilis among men who have sex with men – San Francisco, 2005–2008. Sex Transm Dis 2011; 38 24–9.
| Syphilis testing behavior following diagnosis with early syphilis among men who have sex with men – San Francisco, 2005–2008.Crossref | GoogleScholarGoogle Scholar | 20679965PubMed |
[163] Guy R, Wand H, Holt M, Mao L, Wilson DP, Bourne C, et al High annual syphilis testing rates among gay men in Australia, but insufficient retesting. Sex Transm Dis 2012; 39 268–75.
| High annual syphilis testing rates among gay men in Australia, but insufficient retesting.Crossref | GoogleScholarGoogle Scholar | 22421692PubMed |
[164] Jin F, Prestage GP, Zablotska I, Rawstorne P, Imrie J, Kippax SC, et al High incidence of syphilis in HIV-positive homosexual men: data from two community-based cohort studies. Sex Health 2009; 6 281–4.
| High incidence of syphilis in HIV-positive homosexual men: data from two community-based cohort studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFyqu7fK&md5=706b5b669d02c7ec825760bbbe73a708CAS | 19917195PubMed |
[165] Kalichman SC, Pellowski J, Turner C. Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect 2011; 87 183–90.
| Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention.Crossref | GoogleScholarGoogle Scholar | 21330572PubMed |
[166] Mayer KH, Bush T, Henry K, Overton ET, Hammer J, Richardson J, et al Ongoing sexually transmitted disease acquisition and risk-taking behavior among US HIV-infected patients in primary care: implications for prevention interventions. Sex Transm Dis 2012; 39 1–7.
| Ongoing sexually transmitted disease acquisition and risk-taking behavior among US HIV-infected patients in primary care: implications for prevention interventions.Crossref | GoogleScholarGoogle Scholar | 22183836PubMed |
[167] Rieg G, Lewis RJ, Miller LG, Witt MD, Guerrero M, Daar ES. Asymptomatic sexually transmitted infections in HIV-infected men who have sex with men: prevalence, incidence, predictors, and screening strategies. AIDS Patient Care STDS 2008; 22 947–54.
| Asymptomatic sexually transmitted infections in HIV-infected men who have sex with men: prevalence, incidence, predictors, and screening strategies.Crossref | GoogleScholarGoogle Scholar | 19072101PubMed |
[168] Diaz A, Junquera ML, Esteban V, Martinez B, Pueyo I, Suarez J, et al HIV/STI co-infection among men who have sex with men in Spain. Euro Surveill 2009; 14 19426
| 20003899PubMed |
[169] Lee HC, Ko NY, Lee NY, Chang CM, Liu SY, Ko WC. Trends in sexually transmitted diseases and risky behaviors among HIV-infected patients at an outpatient clinic in southern Taiwan. Sex Transm Dis 2010; 37 86–93.
| Trends in sexually transmitted diseases and risky behaviors among HIV-infected patients at an outpatient clinic in southern Taiwan.Crossref | GoogleScholarGoogle Scholar | 19901862PubMed |
[170] Brosh-Nissimov T, Mor Z, Avramovich E, Katchman E, Avidor B, Mor O, et al Syphilis outbreak among men who have sex with men, Tel Aviv, Israel, 2008–2009. Isr Med Assoc J 2012; 14 152–6.
| 22675853PubMed |
[171] Hoover KW, Butler M, Workowski K, Carpio F, Follansbee S, Gratzer B, et al STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis 2010; 37 771–6.
| STD screening of HIV-infected MSM in HIV clinics.Crossref | GoogleScholarGoogle Scholar | 20585275PubMed |
[172] Baffi CW, Aban I, Willig JH, Agrawal M, Mugavero MJ, Bachmann LH. New syphilis cases and concurrent STI screening in a southeastern U.S. HIV clinic: a call to action. AIDS Patient Care STDS 2010; 24 23–9.
| New syphilis cases and concurrent STI screening in a southeastern U.S. HIV clinic: a call to action.Crossref | GoogleScholarGoogle Scholar | 20095902PubMed |
[173] Callander D, Baker D, Chen M, Guy R. Including syphilis testing as part of standard HIV management checks and improved syphilis screening in primary care. Sex Transm Dis 2013; 40 338–40.
| Including syphilis testing as part of standard HIV management checks and improved syphilis screening in primary care.Crossref | GoogleScholarGoogle Scholar | 23486501PubMed |
[174] Bissessor M, Fairley CK, Leslie D, Chen MY. Use of a computer alert increases detection of early, asymptomatic syphilis among higher-risk men who have sex with men. Clin Infect Dis 2011; 53 57–8.
| Use of a computer alert increases detection of early, asymptomatic syphilis among higher-risk men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 21653303PubMed |
[175] Bourne C, Knight V, Guy R, Wand H, Lu H, McNulty A. Short message service reminder intervention doubles sexually transmitted infection/HIV re-testing rates among men who have sex with men. Sex Transm Infect 2011; 87 229–31.
| Short message service reminder intervention doubles sexually transmitted infection/HIV re-testing rates among men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M3ot1Wqug%3D%3D&md5=96bda2f9d22a39358ee1d28aa9a88e7aCAS | 21296796PubMed |
[176] Zou H, Fairley CK, Guy R, Bilardi J, Bradshaw CS, Garland SM, et al Automated, computer generated reminders and increased detection of gonorrhoea, chlamydia and syphilis in men who have sex with men. PLoS ONE 2013; 8 e61972
| Automated, computer generated reminders and increased detection of gonorrhoea, chlamydia and syphilis in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmvVCktrc%3D&md5=16f5e3ace4e846ecc898330f43188777CAS | 23613989PubMed |
[177] Bissessor M, Fairley CK, Leslie D, Howley K, Chen MY. Frequent screening for syphilis as part of HIV monitoring increases the detection of early asymptomatic syphilis among HIV-positive homosexual men. J Acquir Immune Defic Syndr 2010; 55 211–6.
| Frequent screening for syphilis as part of HIV monitoring increases the detection of early asymptomatic syphilis among HIV-positive homosexual men.Crossref | GoogleScholarGoogle Scholar | 20585261PubMed |
[178] Holt M, Bernard D, Race K. Gay men’s perceptions of sexually transmissible infections and their experiences of diagnosis: ‘part of the way of life’ to feeling ‘dirty and ashamed’. Sex Health 2010; 7 411–6.
| Gay men’s perceptions of sexually transmissible infections and their experiences of diagnosis: ‘part of the way of life’ to feeling ‘dirty and ashamed’.Crossref | GoogleScholarGoogle Scholar | 21062579PubMed |
[179] Barber B, Hellard M, Jenkinson R, Spelman T, Stoove M. Sexual history taking and sexually transmissible infection screening practices among men who have sex with men: a survey of Victorian general practitioners. Sex Health 2011; 8 349–54.
| Sexual history taking and sexually transmissible infection screening practices among men who have sex with men: a survey of Victorian general practitioners.Crossref | GoogleScholarGoogle Scholar | 21851775PubMed |
[180] Baker JR, Arnold-Reed DE, Brett T, Hince DA, O’Ferrall I, Bulsara MK. Perceptions of barriers to discussing and testing for sexually transmitted infections in a convenience sample of general practice patients. Aust J Primary Health 2013; 19 98–101.
| Perceptions of barriers to discussing and testing for sexually transmitted infections in a convenience sample of general practice patients.Crossref | GoogleScholarGoogle Scholar |
[181] Prestage G, Brown G, Keen P. Barriers to HIV testing among Australian gay men. Sex Health 2012; 9 453–8.
| Barriers to HIV testing among Australian gay men.Crossref | GoogleScholarGoogle Scholar | 23380195PubMed |
[182] Fairley CK. Using information technology to control STIs. Sex Transm Infect 2011; 87 ii25–7.
| Using information technology to control STIs.Crossref | GoogleScholarGoogle Scholar | 22110149PubMed |