Vaccination against oncogenic human papillomavirus infection in HIV-infected populations: review of current status and future perspectives
Lars Toft A B , Martin Tolstrup A , Merete Storgaard A , Lars Østergaard A and Ole S. Søgaard AA Department of Infectious Diseases, Aarhus University Hospital, Skejby, 8200 Aarhus, Denmark.
B Corresponding author. Email: larsnise@rm.dk
Sexual Health 11(6) 511-523 https://doi.org/10.1071/SH14015
Submitted: 14 January 2014 Accepted: 5 August 2014 Published: 9 September 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Background: Men and women with HIV infection are at increased risk of developing cancers associated with human papillomavirus (HPV). The two licensed prophylactic HPV vaccines protect against de novo infection with HPV-16 and HPV-18, which cause the majority of HPV-associated cancers. Currently, no vaccine efficacy data are available for persons with HIV infection. Nevertheless, some countries have implemented specific HPV vaccination recommendations for HIV-positive populations. To specifically recommend prophylactic HPV vaccination in people with HIV, the vaccines must be safe and immunogenic in immunosuppressed people at a high risk of HPV infection. This review aims to summarise the current knowledge from published HPV vaccine trials in HIV-infected populations, to compile scheduled and ongoing HPV vaccine trials with HIV-positive study populations and to extrapolate the relevant knowledge about HPV vaccine efficacy in HIV-negative populations to an HIV context. Methods: The databases PubMed, Scopus and ClinicalTrials.gov were searched for peer-reviewed articles and scheduled or ongoing clinical HPV vaccine trials enrolling HIV-positive persons. Results: Current data indicate that prophylactic HPV vaccines are safe and immunogenic in different HIV-positive populations (children, female adolescents, adults). Increased immunogenicity has been reported in persons on antiretroviral therapy compared with antiretroviral-naïve persons, whereas no clear association has been found between CD4+ cell count at immunisation and vaccine response. Several scheduled and ongoing HPV vaccine trials aim to determine vaccine efficacy against disease endpoints in HIV-infected study populations. Conclusion: Prophylactic HPV vaccination appears safe, immunogenic and, by extrapolation, likely to reduce HPV-associated cancer development among persons with HIV infection.
Additional keywords: anal cancer, cervical cancer, genital warts, immunisation.
Introduction
Prior to the widespread introduction of population-based vaccination programs, infection with human papillomavirus (HPV) was the most common sexually transmissible infection worldwide and persistent HPV infection caused more than 600 000 cancers worldwide per year, including cervical cancer, anal cancer, penile cancer, vulval and vaginal cancer and oropharyngeal cancers.1 HIV-infected individuals are at high risk of HPV infection and all types of HPV-associated cancers occur at higher rates in HIV-positive people compared with the general population.2 HIV-infected women are at high risk of persistent HPV infections that may transform into malignant lesions and, ultimately, invasive cervical cancer. The cancer risk is closely related to women’s immune status and, since 1993, invasive cervical cancer has been classified as an AIDS-defining disease.3 Studies linking HIV/AIDS and cancer registries have reported a 2- to 22-fold increased risk of cervical cancer among HIV-positive women compared with the general female population in the same geographical area.4 Among people with HIV infection, the highest rates of anal cancer are found among men who have sex with men (MSM). An 80-fold increased risk of anal cancer development has been reported among HIV-infected MSM compared with the background male population.5
HPV genotypes 16 (HPV-16) and 18 (HPV-18) cause most HPV-associated cancers.6–11 Currently, there are two licensed prophylactic HPV vaccines, a bivalent vaccine12 (Cervarix; GlaxoSmithKline, Middlesex, UK) and a quadrivalent vaccine13 (Gardasil; Merck, Whitehouse Station, NJ, USA). Both vaccines offer protection against infection with HPV-16 and -18 and Gardasil additionally protects against the two most common causative agents of genital warts, HPV-6 and -11. HPV vaccine efficacy has been demonstrated for both vaccines against cervical disease in large trials conducted in female adolescents and young adults.14–18 Furthermore, the bivalent vaccine has demonstrated efficacy against persistent oncogenic anal HPV infection in young women.19 In addition, protection against anal and anogenital disease has been established in a males for the quadrivalent vaccine.20,21 HPV vaccine efficacy has not been evaluated in HIV-positive study populations. However, Australian and US guidelines recommend that HPV vaccination be considered for HIV-positive individuals.22,23
Compared with healthy individuals, HIV-infected individuals often have reduced immune responses to various immunisations (e.g. influenza vaccination,24,25 hepatitis B virus vaccination26 and pneumococcal vaccination27). Nevertheless, vaccination remains a common clinical tool to reduce the risk of secondary infections in people with HIV. Vaccine recommendations for people with HIV-infection have generally been based on data from safety and immunogenicity trials, combined with clinical assessment of need (e.g. high rates of hepatitis B infection or high morbidity associated with influenza in people with HIV). Accordingly, clinical recommendations about HPV vaccination in people with HIV might be established without direct efficacy data. For prophylactic HPV vaccination to be recommended in people with HIV, the vaccines have to be safe and immunogenic despite chronic immunosuppression. A high degree of previous and prevalent HPV-16 and -18-infections in this particular population might decrease the expected vaccine efficacy.28,29
We conducted a systematic search of the present literature to review the available data from published trials of the safety and immunogenicity of HPV vaccines in HIV-positive study populations. In addition, we aimed to describe the ongoing and scheduled HPV vaccine trials in HIV-positive study populations, with enhanced attention given to trials evaluating the study vaccine against disease endpoints. Finally, we discuss the implications of these findings in the context of the current knowledge on HPV vaccine efficacy from HIV-uninfected study populations.
Methods
The review was conducted in concordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.30 Studies were included if they reported data from trials that investigated either one or both of the licensed HPV vaccines in HIV-positive persons. Scheduled and ongoing trials were included if they were designed to test safety, immunogenicity or HPV (bivalent or quadrivalent) vaccine efficacy in HIV-positive persons.
Search strategy
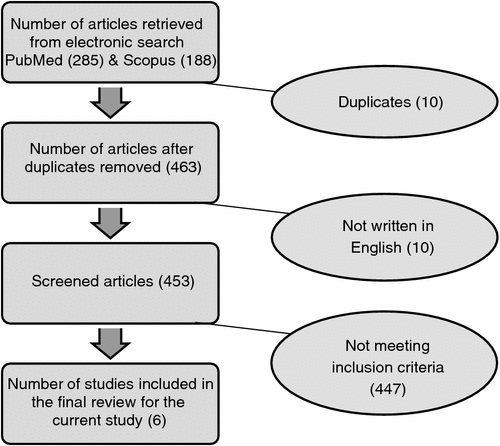
A search of two electronic databases was conducted in November 2013 using standard research procedures. The databases were PubMed and Scopus. Search terms were entered with combined sets of terms relating to HPV vaccine or Gardasil or Cervarix, and HIV. In PubMed, article types were restricted to ‘clinical trials’; in Scopus, document types were restricted to articles. The search results from the two databases were collated and duplicate publications were removed (Fig. 1).
|
Inclusion and exclusion criteria
Two authors reviewed titles and abstracts for the following inclusion criteria:
-
a clinical trial on safety and immunogenicity of either one or both of the HPV vaccines (bivalent or quadrivalent);
-
an HIV-positive study population; and
-
published in English.
Reference lists were not searched. We did not have any exclusion criteria.
Furthermore, published and unpublished studies were investigated through ClinicalTrials.gov (accessed 13 August 2014), which was searched for HPV vaccine immunogenicity or efficacy trials enrolling HIV-positive study subjects up until 18 November 2013. The combination of search terms used was: (1) Gardasil or Cervarix, and (2) HIV. In trials listed as ‘completed’, the ClinicalTrials.gov identifier was entered into the electronic databases PubMed and Scopus to retrieve all published trials.
Results
Published HPV vaccination trials enrolling HIV-infected persons
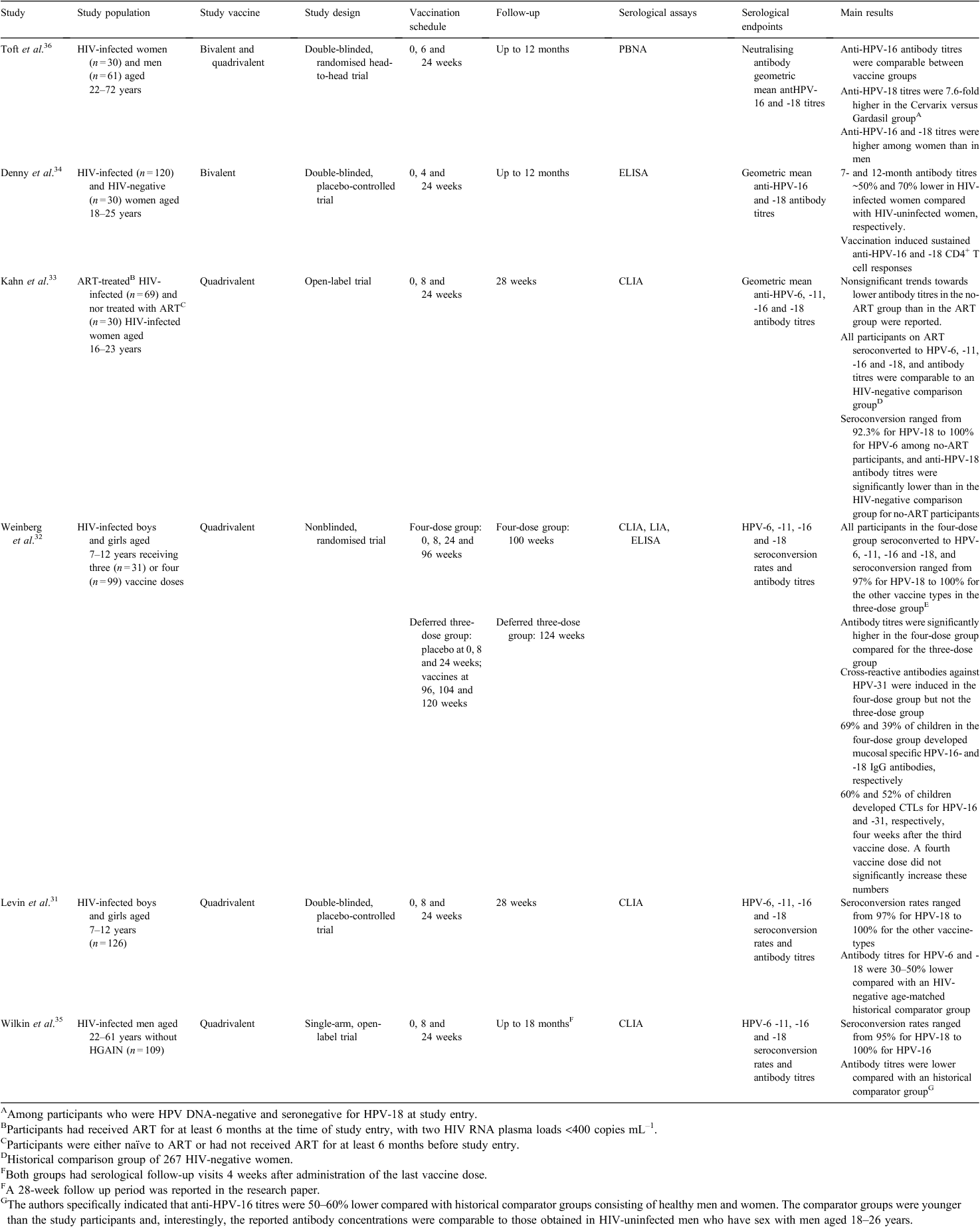
In total, 473 records were identified by searching databases for clinical trials on completed HPV vaccination trials enrolling HIV-infected persons. After removing duplicate publications, 463 remained. Of these, 453 records were written in English. Through screening of abstracts, six independent articles were identified that reported results from relevant clinical trials in HIV-positive study populations and used either one or both of the HPV vaccines as study intervention. Table 1 summarises the study characteristics, immunologic outcome measures and main results from the six identified studies.
Studies in HIV-infected children
Levin et al.31 conducted a double-blinded placebo-controlled safety and immunogenicity trial that enrolled and randomised 126 HIV-infected boys and girls aged 7–12 years to quadrivalent HPV vaccination or placebo injections. Vaccines were administered at 0, 8 and 24 weeks, and the vaccine schedule was well tolerated. Vaccination had no adverse effects on CD4+ cell counts or HIV RNA levels. The vaccine was immunogenic, with seroconversion rates ranging from 97% to 100% for the HPV types included in the vaccine. Antibody geometric mean titres for HPV-6 and -18 were 30–50% lower compared with an HIV-negative age-matched historical comparator group.
Weinberg et al.32 later published additional immunogenicity data from the trial described above, comparing the immunogenicity of a four-vaccine schedule to the standard three vaccines regimen. Vaccines were administered at 0, 8, 24 and 96 weeks in a four-vaccine group whereas study participants in a deferred three-dose arm received a placebo at 0, 8 and 24 weeks, and vaccinations at 96, 100 and 124 weeks. The immune responses were compared at 4 weeks after administration of the final vaccine. All participants (100%) in the four-dose group seroconverted to all four HPV vaccine types, and seroconversion ranged from 97% to 100% in the three-dose group. In the four-vaccine group, anti-HPV-18 antibody concentrations were comparable to those achieved after a standard three-dose regimen in healthy age-matched historical controls. In the four-vaccine group, mucosal anti-HPV-16 and -18 antibodies were detected in 69% and 39%, respectively (no results from the three-vaccine group were presented). Cross-reactive antibodies to HPV-31 were increased from baseline values in the four-vaccine group but not in the standard three-vaccine group.
In conclusion, two studies from the same cohort have documented acceptable safety and solid immunogenicity for the quadrivalent HPV vaccine in HIV-infected children. High seroconversion rates were found using the standard three-vaccine regimen but antibody titres were increased by giving a fourth vaccine dose after 96 weeks. No immunogenicity and safety data were available for the bivalent HPV vaccine in HIV-infected children.
Studies in young HIV-infected women
Kahn et al.33 recently published immunogenicity and safety results from an open-label trial that enrolled and immunised 99 HIV-infected women aged 16–23 years using the quadrivalent vaccine in a standard three-dose regimen. Sixty-nine women had received antiretroviral therapy (ART) for at least 6 months at the time of study entry and 30 were either naïve to ART or had not received ART during the 6 months that preceded study entry. Results were assessed according to ART status and an historical HPV vaccination group of 267 HIV-negative women was used for comparison. Only one Grade 3 adverse event (fatigue) was reported and the vaccine was generally well tolerated. Antibody responses were measured 4 weeks after the final vaccine dose. Seroconversion rates for vaccine HPV types ranged from 92% to 100% among participants not treated with ART, whereas all ART-treated participants seroconverted for all four vaccine serotypes. Neither seroconversion rates nor geometric mean titres differed significantly between the non-ART and the ART groups. Anti-HPV-18 antibody titres were significantly lower for non-ART participants compared with the HIV-negative control group, whereas antibody titres did not differ between ART-treated participants and the HIV-negative control group.
Denny et al.34 recently published data from a partially blinded, placebo-controlled trial investigating the safety and immunogenicity of the bivalent HPV vaccine in young women. HIV-positive women (n = 120) aged 18–25 years were assigned to receive the bivalent vaccine or a placebo in a standard regimen with vaccinations at 0, 1 and 6 months. Thirty HIV-negative women were enrolled as an open-label control group and received bivalent vaccinations at 0, 1 and 6 months. CD4+ cell counts and HIV RNA levels were unaffected by vaccination. Serologic responses were evaluated for up to 12 months. Baseline seropositivity proportions for HPV-16 and -18 were high among HIV-positive women assigned to the bivalent vaccination, 85.4% and 64.3% respectively, compared with 63.5% and 50% among HIV-negative controls. HPV DNA status was not assessed in the trial. All study participants assigned to the bivalent vaccination arm remained seropositive for both serotypes at 12 months. Antibody titres at 7 and12 months for both serotypes were ~50% and 70% lower in HIV-infected compared with HIV-uninfected women, respectively. Baseline CD4+ T-cell count and HIV viral load were not associated with vaccine-induced antibody titres.
In conclusion, both the quadrivalent and the bivalent HPV vaccine appear to be safe and very immunogenic in young HIV-infected women. ART-treated, HIV-infected young women and age-matched HIV-negative women had comparable antibody responses to the quadrivalent HPV vaccine. In contrast, serologic antibody responses to the bivalent HPV vaccine were lower in young HIV-infected women compared with uninfected women. Currently, studies comparing the safety and immunogenicity of the two HPV vaccines in young HIV-infected women have not been conducted.
Studies in HIV-infected adults
Wilkin et al.35 published data from a single-arm open-label trial evaluating the safety and immunogenicity of the quadrivalent vaccine in HIV-infected men (n = 109) aged 22–61 years. Vaccination did not alter CD4+ cell counts or HIV RNA levels. No serious adverse events attributable to vaccination were observed. Seroconversion rates ranged from 95% to 100% for vaccine HPV types. Antibody concentrations were comparable to those previously reported in HIV-uninfected MSM aged 18–26 years but were lower compared with historical comparator groups consisting of young healthy women and heterosexual men. A multivariate linear regression analysis showed that current ART treatment at baseline was the only baseline characteristic associated with increased serologic responses to vaccination.
One article reported the results from a double-blind, randomised head-to-head trial that compared the safety and immunogenicity of the bivalent and quadrivalent vaccines among HIV-infected women and men (n = 91) aged 22–72 years.36 None of the vaccines negatively affected CD4+ cell counts or HIV RNA levels. Injection site reactions were more common in the bivalent compared with the quadrivalent vaccine group, but both vaccines were generally well tolerated. Neutralising anti-HPV-16 and -18 antibody titres were evaluated up to 12 months using a highly sensitive pseudovirion-based neutralisation assay. Anti-HPV-16 antibody titres were comparable between vaccine groups and anti-HPV-18 titres were higher in the Cervarix group compared with the Gardasil group at 7 and 12 months. Anti-HPV-16 and -18 titres were higher among women compared with men. Antibody titres were not compared with HIV-negative controls.
In conclusion, acceptable safety data and convincing immunogenicity data are available for both HPV vaccines in adult HIV-positive study populations. Very high seroconversion rates were reported in HIV-positive men vaccinated with the quadrivalent HPV vaccine. Higher ant-HPV-18 antibody titres were found in HIV-positive men and women following vaccination with the bivalent vaccine compared with the quadrivalent HPV vaccine.
Ongoing, scheduled or complete, unpublished HPV vaccination trials in HIV-positive study populations
Twelve relevant ongoing or scheduled trials were identified from ClinicalTrials.gov. The 12 trial protocols are summarised in Table 2. Four trial protocols were designed to evaluate the efficacy of the quadrivalent vaccine against disease endpoints and will be reviewed below.
NCT00941889 is a scheduled trial sponsored by Washington University School of Medicine. Thirty HIV-infected subjects with anal warts that require surgical treatment will be randomised 1 : 1 to receive either a three-vaccine quadrivalent vaccination series or a placebo. The study is designed to evaluate if quadrivalent HPV vaccination has an effect on the persistence and recurrence of surgically treated anal condylomata during an 18-month follow-up period. The study was last updated on ClinicalTrials.gov on 14 August 2009 and had an estimated study completion date in 2011. We have not been able to find any evidence that the study was actually completed.
NCT01928225 is a scheduled placebo-controlled, randomised, double-blinded trial sponsored by the University of Witwatersrand, South Africa. The trial is not yet recruiting study participants, but 180 HIV-infected women with high-grade squamous intraepithelial cervical lesions on biopsy are planned to be enrolled in a trial designed to evaluate if pretreatment with the quadrivalent HPV vaccine will result in a reduced occurrence of cervical cancer precursors during a 52-week follow-up period after surgical treatment of high-grade squamous intraepithelial lesions. The estimated study completion date is August 2016.
NCT01461096 is an ongoing placebo-controlled, randomised, double-blinded trial sponsored by the National Institute of Allergy and Infectious Diseases. Five hundred and sixty-four HIV-infected men and women over 27 years of age are planned to be enrolled in this trial designed to evaluate if the quadrivalent HPV vaccine can prevent anal HPV infection in HIV-infected men and women. A follow-up period of 3–4 years is scheduled. The main outcome measures are time to first incident; persistent anal HPV-6, -11, -16 and -18 infection; and biopsy-proven high-grade anal intraepithelial neoplasia occurrences or reoccurrences after 52 weeks. The estimated study completion date is August 2016.
NCT01209325 is an ongoing open-label single-arm trial sponsored by the AIDS Malignancy Clinical Trials Consortium. One hundred and fifty HIV-infected MSM aged 13–26 years are planned to be enrolled in this trial designed to evaluate the efficacy of the quadrivalent HPV vaccine against incident anal disease caused by vaccine HPV types. A follow-up period of 2 years is scheduled and the main outcome measures are incident anal intraepithelial neoplasia, anal or perianal condyloma, high-grade anal intraepithelial neoplasia associated with vaccine HPV types and persistent anogenital infection with vaccine HPV types. The estimated study completion date is December 2014.
In summary, four scheduled or ongoing trials aim to investigate vaccine efficacy of the quadrivalent HPV vaccine in different HIV-positive study populations. Two trials focus on anal disease in young HIV-infected MSM, and adult men and women, respectively; two trials investigate the effects of combining HPV vaccination and surgical interventions in HIV-infected persons with established HPV-associated lesions. We found no scheduled or ongoing trials investigating the efficacy of the bivalent HPV vaccine in HIV-positive populations.
Discussion
In the present article, we aimed to review and summarise the current knowledge of HPV vaccination in HIV-positive patients. Six original immunogenicity and safety papers were identified and two of these used the same study cohort. No restrictions of trial outcomes or study population characteristics were applied. Based upon the few available studies, both licensed HPV vaccines appear safe and immunogenic in different HIV-positive populations (children, female adolescents, adults), with very high seroconversion rates but lower antibody responses than HIV-negative populations. ART may increase immune responses to vaccination, but data are quite sparse. Some studies reported lower antibody titres in HIV-positive patients compared with HIV-uninfected study populations. This holds in particular for HPV-18. The clinical significance of this observation remains elusive, as no studies have been able to establish a correlation between serologic antibody titre levels and clinical protection against disease. No long-term follow-up data have been published from HIV-positive study cohorts and thus the duration of potentially protective vaccine-elicited immune responses are currently unknown. Results obtained from efficacy trials in healthy study populations with more than 4 years of follow-up14,37 have not revealed any time-dependent decrease in vaccine-induced protection. As demonstrated by the studies identified in this review, HPV vaccination in people with HIV will result in a robust immunological response, meaning that the vaccinated person is likely to be better protected against future infections.
Clinical implications
The scheduled studies of HPV vaccine efficacy in HIV-positive study populations reviewed in this article may provide useful evidence. However, these trials might be too small to provide definitive answers about vaccine efficacy. Currently, HPV vaccination recommendations in persons with HIV must be based on the best available data as outlined in this review. The expected vaccine efficacy in most HIV-infected populations will be affected by previous HPV-16 and -18 exposure. Table 3 summarizes selected HPV vaccine efficacy trials conducted among HIV-negative, non-HPV-naïve populations. In healthy young women, HPV-vaccination had no therapeutic effects on HPV-16 and -18 infections or associated lesions that were already established at the time of vaccination.15,38 However, prevalent infection by one HPV type included in the vaccines did not reduce the vaccine-induced protection against other HPV vaccine types.39,40
In many countries, HPV vaccination programs primarily target preadolescents around 12 years of age (i.e. before most individuals’ sexual debut). In HIV-infected preadolescent girls, prior exposure to HPV will not be a major issue, and the available safety and immunogenicity data unambiguously favours vaccination. Some countries, including Australia, already offer HPV vaccination to preadolescent boys in national vaccination programs, although substantial protection for boys against HPV-associated disease is expected through herd immunity induced by vaccinating girls. HIV-infected preadolescent boys have increased risk of developing disease if they do get a HPV infection and hence recommending HPV vaccination in this population seems justified. So far, safety and immunogenicity data are only available for the quadrivalent HPV vaccine in HIV-infected preadolescent children.31,32 Including a fourth vaccine dose at 96 weeks increased the immune responses significantly and could be considered for HIV-positive preadolescents, particularly among those who are not on ART at the time of primary immunisation or have low CD4+ cell counts.32,33,41 However, a fourth dose may not be necessary in most cases. Recent data from immunocompetent young women suggest that two doses, or even one dose, of the bivalent HPV vaccine, could be as efficacious as three doses in protecting against persistent HPV-16 and -18 infections.42
Convincing immunogenicity data are available for young HIV-infected women aged 18–25 years for the bivalent vaccine34 and HIV-infected women aged 16–23 years for the quadrivalent vaccine.33 High seroconversion rates were observed for both vaccines. Many countries currently offer catch-up HPV vaccination programs to young women until the mid-20s despite the fact that prior exposure to HPV vaccines types or prevalent infection may become an important issue for women of that age. In healthy young women, prior exposure to the HPV types included in the vaccines did not abolish the beneficial effects of HPV vaccination.37,38 This is an important finding that strongly favours HPV vaccination of young HIV-positive women. Interestingly, even in young HIV-infected women with prevalent HPV infection or cervical dysplasia, HPV vaccination may also be beneficial. In the FUTURE (Females United to Unilaterally Reduce Endo/Ectocervical Disease) studies, some of the young women ended up receiving surgery for precancerous cervical lesions. Among those women, follow-up revealed that persons in the vaccine arm had significantly lower risk of developing subsequent new HPV-related disease than persons in the control arm.43 A scheduled trial (NCT01928225) will evaluate if pretreatment with the quadrivalent HPV vaccine could result in similar reductions of subsequent cervical disease after surgery in HIV-infected women aged >18 years. In the light of the available immunogenicity and efficacy data, HPV vaccination appears highly likely to be at least partly efficacious in young HIV-infected women.
Only one of the immunogenicity trials in HIV-infected study populations enrolled adult women and they were enrolled alongside adult men. Sex-specific seroconversion rates were not evaluated in the study but women immunised by either of the HPV vaccines had high antibody titres against HPV-16 and -18.36 The efficacy of the quadrivalent HPV vaccine was previously investigated in healthy adult women in a randomised, placebo-controlled, double-blinded trial,44 where 3819 women aged 24–45 years with no history of cervical disease or genital warts in the past 5 years before inclusion were enrolled in a study with a mean follow-up period of 4 years. Vaccine efficacy was 47.2% (95% confidence interval (CI): 33.5–58.2) against a combined endpoint: incidence of persistent infection (≥6 months’ duration), cervical intraepithelial neoplasia or external genital lesions related to HPV-6, -11, - 16 and -18. The potential efficacy of HPV vaccination in adult women scheduled to receive surgery for precancerous cervical lesions has not been investigated in women aged 24–45 years. Immunogenicity data from HIV-positive adult women are convincing, albeit very few. Vaccine efficacy in healthy adult women is lower than in healthy young women. There are still many unknowns surrounding the potential effects of vaccinating adult HIV-positive women against HPV.
Only the quadrivalent HPV vaccine is licensed for use in males and the indication differs by geographical region. In the US, the vaccine is licensed against anogenital warts, anal cancer and associated precancerous lesions,45 whereas in Europe46 and Australia,47 the only approved indication for men is prevention of anogenital warts. Solid antibody responses following HPV vaccination were observed in the two trials that included male HIV-positive adults.35,36 Vaccine efficacy has been established against persistent anal HPV-16 and -18 infection among 602 HIV-negative MSM aged 16–26 years.21 During a follow-up with a median of 2.9 years, vaccine efficacy was 57.5% (95% CI: 33.2–73.6) against persistent anal infection with HPV-16 and -18 and 55.2% (95% CI: 8.5–79.3) against anal intraepithelial neoplasia related to HPV-16 and -18. However, only men with a maximum of five lifetime sexual partners were included in the trial and these efficacy results may not be generalisable to MSM populations. In a recent cohort study among 258 HIV-negative MSM aged 26–39 years, the median number of lifetime sexual partners was 50.48 Thus, in the general MSM population, prevalent HPV infections are likely to be more frequent than in this efficacy trial, which may reduce the vaccine’s efficacy in a real-life setting. In addition, HIV-positive MSM are more likely to have prevalent HPV-16 or -18 infections at the time of vaccination than HIV-negative MSM.29 Nevertheless, even with lower vaccine efficacy in this high-risk population, it may still be appropriate to recommend HPV vaccination, since anal cancer rates are worryingly high among HIV-infected MSM.5,49,50 The potential efficacy of vaccinating HIV-positive young and adult MSM will be evaluated against anal intraepithelial neoplasia in two separate trials (NCT01461096 and NCT01209325). The results from these trials will be pivotal in deciding if HPV vaccination also reduces the risk of precancerous anal lesions in this particular high-risk population.
Conclusions
Based on the current available knowledge reviewed in this paper, both HPV vaccines appear to be safe and highly immunogenic in people infected with HIV. In the light of their high risk of HPV-associated cancers, recommending HPV vaccination in HIV-positive individuals of both sexes up to the age of 26 years seems to be very reasonable. Furthermore, it may also be justified to recommend HPV vaccination in HIV-infected women aged 26–45 but the expected vaccine efficacy would be less than among younger women. Finally, HPV vaccination of HIV-infected women before surgical treatment of precancerous cervical lesions may be justified. The current data strongly suggest that HPV vaccination induces protective immunity against vaccine-specific HPV infection in persons with HIV.
Competing interests
MT has received a lecturer fee for an HIV Symposium organised by GlaxoSmithKline. LØ has received a lecturer fee from both GlaxoSmithKline and Sanofi-Pasteur Merck Sharp & Dohme (MSD).
Acknowledgements
The authors thank the Institute of Clinical Medicine at Aarhus University for funding the study.
References
[1] Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al Global burden of human papillomavirus and related diseases. Vaccine 2012; 30 F12–23.| Global burden of human papillomavirus and related diseases.Crossref | GoogleScholarGoogle Scholar | 23199955PubMed |
[2] Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007; 370 59–67.
| Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 17617273PubMed |
[3] 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992; 41 1–19.
| 1480128PubMed |
[4] Denny LA, Franceschi S, de Sanjose S, Heard I, Moscicki AB, Palefsky J. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine 2012; 30 F168–74.
| Human papillomavirus, human immunodeficiency virus and immunosuppression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhsl2jurvO&md5=04bcb7662b4d3f3b57c4f32b81f0dde6CAS | 23199960PubMed |
[5] Silverberg MJ, Lau B, Justice AC, Engels E, Gill MJ, Goedert JJ, et al Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis 2012; 54 1026–34.
| Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America.Crossref | GoogleScholarGoogle Scholar | 22291097PubMed |
[6] de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11 1048–56.
| Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlKmtrnJ&md5=66b98c456ec484cc501a8b788f07e8e0CAS | 20952254PubMed |
[7] Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24 S3/1–10.
| 1:CAS:528:DC%2BD28XptFCns7o%3D&md5=a16ade53e5ebe70a4b3bf7f00dea1b40CAS |
[8] Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, Smith T, et al Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int J Cancer 2014; 134 1389–98.
| Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslOls7vJ&md5=6b9bebdcb3b35841125510ce1c6af7a6CAS | 23929250PubMed |
[9] De Vuyst H, Ndirangu G, Moodley M, Tenet V, Estambale B, Meijer CJ, et al Prevalence of human papillomavirus in women with invasive cervical carcinoma by HIV status in Kenya and South Africa. Int J Cancer 2012; 131 949–55.
| Prevalence of human papillomavirus in women with invasive cervical carcinoma by HIV status in Kenya and South Africa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVektrbM&md5=17369901c70aca12d301f1f7498b7f0aCAS | 21960453PubMed |
[10] Odida M, Sandin S, Mirembe F, Kleter B, Quint W, Weiderpass E. HPV types, HIV and invasive cervical carcinoma risk in Kampala, Uganda: a case–control study. Infect Agent Cancer 2011; 6 8
| HPV types, HIV and invasive cervical carcinoma risk in Kampala, Uganda: a case–control study.Crossref | GoogleScholarGoogle Scholar | 21702999PubMed |
[11] Abramowitz L, Jacquard AC, Jaroud F, Haesebaert J, Siproudhis L, Pradat P, et al Human papillomavirus genotype distribution in anal cancer in France: the EDiTH V study. Int J Cancer 2011; 129 433–9.
| Human papillomavirus genotype distribution in anal cancer in France: the EDiTH V study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXovVCqtr8%3D&md5=08ef1c87016e6961be7760c4c910bd44CAS | 20839262PubMed |
[12] Monie A, Hung CF, Roden R, Wu TC. Cervarix: a vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biologics 2008; 2 97–105.
| 19707432PubMed |
[13] Siddiqui MA, Perry CM. Human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (Gardasil). Drugs 2006; 66 1263–71.
| Human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (Gardasil).Crossref | GoogleScholarGoogle Scholar | 16827602PubMed |
[14] Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, et al Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13 89–99.
| Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XkslSlsQ%3D%3D&md5=aceb26040efa3fe204225f13d88f90f2CAS | 22075171PubMed |
[15] The Future II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356 1915–27.
| Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions.Crossref | GoogleScholarGoogle Scholar | 17494925PubMed |
[16] Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364 1757–65.
| Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXpslGju78%3D&md5=ee7f0a950898842e8542de7e51de11f2CAS | 15541448PubMed |
[17] Herrero R, Wacholder S, Rodriguez AC, Solomon D, Gonzalez P, Kreimer AR, et al Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov 2011; 1 408–19.
| Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica.Crossref | GoogleScholarGoogle Scholar | 22586631PubMed |
[18] Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356 1928–43.
| Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXltFSmtLo%3D&md5=2644876b6d276d35d5eb861584a1a5f5CAS | 17494926PubMed |
[19] Kreimer AR, Gonzalez P, Katki HA, Porras C, Schiffman M, Rodriguez AC, et al Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica vaccine trial. Lancet Oncol 2011; 12 862–70.
| Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica vaccine trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFSqs7%2FF&md5=7e511be17514049464fdf5835036252aCAS | 21865087PubMed |
[20] Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Penny ME, Aranda C, et al Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med 2011; 364 401–11.
| Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFGhsLw%3D&md5=51c4a644d888677ec9f8cdc472f01c08CAS | 21288094PubMed |
[21] Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Aranda C, Jessen H, et al HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365 1576–85.
| HPV vaccine against anal HPV infection and anal intraepithelial neoplasia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVSrsrbN&md5=2cf0bea288639497ec9a39b1d7a55e2eCAS | 22029979PubMed |
[22] Centres for Disease Control and Prevention (CDC). HIV infection and adult vaccination. Atlanta: CDC; 2014. Available online at: http://www.cdc.gov/vaccines/adults/rec-vac/health-conditions/hiv.html [verified 13 August 2014].
[23] National Centre for Immunisation Research & Surveillance (NCIRS). Human papillomavirus (HPV). Westmead, NSW: NCIRS; 2013. Available online at: http://www.ncirs.edu.au/immunisation/fact-sheets/hpv-human-papillomavirus-fact-sheet.pdf [verified 13 August 2014].
[24] Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, et al Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis 2005; 191 1442–50.
| Compromised B cell responses to influenza vaccination in HIV-infected individuals.Crossref | GoogleScholarGoogle Scholar | 15809902PubMed |
[25] Montoya CJ, Toro MF, Aguirre C, Bustamante A, Hernandez M, Arango LP, et al Abnormal humoral immune response to influenza vaccination in pediatric type-1 human immunodeficiency virus infected patients receiving highly active antiretroviral therapy. Mem Inst Oswaldo Cruz 2007; 102 501–8.
| Abnormal humoral immune response to influenza vaccination in pediatric type-1 human immunodeficiency virus infected patients receiving highly active antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVWgtbrF&md5=7d76b9ed48292b7c5675ae41b941fb0cCAS | 17612772PubMed |
[26] Kim HN, Harrington RD, Crane HM, Dhanireddy S, Dellit TH, Spach DH. Hepatitis B vaccination in HIV-infected adults: current evidence, recommendations and practical considerations. Int J STD AIDS 2009; 20 595–600.
| Hepatitis B vaccination in HIV-infected adults: current evidence, recommendations and practical considerations.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1Mrot12isQ%3D%3D&md5=168e0385e529504cf9c032d52f07a470CAS | 19710329PubMed |
[27] Rodriguez-Barradas MC, Groover JE, Lacke CE, Gump DW, Lahart CJ, Pandey JP, et al IgG antibody to pneumococcal capsular polysaccharide in human immunodeficiency virus-infected subjects: persistence of antibody in responders, revaccination in nonresponders, and relationship of immunoglobulin allotype to response. J Infect Dis 1996; 173 1347–53.
| IgG antibody to pneumococcal capsular polysaccharide in human immunodeficiency virus-infected subjects: persistence of antibody in responders, revaccination in nonresponders, and relationship of immunoglobulin allotype to response.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK283hslOgsw%3D%3D&md5=5a4d0e25a9c038e55e74c417a990c203CAS | 8648206PubMed |
[28] Clifford GM, Goncalves MA, Franceschi S. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS 2006; 20 2337–44.
| Human papillomavirus types among women infected with HIV: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 17117020PubMed |
[29] Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, et al Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 2012; 13 487–500.
| Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 22445259PubMed |
[30] Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339 b2535
| Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.Crossref | GoogleScholarGoogle Scholar | 19622551PubMed |
[31] Levin MJ, Moscicki AB, Song LY, Fenton T, Meyer WA, Read JS, et al Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr 2010; 55 197–204.
| Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old.Crossref | GoogleScholarGoogle Scholar | 20574412PubMed |
[32] Weinberg A, Song LY, Saah A, Brown M, Moscicki AB, Meyer WA, et al Humoral, mucosal, and cell-mediated immunity against vaccine and nonvaccine genotypes after administration of quadrivalent human papillomavirus vaccine to HIV-infected children. J Infect Dis 2012; 206 1309–18.
| Humoral, mucosal, and cell-mediated immunity against vaccine and nonvaccine genotypes after administration of quadrivalent human papillomavirus vaccine to HIV-infected children.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWgsrjK&md5=ced9f246992868b295708f935beec37fCAS | 22859825PubMed |
[33] Kahn JA, Xu J, Kapogiannis BG, Rudy B, Gonin R, Liu N, et al Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women. Clin Infect Dis 2013; 57 735–44.
| Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1yrsr7E&md5=2ea25c4cc06c0a6e95acfc0a0f6bbbcdCAS | 23667266PubMed |
[34] Denny L, Hendricks B, Gordon C, Thomas F, Hezareh M, Dobbelaere K, et al Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine 2013; 31 5745–53.
| Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1Wis7vE&md5=05f386f7d8e8e847ed09946f7499e718CAS | 24091311PubMed |
[35] Wilkin T, Lee JY, Lensing SY, Stier EA, Goldstone SE, Berry JM, et al Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis 2010; 202 1246–53.
| Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men.Crossref | GoogleScholarGoogle Scholar | 20812850PubMed |
[36] Toft L, Storgaard M, Muller M, Sehr P, Bonde J, Tolstrup M, et al Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV-infected adults: a randomized, double-blind, clinical trial. J Infect Dis 2014; 209 1165–73.
| Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV-infected adults: a randomized, double-blind, clinical trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXkslCnsLo%3D&md5=9e3d878137e812d61b4a107d745136e5CAS | 24273179PubMed |
[37] The Future I/II Study Group Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ 2010; 341 c3493
| Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial.Crossref | GoogleScholarGoogle Scholar | 20647284PubMed |
[38] Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al Effect of human papillomavirus 16/18 L1 virus-like particle vaccine among young women with preexisting infection: a randomized trial. JAMA 2007; 298 743–53.
| Effect of human papillomavirus 16/18 L1 virus-like particle vaccine among young women with preexisting infection: a randomized trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpt1ais7o%3D&md5=fead34e2f98edf1dd2eae1c5dc7fa7caCAS | 17699008PubMed |
[39] Szarewski A, Poppe WA, Skinner SR, Wheeler CM, Paavonen J, Naud P, et al Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15–25 years with and without serological evidence of previous exposure to HPV-16/18. Int J Cancer 2012; 131 106–16.
| Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15–25 years with and without serological evidence of previous exposure to HPV-16/18.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xlslymtr0%3D&md5=637f1b4d5e6a164650585065a7dbb47eCAS | 21858807PubMed |
[40] The Future II Study Group Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis 2007; 196 1438–46.
| Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection.Crossref | GoogleScholarGoogle Scholar | 18008221PubMed |
[41] Søgaard OS, Schonheyder HC, Bukh AR, Harboe ZB, Rasmussen TA, Ostergaard L, et al Pneumococcal conjugate vaccination in persons with HIV: the effect of highly active antiretroviral therapy. AIDS 2010; 24 1315–22.
| Pneumococcal conjugate vaccination in persons with HIV: the effect of highly active antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 20559037PubMed |
[42] Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst 2011; 103 1444–51.
| Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht12rsrnN&md5=62e0fc9abfcd632d509b92833844764eCAS | 21908768PubMed |
[43] Joura EA, Garland SM, Paavonen J, Ferris DG, Perez G, Ault KA, et al Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ 2012; 344 e1401
| Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data.Crossref | GoogleScholarGoogle Scholar | 22454089PubMed |
[44] Castellsagué X, Muñoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, et al End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer 2011; 105 28–37.
| End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age.Crossref | GoogleScholarGoogle Scholar | 21629249PubMed |
[45] US Food and Drug Administration (USFDA). Gardasil. Silver Spring MD: USFDA; 2014. Available online at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM094042 [verified 13 August 2014].
[46] European Medicines Agency (EMA). Annex I: summary of product characteristics. London: EMA; 2014. Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000703/WC500021142.pdf [verified 13 August 2014].
[47] Therapeutic Goods Administration (TGA). Australian public assessment report for Gardasil. Symonston, ACT: TGA; 2011. Available online at: http://www.tga.gov.au/pdf/auspar/auspar-gardasil.pdf [verified 13 August 2014].
[48] Donà MG, Palamara G, Di Carlo A, Latini A, Vocaturo A, Benevolo M, et al Prevalence, genotype diversity and determinants of anal HPV infection in HIV-uninfected men having sex with men. J Clin Virol 2012; 54 185–9.
| Prevalence, genotype diversity and determinants of anal HPV infection in HIV-uninfected men having sex with men.Crossref | GoogleScholarGoogle Scholar | 22418456PubMed |
[49] de Pokomandy A, Rouleau D, Ghattas G, Trottier H, Vezina S, Cote P, et al HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clin Infect Dis 2011; 52 1174–81.
| HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV.Crossref | GoogleScholarGoogle Scholar | 21364075PubMed |
[50] Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 2008; 148 728–36.
| Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003.Crossref | GoogleScholarGoogle Scholar | 18490686PubMed |
[51] Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin 2009; 5 696–704.
| Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjvF2ksbg%3D&md5=e4173b062398be9299b95787710a4aabCAS | 19855170PubMed |