Neurosyphilis and the impact of HIV infection
Emily L. Ho A B D and Serena S. Spudich CA Department of Neurology, University of Washington, USA.
B Swedish Neuroscience Institute, 550 17th Avenue, 4West, Seattle, WA, 98122, USA.
C Department of Neurology, School of Medicine, PO Box 208018, Yale University, New Haven, CT, 06250, USA.
D Corresponding author. Email: hoel@u.washington.edu
Sexual Health 12(2) 148-154 https://doi.org/10.1071/SH14195
Submitted: 13 October 2014 Accepted: 18 January 2015 Published: 20 April 2015
Journal Compilation © CSIRO Publishing 2015 Open Access CC BY-NC-ND
Abstract
Neurosyphilis is a complication of systemic syphilis. This review of the clinical presentation, diagnostic laboratory findings, treatment and management of neurosyphilis discusses the impact of HIV and the specific challenges it brings, focusing on areas of controversy, and highlighting important questions that remain to be answered.
Introduction
The causative agent of syphilis, Treponema pallidum subspecies pallidum, can invade the central nervous system (CNS) to cause neurosyphilis at any time after infection. Neurosyphilis may be symptomatic or asymptomatic; that is, diagnosed by cerebrospinal fluid (CSF) abnormalities only. Neurosyphilis is of particular concern in HIV-infected patients for several reasons, including: (i) the high rates of HIV and T. pallidum co-infection; (ii) the possibility of increased risk of failing therapy for early syphilis, which may result in the development of early neurosyphilis (i.e. neurorelapse); (iii) challenges to diagnosis of neurosyphilis in these patients; and (iv) the potentially higher rates of treatment failure. All of these factors may contribute to long-term morbidity in HIV-infected patients, a subject undergoing active investigation.
Case 1
A 35-year-old man, otherwise healthy, presented to an outpatient eye clinic complaining of eye pain and blurry vision, first involving the right eye, then involving the left eye. He was diagnosed with iritis by an ophthalmologist and prescribed steroid eye drops. Three months later, he presented to the Emergency Department of a tertiary hospital complaining that his vision had worsened such that he could barely see anything other than hand movements. He had no other known medical problems. He was a man who has sex with men and used methamphetamine several times a month. On ophthalmological examination, the patient had uveitis, choroidal lesions and retinal detachment in both eyes. His general physical examination revealed oral thrush but no genital lesions. His skin exam was otherwise unremarkable. The patient underwent lumbar puncture when initial serum rapid plasma reagin (RPR) was positive. CSF testing showed 40 white blood cell (WBC) (75% lymphocytes), protein 70 mg dL–1, glucose 124 mg dL–1 and a reactive CSF-venereal disease research laboratory (VDRL; 1 : 1 titer). Subsequent laboratory tests showed that the serum VDRL titre was 1:128; the CD4+ T-cell concentration was 169 cells uL–1; and the peripheral blood HIV RNA was greater than 1.3 million copies mL–1. The patient was diagnosed with HIV and neurosyphilis with ocular involvement. He was treated with intravenous (IV) penicillin, 24 million units daily for 10 days. Eight months later, his serum VDRL had decreased to 1 : 16. In the meantime, he underwent surgical repair of the bilateral retinal detachments. While the patient’s vision in the left eye improved significantly, he continued to have severely impaired vision in the right eye.
Syphilis, neurosyphilis and HIV
Since the beginning of the HIV epidemic, there has been a high rate of syphilis in HIV-infected patients. The United States Centers for Disease Control and Prevention (CDC) reported that the incidence rate of syphilis in HIV-infected persons was 77-fold greater than that in the general population.1 In addition, high rates of syphilis occur in regions of high HIV prevalence.2,3 Alarmingly, the number of cases of syphilis has continued to rise worldwide, including Eastern Europe4,5 and, more recently, China.6 In the United States in the past decade, this increase has particularly affected men who have sex with men (MSM) and, more recently, young African-Americans and Hispanics.7,8 Similar trends for MSM have been observed throughout Europe9 and China.3,10,11 Some reports suggest that the increasing incidence of syphilis may be due to high-risk behaviours, such as methamphetamine use,12–14 but as we will discuss later, the higher rates of syphilis and HIV co-infection may also be due to immunological factors.
Neurosyphilis, defined by specific CSF findings (see below) with or without typical clinical symptomatology, is a potential complication of systemic syphilis. Because neurosyphilis is not a reportable disease, the epidemiology of neurosyphilis occurrence in HIV-infected patients is harder to estimate. Many reported studies are retrospective. One study by the CDC using public health records from 2002 to 2004 of four USA cities (Los Angeles, San Diego, Chicago, New York) estimated that among HIV-positive MSM with early syphilis, the risk for having symptomatic early neurosyphilis was 1.7%.15 In the 49 HIV-positive MSM diagnosed with symptomatic early neurosyphilis, symptoms were cranial nerve dysfunction (34 including 25 ocular, six auditory); meningitis (six); meningovascular syndrome (two); and other syndromes (seven). Similar findings were reported in a retrospective review of syphilis cases in Los Angeles county from 2001 to 2004; the estimated incidence of symptomatic neurosyphilis in HIV-infected patients was 2.1% compared with 0.6% in HIV-uninfected patients.16 However, the rate of neurosyphilis in these studies may be underestimated because they are each disadvantaged by ascertainment bias; the patients were offered evaluation for neurosyphilis at the discretion of their healthcare providers. No large, systematic study to date has aimed to document the true prevalence of neurosyphilis in a larger cohort of patients diagnosed with systemic syphilis.
Clinical manifestations of neurosyphilis
The clinical manifestations of neurosyphilis are myriad and include: asymptomatic neurosyphilis, symptomatic meningitis, meningovascular syphilis (affecting the brain or spinal cord), ocular syphilis, otologic syphilis, gumma, general paresis and tabes dorsalis. Neurosyphilis characterised by meningeal inflammation (asymptomatic, meningeal and meningovascular) is most common in the early years of infection, while neurosyphilis involving the parenchyma (gumma, general paresis and tabes dorsalis) occurs years to decades after initial infection. The parenchymal forms are very rare in the modern antibiotic era. Ocular and otological manifestations occur in both early and late stages of neurosyphilis, as observed in our patient case #1, often in combination with meningitis. Given the increased prevalence of syphilis in HIV-infected individuals, in any HIV-infected patient presenting with meningitis, stroke involving the brain or spinal cord, brain mass, transverse myelitis, chronic myelopathy and dementia, neurosyphilis should be considered in the differential diagnosis.
Clinical features of meningeal neurosyphilis include headache, photophobia and stiff neck as well as cranial nerve abnormalities. Some patients may also have encephalopathy. Meningovascular forms of neurosyphilis may affect either the brain or the spinal cord. Patients with strokes affecting the brain may present with confusion, aphasia, hemiplegia or seizure. Patients with strokes involving the spinal cord may manifest with bilateral lower extremity weakness, a spinal sensory level and bowel/bladder incontinence. Because gummas are space-occupying lesions, patients may present with focal neurological signs, seizures, evidence of elevated intracranial pressure or progressive quadriparesis or paraparesis. Historically, general paresis accounted for large proportions of patients admitted to mental asylums (in the 1920s, this was more than 20% of such patients in the USA).17 The clinical manifestations of general paresis include a subacute, chronic, relapsing-remitting change in personality, affect and cognition, in addition to neurological symptoms such as impaired speech, pupillary abnormalities (e.g. Argyll–Robertson pupil), ataxia and hyperreflexia. Patients with tabes dorsalis may complain of lightning-like shooting pain in the lower extremities; on exam, they may have an Argyll–Robertson pupil or ataxic gait due to impaired joint position sense, which is referrable to demyelination of the dorsal columns in the spinal cord.
Case 2
A 41-year-old man with laboratory-confirmed recent (within 6 months) HIV infection enrolled in an observational neurological study with a baseline CD4+ T-cell count of 350 cells uL–1, a plasma HIV RNA level of 404 000 copies mL–1 and non-reactive serum RPR. He was neurologically asymptomatic and antiretroviral naïve at enrolment, and participated in a lumbar puncture solely for study purposes, which revealed CSF WBC of 11 cells uL–1 and a CSF HIV RNA level of 37 500 copies mL–1. After one subsequent lumbar puncture while antiretroviral naïve (CSF WBC of 16 cells uL–1), he initiated antiretroviral therapy (ART). However, during the course of his longitudinal study participation, he discontinued ART and was noted on the subsequent study lumbar puncture to have a marked increase in CSF pleocytosis (CSF WBC of 119 cells uL–1), which prompted repeat serum RPR and CSF VDRL testing. Serum RPR was reactive, with a titre of 1 : 128 and the CSF VDRL was also reactive (titre 1 : 2). Three days after this study visit, he noted aphthous ulcers in his mouth and a rash on his palms, although he remained neurologically asymptomatic. He was treated with 2 weeks’ of IV penicillin and eventually reinitiated combination ART. Longitudinal blood and CSF laboratory results are presented in Fig. 1, demonstrating the impact of HIV infection, treatment with ART, antiretroviral treatment interruption with coincident syphilis and neurosyphilis, treatment for neurosyphilis and reinitiation of ART upon blood and CSF markers. This case provides an example of the complex interplay between HIV, syphilis and treatment for each of these conditions, and the challenges in interpreting blood and CSF laboratory testing for syphilis in a patient with concurrent HIV infection.
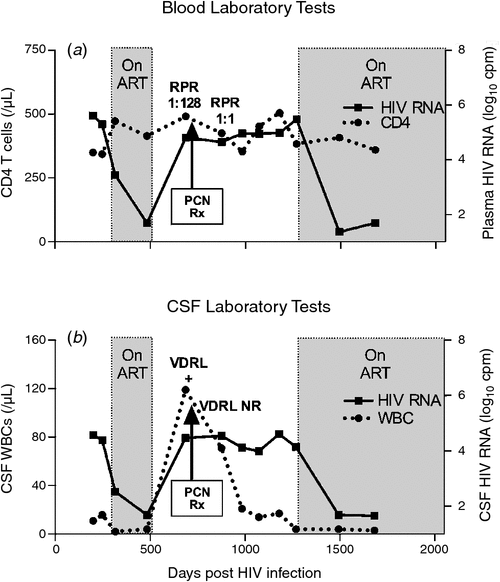
|
Laboratory definitions of neurosyphilis
Neurosyphilis is also diagnosed by CSF abnormalities, including elevated WBC count, elevated CSF protein or reactive CSF-VDRL. The CSF-VDRL is considered to be the ‘gold standard’ diagnostic test. While other forms of neurosyphilis are based on a combination of clinical presentation and CSF abnormalities, diagnosis of asymptomatic neurosyphilis is based upon the presence of CSF abnormalities alone. Several studies indicate that patients with syphilis and abnormal CSF are at greater risk of symptomatic neurosyphilis, especially those patients with greater CSF abnormalities. However, these studies are from the pre-antibiotic era and modern studies, including studies involving HIV-infected patients, are lacking.
In neurosyphilis, CSF WBC concentration is generally >10 cells uL–1 and predominantly lymphocytic. CSF-VDRL has a sensitivity of ~30–70%.18–20 Thus, a reactive CSF-VDRL establishes the diagnosis of neurosyphilis, but a non-reactive test does not exclude the diagnosis. With such an insensitive test, the diagnosis of neurosyphilis in a HIV-infected person may need to be based on CSF pleocytosis alone. In contrast, the CSF treponemal tests (fluorescent treponemal antibody absorbed (FTA-ABS)), T. pallidum Particle Agglutination (TPPA) test, T. pallidum hemagglutination (TPHA) test and various enzyme immunoassays (EIAs)) are less specific for neurosyphilis, but highly sensitive.21,22 Thus, a non-reactive CSF treponemal test excludes the diagnosis of neurosyphilis.
Because HIV itself can cause mild CSF pleocytosis,23 diagnosing neurosyphilis in HIV-infected patients can be particularly challenging. While the patient described in Case #2 had a dramatic rise in his CSF WBC count at the time of diagnosis of incident neurosyphilis, his CSF WBC count in the presence of untreated HIV infection alone had ranged from 11 to 16 cells/uL. Given this, most recent definitions have used a cut-off of >20 WBCs/uL as diagnostic of neurosyphilis in HIV-infected individuals who have a positive blood syphilis titre but a non-reactive CSF-VDRL, as employed in a large prospective study of syphilis in HIV-infected patients.24 Others have used a lower cut-off,25 which may be most appropriate in HIV patients with a low peripheral blood CD4+ T-cell concentration, who are taking combination ART or who have undetectable plasma HIV RNA, because these lower the risk of HIV-related CSF pleocytosis.23,26 Other tests not currently used clinically that may contribute to the diagnosis of asymptomatic neurosyphilis include: (i) the proportion of CSF lymphocytes that are B-cells;27 and (ii) the CSF concentration of the chemokine C-X-C motif ligand 13 (CXCL-13), a B-cell chemoattractant.28 Both tests may be elevated in patients with neurosyphilis compared with patients with uncomplicated syphilis, including those with concurrent HIV infection.28,29
Challenges to the diagnosis of neurosyphilis in HIV-infected patients
Given the complexity of diagnosing neurosyphilis in HIV-infected individuals, several studies have sought out predictors of neurosyphilis in patients with known systemic syphilis that might guide indications for lumbar puncture. Numerous prior studies have shown that the risk of neurosyphilis in HIV-infected patients with systemic syphilis is significantly higher in the setting of neurological symptoms such as headache or ocular symptoms.24,30,31 Additionally, reduced peripheral blood CD4+ T-cell count has been associated with the risk of neurosyphilis: CD4 T-cell counts of ≤350 uL–1 in several studies24,31,32 and ≤500 uL–1 in a more recent study30 have been clearly associated with an increased probability of neurosyphilis in HIV-infected persons, as defined by CSF criteria. Some controversy remains as to whether high serum rapid plasma reagin (RPR) titres also predict neurosyphilis. While some studies routinely assessing all HIV-infected participants presenting with syphilis with a lumbar puncture found that a serum RPR titer of ≥1 : 32 was associated with a higher rate of neurosyphilis,24,25,31 other studies including a recent study of 122 subjects by Dumaresq et al. have not clearly shown this association.30 However, in the latter study, the required enrolment criteria for a study-related lumbar puncture included a serum RPR titre of ≥1:32; with this restricted range of serum RPR titres, it may have been difficult to detect an association between serum RPR and the presence of neurosyphilis. Indeed, in several studies, when HIV-infected patients were offered lumbar puncture if they had RPR ≥1 : 32 and CD4+ T-cells ≤350 uL, rates of diagnosed neurosyphilis were much higher than that reported in retrospective studies with broader entry criteria.15,16,33 The rates of syphilis reported by Marra et al., Leber et al. and Ghanem et al. in individuals undergoing lumbar puncture based on these criteria ranged from 20 to 27%.24,30,32,34
Importantly, HIV-infected patients with syphilis who are virally suppressed on combination ART may be at lower risk of neurosyphilis compared with those not taking combination ART.32 Two retrospective studies have suggested that uncontrolled plasma HIV RNA (>50 copies mL–1) increased the risk of neurosyphilis.30,35 In HIV-infected individuals with detectable plasma HIV RNA, those who have neurosyphilis have higher CSF HIV RNA concentrations compared with those who have uncomplicated syphilis, suggesting there may be an interaction between syphilis and HIV in the CNS.36 The patient described in Case #2 had an increase in his CSF HIV RNA level coincident with acute systemic and neurosyphilis infection, although his rise in HIV RNA in the CSF was also due to antiretroviral treatment interruption. In the absence of syphilis, pleocytosis in the CSF closely associates with the CSF HIV RNA level, most likely due to increased trafficking of lymphocytes into the intrathecal space and resultant amplified local replication of HIV in the CNS compartment.26 The CSF HIV RNA eventually became undetectable on an assay with a lower limit of detection of 40 copies mL–1 after adequate treatment for neurosyphilis as well as reinitiation of combination ART in our patient.
Who to test? Guidelines for lumbar puncture
Outcomes-based data to guide decisions whether patients with HIV infection found to have systemic syphilis should undergo lumbar puncture is currently lacking. Based on prior observational and retrospective studies, experts agree that HIV-infected individuals with systemic syphilis, accompanied by neurologic or ocular symptoms or signs, should undergo CSF testing. The United States CDC only includes this clinical indication in their formal 2010 recommendations regarding which HIV-infected patients should be assessed for neurosyphilis with a lumbar puncture.21 The sexually transmissible disease guidelines from the Public Health Agency of Canada also include CD4 counts <350 cells uL–1, diagnosis of late latent syphilis and a RPR ≥1 : 32 as criteria necessitating lumbar puncture, and point out that some experts consider HIV infection alone as an indication for a lumbar puncture in patients with syphilis.37 European guidelines suggest lumbar puncture can be considered for all HIV-co-infected syphilis patients with a CD4 count <350 cells uL–1 and/or a serum RPR test titre >1 : 32 ‘although robust data are lacking’.38 The range of formal recommendations reflect a lack of consensus as to which patients truly benefit from screening and intervention for potential neurosyphilis, which in turn relates to a dearth of systematic studies in this area. Based on recent studies, and the relatively low-risk of elective lumbar puncture in the controlled setting of an outpatient clinic, it may be reasonable to assess HIV-infected patients with a CSF examination who may be at higher risk of neurosyphilis based on a lower CD4 count range or high serum RPR titres.
Neurorelapse: neurological complications after syphilis treatment
Neurorelapse is the development of neurosyphilis (e.g. ocular or otologic syphilis) after appropriate treatment for early syphilis. Initially recognised in the pre-antibiotic era39 and reported early in the penicillin era,40 several more recent case studies report neurorelapse occurring in HIV-infected patients.19,41–45 While it is difficult to rule out the possibility of reinfection entirely in these case reports, it has also been hypothesised that neurorelapse may be more common in HIV-infected patients with syphilis because benzathine penicillin G, the usual treatment for uncomplicated syphilis, does not clear T. pallidum from the CNS, the eye or the inner ear46,47 and HIV impairs cell-mediated immunity, allowing T. pallidum to persist at these sites.41 Further systematic studies are needed to determine whether HIV co-infection truly increases the odds of neurorelapse with syphilis. If so, it is crucial to understand what mechanisms underlie this heightened risk and whether this apparent neurorelapse in fact represents undetected asymptomatic neurosyphilis, which was present before treatment for systemic disease.
Treatment of neurosyphilis
The United States CDC recommends IV penicillin G, as first-line treatment for neurosyphilis (Table 1). Alternatively, intramuscular (IM) procaine penicillin G with oral probenecid can be used.21 Case reports48–51 and two small studies of HIV-infected patients52,53 suggest that ceftriaxone administered either IM or IV daily may also be an acceptable alternative treatment. As the treatment duration of neurosyphilis is up to 2 weeks, the United States CDC also states that practitioners may consider treating patients with late syphilis with benzathine penicillin, 2.4 million units IM once per week for up to 3 weeks, following treatment for neurosyphilis;21 the European syphilis guidelines make no such suggestion.38 In our practices, we continue treatment according to the CDC guidelines. Neurosyphilis with ocular and otologic manifestations is treated identically, although steroids may be used as an adjunctive therapy.43,54–57 The United States CDC, European syphilis guidelines and Canadian Public Health guidelines recommend penicillin desensitisation for penicillin allergic patients in whom ceftriaxone is not an option.21,37,38
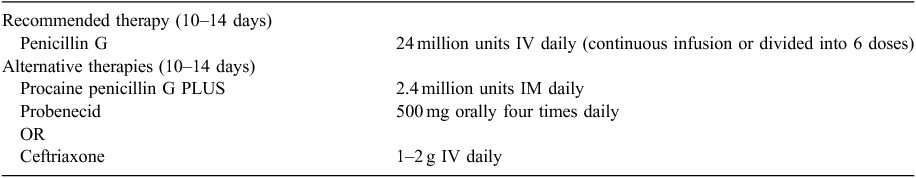
|
In patients who have improvement, resolution or stabilisation of neurological symptoms and normalisation of CSF abnormalities, treatment for neurosyphilis is deemed successful. The CDC recommends that patients undergo repeat lumbar puncture every 6 months until the CSF WBC normalises. If the cell count has not decreased after 6 months or if the CSF cell count or protein is not normal after 2 years, retreatment should be considered. As observed in our patient in Case #2, CSF pleocytosis may persist for a considerable time, and potential for complete resolution will also depend on antiretroviral treatment status in the HIV-co-infected patient. If lumbar puncture is not possible, one study suggested that normalisation of the serum RPR titre predicted normalisation of CSF WBC and clinical abnormalities in >80% of patients at 4 months after neurosyphilis treatment.58 However, the study pointed out that normalisation of the serum RPR titre was consistently less accurate in predicting treatment success in HIV-infected patients who were not receiving CART, compared with those who were receiving such therapy.
Compared with HIV-uninfected patients, HIV-infected patients with symptomatic neurosyphilis may be more likely to fail neurosyphilis therapy based on clinical and serological criteria, as well as on failure to normalise CSF abnormalities.32,59–64 In one prospective study, HIV-infected patients were 2.5-fold less likely to normalise CSF-VDRL after appropriate therapy compared with HIV-uninfected patients.63 In addition, HIV-infected patients with peripheral blood CD4+ T-cells ≤200 cells uL–1 were 3.3-fold less likely to normalise CSF-VDRL.63 Thus, HIV-infected patients should undergo regular follow up with repeat CSF testing despite undergoing adequate treatment for neurosyphilis.
Conclusions
The diagnosis and management of neurosyphilis is challenging, but in the HIV-infected patient, the complexities are amplified. Exact indications for testing CSF in HIV-infected persons for neurosyphilis are still in question, though experts agree that all patients with neurological signs or symptoms in this context should undergo lumbar puncture for further evaluation. In addition, HIV brings on an immunocompromised state, which may make treatment of syphilis and neurosyphilis less reliable in HIV-infected patients, emphasising the need for close follow up after treatment.
Conflicts of interest
None declared.
Acknowledgements
E.L.H. has received funds from a 2014 developmental grant from the University of Washington Center for AIDS Research (CFAR), a NIH-funded program under award number P30AI027757, which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, NIDDK). S.S.S. has received funds from the National Institutes of Health, including The National Institute of Mental Health (NIMH) and National Institute of Neurological Disorders and Stroke (NINDS).
References
[1] Chesson HW, Heffelfinger JD, Voigt RF, Collins D. Estimates of primary and secondary syphilis rates in persons with HIV in the United States, 2002. Sex Transm Dis 2005; 32 265–9.| Estimates of primary and secondary syphilis rates in persons with HIV in the United States, 2002.Crossref | GoogleScholarGoogle Scholar | 15849526PubMed |
[2] World Health Organization. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. Geneva: World Health Organization; 2001.
[3] Men who have Sex with Men (MSM)–Update for ICAAP, Bali; 2009. Available online at: http://www.aidsdatahub.org/sites/default/files/documents/China_MSM_Country_Snapshot__Aug_2009.pdf [verified 12 October 2014].
[4] Herbert LJ, Middleton SI. An estimate of syphilis incidence in Eastern Europe. J Glob Health 2012; 2 010402
| An estimate of syphilis incidence in Eastern Europe.Crossref | GoogleScholarGoogle Scholar | 23198131PubMed |
[5] Kuklova I, Velcevsky P, Kojanova M. Syphilis among STD clinic patients in Prague in 2009. Cent Eur J Public Health 2011; 19 84–90.
| 21739897PubMed |
[6] Tucker JD, Yin YP, Wang B, Chen XS, Cohen MS. An expanding syphilis epidemic in China: epidemiology, behavioural risk and control strategies with a focus on low-tier female sex workers and men who have sex with men. Sex Transm Infect 2011; 87 ii16–8.
| An expanding syphilis epidemic in China: epidemiology, behavioural risk and control strategies with a focus on low-tier female sex workers and men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 22110145PubMed |
[7] Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Atlanta: U.S. Department of Health and Human Services; 2011.
[8] Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2012. Atlanta: U.S. Department of Health and Human Services; 2013.
[9] Savage EJ, Hughes G, Ison C, Lowndes CM. Syphilis and gonorrhoea in men who have sex with men: a European overview. Euro Comm Dis Bull 2009; 14 ii–19417.
[10] Muessig KE, Tucker JD, Wang BX, Chen XS. HIV and syphilis among men who have sex with men in China: the time to act is now. Sex Transm Dis 2010; 37 214–6.
| 20220563PubMed |
[11] Xu JJ, Zhang M, Brown K, Reilly K, Wang H, Hu Q,, Ding H, Chu Z, Bice T, Shang H. Syphilis and HIV seroconversion among a 12-month prospective cohort of men who have sex with men in Shenyang, China. Sex Transm Dis 2010; 37 432–9.
| 20375928PubMed |
[12] Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2005 supplement, syphilis surveillance report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; December 2006.
[13] Spindler HH, Scheer S, Chen SY, Klausner JD, Katz MH, Valleroy LA, Schwarcz SK. Viagra, methamphetamine, and HIV risk: results from a probability sample of MSM, San Francisco. Sex Transm Dis 2007; 34 586–91.
| 17334264PubMed |
[14] Taylor MM, Aynalem G, Smith LV, Montoya J, Kerndt P. Methamphetamine use and sexual risk behaviours among men who have sex with men diagnosed with early syphilis in Los Angeles County. Int J STD AIDS 2007; 18 93–7.
| Methamphetamine use and sexual risk behaviours among men who have sex with men diagnosed with early syphilis in Los Angeles County.Crossref | GoogleScholarGoogle Scholar | 17331279PubMed |
[15] Centers for Disease Control and Prevention Symptomatic early neurosyphilis among HIV-positive men who have sex with men–four cities, United States, January 2002–June 2004. MMWR Morb Mortal Wkly Rep 2007; 56 625–8.
| 17597693PubMed |
[16] Taylor MM, Aynalem G, Olea LM, He P, Smith LV, Kerndt PR. A consequence of the syphilis epidemic among men who have sex with men (MSM): neurosyphilis in Los Angeles, 2001–2004. Sex Transm Dis 2008; 35 430–4.
| A consequence of the syphilis epidemic among men who have sex with men (MSM): neurosyphilis in Los Angeles, 2001–2004.Crossref | GoogleScholarGoogle Scholar | 18446083PubMed |
[17] Brandt AM. No magic bullet: a social history of venereal disease in the United States since 1880. Oxford: Oxford University Press; 1987.
[18] Davis LE, Schmitt JW. Clinical significance of cerebrospinal fluid tests for neurosyphilis. Ann Neurol 1989; 25 50–5.
| Clinical significance of cerebrospinal fluid tests for neurosyphilis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1M7hslWjug%3D%3D&md5=08702a7eda39695b4cff0bbdcfcced5bCAS | 2643918PubMed |
[19] Rolfs RT, Joesoef MR, Hendershot EF, Rompalo AM, Augenbraun MH, Chiu M,, Bolan G, Johnson SC, French P, Steen E, Radolf JD, Larsen S. A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group. N Engl J Med 1997; 337 307–14.
| A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2szmsFSnsg%3D%3D&md5=7e459a6433005d1430c7a1e302b13b3cCAS | 9235493PubMed |
[20] Marra CM, Tantalo LC, Maxwell CL, Ho EL, Sahi SK, Jones T. The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis 2012; 39 453–7.
| The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis.Crossref | GoogleScholarGoogle Scholar | 22592831PubMed |
[21] Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2010. MMWR Morb Mort Wkly Rep 2010; 59 26–39.
[22] Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Morb Mortality Wkly Report 2009; 58 1–207.
[23] Marra CM, Maxwell CL, Collier AC, Robertson KR, Imrie A. Interpreting cerebrospinal fluid pleocytosis in HIV in the era of potent antiretroviral therapy. BMC Infect Dis 2007; 7 37
| Interpreting cerebrospinal fluid pleocytosis in HIV in the era of potent antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 17475004PubMed |
[24] Marra CM, Maxwell CL, Smith SL, Lukehart SA, Rompalo AM, Eaton M, et al Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J Infect Dis 2004; 189 369–76.
| Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features.Crossref | GoogleScholarGoogle Scholar | 14745693PubMed |
[25] Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. Lumbar puncture in HIV-infected patients with syphilis and no neurologic symptoms. Clin Infect Dis 2009; 48 816–21.
| Lumbar puncture in HIV-infected patients with syphilis and no neurologic symptoms.Crossref | GoogleScholarGoogle Scholar | 19187028PubMed |
[26] Spudich SS, Nilsson AC, Lollo ND, Liegler TJ, Petropoulos CJ, Deeks SG, Paxinos EE, Price RW. Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis 2005; 5 98
| Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment.Crossref | GoogleScholarGoogle Scholar | 16266436PubMed |
[27] Marra CM, Tantalo LC, Maxwell CL, Dougherty K, Wood B. Alternative cerebrospinal fluid tests to diagnose neurosyphilis in HIV-infected individuals. Neurology 2004; 63 85–8.
| Alternative cerebrospinal fluid tests to diagnose neurosyphilis in HIV-infected individuals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2czks1Krsw%3D%3D&md5=3f6146a15a74b259cdfcf97633b3724bCAS | 15249615PubMed |
[28] Marra CM, Tantalo LC, Sahi SK, Maxwell CL, Lukehart SA. CXCL13 as a cerebrospinal fluid marker for neurosyphilis in HIV-infected patients with syphilis. Sex Transm Dis 2010; 37 283–7.
| 1:CAS:528:DC%2BC3cXnsVOktbk%3D&md5=3d212143af0505c5d89921ad487fa573CAS | 20393380PubMed |
[29] Rupprecht TA, Kirschning CJ, Popp B, Kastenbauer S, Fingerle V, Pfister HW, et al Borrelia garinii induces CXCL13 production in human monocytes through Toll-like receptor 2. Infect Immun 2007; 75 4351–6.
| Borrelia garinii induces CXCL13 production in human monocytes through Toll-like receptor 2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpvFGhtr8%3D&md5=618d5c64e46dc6742e3e87f8645200e5CAS | 17562761PubMed |
[30] Dumaresq J, Langevin S, Gagnon S, Serhir B, Deligne B, Tremblay C, Tsang RS, Fortin C, Coutlee F, Roger M. Clinical prediction and diagnosis of neurosyphilis in HIV-infected patients with early syphilis. J Clin Microbiol 2013; 51 4060–6.
| Clinical prediction and diagnosis of neurosyphilis in HIV-infected patients with early syphilis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjtlWrtro%3D&md5=b2c812346a9d0f0ce577ede1bd4c88e0CAS | 24088852PubMed |
[31] Libois A, De Wit S, Poll B, Garcia F, Florence E, Del Rio A, Sanchez P, Negredo E, Vandenbruaene M, Gatell JM, Clumeck N. HIV and syphilis: when to perform a lumbar puncture. Sex Transm Dis 2007; 34 141–4.
| HIV and syphilis: when to perform a lumbar puncture.Crossref | GoogleScholarGoogle Scholar | 16865051PubMed |
[32] Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. Neurosyphilis in a clinical cohort of HIV-1-infected patients. AIDS 2008; 22 1145–51.
| Neurosyphilis in a clinical cohort of HIV-1-infected patients.Crossref | GoogleScholarGoogle Scholar | 18525260PubMed |
[33] Agmon-Levin N, Elbirt D, Asher I, Gradestein S, Werner B, Sthoeger Z. Syphilis and HIV co-infection in an Israeli HIV clinic: incidence and outcome. Int J STD AIDS 2010; 21 249–52.
| Syphilis and HIV co-infection in an Israeli HIV clinic: incidence and outcome.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c3kvFSqtA%3D%3D&md5=cb150c6c1ccdbd96dd02a03f94359230CAS | 20378895PubMed |
[34] Leber A, MacPherson P, Lee BC. Epidemiology of infectious syphilis in Ottawa. Recurring themes revisited. Canadian J Public Health 2008; 99 401–5.
[35] Wang YJ, Chi CY, Chou CH, Ho CM, Lin PC, Liao CH, et al Syphilis and neurosyphilis in human immunodeficiency virus-infected patients: a retrospective study at a teaching hospital in Taiwan. J Micro Immunol Infection 2012; 45 337–42.
| Syphilis and neurosyphilis in human immunodeficiency virus-infected patients: a retrospective study at a teaching hospital in Taiwan.Crossref | GoogleScholarGoogle Scholar |
[36] de Almeida SM, Bhatt A, Riggs PK, Durelle J, Lazzaretto D, Marquie-Beck J, McCutchan A, Letendre S, Ellis R. Cerebrospinal fluid human immunodeficiency virus viral load in patients with neurosyphilis. J Neurovirol 2010; 16 6–12.
| Cerebrospinal fluid human immunodeficiency virus viral load in patients with neurosyphilis.Crossref | GoogleScholarGoogle Scholar | 20132081PubMed |
[37] Public Health Agency of Canada. Canadian guidelines on sexually transmitted infections 2013 Available online at: http://www.phac-aspc.gc.ca/std-mts/sti-its/cgsti-ldcits/section-5-10-eng.php [cited 12 October 2014].
[38] Janier M, Hegyi V, Dupin N, Unemo M, Tiplica GS, Potocnik M, French P, Patel R. 2014 European guideline on the management of syphilis. J EuroAcademy Dermatol Venereol 2014; 28 1581–93.
| 2014 European guideline on the management of syphilis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2M3js1Citw%3D%3D&md5=81f54d9f21b643e575d49519756748efCAS |
[39] Moore JEGM. Syphilitic iritis. A study of 249 patients. Am J Ophthalmol 1931; 14 110–26.
| Syphilitic iritis. A study of 249 patients.Crossref | GoogleScholarGoogle Scholar |
[40] Laird SM. Neuro-recurrence after penicillin therapy of secondary syphilis. Urol Cutaneous Rev 1951; 55 475–7.
| 1:STN:280:DyaG3M%2FosFCltg%3D%3D&md5=7f346b125089c56c19a72b27a05bf64fCAS | 14855914PubMed |
[41] Musher DM. Syphilis, neurosyphilis, penicillin, and AIDS. J Infect Dis 1991; 163 1201–6.
| Syphilis, neurosyphilis, penicillin, and AIDS.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M3ksFWlsg%3D%3D&md5=fb42ad98498886862cc83d325c6057deCAS | 2037785PubMed |
[42] Inungu J, Morse A, Gordon C. Neurosyphilis during the AIDS epidemic, New Orleans, 1990–1997. J Infect Dis 1998; 178 1229
| Neurosyphilis during the AIDS epidemic, New Orleans, 1990–1997.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M%2FitlSitA%3D%3D&md5=5953cd7ff807e3c8475af261cfd39b8bCAS | 9806069PubMed |
[43] McLeish WM, Pulido JS, Holland S, Culbertson WW, Winward K. The ocular manifestations of syphilis in the human immunodeficiency virus type 1-infected host. Ophthalmology 1990; 97 196–203.
| The ocular manifestations of syphilis in the human immunodeficiency virus type 1-infected host.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3c3itFCmtw%3D%3D&md5=445723822032a0f76b41d1783d5b23eaCAS | 2326008PubMed |
[44] Shalaby IA, Dunn JP, Semba RD, Jabs DA. Syphilitic uveitis in human immunodeficiency virus-infected patients. Arch Ophthalmol 1997; 115 469–73.
| Syphilitic uveitis in human immunodeficiency virus-infected patients.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s3mtlyrsA%3D%3D&md5=5e3707c9686e431995e30005c33871cdCAS | 9109754PubMed |
[45] Walter T, Lebouche B, Miailhes P, Cotte L, Roure C, Schlienger I, Trepo C. Symptomatic relapse of neurologic syphilis after benzathine penicillin G therapy for primary or secondary syphilis in HIV-infected patients Clin Infect Dis 2006; 43 787–90.
| Symptomatic relapse of neurologic syphilis after benzathine penicillin G therapy for primary or secondary syphilis in HIV-infected patientsCrossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28voslWruw%3D%3D&md5=3b1dc6b60147ed343341059d9e641e43CAS | 16912958PubMed |
[46] Lukehart SA, Hook EW, Baker-Zander SA, Collier AC, Critchlow CW, Handsfield HH. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med 1988; 109 855–62.
| Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1M%2FkvVKktA%3D%3D&md5=16cbf9a1d603c8c687637a3b80f34c71CAS | 3056164PubMed |
[47] Tramont EC. Persistence of Treponema pallidum following penicillin G therapy. Report of two cases. JAMA 1976; 236 2206–7.
| Persistence of Treponema pallidum following penicillin G therapy. Report of two cases.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2s%2FjtF2ltg%3D%3D&md5=ceb4ae3153b78d862b032ea372640832CAS | 789927PubMed |
[48] Hook EW, Baker-Zander SA, Moskovitz BL, Lukehart SA, Handsfield HH. Ceftriaxone therapy for asymptomatic neurosyphilis. Case report and Western blot analysis of serum and cerebrospinal fluid IgG response to therapy. Sex Transm Dis 1986; 13 185–8.
| Ceftriaxone therapy for asymptomatic neurosyphilis. Case report and Western blot analysis of serum and cerebrospinal fluid IgG response to therapy.Crossref | GoogleScholarGoogle Scholar | 3764632PubMed |
[49] Gentile JH, Viviani C, Sparo MD, Arduino RC. Syphilitic meningomyelitis treated with ceftriaxone: case report. Clin Infect Dis 1998; 26 528
| Syphilitic meningomyelitis treated with ceftriaxone: case report.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c7mtFKjtA%3D%3D&md5=3448aacddc065f5db3fd16c156a6715eCAS | 9502500PubMed |
[50] Milger K, Fleig V, Kohlenberg A, Discher T, Lohmeyer J. Neurosyphilis manifesting with unilateral visual loss and hyponatremia: a case report. BMC Infect Dis 2011; 11 17
| Neurosyphilis manifesting with unilateral visual loss and hyponatremia: a case report.Crossref | GoogleScholarGoogle Scholar | 21235811PubMed |
[51] Shann S, Wilson J. Treatment of neurosyphilis with ceftriaxone. Sex Transm Infect 2003; 79 415–6.
| Treatment of neurosyphilis with ceftriaxone.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3svpslaquw%3D%3D&md5=258ea94c6a0de1fd9cd5d44a79695657CAS | 14573840PubMed |
[52] Marra CM, Boutin P, McArthur JC, Hurwitz S, Simpson PA, Haslett JA, van der Horst C, Nevin T, Hook EW. A pilot study evaluating ceftriaxone and penicillin G as treatment agents for neurosyphilis in human immunodeficiency virus-infected individuals. Clin Infect Dis 2000; 30 540–4.
| A pilot study evaluating ceftriaxone and penicillin G as treatment agents for neurosyphilis in human immunodeficiency virus-infected individuals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtVamsb0%3D&md5=893ab1ce26f586f73a51050e35c156c4CAS | 10722441PubMed |
[53] Smith NH, Musher DM, Huang DB, Rodriguez PS, Dowell ME, Ace W, White AC. Response of HIV-infected patients with asymptomatic syphilis to intensive intramuscular therapy with ceftriaxone or procaine penicillin. Int J STD AIDS 2004; 15 328–32.
| Response of HIV-infected patients with asymptomatic syphilis to intensive intramuscular therapy with ceftriaxone or procaine penicillin.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c3hvFyguw%3D%3D&md5=b045daa69453fb4369be0d206da4dbfeCAS | 15117503PubMed |
[54] Dobbin JM, Perkins JH. Otosyphilis and hearing loss: response to penicillin and steroid therapy. Laryngoscope 1983; 93 1540–3.
| Otosyphilis and hearing loss: response to penicillin and steroid therapy.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2c%2Fms12guw%3D%3D&md5=1e0b88ee756057c18a7925acc7f799c5CAS | 6645754PubMed |
[55] Linstrom CJ, Gleich LL. Otosyphilis: diagnostic and therapeutic update. J Otolaryngol 1993; 22 401–8.
| 1:STN:280:DyaK2c3htVGrtA%3D%3D&md5=dcf4c5a80e80c25b00dfc1a546481af3CAS | 8158733PubMed |
[56] Kiss S, Damico FM, Young LH. Ocular manifestations and treatment of syphilis. Semin Ophthalmol 2005; 20 161–7.
| Ocular manifestations and treatment of syphilis.Crossref | GoogleScholarGoogle Scholar | 16282150PubMed |
[57] Tucker JD, Li JZ, Robbins GK, Davis BT, Lobo AM, Kunkel J, Papaliodis GN, Durand ML, Felsenstein D. Ocular syphilis among HIV-infected patients: a systematic analysis of the literature. Sex Transm Infect 2011; 87 4–8.
| Ocular syphilis among HIV-infected patients: a systematic analysis of the literature.Crossref | GoogleScholarGoogle Scholar | 20798396PubMed |
[58] Marra CM, Maxwell CL, Tantalo LC, Sahi SK, Lukehart SA. Normalization of serum rapid plasma reagin titer predicts normalization of cerebrospinal fluid and clinical abnormalities after treatment of neurosyphilis. Clin Infect Dis 2008; 47 893–9.
| Normalization of serum rapid plasma reagin titer predicts normalization of cerebrospinal fluid and clinical abnormalities after treatment of neurosyphilis.Crossref | GoogleScholarGoogle Scholar | 18715154PubMed |
[59] Gordon SM, Eaton ME, George R, Larsen S, Lukehart SA, Kuypers J, Marra CM, Thompson S. The response of symptomatic neurosyphilis to high-dose intravenous penicillin G in patients with human immunodeficiency virus infection N Engl J Med 1994; 331 1469–73.
| The response of symptomatic neurosyphilis to high-dose intravenous penicillin G in patients with human immunodeficiency virus infectionCrossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M%2FlvFamsg%3D%3D&md5=48c42f299612ccb0fe8582f0c01b1c9dCAS | 7969296PubMed |
[60] Malone JL, Wallace MR, Hendrick BB, LaRocco A, Tonon E, Brodine SK,, Bowler WA, Lavin BS, Hawkins RE, Oldfield EC. Syphilis and neurosyphilis in a human immunodeficiency virus type-1 seropositive population: evidence for frequent serologic relapse after therapy. Am J Med 1995; 99 55–63.
| Syphilis and neurosyphilis in a human immunodeficiency virus type-1 seropositive population: evidence for frequent serologic relapse after therapy.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2MzitFemsA%3D%3D&md5=25bd7162130cbcfa627f91d2825e4301CAS | 7598143PubMed |
[61] Dibbern DA, Ray SC. Recrudescence of treated neurosyphilis in a patient with human immunodeficiency virus. Mayo Clin Proc 1999; 74 53–6.
| Recrudescence of treated neurosyphilis in a patient with human immunodeficiency virus.Crossref | GoogleScholarGoogle Scholar | 9987533PubMed |
[62] Marra CM, Longstreth WT, Maxwell CL, Lukehart SA. Resolution of serum and cerebrospinal fluid abnormalities after treatment of neurosyphilis. Influence of concomitant human immunodeficiency virus infection. Sex Transm Dis 1996; 23 184–9.
| Resolution of serum and cerebrospinal fluid abnormalities after treatment of neurosyphilis. Influence of concomitant human immunodeficiency virus infection.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28zjt12ksQ%3D%3D&md5=1e09a8dffb84ee1428b92603ff9b624fCAS | 8724507PubMed |
[63] Marra CM, Maxwell CL, Tantalo L, Eaton M, Rompalo AM, Raines C, Stoner BP, Corbett JJ, Augenbraun M, Zajackowski M, Kee R, Lukehart SA. Normalization of cerebrospinal fluid abnormalities after neurosyphilis therapy: does HIV status matter? Clin Infect Dis 2004; 38 1001–6.
| Normalization of cerebrospinal fluid abnormalities after neurosyphilis therapy: does HIV status matter?Crossref | GoogleScholarGoogle Scholar | 15034833PubMed |
[64] Amaratunge BC, Camuglia JE, Hall AJ. Syphilitic uveitis: a review of clinical manifestations and treatment outcomes of syphilitic uveitis in human immunodeficiency virus-positive and negative patients. Clin Experiment Ophthalmol 2010; 38 68–74.
| Syphilitic uveitis: a review of clinical manifestations and treatment outcomes of syphilitic uveitis in human immunodeficiency virus-positive and negative patients.Crossref | GoogleScholarGoogle Scholar | 20447104PubMed |


