Tillage does not increase nitrous oxide emissions under dryland canola (Brassica napus L.) in a semiarid environment of south-eastern Australia
Guangdi D. Li A C D , Mark K. Conyers A C , Graeme D. Schwenke B , Richard C. Hayes A C , De Li Liu A C , Adam J. Lowrie A , Graeme J. Poile A , Albert A. Oates A and Richard J. Lowrie AA NSW Department of Primary Industries, Wagga Wagga Agricultural Institute, Pine Gully Road, Wagga Wagga, NSW 2650, Australia.
B NSW Department of Primary Industries, Tamworth Agricultural Institute, 4 Marsden Park Road, Tamworth, NSW 2340, Australia.
C Graham Centre for Agricultural Innovation, Albert Pugsley Place, Wagga Wagga, NSW 2650, Australia.
D Corresponding author. Email: guangdi.li@dpi.nsw.gov.au
Soil Research 54(5) 512-522 https://doi.org/10.1071/SR15289
Submitted: 8 October 2015 Accepted: 7 March 2016 Published: 21 June 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Dryland cereal production systems of south-eastern Australia require viable options for reducing nitrous oxide (N2O) emissions without compromising productivity and profitability. A 4-year rotational experiment with wheat (Triticum aestivum L.)–canola (Brassica napus L.)–grain legumes–wheat in sequence was established at Wagga Wagga, NSW, Australia, in a semiarid Mediterranean-type environment where long-term average annual rainfall is 541 mm and the incidence of summer rainfall is episodic and unreliable. The objectives of the experiment were to investigate whether (i) tillage increases N2O emissions and (ii) nitrogen (N) application can improve productivity without increasing N2O emissions. The base experimental design for each crop phase was a split-plot design with tillage treatment (tilled versus no-till) as the whole plot, and N fertiliser rate (0, 25, 50 and 100 kg N/ha) as the subplot, replicated three times. This paper reports high resolution N2O emission data under a canola crop. The daily N2O emission rate averaged 0.55 g N2O-N/ha.day, ranging between –0.81 and 6.71 g N2O-N/ha.day. The annual cumulative N2O-N emitted was 175.6 and 224.3 g N2O-N/ha under 0 and 100 kg N/ha treatments respectively. There was no evidence to support the first hypothesis that tillage increases N2O emissions, a result which may give farmers more confidence to use tillage strategically to manage weeds and diseases where necessary. However, increasing N fertiliser rate tended to increase N2O emissions, but did not increase crop production at this site.
Additional keywords: conservation tillage, greenhouse gases, nitrogen fertiliser, trace gas emission.
Introduction
In Australia, the agriculture sector is the dominant source of anthropogenic nitrous oxide (N2O), accounting for 78.6% of the net national N2O emissions (Commonwealth of Australia 2014). Globally, 70% of annual anthropogenic N2O emissions come from animal and crop production (Mosier 2001). There is a clear need for primary producers to implement methods aimed at reducing overall N2O emissions. Research has attributed the majority of N2O emitted from soil to the following soil microbial processes: (i) nitrification, in which soil bacteria convert ammonium into nitrate (NO3–); (ii) denitrification, in which soil microbes consume soil NO3– under anaerobic conditions; (iii) assimilatory NO3– reduction, in which NO3– is converted to nitrite; and (iv) dissimilatory NO3– reduction, in which NO3– is converted to ammonium (Mosier et al. 1998; Dalal et al. 2003; Harris et al. 2013). Therefore, activities that affect these soil microbial processes could have a significant impact on N2O emissions (Dalal et al. 2003).
No-till farming has been widely adopted for dryland grain production in Australia due to benefits including improved soil structure with better water infiltration and water retention, increased ground cover with less soil erosion and increased soil fertility with more soil organic carbon sequestered (Chan and Heenan 2005; Hobbs 2007; Thomas et al. 2007). In addition, the adoption of no-till farming reduces energy, machinery and labour inputs (Scott et al. 2013), hence increasing on-farm profitability (Williams et al. 1990). A survey conducted across Australia in 2008 showed that nearly 90% of growers were using no-till technology on their farms (Llewellyn and D’Emden 2010). Nationally, 74% of the 27.8 million ha used for crop and pasture production was sown using no-till methods (ABS 2015). Conversely, no-till farming has also contributed to increased incidences of soil- and stubble-borne diseases, and development of more herbicide-resistant weeds (Chauhan et al. 2006). Tillage may be both beneficial and detrimental to many crop diseases, insects, pests and weeds (M. K. Conyers, unpubl. data). The challenge is to find a balance between the negative impacts of tillage on soil structure by tillage and the maintenance of optimum crop agricultural production. In recent years, several field experiments were conducted across northern and southern grain regions in Australia to investigate how much damage is done to soil by occasional tillage, strategically applied, in an otherwise no-till system (Crawford et al. 2015; M. K. Conyers, unpubl. data). It was concluded that strategically timed tillage could be a viable management option to manage diseases and herbicide-resistant weeds in the short-term, but the long-term effect on soil chemical and physical properties requires further investigation. However, there is no published research on the effect of tillage on N2O emission in dryland cropping systems in south-eastern Australia.
Tillage increases the aeration of the topsoil and mixes the crop residues with the soil, typically enhancing soil microbial activity, and therefore potentially increasing N2O emissions (Chatskikh and Olesen 2007). However, many studies have indicated increases in N2O emissions under no-till treatments (Weier et al. 1998; Ball et al. 1999; Skiba et al. 2002). Chatskikh and Olesen (2007) suggested that the greater N2O emissions under no-till treatments may be due to reduced gas diffusivity and air-filled porosity, often caused by high soil moisture, which favours the activity of denitrifying bacteria. Helgason et al. (2005) analysed N2O emissions in Canada using over 400 datasets from a range of farming management regimes, including tillage practices, and found that no-till treatment increased N2O emissions in humid climates but decreased it in semiarid climates. In the USA, Six et al. (2004) found that the conversion from conventional to no-till practice had a strong time dependency on N2O emissions in both humid and dry climates, demonstrating that greenhouse gas mitigation by adoption of no-till technology is much more variable and complex than previously considered. Therefore, there is large uncertainty associated with our current understanding of the influence of tillage practice on N2O emissions.
Increasing application rates of nitrogen (N) fertiliser has been previously shown to increase N2O emissions in broadacre cropping systems across a range of environments (Dalal et al. 2003; Harris et al. 2013; Schwenke and Haigh 2015), attributable to higher availability of N vulnerable to gaseous loss and to an imbalance between N availability in the soil and demand by the crop. Tillage is known to elevate levels of plant-available soil N due to mineralisation of soil organic matter. In situations where tillage is used in conjunction with high application rates of fertiliser N, N2O emissions might be expected to increase further. The objective of this study was to use continuous high resolution N2O emission data collected over 365 days under dryland canola (Brassica napus L.) in a dryland semiarid environment to test (i) whether tillage increases N2O emission and (ii) whether N application improves productivity without increasing N2O emission.
Materials and methods
Site description
The field experiment was conducted at the Wagga Wagga Agricultural Institute, Wagga Wagga, NSW (35°01ʹ45ʹʹS, 147°20ʹ36ʹʹE; 210 m a.s.l.). The soil was a Red Kandosol (Isbell 1996). The baseline soil chemical analysis showed that the site was slightly acidic with a pH of 5.1 in calcium chloride and available phosphorus (P) was 36.1 mg/kg (Colwell P) in the surface 0–0.1 m depth (Table 1). Long-term average rainfall at the site is 541 mm and is relatively evenly distributed throughout the year. Prior to the establishment of the experiment, the site was cropped for at least 5 years with the previous two crops being barley.

|
Experimental design
A 4-year rotation experiment was conducted during 2012–15 with wheat (Triticum aestivum L.)–canola–grain legumes–wheat in sequence. The site was divided into four quadrants with the experimental measurements taken from a different quadrant in each year of measurement. This paper reports the results from a canola crop grown in 2013. A fully automated 12-chamber system was installed and was fully functioning before the crop was sown.
The experiment was a randomised split-plot design with tillage (tilled vs no-till) as the whole plot and N application rates (0, 25, 50 and 100 kg N/ha) as the subplot, replicated three times. Plot size was 5 m × 9 m. The automated chambers were located on 12 plots with two treatment contrasts (tilled vs no-till and 0 vs 100 kg N/ha), replicated three times.
Hyola555 TT canola was sown at 4 kg/ha on 20 May 2013, using an air-seeder with knife points fitted to the front of the tynes, spaced 0.25 m apart. The tilled plots were cultivated at 0.1 m depth with a scarifier in both directions then harrowed twice one day before sowing. At sowing, all plots received 15 kg P/ha as single superphosphate (8.8% P and 11% sulfur) mixed with 5 kg N/ha as urea (46% N) as a base fertiliser. The N treatments were imposed by top-dressing urea at the designed rates before stem elongation on 2 August 2013, 74 days after sowing.
Gas flux measurement
The auto-chamber gas chromatograph (GC) system was installed on the site in March 2013 and tested for 4 weeks before the reporting period (15 April 2013 to 14 April 2014). The system consisted of 12 pneumatically operated static chambers linked to an automated sampling system, an in situ GC (SRI GC8610, Torrance, CA, USA) and an LI-820 infrared gas analyser (LI-COR, Lincoln, NE, USA) as described by Rowlings et al. (2012). The clear acrylic glass chambers (0.5 m × 0.5 m) with a height of 0.15 m were secured to stainless steel bases inserted permanently into the soil to a depth of 0.1 m. Each chamber covered two crop rows. The chamber height was extended to 0.65 m when crop height exceeded 0.15 m. When the crop height exceeded 0.65 m, the plants in the chambers were periodically trimmed above 0.65 m until the crop was harvested. A sampling manifold with three outlets at low, middle and high positions was fitted in each chamber to ensure the system took representative gas samples when the extended chambers were used. Two bases were installed in each plot to enable the chamber to be swapped between two bases every 1–2 weeks during the growing season and every 3–4 weeks at other times. This was done to minimise the glasshouse effect on plant growth and changes on soil moisture in chambers. A thermocouple sensor was fitted inside one chamber in each replicate to monitor chamber air temperature and a theta probe (Theta-probe MK2K, Delta-T Devices Ltd, Burwell, England) was inserted in each plot to measure soil moisture at 0–0.1 m (every 5 min). Water-filled pore space (WFPS) was calculated as described by Linn and Doran (1984). During the measurement cycle, the chambers were programmed to open automatically during high intensity rainfall events (>0.4 mm/5 min) or when air temperature inside the chambers exceeded 45°C during the growing season and 60°C at other times.
A full measurement cycle (of the four chambers per replicate) for greenhouse gas flux determination commenced with lid closure, and finished when the lids were opened 60 min later. During this time, each chamber was sequentially sampled for 3 min followed by a certified calibration standard: 500 ppb N2O, 4.0 ppm methane (CH4) and 800 ppm carbon dioxide (CO2) (Coregas Pty Ltd Australia). Samples passed through the 3-mL sample loop of two separate eight-port valves before injection into the respective gas detectors: electron capture detector for N2O and flame ionisation detector for CH4. The LI-820 was connected to the waste vent of the valve and logged the CO2 concentration every second as described by Rowlings et al. (2012). This process was repeated at 15-min intervals, sampling each chamber four times during the 60-min closure period. The lid then opened for 120 min while the chambers in the other two replicates were sampled. This 3-h cycle allowed eight flux measurements for each chamber to be obtained per 24-h period. The detection limit of the system was approximately –0.43 μg N2O-N/m2.h, 0.26 μg CH4-C/m2.h and 0.48 mg CO2-C/m2.h with 0.15-m head space. Sample dilution via leakage was considered negligible.
N2O emission calculation
Hourly N2O fluxes were calculated from the slope of the linear regression of gas concentration vs measurement time during the chamber lid closure, corrected for air temperature, atmospheric pressure and the ratio of chamber volume to surface area as described by Schwenke and Haigh (2016). The raw data were processed using an Auto GHG System Flux Calculator (Flux.net3.3) developed by the Queensland University of Technology (David Rowlings, pers. comm.). The Pearson’s correlation coefficient (R2) for the linear regression was calculated and used as a quality check for each regression. Flux rates were set to zero if the regression coefficient was <0.8 as per Barton et al. (2011). Daily N2O emission for each chamber was calculated by averaging the eight emission measurements for that day. Annual cumulative N2O emission was then calculated by integrating daily N2O fluxes over 365 days. The emission factor (EF, percentage of applied N emitted) with background emission correction (Type I) was calculated as per Barton et al. (2008).
Agronomic measurements
Seedling establishment was assessed 8 weeks after sowing by counting plants in 1 m of crop row at four random locations in each plot, expressed as plants/m2. Crop aboveground dry matter (DM) was measured at anthesis and at harvest by cutting two adjacent 1-m crop rows at two locations in each plot, and weighing after drying at 70°C for 72 h. The harvest samples were threshed to separate grain from straw and chaff to calculate grain yield and harvest index. The grain samples were sent to the laboratory for quality measurements, including oil content, crude protein, glucosinolates, test weight and 1000-grain weight, as described by AOF (2014).
Soil mineral N measurement
Deep soil cores were taken pre-sowing (April 2013) and post-harvest (December 2013) using a stainless steel tube (44 mm in diameter) inserted to 1.0 m at four locations in each plot. Each core was segmented into 0.1-m increments over the 0–0.40 m depth and 0.2-m increments thereafter. Samples from each plot were bulked for each depth. Surface soil (0–0.1 m) samples (10 cores per plot, bulked) were taken monthly using a foot corer (25-mm diameter) except for November 2013 and January and March 2014 when soil was too dry (i.e. hard) to insert the coring tube. Soil mineral N concentration (NO3–-N and ammonium-N) was analysed in all samples as described by Raymont and Higginson (1992). Soil mineral N was calculated using bulk density estimated from the deep soil coring for each depth at pre-sowing.
N recovery measurement
A micro-plot (1.25 m × 1.0 m, containing five rows of canola) was randomly selected from each plot using a metal frame with 0.1 m inserted into soil and 0.1 m above ground. Each micro-plot received 10% atom enriched urea-15N (prepared from 98 atom% 15N, SerCon, Australia) at one of four N rates (0, 25, 50 and 100 kg N/ha) in both tilled and no-till treatments. The 15N-labelled urea was dissolved in 2000 mL of distilled water and sprayed evenly over the whole area of the micro-plot. The container was then rinsed with 500 mL of distilled water which was also sprayed onto the micro-plot. The total water applied to each micro-plot was equivalent to 2 mm of rainfall.
At crop maturity, aboveground crop biomass, including fallen leaves, was collected from 0.8 m of the middle three crop rows of each micro-plot. The plants in the two outside rows and at 0.1 m each end of the micro-plot were excluded to ensure the plant sample collected had received a uniform rate of 15N. Plant samples were dried at 70°C, and passed through a 2-mm sieve, then finely ground in a puck and ring grinder. A sub-sample (~3.0–3.5 mg) was capsuled into a 1.0 mm × 0.8 mm tin container for total N and 15N isotope analysis (Sercon 20–22 IRMS, UK).
Deep soil samples were taken from all micro-plots after the crop was harvested as described above. The soil samples, including root materials, from 0–0.10, 0.10–0.20 and 0.20–0.3 m, were finely ground through a puck and ring grinder then capsuled for 15N isotope analysis using the same equipment as described for the plant samples.
The N recovery (%) in plant and soil was calculated as follows:
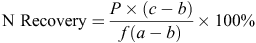
where P is total N in the plant (aboveground biomass) or soil (including roots) in kg/ha, a is 15N atom% in the labelled fertiliser, b is 15N atom% in the plant part or soil receiving no 15N, c is 15N atom% in the plant part or soil receiving 15N and f is the rate of 15N fertiliser applied.
Statistical analysis
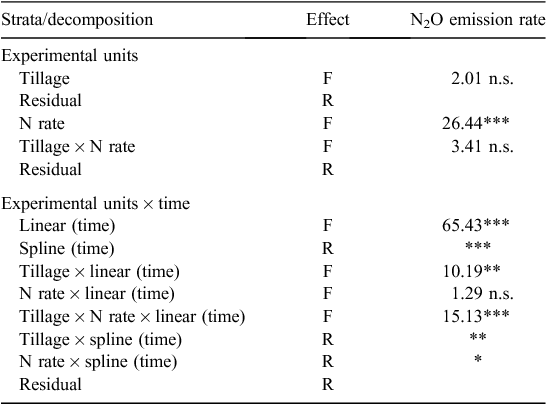
Analysis of variance for seedling density, crop biomass, grain yield, grain quality data and soil data at each depth was performed using a split-plot model in Genstat Release 17.1 (Payne et al. 2014) with tillage as the main plot and N rate as the subplot. Where tillage × N-rate interactions were not significant at P = 0.05, means of main treatment effects are presented and least significant differences (l.s.d.) between treatments presented where appropriate. A multiple linear regression analysis was performed for factors potentially affecting N2O-N emission. The daily N2O-N emission rates over 365 days were spline-fitted using a linear mixed model in ASReml-R (Gilmour et al. 2009). The fixed effects were tillage, N application rate, the linear component of sampling time and their interactions. Random effects were replicates, the spline component of sampling time and associated interactions. All terms were included in the model initially, but terms that failed to achieve statistical significance (P < 0.05) were excluded from the final model. The fixed effects were tested using the Wald statistical test and the random effects were tested using the residual maximum likelihood ratio test when necessary.
Results
Climate and soil moisture
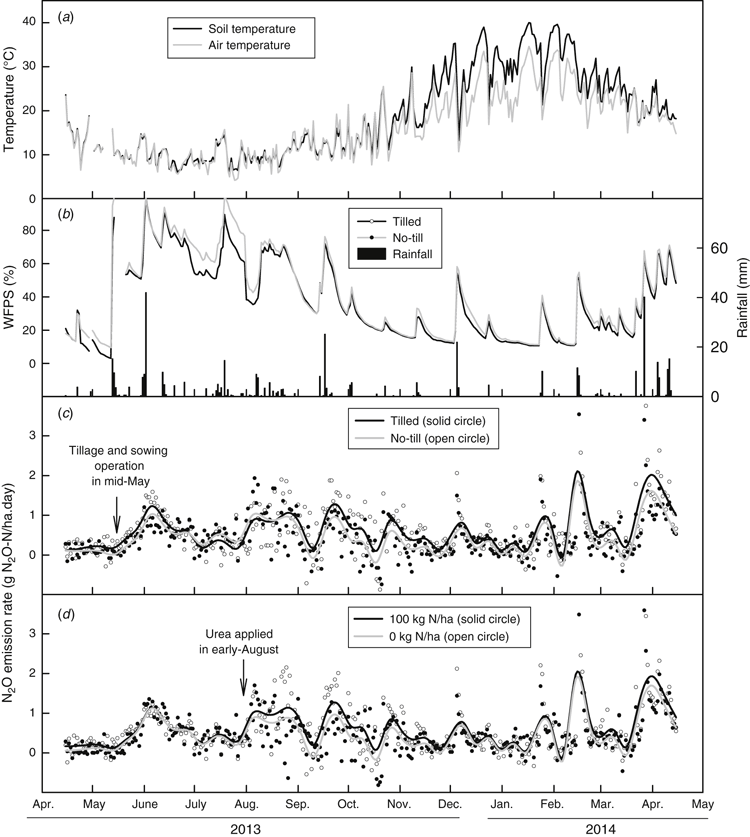
The site received 446 mm of rainfall during the experimental period (15 April 2013 to 14 April 2014), which was 82% of the long-term annual average rainfall (541 mm). There was very little rainfall during late growth in October (16.0 mm) and November (8.2 mm). On five occasions, daily rainfall exceeded 10 mm during December–March (Fig. 1). The minimum average air and soil temperature was 4.2°C and 5.9°C respectively, in July, and the corresponding maxima were 34.6°C and 40.0°C in January. The air and soil temperatures were similar during the growing season, but soil temperatures outside the growing season were much higher than the air temperature (Fig. 1). The soil WFPS in the 0–0.1 m depth closely reflected rainfall patterns. During the winter months (June–August), WFPS exceeded 80% on several occasions when the site received >15 mm of rainfall. Compared with the no-till treatment, WFPS was slightly and non-significantly less in the tilled treatment for the first two months after the tillage and sowing operations. Overall, there was no significant difference in WFPS between tilled and no-till treatments (Fig. 1).
Crop agronomy and grain quality
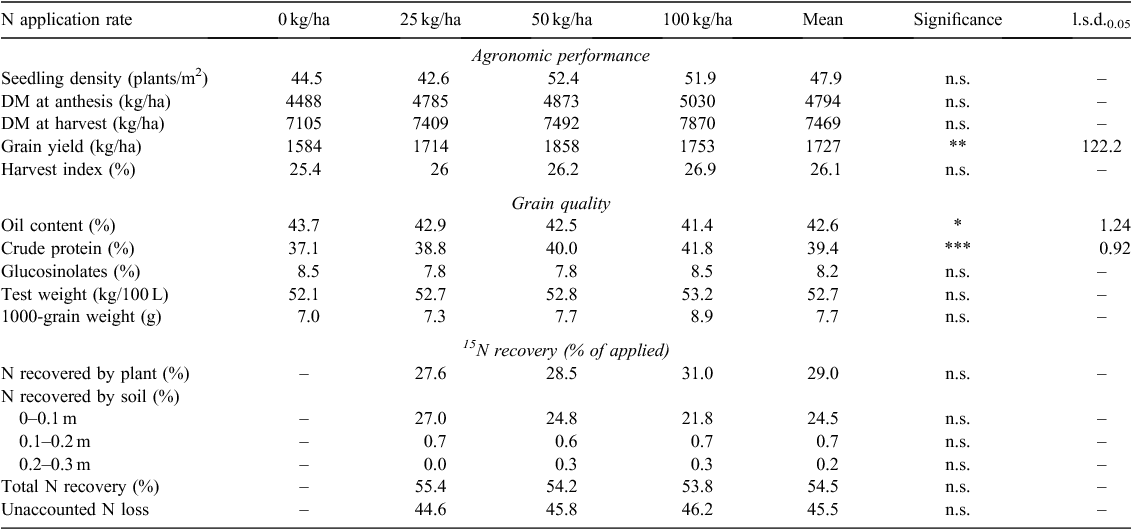
There were no significant differences in any agronomy or grain quality parameters measured between tilled and no-till treatments, neither for interaction of tillage and N rate treatments (P > 0.05). However, there was a significant difference in grain yield between N application rates (P < 0.01), with the highest grain yield recorded at the 50 kg N/ha rate. There were also significant differences in oil content (P < 0.05), crude protein (P < 0.001) and glucosinolates (P < 0.01) between N rate treatments. The canola had the highest crude protein and the lowest oil content in the 100 kg N/ha treatment, but the lowest crude protein and the highest oil content in the nil-N treatment (Table 2). The N application rate had no significant effect on seedling density at establishment, DM at anthesis or DM at harvest.
Soil mineral N and N recovery
The soil contained 52 kg/ha mineral N in the 0–0.1 m soil depth before sowing in April 2013. As the season progressed, soil mineral N decreased to 14 kg/ha by July 2013 (Fig. 2). There were significant differences in soil mineral N between N rate treatments after fertiliser N was top-dressed in early August (Fig. 2), but soil mineral N subsequently declined in all treatments during August–October as crop growth progressed. From October onwards, soil mineral N increased greatly (Fig. 2), indicating that mineralisation of N in soil exceeded plant uptake as the soil dried and the crop matured. Soil mineral N peaked in February 2014 and then decreased to 33 kg/ha with no treatment difference by April 2014 (Fig. 2).
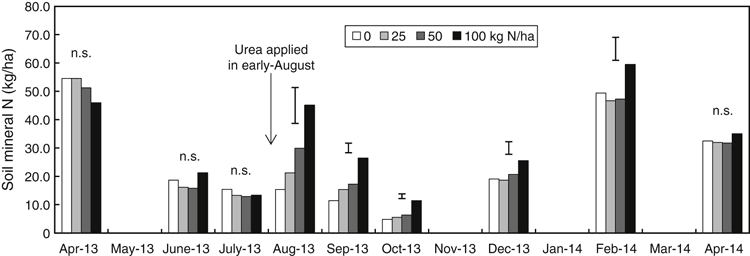
|
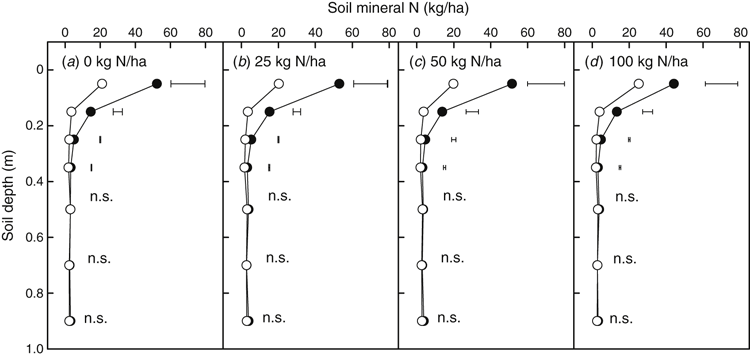
Tillage had no significant effect on soil mineral N in any depths in the soil profile (0–1.0 m) at any sampling time (data not shown). However, there were significant differences in soil mineral N within 0–0.4 m due to different N rates between the pre-sowing (April 2013) and post-harvest (December 2013) sampling dates. Below 0.4 m, there was no difference in soil mineral N between the two sampling times (Fig. 3). The soil had 83 kg/ha soil mineral N in the 0–1.0 m profile before sowing with no difference between tillage or N application treatments. After harvest, the soil had only 37 kg/ha soil mineral N in the whole profile with the highest mineral N in the 100 kg N/ha treatment (Fig. 3).
There was no significant difference in 15N recovery percentage between N rate either by plant or soil in 0–0.3 m (Table 2). The majority of N retained in soil (96%) was found in the 0–0.1 m depth, with <1% of N recovered in the 0.2–0.3 m depth, indicating negligible leaching of 15N beyond 0.3 m. The total N recovery in plant and soil was 54% across N rates, with ~46% of N unaccounted (Table 2), presumably lost to the atmosphere as gases, such as ammonia (NH3), N2 and N2O.
Seasonal N2O emission
There was a distinct seasonal variation in N2O emission rates which followed the rainfall pattern, although temperature change may have also contributed. Significant rainfall events stimulated N2O emissions, particularly summer storms of >10 mm (Fig. 1). Immediately after tillage and sowing operations (tillage on the tilled treatments was carried out one day before sowing), N2O emission from both tilled and no-till treatments increased, coinciding with two significant rainfall events in late May and June (Fig. 1). The N2O emissions were slightly higher in the tilled treatment during winter, late spring, early summer and early in the following autumn compared with no-till treatment. However, there was no overall significant difference in daily N2O emitted between tilled and no-till treatments (P > 0.05, Table 3).
|
The N2O emission rate in the 100 kg N/ha treatment was higher than in the nil-N treatment after N fertiliser was top-dressed and remained higher throughout the growing season (Fig. 1). Over the year of measurement, there was a significant difference in daily N2O emission rate between 0 and 100 kg N/ha rate treatments (P < 0.001, Table 3). There was also a significant interaction between tillage treatments and the linear component of time trend (P < 0.01), but no difference between N rate and the linear component of time trend (P > 0.05), indicating that the slopes of time trends significantly differed between tilled and no-till treatments, but were similar between N rates in terms of daily N2O emission rate (Table 3).
Cumulative N2O emitted and EF
There was no significant difference in cumulative N2O-N emitted between tilled and no-till treatments. However, the cumulative N2O-N emitted under the 100 kg N/ha treatment (224.3 g N2O-N/ha.year) was higher than that under the nil-N treatment (175.6 g N2O-N/ha.year) at P = 0.062 (Table 4). The average daily N2O emission rate was 0.48 and 0.61 g N2O-N/ha.day under 0 and 100 kg N/ha treatments respectively, ranging between –0.81 and 6.71 g N2O-N/ha.day (Table 4). The EF, corrected with background emission, was 0.05% averaged across tilled and no-till treatments (Table 4).

|
Relationship between daily N2O-N emission rate and environmental factors
Multiple linear regression analysis showed that daily N2O emission rate was closely related to air temperature (P < 0.01) and WFPS (P < 0.001), with N2O-N emitted = 0.0273 air temperature + 0.0156 WFPS – 0.499 (Fig. 4). The greatest daily N2O emission rates (3.8–7.8 g N2O-N/ha.day) occurred during February and March 2014 when average air temperature was 19−24°C and WFPS was 51–67% (Figs 1 and 4). During the growing season of May–November, N2O-N was <3.0 g N2O-N/ha.day with air temperature <20°C, while WFPS varied within 40–100% depending on rainfall intensity and frequency (Figs 1 and 4). No relationship was found between N2O emission rate and soil mineral N at 0–0.1 m based on data from monthly soil sampling (P > 0.05, data not shown).
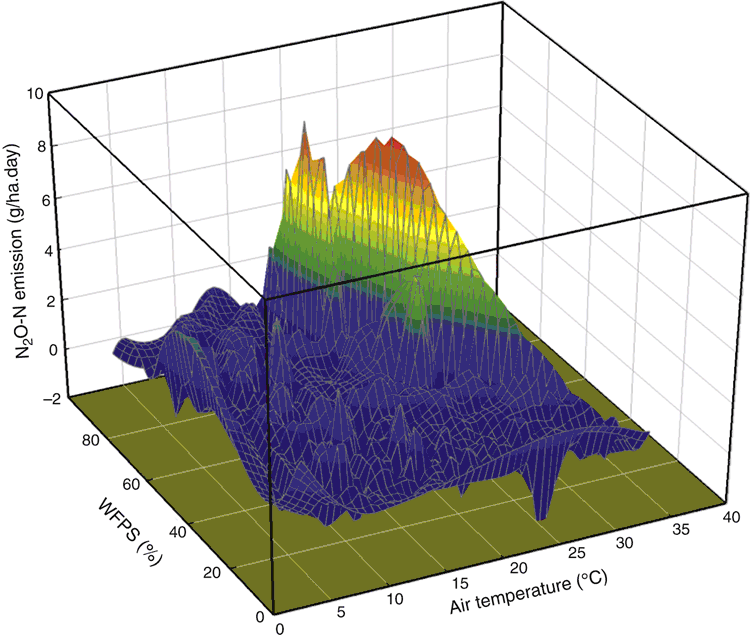
|
Discussion
The daily N2O emission rate was relatively low, ranging from –0.81 to 6.71 g N2O-N/ha.day. Total N2O emitted over the 365-day experiment was 176 and 224 g N2O-N/ha from 0 and 100 kg N/ha treatments respectively, under a canola crop in dryland conditions in southern Australia (Table 4). These losses are comparable to those recorded for rain-fed wheat from a strongly acid soil in a temperate region in south-eastern Australia, where emissions were in the range of 0–12.5 g N2O-N/ha.day and the annual losses were 200–270 g N2O-N/ha (Barker-Reid et al. 2005), but nearly double those from a canola crop grown in a sandy soil in a semiarid Mediterranean environment in Western Australia (Barton et al. 2010) where annual N2O emission was 80 and 128 g N2O-N/ha from non-N- and N-fertilised canola respectively. In contrast, the average daily N2O emission rate measured at the current site (0.48–0.61 g N2O-N/ha.day) was only a fraction of that measured under a canola crop on raised and flat seedbeds in a high-rainfall environment in south-west Victoria (Harris et al. 2013) where the average daily N2O emission rate was up to 215 g N2O-N/ha during July–September 2011. Harris et al. (2013) concluded that denitrification was the main process leading to high N2O emissions during the wet period in the high-rainfall cropping system. No obvious periods of waterlogging were observed at the current site, reflecting the low rainfall and a lighter textured soil than that of Harris et al. (2013). The EF (0.05%, Table 4) at the current site was nearly identical to that measured from a canola crop grown on a sandy soil (0.06%) in Western Australia (Barton et al. 2010), but only one-quarter of the current EF for all non-irrigated N-fertilised crop in Australia (0.2%, ANGA 2015). As a comparison, the EF of 0.15% obtained by Officer et al. (2015) on an alkaline Vertosol is consistent with the latter (0.2%, ANGA 2015). We suggest that soil properties such as texture and pH need to be considered when estimating the potential EFs that might be applied in models.
In the current study, the site received 446 mm of rainfall during the experimental period, which was a decile-4 year, slightly drier than the long-term average (541 mm). Although WFPS was over 70% for short periods after significant rainfall events in winter (Fig. 1), soil moisture was not high enough to initiate much denitrification. In addition, active plant growth during the growing season utilised the majority of plant-available soil mineral N and kept the soil mineral N status low (Fig. 2), which reduced the chance for N2O emission from denitrification. Outside of the growing season, when the air temperature was high, significant rainfall events (>10 mm) stimulated soil microbial activity and resulted in brief spikes in N2O emission (Fig. 1). The highest N2O daily emission rate for the year of measurement was in summer at an average air temperature of 19−24°C and WFPS of 51–67% (Fig. 4). Therefore, out-of-season N2O emissions over the summer fallow period appear to require more attention in this dryland cropping environment, which is consistent with the observations of Barton et al. (2008). The current findings highlight the need to monitor N2O emissions from rotations which include grain and pasture legume break-crops in this environment, as carry-over of N from one growing season to the next has been previously shown to be substantial (Evans et al. 2006), potentially elevating gaseous losses of N2O, particularly during wet summers.
The inclusion of perennial species into the cropping systems (Robertson and Revell 2014) is one option that could reduce the risk of N2O emission in this environment. Perennial pasture, with its deep root system, responds quickly to out-of-season rainfall and can use excess water and NO3– efficiently (Heng et al. 2001; Ridley et al. 2001) rather than accumulating in a post-crop fallow period. This also reduces the risk of on-site soil acidification (Scott et al. 2000; Dear et al. 2009) and off-site soil salinity (Dear and Ewing 2008). An even more progressive option may be to develop perennial-based cropping systems where grain is harvested directly from the perennial crop rather than growing sequences of annual crops in phased rotations with perennial pasture species. Early generation perennial crop germplasm currently exists which, although not yet commercially deployable, holds promise for future cropping systems to further improve resource use efficiencies and reduce losses such as N2O emissions (Hayes et al. 2012; Crews and Dehaan 2015). Further research is warranted to progress perennial plant technologies to reduce N2O emissions from cropping soils.
No-till technology has been widely adopted in dryland farming systems in south-eastern Australia (Llewellyn and D’Emden 2010; ABS 2015). However, with increased pressure from soil- and stubble-borne diseases and the development of herbicide-resistant weeds under a strict no-till farming system, farmers are tending to use tillage as a strategic tool to manage problematic weeds and break disease life cycles (Crawford et al. 2015). In the current study, the site had been cropped using no-till or minimum tillage for at least 5 years before the experiment commenced. The plots under the tilled treatment were cultivated over two years in 2012 and 2013. Results from the current study showed that the tilled treatment did not significantly increase N2O emissions compared with the no-till treatment (P > 0.05, Table 3); although slightly higher N2O emission flux was recorded for several months after tillage and sowing operations (Fig. 1), probably due to improved soil aeration enhancing soil nitrification rates (Chatskikh and Olesen 2007). No difference in surface soil mineral N was found in any months sampled between tilled and no-till treatments (Fig. 2). Our results, therefore, indicated that short-term tillage (over two years) had a negligible effect on N2O emission. However, Chatskikh and Olesen (2007) reported that conventional tillage doubled the N2O emission compared with direct drilling in spring and summer 2004 under spring barley from loamy sand soil at Foulum, Denmark. Chatskikh and Olesen (2007) suggested that it is likely that tillage affected N2O emissions and crop growth through different processes, where effects of soil compactness reduced root penetration and soil aeration and associated gas diffusivity increased soil organic matter turnover. In contrast, Ball et al. (1999) and Skiba et al. (2002) reported that direct drilling enhanced N2O emission by reducing gas diffusivity and air-filled porosity under heavy rainfall. More research is needed to give farmers more confidence to use tillage strategically to manage herbicide-resistant weeds and reduce incidence of soil-borne diseases.
There is ample evidence that increased N application increases N2O emission in many environments (Wang et al. 2011; Harris et al. 2013; Schwenke et al. 2015). In the current study, the N2O emission increased significantly immediately after fertiliser N was top-dressed compared with the nil-N treatment (Fig. 1). After crop harvest, soil mineral N increased and peaked in February 2014 (Fig. 2). High soil temperature, coupled with episodic summer rainfall events, stimulated soil microbial activity, resulting in N mineralisation from crop residues and soil organic matter with concomitant increased N2O emissions. The significantly higher soil mineral N under the 100 kg N/ha treatment, particularly during the fallow period, was either due to over-supply of fertiliser N under that treatment, or rapid breakdown of the N-rich canola residues (Schwenke et al. 2015). Over the 365-day measurement period, applying 100 kg N/ha increased the annual N2O emission by 28% compared with the nil-N treatment, most probably due to increased soil nitrification and denitrification processes resulting from the higher soil N status (Fig. 2). However, there was no relationship between daily N2O emission rate with soil mineral N, probably because the soil sampling was not frequent enough to document the rapid changes in soil mineral N content occurring due to rainfall events.
Results from 15N recovery measurements showed that the total recovery of applied 15N from plant and soil was ~54% with no difference between N rates. This result is similar to the result on a neutral-alkaline Black Vertosol in the Liverpool Plains where Schwenke and Haigh (2016) reported that 55–57% of the applied N was recovered from the 40 and 120 kg N ha–1 treatments, but substantially less than the 76% recovery measured after sorghum crops grown on Vertosols in the northern Australian grains region (Armstrong et al. 1996). The unaccounted N (~46%) at the current site is presumably lost to the atmosphere as gases, such as NH3, N2 and N2O as N leaching beyond 0.30 m was negligible (Table 2). With an extremely low EF (0.05%) for N2O in this dry environment (Table 4), the majority of the gaseous loss was probably from NH3 and N2 loss. Volatilisation of NH3 could be significant (Freney et al. 1983; Turner et al. 2012) as N was top-dressed and no significant rainfall fell until 5 days after N was applied (17 mm, Fig. 1). Emission of N2 by denitrification during the rest of the season may be responsible for the remainder of the total N loss. An incubation study with intact soil cores from tropical savanna and grasslands of northern Australia also indicated that N2 emissions accounted for 82.4–99.3% of total N lost (Werner et al. 2014). Therefore, we suggest that measurement of gaseous NH3 and N2 losses could provide greater insight into N losses from semiarid Mediterranean cropping environments and ultimately help identify agronomic options for improving crop N recovery to minimise losses to the environment.
Acknowledgements
The authors are grateful to Binbin Xu and Vince van der Rijt from the NSW Department of Primary Industries for the field assistance, and Christian Brunk from the Queensland University of Technology (QUT) for technical support during the setup of the automated chamber system. An Auto GHG System Flux Calculator (Flux.net3.3), developed by Dr David Rowlings (QUT), was used to calculate the gas fluxes. We appreciate the guidance and advice from Professor Peter Grace (QUT), the coordinator of the National Agricultural Nitrous Oxide Research Program (NANORP), and all other network participants during the entire course of the study. The project was conducted on the land of Wagga Wagga Agricultural Institute and funded by NSW Department of Primary Industries with financial support from Federal Department of Agriculture and the Grains Research and Development Corporation.
References
ABS (2015) Land management and farming in Australia, 2013–14. Cat No. 4627.0. Available at http://www.abs.gov.au/ausstats/abs@.nsf/mf/4627.0 [accessed 23 May 2016]ANGA (2015) Australian National Greenhouse Accounts: National Inventory Report 2013 Volume 1. Available at http://www.environment. gov.au/system/files/resources/7d7f7ef6-e028-462e-b15c-ede14e222e65/ files/national-inventory-report-2013-vol1.pdf [accessed 23 May 2016]
AOF (2014) Australian Oilseeds Federation. Section 1: Quality standards, technical information & typical analysis. Issue 13. Available at http://brounandco.com.au/wp-content/uploads/2014/10/Oilseed-Standards-2014-15.pdf [accessed 23 May 2016]
Armstrong R, Halpin N, McCosker K, Standley J, Lisle A (1996) Applying nitrogen to grain sorghum in central Queensland: residual value and effect of fallowing and tillage practice. Australian Journal of Agricultural Research 47, 81–95.
| Applying nitrogen to grain sorghum in central Queensland: residual value and effect of fallowing and tillage practice.Crossref | GoogleScholarGoogle Scholar |
Ball BC, Scott A, Parker JP (1999) Field N2O, CO2 and CH4 fluxes in relation to tillage, compaction and soil quality in Scotland. Soil & Tillage Research 53, 29–39.
| Field N2O, CO2 and CH4 fluxes in relation to tillage, compaction and soil quality in Scotland.Crossref | GoogleScholarGoogle Scholar |
Barker-Reid F, Gates WP, Wilson K, Baigent R, Galbally IE, Meyer CP, Weeks IA, Eckard RJ (2005) Soil nitrous oxide emissions from rainfed wheat in SE Australia. In ‘Fourth International Symposium on Non-CO2 Greenhouse Gases (NCGG-4) Science, Control, Policy and Implementation’. Utrecht, Netherlands. (Ed. A van Amstel) p. 8. (Mill Press: Utrecht)
Barton L, Kiese R, Gatter D, Butterbach-Bahl K, Buck R, Hinz C, Murphy DV (2008) Nitrous oxide emissions from a cropped soil in a semi-arid climate. Global Change Biology 14, 177–192.
Barton L, Murphy DV, Kiese R, Butterbach-Bahl K (2010) Soil nitrous oxide and methane fluxes are low from a bioenergy crop (canola) grown in a semi-arid climate. Global Change Biology. Bioenergy 2, 1–15.
| Soil nitrous oxide and methane fluxes are low from a bioenergy crop (canola) grown in a semi-arid climate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXms1antb0%3D&md5=d1c7d24d4264220dbb973917781d5336CAS |
Barton L, Butterbach-Bahl K, Kiese R, Murphy DV (2011) Nitrous oxide fluxes from a grain-legume crop (narrow-leafed lupin) grown in a semiarid climate. Global Change Biology 17, 1153–1166.
| Nitrous oxide fluxes from a grain-legume crop (narrow-leafed lupin) grown in a semiarid climate.Crossref | GoogleScholarGoogle Scholar |
Chan KY, Heenan DP (2005) The effects of stubble burning and tillage on soil carbon sequestration and crop productivity in southeastern Australia. Soil Use and Management 21, 427–431.
| The effects of stubble burning and tillage on soil carbon sequestration and crop productivity in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Chatskikh D, Olesen JE (2007) Soil tillage enhanced CO2 and N2O emissions from loamy sand soil under spring barley. Soil & Tillage Research 97, 5–18.
| Soil tillage enhanced CO2 and N2O emissions from loamy sand soil under spring barley.Crossref | GoogleScholarGoogle Scholar |
Chauhan BS, Gill GS, Preston C (2006) Tillage system effects on weed ecology, herbicide activity and persistence: a review. Australian Journal of Experimental Agriculture 46, 1557–1570.
| Tillage system effects on weed ecology, herbicide activity and persistence: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFyitLbI&md5=fbdba5ce10ab834b2bdf9e1f629c6215CAS |
Commonwealth of Australia (2014) Agriculture. In ‘National Inventory Report 2012’. pp. 257–351. (Commonwealth of Australia: Canberra, ACT)
Crawford MH, Rincon-Florez V, Balzer A, Dang YP, Carvalhais LC, Liu H, Schenk PM (2015) Changes in the soil quality attributes of continuous no-till farming systems following a strategic tillage. Soil Research 53, 263–273.
| Changes in the soil quality attributes of continuous no-till farming systems following a strategic tillage.Crossref | GoogleScholarGoogle Scholar |
Crews TE, Dehaan LR (2015) The strong perennial vision: a response. Agroecology and Sustainable Food Systems 39, 500–515.
| The strong perennial vision: a response.Crossref | GoogleScholarGoogle Scholar |
Dalal RC, Wang WJ, Robertson GP, Parton WJ (2003) Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Australian Journal of Soil Research 41, 165–195.
| Nitrous oxide emission from Australian agricultural lands and mitigation options: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktFKisr8%3D&md5=82cb3d74aaae67b11972b27b0ea300f6CAS |
Dear BS, Ewing MA (2008) The search for new pasture plants to achieve more sustainable production systems in southern Australia. Australian Journal of Experimental Agriculture 48, 387–396.
| The search for new pasture plants to achieve more sustainable production systems in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Dear BS, Virgona JM, Sandral GA, Swan AD, Morris S (2009) Changes in soil mineral nitrogen, nitrogen leached, and surface pH under annual and perennial pasture species. Crop and Pasture Science 60, 975–986.
| Changes in soil mineral nitrogen, nitrogen leached, and surface pH under annual and perennial pasture species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFCntr%2FP&md5=4e9bb973da0ab03ea60b2423898dad0bCAS |
Evans J, Murray GM, Scott G, Orchard B, Brennan J, Lemerle D, Kaiser A, Armstrong EL (2006) Impact of annual legume ‘break’ crops on the yield and quality of canola in comparison with the impact on yield of wheat. Australian Journal of Experimental Agriculture 46, 1489–1497.
| Impact of annual legume ‘break’ crops on the yield and quality of canola in comparison with the impact on yield of wheat.Crossref | GoogleScholarGoogle Scholar |
Freney JR, Simpson JR, Denmead OT (1983) Volatilization of ammonia. In ‘Gaseous loss of nitrogen from plant-soil systems’. (Eds JR Freney, JR Simpson) pp. 1–32. (Springer: Netherlands)
Gilmour AR, Gogel BJ, Cullis BR, Thompson R (2009) ‘ASReml User Guide, Release 3.’ (VSN International: Hemel Hempstead, UK)
Harris RH, Officer SJ, Hill PA, Armstrong RD, Fogarty KM, Zollinger RP, Phelan AJ, Partington DL (2013) Can nitrogen fertiliser and nitrification inhibitor management influence N2O losses from high rainfall cropping systems in South Eastern Australia? Nutrient Cycling in Agroecosystems 95, 269–285.
| Can nitrogen fertiliser and nitrification inhibitor management influence N2O losses from high rainfall cropping systems in South Eastern Australia?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXotV2ru70%3D&md5=dfcb3a9185e64759c620914c4cfd4732CAS |
Hayes RC, Newell MT, DeHaan LR, Murphy KM, Crane S, Norton MR, Wade LJ, Newberry M, Fahim M, Jones SS, Cox TS, Larkin PJ (2012) Perennial cereal crops: An initial evaluation of wheat derivatives. Field Crops Research 133, 68–89.
| Perennial cereal crops: An initial evaluation of wheat derivatives.Crossref | GoogleScholarGoogle Scholar |
Helgason BL, Janzen HH, Chantigny MH, Drury CF, Ellert BH, Gregorich EG, Lemke RL, Pattey E, Rochette P, Wagner-Riddle C (2005) Toward improved coefficients for predicting direct N2O emissions from soil in Canadian agroecosystems. Nutrient Cycling in Agroecosystems 72, 87–99.
| Toward improved coefficients for predicting direct N2O emissions from soil in Canadian agroecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVKitrfK&md5=5e7500a1f945cf1f942399fdd0978636CAS |
Heng LK, White RE, Helyar KR, Fisher R, Chen D (2001) Seasonal differences in the soil water balance under perennial and annual pastures on an acid Sodosol in southeastern Australia. European Journal of Soil Science 52, 227–236.
| Seasonal differences in the soil water balance under perennial and annual pastures on an acid Sodosol in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Hobbs PR (2007) Conservation agriculture: what is it and why is it important for future sustainable food production? The Journal of Agricultural Science 145, 127–137.
| Conservation agriculture: what is it and why is it important for future sustainable food production?Crossref | GoogleScholarGoogle Scholar |
Isbell RF (1996) ‘The Australian soil classification.’ (CSIRO Publishing: Melbourne)
Linn DM, Doran JW (1984) Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Science Society of America Journal 48, 1267–1272.
| Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhtFaitL4%3D&md5=0b26b0412d09d2e99729b94c3b26fe9aCAS |
Llewellyn RS, D’Emden FH (2010) ‘Adoption of no-till cropping practices in Australian grain growing regions.’ (Grains Research and Development Corporation and CSIRO: Canberra)
Mosier AR (2001) Exchange of gaseous nitrogen compounds between agricultural systems and the atmosphere. Plant and Soil 228, 17–27.
| Exchange of gaseous nitrogen compounds between agricultural systems and the atmosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtlWkur4%3D&md5=aac3d8727a195029013cc81f60b2be05CAS |
Mosier AR, Duxbury JM, Freney JR, Heinemeyer O, Minami K (1998) Assessing and mitigating N2O emissions from agricultural soils. Climatic Change 40, 7–38.
| Assessing and mitigating N2O emissions from agricultural soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmslWgu7w%3D&md5=b62628868bce2c12bee245d1ab7f13a7CAS |
Officer SJ, Phillips F, Kearney G, Armstrong R, Graham J, Partington D (2015) Response of soil nitrous oxide flux to nitrogen fertiliser application and legume rotation in a semi-arid climate, identified by smoothing spline models. Soil Research 53, 227–241.
| Response of soil nitrous oxide flux to nitrogen fertiliser application and legume rotation in a semi-arid climate, identified by smoothing spline models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXosFGntL0%3D&md5=1f110bcfecd3b8de81e4f80e9df39fffCAS |
Payne R, Murray D, Harding S, Baird D, Soutar D (2014) ‘Introduction to GenStat® for Windows™.’ 17th Edn. (VSN International: Hemel Hempstead, UK)
Raymont GE, Higginson FR (1992) ‘Australian laboratory handbook of soil and water chemical methods.’ (Inkata Press: Melbourne).
Ridley AM, White RE, Helyar KR, Morrison GR, Heng LK, Fisher R (2001) Nitrate leaching loss under annual and perennial pastures with and without lime on a duplex (texture contrast) soil in humid southeastern Australia. European Journal of Soil Science 52, 237–252.
| Nitrate leaching loss under annual and perennial pastures with and without lime on a duplex (texture contrast) soil in humid southeastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltVKku7w%3D&md5=530a4cd83632645fcb9c53e43e05d05dCAS |
Robertson M, Revell C (2014) Perennial pastures in cropping systems of southern Australia: an overview of present and future research. Crop and Pasture Science 65, 1084–1090.
| Perennial pastures in cropping systems of southern Australia: an overview of present and future research.Crossref | GoogleScholarGoogle Scholar |
Rowlings DW, Grace PR, Kiese R, Weier KL (2012) Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest. Global Change Biology 18, 726–738.
| Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest.Crossref | GoogleScholarGoogle Scholar |
Schwenke GD, Haigh BM (2016) The interaction of seasonal rainfall and nitrogen fertiliser rate on soil N2O emission, total N loss and crop yield of dryland sorghum and sunflower grown on sub-tropical Vertosols. Soil Research 54, 604–618.
| The interaction of seasonal rainfall and nitrogen fertiliser rate on soil N2O emission, total N loss and crop yield of dryland sorghum and sunflower grown on sub-tropical Vertosols.Crossref | GoogleScholarGoogle Scholar |
Schwenke GD, Herridge DF, Scheer C, Rowlings DW, Haigh BM, McMullen KG (2015) Soil N2O emissions under N2-fixing legumes and N-fertilised canola: a reappraisal of emissions factor calculations. Agriculture, Ecosystems & Environment 202, 232–242.
| Soil N2O emissions under N2-fixing legumes and N-fertilised canola: a reappraisal of emissions factor calculations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtVOgsL4%3D&md5=5e74db16ebc6b862a9ac74b9a0396a34CAS |
Scott BJ, Ridley AM, Conyers MK (2000) Management of soil acidity in long-term pastures of south-eastern Australia: a review. Australian Journal of Experimental Agriculture 40, 1173–1198.
| Management of soil acidity in long-term pastures of south-eastern Australia: a review.Crossref | GoogleScholarGoogle Scholar |
Scott BJ, Martin P, Riethmuller GP (2013) ‘Graham Centre Monograph No. 3: Row spacing of winter crops in broad scale agriculture in southern Australia.’ (NSW Department of Primary Industries: Orange).
Six J, Ogle SM, Breidt FJ, Conant RT, Mosier AR, Paustian K (2004) The potential to mitigate global warming with no-tillage management is only realized when practised in the long term. Global Change Biology 10, 155–160.
| The potential to mitigate global warming with no-tillage management is only realized when practised in the long term.Crossref | GoogleScholarGoogle Scholar |
Skiba U, van Dijk S, Ball BC (2002) The influence of tillage on NO and N2O fluxes under spring and winter barley. Soil Use and Management 18, 340–345.
| The influence of tillage on NO and N2O fluxes under spring and winter barley.Crossref | GoogleScholarGoogle Scholar |
Thomas GA, Titmarsh GW, Freebairn DM, Radford BJ (2007) No-tillage and conservation farming practices in grain growing areas of Queensland – a review of 40 years of development. Australian Journal of Experimental Agriculture 47, 887–898.
| No-tillage and conservation farming practices in grain growing areas of Queensland – a review of 40 years of development.Crossref | GoogleScholarGoogle Scholar |
Turner DA, Edis RE, Chen D, Freney JR, Denmead OT (2012) Ammonia volatilization from nitrogen fertilizers applied to cereals in two cropping areas of southern Australia. Nutrient Cycling in Agroecosystems 93, 113–126.
| Ammonia volatilization from nitrogen fertilizers applied to cereals in two cropping areas of southern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpvVyqtb8%3D&md5=73095ed40add5839dbc8a2f4977a7d79CAS |
Wang WJ, Dalal RC, Reeves SH, Butterbach-Bahl K, Kiese R (2011) Greenhouse gas fluxes from an Australian subtropical cropland under long-term contrasting management regimes. Global Change Biology 17, 3089–3101.
| Greenhouse gas fluxes from an Australian subtropical cropland under long-term contrasting management regimes.Crossref | GoogleScholarGoogle Scholar |
Weier KL, Rolston DE, Thorburn PJ (1998) The potential for N losses via denitrification beneath a green cane trash blanket. In ‘Proceedings of the 20th Conference of the Australian Society of Sugar Cane Technologists’, Ballina, NSW. (Ed. DM Hogarth) pp. 169–175. (Australian Society of Sugarcane Technologists)
Werner C, Reiser K, Dannenmann M, Hutley LB, Jacobeit J, Butterbach-Bahl K (2014) N2O, NO, N2 and CO2 emissions from tropical savanna and grassland of northern Australia: an incubation experiment with intact soil cores. Biogeosciences 11, 6047–6065.
| N2O, NO, N2 and CO2 emissions from tropical savanna and grassland of northern Australia: an incubation experiment with intact soil cores.Crossref | GoogleScholarGoogle Scholar |
Williams JR, Gross LK, Claassen MM, Llewelyn RV (1990) Economic analysis of tillage for corn and soybean rotations with government commodity programs. Journal of Production Agriculture 3, 308–316.
| Economic analysis of tillage for corn and soybean rotations with government commodity programs.Crossref | GoogleScholarGoogle Scholar |