Effect of nitrogen fertiliser management on soil mineral nitrogen, nitrous oxide losses, yield and nitrogen uptake of wheat growing in waterlogging-prone soils of south-eastern Australia
Robert H. Harris A B E , Roger D. Armstrong C D , Ashley J. Wallace C and Oxana N. Belyaeva AA Agriculture Victoria, Department of Economic Development, Jobs, Transport and Resources, Hamilton Centre, PO Box 105, Hamilton, Vic. 3300, Australia.
B Present address: 70 Martin St, Dunkeld, Vic. 3294, Australia.
C Agriculture Victoria, Department of Economic Development, Jobs, Transport and Resources, Horsham Centre, PO Box 260 Horsham, Vic. 3400, Australia.
D Department of Animal, Plant and Soil Sciences, LaTrobe University, Bundoora, Vic. 3086, Australia.
E Corresponding author. Email: rob.h.harris74@gmail.com
Soil Research 54(5) 619-633 https://doi.org/10.1071/SR15292
Submitted: 9 October 2015 Accepted: 15 February 2016 Published: 11 July 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Some of the highest nitrous oxide (N2O) emissions arising from Australian agriculture have been recorded in the high-rainfall zone (>650 mm) of south-western Victoria. Understanding the association between nitrogen (N) management, crop N uptake and gaseous losses is needed to reduce N2O losses. Field experiments studied the effect of N-fertiliser management on N2O emissions, crop N uptake and crop productivity at Hamilton and Tarrington in south-western Victoria. Management included five rates of urea-N fertiliser (0, 25, 50, 100 and 200 kg N/ha) topdressed at either mid-tillering or first-node growth stages of wheat development; urea-N deep-banded 10 cm below the seed at sowing; and urea coated with the nitrification inhibitor DMPP (3,4-dimethylpyrazole phosphate) was either topdressed or deep-banded. Pre-sowing soil profile chemical properties were determined before static chambers were installed to measure N2O losses, accompanied by wheat dry matter, crop N uptake and grain yield and quality, to measure treatment differences. N2O losses increased significantly (P ≤ 0.10) where urea-N was deep-banded, resulting in a 2–2.5-fold increase in losses, compared with the nil N control. The high N2O losses from deep-banding N appeared to result from winter waterlogging triggering gaseous or drainage losses before wheat reached peak growth and demand for N in spring. Despite the high losses from deep-banding urea-N, grain yields were largely unaffected by N management, except at Hamilton in 2012, where topdressed wheat growing in a soil with large reserves of NO3–-N, and later experiencing post-anthesis water deficit resulted in a negative grain yield response. All sites had high concentrations of soil organic carbon (>2.8%) and the potential for large amounts of N mineralisation throughout the growing season to supplement low N fertiliser recovery. However, topdressed urea-N resulted in significant enrichment of crop tissue (P ≤ 0.004) and associated positive response in grain protein compared with the deep banded and nil N treatments. 3,4-Dimethylpyrazole phosphate (DMPP)-coated urea provided no additional benefit to crop yield over conventional urea N. Our study highlighted the importance of synchronising N supply with peak crop N demand to encourage greater synthetic N uptake and mitigation of N2O losses.
Additional keywords: crop nitrogen recovery, 3,4-dimethylpyrazole phosphate, nitrification inhibitor, raised bed, static chamber, water-filled pore space, Triticum aestivum.
Introduction
Nitrous oxide (N2O) has a warming potential ~300 times greater than that of carbon dioxide (CO2) (Crutzen 1981) and is considered an important greenhouse gas (IPCC 2013). Atmospheric N2O concentrations have risen by ~50 ppb since the beginning of the industrial revolution, and a significant proportion of this increase has been attributed to the loss of N2O from agricultural soils (IPCC 2013), largely due to the increased use of synthetic nitrogen (N) fertilisers (Van Groenigen et al. 2010). Second to water, N is an essential requirement for crop growth, and as human populations continue to rise, so too global demand for N fertiliser, forecast to increase by 1.4% annually over the previous year from 2014 to 2018 (FAO 2015). Consequently, global emissions of N2O are also likely to increase.
Over the past 20 years, demand for N fertiliser in Australian cropping systems has doubled (Lake 2012), with a shift away from traditional pasture legumes grown in sequence with annual crops to more intensified, continuous cropping with greater reliance on synthetic N (Rovira 1994; Hooper et al. 2003; Edwards et al. 2012). Unfortunately, N supplied through synthetic sources is likely to produce more N2O than N supplied through the mineralisation of legume residues (Jensen et al. 2012; Schwenke et al. 2015). Both Barton et al. (2008) and Officer et al. (2015) showed small but slightly elevated N2O emissions from the addition of N fertiliser to dryland wheat in semi-arid climatic zones (<450 mm) of southern Australia. In the high-rainfall zone (>650 mm) of south-western Victoria Harris et al. (2013) measured large losses of N2O (<600 g N2O-N/ha.day) where high inputs of N fertiliser were applied to soils prone to prolonged periods of saturation, with high background levels of mineral N (>200 kg/ha to 100 cm depth) and organic carbon (C, >3.5%).
Although many studies have shown higher rates of N fertiliser produce greater soil-based N2O losses (Grant et al. 2006; Mosier et al. 2006; Halvorson et al. 2008; Ma et al. 2010; Hoben et al. 2011; Liu et al. 2012), few have attempted to link agronomic N efficiencies with N2O production (Van Groenigen et al. 2010). Best management practices that balance N supply to meet crop demand have been shown to produce lower N2O emissions (Snyder et al. 2007; Scheer et al. 2008; Scheer et al. 2013; Macdonald et al. 2015). Because N fertiliser input is known to influence both crop yield and N2O loss, it seems reasonable that improving crop N uptake may help to minimise greenhouse-gas emissions from cropping systems. Mosier et al. (2006) argued that by linking crop productivity with greenhouse-gas output, it was possible to determine appropriate levels of N fertiliser input for optimising economic return and environmental sustainability.
Another possible strategy for improving crop N utilisation is the use of nitrification inhibitors such as DMPP (3,4-dimethylpyrazole phosphate; BASF Germany), the active ingredient in ENTEC fertilisers. Nitrification inhibitors are designed to delay the oxidation of ammonium (NH4+-N) to nitrite (NO2–-N) (Zerulla et al. 2001), to keep N in a form less able to escape to the atmosphere as N2O (Weiske et al. 2001; Pfab et al. 2012), or leached deeper into the soil profile (Di and Cameron 2012). Greater retention of soil inorganic N implies potentially greater opportunities for plant N uptake (Liu et al. 2013). Studies have shown a reduction in N2O production in response to the application of DMPP (Weiske et al. 2001; Belastegui-Macadam et al. 2003; Merino et al. 2005; Chen et al. 2010; Pfab et al. 2012; Di and Cameron 2012); however, few have demonstrated improved crop N uptake and grain yield (Liu et al. 2013).
This paper examines the role of N-fertiliser management in crop N utilisation, crop productivity and soil-based N2O losses from wheat growing in waterlogging-prone soils with high concentrations of organic C in south-western Victoria. The fertiliser strategies included different rate, placement (deep-banded v. topdressing) and DMPP-coated N fertiliser, imposed in three separate field experiments; to test the hypothesis that improving crop N fertiliser uptake can reduce N2O losses from crops growing in waterlogging-prone soil.
Materials and methods
Experimental sites
Field experiments were conducted at Hamilton (142°4ʹ15ʹʹE, 37°49ʹ27ʹʹS) in 2012 and 2014 and at Tarrington (142°6ʹ9ʹʹE, 37°47ʹ6ʹʹS) in 2013, in south-western Victoria on Ferric-Eutrophic Brown Chromosol soil (Isbell 2002). Soils at the Hamilton and Tarrington sites were characterised by moderate levels of clay (10–20%) in the topsoil (0–30 cm), but thereafter clay content abruptly increased and remained high (60–70%) throughout the rest of the soil profile.
Before the study, raised beds were formed at the Hamilton site. The site was sprayed with a tank mix of glyphosate (1080 g a.i./ha) and carfentrazone-ethyl (18 g a.i./ha) on 1 September, 5 October and 14 December 2011, to impose a chemical fallow, followed by deep-ripping on 13 January and 7 March and power-harrowing on 15 March. The beds were formed on 21 March 2012. Beds were 1.35 m wide and adjacent furrows 15 cm deep by 35 cm wide. Canola (Brassica napus L.) cv. Stingray was sown at 3 kg/ha with a basal application of mono-ammonium phosphate fertiliser (10 kg N and 22 kg phosphorus (P)/ha) with the seed in 2013, the intervening year between experiments. By contrast, the Tarrington site was conventionally flat with a slight slope (1%); canola stubble was incorporated by tillage on 12 March 2013. Winter wheat (Triticum aestivum L.) and canola had been rotated at both sites before the study.
Experimental design
All of the three field experiments comprised a completely randomised block design with seven treatments replicated five times. Plots were 10 m long by 3.4 m wide; or two raised beds with one dividing furrow per plot at the Hamilton sites. The treatments included: a nil N experimental control (0N); four rates of granular urea-N fertiliser topdressed at 10 (TD10N), 35 (TD35N) and 85 (TD85N) and 185 (TD185N) kg N/ha at first-node (Zadoks growth stage Z31) or mid-till (Z25) stage of wheat growth (Zadoks et al. 1974); 85 kg N/ha of urea coated with DMPP (ENTEC, Incitec Pivot Ltd, Melbourne), either topdressed at first-node (DMPP85N@Z31) or deep-banded at sowing (DMPP85N@Z00); and 85 kg N/ha of urea-N deep-banded at sowing (DB85N@Z00).
Deep banding involved drilling either urea or DMPP-coated urea to a depth of 10 cm below the intended wheat seeding depth (~5 cm) on the day before planting. All treatments received a basal application of 15 kg P/ha at sowing. With the exception of the 0N treatment, a basal application of 15 kg N/ha was also applied with the seed when wheat cv. Bolac was sown on 31 May 2012 and 7 May 2014 at the Hamilton sites, and 9 May 2013 at the Tarrington site. Fertiliser was topdressed to wheat on 10 September 2012 and 19 August 2013, coinciding with the appearance of the first node at the respective Hamilton and Tarrington sites, and on 18 July 2014 around mid-tillering at Hamilton. Urea and DMPP-coated urea were topdressed by hand to the treatments allocated for topdressing at all sites, and fertiliser was not applied to the adjacent furrows at the Hamilton sites.
Crop management
Before sowing wheat at the Hamilton and Tarrington sites, a tank mix of glyphosate (1080 g a.i./ha) and carfentrazone-ethyl (18 g a.i./ha) was sprayed to eradicate weeds. Pyroxasulfone (100 g a.i./ha) was sprayed shortly before sowing. Plots were sown by using a cone seeder with knife-points and press-wheels spaced 15 cm apart; furrows were not planted on the raised bed sites. A basal application of N and P was applied with the seed as di-ammonium phosphate (15 N and 15 kg P/ha) to all treatments except for 0N, which received double superphosphate (15 kg P/ha). Fertiliser was treated with flutriafol (125 g a.i./ha) to combat threats of stripe rust (caused by Puccinia striiformis). Control of germinating wild radish (Raphanus raphanistrum L.) and capeweed (Arctotheca calendula) populations involved the use of MCPA and diflufenican (188 g and 19 g a.i./ha, respectively). Annual ryegrass (Lolium rigidum) populations were controlled with tralkoxydim (200 g a.i./ha). Propiconazole (125 g a.i./ha) fungicide was applied to control outbreaks of Septoria leaf blotch (Mycosphaerella graminicola). Stubbles remained standing on the sites, over the fallow during the experimental period.
Climatic and soil temperature measurements
An automated tipping-bucket rain gauge (Hastings Dataloggers, Port Macquarie, NSW) installed in close proximity to the experimental sites measured hourly rainfall at the Hamilton and Tarrington sites. Hourly topsoil water was monitored by theta probes (Theta-Probe MK2x; Delta-T Devices Ltd, Burwell, UK) installed to 6 cm depth. Theta probes were installed in the TD10N treatments at the Hamilton and Tarrington sites, and were placed on top of the raised bed and in the middle of the adjacent furrow at the Hamilton sites.
Soil sample collection for chemical analysis and bulk density
Five deep soil cores (internal diameter 42 mm) were randomly collected from each replicate of the Hamilton and Tarrington sites before experimentation. On each occasion, the cores were divided into 10-cm increments to 40 cm depth, and thereafter into 20-cm increments. Four of the five cores collected were combined for each layer within each replicate. Samples were then oven-dried at 40°C for 48 h and passed through a 2-mm sieve before chemical analysis. The remaining core collected from each replicate was weighed, oven-dried at 105°C for 48 h and weighed again to determine gravimetric water, bulk density and volumetric water.
Surface soil mineral N (NH4+ and NO3–) was measured monthly at the Hamilton and Tarrington sites, with more frequent samples taken before and after N fertiliser applications. On each occasion, 12–15 soil cores (internal diameter 20 mm) were randomly collected to a depth of 10 cm from each plot; at the Hamilton sites, separate soil samples were taken from the beds and from the adjacent furrows. During the Hamilton 2014 experiment, additional soil samples were taken from the 10–20 cm soil layer, from the bed tops over the winter period. Samples were oven-dried at 40°C for 48 h, and passed through a 2 mm sieve in preparation for soil mineral N analysis.
Additional topsoil samples were taken by hand to measure bulk density by using stainless-steel rings (internal diameter 50 mm) to 10 cm depth from each replicate at the Hamilton and Tarrington sites. In each replicate 10 random samples were collected; at the Hamilton sites, separate soil samples were taken from the beds and from the adjacent furrows. Samples were weighed and then oven-dried at 105°C for 48 h before reweighing.
Baseline soil chemical and physical properties
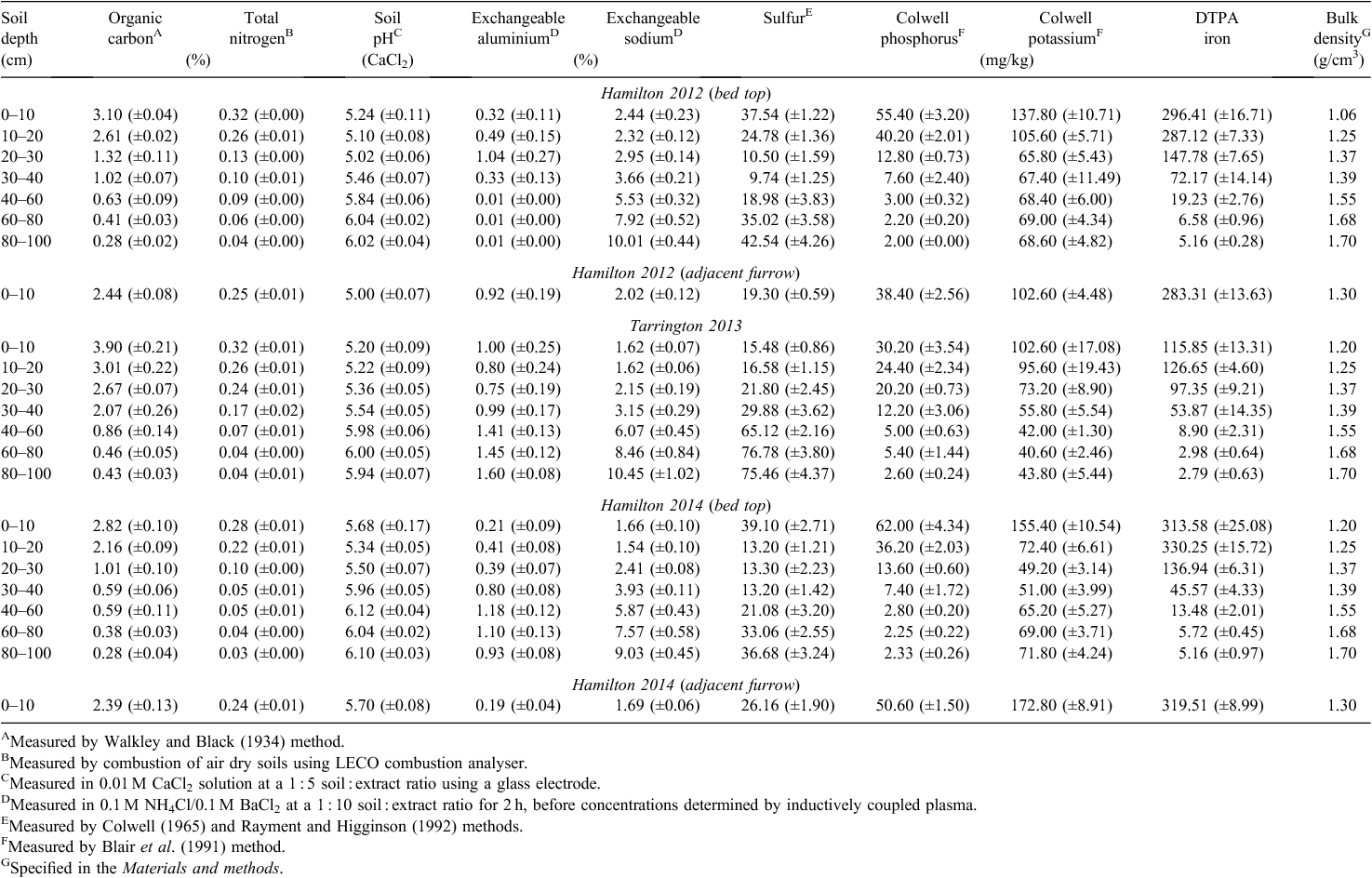
Analysis of soil before experimentation showed that organic C, total N and P and iron (Fe) concentrations generally declined with depth and that soil pH, exchangeable sodium and bulk density increased with depth at both the Hamilton and Tarrington sites (Table 1). Exchangeable aluminium (Al), soil P and sulphur (S) concentrations were unlikely to inhibit crop growth; however, concentrations of potassium (K) were generally marginal in the surface soil layers at both sites. In the topsoil layer (0–10 cm) of the furrow at the Hamilton site, all soil parameters were lower than in the adjacent beds, with the exception of exchangeable Al and bulk density (Table 1).
Crop measurements
Crop emergence was measured at the second-leaf stage (Z12) of wheat growth, by counting plants on both sides of a 0.5-m stick, randomly placed 10 times within each plot. Wheat biomass was measured at first node (Z31), anthesis (Z65) and maturity (Z93) by cutting a 1-m row of crop at two random locations within each plot. On each occasion the crop was cut at ground level and bulked within each plot, and subsamples retained and oven-dried at 65°C until constant weight was reached. After drying, spike density was measured by counting fully emerged wheat ears collected from anthesis (Z65) biomass samples, and maturity (Z93) biomass samples were threshed to separate grain from straw to determine respective N concentrations.
Grain yield was measured by mechanically harvesting each plot. A subsample of grain was retained to assess grain quality. Wheat grain protein was calculated by multiplying grain N concentration by 5.7 (Terman 1979). Grain weight was quantified by the mass of 1000 grains, and grain screenings by measuring the proportion of a hectolitre (hL) of grain passing through a 2-mm sieve.
Collection of N2O gas samples
Concentrations of N2O gas were measured on a 1–2-week frequency over the growing season, with more intensive samplings around N fertilisation at sowing (Z00) and topdressing (Z25 and Z31). Over the fallow period as topsoil moisture levels declined, gas collection frequency was extended to monthly. Gas was captured in vented static chambers constructed from 25-L PVC drums (internal diameter of 300 mm), with the bases cut off and fitted with one-way valves, rubber septa and battery-powered computer fans to provide continuous air circulation (Harris et al. 2013). At sampling, the chambers were placed into in-situ ground-level metal troughs filled with water, with each plot containing two troughs; except at the Hamilton sites, where two troughs were installed on the beds and two more in the adjacent furrows. The close proximity of troughs was necessary to place raised platforms between troughs to minimise soil compaction and plot damage from frequent samplings. On all occasions, fluxes were measured between 0900 and 1500 hours. Samples were collected by syringe at 0, 20, 40 and 60 min after chamber emplacement. Air samples (20 mL) were injected into 12-mL evacuated Exetainers (Labco Ltd, High Wycombe, UK) and analysed by gas chromatography. Tinytag Plus 2 temperature data loggers (Hastings Dataloggers, Port Macquarie, NSW) were placed inside one chamber in each replicate to monitor changes in air temperature during gas sampling.
Calibration of the theta probe and conversion to water-filled pore space (WFPS)
When topsoil samples were collected for soil mineral N analysis, a subsample was retained, weighed and oven-dried at 105°C for 48 h, then weighed again to determine gravimetric soil water, before multiplying by bulk density to determine volumetric water (VSW). A regression analysis determined an equation, used to convert raw theta probe water-content data to VSW. Calibration equations used at the Hamilton sites included the following:
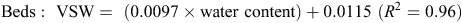
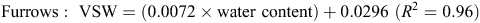
The calibration equation used at the Tarrington site was
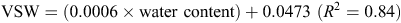
The WFPS was then determined by dividing VSW by total porosity (Linn and Doran 1984).
Chemical analyses and calculations
Soil mineral N was determined by extracting soils with 2 M KCl at a soil : solution ratio of 1 : 10 for 1 h at 25°C, and measuring the concentration of NO3–-N and NH4+-N in this extract on a Lachat Flow Injection Analyzer (Lachat Instruments, Milwaukee, WI, USA) (Searle 1984). Grain and plant N concentrations were measured using a LECO CNS 2000 analyser apparatus (LECO Corp., St. Joseph, MI, USA). Gas samples were analysed by a fully automated gas chromatograph (Agilent 7890A; Agilent Technologies Inc., Wilmington, DE, USA) equipped with a micro electron capture detector to quantify N2O (N2O(g)) concentration and then convert to gas density (N(g)) by
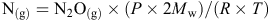
where P is atmospheric standard air pressure of 101.31 kPa, Mw is the molecular weight of N, R is the universal gas constant (8.314 J/K.mol), and T is chamber air temperature (K). Gas density was then adjusted for chamber volume. Fluxes were calculated from the linear increase in gas density in the chamber headspace with time (Barton et al. 2008). Cumulative flux was estimated by taking the mean between adjacent gas sampling points in time and multiplying by the number of days between samplings. In the TD35N@Z31 and TD85N@Z31 treatments, cumulative flux was assumed identical to the 0N control in the period before topdressing N fertiliser at the Hamilton 2012 and Tarrington sites.
Statistical analyses
Separate statistical analyses were performed to determine the effect of N rate, placement (deep banding v. topdressing) and fertiliser type (urea v. DMPP-coated urea) on topsoil NO3–-N and NH4+-N supply, N2O fluxes and crop productivity from each experiment. Data collected from the beds and furrows at the Hamilton sites were analysed separately. Treatment differences in topsoil NO3–-N and NH4+-N, cumulative N2O flux and crop productivity were tested by using analysis of variance (ANOVA) appropriate for a completely randomised block design. Logarithmic (base 10) transformations were used to normalise the cumulative N2O flux data before analyses. All analyses were undertaken using Genstat 14 Edition (Lawes Agricultural Trust, Harpenden, UK).
Results
Rainfall
During the 2012, 2013 and 2014 seasons, annual rainfall in the Hamilton region was, respectively, 44, 65 and 187 mm below the long-term (1962–2014) mean of 685 mm. Despite lower-than-average annual rainfall, growing season (April–November) rainfall was within 9 mm of the long-term mean of 538 mm in the 2012 and 2013 seasons, whereas the 2014 season experienced 126 mm less than the long-term mean. Winter rainfall (June–August) was 52 and 22 mm above the long-term average of 233 mm for the 2012 and 2013 seasons, respectively, and 33 mm below average during the 2014 season. Subsequent spring rainfall (September–November) was 50 and 109 mm below the long-term average of 193 mm for the 2012 and 2014 seasons, respectively, and 5 mm above average during the 2013 season.
Autumn profile soil NO3–-N at the beginning of each experiment
Before imposing treatments, the soil profile at the Hamilton 2012, Tarrington 2013 and Hamilton 2014 experimental sites contained (mean ± s.e.) 297 (±12), 145 (±12) and 116 (±6) kg soil NO3–-N/ha, respectively, in the top 100 cm. At the Hamilton 2012 site, 90% of the NO3–-N was stored in the top 40 cm of the profile, compared with 48% at the Tarrington site and 70% at the Hamilton 2014 site (Fig. 1).
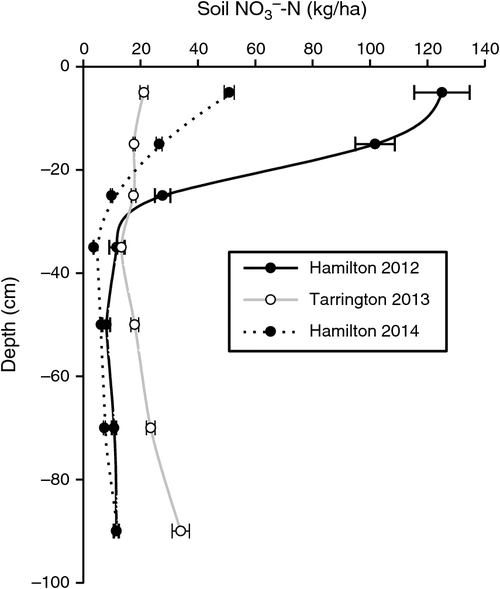
|
Temporal changes in surface soil NO3–-N in each experiment
At all experimental sites, soil NO3–-N concentrations increased significantly (P < 0.05) after topdressing urea at 85 kg N/ha at either first node (TD85N@Z31) or mid tillering (TD85N@Z25) compared with the 0N control (Table 2). Higher NO3–-N concentrations persisted under the TD85N treatments throughout the rest of the growing season at all sites and into the fallow at the Hamilton sites in both the 2012–13 and 2014–15 seasons compared with the 0N control. When DMPP was topdressed at first node (DMPP85N@Z31), soil NO3–-N concentration was significantly (P < 0.05) lower in the two samplings after application than in the TD85N@Z31 treatment at the Hamilton 2012 and Tarrington sites (Table 2). The only time that NO3–-N concentrations were found to be significantly higher under the DB85N@Z00 treatment than the topdressed treatments, or where DMPP was deep-banded at sowing, was when deeper (10–20 cm) samples were collected from the Hamilton site in 2014 (Table 2).
Temporal changes in surface soil NH4+-N in each experiment
Generally, concentrations of NH4+-N in the top 10 cm of soil at the first sampling after application were significantly (P < 0.05) higher after 85 kg N/ha was topdressed at first node (TD85N@Z31 and DMPP85N@Z31) than in the 0N control at both the Hamilton 2012 and Tarrington sites (Table 3). Thereafter, NH4+-N concentrations decreased more rapidly under TD85N@Z31 than DMPP85N@Z31 at both the Hamilton 2012 and Tarrington sites (Table 3). In the Hamilton 2014 experiment, significantly (P < 0.05) higher concentrations of NH4+-N were measured in the top 10 cm of soil on 16 June in the deep-banded urea (DB85N@Z00) treatment than in topdressed and 0N treatments, and on 30 July and 15 August in the TD85N@Z25 than all other treatments (Table 3).
Temporal changes in WFPS and N2O flux in each experiment
In all experiments, topsoil WFPS responded to periods of high rainfall (>15 mm), associated runoff, surface pondage, evapotranspiration and evaporation. WFPS peaked at 90% on 17 August 2012 in the beds at the Hamilton 2012 site (Fig. 2a), at 108% on 23 August 2013 at the flatter Tarrington site (Fig. 2b), and at 85% on 1 August 2014 in the beds at the Hamilton 2014 site (Fig. 2c).
At all sites, peak N2O production coincided with the initial stages of the topsoil drying out, after WFPS had peaked in August, with peak flux reaching 372 g N2O-N/ha.day on 25 September 2012 at the Hamilton 2012 site (Fig. 2a), 65 g N2O-N/ha.day on 3 September 2013 at the Tarrington site (Fig. 2b), and 129 g N2O-N/ha.day on 19 August 2014 at the Hamilton 2014 site (Fig. 2c). At all sites, soil N2O production was <1 g N2O-N/ha.day once WFPS dropped below 40% (Fig. 2a, b, c), or when WFPS exceeded 80% (Fig. 2b). At all sites, fluxes were higher where urea-N was either deep-banded (DB85N@Z00) or topdressed (TD85N@Z31 and TD85N@Z25) (Fig. 2a–c) than from the 0N control.
Cumulative losses of N2O from each experiment
Cumulative N2O flux was approximately 2–2.5-fold higher where urea was deep-banded (DB85N@Z00) at sowing than in the 0N control on the beds of the Hamilton sites in 2012 and 2014 (Fig. 3a, c) and the Tarrington site (Fig. 3b). The cumulative N2O flux from the DB85N@Z00 treatment was significantly (P ≤ 0.10) greater than from the 0N control at all sites, and from both DMPP deep-banded and topdressed treatments at the Hamilton 2012 and 2014 sites, and some of the topdressed treatments at the Hamilton 2012 and Tarrington sites (Fig. 3). There was no effect of treatment in the adjacent furrows at the Hamilton sites during the 2012–13 and 2014–15 seasons (data not shown).
Crop biomass, N uptake, and grain yield and quality
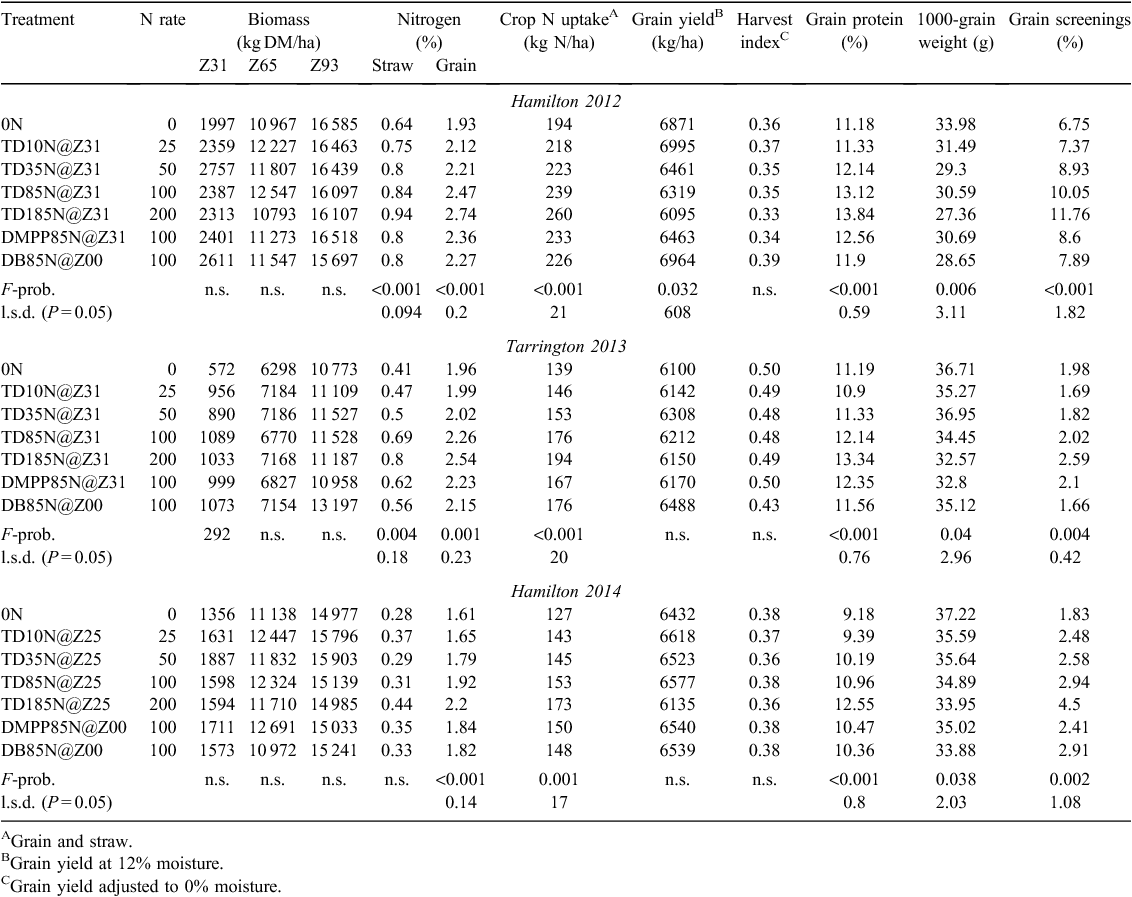
Throughout the three seasons of experimentation, N-fertiliser management had no effect on crop emergence and ear density at anthesis (data not shown), biomass at anthesis and maturity, or harvest index. The exception was biomass measured at first node at the Tarrington site in 2013, where treatments receiving N fertiliser produced 35–47% more biomass than the 0N treatment (Table 4). Grain yield was also unaffected by N-fertiliser management, except at the Hamilton 2012 site, where a significant (P = 0.038) incremental decrease in grain yield occurred when urea-N was topdressed compared with the 0N treatment (Table 4). However, at all sites, significant (P ≤ 0.001) incremental increases in crop N uptake were observed in response to increasing rates of urea-N application (Table 4). The additional crop N uptake resulting from increasing rates of urea-N corresponded to significantly (P ≤ 0.001) higher grain N concentrations at all sites and significantly (P ≤ 0.004) higher concentrations of straw N at the Hamilton 2012 and Tarrington sites (Table 4). At all sites, significant (P < 0.001) incremental increases in grain protein were observed from increasing urea-N supply (Table 4). However, the positive grain protein responses to increasing urea-N supply corresponded to significantly (P ≤ 0.04) lower grain weight, and conversely significantly (P ≤ 0.004) higher grain screenings, in all experiments (Table 4). Furthermore, in all experiments, topdressing DMPP or deep banding N at sowing did not significantly improve wheat yield compared with topdressing urea-N at the equivalent rate (Table 4).
Discussion
Effect of N management on topsoil mineral N supply and associated N2O losses
Placement and/or the timing of urea-N application had a significant effect on N2O losses; deep-banding urea-N at sowing consistently produced higher losses than the 0N and topdressed urea-N treatments (Fig. 3). Peak crop demand for N in dryland wheat grown in southern Australia occurs during spring, from stem extension until anthesis (Angus 2001). Supplying high rates of urea-N early in the growing season, even when deep-banded in the soil, caused a temporal mismatch between supply and peak crop demand for N, providing greater opportunities for N2O losses than topdressing in-season around the commencement of stem extension.
Nitrification inhibitors are designed to delay the oxidation of NH4+-N to nitrite (NO2–-N) by depressing the activity of soil bacteria (e.g. Nitrosomonas) during the nitrification process (Zerulla et al. 2001). Addition of DMPP to urea slowed the conversion of NH4+-N to NO3–-N (Tables 2 and 3), consistent with observations of other researchers (Weiske et al. 2001; Belastegui-Macadam et al. 2003; Merino et al. 2005; Chen et al. 2010; Di and Cameron 2012; Pfab et al. 2012). Slowing the process of nitrification through the use of nitrification inhibitors such as DMPP can reduce subsequent N2O losses (Weiske et al. 2001; Liu et al. 2013). The greatest reduction in N2O emissions observed in our study was when DMPP-coated urea was deep-banded at sowing, compared with deep-banded untreated urea, the former management effectively mitigating emissions during the high-risk winter waterlogging period.
Effect of soil moisture and background soil NO3– on N2O losses
Soils with abrupt textual changes between the A (topsoil) and B (subsoil) horizons frequently develop surface and subsurface waterlogging caused by rapid saturation of the A horizon from excess winter rainfall and poor infiltration into the B horizon (MacEwan et al. 1992). The Brown Chromosol (Isbell 2002) soils used in our studies exhibit strong textural contrast at the junction of the A and B horizons, resulting in prolonged periods of elevated WFPS where raised beds were used at Hamilton in 2012 and 2014 (Fig. 2a, c) and saturated conditions at Tarrington in the absence of surface drainage (Fig. 2b). Although peak N2O losses from the soil coincided with the topsoil drying in late winter–early spring (Fig. 2), the N2O may have formed earlier, before becoming entrapped in the topsoil where it accumulated before release later in the season (Samson et al. 1990). Unfortunately, only topsoil (0–10 cm) moisture was monitored in our studies, the placement of theta probes on the junction of the A and B horizons may have enhanced data interpretation.
The magnitude of N2O losses varied markedly between sites and seasons, due in part to differences in the amounts of soil NO3–-N, stored in the soil profile at the beginning of the growing season at the respective sites (Fig. 1). The Hamilton 2012 experiment was imposed after a 6-month chemical fallow, during which soil disturbance occurred when forming raised beds, and in combination with high background soil organic C concentration, this resulted in large amounts of mineral N accumulating before sowing. By contrast, the Tarrington experiment was preceded by ~20 years of annual crops (B. Herrmann pers. comm.), with the previous 2012 canola residue incorporated approximately 2 months before the experiment was sown; the site also received well below-average summer rainfall. Bending and Lincoln (2000) reported that glucosinolates sourced from residues of brassicas could alter soil microbiota, causing a decline in both NH3-oxidising and nitrifying bacteria, and slowing mineral N transformations for a period. More recently, in laboratory soil incubation experiments, Begum et al. (2014) also showed initial N immobilisation and associated reductions in N2O emissions from the addition of canola (Brassica napus L.) residue; however, later in the experiment, the low C : N ratio of canola residue produced greater N2O losses. Given the absence of a significant yield response to urea-N application at the Tarrington site (Table 4), we speculate that our wheat crop may have accessed a significant proportion of its N supply from mineralisation of the previously incorporated canola residue. Furthermore, frequent wetting and drying of the topsoil (Fig. 2b) over spring at Tarrington in the presence of potentially high amounts of soluble C could have been ideal for rapid mineralisation of fresh residue (Fillery 2001). At the Hamilton site in 2014, the previous surface canola residue was removed before experimentation, possibly reducing immobilisation of NO3–-N.
Differences in the magnitude of N2O losses between sites and seasons may also be due to the drainage characteristics of the respective sites. The N2O : N2 ratios associated with denitrification are strongly influenced by WFPS percentage (Ciarlo et al. 2007). At the wetter Tarrington site, WFPS levels exceeded 80% for longer, and therefore, conditions were more conducive to greater N2 production compared with the raised beds at the Hamilton sites, where WFPS rarely exceeded 80% in both the 2012 and 2014 growing seasons (Fig. 2). However, this theory assumes that denitrification was the source of the N2O production, and we acknowledge that dissimilatory NO3–-N reduction, another possible pathway for N2O losses, may be occurring in our soils with high concentrations of C (Yin et al. 2002), and if so, N2 gas losses may be less consequential. Although our research has quantified losses of N2O under different N-fertiliser management, we remain uncertain about the origin of the N2O and, therefore, total gaseous N losses and the associated agronomic implications for high-rainfall cropping soils with high background soil C supply.
Effect of management on crop N-fertiliser recovery and productivity
Both Edwards (1992) and Anderson et al. (1992) showed that establishment of early-sown, well-fertilised, dense, vigorously growing wheat crops on texture-contrast soils was an important strategy for optimising grain yields. Passioura (1992) suggested two main reasons for the success of this strategy. First, it allows roots more time to colonise the subsoil before the onset of winter waterlogging where anoxic conditions were less likely to impede growth, even though the topsoil might be saturated above. Second, early growth promotes greater utilisation of soil NO3–-N after opening autumn rains stimulate the flush of organic matter mineralisation. In our studies, only a small benefit of N fertiliser, applied with the seed or deep-banded at sowing, was observed at the Tarrington site, and this was evident only in the dry matter data collected at first node (Table 4). Thereafter, no biomass or grain yield response to this added N was found. This finding contradicts the conclusions reached by Watson et al. (1976), Drew et al. (1979) and Huang et al. (1994), who reported that additional N at sowing, or increased supply of N to waterlogged crops, could increase yield and mitigate the harmful effects of waterlogging. Bell et al. (2013) reported a critical soil test value at the beginning of the growing season for wheat in Victoria of 62 kg NO3–-N/ha to 60 cm depth; below this concentration, wheat was likely to respond to additional synthetic N supply. Although the value from Bell et al. (2013) is based mainly on drier Victorian environments with much lower yield potentials and associated demand for N, the NO3–-N concentrations to 60 cm depth in our studies approached this critical concentration only at the Tarrington site (Fig. 1b).
In our experiments, the wheat crops must have had access to more N than just the NO3–-N stored in the profile around seeding. The large losses of N2O observed in our studies, by southern Australian cropping standards (Barton et al. 2008; Officer et al. 2015) and in the absence of any positive grain yield response, seem a contradictory outcome. In-crop N mineralisation has been shown to be an important source of non-synthetic N supply to crops in medium–high-rainfall zones of southern New South Wales (Angus et al. 1998). Although soil mineralisation rates were not measured in our studies, applying the model developed by Baldock (2003), accounting for high organic C concentrations at the Tarrington (3.90%) and Hamilton (3.10–2.82%) sites, we estimate that 99–195 kg N/ha may have mineralised over the respective growing seasons. The high in-crop mineralisation potential at both the Hamilton and Tarrington sites partly explains why yields remained unaffected despite the concurrent large losses of N2O (Fig. 3).
Mineralisation of organic matter only partly explains the outcomes of N-fertiliser management on crop productivity. We estimate the water use efficiency (WUE) at the Hamilton 2012, Tarrington 2013 and Hamilton 2014 sites, respectively, to be 16, 15 and 20 kg grain/mm plant-available water. The benchmark of 20 kg grain/mm plant-available water suggested by French and Schultz (1984) was reached only in 2014. We cautiously rule out other potential nutritional constraints, with S and K concentrations above levels considered to limit yield (Anderson et al. 2013; Brennan and Bell 2013), although K appeared marginal in the lower layers of the topsoil (Table 1). Research elsewhere has shown that applications of K to waterlogged wheat can significantly increase yield (Belford et al. 1992). Likewise, we cannot rule out that ion (e.g. Fe) toxicities often associated with transient waterlogging (Khabaz-Saberi et al. 2014) may have played some role in reducing WUE.
The large reduction in WUE at Tarrington in 2013 appeared largely due the site becoming inundated with water in mid-August (Fig. 2b), reducing soil oxygen diffusion (Bollmann and Conrad 1998) and causing crop damage from transient waterlogging. McDonald and Gardner (1987), MacEwan et al. (1992) and Harris et al. (2013) have collectively reported reductions in grain yields from 25% to 85% compared with crops grown under improved drainage in south-western Victoria. McDonald and Gardner (1987) observed that waterlogging resulted in shoot N concentrations declining to low levels between stem elongation and anthesis. In our study, topdressing urea-N at around first node resulted in greater crop uptake of N, but had no effect on anthesis and maturity biomass or grain yield (Table 4). Although topdressing N at around first node reduced N2O emissions, the application to cv. Bolac, a slow-maturing spring-type wheat, may have been too late to influence yield; instead, the extra N was directed into the kernel, thereby increasing grain protein concentrations (Fischer et al. 1993).
Surface waterlogging was less of a constraint to crop yield on the raised beds at the Hamilton sites, because the beds are designed to drain excess winter rainfall into the adjacent furrow (Bakker et al. 2005). However, yellowing of older leaves was still observed in mid–late winter in both years at Hamilton, symptoms associated with transient N deficiency (Robertson et al. 2009), raising the possibility of subsoil waterlogging at the junction of the A and B horizons below the depth of the furrow. The topdressing of urea-N resulted in greater crop N uptake and the alleviation of temporary N deficiency; however, a positive yield response was not observed, despite improved surface drainage. Although our experiments were conducted in three different seasons, anthesis and maturity biomass were greater and conversely harvest index (HI) was much lower on the beds at Hamilton than the undrained Tarrington site. Passioura (1977) illustrated how HI can be a function of water used after anthesis, when biomass is partitioned into grain. Later, Fischer (1979) described a physiological framework for dryland wheat yields in Australia, suggesting ‘that grain number in the wheat crop is largely determined by the end of anthesis’, but that grain weight appeared largely determined by post-anthesis grain-filling conditions. The low HI at the Hamilton sites suggests post-anthesis water stress resulting from below-average spring rainfall in both seasons, and perhaps excessive pre-anthesis growth from improved surface drainage, contributing to the crops experiencing water deficit late in the growing season.
The higher N concentration in response to the extra urea-N topdressed at around first node to the 2012 crop, along with large reserves of soil NO3–-N (Fig. 1a), probably resulted in greater accumulation of stem and leaf protein and an associated reduction in water-soluble carbohydrates (van Herwaarden et al. 1998a). Although no measurements of kernel density were made in our experiments, van Herwaarden et al. (1998b) also showed no significant increase in anthesis and maturity biomass yet a significant increase in spike and kernel density when they applied increasing rates of N fertiliser to wheat growing at Wagga Wagga. The van Herwaarden et al. (1998b) crop subsequently experienced water deficit over the grain-filling period and a shortfall of assimilate for translocation into the kernel. The grain yield reduction and associated loss of grain quality, described by van Herwaarden et al. (1998b) as ‘haying-off’, was the likely mechanism for the grain yield reduction observed at the Hamilton 2012 site.
Even less spring rainfall fell at Hamilton in 2014, and yet haying-off was less pronounced with marginal penalty to grain quality, and WUE was much higher than previous seasons. The 2014 season was also characterised by a drier winter, especially during August, with topsoil drying and release of N2O observed earlier than in 2012 (Fig. 2a, b). Perhaps a longer duration between topsoil drying out and anthesis provided more time for crop root penetration into the subsoil and greater access to subsoil moisture compared with the 2012 crop. Alternatively, the 2014 crop was sown earlier followed by unseasonably warm conditions during May, which may have led to deeper root penetration into the subsoil before the onset of waterlogging. Passioura (1992) suggested that despite roots in the waterlogged soil layers losing their ability to extract water and nutrients, the cortex of the anoxic roots may still act as a conduit for moving shoot-produced assimilates to active roots in the subsoil, and in turn water and nutrients from the subsoil to the shoot. Kirkegaard et al. (2007) highlighted that ‘relatively small amounts of subsoil water can be highly valuable to grain yield’, reporting efficiencies from subsoil water use three times that typically expected for total seasonal water use. Although we remain uncertain about when the roots colonised the subsoil in 2014, access to deeper subsoil moisture is the only reasonable explanation for the wheat production and grain quality achieved. Perhaps the later sown 2012 crop, with greater N supply and a longer duration of subsoil winter waterlogging confining roots largely to the topsoil, resulted in more severe water deficit at grain fill than that experienced by the 2014 crop.
The absence of a grain yield response to urea-N may not be entirely explained by high rates of organic matter mineralisation, waterlogging, water deficits during grain filling or timing of N application. Riffkin et al. (2012) reported significant grain-yield increases from applying high rates of N fertiliser to early-sown, late-maturing winter-type canola at Hamilton during the 2008 growing season. Although canola is a different crop from wheat, longer term results from National Variety Trials testing of wheat varieties in south-western Victoria have also shown longer seasoned winter types out yielding spring-type wheats (http://www.nvtonline.com.au/google-maps/2014/WheatEaSouth%20West.pdf). Early-sown, longer season winter-type crops possessing longer vegetative growth phases are likely to utilise more radiation (Riffkin et al. 2012), potentially resulting in higher demands for synthetic N and associated yield responsiveness in the south-western Victorian environment.
Yield and grain quality were unaffected by the use of DMPP-treated urea, as there was no evidence of increased retention of the applied N for crop N uptake (Table 4). Furthermore, although nitrification was slowed, no subsequent delayed increase in soil NO3–-N concentrations was observed after the application of DMPP (Table 2). Soares et al. (2012) reported that the nitrification inhibitor dicyandiamide (DCD) coated to urea increased ammonia (NH3) volatilisation by 5–16%; this was caused by maintaining higher soil NH4+-N and pH for a longer time than with urea. Although our study involved the use of a different type of nitrification inhibitor, we speculate that higher NH3 losses from the use of DMPP may have resulted in no greater net retention of N for crop uptake or subsequent improvements in yield or grain protein over the use of conventional urea (Table 4).
Conclusion
This study has shown that synchronising N fertiliser supply with peak crop demand can significantly reduce N2O losses from waterlogging-prone soils. Excessive N supplied early in the growing season provides greater opportunity for N loss over winter, because wheat utilises most of the available N over the early–mid-spring period. Although the supply of N at around the commencement of stem extension enhanced crop N uptake, no improvement in grain yield was found, likely from high rates of in-season organic matter mineralisation, and/or crops encountering water deficit during grain fill, especially during the 2012 growing season. However, the enhanced crop N uptake from urea topdressed around stem extension is likely to benefit grain quality, especially protein, provided no water deficit is experienced during grain fill. Although DMPP reduced N2O losses, especially when applied at sowing, there was no evidence of improved yield or grain quality over the use of conventional urea N.
Acknowledgements
We thank Brent Herrmann for allowing us to conduct the Tarrington experiment on his property, and Kirsten Fogarty, the late Michael Byron and Reto Zollinger for technical assistance. This research was funded through the federal Department of Agriculture, the Grains Research and Development Corporation, and the Victorian State Government.
References
Anderson WK, French RJ, Seymour M (1992) Yield responses of wheat and other crops to agronomic practices on duplex compared to other soils in Western Australia. Australian Journal of Experimental Agriculture 32, 963–970.| Yield responses of wheat and other crops to agronomic practices on duplex compared to other soils in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Anderson GC, Peverill KI, Brennan RF (2013) Soil Sulfur-crop response calibration relationships and criteria for field crops grown in Australia. Crop & Pasture Science 64, 523–530.
| Soil Sulfur-crop response calibration relationships and criteria for field crops grown in Australia.Crossref | GoogleScholarGoogle Scholar |
Angus JF (2001) Nitrogen supply and demand in Australian agriculture. Australian Journal of Experimental Agriculture 41, 277–288.
| Nitrogen supply and demand in Australian agriculture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkt1CrsbY%3D&md5=6be1af538eb01fe009f2863ce1cb2854CAS |
Angus JF, van Herwaarden AF, Fischer RA, Howe GN, Heenan DP (1998) The source of mineral nitrogen for cereals in south-eastern Australia. Australian Journal of Agricultural Research 49, 511–522.
| The source of mineral nitrogen for cereals in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXisFGqt74%3D&md5=c0b1ca62eec334c2182dc7e7a2d69669CAS |
Bakker DM, Hamilton GJ, Houlbrooke DJ, Spann C (2005) The effect of raised beds on soil structure, waterlogging, and productivity on duplex soils in Western Australia. Australian Journal of Soil Research 43, 575–585.
| The effect of raised beds on soil structure, waterlogging, and productivity on duplex soils in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Baldock J (2003) Match fertiliser rates with available water. Farming Ahead 140, 40–43.
Barton L, Kiese R, Gatter D, Butterbach-Bahl K, Buck R, Hinz C, Murphy DV (2008) Nitrous oxide emissions from a cropped soil in a semi-arid climate. Global Change Biology 14, 177–192.
Begum N, Guppy C, Herridge D, Schwenke G (2014) Influence of source and quality of plant residue on emissions and N2O and CO2 from a fertile, acidic Black Vertisol. Biology and Fertility of Soils 50, 499–506.
| Influence of source and quality of plant residue on emissions and N2O and CO2 from a fertile, acidic Black Vertisol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXktlertb0%3D&md5=9ee4aa49cf6fbfe41798c67f05e44be5CAS |
Belastegui-Macadam XM, del Prado A, Merino P, Estavillo JM, Pinto M, Gonzalez-Murua C (2003) Dicyandiamide and 3,4-dimethylpyrazole phosphate decrease N2O emissions from grassland but dicyandiamide produces deleterious effects in clover. Journal of Plant Physiology 160, 1517–1523.
| Dicyandiamide and 3,4-dimethylpyrazole phosphate decrease N2O emissions from grassland but dicyandiamide produces deleterious effects in clover.Crossref | GoogleScholarGoogle Scholar |
Belford RK, Dracup M, Tennant D (1992) Limitations to growth and yield of cereal and lupin crops on duplex soils. Australian Journal of Experimental Agriculture 32, 929–945.
| Limitations to growth and yield of cereal and lupin crops on duplex soils.Crossref | GoogleScholarGoogle Scholar |
Bell MJ, Strong W, Elliot D, Walker C (2013) Soil nitrogen-crop response calibration relationships and criteria for winter cereal crops grown in Australia. Crop & Pasture Science 64, 442–460.
| Soil nitrogen-crop response calibration relationships and criteria for winter cereal crops grown in Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlakt73I&md5=dba2e142bd51aa7f6a94716d4a6cc20fCAS |
Bending GD, Lincoln SD (2000) Inhibition of soil nitrifying bacteria communities and their activities by glucosinolate hydrolysis products. Soil Biology & Biochemistry 32, 1261–1269.
| Inhibition of soil nitrifying bacteria communities and their activities by glucosinolate hydrolysis products.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlslyhs70%3D&md5=464f9438b33ed1fa11fb2ffb960fd3c5CAS |
Blair GJ, Chinoim N, Lefroy RDB, Anderson GC, Crocker GJ (1991) A soil sulfur test for pastures and crops. Australian Journal of Soil Research 29, 619–626.
| A soil sulfur test for pastures and crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmsFGjtr4%3D&md5=16bb4cd301f8761e0a29901bc162ad4bCAS |
Bollmann A, Conrad R (1998) Influence of O2 availability of NO and N2O release by nitrification and denitrification in soils. Global Change Biology 4, 387–396.
| Influence of O2 availability of NO and N2O release by nitrification and denitrification in soils.Crossref | GoogleScholarGoogle Scholar |
Brennan RF, Bell MJ (2013) Soil potassium-crop response calibration relationships and criteria for field crops grown in Australia. Crop & Pasture Science 64, 514–522.
| Soil potassium-crop response calibration relationships and criteria for field crops grown in Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlakt73L&md5=e819c1ecedc80c8881b203b74f5dc34bCAS |
Chen D, Suter HC, Islam A, Edis R (2010) Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emissions from a clay loam soil fertiliser with urea. Soil Biology & Biochemistry 42, 660–664.
| Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emissions from a clay loam soil fertiliser with urea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXis1Sgtrw%3D&md5=cb0fbf1d12401ca061462b81b933ca1fCAS |
Ciarlo E, Conti M, Bartoloni N, Rubio G (2007) The effect of moisture on nitrous oxide emissions from soil and the N2O/(N2O+N2) ratio under laboratory conditions. Biology and Fertility of Soils 43, 675–681.
| The effect of moisture on nitrous oxide emissions from soil and the N2O/(N2O+N2) ratio under laboratory conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntlajs78%3D&md5=650aa031dc2136c3bc6f63aed8e83d39CAS |
Colwell JD (1965) An automatic procedure for the determination of phosphorus in sodium hydrogen carbonate extracts of soils. Chemistry & Industry 893–895.
Crutzen PJ (1981) Atmospheric chemical processes of the oxides of nitrogen, including nitrous oxide. In ‘Denitrification, nitrification, and atmospheric nitrous oxide’. (Ed. CC Delwiche) pp. 17–44. (John Wiley and Son: New York)
Di HJ, Cameron KC (2012) How does the application of different nitrification inhibitors affect nitrous oxide emissions and nitrate leaching from cow urine in grazed pastures. Soil Use and Management 28, 54–61.
| How does the application of different nitrification inhibitors affect nitrous oxide emissions and nitrate leaching from cow urine in grazed pastures.Crossref | GoogleScholarGoogle Scholar |
Drew MC, Sisworo EJ, Saker LR (1979) Alleviation of waterlogging damage to young barley plants by application of nitrate and asynthetic cytokinin, and comparison between the effects of waterlogging, nitrogen deficiency and root extension. New Phytologist 82, 315–329.
| Alleviation of waterlogging damage to young barley plants by application of nitrate and asynthetic cytokinin, and comparison between the effects of waterlogging, nitrogen deficiency and root extension.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXlsVGhsr8%3D&md5=fe98b6c3198d74333188af2696c3448aCAS |
Edwards I (1992) Farming duplex soils- a farmer’s perspective (1992) Australian Journal of Experimental Agriculture 32, 811–814.
| Farming duplex soils- a farmer’s perspective (1992)Crossref | GoogleScholarGoogle Scholar |
Edwards J, Umbers A, Wentworth S (2012) Farm practices survey report 2012. Grains Research and Development Corporation. Available at http://www.grdc.com.au/Resources/Publications/2012/11/GRDC-Farm-Practices-Survey-2012 [verified 22 May 2016]
FAO (2015) World fertilizer trends and outlook to 2018. Food and Agricultural Organization of the United Nations, Rome. Available at http://www.fao.org/3/a-i4324e.pdf [verified 7 June 2016]
Fillery IRP (2001) The fate of biologically fixed nitrogen in legume-based dryland farming systems: a review. Australian Journal of Experimental Agriculture 41, 361–381.
| The fate of biologically fixed nitrogen in legume-based dryland farming systems: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkt1Crtro%3D&md5=8f49c78c2ca27ba1a36468adefaba3f1CAS |
Fischer RA (1979) Growth and water limitation to dryland wheat yield in Australia: a physiological framework. The Journal of the Australian Institute of Agricultural Science 45, 83–94.
Fischer RA, Howe GN, Ibrahim Z (1993) Irrigated spring wheat and timing and amount of nitrogen fertilizer. I. Grain yield and protein content. Field Crops Research 33, 37–56.
| Irrigated spring wheat and timing and amount of nitrogen fertilizer. I. Grain yield and protein content.Crossref | GoogleScholarGoogle Scholar |
French RJ, Schultz JE (1984) Water use efficiency of wheat in a Mediterranean-type environment I. The relationship between yield, water use and climate. Australian Journal of Agricultural Research 35, 743–764.
| Water use efficiency of wheat in a Mediterranean-type environment I. The relationship between yield, water use and climate.Crossref | GoogleScholarGoogle Scholar |
Grant RF, Pattery E, Goddard TW, Kryanowski LM, Puurveen H (2006) Modeling the effects of fertilizer application rate on nitrous oxide emissions. Soil Science Society of America Journal 70, 235–248.
| Modeling the effects of fertilizer application rate on nitrous oxide emissions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1Wnsr0%3D&md5=7bdca83f9963d232213180ddece7f8f0CAS |
Halvorson AD, Del Grosso SJ, Reule CA (2008) Nitrogen, tillage, and crop rotation effects on nitrous oxide emissions from irrigated cropping systems. Journal of Environmental Quality 37, 1337–1344.
| Nitrogen, tillage, and crop rotation effects on nitrous oxide emissions from irrigated cropping systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXos1eksrc%3D&md5=669a7dd0b0a59f1472689e0d05a7a5a6CAS | 18574163PubMed |
Harris RH, Officer SJ, Hill PA, Armstrong RD, Fogarty KM, Zollinger RP, Phelan AJ, Partington DL (2013) Can nitrogen fertiliser and nitrification inhibitor management influence N2O losses from high rainfall cropping systems in South Eastern Australia? Nutrient Cycling in Agroecosystems 95, 269–285.
| Can nitrogen fertiliser and nitrification inhibitor management influence N2O losses from high rainfall cropping systems in South Eastern Australia?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXotV2ru70%3D&md5=dfcb3a9185e64759c620914c4cfd4732CAS |
Hoben JP, Gehl RJ, Millar N, Grace PR, Robertson GP (2011) Nonlinear nitrous oxide (N2O) response to nitrogen fertilizer in on-farm corn crops of the US midwest. Global Change Biology 17, 1140–1152.
| Nonlinear nitrous oxide (N2O) response to nitrogen fertilizer in on-farm corn crops of the US midwest.Crossref | GoogleScholarGoogle Scholar |
Hooper S, Barrett D, Martin P (2003) ‘Australian Grains Industry 2003. Performance and outlook.’ (ABARE: Canberra, ACT) Available at http://www.airc.gov.au/safetynet_review/actu/actu_exhibit2003_11.pdf [verified 7 June 2016]
Huang B, Johnson JW, Nesmith S, Bridges DC (1994) Growth physiological and anatomical responses of two wheat genotypes to waterlogging and nutrient supply. Journal of Experimental Botany 45, 193–202.
| Growth physiological and anatomical responses of two wheat genotypes to waterlogging and nutrient supply.Crossref | GoogleScholarGoogle Scholar |
IPCC (2013) ‘Climate change 2013: the physical science basis.’ (Intergovernmental Panel on Climate Change: Geneva, Switzerland) Available at https://www.ipcc.ch/pdf/assessment-report/ar5/wg1/WGIAR5_SPM_brochure_en.pdf [verified 7 June 2016]
Isbell RF (2002) ‘The Australian soil classification.’ Revised edn. (CSIRO Publishing: Melbourne)
Jensen ES, Peoples MB, Boddy RM, Gresshoff PM, Hauggaard-Nielsen H, Alves JR, Morrison MJ (2012) Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries. A review. Agronomy for Sustainable Development 32, 329–364.
| Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries. A review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XksVOgt78%3D&md5=f45ebd9c47ed82046555ee217a464e1cCAS |
Khabaz-Saberi H, Barker SJ, Rengel Z (2014) Tolerance to ion toxicities enhances wheat grain yield in acid soils prone to drought and transient waterlogging. Crop and Pasture Science 65, 862–867.
| Tolerance to ion toxicities enhances wheat grain yield in acid soils prone to drought and transient waterlogging.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsFKnsr%2FP&md5=70c38b853297ab11a745ee9a7ed93f91CAS |
Kirkegaard JA, Lilley JM, Howe GN, Graham JM (2007) Impact of water use on wheat yield. Australian Journal of Agricultural Research 58, 303–315.
| Impact of water use on wheat yield.Crossref | GoogleScholarGoogle Scholar |
Lake A (2012) Australia’s declining crop yield trends II: The role of nitrogen nutrition. In ‘Proceedings 16th Australian Agronomy Conference’. Armidale, NSW. (Australian Society of Agronomy, The Regional Institute: Gosford, NSW) Available at http://www.regional.org.au/au/asa/2012/nutrition/8166_lakea.htm[verified 7 June 2016]
Linn DM, Doran JW (1984) Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Science Society of America Journal 48, 1267–1272.
| Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhtFaitL4%3D&md5=0b26b0412d09d2e99729b94c3b26fe9aCAS |
Liu C, Wang K, Zheng X (2012) Responses of N2O and Ch4 fluxes to fertilizer nitrogen addition rates in an irrigated wheat–maize cropping system in northern China. Geosciences 9, 839–850.
Liu C, Wang K, Zheng X (2013) Effects of nitrification inhibitors (DCD and DMPP) on nitrous oxide emission, crop yield and nitrogen uptake in a wheat-maize cropping system. Biogeosciences 10, 2427–2437.
| Effects of nitrification inhibitors (DCD and DMPP) on nitrous oxide emission, crop yield and nitrogen uptake in a wheat-maize cropping system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXltlGktLg%3D&md5=7ae6f766dbe0c2a108f66906ecb09266CAS |
Ma BL, Wu TY, Tremblay N, Deen W, Morrison MJ, McLaughlin NB, Gregorich EG, Stewart G (2010) Nitrous oxide fluxes from corn fields: on farm assessment of the amount and timing of nitrogen fertilizer. Global Change Biology 16, 156–170.
| Nitrous oxide fluxes from corn fields: on farm assessment of the amount and timing of nitrogen fertilizer.Crossref | GoogleScholarGoogle Scholar |
Macdonald BCT, Rochester IJ, Nadelko A (2015) High yielding cotton produced without excessive nitrous oxide emissions. Agronomy Journal 107, 1673–1681.
| High yielding cotton produced without excessive nitrous oxide emissions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xlt1Wksbc%3D&md5=7e5a00040fd5c2f276c7dc4fcd68cbf1CAS |
MacEwan RJ, Gardner WK, Ellington A, Hopkins DG, Bakker AC (1992) Tile and mole drainage for control of waterlogging in duplex soils of south-eastern Australia. Australian Journal of Experimental Agriculture 32, 865–878.
| Tile and mole drainage for control of waterlogging in duplex soils of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
McDonald GK, Gardner WK (1987) Effect of waterlogging on the grain yield response of wheat to sowing date in south-western Victoria. Australian Journal of Experimental Agriculture 27, 661–670.
| Effect of waterlogging on the grain yield response of wheat to sowing date in south-western Victoria.Crossref | GoogleScholarGoogle Scholar |
Merino P, Menendez S, Pinto M, Gonzalez-Murua C, Estavillo JM (2005) 3,4-Dimethylpyrazole phosphate reduces N2O emissions from grassland after slurry application. Soil Use and Management 21, 53–57.
| 3,4-Dimethylpyrazole phosphate reduces N2O emissions from grassland after slurry application.Crossref | GoogleScholarGoogle Scholar |
Mosier AR, Halvorsen AD, Reule CA, Lui XJ (2006) Net global warming potential and greenhouse gas intensity in irrigated cropping systems in Northeastern Colorado. Journal of Environmental Quality 35, 1584–1598.
| Net global warming potential and greenhouse gas intensity in irrigated cropping systems in Northeastern Colorado.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xns1Cku70%3D&md5=86c855d92a120c59ebcfcb09d3bc380bCAS | 16825479PubMed |
Officer SJ, Phillips F, Kearney G, Armstrong RD, Graham J, Partington DL (2015) Response of soil nitrous oxide flux to nitrogen fertiliser application and legume rotation in a semi-arid climate, identified by smoothing spline models. Soil Research 53, 227–241.
| Response of soil nitrous oxide flux to nitrogen fertiliser application and legume rotation in a semi-arid climate, identified by smoothing spline models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXosFGntL0%3D&md5=1f110bcfecd3b8de81e4f80e9df39fffCAS |
Passioura JB (1977) Grain yield, harvest index and water use of wheat. The Journal of the Australian Institute of Agricultural Science 43, 117–120.
Passioura JB (1992) Overview of the processes limiting crop production on duplex soils. Australian Journal of Experimental Agriculture 32, 987–990.
| Overview of the processes limiting crop production on duplex soils.Crossref | GoogleScholarGoogle Scholar |
Pfab H, Palmer I, Buegger F, Fiedler S, Muller T, Ruser R (2012) Influence of a nitrification inhibitor and placed N fertilization on N2O fluxes from a vegetable cropped loamy soil. Agriculture, Ecosystems & Environment 150, 91–101.
| Influence of a nitrification inhibitor and placed N fertilization on N2O fluxes from a vegetable cropped loamy soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsVCksb8%3D&md5=21f8aeda4dd0f41f8a925bb293d83492CAS |
Rayment GE, Higginson FR (1992) Ion exchange properties. In ‘Australian laboratory handbook of soil and water chemical methods’. (Inkata Press: Melbourne)
Riffkin P, Potter T, Kearney G (2012) Yield performance of late-maturing winter canola (Brassica napus L.) types in the High Rainfall Zone of southern Australia. Crop and Pasture Science 63, 17–32.
| Yield performance of late-maturing winter canola (Brassica napus L.) types in the High Rainfall Zone of southern Australia.Crossref | GoogleScholarGoogle Scholar |
Robertson D, Zhang H, Palta JA, Colmer T, Turner NC (2009) Waterlogging affects the growth, development of tillers, and yield of wheat through a severe, but transient, N deficiency. Crop and Pasture Science 60, 578–586.
| Waterlogging affects the growth, development of tillers, and yield of wheat through a severe, but transient, N deficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntFequr8%3D&md5=645b2447be2ab2d2b83b59164e83b47eCAS |
Rovira AD (1994) The effect of farming practices on the soil biota. In ‘Soil biota: management in sustainable farming systems’. (Eds CE Pankhurst, DM Double, VVSR Gupta, PR Grace). (CSIRO: Australia)
Samson MI, Buresh RI, Dalta SK (1990) Evolution and soil entrapment of nitrogen gases formed by denitrification in flooded soil. Soil Science and Plant Nutrition 36, 299–307.
| Evolution and soil entrapment of nitrogen gases formed by denitrification in flooded soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXlvFShu7k%3D&md5=58df0404ec6c3a003f6480d3b88d3f48CAS |
Scheer C, Wassmann R, Kienzler K, Ibragimov N, Eschanov R (2008) Nitrous oxide emissions from fertilized, irrigated cotton (Gossypium hirsutum L.) in the Aral Sea Basin, Uzbekistan: Influence of nitrogen applications and irrigation practices. Soil Biology & Biochemistry 40, 290–301.
| Nitrous oxide emissions from fertilized, irrigated cotton (Gossypium hirsutum L.) in the Aral Sea Basin, Uzbekistan: Influence of nitrogen applications and irrigation practices.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlajs7bN&md5=632f84bcfab0a6dead24d4cc75a89447CAS |
Scheer C, Grace P, Rowlings D, Payero J (2013) Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management. Nutrient Cycling in Agroecosystems 95, 43–56.
| Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitVOjsr8%3D&md5=428d497322a79f52965f9aeb6e3e7a96CAS |
Schwenke GD, Herridge DF, Scheer C, Rowlings DW, Haigh BM, McMullen KG (2015) Soil N2O emissions under N2-fixing legumes and N-fertilised canola: A reappraisal of emission factor calculations. Agriculture, Ecosystems & Environment 202, 232–242.
| Soil N2O emissions under N2-fixing legumes and N-fertilised canola: A reappraisal of emission factor calculations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtVOgsL4%3D&md5=5e74db16ebc6b862a9ac74b9a0396a34CAS |
Searle PL (1984) The Bertholet or indophenol reaction and its use in the analytical chemistry of Nitrogen. Analyst (London) 109, 549–568.
| The Bertholet or indophenol reaction and its use in the analytical chemistry of Nitrogen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXlsVartbk%3D&md5=d7a93d0d133c8567acf4e496090c6b3aCAS |
Snyder CS, Bruullsema TW, Jensen TL (2007) ‘Greenhouse gas emissions from cropping systems and the influence of fertilizer management—a literature review.’ (International Plant Nutrition Institute: Norcross, GA, USA)
Soares JR, Cantarella H, de Campos Menegale M (2012) Ammonia volatilisation losses from surface-applied urea with urease and nitrification inhibitors. Soil Biology & Biochemistry 52, 82–89.
| Ammonia volatilisation losses from surface-applied urea with urease and nitrification inhibitors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XosVagsrc%3D&md5=a32a8f4e9d338fc6b015642a2945548fCAS |
Terman GL (1979) Yields and protein content of wheat grain as affected by cultivar, N, and environmental growth factors. Agronomy Journal 71, 437–440.
| Yields and protein content of wheat grain as affected by cultivar, N, and environmental growth factors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXktlyit7c%3D&md5=132bee09c905ba419eb15316d4467897CAS |
Van Groenigen JW, Velthof GL, Oenema O, Van Groenigen KJ, Van Kessel C (2010) Towards an agronomic assessment of N2O emissions: a case study for arable crops. European Journal of Soil Science 61, 903–913.
| Towards an agronomic assessment of N2O emissions: a case study for arable crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXks1Gr&md5=eeb4cb3f453a9f9bab1a9285109f287cCAS |
van Herwaarden AF, Angus JF, Richards RA, Farquhar GD (1998a) ‘Haying off’, the negative grain yield response of dryland wheat to nitrogen fertiliser II. Carbohydrate and protein dynamics. Australian Journal of Agricultural Research 49, 1083–1093.
| ‘Haying off’, the negative grain yield response of dryland wheat to nitrogen fertiliser II. Carbohydrate and protein dynamics.Crossref | GoogleScholarGoogle Scholar |
van Herwaarden AF, Farquhar GD, Angus JF, Richards RA, Howe GN (1998b) ‘Haying off’, the negative grain yield response of dryland wheat to nitrogen fertiliser I. Biomass, grain yield and water use. Australian Journal of Agricultural Research 49, 1067–1081.
| ‘Haying off’, the negative grain yield response of dryland wheat to nitrogen fertiliser I. Biomass, grain yield and water use.Crossref | GoogleScholarGoogle Scholar |
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science 37, 29–38.
| An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaA2cXitlGmug%3D%3D&md5=228f3c4b336b2a210f0fcda1e493832aCAS |
Watson ER, Lapins P, Barron RJW (1976) Effect of waterlogging on the growth, grain yield and straw yield of wheat, barley and oats. Australian Journal of Experimental Agriculture and Animal Husbandry 16, 114–122.
| Effect of waterlogging on the growth, grain yield and straw yield of wheat, barley and oats.Crossref | GoogleScholarGoogle Scholar |
Weiske A, Benckiser G, Herbert T, Ottow JCG (2001) Influence of the nitrification inhibitor 3,4-dimethylppyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments. Biology and Fertility of Soils 34, 109–117.
| Influence of the nitrification inhibitor 3,4-dimethylppyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltlyqtL4%3D&md5=b2d586c1d498dafda647d5eaea7be43cCAS |
Yin SX, Chen D, Chen LM, Edis R (2002) Dissimilatory nitrate reduction to ammonium and responsible microorganisms in two Chinese and Australian paddy soils. Soil Biology & Biochemistry 34, 1131–1137.
| Dissimilatory nitrate reduction to ammonium and responsible microorganisms in two Chinese and Australian paddy soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xlt1Oitro%3D&md5=6a782619fa5a1922605e5374d86c3e4cCAS |
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals Weed Research 14, 415–421.
| A decimal code for the growth stages of cerealsCrossref | GoogleScholarGoogle Scholar |
Zerulla W, Barth T, Dressel J, von Locquenghien KEKH, Pasda G, Rädle M, Wissemeier AH (2001) 3,4-Dimethlypyrazole phosphate (DMPP) a new nitrification inhibitor for agriculture and horticulture. Biology and Fertility of Soils 34, 79–84.
| 3,4-Dimethlypyrazole phosphate (DMPP) a new nitrification inhibitor for agriculture and horticulture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltlyqt7g%3D&md5=a15ee234ef5ce9013521b5fc5b89aeccCAS |