Nitrous oxide emission and fertiliser nitrogen efficiency in a tropical sugarcane cropping system applied with different formulations of urea
Weijin Wang A B E , Glen Park C , Steven Reeves A , Megan Zahmel C , Marijke Heenan A and Barry Salter DA Department of Science, Information Technology and Innovation, GPO Box 5078, Brisbane, Qld 4001, Australia.
B Environmental Futures Research Institute, Griffith University, Nathan, Qld 4111, Australia.
C Sugar Research Australia, Ingham, PO Box 135, Qld 4850, Australia.
D Sugar Research Australia, Te Kowai, Qld 4741, Australia.
E Corresponding author. Email: weijin.wang@qld.gov.au
Soil Research 54(5) 572-584 https://doi.org/10.1071/SR15314
Submitted: 28 October 2015 Accepted: 17 March 2016 Published: 11 July 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Nitrous oxide (N2O) emissions from sugarcane cropped soils are usually high compared with those from other arable lands. Nitrogen-efficient management strategies are needed to mitigate N2O emissions from sugarcane farming whilst maintaining productivity and profitability. A year-long field experiment was conducted in wet tropical Australia to assess the efficacy of polymer-coated urea (PCU) and nitrification inhibitor (3,4-dimethylpyrazole phosphate)-coated urea (NICU). Emissions of N2O were measured using manual and automatic gas sampling chambers in combination. The nitrogen (N) release from PCU continued for >5–6 months, and lower soil NO3– contents were recorded for ≥ 3 months in the NICU treatments compared with the conventional urea treatments. The annual cumulative N2O emissions were high, amounting to 11.4–18.2 kg N2O-N ha–1. In contrast to findings in most other cropping systems, there were no significant differences in annual N2O emissions between treatments with different urea formulations and application rates (0, 100 and 140 kg N ha–1). Daily variation in N2O emissions at this site was driven predominantly by rainfall. Urea formulations did not significantly affect sugarcane or sugar yield at the same N application rate. Decreasing fertiliser application rate from the recommended 140 kg N ha–1 to 100 kg N ha–1 led to a decrease in sugar yield by 1.3 t ha–1 and 2.2 t ha–1 for the conventional urea and PCU treatments, respectively, but no yield loss occurred for the NICU treatment. Crop N uptake also declined at the reduced N application rate with conventional urea, but not with the PCU and NICU. These results demonstrated that substituting NICU for conventional urea may substantially decrease fertiliser N application from the normal recommended rates whilst causing no yield loss or N deficiency to the crop. Further studies are required to investigate the optimal integrated fertiliser management strategies for sugarcane production, particularly choice of products and application time and rates, in relation to site and seasonal conditions.
Additional keywords: controlled-release fertiliser, DMPP, greenhouse gas, N2O, nitrification inhibitor, urea.
Introduction
The efficiency of fertiliser nitrogen (N) use by sugarcane (Saccharum officinarum L.) crops is generally low, with ~40–60% of the applied N lost from the plant–soil system (Vallis et al. 1996; Prasertsak et al. 2002). Given that N fertiliser is mostly applied below the soil surface (8–10 cm) on Australian sugarcane farms, ammonia volatilisation is no longer considered a major issue. However, N losses through denitrification, leaching and runoff are still of great concern as sugarcane farms are mostly located in subtropical or tropical regions with high rainfall (>1200 mm year–1) or irrigation.
Excessive fertiliser N loss not only represents an economic cost to farmers but also introduces large quantities of reactive N compounds into the environment, which can further alter natural N cycles imposing great pressure on ecosystem health and contributing to climate change (Galloway et al. 2003). For example, nitrous oxide (N2O), which can be produced from fertiliser N during the nitrification and denitrification processes in soil, is a powerful greenhouse gas with a global warming potential 298 times that of carbon dioxide on an equivalent mass basis (IPCC 2007). Agriculture is responsible for ~74% and 80% of the anthropogenic N2O emissions in Australia and the world, respectively (IPCC 2014; Department of Environment 2014; FAO 2015). Previous studies show that annual N2O emissions from Australian sugarcane cropping soils were high, mostly in the range of 3–15 kg N2O-N ha–1 year–1 with the emission factors of fertiliser N (EF = % of fertiliser N lost as N2O) generally between 1.3% and 4.5% (Allen et al. 2010; Wang et al. 2012; Wang et al. 2015; Wang et al. 2016). Extraordinarily high N2O emissions (28.2 kg N2O-N ha–1 year–1 measured with manual chambers and 45.9 kg N2O-N ha–1 year–1 using a micrometeorological method) were observed from a low-lying acid sulfate soil containing 9.8% organic carbon (C) on a sugarcane farm in northern New South Wales, Australia (Denmead et al. 2010; Wang et al. 2016). Of the limited number of year-long measurements in sugarcane fields in other countries, 1.9 and 4.5 kg N2O-N ha–1 year–1 (EF = 0.69% and 2.03%) were recorded in Brazil where 120 kg N ha–1 was applied as urea and ammonium nitrate, respectively (Carmo et al. 2013; Soares et al. 2015). The higher N2O emissions from Australian sugarcane cropping systems may be attributed to higher fertiliser N application rates (mostly ≥140 kg N ha–1), higher rainfall or retention of crop residues (trash) compared with those in Brazil. With increasing concerns over anthropogenic greenhouse gas emissions and climate change, development of N-efficient farming strategies with decreased N2O emissions and sustained productivity is important for the sugar industry.
Many studies have demonstrated that ‘enhanced efficiency fertilisers’, including slow-release formulations and ammonium (NH4+)-based fertilisers containing nitrification inhibitors, can substantially increase crop yield and/or fertiliser N efficiency or decrease N losses, such as N2O emissions (Chen et al. 2008; Akiyama et al. 2010). Of the slow-release techniques, polymer-coating is relatively new and does not cause a substantial reduction in N content of the product. Polymer-coated fertiliser relies on a physical barrier around the fertiliser prills to control the release of N to the surrounding soil, with the potential to better match crop N demand (Timilsena et al. 2014). The release rate can be manipulated by changing characteristics of the coating, including polymer type and thickness. Of the nitrification inhibitors that have gained commercial importance, DMPP (3,4-dimethylpyrazole phosphate) offers several advantages including high efficiency, low application rate and low toxicity (Zerulla et al. 2001).
Efficacy of the ‘enhanced efficiency fertilisers’ varies with the fertiliser formulation, soil properties, climatic conditions and management practices (Akiyama et al. 2010). In the wet, tropical sugarcane growing region of Australia, fertiliser N is more susceptible to losses by denitrification, leaching and runoff than many other areas due to the high seasonal rainfall. Recent studies in this region by Di Bella et al. (2013) demonstrated that polymer- and sulfur-coated urea increased sugarcane/sugar yield at the same N application rate and maintained productivity at substantially decreased application rates compared to conventional urea. However, there have been no year-long measurements of N2O emissions in the wet tropics of Australia and the impacts of the ‘enhanced efficiency fertilisers’ on annual N2O emissions remain unknown. The major objectives of this study were to: (i) measure annual N2O emissions under normal management practices to provide data for the national greenhouse gas inventory; and (ii) investigate the efficacy of polymer- or DMPP-coated urea for mitigating N2O emissions, increasing fertiliser N use efficiency and enhancing or maintaining productivity at a decreased fertiliser application rate.
Materials and methods
Experimental site
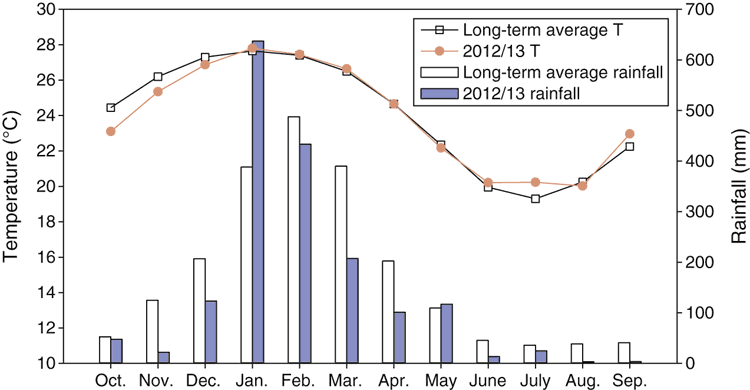
The experiment was conducted at Ingham in north Queensland, Australia (18°42ʹ43ʹʹS, 146°08ʹ48ʹʹE). This region has a tropical monsoon climate. Long-term (1968–2013) annual mean temperature is 24.0°C, with the lowest monthly mean temperature in July (19.3°C) and the highest in January (27.7°C). Mean annual rainfall is 2110 mm, with ~60% received between January and March (Bureau of Meteorology, Australia; Station 032078, 7 km north of the experimental site). An on-site weather station (Campbell Scientific Australia Pty Ltd, Qld, Australia) was installed during the experiment to record rainfall and air temperature. The rain gauge did not function properly from 1 February to 12 May 2013. As a consequence, the rainfall data for this period were drawn from the weather station as described above. The accumulative rainfall during the 365-day gas measurement period (5 October 2012 to 4 October 2013) was 1717 mm, and the observed monthly rainfall and temperature in comparison to the long-term averages are shown in Fig. 1.
|
The soil was classified as a Redoxic Hydrosol (Isbell 2002) or Oxygleyic Gleysol (IUSS Working Group WRB 2014) and had a silty, clay loam layer at 0–25 cm, underlain by a silty clay layer at 25–60 cm depth (Table 1). The crop was the first ratoon, i.e. regrown from the stool of a crop (cv. Q208 ) planted in the previous year. ‘Green cane trash blanketing’, a practice where the cane is harvested green and the trash (crop residues) is spread over the ground, has been practiced on this farm since 1985. The crop was initially planted in late August 2011 with a row spacing of 165 cm and harvested on 27 September 2012, with a low fresh cane yield of ~55 t ha–1 due to poor seasonal conditions. Based on the relationship: trash dry matter = 0.046 × fresh cane yield + 5.84 (r2 = 0.20; n = 325) and the average N content of 0.64% in the dry matter of trash (N. Halpin, pers. comm.), the cane trash present on the ground at the commencement of this experiment was estimated to be ~8.4 t dry matter equivalent per ha, containing 54 kg N ha–1.
) planted in the previous year. ‘Green cane trash blanketing’, a practice where the cane is harvested green and the trash (crop residues) is spread over the ground, has been practiced on this farm since 1985. The crop was initially planted in late August 2011 with a row spacing of 165 cm and harvested on 27 September 2012, with a low fresh cane yield of ~55 t ha–1 due to poor seasonal conditions. Based on the relationship: trash dry matter = 0.046 × fresh cane yield + 5.84 (r2 = 0.20; n = 325) and the average N content of 0.64% in the dry matter of trash (N. Halpin, pers. comm.), the cane trash present on the ground at the commencement of this experiment was estimated to be ~8.4 t dry matter equivalent per ha, containing 54 kg N ha–1.

|
Seven treatments were applied to compare polymer- or DMPP-coated urea with conventional urea as follows:
-
0N: Control, nil N fertiliser applied
-
140N_U: Conventional urea applied at 140 kg N ha–1 (recommended rate)
-
140N_PCU: Polymer-coated urea (Incitec Pivot Fertilisers, Australia) at 140 kg N ha–1
-
140N_NICU: Nitrification inhibitor DMPP-coated urea (Entec®) at 140 kg N ha–1
-
100N_U: Conventional urea at 100 kg N ha–1 (suboptimal rate; not measured for N2O emissions)
-
100N_PCU: Polymer-coated urea at 100 kg N ha–1
-
100N_NICU: Nitrification inhibitor DMPP-coated urea at 100 kg N ha–1.
Inclusion of the 100 kg N ha–1 treatments was based on the assumption that the potential of the PCU or NICU fertiliser to improve crop N use efficiency and yield should be more evident at a suboptimal N application rate. The treatments were arranged in a randomised block design with four replicates. The plots were 20 m long and 8.4 m wide containing five crop rows, each planted in the middle of a ~1 m-wide bed. The blocks were separated with a 1 m-wide buffer zone. Nitrogen fertiliser was applied on 4 October 2012 at ~8 cm below the soil surface in slits cut in the middle of the sugarcane crop rows – a practice known as ‘stool-splitting’. The crop was harvested on 6 August 2013.
Measurement of greenhouse gas fluxes
Nitrous oxide emissions were measured for all treatments except treatment 5 (100N_U) using a combination of manual and automatic gas sampling chambers. The manual chambers (Wang et al. 2011) consisted of a square stainless steel base (50 cm W × 50 cm L × 15 cm H) and a cover box with aluminium frames covered with white plastic panels (50 cm L × 50 cm W × 55 cm H). The cover box was fitted with a sampling outlet in the middle of the top panel, a mini fan inside the top panel for mixing the air, and a closed-cell foam seal at the bottom. Two chamber bases were installed in each plot with one covering across the bed centre and another covering one side of the bed shoulder and half of the furrow area, inserted to a depth of 10–15 cm in the soil (Fig. 2). Sugarcane crops inside the chambers in the middle of the beds were cut to <50 cm when needed to ensure proper closure of the chambers during gas sampling. The chamber bases were occasionally relocated to minimise the impact of chambers on soil moisture and crop growth and to obtain better spatial representation. To measure greenhouse gas fluxes between soil and the atmosphere, the chambers were closed by clamping the cover boxes on to the bases for 1–1.5 h between 0900 and 1130 hours. Gas samples were taken with a 50 mL syringe at the beginning and end of the enclosure period, and occasionally in the interim to check the linearity of gas concentration increase with time during the enclosure. The gas samples were injected into pre-evacuated glass vials (Exetainer, Labco Ltd, High Wycombe, UK) for storage before analysis. The cover boxes were removed from the bases immediately after the gas sampling to minimise potential microclimatic modification of the sampling area. Gas samples were taken approximately two to three times per week during the high emission periods (e.g. following substantial rainfall) and less frequently at low emission times (e.g. dry periods). Gas sampling with the manual chamber method concluded at sugarcane harvest. The gas samples were analysed in the laboratory with a gas chromatograph (Varian CP-3800, Varian Inc., Middelburgh, The Netherlands) for N2O concentrations as described by Wang et al. (2011).
Automatic gas sampling chambers were used to measure greenhouse gas fluxes at a sub-daily frequency (10 samplings per day). Limited by the number of chambers (nine in total) and the length of gas sampling lines, the automatic chambers were installed only for treatments 1 (0N) and 2 (140N_U) with four and five chambers per treatment, respectively. This was expected to give more temporally representative data for estimating the N2O emission factor of fertiliser N under normal fertilisation practices. Each automatic chamber (Wang et al. 2011) consisted of a stainless steel base that was identical to the manual chamber base, an extension (30 cm high) and a cover box (30 cm high) with stainless steel frames and two lids on the top panel that could be opened and closed automatically at pre-set intervals. Placement and management of the chamber bases were similar to those described above for the manual chambers. Air samples were automatically extracted from the headspace of the chamber into a gas chromatograph fitted with ECD and FID detectors (SRI 8610C, SRI Instruments, CA, USA) that analysed the concentrations of N2O and CH4 simultaneously (Wang et al. 2011). Measurements with the automatic chambers continued after sugarcane harvest until 4 October 2013.
Determination of soil mineral N contents
Soil samples were taken seven times during the cropping season from the centre of the sugarcane bed (fertilisation band) and the bed shoulder/furrow areas at 0–10 and 10–30 cm depths in all plots except those of treatment 5 (100N_U). Soil samples were also taken to a depth of 1 m (divided into 0–10, 10–30, 30–60 and 60–100 cm depths) at pre-fertilisation and post-harvest. Three samples were taken from each location, bulked and immediately air-dried at 40°C in a ventilated oven, before being ground and sieved to <2 mm. Soil mineral N content, including NH4+ and NO2– + NO3– (collectively referred to as NO3– hereafter), in air-dried samples was determined using 2 M KCl extraction and colourimetric techniques (Rayment and Lyons 2010). Gravimetric soil water content was determined by oven-drying at 105°C for >24 h. Soil mineral N contents were expressed on a dry mass basis.
Other measurements
Sugarcane yield was measured by harvesting the entire middle row (20 m) with a plot harvester and truck that contained a bin mounted on load cells. An additional 5-m section was manually harvested from an adjacent row, and the millable cane, and leaf and cabbage (immature stalk) were separated, weighed, cut into small pieces and sub-sampled. Dry matter contents of the fresh cane, and leaf and cabbage were determined by placing a portion of each sample in an oven at 60°C for >48 h. Total N content was determined using the Kjeldahl digestion and colorimetric method (Rayment and Lyons 2010). Total N uptake of the millable cane, and leaf and cabbage were calculated by multiplying the N content in the plant samples by the dry biomass yield. Total aboveground N uptake was the sum of the total N uptake in the millable cane, and leaf and cabbage.
Soil bulk density (BD) was measured separately for the bed and furrow areas using stainless steel corers with a diameter of 10 cm for the 0–10, 10–20 and 20–30 cm depths and smaller steel corers (5 cm dia.) for the 30–60 and 60–100 cm depths. Soil temperature at 5 cm and volumetric moisture content at 7–13 cm below the soil surface were measured with in situ probes and recorded with a data logger (ThetaProbe, Delta-T Devices Ltd, UK). The soil moisture probe readings were calibrated against the volumetric soil moisture contents determined from the gravimetric measurements and bulk densities.
Data processing and statistical analysis
Hourly emission rates during the chamber closure period were calculated from the increase of gas concentration in the headspace (Wang et al. 2011). Daily emission rates for the automatic chamber measurements were estimated by multiplying the averages of all hourly emission rates for that day by 24. Daily emission rates for the manual chamber measurements were estimated by multiplying the hourly emission rates measured between 0900 and 1130 hours by 24 without correction for diurnal fluctuations because the automatic chamber measurements indicated that N2O emission rates measured during this time (E9 a.m.–12 a.m.) were generally close to the daily averages (Edaily = 1.027 E9 a.m.–12 a.m., r2 = 0.92, n = 340). The daily emission rates between the days of manual chamber measurements were estimated by linear interpolation. In rare cases where a major rainfall event occurred following a prolonged (>1 week) dry period after a gas sampling, the emission rates measured before the rainfall were used for the dry period.
The water-filled pore space (WFPS) in soil was calculated from the volumetric water contents (%, v/v), BD (g cm–3) and a particle density of 2.65 g cm–3 as follows in Eqn 1:

All statistical analyses were performed using Genstat V.14 (VSN International Ltd, UK). Prior to analysis of variance (ANOVA), data were tested for normal distribution and log-transformed where appropriate. Differences and interactions among treatments were assessed using the ANOVA procedure and Duncan’s multiple range test at P < 0.05. To quantify the relationships of daily N2O emission rates to possible driving factors, stepwise regression analyses were conducted using the automatic chamber measurements against soil NH4+, NO3– and total mineral N (NH4+ + NO3) contents, soil temperature at 5 cm and WFPS at 7–13 cm depth in the 0N and 140N_U treatments. The soil NH4+ and NO3– contents between the two closest consecutive soil sampling days were estimated by linear interpolation.
Results
Dynamics of soil mineral nitrogen
Soil NH4+ and NO3– contents (0–30 cm depth) were consistently low (≤5 mg N kg–1) in the bed shoulder and furrow area throughout the cropping season, with no significant differences between treatments (Fig. 3a, c, e). The NH4+ contents in the bed centre of the 140N_U treatment increased dramatically in the three weeks following fertilisation and remained high (>95 mg N kg–1) for about two and a half months until early January 2013 (Fig. 3b). In contrast, soil NO3– contents increased steadily and slowly after fertilisation until early January 2013 and then declined substantially following several high rainfall events (Fig. 3d). The NICU treatments had consistently lower NO3– contents in the fertiliser band than the other fertilised treatments in the first three to four months. The PCU treatments had lower NH4+ concentrations during the early stage of the experiment compared with other fertilised treatments, consistent with the expected slow-release feature of this fertiliser. After the high rainfall events in January and February 2013, NH4+ concentrations in the PCU treatments exceeded those in other fertilised treatments. Soil mineral N contents in the bed of the unfertilised control treatment remained in the range of 8–13 mg N kg–1, significantly lower than those in the fertilised treatments during the first four months after fertiliser application.
Dynamics of N2O emissions in relation to key driving factors
All high N2O emission spikes occurred following major rainfall events, but the magnitude of daily emissions were not always proportional to the amount of rainfall received. The N2O emissions generally remained low during the initial two and a half months with low rainfall (Fig. 4), in spite of high soil mineral N contents in the fertilised area (Fig. 2). The manual chamber measurements showed no significant differences in the N2O emission rates between different treatments, but the automatic chambers recorded higher emissions for the fertilised treatment than the unfertilised control during this low rainfall period (P < 0.05). High N2O emissions (>200 g N2O-N ha–1 day–1) occurred following high rainfall events in late December 2012 and the highest daily emissions (>500 g N2O-N/ha–1 day–1) were observed immediately after 140 mm of rainfall on 1 January 2013. The high rainfall events in late January 2013 accelerated N2O emissions again but at lower magnitudes compared with the previous emission spikes. From February to April 2013, several moderate emission peaks were recorded following rainfall events. Nitrous oxide emissions remained low during the last two to three months of the cropping season and in the two months following harvest when rainfall was consistently low.
The automatic chamber measurements in the 0N and 140N_U treatments demonstrated that daily N2O emissions greater than 100 g N ha–1 day–1 occurred only when the soil WFPS in the 7–13 cm depth was >55% and soil temperature at 5 cm was >27°C, irrespective of the application of fertiliser and position in a plot (Fig. 5). Stepwise regression analyses of daily N2O emission rates against possible driving factors indicated that soil WFPS (%) and temperature (°C) together accounted for 66% and 59% of the variation in the logarithm values of N2O emission rates (g N ha–1 day–1) in the bed centre (Eqn 2) and the bed shoulder and furrow areas (Eqn 3), respectively:
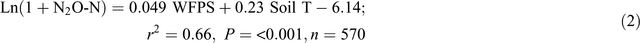
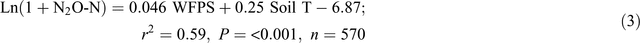
Adding soil mineral N contents (Nmin = NH4+ + NO3–, mg N kg–1) into the equations improved the prediction of N2O emissions from the bed centre area (Eqn 4) but not from the bed shoulder and furrow areas (Eqn 5), where soil mineral N contents varied little over time:
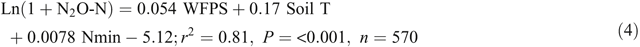
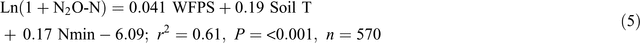
Note that Nmin in Eqn 4 refers to soil mineral N concentrations (0–30 cm) in the middle of beds and thus is only a relative indication rather than an average of the enclosed area. The temperature effects were also evident from diurnal variations in N2O emissions of the automatic chamber measurements, with a general sinusoidal trend being highest in the afternoon and lowest from night to early morning during the high emission days (Reeves et al. 2016).
Annual cumulative N2O emissions
Cumulative N2O emissions during the 365 days were 14.0 and 18.2 kg N ha–1 for the unfertilised (0N) and conventionally fertilised (140N_U) treatments, respectively, based on the automatic chamber measurements (Fig. 6a). The difference in the annual N2O emissions between these two treatments, which was not statistically different at P < 0.05, was mainly due to higher emissions for the fertilised treatment from October to mid-December 2012. During this low rainfall period, no substantial N2O emissions were recorded from the manual chamber samplings (Fig. 6b). The annual cumulative N2O emissions measured with the manual chambers were 13.6 and 13.2 kg N ha–1 for the 0N and 140N_U treatments, respectively, and ranged between 11.4 and 16.1 kg N ha–1 for all treatments (after adding 0.07 kg N ha–1 for the period between 4 August and 4 October 2013, based on the automatic chamber measurements). The manual chamber measurements showed no significant differences in the annual N2O emissions between different N fertiliser application rates (0, 100 and 140 kg N ha–1) or between conventional urea, PCU and NICU (Fig. 6b). Using the average of annual N2O emissions measured with automatic and manual chambers for the 0N and 140N_U treatments, the emission factor of fertiliser N was 1.36% under the normal fertilisation practice.
Sugar yield, crop N uptake and N balance
The sugar yield of this first ratoon crop ranged from 9.0 to 13.3 t ha–1 (Table 2), and was significantly greater for the fertilised treatments than the unfertilised control. There were no significant differences in the sugar yield between different urea formulations at the recommended N application rate of 140 kg N ha–1. A decrease in the fertiliser N application rate from 140 kg N ha–1 to 100 kg N ha–1 decreased sugar yield by 1.3 t ha–1 and 2.2 t ha–1 for the conventional urea (P > 0.05) and PCU (P < 0.05) treatments, respectively, but not for the NICU treatment. The efficiency of fertiliser N (sugar yield increase per unit of N applied) for conventional urea, PCU and NICU was 27, 31 and 22 kg sugar/kg N at 140 kg N ha–1, and 25, 21 and 34 kg sugar/kg N at 100 kg N ha–1, respectively.
Substantial amounts of fertiliser N were unrecovered in the 0–100 cm soil depth and the aboveground plant biomass. Total N uptake in the aboveground biomass was ~80–81 kg N ha–1 at harvest for treatments receiving 140 kg N ha–1, regardless of the fertiliser formulations (Table 2). Decreasing fertiliser application rate from 140 kg N ha–1 to 100 kg N ha–1 decreased the crop N uptake by 21% (17 kg N ha–1) when conventional urea was used. However, use of PCU or NICU at 100 kg N ha–1 did not significantly affect the cumulative crop N uptake compared with that at 140 kg N ha–1. A positive N balance of 55 kg N ha–1 was recorded for the 0N treatment, representing a substantial N input from non-fertiliser N sources (mainly N mineralisation from soil organic matter and possibly cane trash) during the crop growing season. All the fertilised treatments had negative N balance values. Assuming that the amount of N absorption into the roots and the amount of N release from the roots (living or dead) were similar, the negative N balance values demonstrate N losses from the soil-crop system. Also assuming that the crop N uptake from the non-fertiliser sources was the same for the unfertilised and fertilised treatments, the amount of fertiliser N unaccounted for in soil (0–100 cm) and plants was equivalent to 88, 70 and 73 kg N ha–1 for the 100 kg fertiliser N ha–1 applied as conventional urea, PCU and NICU, respectively. At the recommended N application rate of 140 kg N ha–1, 108–118 kg N ha–1 was unrecovered, significantly greater than that at the lower N application rate. Overall, only 12–30% of the fertiliser N could be accounted for in the soil and aboveground biomass, with the highest percentage recovery achieved under the 100N_PCU treatment (P > 0.05; Table 2).
Discussion
Processes and factors affecting soil mineral N dynamics and N2O emissions
The prolonged dominance of NH4+ over NO3– following application of conventional urea (Fig. 3b, d) demonstrated a slow nitrification process in this soil. High ratios of NH4+ : NO3– were also noted previously in tropical Queensland soils under sugarcane (Meier et al. 2006), but the cause has not been fully explained. In an incubation experiment, Meier et al. (2006) could not replicate the low nitrification rates that were observed in the field, and therefore concluded that instead of an inherent low nitrification capacity, increased losses of NO3– via leaching or denitrification caused the disparity between NH4+ and NO3– contents. However, the initial dominant soil NH4+ concentrations during the two and a half months after fertilisation in the present study occurred in a relatively dry period (soil WFPS <55%) with limited rainfall. Thus, substantial NO3– losses via leaching or denitrification were unlikely during this time. The slow nitrification might be attributable to the ongoing inhibitory effects of low pH (5.06–5.33) in the top 20 cm of soil (Morrill and Dawson 1967; Sahrawat 2008). In the subsequent two months (24 December 2012 to 1 March 2013) when heavy rainfall and the majority of high N2O emission episodes occurred, substantial loss of N was most likely to have occurred as a result of leaching or denitrification. This juxtaposition of a dry and low nitrifying period with slow NO3– accumulation followed by a wet and high NO3– loss environment together might have contributed to the continued dominance of NH4+ over NO3– observed in this environment.
In spite of the large differences in soil mineral N content between the 0N and 140N_U treatments, the lack of consistent and substantial differences in N2O emissions between these treatments during the high emission periods, as demonstrated consistently by both automatic and manual chamber measurements (Fig. 4c, d), suggested that mineral N availability was not the primary driving factor of N2O production in this cropping system. This differed from previous observations in other Australian sugarcane cropping systems where N fertiliser application significantly increased N2O emissions from soil (Allen et al. 2010; Wang et al. 2012; Wang et al. 2016). The dry weather conditions during the initial two and a half months of the cropping season (Fig. 1) most likely favoured soil mineral N accumulation before the wet season by limiting crop growth and N uptake, as well as preventing marked N losses from soil. The fact that all high N2O emission spikes occurred following high rainfall events (Fig. 4) suggested that soil water content was the key driving factor of daily variations in N2O emissions. This conclusion is supported by Eqns 2–5, which suggest that soil water and temperature were more important than soil mineral N contents in regulating N2O emissions at this site. The similar N2O emissions in the 0N and 140N_U treatments also indicated that mineral N supply from non-fertiliser sources, such as those carried over from the previous season and mineralisation of soil organic matter and cane trash in the current season, should have supplied sufficient substrates relative to the N2O production capacity through denitrification and/or nitrification under the particular climatic conditions.
Very high daily N2O emissions (>500 g N ha–1 day–1) like those in early January 2013 (Fig. 4c, d) have also been observed in other sugarcane cropping systems in Australia (Allen et al. 2010; Denmead et al. 2010; Wang et al. 2012; Wang et al. 2016) but seldom recorded in subtropical cereal or cotton cropping systems even after similar rainfall or irrigation events (Wang et al. 2011; Scheer et al. 2013). The concurrence of the warm and wet soil conditions in sugarcane fields in the months after N fertiliser application around the summer season are conducive to high N2O emissions. In addition, the large amounts of cane trash retained on the soil surface were found to increase N2O, most likely by acting as a source of biologically available C and N and maintenance of wet soil conditions due to reduced evaporative water loss (Wang et al. 2016). Furthermore, poor drainage (e.g. >40% clay in subsoil at the present site) and low pH (increasing N2O:N2 ratios in the denitrification products), which are common for sugarcane cropping soils, may also have contributed to the observed high N2O emissions.
Automatic versus manual chamber measurements
The automatic chambers recorded higher annual N2O emissions (18.2 kg N ha–1 on average) for the 140N_U treatment but similar magnitudes (14.0 kg N ha–1 on average) for the 0N treatment, compared with the manual chamber measurements (13.2 and 13.6 kg N ha–1, respectively). Higher annual N2O emissions were also obtained with automatic chambers than manual chambers in a N-fertilised canola crop, while similar results were recorded with the different methods in a parallel, unfertilised chickpea crop (Schwenke et al. 2015). During the first two and half months in the present study (Fig. 6a, b), the automatic chambers might have decreased the soil water evaporation inside the enclosed area due to restricted air flow. As a result, the wetter soil conditions inside the automatic chambers could have promoted N2O emissions from nitrification and/or denitrification of the fertiliser N. In addition, the automatic chambers could cause uneven rainwater distribution in the enclosed area due to blockage of raindrops by the vertical panels and top lids. During and after heavy rainfall, the chambers could block lateral rainwater movement, resulting in more frequent and prolonged waterlogged conditions than the ambient area. Relocation of the chambers from time to time can alleviate, but unlikely eliminate, such chamber impacts on soil conditions. In comparison, these problems could be largely overcome by the manual chambers because the chamber bases were short and the cover boxes were removed after gas sampling. In addition, an automatic sampling system usually has only a limited number of chambers (generally ~12), which restricts the number of treatments and replicates included for measurement. This is particularly true for sugarcane cropping systems where the plants are usually grown on raised beds with wide row spacing and fertiliser applied in bands. In such circumstances, more chambers are needed for each plot to cover the different positions, compared with uniform farming systems such as pasture. Because of the lower setup costs, manual sampling chambers can usually be deployed in much larger numbers and thus allow more treatments to be included in a study with more replicates, minimising the impacts of spatial variability. However, the intermittent measurements with manual chambers can potentially under-represent the diurnal and daily variations in N2O emissions, and thus result in over- or underestimation of daily and annual emissions, particularly when sampling at an inappropriate time of day and with inadequate sampling frequency over a season. A temporal analysis of the automatic chamber measurements in the present study suggested that weekly gas sampling plus bi-weekly and/or tri-weekly sampling for 1–2 weeks after >20 mm rainfalls should yield annual cumulative N2O emissions within ± 10% of the sub-daily measurements (Reeves et al. 2016). Taking into account the above advantages and disadvantages of the two methods, the large spatial variability in N2O emissions (Fig. 4) and the similar annual N2O emissions for the 0N treatment measured with the two gas sampling techniques (Fig. 6), we consider that the manual chamber method, with the sampling frequencies used in this study, provided robust and satisfactory data for comparing different fertilisation treatments and remains a useful tool for measuring N2O emissions in sugarcane cropping systems.
High annual N2O emissions and low fertiliser N recovery
The annual cumulative N2O emissions of 13.2 kg N ha–1 (with manual chambers) and 18.2 kg N ha–1 (with automatic chambers) for the 140N_U treatment were comparable to those (12.3–15.3 kg N ha–1) observed in a sugarcane cropping system applied with 150 kg N ha–1 in central Queensland, Australia (Wang et al. 2012). Substantially higher annual emissions (28–46 kg N ha–1) were recorded on a sugarcane farm with high organic C content (9.8%) in northern New South Wales, Australia (Denmead et al. 2010; Wang et al. 2016). Although the N2O emissions can usually account for only part of the unrecovered N, they demonstrate the potential of possible gaseous N loss as N2, which is generally released simultaneously with N2O during denitrification, often in greater magnitudes than N2O (Weier 1999). In addition to the gaseous N losses, the low fertiliser N recoveries were attributable to other possible N loss pathways, such as deep leaching and lateral runoff resulting from high rainfall events.
Fertiliser N management remains a challenging topic for the Australian sugar industry. The sugarcane yield of 76.6 t ha–1 under the 140N_U treatment was relatively low in comparison to historical records, most likely due to the dry seasonal conditions from November to December 2012 and from March to April 2013 (Fig. 1). The dry weather conditions might also have negatively affected crop N uptake. Nonetheless, the low percentage of fertiliser N that could be accounted for in the aboveground biomass and soil mineral N pool (e.g. ~23% for the 140N_U treatment) was also observed in other sugarcane cropping systems (Halpin et al. 2013). Although part of the unrecovered fertiliser N might have been transformed into sugarcane roots, soil organic matter or below the 100 cm soil depth, these low N recoveries highlighted the continued need to develop more efficient N management strategies for sugarcane farming in Australia. Among many possible strategies, avoiding excessive fertiliser N application generally provides an efficient and effective means of decreasing fertiliser N loss. The lower aboveground N uptake and fertiliser N recovery for the 100N_U treatment compared with the 140N_U treatment (Table 2) could be due to high fertiliser N loss from leaching, runoff or denitrification over the wet summer period leaving lower proportions of fertiliser N for crop use at the lower N application rate. Thus, climatic conditions, particularly rainfall quantity and pattern, are important factors in determining the optimum fertiliser application rates for different regions and years.
Efficacy of polymer-coated urea
Both decreased and increased N2O emissions from the use of PCU compared with conventional urea have been observed in other sugarcane cropping systems (Wang et al. 2016). In the present study, soil mineral N dynamics in the PCU treatments displayed a delayed release pattern, with soil mineral N concentration initially increasing more slowly and then remaining at higher levels during the late cropping season, compared with the conventional 140N_U treatment (Fig. 3b, d). However, the delayed release of N from PCU was not translated into lower or higher N2O emissions compared with the conventional urea treatments, again demonstrating that N2O emission rates were not sensitive to variation in mineral N contents in this specific cropping system. Zebarth et al. (2012) also found no significant difference in annual cumulative N2O emissions between PCU and conventional fertiliser. Polymer-coated urea would more likely reduce N2O emissions if applied at a lower rate, without causing a loss in crop yield. However, this was not observed in this study, possibly due to soil mineral N concentration being less important among all the driving factors for N2O production. The divergent effects of PCU on N2O emissions may be related to the interactive relationships between its N release dynamics, crop N uptake and other N losses as a function of fertiliser formulation, application method (e.g. timing and rate), soil properties (e.g. drainage), and rainfall quantity and distribution patterns.
The application of PCU did not significantly increase sugarcane or sugar yield at either the recommended rate or the suboptimal rate compared with conventional urea (Table 2). In contrast, Di Bella et al. (2013) reported higher sugarcane yields using PCU compared with conventional urea at the same N application rate and a similar yield using PCU at a half the application rate of conventional urea. Yield increases have also been observed when using PCU in other cropping systems (Chen et al. 2008). The contrasting results showed that the efficacy of PCU for yield increase varied with the specific circumstances.
Decreasing the application rate of PCU (unlike conventional urea) from 140 kg N ha–1 to 100 kg N ha–1 did not decrease crop N uptake and, as a consequence, reduced the amount of fertiliser N unrecovered in the soil mineral N and aboveground biomass by ~40 kg N ha–1 (Table 2). In addition, the apparent fertiliser N recovery at 100 kg N ha–1 tended to be higher for PCU than conventional urea but this was not significant (Table 2; P > 0.05). The similar crop N uptake but lower cane yield for 100N_PCU compared with 140N_PCU, and the higher crop N uptake but similar cane yield for 100N_PCU compared with 100N_U might be a result of: (i) inadequate N supply owing to the slow release of N from the PCU at 100 kg N ha–1 restricting crop growth during the early stages; and (ii) sustained N supply from PCU during the late crop growing season (Fig. 3b) increasing the N concentration in the plant biomass under 100N_PCU (3.3 g N kg–1; weighted average) due to the lower biomass compared with that under 140N_PCU (2.7 g N kg–1) and 100N_U (2.6 g N kg–1). The improved fertiliser N recovery under 100N_PCU demonstrated the potential of PCU to meet crop N demand at a substantially lower rate than the current recommendation, but improved PCU management techniques (e.g. optimal application time and combined use with conventional urea) are required to ensure sugar yield is maintained.
As the majority of sugarcane crop N uptake occurs in the first six months following planting or ratooning (Wood et al. 1996), surplus soil mineral N during the late stage of the cropping season would be susceptible to losses through denitrification, leaching or runoff if heavy rainfall is received. Therefore, it appears important to select an appropriate PCU formulation that best matches the N release pattern to plant N demand whilst avoiding excessive N accumulation before the wet season or after the peak N uptake stage. Alternatively, a custom blend of conventional urea with PCU might offer a solution to meet the N requirements by the crops during the early fast-growing season whilst reducing the fertiliser cost by using less PCU.
Efficacy of nitrification inhibitor-coated urea
Despite the slower nitrification process in the NICU treatments, especially in the first three months following fertilisation (Fig. 3d), the capability of the nitrification inhibitor to mitigate N2O emissions was not evident. DMPP is most effective in the initial weeks after application (Zerulla et al. 2001). However, emissions during this period were low for all treatments in the present study, most likely due to the dry soil conditions. The less important role of soil NH4+ and NO3– contents in the regulation of N2O production in this soil could also have contributed to the ineffectiveness of NICU to mitigate N2O emissions.
The capacity of NICU to abate N2O emissions has been proven in numerous studies, with decreases in N2O emissions between 37–77% in various cropping systems (Liu et al. 2013; De Antoni Migliorati et al. 2014; Scheer et al. 2014; Lam et al. 2015). A field study on a sugarcane farm in Central Queensland, Australia showed that DMPP-coated urea decreased post-fertilisation N2O emissions by ~44% (Wang et al. 2012), while a decrease of ~38% in N2O emissions from the fertilised area was recorded in another Australian sugarcane cropping system (Wang et al. 2016). Studies in a Brazilian sugarcane cropping system found that addition of DMPP to urea reduced N2O emissions by 94–100% in two consecutive cropping seasons (Soares et al. 2015). These results suggest that the use of DMPP can be an effective N2O mitigation technique in many circumstances.
Another potential benefit of nitrification inhibitors is to maintain or increase yield at the same or decreased fertilisation rate. A meta-analysis by Abalos et al. (2014) showed an overall increase in crop yield and N use efficiency of 7.5% and 12.9%, respectively. The higher plant N uptake under the 100N_NICU treatment than the 100N_U treatment (P > 0.05; Table 2) suggested that the nitrification inhibitor might increase the availability of fertiliser N to plants. This could be due to reduced NO3– leaching during the high rainfall period in January 2013 (Fig. 3f) as gaseous N losses, indicated by N2O emissions were not affected by the urea formulations. It might also be partly attributable to the preference of NH4+ over NO3– by sugarcane crops (Robinson et al. 2011). The increased N uptake could have contributed to the increase in sugarcane yield for the 100N_NICU treatment compared with the 100N_U treatment. However, no significant increases in yield and crop N uptake were found at 140 kg N ha–1 possibly because N was not a limiting factor when applied at this rate.
The similar sugarcane/sugar yields and aboveground plant N uptakes between the recommended and the suboptimal fertiliser application rates (Table 2) with NICU demonstrated the potential of fertiliser N reduction without sacrificing crop yield. These results are in agreement with previously reported observations in other cropping systems (Zerulla et al. 2001; Chen et al. 2008). Further research is required to investigate appropriate decreases in fertiliser N if NICU is used, particularly in relation to soil properties, environmental conditions and other management practices.
Conclusions
Annual N2O emissions from soil under conventional N fertilisation (140N_U) were high in this sugarcane cropping system, amounting to 13.2 kg N ha–1 when measured with manual chambers or 18.2 kg N ha–1 with the automatic chambers. High rainfall was the triggering factor for large daily N2O emissions (>200 g N ha–1 day–1), but other factors, including concurrence of warm and wet weather conditions, presence of abundant crop residues on the soil surface, poor drainage, moderately low soil pH and high availability of soil mineral N, might also have interactively contributed to the high cumulative N2O emissions observed. In contrast to most previous observations, different N fertiliser formulations (urea, PCU and NICU) or application rates (0, 100 and 140 kg N ha–1) had no significant impacts on the annual cumulative N2O emissions at this site, demonstrating the lesser importance of fertiliser N among the possible driving factors.
PCU did not significantly increase sugarcane or sugar yield compared with conventional urea at the same N application rates. In addition, the sugarcane or sugar yield decreased significantly at the suboptimal N application rate (100N_PCU) compared with the recommended rate (140N_PCU). In spite of these, PCU displayed the capacity to maintain crop N uptake at a substantially reduced application rate (100 kg N ha–1) compared with the normal application rate (140 kg N ha–1), and enhance the aboveground plant N uptake and reduce fertiliser N loss at 100 kg N ha–1 compared with conventional urea. Selecting an appropriate PCU formulation and blending with conventional urea to better match the fertiliser N supply to plant N demand may provide an effective and economic management strategy for PCU fertilisers.
Application of NICU resulted in lower soil NO3– concentrations for at least three months compared to conventional urea. However, substitution of NICU for urea at the normal application rate (140 kg N ha–1) did not significantly increase sugarcane or sugar yield. Decreasing NICU application from 140 kg N ha–1 to 100 kg N ha–1 did not affect sugar yield and crop N uptake, offering a saving of 40 kg fertiliser N ha–1 without yield loss. The efficacy of NICU and the extent of reduction in the application rate in relation to the N-supplying capacity of soil and the climatic conditions need to be investigated further.
Acknowledgements
This project was supported by funding from the Australian Government through the Department of Agriculture, Fisheries and Forestry. The authors would like to thank Mr V. Blanco for providing the experimental site. We are grateful to colleagues of the Chemistry Centre, DSITI, for the analyses of soil and plant samples. We sincerely thank the anonymous reviewers for their constructive comments which greatly improved the quality of this paper.
References
Abalos D, Jeffery S, Sanz-Cobena A, Guardia G, Vallejo A (2014) Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agriculture, Ecosystems & Environment 189, 136–144.| Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXnslGqs7Y%3D&md5=05c0ff60ecf5bb5ce2cf3b91bc7194b9CAS |
Akiyama H, Yan X, Yagi K (2010) Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis. Global Change Biology 16, 1837–1846.
| Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Allen DE, Kingston G, Rennenberg H, Dalal RC, Schmidt S (2010) Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils. Agriculture, Ecosystems & Environment 136, 209–217.
| Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFentb8%3D&md5=3d8ca5935f97bbe807b7a0c7de966c8dCAS |
Carmo JBD, Filoso S, Zotelli LC, De Sousa Neto ER, Pitombo LM, Duarte-Neto PJ, Vargas VP, Andrade CA, Gava GJC, Rossetto R, Cantarella H, Neto AE, Martinelli LA (2013) Infield greenhouse gas emissions from sugarcane soils in Brazil: Effects from synthetic and organic fertilizer application and crop trash accumulation. GCB Bioenergy 5, 267–280.
| Infield greenhouse gas emissions from sugarcane soils in Brazil: Effects from synthetic and organic fertilizer application and crop trash accumulation.Crossref | GoogleScholarGoogle Scholar |
Chen D, Suter H, Islam A, Edis R, Freney JR, Walker CN (2008) Prospects of improving efficiency of fertiliser nitrogen in Australian agriculture: a review of enhanced efficiency fertilisers. Australian Journal of Soil Research 46, 289–301.
| Prospects of improving efficiency of fertiliser nitrogen in Australian agriculture: a review of enhanced efficiency fertilisers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXns1OktLw%3D&md5=fec61f7dd15fe4ea2a2bc5ea7113200cCAS |
De Antoni Migliorati M, Scheer C, Grace PR, Rowlings DW, Bell M, McGree J (2014) Influence of different nitrogen rates and DMPP nitrification inhibitor on annual N2O emissions from a subtropical wheat–maize cropping system. Agriculture, Ecosystems & Environment 186, 33–43.
| Influence of different nitrogen rates and DMPP nitrification inhibitor on annual N2O emissions from a subtropical wheat–maize cropping system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmsVCjtbo%3D&md5=0844cd1e106bc3431f689d097fcc0b36CAS |
Denmead OT, Macdonald BCT, Bryant G, Naylor T, Wilson S, Griffith DWT, Wang WJ, Salter B, White I, Moody PW (2010) Emissions of methane and nitrous oxide from Australian sugarcane soils. Agricultural and Forest Meteorology 150, 748–756.
| Emissions of methane and nitrous oxide from Australian sugarcane soils.Crossref | GoogleScholarGoogle Scholar |
Department of Environment (2014) ‘National Inventory Report 2012, Volume 1.’ (The Commonwealth of Australia: Canberra)
Di Bella LP, Stacey SP, Benson A, Royle A, Holzberger G (2013) An assessment of controlled release fertiliser in the Herbert cane growing region. International Sugar Journal 115, 784–788.
FAO (2015) FAOSTAT Online Database. Available at http://faostat3.fao.org/compare/E
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. Bioscience 53, 341–356.
| The nitrogen cascade.Crossref | GoogleScholarGoogle Scholar |
Halpin NV, Bell MJ, Rehbein WE, Short KS (2013) The impact of combinations of trash management and tillage on grain legume productivity and subsequent sugarcane productivity in the Bundaberg/Childers district. In ‘Proceedings of the Australian Society of Sugar Cane Technologists’. (Ed. RC Bruce) p. 9. (Australian Society of Sugar Cane Technologists Ltd: Mackay, Qld) Available at https://www.assct.com.au/media/pdfs/2013-Ag38-Halpin.pdf [verified 31 May 2016]
IPCC (2007) ‘Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change.’ (Cambridge University Press: Cambridge, UK and New York, USA)
IPCC (2014) Climate Change 2014: Synthesis Report, Summary for Policymakers. In ‘IPCC AR5 Synthesis Report’. p. 35. (IPCC: Geneva, Switzerland)
Isbell RF (2002) ‘Australian soil classification.’ Revised Edition. (CSIRO Publishing: Melbourne)
IUSS Working Group WRB (2014) World Reference Base for Soil Resources 2014. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Report No. 106. (FAO: Rome)
Lam SK, Suter H, Davies R, Bai M, Sun J, Chen D (2015) Measurement and mitigation of nitrous oxide emissions from a high nitrogen input vegetable system. Scientific Reports 5, 8208.
| Measurement and mitigation of nitrous oxide emissions from a high nitrogen input vegetable system.Crossref | GoogleScholarGoogle Scholar | 25644694PubMed |
Liu C, Wang K, Zheng X (2013) Effects of nitrification inhibitors (DCD and DMPP) on nitrous oxide emission, crop yield and nitrogen uptake in a wheat-maize cropping system. Biogeosciences 10, 2427–2437.
| Effects of nitrification inhibitors (DCD and DMPP) on nitrous oxide emission, crop yield and nitrogen uptake in a wheat-maize cropping system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXltlGktLg%3D&md5=7ae6f766dbe0c2a108f66906ecb09266CAS |
Meier EA, Thorburn PJ, Probert ME (2006) Occurrence and simulation of nitrification in two contrasting sugarcane soils from the australian wet tropics. Australian Journal of Soil Research 44, 1–9.
| Occurrence and simulation of nitrification in two contrasting sugarcane soils from the australian wet tropics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlWjsr8%3D&md5=c819dc6010aa33371a24bc412fc4c52aCAS |
Morrill LG, Dawson JE (1967) Patterns observed for the oxidation of ammonium to nitrate by soil organisms. Soil Science Society of America Proceedings 31, 757–760.
| Patterns observed for the oxidation of ammonium to nitrate by soil organisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1cXnslalsQ%3D%3D&md5=d53d4160f471306f3f07369ffe1b9f78CAS |
Prasertsak P, Denmead OT, Freney JR, Saffigna PG, Prove BG, Reghenzani JR (2002) Effect of fertilizer placement on nitrogen loss from sugarcane in tropical Queensland. Nutrient Cycling in Agroecosystems 62, 229–239.
| Effect of fertilizer placement on nitrogen loss from sugarcane in tropical Queensland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XovFShtr8%3D&md5=f81a8d22102f35716013fd51cb096062CAS |
Rayment GE, Lyons DJ (2010) ‘Soil chemical methods – Australasia.’ (CSIRO Publishing: Melbourne)
Reeves S, Wang WJ, Salter B, Halpin N (2016) Quantifying nitrous oxide emissions from sugarcane cropping systems: Optimum sampling time and frequency. Atmospheric Environment 136, 123–133.
| Quantifying nitrous oxide emissions from sugarcane cropping systems: Optimum sampling time and frequency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xms1Ort74%3D&md5=ab4d434396a229500a31cd7c5ab4f54bCAS |
Robinson N, Brackin R, Vinall K, Soper F, Holst J, Gamage H, Paungfoo-Lonhienne C, Lakshmanan P, Schmidt S (2011) Nitrate paradigm does not hold up for sugarcane. PLoS One 6, e19045.
| Nitrate paradigm does not hold up for sugarcane.Crossref | GoogleScholarGoogle Scholar | 21552564PubMed |
Sahrawat KL (2008) Factors affecting nitrification in soils. Communications in Soil Science and Plant Analysis 39, 1436–1446.
| Factors affecting nitrification in soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXltFyitbc%3D&md5=a30169d76ba6458124abc244bc4f7d56CAS |
Scheer C, Grace PR, Rowlings DW, Payero J (2013) Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management. Nutrient Cycling in Agroecosystems 95, 43–56.
| Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitVOjsr8%3D&md5=428d497322a79f52965f9aeb6e3e7a96CAS |
Scheer C, Rowlings DW, Firrel M, Deuter P, Morris S, Grace PR (2014) Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical Australia. Soil Biology & Biochemistry 77, 243–251.
| Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht12ntL3M&md5=ee89302220febfea18060a4a6d021cc2CAS |
Schwenke GD, Herridge DF, Scheer C, Rowlings DW, Haigh BM, McMullen KG (2015) Soil N2O emissions under N2-fixing legumes and N-fertilised canola: A reappraisal of emissions factor calculations. Agriculture, Ecosystems & Environment 202, 232–242.
| Soil N2O emissions under N2-fixing legumes and N-fertilised canola: A reappraisal of emissions factor calculations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtVOgsL4%3D&md5=5e74db16ebc6b862a9ac74b9a0396a34CAS |
Soares JR, Cantarella H, Vargas VP, Carmo JB, Martins AA, Sousa RM, Andrade CA (2015) Enhanced-efficiency fertilizers in nitrous oxide emissions from urea applied to sugarcane. Journal of Environmental Quality 44, 423–430.
| Enhanced-efficiency fertilizers in nitrous oxide emissions from urea applied to sugarcane.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXkvFSjs74%3D&md5=5c1fe7c445e5e38b52ccf9660ba2ecf9CAS | 26023961PubMed |
Timilsena YP, Adhikari R, Casey P, Muster T, Gill H, Adhikari B (2014) Enhanced efficiency fertilisers: A review of formulation and nutrient release patterns. Journal of the Science of Food and Agriculture 95, 1131–1142.
| Enhanced efficiency fertilisers: A review of formulation and nutrient release patterns.Crossref | GoogleScholarGoogle Scholar | 25043832PubMed |
Vallis I, Catchpoole VR, Hughes RM, Myers RJK, Ridge DR, Weir KL (1996) Recovery in plants and soils of 15N applied as subsurface bands of urea to sugarcane. Australian Journal of Agricultural Research 47, 355–370.
| Recovery in plants and soils of 15N applied as subsurface bands of urea to sugarcane.Crossref | GoogleScholarGoogle Scholar |
Wang WJ, Dalal RC, Reeves SH, Butterbach-Bahl K, Kiese R (2011) Greenhouse gas fluxes from an Australian subtropical cropland under long-term contrasting management regimes. Global Change Biology 17, 3089–3101.
| Greenhouse gas fluxes from an Australian subtropical cropland under long-term contrasting management regimes.Crossref | GoogleScholarGoogle Scholar |
Wang WJ, Salter B, Reeves SH, Brieffies TC, Perna J (2012) Nitrous oxide emissions from a sugarcane soil under different fallow and nitrogen fertiliser management regimes. In ‘Proceedings of the Australian Society of Sugar Cane Technologists’. p. 9 (Australian Society of Sugar Cane Technologists Ltd: Mackay, Qld) Available at https://www.assct.com.au/media/pdfs/Ag%2025%20Wang%20et%20al.pdf [verified 31 May 2016]
Wang WJ, Halpin NV, Reeves SH, Rehbein WE (2015) Soybean rotation and crop residue management to reduce nitrous oxide emissions from sugarcane soils. Proceedings of the Australian Society of Sugar Cane Technologists 37, 33–41.
Wang WJ, Reeves SH, Salter B, Moody PW, Dalal RC (2016) Effects of urea formulations, application rates and crop residue retention on N2O emissions from sugarcane fields in Australia. Agriculture, Ecosystems & Environment 216, 137–146.
| Effects of urea formulations, application rates and crop residue retention on N2O emissions from sugarcane fields in Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhs1GjsrvN&md5=2140ce5f0efc823ce2b1c0a3fcf4b389CAS |
Weier KL (1999) N2O and CH4 emission and CH4 consumption in a sugarcane soil after variation in nitrogen and water application. Soil Biology & Biochemistry 31, 1931–1941.
| N2O and CH4 emission and CH4 consumption in a sugarcane soil after variation in nitrogen and water application.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXntVOitb0%3D&md5=89a90fa50e7c1a682e5539149248b5aeCAS |
Wood AW, Muchow RC, Robertson MJ (1996) Growth of sugarcane under high input conditions in tropical Australia. III. Accumulation, partitioning and use of nitrogen. Field Crops Research 48, 223–233.
| Growth of sugarcane under high input conditions in tropical Australia. III. Accumulation, partitioning and use of nitrogen.Crossref | GoogleScholarGoogle Scholar |
Zebarth BJ, Snowdon E, Burton DL, Goyer C, Dowbenko R (2012) Controlled release fertilizer product effects on potato crop response and nitrous oxide emissions under rain-fed production on a medium-textured soil. Canadian Journal of Soil Science 92, 759–769.
| Controlled release fertilizer product effects on potato crop response and nitrous oxide emissions under rain-fed production on a medium-textured soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVensrzL&md5=d1d0de54d48afb0d4029b6de3c9f1c76CAS |
Zerulla W, Barth T, Dressel J, Erhardt K, Locquenghien KH, Pasda G, Rädle M, Wissemeier AH (2001) 3,4-Dimethylpyrazole phosphate (DMPP) – a new nitrification inhibitor for agriculture and horticulture: An introduction. Biology and Fertility of Soils 34, 79–84.
| 3,4-Dimethylpyrazole phosphate (DMPP) – a new nitrification inhibitor for agriculture and horticulture: An introduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltlyqt7g%3D&md5=a15ee234ef5ce9013521b5fc5b89aeccCAS |








