Influence of enhanced efficiency fertilisation techniques on nitrous oxide emissions and productivity response from urea in a temperate Australian ryegrass pasture
H. C. Suter A D , H. Sultana A , R. Davies B , C. Walker C and D. Chen AA Faculty of Veterinary and Agricultural Sciences, The University of Melbourne, Parkville, Vic. 3010, Australia.
B BASF Australia, Freshwater Place, Southbank, Vic. 3006, Australia.
C Incitec Pivot Fertilisers, PO Box 54, North Geelong, Vic. 3215, Australia.
D Corresponding author. Email: helencs@unimelb.edu.au
Soil Research 54(5) 523-532 https://doi.org/10.1071/SR15317
Submitted: 29 October 2015 Accepted: 17 March 2016 Published: 6 July 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
The effect of a nitrification inhibitor on nitrous oxide (N2O) emissions across seasons, the effect of a urease inhibitor and a fine particle spray (both targeting ammonia (NH3) loss) on N2O emissions, and the potential for productivity benefits and efficiencies by using these enhanced efficiency fertilisers (EEFs) were investigated in temperate pastures. The study compared three treatments over an eight month period (April to December 2010): (1) urea (U), (2) urea with a nitrification inhibitor (3,4-dimethylpyrazole phosphate) (DMPP), and (3) urea with a urease inhibitor (N-(n-butyl) thiophosphoric triamide (NBTPT)) (GU). In autumn, when NH3 loss was predicted to be high, the effect of urea applied as a fine particle spray (containing urea, NBTPT and gibberellic acid (10 g ha–1)) (FPA) on N2O emissions and productivity was determined.
N2O emissions from urea applied to pastures were low, and were larger in spring than autumn due to soil moisture and temperature. DMPP was an effective tool for mitigating N2O emissions, decreasing fertiliser-induced N2O emissions relative to urea by 76% over eight months. However, the urease inhibitor (NBTPT) (GU) increased N2O emissions from urea by 153% over eight months. FPA had no impact on N2O, but was only examined during periods of low emission (autumn). No significant biomass productivity, agronomic efficiency benefits, or improvements in apparent fertiliser recovery were observed with the DMPP and GU treatments. A significant biomass productivity benefit was observed with the FPA treatment 55 days after fertiliser was applied, most likely because of the gibberellic acid. The outcomes highlight that although DMPP effectively decreased N2O emissions it had no impact on biomass productivity compared with urea. The use of the GU increased N2O emissions by preserving NH3 in the soil. To avoid this a lower rate of N should be applied with the urease inhibitor.
Additional keywords: 3,4-dimethylpyrazolephosphate, fine particle spray, nitrification inhibitor, N-(n-butyl) thiophosphoric triamide, urease inhibitor.
Introduction
High nitrogen (N) fertiliser inputs, commonly as surface-applied granular urea, are typical in pasture-based dairy systems in Australia, resulting in low N use efficiency (NUE). Application of N fertilisers, such as urea, can lead to emissions of the greenhouse gas nitrous oxide (N2O). Fertiliser-induced N2O emissions from crops and grasslands total 0.9 million tonnes of N per year globally (IFA 2001). In Australia, agriculture represents 76% of the national total N2O emissions, with around 50% of this from mineral fertiliser application (DCCEE 2011). Enhanced efficiency fertilisers (EEFs), including urease inhibitors, nitrification inhibitors, fine particle sprays, and controlled-release fertilisers, have the potential to decrease N losses from agriculture by either altering the rate of N transformations or slowing the release of N from fertiliser granules (Chen et al. 2008).
Various EEFs have been developed to target particular N loss pathways (Chen et al. 2008). Urease inhibitors, such as N-(n-butyl) thiophosphoric triamide (NBTPT), slow urea hydrolysis and are designed to decrease ammonia (NH3) volatilisation from surface-applied granular urea. Nitrification inhibitors, such as 3,4-dimethylpyrazole phosphate (DMPP) and dicyandiamide (DCD), slow nitrification and are designed to decrease N loss as nitrate (NO3–), N2O, oxides of nitrogen (NOx) and dinitrogen (N2). Liquid or fine particle spray fertilisers are designed to decrease NH3 volatilisation and increase plant utilisation through enhanced foliar uptake (Dawar et al. 2012). The greatest benefit of the EEFs is expected in spring and autumn (urease inhibitor) and late winter–spring (nitrification inhibitor) when climatic conditions favour NH3 volatilisation and denitrification losses, respectively. However, when a loss pathway is decreased as a consequence of EEF use and the rate of applied N remains the same, there is a risk of increased loss from an alternate pathway unless the additional N is used by the plant.
The nitrification inhibitors DMPP and DCD have been reported to decrease N2O emissions in laboratory studies on Australian soils (DMPP) (Chen et al. 2010) and in field studies in pasture systems (DCD) (Di et al. 2007; Kelly et al. 2008; Di et al. 2010; Gilsanz et al. 2016). A recent review found them effective in temperate grassland-based agriculture (Li et al. 2013a). Rowlings et al. (2016) studied the impact of DMPP on NUE in sub-tropical pastures, but no studies have investigated its impact on N2O emissions and NUE in Australian temperate pasture systems to date. Although urease inhibitors are effective at decreasing NH3 volatilisation by slowing the rate of urea hydrolysis and reducing the risk of elevated pH that drives NH3 formation, their impact can be variable due to the influence of climatic conditions, soil type, and land use on NH3 volatilisation. A review of the literature shows that across a range of crops and pastures, use of a urease inhibitor led to a 20% to 88% decrease in NH3 volatilisation compared with urea (Watson et al. 1990; Rawluk et al. 2001; Turner et al. 2010; Suter et al. 2013). Due to the impacts on NH3 volatilisation, it is expected that N2O emissions may increase relative to urea because of greater N in the system, but some studies have reported decreases in N2O with the urease inhibitor (Zaman et al. 2009; Singh et al. 2013; Ding et al. 2014). Use of fine particle sprays and suspensions have been shown to decrease NH3 and N2O loss and increase productivity relative to urea when applied with and without urease or nitrification inhibitors (Di and Cameron 2006; Dawar et al. 2011). To develop effective strategies to mitigate N2O emissions and provide productivity benefits to facilitate adoption of these strategies in Australian pasture systems, greater knowledge of the impact of EEFs under field conditions is required.
This paper reports on a field experiment using EEFs in a ryegrass seed crop. Surface applications of granular urea (40 kg N ha–1) with and without EEFs (urease inhibitor NBTPT, nitrification inhibitor DMPP) were made regularly over an eight month period (autumn to summer) in 2010. A fine particle spray containing NBTPT and gibberellic acid was also used during autumn when expected NH3 loss is high. The impact of these amendments on soil mineral N, N2O emissions, and biomass production and N utilisation was determined.
Materials and method
Site details
The experiment was conducted at a ryegrass (Lolium perenne L.) seed crop site at Murroon in south-western Victoria, Australia (38°26ʹ10.18ʹʹS, 143°47ʹ34.57ʹʹE). Details of the site characteristics are described in Suter et al. (2013). Briefly, the Chromosol soil (Isbell 1996) has a topsoil (0–10 cm) with a silty loam texture (22% clay, 38% silt, 40% sand, from 0–25 cm depth), a pHCaCl2 of 4.6, 0.2% total N, 2.7% total C, a bulk density of 1.23 g cm–3, and a cation exchange capacity (CEC) of 4.98 cmol (+) kg soil−1. Initial mineral N content (0–10 cm) was 13.4 kg N ha–1 as ammonium (NH4+) and 0.84 kg N ha–1 as nitrate (NO3–). The soil porosity at 0–10 cm depth was 0.54. The site was used for ryegrass seed production and fenced off from sheep that grazed the site intermittently before (>3 weeks) establishing the experiment. The experiment was a replicated split block design of five blocks, separated by a 0.5 m buffer zone, with each treatment randomly assigned within each block to account for site spatial heterogeneity. Each treatment plot was 1 m × 2 m. The experiment commenced 12 April 2010 and finished 23 December 2010.
Local rainfall measured on site in 2010 was 803 mm. Climatic variables were measured on site with a weather station (model WXT510; Vaisala, Helsinki, Finland) until 3 October 2010 and afterwards from the two closest Bureau of Meteorology stations (Colac, 38.23°S, 143.79°E and Cape Otway, 38.86°S, 143.51°E) due to issues with the on-site weather station. Soil moisture and temperature were measured using capacitance probes (EnviroPro®) inserted vertically into the ground and logging at 10 cm intervals.
Treatments
Fertiliser (40 kg N ha–1) was applied six times from April to October 2010 on 12 April, 7 June, 16 July, 3 September, 27 September and 21 October. Treatments were as follows:
-
control (C) (no fertiliser)
-
granular urea (U) (46% urea-N)
-
granular urea with the urease inhibitor, NBTPT (GU) (Green Urea 14™ (45.8% urea-N with ‘Agrotain®’ @ 5 L t–1 urea))
-
granular urea with the nitrification inhibitor, DMPP (DMPP) (Urea with ENTEC (46% urea-N with 0.4% of DMPP per unit of ammonium-N))
-
urea applied as a fine particle spray containing NBTPT and gibberellic acid (FPA) (46% urea-N with ‘Agrotain’ @ 1 L t–1 of urea and gibberellic acid (10 g ha–1)). Treatment 5 was applied once on 12 April 2010 to target NH3 loss, which was expected to be high in autumn.
Soil mineral N
Three composite soil samples (0–10 cm depth) were collected from each plot using a corer (2.5 cm internal diameter) at regular intervals (~fortnightly), immediately dried (40°C) and sieved (<2 mm). Subsamples (20 g of 105°C dried soil equivalent) were extracted with 2 M KCl (1 : 5 soil solution), by shaking for 1 h, filtering though Whatman No. 42 filter papers and were analysed for NH4+-N, and NO3–-N using a SAN++ segmented flow analyser (Skalar Analytical B.V. 2005).
N2O emissions
Nitrous oxide (N2O) emissions were measured using manual chambers (23 cm diameter × 25 cm high) similar to those reported in Saggar et al. (2004). Open-topped chambers were inserted into the ground (5 cm) for the entire course of the experiment and were capped for 1 h during sample collection times. Gas flux measurements were collected at regular intervals (every second day for one week after fertilisation, and then weekly) from the capped chambers commencing between 1000 and 1200 hours. Three samples were collected at 0, 30 and 60 min after the chamber was capped. Collected samples (20 mL, injected into a 12 mL evacuated Exetainer®, (Labco Ltd, United Kingdom)) were analysed by gas chromatograph (Agilent 6890) using an electron capture detector (ECD) with a lower detection limit of 0.2 ± 0.02 μL L–1. Temperature was logged within the closed chamber (Tinytag Transit 2 TG-4080, Gemini Data Loggers). Soil moisture (ML2x theta probes (Delta-T Devices Ltd, Cambridge, UK)) and temperature (small portable temperature probe) were recorded at each sampling time.
From 3 August to 30 August 2010 free water was observed on the soil surface and gas samples were not collected during this time due to problems accessing the area and collecting samples without disturbing the soil and chambers. We hypothesised that any denitrification would be largely N2 during this time and N2O emissions would be minimal (Ciarlo et al. 2008). Recent work by Friedl et al. (2016) found that the N2/(N2+N2O) ratio increased with increasing soil moisture (to 100% water filled pore space, WFPS) in sub-tropical pastures, and Harris et al. (2013) hypothesised that this was the reason for low N2O emissions from cropping sites during periods when soil WFPS exceeded 90%.
Soil N2O flux (kg N ha–1 h–1) was calculated using the linear regression (LR) model recommended by Venterea et al. (2012) and extrapolated to a daily N2O emission. To compare treatment effects, cumulative emission was calculated by integration of the area under the daily flux curve for the period of measurement.
Biomass production
Biomass was measured on samples (2 m length × 0.4 m width; 0.8 m2 cut per plot) collected using a lawn mower to simulate grazing rotations typical of the area at 24–28 days after fertiliser (DAF) (autumn and spring) and 42–46 DAF (winter). Biomass samples were collected on 10 May (28 DAF), 7 June (55 DAF), 19 July (42 DAF), 3 September (46 DAF), 27 September (24 DAF), 21 October (24 DAF), and 23 December (63 DAF) in 2010. The harvest on 7 June 2010 was included to assess the longevity of the impact of the EEFs following a time of expected high ammonia loss. The harvest on 23 December 2010 was 63 DAF, as the pasture was grown to seed production stage, and included both the stem and grain. All other harvests were vegetative only. Biomass was removed from the entire experimental area when each harvest was collected. Pasture N content was determined by the Kjeldahl digestion method with colourimetric analysis using a Lachat 8500 Flow Injection Analyser after samples were dried at 70°C for 72 h (Rayment and Lyons 2011).
Statistics
Statistical analysis of treatment effects at each harvest was performed using the Fisher l.s.d. analysis of variance (ANOVA, P < 0.05) with Minitab17. In addition, significance of soil properties (mineral N, temperature and moisture), sample time and treatment, and the interaction between these factors, on daily N2O flux (log10 transformed) and dry matter production was assessed using the program RStudio, Version 0.97.248.
Results and discussion
Climatic conditions
Rainfall and temperature data (Fig. 1) showed an initially dry autumn followed by a wet, cool winter (August) and a warm, moist spring (October–November). Air temperature ranged from a low of 0°C to a maximum of 31°C. Soil moisture increased from 12 April 2010 through to late August when the site became saturated, before drying off towards the end of the year (Fig. 2). Rainfall during spring (Fig. 1) caused soil moisture contents to fluctuate between mid-September through to December. The measured volumetric soil moisture (ϴv) ranged from 10% (19% WFPS) to 53% (98% WFPS) at 10 cm depth, 13% to 58% at 20 cm depth and 13% to 56% at 30 cm depth during the study. The upper reported ϴv for each depth represents saturation.

|
Soil temperatures ranged from 4.7 to 28°C at 10 cm depth, 6.7 to 24°C at 20 cm depth and 8 to 22°C at 30 cm depth (Fig. 2), following a similar pattern to the ambient temperature (Fig. 1). There was a strong diurnal pattern of soil temperature with minimum temperature recorded between 0630 and 0900 hours and maximum between 1700 and 1800 hours.
Soil mineral N transformations
Soil ammonium (NH4+) levels fluctuated in response to fertiliser additions (Fig. 3), plant uptake and mineralisation. In spring, increased NH4+-N was observed in all treatments, including the control, indicating that the increased soil temperature and moist conditions (Fig. 2) stimulated mineralisation from the abundant organic matter pool (total N and C contents of 0.2 and 2.7% respectively) (Hu et al. 2014). There was no overall significant difference in NH4+-N between treatments. Slightly greater NH4+-N was measured in the DMPP treatment compared with U many times before 27 September 2010, most noticeably on 19 July 2010 (23.2 ± 10.0 kg N ha–1 for DMPP and 4.1 ± 0.2 kg N ha–1 for U) and 11 August 2010 (49.0 ± 20.6 kg N ha–1 for DMPP and 8.2 ± 1.1 kg N ha–1 for U) (P < 0.1) (Fig. 3a). This is expected as the inhibitor slows ammonification, and Fangueiro et al. (2009) found this inhibitory effect can occur for extended periods (e.g. 100 days for DMPP). In this study, the suppression of nitrification over the wetter months (July to September) when there is an increased risk of NO3– leaching and denitrification losses, indicates that the inhibitor could provide real benefits in decreasing gaseous emissions (N2O and N2) and increasing biomass production. This is reflected in the lower NO3–-N levels in the DMPP treatment during July and August (see below). Lower NH4+-N in the DMPP treatment after fertilisation on 27 September 2010 compared with U and GU was not expected but the reason for this is not clear.
Addition of NBTPT to urea (GU) increased the quantity of NH4+-N in the soil compared with U in autumn (19 April), early winter (5 July) and for most of spring (8 October, 21 October and 19 November). This results from decreased NH3 loss, leading to greater N remaining in the soil. A concurrent study of NH3 loss at the site found that using NBTPT decreased N loss as NH3 in autumn to 3.7 kg N from 12 kg N with urea (Suter et al. 2013). However, in July and September 2010 the higher rainfall and soil moisture (Figs 1 and 2) would lower the potential for NH3 loss from urea so no additional N would be ‘saved’ in the urease inhibitor treatment, indicating no benefit from using the urease inhibitor at this time.
Applying FPA once on 12 April 2010 did not alter soil NH4+-N levels compared with U, and the soil NH4+-N returned to baseline levels by 24 May 2010 (5.9 ± 0.6 kg N ha–1 compared with 5.3 ± 0.5 kg N ha–1 for C). Samples collected on 27 September 2010 showed all treatments had similar background levels of NH4+-N (1.9 ± 0.2 kg N ha–1 for C; between 2.2 ± 0.4 and 3.4 ± 0.2 kg N ha–1 for the fertiliser plots (Fig. 3a)).
Soil NO3–-N levels also fluctuated in response to applied fertiliser N (Fig. 3b) and were lower than NH4+-N. This indicates that NO3–-N was either: (1) being removed by the plant material, with NO3–-N the favoured form of N for plant uptake (Li et al. 2013b); or (2) was denitrified. Denitrification losses are expected to occur mostly over winter under anaerobic soil conditions (Butterbach-Bahl et al. 2013). In the early stages of the study, conditions were dry so biomass production was low (see Biomass section below), and plant uptake of NO3–-N would also be low. At this time the DMPP and FPA treatments decreased the amount of NO3–-N produced relative to U and GU thereby retaining N in the soil for subsequent plant uptake. In the FPA treatment, NO3–-N levels decreased to baseline levels one month after application, indicating plant uptake or immobilisation. The urease inhibitor (GU) increased NO3–-N levels compared with U throughout the study due to decreased NH3 loss, and this was significantly more than that observed in the C and DMPP treatments (P < 0.01). During spring when high biomass production occurred, NO3–-N did not appear to respond to fertiliser application due to plant uptake, which is supported by the lack of difference between the NH4+-N levels in the treatments at this time (Fig. 3).
N2O emissions
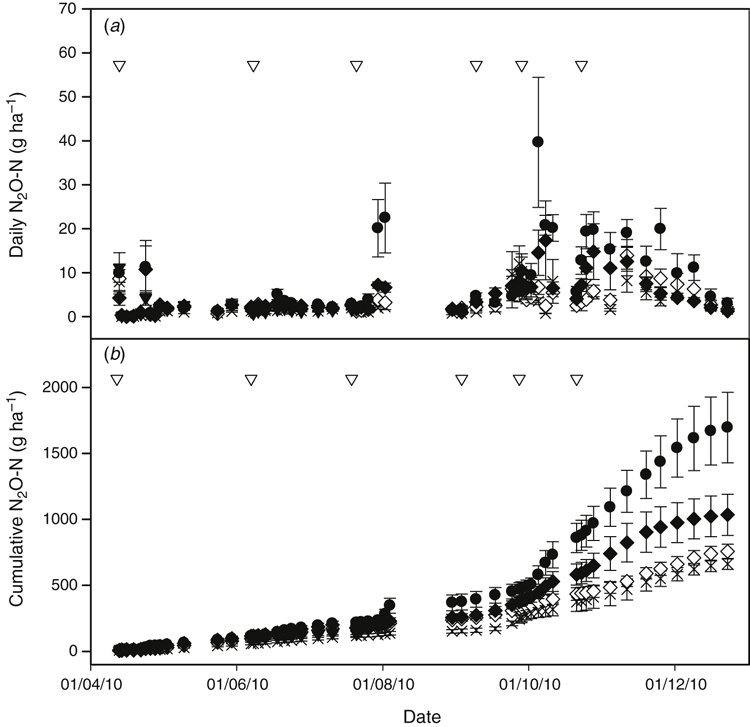
There was a significant relationship between daily N2O flux, day of sample collection, soil temperature, soil moisture, and the interaction between temperature and soil moisture, and temperature and day of sample collection, and GU (F(16, 251) = 36.65, P < 0.005). Daily N2O emissions were comparatively low throughout autumn and early winter (April to early August) and higher in spring (Fig. 4a), reflecting soil moisture and temperature conditions (Fig. 2). This trend (Figs 2 and 4) is expected based on our understanding of the drivers of N2O emissions (Rafique et al. 2012; Butterbach-Bahl et al. 2013; Huang et al. 2013; Bell et al. 2015). Nitrate, the product of nitrification and the substrate for denitrification, had a significant (P < 0.001) influence on daily N2O flux. The lower N2O emissions in autumn, despite NO3– being higher in all treatments (Fig. 3b) compared with the rest of the year, is due to lower soil moisture (Fig. 2), which limits denitrification.
|
Minimum and maximum daily N2O flux for each treatment was –0.28 to 24.56 g N2O-N ha–1 day–1 for C; –0.6 to 50.93 g N2O-N ha–1 day–1 for U; 0.07 to 94.51 g N2O-N ha–1 day–1 for GU; –1.89 to 23.56 g N2O-N ha–1 day–1 for DMPP; –0.28 to 15.95 g N2O-N ha–1 day–1 for FPA. The lowest N2O emission recorded occurred on 16 April 2010, four days after the first fertiliser application when soil moisture was low. For all but the FPA treatment (which was only used in autumn) the maximum N2O flux occurred during spring (September to November), seven or more days after fertiliser was applied. This was due to the time required for urea hydrolysis, nitrification and denitrification and the warm, moist conditions. Similar time delays in N2O production have been observed when cattle urine is added to soil (Bell et al. 2015).
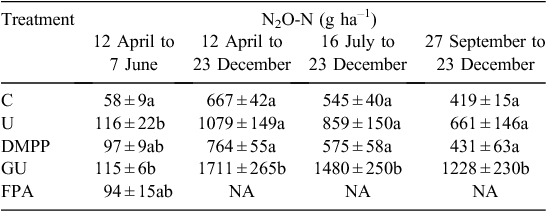
The cumulative N2O data showed that over eight months the emission of N2O from C and U were 0.6 and 1 kg N2O-N ha–1. This represents one tenth of the denitrification loss reported by Eckard et al. (2003) in temperate Australian pastures (6 and 13 kg N ha–1 year–1 (N2 and N2O combined) from control and urea (200 kg N ha–1 year–1) treatments). Although the N2O : N2 ratio can vary widely depending on soil type and environmental conditions (Saggar et al. 2013), our reported results provide a reasonable estimate of N2O emissions from temperate Australian pastures when compared with those of Eckard et al. (2003). The cumulative N2O data show that although the fertiliser treatments caused differences in N2O emissions relative to the control during the earlier part of the experiment (12 April to 7 June 2010) (Table 1), the absolute difference was small because of the low level of emissions occurring at that time (Fig. 4b) compared with the remainder of the year. At that time there was no significant difference in N2O emissions between fertiliser treatments (Table 1).
|
DMPP decreased fertiliser-induced N2O emissions by 76% during the study relative to U by decreasing NO3–-N production, the substrate for denitrification (Fig. 3). The impact of DMPP on N2O emissions was most noticeable during spring (September to November) when fertiliser-induced emissions with DMPP decreased by 95% (to 9 ± 63 g N2O-N ha–1) compared with U (240 ± 146 g N2O-N ha–1), with N2O essentially reaching background (control) levels (419 ± 146 g N2O-N ha–1 for C, 431 ± 64 g N2O-N ha–1 for DMPP) (Table 1, Fig. 4a). This occurred despite low soil NO3– levels for all treatments during spring (Fig. 3b), indicating increased plant uptake due to the spring growing conditions. The calculated reductions in N2O emissions with DMPP were similar to those reported elsewhere (Menéndez et al. 2009; Di and Cameron 2012; Misselbrook et al. 2014). The observed results indicate that DMPP is an effective N2O mitigation tool for temperate Australian pasture systems.
Addition of NBTPT (GU) resulted in a 153% increase in fertiliser-induced N2O emissions (1044 ± 265 g N2O-N ha–1) relative to U (412 ± 149 g N2O-N ha–1), with the greatest impact occurring from 16 July to 23 December 2010 (GU; 935 ± 250 g N2O-N ha–1, U; 314 ± 150 g N2O-N ha–1) (Table 1). Other studies have reported decreased N2O emissions with urease inhibitors (Dawar et al. 2011; Sanz-Cobena et al. 2012; Singh et al. 2013). From previous work, urease inhibitors can stimulate or decrease denitrification depending on the inhibitor type and concentration used (Yeomans and Bremner 1986; Zhengping et al. 1991). Zhengping et al. (1991) observed no inhibitory effect on denitrification with NBTPT. If the urease inhibitor prevents NH3 loss and the rate of N applied is the same as for urea, as is the case here (40 kg N ha–1), then there is more NH4+-N retained in the soil that can undergo nitrification and denitrification to produce N2O. The greatest impact of elevated emissions from the urease inhibitor occurred after the 16 July 2010 application. This was unexpected because in July NH3 loss was assumed to be low, based on winter climatic conditions, and in September the measured NH3 loss was low (Suter et al. (2013). So, the applied N remaining in the soil for the U and GU treatments should have been similar, but for GU the urea would have been released more slowly making the substrate for nitrification and N2O production available for longer compared with urea. The impact of GU on N2O, therefore, depends on the temporal synchronisation of the substrates for N2O production (NH4+ for nitrification and NO3– for denitrification) and conditions conducive for denitrification. This explains the differences reported here and in the literature (Ding et al. 2014). Whereas the mineral N data (Fig. 3) shows similar levels of NH4+-N for U and GU, there is a trend for greater NO3– with GU, supporting our hypothesis.
Biomass
Average biomass production responded to N application but, excluding spring, the response was slightly lower than that reported for long-term N studies in the same region (McKenzie et al. 2003) (Table 2). There was a significant relationship between dry matter production, date of sample collection, treatment, and the interaction between sample date and soil NH4+ levels and pasture dry matter (F(33, 116) = 45.35, P < 0.005). Total plant production increased and was significantly greater (P < 0.001) for the final harvest on 23 December 2010 (Table 2) because this contained both vegetative biomass and seed. Biomass production was significantly lower (P < 0.001) at the 7 June 2010 harvest as a result of the period between fertilisation and harvest (55 DAF) and the removal of part of the applied N in the first pasture harvest (10 May 2010, 28 DAF). Applying urea did not significantly increase biomass relative to the control at this harvest indicating insufficient N remaining in the soil (Fig. 3a, b). DMPP, GU and FPA significantly (P < 0.05) increased biomass production compared with C by an average of 81 ± 15 kg dry matter (DM) at this harvest. FPA significantly increased biomass relative to U at this harvest, which may be due to the presence of gibberillic acid (Biddiscombe et al. 1962). The greater response to the inhibitors in autumn, when conditions were drier, is similar to the results reported by Rowlings et al. (2016). The lack of a consistent productivity benefit with the nitrification inhibitor, despite the decrease in N2O emissions, was due to little N being lost via denitrification (measured as N2O), limited leaching in these texture contrast soils (Chromosols) (Isbell 1996), and the presence of sufficient N from fertilisation in all treatments.
The agronomic efficiency (kg pasture increase per kg N applied) and apparent recovery of N (net kg N taken up per kg N applied) were significantly (P < 0.05) greater in spring (September to October) and December than in autumn and winter, reflecting the seasonal pattern of pasture production for the region as reported by Suter et al. (2013) (Table 2). The lowest response to N occurred in July (average 7.7 ± 0.3 kg DM per kg of N for fertiliser treatments) where pasture was cut at 42 DAF (Table 2) due to low mineral N available for pasture growth (Fig. 3). There was a trend for increased agronomic efficiency and recovery of applied N with DMPP and GU relative to U for the May, June, July and 3 September harvests (Table 2), as well as for FPA in May and June. At other harvests this was not observed. The lack of significant difference in the agronomic efficiency and apparent recovery of N with the EEFs corresponds with the response seen in other studies (O’Connor et al. 2012; Misselbrook et al. 2014; Bell et al. 2015). This is most likely a result of a combination of sufficient N being available for pasture growth across all treatments and other factors (water, temperature, environment) limiting pasture production. This, plus the impact of management, was concluded as the main reason for variable responses in productivity and agronomic efficiency trials using the inhibitors in a meta-analysis by Abalos et al. (2014). Previous research has found productivity benefits from the use of urease and nitrification inhibitors, with the greatest response from the urease inhibitor and greater efficiency observed where lower N rates were used (30 kg N ha–1 compared with 60 kg N ha–1) (Zaman et al. 2013). For example, Watson et al. (1990) found that NBTPT increased both yield and N efficiency by 11%. Dawar et al. (2011) concluded that using a fine particle spray instead of granular urea and addition of NBTPT, both increased biomass production (by 27% and 38%, respectively) and N response efficiency (by 9 and 13 kg DM kg N–1, respectively) compared with granular urea. This is a much greater response than observed here, which may indicate the importance of season, with Dawar et al. (2011) applying the fertiliser in spring. The June harvest showed that GU, DMPP and FPA all gave a production benefit when the time since fertilisation was extended, by either providing greater mineral N due to decreased NH3 loss (GU and FPA) or retaining N in a form less prone to loss (DMPP) (Fig. 3). This reflects the ability of the inhibitors to work for extended periods (Fangueiro et al. 2009). However, the results reported are for a single event and due to the importance of environment and management on the performance of the EEFs (Abalos et al. 2014) extrapolation to different seasons and conditions is problematic.
Urease inhibitors target NH3 loss and greatest benefits from these products are expected under conditions where NH3 loss is high, i.e. moist, warm, windy conditions (Suter et al. 2013). No benefit from these products is expected during high rainfall periods, particularly when rain falls soon after fertiliser application, as NH3 loss is low. Conversely, for the nitrification inhibitors we would expect to see the greatest benefit at times when there is high leaching and denitrification losses, i.e. during the wetter months. Studies on corn (Zea mays L.) found variable yield responses with a nitrification inhibitor concluding that profitable yield benefits were only likely to occur under conditions where spring and summer rainfalls were greater (40%) than the long-term average (Kyveryga and Blackmer 2014). Another study reported that one inhibitor (urease) was effective in increasing yield but not the other (nitrification) in the studied cropping system (Kawakami et al. 2012). This indicates that the dominant loss pathways will differ between systems, years and seasons. A synopsis of pasture trials conducted on 132 paddocks in 37 farms across New Zealand found overall biomass benefits from the use of a nitrification inhibitor (DCD) (19% increase), with variation around this dependent upon the region (Carey et al. 2012). The authors of the New Zealand study point out that this benefit is greater than reported on previous individual small plot studies (Carey et al. 2012).
Conclusions
This study found that urea boosted pasture productivity in southern Australian rainfed pasture systems by between 0.7 and 18 kg DM (vegetative) per kg of N applied, with soil moisture and climate dictating the seasonal response to N. Overall, N2O emissions from applied fertiliser were low, but greater when conditions were conducive for denitrification (spring). Use of the nitrification inhibitor, DMPP, was consistently effective as a tool for mitigating N2O emissions from urea across all seasons (autumn, winter and spring) with a 76% reduction recorded over eight months. However, DMPP did not significantly boost pasture productivity, with any observed biomass response influenced by seasonal conditions. The urease inhibitor, NBTPT (GU), is not an effective tool for mitigating N2O emissions from urea, particularly during periods of high emissions (spring), with 53% more fertiliser-induced N2O emissions produced relative to granular urea over the eight months. The importance of climate conditions for ammonia loss caused inconsistent productivity benefits from the use of GU. The potential for FPA to mitigate N2O and increase pasture production requires investigation during periods of high emissions. These results highlight the need to use EEFs at times when their targeted loss pathway is important. This can be achieved by identifying the temporal changes in loss pathways (volatilisation, leaching and denitrification) based on climatic conditions and soil background N and C levels. To gain maximum productivity benefits from the use of EEFs other factors that restrict pasture growth, such as soil moisture and temperature, need to be effectively managed and N inputs should be lowered also.
Acknowledgements
The authors acknowledge financial support from Incitec Pivot Fertiliser, the Department of Agriculture, Fisheries and Forestry, and the Grains Research & Development Corporation. We also acknowledge the generosity of the landowners, the Gannon family, in providing us with the land and supporting us with maintenance of the field site. We also acknowledge the contribution of Mr Matt Mahoney, Ms Marion Benfell, Mr David Hopkins and Dr Xing Chen for assistance in the collection of field data.
References
Abalos D, Jeffery S, Sanz-Cobena A, Guardia G, Vallejo A (2014) Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agriculture, Ecosystems & Environment 189, 136–144.| Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXnslGqs7Y%3D&md5=05c0ff60ecf5bb5ce2cf3b91bc7194b9CAS |
Bell MJ, Rees RM, Cloy JM, Topp CFE, Bagnall A, Chadwick DR (2015) Nitrous oxide emissions from cattle excreta applied to a Scottish grassland: Effects of soil and climatic conditions and a nitrification inhibitor. The Science of the Total Environment 508, 343–353.
| Nitrous oxide emissions from cattle excreta applied to a Scottish grassland: Effects of soil and climatic conditions and a nitrification inhibitor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXitV2isL%2FK&md5=1a116667f44fcf1d282b4415b337e0a9CAS | 25497356PubMed |
Biddiscombe E, Arnold G, Scurfield G (1962) Effects of Gibberellic acid on pasture and animal production in winter. Australian Journal of Agricultural Research 13, 400–413.
| Effects of Gibberellic acid on pasture and animal production in winter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3sXhtFWitA%3D%3D&md5=0045962f63f090a7ff43ca92474282b5CAS |
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philosophical Transactions of the Royal Society, B: Biological Sciences 368, 20130122.
| Nitrous oxide emissions from soils: how well do we understand the processes and their controls?Crossref | GoogleScholarGoogle Scholar |
Carey PL, Jiang S, Roberts AH (2012) Pasture dry matter responses to the use of a nitrification inhibitor: a national series of New Zealand farm trials. New Zealand Journal of Agricultural Research 55, 63–72.
| Pasture dry matter responses to the use of a nitrification inhibitor: a national series of New Zealand farm trials.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtFyitrs%3D&md5=6cdab99d20156d2208361fc393468bceCAS |
Chen D, Suter HC, Islam A, Edis R, Freney JR, Walker CN (2008) Prospects of improving efficiency of fertilizer nitrogen in Australian agriculture; a review of enhanced efficiency fertilizers. Australian Journal of Soil Research 46, 289–301.
| Prospects of improving efficiency of fertilizer nitrogen in Australian agriculture; a review of enhanced efficiency fertilizers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXns1OktLw%3D&md5=fec61f7dd15fe4ea2a2bc5ea7113200cCAS |
Chen D, Suter HC, Islam A, Edis R (2010) Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea. Soil Biology & Biochemistry 42, 660–664.
| Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXis1Sgtrw%3D&md5=cb0fbf1d12401ca061462b81b933ca1fCAS |
Ciarlo E, Conti M, Bartoloni N, Rubio G (2008) Soil N2O emissions and N2O/(N2O+N2) ratio as affected by different fertilization practices and soil moisture. Biology and Fertility of Soils 44, 991–995.
| Soil N2O emissions and N2O/(N2O+N2) ratio as affected by different fertilization practices and soil moisture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXos1WltLw%3D&md5=e685e7c6c7cc18cb089a89111f845c6cCAS |
Dawar K, Zaman M, Rowarth JS, Blennerhassett J, Turnbull MH (2011) Urease inhibitor reduces N losses and improves plant-bioavailability of urea applied in fine particle and granular forms under field conditions. Agriculture, Ecosystems & Environment 144, 41–50.
| Urease inhibitor reduces N losses and improves plant-bioavailability of urea applied in fine particle and granular forms under field conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFCqsLnL&md5=fb5302fdd4252b37927c715ce62910fbCAS |
Dawar K, Zaman M, Rowarth JS, Turnbull MH (2012) Applying urea with urease inhibitor (N-(n-butyl) thiophosphoric triamide) in fine particle application improves nitrogen uptake in ryegrass (Lolium perenne L.). Soil Science and Plant Nutrition 58, 309–318.
| Applying urea with urease inhibitor (N-(n-butyl) thiophosphoric triamide) in fine particle application improves nitrogen uptake in ryegrass (Lolium perenne L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpvV2isr8%3D&md5=052c8ee7d1491ffa5947842a3efae363CAS |
Department of Climate Change and Energy Efficiency (DCCEE) (2011) ‘Australian national greenhouse accounts: national greenhouse gas inventory accounting for the Kyoto target December Quarter 2010.’ (DCCEE, Australian National Greenhouse Accounts: Canberra)
Di HJ, Cameron KC (2006) Nitrous oxide emissions from two dairy pasture soils as affected by different rates of a fine particle suspension nitrification inhibitor, dicyandiamide. Biology and Fertility of Soils 42, 472–480.
| Nitrous oxide emissions from two dairy pasture soils as affected by different rates of a fine particle suspension nitrification inhibitor, dicyandiamide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntlGhtrw%3D&md5=53d42143fb6fc179604517b10ed6d4ebCAS |
Di HJ, Cameron KC (2012) How does the application of different nitrification inhibitors affect nitrous oxide emissions and nitrate leaching from cow urine in grazed pastures? Soil Use and Management 28, 54–61.
| How does the application of different nitrification inhibitors affect nitrous oxide emissions and nitrate leaching from cow urine in grazed pastures?Crossref | GoogleScholarGoogle Scholar |
Di HJ, Cameron KC, Sherlock RR (2007) Comparison of the effectiveness of a nitrification inhibitor, dicyandiamide (DCD), in reducing nitrous oxide emissions in four different soils under different climatic and management conditions. Soil Use and Management 23, 1–9.
| Comparison of the effectiveness of a nitrification inhibitor, dicyandiamide (DCD), in reducing nitrous oxide emissions in four different soils under different climatic and management conditions.Crossref | GoogleScholarGoogle Scholar |
Di HJ, Cameron KC, Sherlock RR, Shen JP, He JZ, Winefield CS (2010) Nitrous oxide emissions from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia-oxidizing bacteria and archaea. Journal of Soils and Sediments 10, 943–954.
| Nitrous oxide emissions from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia-oxidizing bacteria and archaea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXntlKqt7c%3D&md5=fabbb724d0b823c1294c4a5796290492CAS |
Ding WX, Chen ZM, Yu HY, Luo JF, Yoo GY, Xiang J, Zhang HJ, Yuan JJ (2014) Nitrous oxide emission and nitrogen use efficiency in response to nitrophosphate, N-(n-butyl) thiophosphoric triamide and dicyandiamide of a wheat cultivated soil under sub-humid monsoon conditions. Biogeosciences Discussions 11, 13571–13603.
| Nitrous oxide emission and nitrogen use efficiency in response to nitrophosphate, N-(n-butyl) thiophosphoric triamide and dicyandiamide of a wheat cultivated soil under sub-humid monsoon conditions.Crossref | GoogleScholarGoogle Scholar |
Eckard RJ, Chen D, White RE, Chapman DF (2003) Gaseous nitrogen loss from temperate perennial grass and clover dairy pastures in south-eastern Australia. Australian Journal of Agricultural Research 54, 561–570.
| Gaseous nitrogen loss from temperate perennial grass and clover dairy pastures in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Fangueiro D, Fernandes A, Coutinho J, Moreira N, Trindade H (2009) Influence of two nitrification inhibitors (DCD and DMPP) on annual ryegrass yield and soil mineral N dynamics after incorporation with cattle slurry. Communications in Soil Science and Plant Analysis 40, 3387–3398.
| Influence of two nitrification inhibitors (DCD and DMPP) on annual ryegrass yield and soil mineral N dynamics after incorporation with cattle slurry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXksVyntA%3D%3D&md5=2b68ec8c016e5846918076c577cc3fafCAS |
Friedl J, Scheer C, Rowlings DW, McIntosh HV, Strazzabosco A, Warner DI, Grace PR (2016) Denitrification losses from an intensively managed sub-tropical pasture – Impact of soil moisture on the partitioning of N2 and N2O emissions. Soil Biology & Biochemistry 92, 58–66.
| Denitrification losses from an intensively managed sub-tropical pasture – Impact of soil moisture on the partitioning of N2 and N2O emissions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhs1Cqtb%2FP&md5=e98fb9ecc5eb6f692b381b550a1ff26bCAS |
Gilsanz C, Baez D, Misselbrook TH, Dhanoa MS, Cardenas LM (2016) Development of emission factors and efficiency of two nitrification inhibitors, DCD and DMPP. Agriculture, Ecosystems & Environment 216, 1–8.
Harris RH, Officer SJ, Hill PA, Armstrong RD, Fogarty KM, Zollinger RP, Phelan AJ, Partington DL (2013) Can nitrogen fertiliser and nitrification inhibitor management influence N2O losses from high rainfall cropping systems in South Eastern Australia? Nutrient Cycling in Agroecosystems 95, 269–285.
| Can nitrogen fertiliser and nitrification inhibitor management influence N2O losses from high rainfall cropping systems in South Eastern Australia?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXotV2ru70%3D&md5=dfcb3a9185e64759c620914c4cfd4732CAS |
Hu R, Wang XP, Pan YX, Zhang YF, Zhang H (2014) The response mechanisms of soil N mineralization under biological soil crusts to temperature and moisture in temperate desert regions. European Journal of Soil Biology 62, 66–73.
| The response mechanisms of soil N mineralization under biological soil crusts to temperature and moisture in temperate desert regions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXosFartLc%3D&md5=3fdacd67c85e5a11ce43d3a4e3e58d33CAS |
Huang X, Grace P, Rowlings D, Mengersen K (2013) A flexible Bayesian model for describing temporal variability of N2O emissions from an Australian pasture. The Science of the Total Environment 454–455, 206–210.
| A flexible Bayesian model for describing temporal variability of N2O emissions from an Australian pasture.Crossref | GoogleScholarGoogle Scholar | 23542492PubMed |
International Fertilizer Industry Association (IFA) (2001) ‘Global estimates of gaseous emissions of NH3, NO and N2O from agricultural land.’ (IFA and Food and Agriculture Organisation (FAO): Rome)
Isbell RF (1996) ‘The Australian soil classification.’ Revised edn. (CSIRO Publishing: Melbourne)
Kawakami EM, Oosterhuis DM, Snider JL, Mozaffari M (2012) Physiological and yield responses of field-grown cotton to application of urea with the urease inhibitor NBPT and the nitrification inhibitor DCD. European Journal of Agronomy 43, 147–154.
| Physiological and yield responses of field-grown cotton to application of urea with the urease inhibitor NBPT and the nitrification inhibitor DCD.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1Gmur7N&md5=1da09bc2c2b3a2530875181aff9b87ddCAS |
Kelly KB, Phillips FA, Baigent R (2008) Impact of dicyandiamide application on nitrous oxide emissions from urine patches in northern Victoria, Australia. Australian Journal of Experimental Agriculture 48, 156–159.
| Impact of dicyandiamide application on nitrous oxide emissions from urine patches in northern Victoria, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovVyh&md5=661475009eb8656b0ea56c81368374caCAS |
Kyveryga P, Blackmer T (2014) Probability of profitable yield response to nitrification inhibitor used with liquid swine manure on corn. Precision Agriculture 15, 133–146.
| Probability of profitable yield response to nitrification inhibitor used with liquid swine manure on corn.Crossref | GoogleScholarGoogle Scholar |
Li D, Watson CJ, Yan MJ, Lalor S, Rafique R, Hyde B, Lanigan G, Richards KG, Holden NM, Humphreys J (2013a) A review of nitrous oxide mitigation by farm nitrogen management in temperate grassland-based agriculture. Journal of Environmental Management 128, 893–903.
| A review of nitrous oxide mitigation by farm nitrogen management in temperate grassland-based agriculture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVWjtbrI&md5=69235b7507a46b937a90ab5d23de2555CAS | 23880433PubMed |
Li S-X, Wang Z-H, Stewart BA (2013b) Responses of crop plants to ammonium and nitrate N. In ‘Advances in agronomy’. (Ed. DL Sparks) Ch. 5, pp. 205–397. (Academic Press).
McKenzie FR, Jacobs JL, Kearney G (2003) Long-term effects of multiple applications of nitrogen fertiliser on grazed dryland perennial ryegrass/white clover dairy pastures in south-west Victoria. 2. Growth rates, dry matter consumed, and nitrogen response efficiencies. Australian Journal of Agricultural Research 54, 471–476.
| Long-term effects of multiple applications of nitrogen fertiliser on grazed dryland perennial ryegrass/white clover dairy pastures in south-west Victoria. 2. Growth rates, dry matter consumed, and nitrogen response efficiencies.Crossref | GoogleScholarGoogle Scholar |
Menéndez S, Merino P, Pinto M, González-Murua C, Estavillo JM (2009) Effect of N-(n-butyl) thiophosphoric triamide and 3,4 dimethylpyrazole phosphate on gaseous emissions from grasslands under different soil water contents. Journal of Environmental Quality 38, 27–35.
| Effect of N-(n-butyl) thiophosphoric triamide and 3,4 dimethylpyrazole phosphate on gaseous emissions from grasslands under different soil water contents.Crossref | GoogleScholarGoogle Scholar | 19141792PubMed |
Misselbrook TH, Cardenas LM, Camp V, Thorman RE, Williams HM, Rollett AJ, Chambers BJ (2014) An assessment of nitrification inhibitors to reduce nitrous oxide emissions from UK agriculture. Environmental Research Letters 9, 115006
| An assessment of nitrification inhibitors to reduce nitrous oxide emissions from UK agriculture.Crossref | GoogleScholarGoogle Scholar |
O’Connor PJ, Hennessy D, Brophy C, O’Donovan M, Lynch MB (2012) The effect of the nitrification inhibitor dicyandiamide (DCD) on herbage production when applied at different times and rates in the autumn and winter. Agriculture, Ecosystems & Environment 152, 79–89.
| The effect of the nitrification inhibitor dicyandiamide (DCD) on herbage production when applied at different times and rates in the autumn and winter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XkvFSns7k%3D&md5=8579dbc6a095dd3f0e9ca967a270bf96CAS |
Rafique R, Anex R, Hennessy D, Kiely G (2012) What are the impacts of grazing and cutting events on the N2O dynamics in humid temperate grassland? Geoderma 181–182, 36–44.
| What are the impacts of grazing and cutting events on the N2O dynamics in humid temperate grassland?Crossref | GoogleScholarGoogle Scholar |
Rawluk CDL, Grant CA, Racz GJ (2001) Ammonia volatilization from soils fertilized with urea and varying rates of urease inhibitor NBPT. Canadian Journal of Soil Science 81, 239–246.
| Ammonia volatilization from soils fertilized with urea and varying rates of urease inhibitor NBPT.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvVGqsrc%3D&md5=28fd754abc7da7993c5dedc04fa3f362CAS |
Rayment GE, Lyons DJ (2011) 7A2b Total soil-N, semi micro Kjeldahl - automated colour, FIA. In ‘Soil chemical methods – Australasia’. (Eds GE Rayment, DJ Lyons) pp. 104–108. (CSIRO Publishing: Melbourne)
Rowlings DW, Scheer C, Liu S, Grace PR (2016) Annual nitrogen dynamics and urea fertilizer recoveries from a dairy pasture using 15N; effect of nitrification inhibitor DMPP and reduced application rates. Agriculture, Ecosystems & Environment 216, 216–225.
| Annual nitrogen dynamics and urea fertilizer recoveries from a dairy pasture using 15N; effect of nitrification inhibitor DMPP and reduced application rates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhs1yjtb7K&md5=96447a2be2ec2cdc3b0046fcba10d9a5CAS |
Saggar S, Andrew RM, Tate KR, Hedley CB, Rodda NJ, Townsend JA (2004) Modelling nitrous oxide emissions from dairy-grazed pastures. Nutrient Cycling in Agroecosystems 68, 243–255.
| Modelling nitrous oxide emissions from dairy-grazed pastures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhvF2lsr8%3D&md5=bb8d0458e4c7bf55fc3d8dbfe9f9738cCAS |
Saggar S, Jha N, Deslippe J, Bolan NS, Luo J, Giltrap DL, Kim DG, Zaman M, Tillman RW (2013) Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. The Science of the Total Environment 465, 173–195.
| Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVKlt73L&md5=2ed2f15654c742395c79bc89669715e7CAS | 23260378PubMed |
Sanz-Cobena A, Sánchez-Martín L, García-Torres L, Vallejo A (2012) Gaseous emissions of N2O and NO and NO3 − leaching from urea applied with urease and nitrification inhibitors to a maize (Zea mays) crop. Agriculture, Ecosystems & Environment 149, 64–73.
| Gaseous emissions of N2O and NO and NO3 − leaching from urea applied with urease and nitrification inhibitors to a maize (Zea mays) crop.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XitlGiu7o%3D&md5=2d7194fc1c2ba4a74f292707cc48eeeeCAS |
Singh J, Kunhikrishnan A, Bolan NS, Saggar S (2013) Impact of urease inhibitor on ammonia and nitrous oxide emissions from temperate pasture soil cores receiving urea fertilizer and cattle urine. The Science of the Total Environment 465, 56–63.
| Impact of urease inhibitor on ammonia and nitrous oxide emissions from temperate pasture soil cores receiving urea fertilizer and cattle urine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjsFygsb4%3D&md5=8d63b3b9a5eb463ccf833f8dc11e6f91CAS | 23473618PubMed |
Skalar Analytical B.V. (2005) ‘The SANplus segmented flow analyser: soil and plant analysis.’ (Skalar Analytical B.V.: Netherlands).
Suter HC, Sultana H, Turner DA, Davies R, Walker C, Chen D (2013) Influence of urea fertiliser formulation, urease inhibitor and season on ammonia loss from ryegrass. Nutrient Cycling in Agroecosystems 95, 175–185.
| Influence of urea fertiliser formulation, urease inhibitor and season on ammonia loss from ryegrass.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXotV2qsrs%3D&md5=40e8195f3535700e0b7bc491dc38928aCAS |
Turner DA, Edis RB, Chen D, Freney JR, Denmead OT, Christie R (2010) Determination and mitigation of ammonia loss from urea applied to winter wheat with N-(n-butyl) thiophosphorictriamide. Agriculture, Ecosystems & Environment 137, 261–266.
| Determination and mitigation of ammonia loss from urea applied to winter wheat with N-(n-butyl) thiophosphorictriamide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltFOms7k%3D&md5=d6b1cfdc9688ec61f49372905b72edc7CAS |
Venterea RT, Parkin TB, Cardenas L, Petersen SO, Pedersen AR (2012) Data analysis considerations. In ‘Nitrous oxide chamber methodology guidelines version 1’. (Eds CAM de Klein, MJ Harvey) pp. 95–121. (Ministry for Primary Industries: New Zealand)
Watson CJ, Stevens RJ, Laughlin RJ (1990) Effectiveness of the urease inhibitor NBPT (N-(n-butyl) thiophosphoric triamide) for improving the efficiency of urea for ryegrass production. Fertilizer Research 24, 11–15.
| Effectiveness of the urease inhibitor NBPT (N-(n-butyl) thiophosphoric triamide) for improving the efficiency of urea for ryegrass production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXhtVSgur0%3D&md5=8aa2ea451e5aad250b3fd332c47b59e0CAS |
Yeomans JC, Bremner JM (1986) Effects of urease inhibitors on denitrification in soil. Communications in Soil Science and Plant Analysis 17, 63–73.
| Effects of urease inhibitors on denitrification in soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28Xhs1OnurY%3D&md5=5780b13cf7a1ca49f62fba3e5ef88f9cCAS |
Zaman M, Saggar S, Blennerhassett JD, Singh J (2009) Effect of urease and nitrification inhibitors on N transformation, gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system. Soil Biology & Biochemistry 41, 1270–1280.
| Effect of urease and nitrification inhibitors on N transformation, gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtVGkur0%3D&md5=c5d7fdefaa7b8b225149bc11d4d8d361CAS |
Zaman M, Zaman S, Adhinarayanan C, Nguyen ML, Nawaz S, Dawar KM (2013) Effects of urease and nitrification inhibitors on the efficient use of urea for pastoral systems. Soil Science and Plant Nutrition 59, 649–659.
| Effects of urease and nitrification inhibitors on the efficient use of urea for pastoral systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlansr7E&md5=9dac0b9659910604c3c3aeaad1d4ccb1CAS |
Zhengping W, Liantie L, Van Cleemput O, Baert L (1991) Effect of urease inhibitors on denitrification in soil. Soil Use and Management 7, 230–233.
| Effect of urease inhibitors on denitrification in soil.Crossref | GoogleScholarGoogle Scholar |



 ) showing timing of each fertilisation event (
) showing timing of each fertilisation event ( ) and harvest (+) with standard error of the mean of five replicates.
) and harvest (+) with standard error of the mean of five replicates.

