Effect of enhanced efficiency fertilisers on nitrous oxide emissions in a sub-tropical cereal cropping system
Clemens Scheer A D , David W. Rowlings A , Massimiliano De Antoni Migliorati A , David W. Lester B , Mike J. Bell C and Peter R. Grace AA Institute for Future Environments, Queensland University of Technology, Brisbane, Qld 4000, Australia.
B Queensland Department of Agriculture and Fisheries, PO Box 2282, Toowoomba, Qld 4350, Australia.
C Queensland Alliance for Agriculture and Food Innovation, School of Crop and Food Sciences, University of Queensland, Gatton, Qld 4343, Australia.
D Corresponding author. Email: clemens.scheer@qut.edu.au
Soil Research 54(5) 544-551 https://doi.org/10.1071/SR15332
Submitted: 10 November 2015 Accepted: 29 February 2016 Published: 6 July 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
To meet the global food demand in the coming decades, crop yields per unit area must increase. This can only be achieved by a further intensification of existing cropping systems and will require even higher inputs of N fertilisers, which may result in increased losses of nitrous oxide (N2O) from cropped soils. Enhanced efficiency fertilisers (EEFs) have been promoted as a potential strategy to mitigate N2O emissions and improve nitrogen use efficiency (NUE) in cereal cropping systems. However, only limited data are currently available on the use of different EEF products in sub-tropical cereal systems. A field experiment was conducted to investigate the effect of three different EEFs on N2O emissions, NUE and yield in a sub-tropical summer cereal cropping system in Australia. Over an entire year soil N2O fluxes were monitored continuously (3 h sampling frequency) with a fully-automated measuring system. The experimental site was fertilised with different nitrogen (N) fertilisers applied at 170 kg N ha–1, namely conventional urea (Urea), urea with the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP), polymer-coated urea (PCU), and urea with the nitrification inhibitor nitrapyrin (Nitrapyrin). Nitrous oxide emissions were highly episodic and mainly controlled by heavy rainfall events within two months of planting and fertiliser N application. Annual N2O emissions in the four treatments amounted to 2.31, 0.40, 0.69 and 1.58 kg N2O-N ha–1 year–1 for Urea, DMPP, PCU and Nitrapyrin treatments, respectively, while unfertilised plots produced an average of 0.16 kg N2O-N ha–1 year–1. Two of the tested products (DMPP and PCU) were found to be highly effective, decreasing annual N2O losses by 83% and 70%, respectively, but did not affect yield or NUE. This study shows that EEFs have a high potential to decrease N2O emissions from sub-tropical cereal cropping systems. More research is needed to assess if the increased costs of EEFs can be compensated by lower fertiliser application rates and/or yield increases.
Introduction
Nitrogen (N) fertiliser is a key input to achieve global food security and has made a crucial contribution to increasing food production over the past 50 years. Today the yield increases obtained from N fertilisers are responsible for feeding almost half of the world’s population (Erisman et al. 2008). Cereal production systems provide more than 60% of human dietary calories and account for the majority of global fertiliser N consumption (Heffer 2013). By 2050 it is estimated that the world’s population will reach 9–10 billion (UNFPA 2011) and cereal demand is predicted to double (Ladha et al. 2005). Since little new land is available for crop production, crop yields per unit area must increase to meet the global food demand. This can only be achieved by intensification of existing cropping systems and will require even higher inputs of fertiliser N into these systems. However, a large proportion of this N is currently lost to the environment (Mosier et al. 2004), causing environmental pollution and increases in greenhouse gas (GHG) emissions, especially nitrous oxide (N2O). Nitrous oxide is a potent GHG with a global warming potential of nearly 300 times that of carbon dioxide (CO2) and is also the primary contributor to stratospheric ozone depletion (Ravishankara et al. 2009). It is estimated that agricultural activities are responsible for ~70% of all anthropogenic N2O emissions, to which increased fertiliser and manure N inputs have a large contribution (Davidson 2009).
Sustaining yield increases on the same area of land while reducing the environmental impact is a major challenge and requires significantly improved N use efficiency (NUE) in global cropping systems. To achieve this a broad range of technological innovations need to be pursued simultaneously. One effective option could be the use of enhanced efficiency fertilisers (EEFs) for their potential to improve fertiliser NUE and decrease N2O emissions.
Enhanced efficiency fertilisers are defined as fertiliser products with characteristics that allow increased plant uptake and reduce the potential of nutrient losses to the environment (e.g. gaseous losses, leaching or runoff) when compared with an appropriate reference product (AAPFCO 2013). Enhanced efficiency fertilisers control the rate of N release or N availability from the fertiliser via the addition of nitrification and/or urease inhibitors, or through coating and chemical modification. Several studies have shown that the use of EEFs can potentially increase crop NUE, decrease N leaching, and mitigate N2O emissions from cropping soils (Abalos et al. 2014; Halvorson et al. 2014). In a recent meta-analysis Akiyama et al. (2010) showed that EEFs containing nitrification inhibitors (NIs) and polymer-coated fertilisers (PCFs) significantly decreased N2O emissions by 38% and 35%, respectively; whereas urease inhibitors (UIs) were not effective in decreasing N2O. The combined effect of EEFs on N2O emissions and crop production has been shown to strongly depend on site-specific conditions, such as soil texture and climate, and a significant decrease in the effectiveness of NIs at increased soil temperatures has been reported (Irigoyen et al. 2003; Merino et al. 2005).
However, most data on the effect of EEFs on N2O emissions and productivity in cereal systems refer to temperate regions or laboratory experiments and the efficacy of many EEFs in sub-tropical environments remains unknown. In Australia, two studies investigated the effect of the NI 3,4-dimethylpyrazole phosphate (DMPP) on N2O emissions in sub-tropical cropping systems (De Antoni Migliorati et al. 2014; Scheer et al. 2014). These studies showed that DMPP has the potential to substantially decrease N2O emissions but no data is available on the effect of other NIs or PCFs under sub-tropical conditions in Australia. Such data is urgently needed to identify management strategies that maximise the efficient use of fertiliser N while minimising environmental impacts in Australian cropping systems.
The aims of this study were to investigate the effect of EEF products with different modes of action (controlled release versus nitrification inhibition) on soil N2O emissions and fertiliser NUE in a grain cropping system in sub-tropical Queensland, Australia. Nitrous oxide emissions were monitored using a fully-automated high frequency (3 h sampling frequency) GHG measuring system deployed for a full calendar year, during which a summer cereal (sorghum) crop was followed by a winter cover crop (barley). In this field study we investigated the hypotheses:
-
N2O emissions from a sub-tropical cereal cropping system can be decreased by the use of EEFs; and
-
The use of EEFs will increase the NUE in this sub-tropical cereal cropping system.
Material and methods
Study site
The field experiment was conducted over one full year from 25 November 2012 to 24 November 2013 at the Kingsthorpe Research Station, 20 km west of Toowoomba, Queensland. The station is located in the Darling Downs region ~140 km west of Brisbane (27°31ʹS, 151°47ʹE, 431 m above mean sea level). The region has a sub-tropical climate (classified as Cfa, according to Köppen climate classification) with warm, humid summers and mild winters. Daily mean minimum and maximum temperatures are 16.3°C and 27.2°C, respectively in the summer, and 5.9°C and 17.0°C, respectively in winter. Mean annual precipitation is 630 mm (1990–2010) (Commonwealth Bureau of Meteorology; http://www.bom.gov.au/climate), and most of the rainfall occurs between October and March, during the summer crop growing season.
The soil at the site is surveyed as a Craigmore (Powell et al. 1988), which classifies as a haplic, self-mulching, black Vertosol (Isbell 2002). It has a heavy clay texture (76% clay) in the 1.5 m root zone profile, with a distinct change in soil colour from brownish black (10YR22) in the top 90 cm to dark brown (7.5YR33) deeper in the profile. The soil is formed in a colluvial fan of basalt rock origin, is slowly permeable, and the experimental site had a surface slope of ~0.5%. Soil samples to 120 cm were collected using the depth increments shown in Table 1. Chemical methods for soil analysis refer to those described in Rayment and Lyons (2011).
Experimental design
The experiment was conducted to compare the standard N fertiliser urea against three EEF treatments with three replications arranged in a completely randomised block design. Each experimental plot was 3.0 m wide (4 × 0.76 m crop rows) × 10 m in length, with the crop planted in the north–south orientation. A 1.52 m-wide buffer zone (2 × 0.76 m crop rows) was planted between plots. During the experiment the site was cropped to grain sorghum (Sorghum bicolour L. Pacific Seeds MR Taurus) with sowing on 25 November 2012 and grain harvest on 06 May 2013. Sowing rate was 60 000 seeds per hectare. Before the experimental phase, the site was prepared by cover cropping with barley (Hordeum vulgare L.) to decrease the soil mineral N profile. The crop was sprayed out with glyphosate and stubble left intact into which the experimental sorghum crop was direct drilled. Following this, a barley (Hordeum vulgare L.) cover crop was grown from June to November 2013.
Four fertiliser treatments were compared with an untreated control (0N). Treatments were as follows:
-
Zero nitrogen fertiliser (0N) – i.e. no added fertiliser
-
Urea
-
Urea + DMPP – urea-coated with DMPP nitrification inhibitor, commercially available as Entec® (Incitec Pivot fertiliser, Australia)
-
PCU – polymer-coated urea prills (CoteN™ four month release, Haifa-group)
-
Urea + Nitrapyrin – urea with the nitrification inhibitor nitrapyrin, commercially available as eNtrench™ (Dow Chemical Co., Australia) injected in solution onto the urea band before closing the fertiliser trench. The product was applied at 2.5 L ha–1 in a 5% solution (i.e. 50 L solution ha–1).
Fertiliser application rate was 170 kg N ha–1 and the fertilisers were band applied at planting beside the crop row.
Continuous N2O flux measurement
Nitrous oxide fluxes were measured over an entire year from 25 November 2012 to 24 November 2013. Measurements were taken from every plot using a fully-automated measuring system similar to the one described in Scheer et al. (2014). Briefly, the system consisted of 12 acrylic static chambers (50 cm × 50 cm × 15 cm) that were equipped with pneumatically operated lids and fixed on stainless steel bases inserted 10 cm into the soil. The chambers were positioned next to the plant rows to account for N2O emissions from a localised source (banded fertiliser) and background emissions from residual soil N. Adapting the methodology described by De Antoni Migliorati et al. (2015), for each treatment two of the three replicate chambers were positioned over the fertiliser band and the third one in the inter-band. The 12 chambers allowed for the direct measurement of N2O fluxes in the four fertilised treatments. Cumulative N2O emissions from the unfertilised control treatment (0N) were derived from the results of the chambers positioned in the unfertilised inter-band.
The chambers were linked to a fully-automated system comprised of a computerised sampling unit and an in situ gas chromatograph (SRI GC 8610C) equipped with a 63Ni electron capture detector (ECD) for N2O concentration analysis. Sample gas measurements were calibrated automatically by a single-point calibration using a certified gas standard of 0.5 ppm N2O. During the measuring season a multi-point calibration was performed using certified gas standards of 500, 980, 5030 ppb N2O (BOC; Munich, Germany) and the GC response over this range was determined to be linear. The detection limit of the system was ~1.0 µg N2O-N m–2 h–1 and sample dilution via leakage was considered negligible.
Flux calculations
Fluxes of N2O from the automated chambers were calculated from the slope of the linear increase or decrease over the four concentrations measured during the closure time, as described in detail in Scheer et al. (2014). The coefficient of determination was used to quality check the flux measurements. Fluxes above the detection limit were discarded if the regression coefficient (r2) was <0.80. Mean daily fluxes were calculated using weighted averages of hourly data from the three replicates. For each treatment, fluxes from the two chambers over the fertiliser band (covering 50 cm of the crop inter-row) were averaged together and then weighted with the fluxes measured by the chamber in the inter-band (covering 26 cm of the inter-row). With this method it was possible to accurately calculate the average N2O emissions of each treatment, accounting for the spatial variability occurring between two crop rows (76 cm). Daily fluxes were calculated by averaging sub-daily measurements over the 24 h period (midnight to midnight). To calculate seasonal cumulative fluxes, calculated daily fluxes were summed according to the measurement period. Gaps in the dataset were filled by linear interpolation across missing days.
Emission factors of the N fertiliser applied to the soil were calculated using the following equation:

where EF is the emission factor (percentage of the total fertiliser N applied that was emitted as N2O-N); N2O-N is the total N2O over one year (kg N ha–1 year–1) for each treatment; total N applied is the amount of N fertiliser applied (kg N ha–1 year–1).
Auxiliary measurements
Soil temperature (at a depth of 10 cm) and chamber temperature were measured every minute in conjunction with the automatic sampling system using a PT100 probe (Temperature Controls Pty, Australia). An electronic weather station was installed at the experimental site to measure local weather variables. The station recorded daily values of air temperature (maximum, minimum, and average), relative humidity, wind speed, and rainfall. Soil moisture was measured from 0–5 cm continuously in one plot per treatment using a MP406 standing wave soil moisture probe (ICT International Pty Ltd, Armidale, NSW, Australia) that was calibrated for the soil type at the research site.
The mineral N content of the surface soil (0–10 cm) was measured immediately before planting and at selected dates over the experiment. At each sampling date, four samples were taken randomly from each replicate plot with a soil auger and combined to a bulk sample, resulting in three replicate samples per treatment. To assess the mineral N dynamics in the fertiliser band versus off-the-band, separate samples were taken from the banded fertiliser area and from the unfertilised inter-band area at one sampling occasion (18 January 2013). The ammonium (NH4+) and nitrate (NO3–) were extracted from the soil samples by adding 100 mL of 1M KCl to 20 g of soil and shaking for 1 h. The solution was filtered and stored frozen until analysed colourimetrically for NH4-N and NO3-N using an AQ2+ discrete analyser (SEAL Analytical WI, USA).
At physiological maturity, two crop rows of one metre were sampled, dried and weighed. Grain yield was measured in each plot by machine harvesting two crop rows for the plot length (10 m). Above ground biomass samples were ground and analysed for total N content using the Dumas combustion method by a TruMac Series Macro Determinator (LECO Corporation, St Joseph, MI, USA).
Statistical analysis
Statistical analysis was undertaken using SPSS 16.0 (SPSS Inc., USA). Analysis of variance (ANOVA) was performed to determine whether the fertiliser product had a significant influence on N2O emissions and crop yields. The Bonferroni post hoc test was used to compare cumulative N2O emissions, grain yields and total N uptake across treatments.
Results
Seasonal variability of environmental and soil conditions
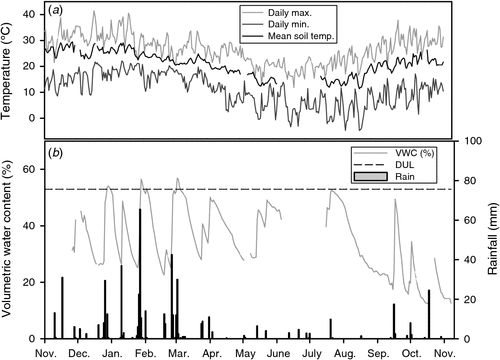
Over the 2012–13 cropping cycle a total of 532 mm of rainfall was recorded at the study site including two heavy events where weekly rainfall exceeded 100 mm in late January and early March 2013. The highest daily rainfall occurred in the January event where 65 mm fell on 27 January 2013. The rainfall at the site was slightly lower than the long-term mean annual rainfall (630 mm) although good summer crop rainfall was received from sowing in late November 2012 through to early March 2013. Maximum hourly air temperature (41.5°C) was recorded in December 2012, while minimum hourly air temperature (–4.7°C) was recorded in August 2013. Hourly soil temperatures (10 cm) ranged from 11.4°C to 29.4°C with the lowest soil temperatures during July–August and the highest during November–December (Fig. 1). Volumetric soil moisture (VSM) content of the upper soil (0–5 cm) varied over the season in response to rainfall. Due to frequent rainfall over the sorghum growing phase and the high water holding capacity of the clay, soil moisture levels stayed relatively high with VSM ranging from 23% to 54% (Fig. 1). The highest values (54% VSM) were observed after the high rainfall events in late January and early March. The lowest values were recorded at the end of the observation period (12% VSM) in late October 2013.
|
Soil mineral N dynamics were significantly affected by the fertiliser treatments over the sorghum growing period, while there was no significant difference over the post-harvest fallow and the barley cover crop period (data not shown). Mineral N dynamics were significantly elevated in the fertiliser band, while there was no significant difference between the inter-band sampling and the unfertilised control. Fifty days after fertiliser application, NH4+ concentrations in the fertiliser band were significantly higher in the DMPP treatment compared with the Urea treatment, while there was no significant effect of fertiliser product on NO3– concentrations (Fig. 2).
N2O emissions and emission factors
The majority of N2O fluxes occurred in the first two months of the sorghum growing period while N2O emissions over the post-harvest fallow and the barley cover crop period were negligible (Fig. 3). There were small emission pulses in response to rainfall events in December and early January but a heavy rainfall event in late January (106 mm over six days with 65 mm during a single event), triggered an extremely high emission pulse with peak fluxes in the Urea treatment reaching 600 g N2O-N ha–1 day–1. This emission pulse lasted seven days and was responsible for 32% to 79% of the total annual emissions across all treatments. A heavy rainfall event in early March (99 mm over seven days with 43 mm during a single event) only resulted in minor N2O emissions in the PCU and Urea treatments.
Relative to Urea, all EEF products reduced the intensity of emission peaks in the early season events. However, while DMPP and PCF decreased peak emissions by as much as a factor of 10 in the late January event, there was no significant effect of the Nitrapyrin on N2O emissions compared with Urea.
Cumulative N2O emissions were estimated to be 2.31 ± 0.16, 0.40 ± 0.02, 0.69 ± 0.24 and 1.58 ± 0.74 kg N2O-N ha–1 year–1 for Urea, DMPP, PCU and Nitrapyrin, respectively, while from the unfertilised plots an average of 0.16 ± 0.03 kg N2O-N ha–1 year–1 was emitted (Table 2). The use of DMPP or PCU fertiliser significantly decreased annual emissions by 1.91 and 1.62 kg N2O-N ha–1 year–1, respectively (equivalent to an 83% and 70% reduction compared with the Urea treatment). There was no significant effect of Nitrapyrin on annual N2O emissions compared with the Urea treatment. Corresponding N fertiliser-induced annual N2O emission factors (EFs) found in the present study were 1.27%, 0.14%, 0.31% and 0.83% for Urea, DMPP, PCU and Nitrapyrin, respectively (Table 2).
Grain yield, N uptake and emissions intensity
The site was extremely responsive to N fertiliser application, with yields increasing from 2.0 ± 0.15 t ha–1 without fertiliser N to >6 t ha–1 with the various N fertilisers (Table 2). The highest recorded yield was 6.7 ± 0.59 t ha–1 in the DMPP treatment, but there were no statistically significant differences in crop yields between fertiliser products. Total N uptake increased from 46 ± 8.1 kg N ha–1 with 0N fertiliser applied to a range of 134–167 kg N ha–1 with the different fertiliser treatments. The effect of EEFs on NUE was evaluated using the apparent N recovery calculated by subtracting the N yields of the 0N plots from that of the fertilised plots and expressed as a percentage of the total fertiliser N applied (Henzell 1971). The apparent fertiliser N recovery in crop biomass was 57 ± 6%, 69 ± 14%, 55 ± 9%, and 49 ± 14% for the Urea, DMPP, PCU and Nitrapyrin treatments, respectively. DMPP significantly increased total N uptake and fertiliser N recovery compared with the Nitrapyrin treatment.
Discussion
Temporal variability of N2O emissions
Nitrous oxide emissions in this sub-tropical cereal cropping system were highly episodic and mainly in response to heavy rainfall events within two months after planting and fertiliser N application when soil mineral N content was still high (Fig. 3). This is in good agreement with results from studies in other sub-tropical cereal systems in Australia (De Antoni Migliorati et al. 2014; Jamali et al. 2015; Schwenke et al. 2015; De Antoni Migliorati et al. 2016) where emission pulses were generally observed after heavy rainfall or irrigation. These emission pulses are typically triggered by the combination of high soil moisture, high availability of NO3–-N following fertilisation and high soil temperatures. Previous studies have shown that the amount of rainfall or irrigation affects the magnitude of N2O losses and that events greater than 50–60 mm are typically required to trigger such pulses (Jamali et al. 2015; Scheer et al. 2012). In this study several smaller rainfall events (up to 36 mm) in December and early January stimulated N2O emissions (up to 46 g N2O-N ha–1 day–1), but those emissions were dwarfed by the fluxes recorded with the heavy rainfall event that occurred in late January, when 106 mm fell over six days and the upper soil stayed near saturation for almost 10 days (Fig. 1). Heavy rainfall that occurred later in the growing season (99 mm over seven days in early March) did not generate high N2O emissions, demonstrating that most of the applied N was either in plant or microbial biomass, deeper in the soil profile and less vulnerable to denitrification, or was already lost to the environment. This highlights the importance of the early stage of the summer growing season for N2O mitigation in this particular cropping system. Mitigation methodologies need to target this emission window, when conditions are highly conducive for elevated N2O losses following heavy rainfall events.
Effect of EEFs on N2O emissions and plant production
Enhanced efficiency fertiliser technology has been promoted as a strategy to decrease N2O emissions, total denitrification losses and N leaching by avoiding high concentrations of NO3–-N in the soil profile. Our hypothesis that N2O emissions from a sub-tropical cereal cropping system can be decreased by the use of EEFs was confirmed for DMPP and PCU fertiliser, while Nitrapyrin resulted in a much lower, but not statistically significant, decrease in N2O emissions. These results are consistent with those reported by De Antoni Migliorati et al. (2016) who reported a 60% decrease in N2O emissions by the use of DMPP-coated urea from a sub-tropical cereal crop (sorghum) grown on a Vertisol and an Oxisol. In soils N2O is produced during nitrification and denitrification. Nitrification inhibitors block the enzyme ammonia mono-oxygenase which catalyses the first step of nitrification, i.e. the oxidation of ammonium (NH4+) to nitrite (NO2–) (Zerulla et al. 2001). This inhibition delays soil microbial nitrification, resulting in lower soil NO3– levels and consequently decreasing potential denitrification losses. PCU slows the release of fertiliser through coating of the fertiliser granule and is designed to release more N later in crop growth as the N demand increases, thus decreasing the quantity of soil N available for nitrification and denitrification processes and therefore N2O formation (Halvorson et al. 2014). In this study, fertiliser N at planting was applied well in advance of maximum crop demand. The combination of wide crop row spacing and relatively high fertiliser N rates resulted in high in-band concentrations of mineral N which, with conventional fertilisers, were rapidly nitrified into NO3–-N (Fig. 2). These localised bands of high NO3–-N concentration were situated in the relatively carbon-rich top soil layers where microbial activity is most pronounced, and so are especially vulnerable to denitrification from high intensity rainfall events early in the crop growing season. This explains the extremely high N2O emissions observed in the Urea treatment in late January 2013. Both DMPP and PCU were very efficient at inhibiting the conversion of urea into NO3–-N, thus avoiding high in-band concentrations of NO3–-N and substantially decreasing the denitrification potential. These results also show that DMPP and PCU fertiliser products continued to be effective until early February 2013. This was unexpected for the DMPP treatment since the period of time during which DMPP is effective strongly depends on soil temperature, and it has been shown that at 20°C this effect lasts ~7 weeks (Zerulla et al. 2001). In the current study, with an average soil temperature of 25°C, there was still an effect of DMPP nine weeks after the last application. Overall, these findings indicate that DMPP and PCU fertiliser products have substantial scope to abate N2O emissions that occur in the first 6–8 weeks after fertiliser application and/or planting, before the plant can accumulate the fertiliser N in crop biomass. This is particularly important in summer-dominated cropping systems where large rainfall events can occur.
The second hypothesis, that EEFs will increase the NUE, cannot be confirmed from this study. The highest N uptake and apparent N fertiliser recovery was observed in the DMPP treatment, but this was not significantly different to the conventional urea treatment and there were no differences in crop yields between fertiliser products. This was unexpected, given the substantial decrease in N2O emissions, but is most likely explained by the high fertiliser N application rates used in this product comparison. There was surplus N to meet crop demands in all treatments and hence there was no significant scope for the EEF products to increase NUE or increase crop yield. As shown by Lester et al. (2016), DMPP might have the greatest scope to increase NUE under high-intensity cropping when high rates of N fertiliser are necessary to meet crop demand. Future EEF studies should involve a range of N rates to identify if decreased N rates can be used with EEF products to achieve the target yield.
N2O emission factors and N2O intensities
Nitrous oxide emission factors ranged from 0.14 to 1.27% of total fertiliser N applied for the different fertiliser products. The value for the conventional urea treatment (1.27%) is at the upper level of emission factors reported for other sub-tropical cereal production systems in Australia, which may be related to the substantial in-crop rainfall early in the season. De Antoni Migliorati et al. (2014) reported emission factors of 0.69% for a wheat–maize crop rotation fertilised with urea, while Schwenke et al. (2015) reported 0.48% to 0.78% from a canola–chickpea rotation. It is also significantly higher than the current type I emission factor for all non-irrigated N-fertilised crops in Australia (0.3%) (DCCEE 2010). The significantly lower emission factors in the DMPP and PCU treatments reflect the efficacy of these products in mitigating N2O emissions. The annual emission factor of 0.14% measured in the DMPP treatment represents one of the lowest annual emission factors reported from fertilised sub-tropical cereal systems. Wang et al. (2011) reported emission factors ranging from 0.39% to 1.78% in a sub-tropical cereal crop in Australia, while Zhou et al. (2014) reported 0.39% to 0.72% from a sub-tropical wheat–maize rotation in China. This significant decrease of emission factors in the EEF treatments highlights the potential for decreased emission factors for these products. The efficacy of different EEF products in maximising crop yield and decreasing N2O losses was assessed through the emissions intensity, defined as the ratio of N2O emitted in relation to the grain produced. The use of EEF decreased the emissions intensity in all treatments. DMPP had the lowest emissions intensity (0.06 kg N2O-N emitted per t grain yield) of all treatments, producing a 6-fold reduction in the emissions intensity compared with the Urea treatment. These findings are similar to other studies that tested the effect of DMPP fertilisers in sub-tropical cereal systems (De Antoni Migliorati et al. 2014, 2016), and indicate that the use of EEFs is a feasible strategy to decrease N2O emissions whilst sustaining high crop yields. The use of EEFs (DMPP and PCU) can therefore be recommended in sub-tropical cereal systems. However, more research is required to assess the effect of EEFs on other N loss pathways, namely N2 losses from denitrification and ammonia volatilisation, and field studies are required to optimise N rates and timing for different EEF products. Further research is also needed to assess if the increased costs of using EEFs can be compensated by decreased fertiliser application rates and yield increases.
Conclusion
Automated high frequency measurements were conducted over a full year to study the effect of three different EEFs on N2O emissions, NUE and yield in a sub-tropical cereal cropping system in Australia. Nitrous oxide emissions were highly episodic and controlled mainly by heavy rainfall events occurring up to two months after planting and fertiliser N application. Two of the tested products (DMPP and PCU) were found to be highly effective, decreasing annual N2O losses by 83% and 70%, respectively, but did not affect yield or NUE. This study shows that N2O emissions from a sub-tropical cropping system fertilised with conventional urea can be high if heavy rainfall events occur early in the season. EEFs offer a substantial potential to abate N2O emissions but need to be tested for their efficacy in different cropping systems. The application of DMPP and PCU fertiliser products for summer grain crops in sub-tropical Australia can be recommended, while Nitrapyrin did not show any significant effect on N2O emissions. Despite the large decrease in N2O emissions, there was no real evidence of improved NUE or grain yields in the DMPP and PCU treatments. Further research is needed to quantify total fertiliser N losses from EEFs and to identify if lower N rates could be used with these EEF products to achieve the target yield. Such data is needed to evaluate if the increased costs of EEF products can be compensated by lower fertiliser application rates.
Acknowledgements
This research was undertaken as part of the National Agricultural Nitrous Oxide Research Program funded by the Australian Department of Agriculture (Project 1202.004). We would also like to thank Patricia Balzer and Lawrence Smith for their valuable support during the field campaign, the Department of Agriculture, Fisheries and Forestry (DAFF) for providing the field site, machinery and staff for the farming operations.
References
Abalos D, Jeffery S, Sanz-Cobena A, Guardia G, Vallejo A (2014) Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agriculture, Ecosystems & Environment 189, 136–144.| Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXnslGqs7Y%3D&md5=05c0ff60ecf5bb5ce2cf3b91bc7194b9CAS |
Akiyama H, Yan X, Yagi K (2010) Evaluation of effectiveness of enhanced‐efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta‐analysis. Global Change Biology 16, 1837–1846.
| Evaluation of effectiveness of enhanced‐efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta‐analysis.Crossref | GoogleScholarGoogle Scholar |
Association of American Plant Food Control Officials (AAPFCO) (2013) Official Publication 65. Little Rock, AR, USA
Davidson EA (2009) The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nature Geoscience 2, 659–662.
| The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVKktb7P&md5=92801f81686accec31bdad9a5ca2b8c2CAS |
De Antoni Migliorati M, Scheer C, Grace PR, Rowlings DW, Bell M, McGree J (2014) Influence of different nitrogen rates and DMPP nitrification inhibitor on annual N2O emissions from a subtropical wheat–maize cropping system. Agriculture, Ecosystems & Environment 186, 33–43.
| Influence of different nitrogen rates and DMPP nitrification inhibitor on annual N2O emissions from a subtropical wheat–maize cropping system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmsVCjtbo%3D&md5=0844cd1e106bc3431f689d097fcc0b36CAS |
De Antoni Migliorati M, Bell M, Grace PR, Scheer C, Rowlings DW, Liu S (2015) Legume pastures can reduce N2O emissions intensity in subtropical cereal cropping systems. Agriculture, Ecosystems & Environment 204, 27–39.
| Legume pastures can reduce N2O emissions intensity in subtropical cereal cropping systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjtFegsL4%3D&md5=1e18334b81692abfbeea6425732375d5CAS |
De Antoni Migliorati M, Bell M, Lester D, Rowlings DW, Scheer C, de Rosa D, Grace PR (2016) Comparison of grain yields and N2O emissions on Oxisol and Vertisol soils in response to fertiliser N applied as urea or urea coated with nitrification inhibitor 3,4-dimethylpyrazole phosphate. Soil Research 54, 552–564.
| Comparison of grain yields and N2O emissions on Oxisol and Vertisol soils in response to fertiliser N applied as urea or urea coated with nitrification inhibitor 3,4-dimethylpyrazole phosphate.Crossref | GoogleScholarGoogle Scholar |
Department of Climate Change and Energy Efficiency (DCCEE) (2010) Australian national greenhouse accounts. National inventory report 2008, Department of Climate Change and Energy Efficiency, Australia. Available at https://www.environment.gov.au/system/files/resources/add6a870-0846-4b62-85ac-78527144e370/files/national-inventory-report-2010-1.pdf [verified 7 June 2016]
Erisman JW, Sutton MA, Galloway J, Klimont Z, Winiwarter W (2008) How a century of ammonia synthesis changed the world. Nature Geoscience 1, 636–639.
| How a century of ammonia synthesis changed the world.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFOlur%2FM&md5=a1dcf08a1b0a398a2509f5733d4c3568CAS |
Halvorson AD, Snyder CS, Blaylock AD, Del Grosso SJ (2014) Enhanced-efficiency nitrogen fertilizers: Potential role in nitrous oxide emission mitigation. Agronomy Journal 106, 715–722.
| Enhanced-efficiency nitrogen fertilizers: Potential role in nitrous oxide emission mitigation.Crossref | GoogleScholarGoogle Scholar |
Heffer P (2013) ‘Assessment of fertilizer use by crop at the global level 2010–2011.’ (International Fertilizer Industry Association: Paris, France)
Henzell E (1971) Recovery of nitrogen from four fertilizers applied to Rhodes grass in small plots. Australian Journal of Experimental Agriculture 11, 420–430.
| Recovery of nitrogen from four fertilizers applied to Rhodes grass in small plots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE38XivVOl&md5=ff63e74a8ee7b65622186b0e5ac81089CAS |
Irigoyen I, Muro J, Azpilikueta M, Aparicio-Tejo P, Lamsfus C (2003) Ammonium oxidation kinetics in the presence of nitrification inhibitors DCD and DMPP at various temperatures. Australian Journal of Soil Research 41, 1177–1183.
| Ammonium oxidation kinetics in the presence of nitrification inhibitors DCD and DMPP at various temperatures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXot1Cgt70%3D&md5=92d1b1fadc60b4d0482344e6dd14bd3cCAS |
Isbell RF (2002) ‘The Australian soil classification.’ (CSIRO Publishing: Melbourne)
Jamali H, Quayle WC, Baldock J (2015) Reducing nitrous oxide emissions and nitrogen leaching losses from irrigated arable cropping in Australia through optimized irrigation scheduling. Agricultural and Forest Meteorology 208, 32–39.
| Reducing nitrous oxide emissions and nitrogen leaching losses from irrigated arable cropping in Australia through optimized irrigation scheduling.Crossref | GoogleScholarGoogle Scholar |
Ladha JK, Pathak H, Krupnik TJ, Six J, van Kessel C (2005) Efficiency of fertilizer nitrogen in cereal production: retrospects and prospects. Advances in agronomy 87, 85–156.
| Efficiency of fertilizer nitrogen in cereal production: retrospects and prospects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitlahsLg%3D&md5=e0f949b1a934047e4fd867c7ed8eabe2CAS |
Lester DW, Bell MJ, Bell KL, De Antoni Migliorati M, Scheer C, Rowlings D, Grace PR (2016) Agronomic responses of grain sorghum to DMPP-treated urea on contrasting soil types in north-eastern Australia. Soil Research 54, 565–571.
| Agronomic responses of grain sorghum to DMPP-treated urea on contrasting soil types in north-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Merino P, Menéndez S, Pinto M, González-Murua C, Estavillo JM (2005) 3,4-Dimethylpyrazole phosphate reduces nitrous oxide emissions from grassland after slurry application. Soil Use and Management 21, 53–57.
| 3,4-Dimethylpyrazole phosphate reduces nitrous oxide emissions from grassland after slurry application.Crossref | GoogleScholarGoogle Scholar |
Mosier A, Syers JK, Freney JR (eds) (2004) ‘Agriculture and the nitrogen cycle. Assessing the impacts of fertilizer use on food production and the environment.’ (Island Press: Washington DC)
Powell BS, Baker DE, Christianos NG (1988) Soils of the Kingsthorpe Field Station, eastern Darling Downs. Project Report QR88003. (Ed. QDoP Industries) p. 20. (Land Resources Branch, Queensland Department of Primary Industries: Brisbane, Qld)
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326, 123–125.
| Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtF2hs7jF&md5=d412beb03e751c999a0044beb213d53fCAS | 19713491PubMed |
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods: Australasia.’ (CSIRO Publishing: Collingwood)
Scheer C, Grace PR, Rowlings DW, Payero J (2012) Nitrous oxide emissions from irrigated wheat in Australia: impact of irrigation management Plant and Soil. 359, 351–362.
| Nitrous oxide emissions from irrigated wheat in Australia: impact of irrigation managementCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlGmsrrJ&md5=0a0042c45a025374b5e699c821e096f1CAS |
Scheer C, Rowlings DW, Firrel M, Deuter P, Morris S, Grace PR (2014) Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical Australia. Soil Biology & Biochemistry 77, 243–251.
| Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht12ntL3M&md5=ee89302220febfea18060a4a6d021cc2CAS |
Schwenke GD, Herridge DF, Scheer C, Rowlings DW, Haigh BM, McMullen KG (2015) Soil N2O emissions under N2-fixing legumes and N-fertilised canola: A reappraisal of emissions factor calculations. Agriculture, Ecosystems & Environment 202, 232–242.
| Soil N2O emissions under N2-fixing legumes and N-fertilised canola: A reappraisal of emissions factor calculations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtVOgsL4%3D&md5=5e74db16ebc6b862a9ac74b9a0396a34CAS |
United Nations Population Fund (UNFPA) (2011) ‘State of the world population 2011.’ (United Nations Population Fund: New York, NY, USA)
Wang W, Dalal RC, Reeves SH, Butterbach-Bahl K, Kiese R (2011) Greenhouse gas fluxes from an Australian subtropical cropland under long‐term contrasting management regimes. Global Change Biology 17, 3089–3101.
| Greenhouse gas fluxes from an Australian subtropical cropland under long‐term contrasting management regimes.Crossref | GoogleScholarGoogle Scholar |
Zerulla W, Barth T, Dressel J, Erhardt K, von Locquenghien KH, Pasda G, Rädle M, Wissemeier A (2001) 3, 4-Dimethylpyrazole phosphate (DMPP)–a new nitrification inhibitor for agriculture and horticulture. Biology and Fertility of Soils 34, 79–84.
| 3, 4-Dimethylpyrazole phosphate (DMPP)–a new nitrification inhibitor for agriculture and horticulture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltlyqt7g%3D&md5=a15ee234ef5ce9013521b5fc5b89aeccCAS |
Zhou M, Zhu B, Brüggemann N, Bergmann J, Wang Y, Butterbach-Bahl K (2014) N2O and CH4 emissions, and NO3 – leaching on a crop-yield basis from a subtropical rain-fed wheat–maize rotation in response to different types of nitrogen fertilizer. Ecosystems 17, 286–301.
| N2O and CH4 emissions, and NO3 – leaching on a crop-yield basis from a subtropical rain-fed wheat–maize rotation in response to different types of nitrogen fertilizer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjslWntrY%3D&md5=c9e7aee3ba9b25329389a095a7d07169CAS |






