The composition of organic phosphorus in soils of the Snowy Mountains region of south-eastern Australia
Ashlea L. Doolette A C , Ronald J. Smernik A and Timothy I. McLaren A BA Soils Group, School of Agriculture, Food and Wine and Waite Research Institute, Davies Building, The University of Adelaide, Waite Campus, Urrbrae, SA 5064, Australia.
B Present address: Group of Plant Nutrition, Institute of Agricultural Sciences, Swiss Federal Institute of Technology (ETH) Zurich, Eschikon 33, CH-8315 Lindau, Switzerland.
C Corresponding author. Email: ashlea.doolette@adelaide.edu.au
Soil Research 55(1) 10-18 https://doi.org/10.1071/SR16058
Submitted: 1 March 2016 Accepted: 6 July 2016 Published: 10 October 2016
Journal Compilation © CSIRO Publishing 2017 Open Access CC BY-NC-ND
Abstract
Few studies have considered the influence of climate on organic phosphorus (P) speciation in soils. We used sodium hydroxide–ethylenediaminetetra-acetic acid (NaOH–EDTA) soil extractions and solution 31P nuclear magnetic resonance spectroscopy to investigate the soil P composition of five alpine and sub-alpine soils. The aim was to compare the P speciation of this set of soils with those of soils typically reported in the literature from other cold and wet locations, as well as those of other Australian soils from warmer and drier environments. For all alpine and sub-alpine soils, the majority of P detected was in an organic form (54–66% of total NaOH–EDTA extractable P). Phosphomonoesters comprised the largest pool of extractable organic P (83–100%) with prominent peaks assigned to myo- and scyllo-inositol hexakisphosphate (IP6), although trace amounts of the neo- and d-chiro-IP6 stereoisomers were also present. Phosphonates were identified in the soils from the coldest and wettest locations; α- and β-glycerophosphate and mononucleotides were minor components of organic P in all soils. The composition of organic P in these soils contrasts with that reported previously for Australian soils from warm, dry environments where inositol phosphate (IP6) peaks were less dominant or absent and humic-P and α- and β-glycerophosphate were proportionally larger components of organic P. Instead, the soil organic P composition exhibited similarities to soils from other cold, wet environments. This provides preliminary evidence that climate is a key driver in the variation of organic P speciation in soils.
Additional keywords: climate, organic matter, solution 31P NMR spectroscopy.
Introduction
The soil organic matter (SOM) content of soils is influenced by all the key soil-forming factors, i.e. parent material, topography, climate, vegetation and time. In broad terms, the influence of climate on SOM levels is two-fold: carbon (C) and nitrogen (N) contents of soils are positively correlated with precipitation and negatively correlated with temperature (Post et al. 1982; Oades 1988). Changes in SOM are often examined along elevation gradients as they reflect differences in climatic conditions. Chatterjee and Jenerette (2015) investigated changes in SOM along an elevation gradient from a sub-alpine through to a desert environment. The authors observed an increase in SOM with increasing elevation, which they attributed to a greater input of organic matter with a constant rate of SOM decomposition. Similarly, Trumbore et al. (1996) showed that the rate of C turnover increased with decreasing temperature (increasing elevation) along a gradient in Sierra Nevada, California. In contrast, Yang et al. (2011) found soil organic C and total N decreased with increasing drought-stress as primary productivity decreased along an aridity gradient in China. In most cases the influence of climate on SOM can be attributed to its effect on soil biological activity, both primary productivity and microbial breakdown. While primary productivity is most affected by low temperatures and dry conditions, the breakdown of organic matter by microbial processes is slowed when soils are either too dry or too wet, or under cold (Van Meeteren et al. 2007) or acidic (Hollings et al. 1969) conditions. Thus under warmer climates, so long as there is adequate moisture, the rate of microbial processes is greater and rapid decomposition does not allow for the accumulation of organic matter.
Though studies of SOM cycling usually focus on C and N, consideration should also be given to phosphorus (P) (Kirkby et al. 2011) as organic P can constitute a large proportion of total P in many soils (Williams and Steinbergs 1958), and although not directly plant available, can provide a source of P for plant uptake once mineralised. However, surprisingly few researchers have examined the role of climate on soil organic P. Turner et al. (2003a) suggested that climate was a key driver of the low SOM concentrations reported for a series of semiarid arable cropping soils. The authors found that decreasing organic P concentrations correlated with increasing temperatures, whereas soil organic P increased with increasing precipitation. Sumann et al. (1998) reported an effect of climate on organic P speciation. They observed a strong correlation between climate and phosphodiesters, but a weak correlation with phosphomonesters. Furthermore, the proportion of phosphodiesters increased with increasing mean annual temperature and precipitation whereas the proportion of phosphomonoesters declined. Generally under cold, wet conditions, low decomposition rates result in the persistence and accumulation of organic P species thought to be labile, such as phosphodiesters, notably DNA (Makarov et al. 2002a) and phosphonates (Tate and Newman 1982). However, seasonal differences in organic P content have also been observed by Sharpley (1985), who found that concentrations of organic P in soils were higher in winter than in spring, which he attributed to a slower rate of mineralisation and removal of P from the surface through plant uptake in winter. In a study of cultivated and uncultivated soils under different environmental conditions, Condron et al. (1990) reasoned that the accumulation of phosphodiesters occurred when there was limited microbial decomposition due to waterlogging of the soil. McKercher and Anderson (1968) identified climate as the reason for lower levels of inositol P in Canadian compared with British soils (Anderson 1964).
Recently, we hypothesised that the generally low concentrations of inositol phosphates reported for most Australian soils could be related to the generally warm conditions across most of Australia, and in particular, the warm environments from which the soils analysed to date have been sourced (McLaren et al. 2015a; Moata et al. 2016).
In this study we have targeted soils from the Australia-wide Australian Soil Resources Information System (ASRIS) database that were sampled from the only alpine and sub-alpine environments in mainland Australia. The aim was to compare the P speciation of this set of soils with those previously reported in the literature of soils from other cold and wet locations around the world, as well as those of other Australian soils from warmer and drier environments.
Materials and methods
Soil selection and classification
A digital dataset of soils (ASRIS) was created as part of the National Land and Water Resources Audit of Australia in 2001 (Johnston et al. 2003). The data are a collation of soil and vegetation survey data collected from the 1950s to the 1990s. While these data were collected for differing purposes and by different agencies, in some instances the original samples are still in existence and are stored in the CSIRO National Soils Archive. This provided us access to soils that are otherwise difficult to obtain and also an opportunity to add information to the widely used ASRIS database.
In this study, we employed the spatial search feature of ASRIS to select the only soils available in the CSIRO Soil Archive from alpine and sub-alpine regions of mainland Australia; the soils were all collected from the Snowy Mountains region of south-eastern Australia, and within the Kosciusko National Park. All five soils were collected in 1968 by Wood (1970, 1974), who reported on rates of biomass decomposition (Wood 1970) and earthworm communities (Wood 1974); these papers also contain detailed information about the formation and properties of soils of this region. At this time, the soil taxonomic scheme in use in Australia was the classification scheme of Great Soil Groups (Stace et al. 1968). Under this scheme, soils A1053, A1054 and A1055 are classified as Alpine Humus and soils A1056 and A1507 are classified as Podosols. However, all five soils are classified as Podosols under the current Australian Soil Classification (Isbell 2002). Vegetation and parent material at the collection sites are reported in Wood (1970, 1974). Soils A1053, A1054 and A1055 were collected under tall alpine herbfields, whereas soils A1056 and A1057 were collected under dry sclerophyll forest. All soils were developed on gneissic granite parent material (Wood 1974).
Soil characterisation
Soil samples were collected from the 0–10 cm layer and ground to pass through a 2-mm sieve before analysis. Total soil C and N were determined on the archived soils using a dry combustion CNS-2000 analyser (LECO Corporation, St. Joseph, MI, USA) (Matejovic 1997). Total P concentrations were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) following aqua regia (HNO3/HCl; 1 : 3) digestion (Zarcinas et al. 1996). Texture, soil pH and electrical conductivity (EC) were not determined in this study as there was insufficient soil available to repeat this analysis; values reported here are taken directly from the ASRIS database.
NaOH–EDTA extraction
Soil samples were ground to pass through a 2-mm sieve before extraction. Soils were extracted with sodium hydroxide–ethylenediaminetetra-acetic acid (NaOH–EDTA) using standard procedures for nuclear magnetic resonance (NMR) analysis based on those of Cade-Menun and Preston (1996) and Turner (2008). In summary, 4 g (±0.10) of soil was extracted with 0.25 M NaOH + 0.05 M Na2EDTA at a 1 : 10 soil to solution ratio and shaken for 16 h. The extracts were then centrifuged at 1400g for 20 min at 20°C and the supernatant collected after filtration through a Whatman number 42 filter paper. A 20-mL aliquot of the filtrate was frozen in liquid N2 and freeze-dried to concentrate the extracts before spectroscopic analysis. This resulted in isolation of ~600 mg of freeze-dried material.
Solution 31P NMR spectroscopy
A 500-mg subsample of each freeze-dried extract was dissolved in 5 mL of deionised water and centrifuged at 1400g for 20 min. A 3.5-mL aliquot of the redissolved NaOH–EDTA extract, 0.3 mL of deuterium oxide and 0.1 mL of a methylenediphosphonic acid (MDP) (Sigma-Aldrich; M9508; ≥99%) solution containing 6.0 g MDP L–1 were then placed in a 10-mm diameter NMR tube. Solution 31P NMR spectra were obtained at 24°C on a Varian INOVA 400 NMR spectrometer (Varian, Palo Alto, CA) at a 31P frequency of 161.9 MHz. Samples were analysed using a 30-µs pulse (90°), a recycle delay of 14–22 s (set to ≥5 × T1 based on a preliminary inversion-recovery experiment), an acquisition time of 1.0 s and gated broadband 1H decoupling. Between 2851 and 5580 scans were acquired for each sample. Spectra are plotted with a line broadening of 2 Hz. The chemical shift of signals is reported in parts per million (ppm) relative to an external standard of 85% H3PO4.
Quantification of P species in NaOH–EDTA extracts by integration and deconvolution of 31P NMR spectra
Quantification of soil P forms in NaOH–EDTA extracts using spectral integration was based on the addition of a known amount of MDP to NaOH–EDTA extracts, which gave a unique spectral signal separate from all other resonances. The peak area of the MDP signal is proportional to the absolute concentration added, which was then compared with the peak areas of all other resonances contained in the solution 31P NMR spectrum.
The following classes of P species were quantified using spectral integration based on previous studies (Smernik and Dougherty 2007; Doolette et al. 2009): phosphonates (δ 18–22 ppm), MDP (δ 16–18 ppm) orthophosphate (δ 7.0–5.4 ppm), phosphomonoesters (δ 5.4–3.5 ppm), phosphodiesters (δ 2 to –1.0 ppm) and pyrophosphate (δ –4.5 to –5.5 ppm). Owing to several overlapping peaks within the orthophosphate and phosphomonoester region of the 31P NMR spectra, the relative concentrations of P species were determined using the integration and deconvolution technique described in McLaren et al. (2015a). Briefly, this method involved first quantifying the broad feature and then quantifying the individual sharp resonances. Consequently, the orthophosphate and phosphomonoester region of the NMR spectra were partitioned into the following components: (i) orthophosphate; (ii) a broad feature (hereafter termed humic-P); (iii) myo-inositol hexakisphosphate (phytate, myo-IP6); (iv) scyllo-inositol hexakisphosphate (scyllo-IP6); (v) lipid P, identified as the sum of α- and β-glycerophosphate (Baer and Kates 1948; Doolette et al. 2009); and (vi) RNA P, identified as the sum of up to four peaks assigned to mononucleotides derived from alkaline hydrolysis of RNA (Smernik et al. 2015). The relatively poor quality of the 31P NMR spectrum for soil A1056 precluded the application of deconvolution for this soil.
Results
General description of soils
Alpine regions are rare in Australia and comprise only 0.15% of the total land area, occurring mainly in the Snowy Mountains in south-eastern Australia and Tasmania (Williams et al. 2008). Australian alpine and sub-alpine ecosystems are traditionally defined as landscapes situated above elevations of ~1000 m (Williams et al. 2008) where the ground is usually covered with snow for at least one month of the year (Costin 1957). The division of alpine and sub-alpine environments is related to the average length of snow cover throughout the year. Alpine regions are those with longer periods of snow cover (>4 months) and occur above the tree line; in mainland Australia they generally occur at an elevation greater than 1800 m. In sub-alpine regions the ground is covered with snow for 1–4 months of the year and are generally found below the tree line although tree development is limited. In mainland Australia sub-alpine environments generally occur at an elevation of 1400–1800 m (Costin 1957; Williams and Costin 1994; Williams et al. 2008). Soils A1053 and A1054 were collected from within 1800–2000 m above sea level (Table 1) and can be considered alpine soils. Soil A1055 is classified as sub-alpine as it was collected from a site at over 1500 m above sea level. Soils A1056 and A1057 were collected at sites at ~1200 m above sea level in an environment better described as montane; that is environments at elevations of 1100–1400 m, which are typically below the snow line and where the climate is still cool but drier than alpine and sub-alpine regions (Costin 1954).
In the Snowy Mountains region there is a large increase in rainfall with increasing elevation, ranging from ~900 mm in the montane–lower sub-alpine areas to >2000 mm in the higher elevation alpine areas. Also, the annual minimum temperature ranges from –0.4 to 4°C and the annual maximum temperature ranges from 9.8 to 18.1°C (Table 1).
Soil pH varied from moderately (4.7) to slightly (6.2) acidic. The range of soil organic C was 2.4–12%, total soil N was 0.1–0.98% and total soil P was 195–1190 mg kg–1. Soil texture varied from loamy sand to clay loam.
Quantitative analysis and interpretation of 31P NMR spectra on NaOH–EDTA soil extracts
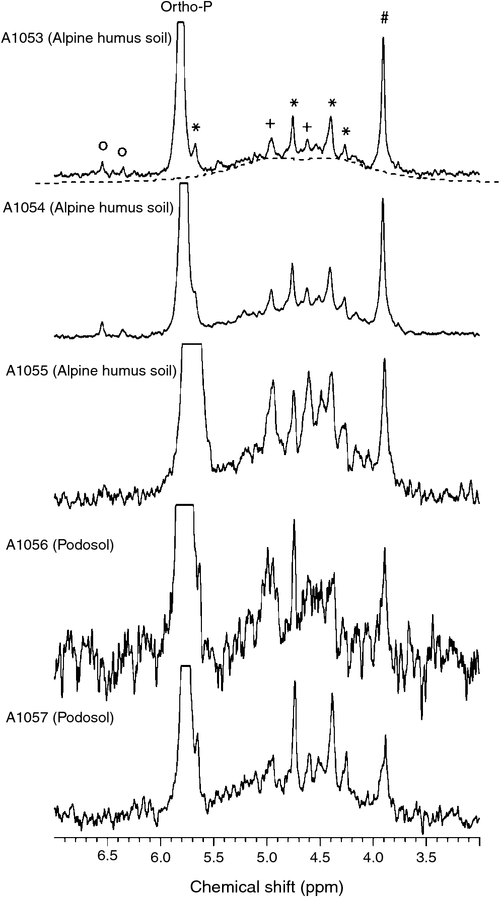
The 31P NMR spectra of NaOH–EDTA extracts of the five soils are displayed in Fig. 1 to highlight the spectral features of the orthophosphate and phosphomonoester regions. For each soil, the orthophosphate resonance centred at δ 5.7–5.8 ppm is the most prominent peak in the spectrum and is consequently off-scale in this representation (see Fig. 2 for another representation of the NMR spectra in which the orthophosphate peak is on-scale). For the three Alpine Humus soils (A1053, A1054 and A1055), the most prominent peak within the phosphomonoester region appeared at δ 3.9 ppm and this can be assigned to scyllo-IP6 (Turner and Richardson 2004; Doolette et al. 2009). This peak was also clearly present in the 31P NMR spectra of the other two soils (A1056 and A1057). For soils A1053 and A1054, the next most prominent peaks within the phosphomonoester region were at δ 4.8 and δ 4.4 ppm, and can be assigned to myo-IP6. Again, these peaks were also clearly seen in the 31P NMR spectra of the other soils, A1055, A1056 and A1057 and in fact, these two myo-IP6 peaks were the most prominent peaks in the phosphomonoester region of soil A1057. Two smaller peaks, at δ 5.7 ppm (where it appeared as a shoulder on the much larger orthophosphate peak) and at δ 4.3 ppm can also be attributed to myo-IP6, a molecule that contains six phosphate groups and has an axis of symmetry that results in a 1 : 2 : 2 : 1 pattern of 31P NMR peaks (Johnson and Tate 1969).
|
|
Peaks at δ 5.0 and δ 4.6 ppm were also present in all spectra, and were particularly prominent for soil A1055. These peaks can be assigned as α- and β-glycerophosphate, respectively, which are most likely primarily derived from the alkaline hydrolysis of phospholipids (Baer and Kates 1950; Doolette et al. 2009). Other small peaks were evident within the phosphomonoester region: (i) at δ 6.5 and δ 6.3 ppm in the 31P NMR spectra of soils A1053 and A1054, most likely due to the neo- and d-chiro-stereoisomers of myo-IP6 (Turner et al. 2012) and (ii) several small peaks between δ 4.0 and δ 4.8 ppm, which are likely mononucleotides produced by hydrolysis of RNA under alkaline conditions (Smernik et al. 2015). All 31P NMR spectra in Fig. 1 also contained a broad signal extending from around δ 6.0 to δ 3.5 ppm that has been attributed to phosphate groups attached via monoester linkages in high molecular weight material and is referred to as humic-P (Doolette et al. 2011; McLaren et al. 2015b).
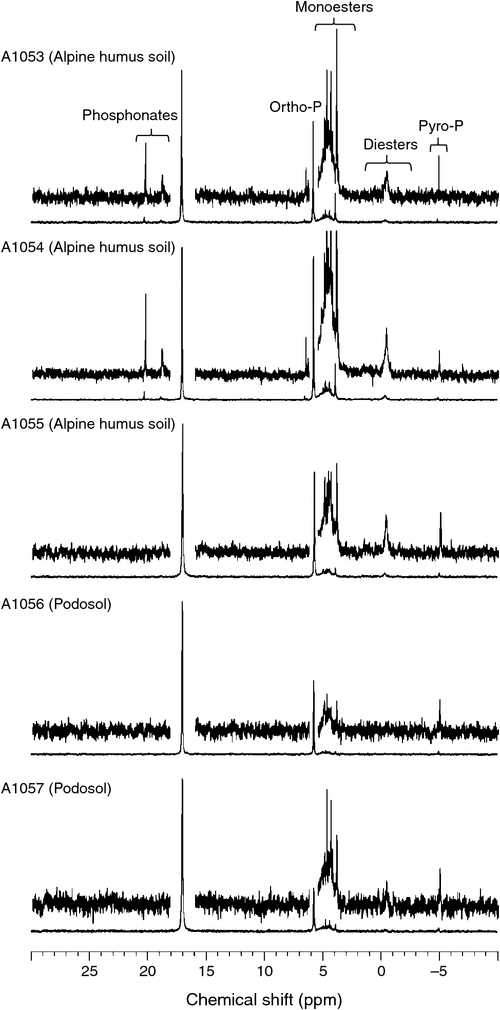
Several 31P NMR signals appear outside the chemical shift region shown in Fig. 1; these can be seen in Fig. 2. Two small peaks that appeared at δ 18.8 and δ 17.2 ppm in the 31P NMR spectrum of soils A1053 and A1054 are attributed to phosphonates (Turner et al. 2003b), compounds which contain a direct C–P bond. A broad signal centred at δ –0.5 ppm is attributed to phosphodiesters and is most likely due to DNA (Newman and Tate 1980; Makarov et al. 2002b). Finally, a sharp peak that appeared in all spectra at δ –5.0 ppm can be assigned to pyrophosphate.
Concentrations of P species determined by 31P spectroscopy on NaOH–EDTA extracts are shown in Table 2. Comparison of total detected NMR signal against total P determined by ICP-OES following aqua regia digestion indicated extraction efficiencies by NaOH–EDTA of 46–73% of total soil P. A portion of the undetected P is likely to be orthophosphate in mineral forms that are not soluble in the NaOH–EDTA extractant, including for example fluorapatite, xenotime and monazite (Williams et al. 1980). It is also likely that some organic P present in the soils was not extracted into NaOH–EDTA (Doolette et al. 2011; McLaren et al. 2015c). Concentrations of extractable orthophosphate were in the range of 46–179 mg P kg–1 (Table 2) and represented 32–45% of P detected in the NaOH–EDTA extracts. In addition, a small proportion of P (3–6 mg P kg–1) was detected as pyrophosphate. Thus, for all soils the majority (54–66%) of P detected in the NaOH–EDTA extracts was in an organic form.
Concentrations of humic-P were in the range of 52–190 mg P kg–1 (Table 2). Humic-P was the most abundant P species detected for soils A1053, A1054 and A1057, with concentrations slightly higher than for orthophosphate. The concentration of humic-P was lower than that of orthophosphate for soil A1055; for soil A1056 the poor quality of the 31P NMR spectrum precluded the application of deconvolution, but the concentration of total phosphomonoester P was less than half that of orthophosphate (Table 2).
Concentrations of myo-IP6 were in the range of 17–34 mg P kg–1 (Table 2), whereas concentrations of its stereoisomer, scyllo-IP6, were 6–36 mg P kg–1. The remaining phosphomonesters comprised only 9–13% of total organic P and included up to 7 mg P kg–1 as neo- and d-chiro-IP6, up to 14 mg P kg–1 as α- and β-glycerophosphate and up to 18 mg P kg–1 as RNA mononucleotides. Phosphodiesters (up to 10% of total NaOH–EDTA extractable P) and phosphonates (up to 4% of total NaOH–EDTA extractable P) were minor components of P in all soil extracts where they were detected. Polyphosphate (<2% total NaOH–EDTA extractable P) was only detected in sample A1053.
Discussion
The total C (2.4–12%) and N (0.10–0.98%) contents of the soils in the current study (Table 1) are similar to those of 166 Australian alpine soils reported by Kirkpatrick et al. (2014) where organic C content was in the range of 6–35% and the total N content was 0.3–1.2%. The C and N contents are also in the range of those reported for alpine soils in Utah, USA (up to 16% C and 1.3% N) (Bockheim and Koerner 1997), British Columbia, Canada (13.2% C and 0.82% N) (van Ryswyk and Okazaki 1979) and in Valais, Switzerland (38% and 1.8% N) (Egli et al. 2001). Importantly, the C and N contents of the soils investigated here are clearly higher than those typical for most soils in Australia (Williams and Steinbergs 1958; Bui and Henderson 2013). Further, the differences in C and N content are most noticeable when comparing the soils in this study to those in agro-ecosystems, for which soils typically contain organic C contents <3% and N contents <0.3% (Kirkby et al. 2011). These differences can be largely explained by climate, in particular the contrast between the cold, wet conditions that are experienced in alpine environments, which slow microbial degradation of organic matter and are in contrast with the seasonally warm conditions experienced more broadly across most of Australia.
By targeting soils from within the Australia-wide ASRIS database, samples from the highest altitudes of Australia were chosen to represent a series of typical alpine soils, which we hypothesised would exhibit spectral features in their 31P NMR spectra more akin to those of soils from other relatively cold and wet locations. The composition of organic P in these alpine soils appears to differ to those previously reported for Australian soils (Doolette et al. 2011; McLaren et al. 2014; Moata et al. 2016). In particular, the presence of phosphonates, which we identified in two of the soils at the coldest and wettest locations, have not been reported before in Australian soils but have been identified in cold, wet and acidic soils elsewhere where microbial activity is limited (Tate and Newman 1982; Zech et al. 1987; Gil-Sotres et al. 1990). Similarly, neo- and d-chiro-IP6 are also often associated with soils from cooler, rather than warmer climates (Omotoso and Wild 1970; Irving and Cosgrove 1982; Turner et al. 2014; Jarosch et al. 2015), although the origins and dynamics of these compounds are still not well understood.
Of particular interest is the fact that the phosphomonoester region of the 31P spectra shown in Fig. 1 contains prominent IP6 peaks, especially for the two soils collected from above 1800 m above sea level (A1053 and A1054). This is in contrast to our previous studies on Australian soils where we noted the absence of, or relatively low intensity of, IP6 peaks, which account for a relatively minor component (typically <12%) of the organic P in most Australian soils (Doolette et al. 2011; McLaren et al. 2014; Moata et al. 2016). In previous studies, we reported that peaks due to α- and β-glycerophosphate have usually been larger than those of the IP6; this is especially evident in the 31P NMR spectra of Vertosols, for which IP6 peaks were barely discernible (McLaren et al. 2014).
Interestingly, the characteristic features of the spectra of the soils presented here, especially the prominent IP6 peaks, are very similar to those reported in other studies of soils from cold, wet climates (e.g. Turner et al. 2012; Vincent et al. 2012; Ahlgren et al. 2013; Vincent et al. 2013; Turner et al. 2014). This supports our hypothesis that climate has a strong influence on organic P speciation, with IP6 comprising a larger proportion of organic P in cold and wet environments, whereas humic-P comprises most of the organic P in warm and dry environments. The soils along a chronosequence in New Zealand, where mean annual rainfall is 3455 mm and the mean monthly temperature range is 7–15°C, displayed similar organic P compositions to those of the soils presented in this study (Turner et al. 2014) in that the phosphomonoester region in the New Zealand soils was also was dominated by myo- and scyllo-IP6 and smaller quantities of the neo- and d-chiro-IP6 stereoisomers were also detected. Similarly, pasture soils from the Falkland Islands were particularly rich in scyllo-IP6 and contained detectable amounts of the neo- and chiro-stereoisomers (Turner et al. 2012). Furthermore, in three separate studies, soils from cold temperate (<8°C) and humid (520–600 mm rainfall) climates in Sweden were found to contain considerable amounts of IP6; myo-IP6 comprised 19–36% of organic P and scyllo-IP6 comprised 3% of organic P along a chronosequence (Vincent et al. 2013) and myo-IP6 was also a prominent form of IP6 in both a long-term fertiliser experiment (Ahlgren et al. 2013) and in organic soils (humus) in a boreal forest (Vincent et al. 2012). The myo- and scyllo-IP6 were predominant in non-basaltic grassland soils in north-east Ireland (Murphy et al. 2009) and in permanent grassland and cropped soils from Switzerland, where average annual temperatures were in the range of 2.2–9.4°C and annual rainfall was 1042–2400 mm, myo- and scyllo-IP6 comprised up to 25% of soil organic P (Jarosch et al. 2015).
Although the distinctiveness of the 31P NMR spectra of these five alpine soils relative to the 31P NMR spectra of other Australian soils we have analysed and their greater similarity to the 31P NMR spectra of other soils from cold and wet climates supports our hypothesis that climate has a major influence on organic P speciation in soils, more research is needed to further test this contention. In particular, the five soils analysed here do not span the full range of Australian alpine soils identified by Kirkpatrick et al. (2014), and 31P NMR analysis of further alpine soils is warranted. Unfortunately, P analysis in Kirkpatrick et al. (2014) was limited to bicarbonate extractable and total P, neither of which were significantly correlated with mean annual temperature. More promisingly, organic C and total N (both measures of organic matter levels) were significantly correlated with climate, along with geological and vegetation factors, and this could assist in choosing target soils for analysis.
It must also be acknowledged that although the soils in this study were chosen on the basis that they included the only alpine soils from mainland Australia in the large ASRIS database of Australian soils, and hence represent an extreme in Australian climate of relative cold and wet conditions, the possibility of co-variation with other soil-forming factors (i.e. parent material, topography, vegetation and time) means the relationship we found between climate and organic P composition is not necessarily causal. The vegetation of the region does co-vary with climate. The three highest elevation sites – the alpine and sub-alpine sites where soils A1053 A1054 and A1055 were collected – are characterised by tall alpine herbfield vegetation, whereas the two lowest elevation (montane) sites, where soils A1056 and A1057 were collected, are under dry sclerophyll forest dominated by Eucalyptus species (Wood 1974). Thus it is possible that the relationship between climate and organic P composition is an indirect one and is driven by differences in vegetation. However, this does not explain the similarity of organic P composition of these alpine soils to alpine soils elsewhere in the world, given that these widely separated sites would have very different species composition. In terms of parent material, all soils were developed on a uniform gneissic granite bedrock (Wood 1974), although it has been suggested that the region’s soils are also influenced by historic and contemporary dust deposition, the distribution of which may be non-uniform (Kirkpatrick et al. 2014). Topography is considered an unlikely driver of differences in organic P composition; although the Kosciusko region is the highest in Australia, it is characterised by relatively gentle slopes. Time is also not likely to be a strong driver as the soils are not particularly young and do not form a clear chronosequence.
Management is another point of difference between the five soils analysed in this study and the majority of Australian soils we have previously reported on (Doolette et al. 2011; McLaren et al. 2014, 2015b; Moata et al. 2016). In particular, the alpine soils reported here are all unmanaged and under native vegetation, whereas the majority of soils we investigated previously are agricultural soils. However, several recent studies have reported agricultural management to have surprisingly little impact on organic P composition. Turner and Blackwell (2013) reported a relatively minor influence of liming on organic P composition in a long-term field trial at Rothamsted. This study utilised a management-induced gradient in lime addition and hence pH, and found accumulation of some specific organic P species only occurred under extremely acidic conditions of pH < 4. Similarly, Annaheim et al. (2015) reported that 62 years of application of three organic fertilisers (dairy manure, compost and dried sewage sludge) had no discernible effect on organic P speciation, despite substantially increasing both extractable and total P, in some cases by a factor of two or more. In another study, 100 years of superphosphate addition to a pasture soil had virtually no impact on organic P speciation, despite having profound effects on many soil properties, including total and extractable P and pH, and also pasture composition (Schefe et al. 2015). Based on these findings, it seems unlikely that the difference in organic P composition between the five alpine soils and other Australian soils is due to management.
This study also serves to shed some light on a point of contention among researchers who use 31P NMR spectroscopy to determine the speciation of organic P. It has been suggested that the broad feature we have consistently found to dominate 31P NMR spectra of NaOH–EDTA extracts of Australian soils (Doolette et al. 2011; McLaren et al. 2014, 2015b; Moata et al. 2016) and identify as humic-P, but which is less obvious in many other 31P NMR studies, may result from slight differences in methodology, in particular that we redissolved our freeze-dried extracts in water rather than NaOH–EDTA (Cade-Menun and Liu 2014; Kruse et al. 2015). However, here we have shown that we can obtain 31P NMR spectra very similar to those reported in other studies. We suggest that the humic-P peaks are in fact less prominent in the spectra of soils from cold, wet environments due to the higher proportion of IP6 compounds present.
Conclusions
The characterisation of organic P in the alpine and sub-alpine soils of the Snowy Mountain region presented in this study provides preliminary support for the hypothesis that climate has a strong influence on the speciation and composition of soil organic P. The 31P NMR spectra of NaOH–EDTA soil extracts revealed that the organic P composition of these alpine soils markedly differed to that reported for other Australia soils from warm, dry environments, but was consistent with the P composition of soils from cold, wet environments outside Australia. In particular, we identified the presence of phosphonates and neo- and d-chiro-isomers of IP6 in the soils from the colder and wetter locations, as well as prominent peaks associated with myo- and scyllo-IP6, which are often minor or absent in the 31P NMR spectra of other Australian soils from warm and dry environments. A more comprehensive study including a more substantial number of soils from varied climates is needed to investigate the mechanisms that are affected by climate and how this influences the abundance of complex humic-P relative to individual molecular organic P species.
Acknowledgements
We thank the Waite Research Institute (WRI) for the funding provided to complete this research and the CSIRO National Soils Archive for providing the soil samples.
References
Ahlgren J, Djodjic F, Börjesson G, Mattsson L (2013) Identification and quantification of organic phosphorus forms in soils from fertility experiments. Soil Use and Management 29, 24–35.| Identification and quantification of organic phosphorus forms in soils from fertility experiments.Crossref | GoogleScholarGoogle Scholar |
Anderson G (1964) Investigations on the analysis of inositol hexaphosphate in soils. In ‘Transactions of the 8th International Congress of Soil Science’, 31 August–9 September 1964, Bucharest, Romania. Vol. 4, pp. 563–571. (Publishing House of the Academy of The Socialist Republic of Romania)
Annaheim KE, Doolette AL, Smernik RJ, Mayer J, Oberson A, Frossard E, Bünemann EK (2015) Long-term addition of organic fertilizers has little effect on soil organic phosphorus as characterized by 31P NMR spectroscopy and enzyme additions. Geoderma 257–258, 67–77.
| Long-term addition of organic fertilizers has little effect on soil organic phosphorus as characterized by 31P NMR spectroscopy and enzyme additions.Crossref | GoogleScholarGoogle Scholar |
Baer E, Kates M (1948) Migration during hydrolysis of esters of glycerophosphoric acid. I. The chemical hydrolysis of l-α-glycerylphosphorylcholine. The Journal of Biological Chemistry 175, 79–88.
Baer E, Kates M (1950) Migration during hydrolysis of esters of glycerophosphoric acid. II. The acid and alkaline hydrolysis of l-α-lecithins. The Journal of Biological Chemistry 185, 615–623.
Bockheim JG, Koerner D (1997) Pedogenesis in alpine ecosystems of the Eastern Uinta Mountains, Utah, U.S.A. Arctic and Alpine Research 29, 164–172.
| Pedogenesis in alpine ecosystems of the Eastern Uinta Mountains, Utah, U.S.A.Crossref | GoogleScholarGoogle Scholar |
Bui E, Henderson B (2013) C:N:P stoichiometry in Australian soils with respect to vegetation and environmental factors. Plant and Soil 373, 553–568.
| C:N:P stoichiometry in Australian soils with respect to vegetation and environmental factors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVGrsrzI&md5=a8921b21826bab215570d0e93b9ac25dCAS |
Cade-Menun B, Liu CW (2014) Solution phosphorus-31 nuclear magnetic resonance spectroscopy of soils from 2005 to 2013: a review of sample preparation and experimental parameters. Soil Science Society of America Journal 78, 19–37.
| Solution phosphorus-31 nuclear magnetic resonance spectroscopy of soils from 2005 to 2013: a review of sample preparation and experimental parameters.Crossref | GoogleScholarGoogle Scholar |
Cade-Menun BJ, Preston CM (1996) A comparison of soil extraction procedures for 31P NMR spectroscopy. Soil Science 161, 770–785.
| A comparison of soil extraction procedures for 31P NMR spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XntVOitLo%3D&md5=31dc5dbb69d43b0abeac6c57e5ded86bCAS |
Chatterjee A, Jenerette G (2015) Variation in soil organic matter accumulation and metabolic activity along an elevation gradient in the Santa Rosa Mountains of Southern California, USA. Journal of Arid Land 7, 814–819.
| Variation in soil organic matter accumulation and metabolic activity along an elevation gradient in the Santa Rosa Mountains of Southern California, USA.Crossref | GoogleScholarGoogle Scholar |
Condron LM, Frossard E, Tiessen H, Newman RH, Stewart JWB (1990) Chemical nature of organic phosphorus in cultivated and uncultivated soils under different environmental conditions. Journal of Soil Science 41, 41–50.
| Chemical nature of organic phosphorus in cultivated and uncultivated soils under different environmental conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXitFGlsr8%3D&md5=924dc5b0c4ee2fdf5292b84cdb204012CAS |
Costin AB (1954) ‘A study of the ecosystems of the Monaro Region of New South Wales.’ (Government Printer: Sydney)
Costin A (1957) The high mountain vegetation of Australia. Australian Journal of Botany 5, 173–189.
| The high mountain vegetation of Australia.Crossref | GoogleScholarGoogle Scholar |
Doolette AL, Smernik RJ, Dougherty WJ (2009) Spiking improved solution phosphorus-31 nuclear magnetic resonance identification of soil phosphorus compounds. Soil Science Society of America Journal 73, 919–927.
| Spiking improved solution phosphorus-31 nuclear magnetic resonance identification of soil phosphorus compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsFylsLk%3D&md5=5507554d66109cd2e9aca0dfeaae56adCAS |
Doolette AL, Smernik RJ, Dougherty WJ (2011) A quantitative assessment of phosphorus forms in some Australian soils. Soil Research 49, 152–165.
| A quantitative assessment of phosphorus forms in some Australian soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXivFylsrk%3D&md5=93dc20694f8b78d2a8120cdf552b1989CAS |
Egli M, Fitze P, Mirabella A (2001) Weathering and evolution of soils formed on granitic, glacial deposits: results from chronosequences of Swiss alpine environments. Catena 45, 19–47.
| Weathering and evolution of soils formed on granitic, glacial deposits: results from chronosequences of Swiss alpine environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjsFeht7c%3D&md5=2a1eae4f135e2a063e26cf38b17c3cd3CAS |
Gil-Sotres F, Zech W, Alt HG (1990) Characterization of phosphorus fractions in surface horizons of soils from Galicia (N.W. Spain) by 31P NMR spectroscopy. Soil Biology & Biochemistry 22, 75–79.
| Characterization of phosphorus fractions in surface horizons of soils from Galicia (N.W. Spain) by 31P NMR spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXhsFOitLY%3D&md5=a3bf85d29e2b80f3224c6d7069c0ea0dCAS |
Hollings PE, Dutch ME, Stout JD (1969) Bacteria of four tussock grassland soils on the Old Man Range, Central Otago, New Zealand. New Zealand Journal of Agricultural Research 12, 177–192.
| Bacteria of four tussock grassland soils on the Old Man Range, Central Otago, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Irving GCJ, Cosgrove DJ (1982) The use of gas-liquid chromatography to determine the proportions of inositol isomers present as pentakis- and hexakisphosphates in alkaline extracts of soils. Communications in Soil Science and Plant Analysis 13, 957–967.
| The use of gas-liquid chromatography to determine the proportions of inositol isomers present as pentakis- and hexakisphosphates in alkaline extracts of soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXhsFymsQ%3D%3D&md5=1d1014824435c048256f120f2de8f7f6CAS |
Isbell RF (2002) ‘The Australian soil classification.’ (CSIRO Publishing: Melbourne)
Jarosch KA, Doolette AL, Smernik RJ, Tamburini F, Frossard E, Bünemann EK (2015) Characterisation of soil organic phosphorus in NaOH-EDTA extracts: a comparison of 31P NMR spectroscopy and enzyme addition assays. Soil Biology & Biochemistry 91, 298–309.
| Characterisation of soil organic phosphorus in NaOH-EDTA extracts: a comparison of 31P NMR spectroscopy and enzyme addition assays.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsFOrsrfL&md5=1ae1749103deb1cffd276824a500002eCAS |
Johnson LF, Tate ME (1969) Structure of ‘phytic acids’. Canadian Journal of Chemistry 47, 63–73.
| Structure of ‘phytic acids’.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1MXjvVSiug%3D%3D&md5=6b1143bad377bf4290c96c2262cad06bCAS |
Johnston RM, Barry SJ, Bleys E, Bui EN, Moran CJ, Simon DAP, Carlile P, McKenzie NJ, Henderson BL, Chapman G, Imhoff M, Maschmedt D, Howe D, Grose C, Schoknecht N, Powell B, Grundy M (2003) ASRIS: the database. Soil Research 41, 1021–1036.
| ASRIS: the database.Crossref | GoogleScholarGoogle Scholar |
Kirkby CA, Kirkegaard JA, Richardson AE, Wade LJ, Blanchard C, Batten G (2011) Stable soil organic matter: a comparison of C:N:P:S ratios in Australian and other world soils. Geoderma 163, 197–208.
| Stable soil organic matter: a comparison of C:N:P:S ratios in Australian and other world soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnt1amsr0%3D&md5=2468d70c13ed22f09cb1c1b41052f24bCAS |
Kirkpatrick JB, Green K, Bridle KL, Venn SE (2014) Patterns of variation in Australian alpine soils and their relationships to parent material, vegetation formation, climate and topography. Catena 121, 186–194.
| Patterns of variation in Australian alpine soils and their relationships to parent material, vegetation formation, climate and topography.Crossref | GoogleScholarGoogle Scholar |
Kruse J, Abraham M, Amelung W, Baum C, Bol R, Kühn O, Lewandowski H, Niederberger J, Oelmann Y, Rüger C, Santner J, Siebers M, Siebers N, Spohn M, Vestergren J, Vogts A, Leinweber P (2015) Innovative methods in soil phosphorus research: a review. Journal of Plant Nutrition and Soil Science 178, 43–88.
| Innovative methods in soil phosphorus research: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXmtVOjsQ%3D%3D&md5=4d3c16874bc2688058852104d4a54db8CAS | 26167132PubMed |
Makarov MI, Haumaier L, Zech W (2002a) The nature and origins of diester phosphates in soils: a 31P-NMR study. Biology and Fertility of Soils 35, 136–146.
| The nature and origins of diester phosphates in soils: a 31P-NMR study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XisF2qtL0%3D&md5=b2c361e7ca8b2afeb1ef8f3cce056108CAS |
Makarov MI, Haumaier L, Zech W (2002b) Nature of soil organic phosphorus: an assessment of peak assignments in the diester region of 31P NMR spectra. Soil Biology & Biochemistry 34, 1467–1477.
| Nature of soil organic phosphorus: an assessment of peak assignments in the diester region of 31P NMR spectra.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnsVWrs78%3D&md5=7f3b74ba5dfdbf92780a35050fe59e38CAS |
Matejovic I (1997) Determination of carbon and nitrogen in samples of various soils by the dry combustion. Communications in Soil Science and Plant Analysis 28, 1499–1511.
| Determination of carbon and nitrogen in samples of various soils by the dry combustion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXnsVCltbg%3D&md5=0561b2954a70c273acd16f2a5ba40b5aCAS |
McKercher RB, Anderson G (1968) Content of inositol penta- and hexaphosphates in some Canadian soils. European Journal of Soil Science 19, 47–55.
| Content of inositol penta- and hexaphosphates in some Canadian soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1cXktV2qsbs%3D&md5=1a91fee57ca796e417698992cda98f31CAS |
McLaren TI, Smernik RJ, Guppy CN, Bell MJ, Tighe MK (2014) The organic P composition of vertisols as determined by 31P NMR spectroscopy. Soil Science Society of America Journal 78, 1893–1902.
| The organic P composition of vertisols as determined by 31P NMR spectroscopy.Crossref | GoogleScholarGoogle Scholar |
McLaren TI, Smernik RJ, Simpson RJ, McLaughlin MJ, McBeath TM, Guppy CN, Richardson AE (2015a) Spectral sensitivity of solution 31P NMR spectroscopy is improved by narrowing the soil to solution ratio to 1 : 4 for pasture soils of low organic P content. Geoderma 257–258, 48–57.
| Spectral sensitivity of solution 31P NMR spectroscopy is improved by narrowing the soil to solution ratio to 1 : 4 for pasture soils of low organic P content.Crossref | GoogleScholarGoogle Scholar |
McLaren TI, Smernik RJ, McLaughlin MJ, McBeath TM, Kirby JK, Simpson RJ, Guppy CN, Doolette AL, Richardson AE (2015b) Complex forms of soil organic phosphorus – a major component of soil phosphorus. Environmental Science & Technology 49, 13238–13245.
| Complex forms of soil organic phosphorus – a major component of soil phosphorus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhslSlsbfP&md5=09987035b965e39e795a945c2c928713CAS |
McLaren TI, Simpson RJ, McLaughlin MJ, Smernik RJ, McBeath TM, Guppy CN, Richardson AE (2015c) An assessment of various measures of soil phosphorus and the net accumulation of phosphorus in fertilized soils under pasture. Journal of Plant Nutrition and Soil Science 178, 543–554.
| An assessment of various measures of soil phosphorus and the net accumulation of phosphorus in fertilized soils under pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtVGmu7jO&md5=ec55cdbd1f87231e6dde91bfb554a2bdCAS |
Moata M, Doolette A, Smernik R, McNeill A, Macdonald L (2016) Organic phosphorus speciation in Australian red chromosols: stoichiometric control. Soil Research 54, 11–19.
| Organic phosphorus speciation in Australian red chromosols: stoichiometric control.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XisVyhsb4%3D&md5=3ab3158b164769d1cafb30df3beb5cddCAS |
Murphy PNC, Bell A, Turner BL (2009) Phosphorus speciation in temperate basaltic grassland soils by solution 31P NMR spectroscopy. European Journal of Soil Science 60, 638–651.
| Phosphorus speciation in temperate basaltic grassland soils by solution 31P NMR spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVGis7zF&md5=ae76041e3eae7f891366211ee38f6ad6CAS |
Newman RH, Tate KR (1980) Soil phosphorus characterization by 31P nuclear magnetic resonance. Communications in Soil Science and Plant Analysis 11, 835–842.
| Soil phosphorus characterization by 31P nuclear magnetic resonance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXlvVCrtbw%3D&md5=56d9cb47425dc141a41398810a979adaCAS |
Oades JM (1988) The retention of organic matter in soils. Biogeochemistry 5, 35–70.
| The retention of organic matter in soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXitVeksb0%3D&md5=86fa1a5b31abd3d6601b19375be33de2CAS |
Omotoso TI, Wild A (1970) Content of inositol phosphates in some English and Nigerian soils. Journal of Soil Science 21, 216–223.
| Content of inositol phosphates in some English and Nigerian soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3MXjtFWgsg%3D%3D&md5=3849698d3f8fd18716988985301a0060CAS |
Post WM, Emanuel WR, Zinke PJ, Stangenberger AG (1982) Soil carbon pools and world life zones. Nature 298, 156–159.
| Soil carbon pools and world life zones.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XlsVyju7w%3D&md5=1f64ddd286d6937080d11e5a10d1e607CAS |
Schefe CR, Barlow KM, Robinson N, Crawford D, McLaren TI, Smernik RJ, Croatto G, Walsh R, Kitching M (2015) 100 years of superphosphate addition to pasture in an acid soil – current nutrient status and future management. Soil Research 53, 662–676.
| 100 years of superphosphate addition to pasture in an acid soil – current nutrient status and future management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsFart7bF&md5=529df45cc03bbf4d13d7d32e88bcd995CAS |
Sharpley AN (1985) Phosphorus cycling in unfertilized and fertilized agricultural soils. Soil Science Society of America Journal 49, 905–911.
| Phosphorus cycling in unfertilized and fertilized agricultural soils.Crossref | GoogleScholarGoogle Scholar |
Smernik RJ, Dougherty WJ (2007) Identification of phytate in phosphorus-31 nuclear magnetic resonance spectra – the need for spiking. Soil Science Society of America Journal 71, 1045–1050.
| Identification of phytate in phosphorus-31 nuclear magnetic resonance spectra – the need for spiking.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlvFGgurc%3D&md5=5f27bf49ba6631ca531289d752dd46f9CAS |
Smernik RJ, Doolette AL, Noack SR (2015) Identification of RNA hydrolysis products in NaOH-EDTA extracts using 31P NMR spectroscopy. Communications in Soil Science and Plant Analysis 46, 2746–2756.
| Identification of RNA hydrolysis products in NaOH-EDTA extracts using 31P NMR spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhvVKmurjI&md5=86190eb72b75206a4ce174c6d6a603d3CAS |
Stace HTC, Hubble GD, Brewer R, Northcote KH, Sleeman JR, Mulcahy MJ, Hallsworth EG (1968) ‘A handbook of Australian soils.’ (Rellim Technical Publications for the CSIRO and the International Society of Soil Science: Adelaide, SA)
Sumann M, Amelung W, Haumaier L, Zech W (1998) Climatic effects on soil organic phosphorus in the North American Great Plains identified by phosphorus-31 nuclear magnetic resonance. Soil Science Society of America Journal 62, 1580–1586.
| Climatic effects on soil organic phosphorus in the North American Great Plains identified by phosphorus-31 nuclear magnetic resonance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjslygtA%3D%3D&md5=77d9056088281f1ae39e5ed4bd5335ecCAS |
Tate KR, Newman RH (1982) Phosphorus fractions of a climosequence of soils in New Zealand tussock grassland. Soil Biology & Biochemistry 14, 191–196.
| Phosphorus fractions of a climosequence of soils in New Zealand tussock grassland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XoslGksA%3D%3D&md5=bfa920221bf6b78bf231bf65d70de640CAS |
Trumbore SE, Chadwick OA, Amundson R (1996) Rapid exchange between soil carbon and atmospheric carbon dioxide driven by temperature change. Science 272, 393–396.
| Rapid exchange between soil carbon and atmospheric carbon dioxide driven by temperature change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XisVehu7g%3D&md5=bf08dfc70b2da6a0d41f8e6e6502fe36CAS |
Turner B (2008) Soil organic phosphorus in tropical forests: an assessment of the NaOH-EDTA extraction procedure for quantitative analysis by solution 31P NMR. European Journal of Soil Science 59, 453–466.
| Soil organic phosphorus in tropical forests: an assessment of the NaOH-EDTA extraction procedure for quantitative analysis by solution 31P NMR.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvVOju7g%3D&md5=2beb5f6821773a6b9eb610aa9fb098dbCAS |
Turner BL, Blackwell MSA (2013) Isolating the influence of pH on the amounts and forms of soil organic phosphorus. European Journal of Soil Science 64, 249–259.
| Isolating the influence of pH on the amounts and forms of soil organic phosphorus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXksVKktbw%3D&md5=ab4a3deecf3fd1b8435cf9ddb120b8c2CAS |
Turner BL, Richardson AE (2004) Identification of scyllo-inositol phosphates in soils by solution phosphorus-31 nuclear magnetic resonance spectroscopy. Soil Science Society of America Journal 68, 802–808.
| Identification of scyllo-inositol phosphates in soils by solution phosphorus-31 nuclear magnetic resonance spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXktV2gtb8%3D&md5=00838635fc9ba14a430efd515f8daf09CAS |
Turner BL, Cade-Menun BJ, Westermanm DT (2003a) Organic phosphorus composition and potential bioavailability in semi-arid arable soils of the western United States. Soil Science Society of America Journal 67, 1168–1179.
| Organic phosphorus composition and potential bioavailability in semi-arid arable soils of the western United States.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlslCqtb0%3D&md5=d06e2b410b31bd23afcee40f3df569f6CAS |
Turner BL, Mahieu N, Condron LM (2003b) Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH-EDTA extracts. Soil Science Society of America Journal 67, 497–510.
| Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH-EDTA extracts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkslChsLo%3D&md5=9b838b544107d6e31f66f5beba60b205CAS |
Turner BL, Cheesman AW, Godage HY, Riley AM, Potter BVL (2012) Determination of neo- and d-chiro-inositol hexakisphosphate in soils by solution 31P NMR spectroscopy. Environmental Science & Technology 46, 4994–5002.
| Determination of neo- and d-chiro-inositol hexakisphosphate in soils by solution 31P NMR spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xltlags7w%3D&md5=8c5cb90d54e0f8722222a9f7112ec7edCAS |
Turner B, Wells A, Condron L (2014) Soil organic phosphorus transformations along a coastal dune chronosequence under New Zealand temperate rain forest. Biogeochemistry 121, 595–611.
| Soil organic phosphorus transformations along a coastal dune chronosequence under New Zealand temperate rain forest.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVCisbrE&md5=350603b2931f8c413deee9726c3b66fbCAS |
Van Meeteren MKM, Tietema A, Westerveld JW (2007) Regulation of microbial carbon, nitrogen, and phosphorus transformations by temperature and moisture during decomposition of Calluna vulgaris litter. Biology and Fertility of Soils 44, 103–112.
| Regulation of microbial carbon, nitrogen, and phosphorus transformations by temperature and moisture during decomposition of Calluna vulgaris litter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVGmt73O&md5=46bdaa59ef3d44ae35805e277bd52cd7CAS |
van Ryswyk AL, Okazaki R (1979) Genesis and classification of modal subalpine and alpine soil pedons of south-central British Columbia, Canada. Arctic and Alpine Research 11, 53–67.
| Genesis and classification of modal subalpine and alpine soil pedons of south-central British Columbia, Canada.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXhvVGnu7w%3D&md5=af9b4f8cafe8c45988e853dd7910cee7CAS |
Vincent A, Schleucher J, Gröbner G, Vestergren J, Persson P, Jansson M, Giesler R (2012) Changes in organic phosphorus composition in boreal forest humus soils: the role of iron and aluminium. Biogeochemistry 108, 485–499.
| Changes in organic phosphorus composition in boreal forest humus soils: the role of iron and aluminium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XivFOnt78%3D&md5=6dd0215f1e842559da0aa4bcb2f86ea9CAS |
Vincent A, Vestergren J, Gröbner G, Persson P, Schleucher J, Giesler R (2013) Soil organic phosphorus transformations in a boreal forest chronosequence. Plant and Soil 367, 149–162.
| Soil organic phosphorus transformations in a boreal forest chronosequence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXntVaksbg%3D&md5=3eb139ea04b133cddf6eea87d28dec09CAS |
Williams CH, Costin AB (1994) Alpine and subalpine vegetation. In ‘Australian vegetation’. (Ed. RH Groves) pp. 467–500. (Cambridge University Press: Melbourne)
Williams CH, Steinbergs A (1958) Sulphur and phosphorus in some eastern Australian soils. Australian Journal of Agricultural Research 9, 483–491.
| Sulphur and phosphorus in some eastern Australian soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG1cXhtVOht7k%3D&md5=16f454298b679f032e2e6ebe11becdc7CAS |
Williams JDH, Mayer T, Nriagu JO (1980) Extractability of phosphorus from phosphate minerals common in coils and sediments. Soil Science Society of America Journal 44, 462–465.
| Extractability of phosphorus from phosphate minerals common in coils and sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXkvFyqtr0%3D&md5=7bffe1729200537824a182bf657da574CAS |
Williams RJ, Wahren C, Tolsma AD, Sanecki GM, Papst WA, Myers BA, McDougall KL, Heinze DA, Green K (2008) Large fires in Australian alpine landscapes: their part in the historical fire regime and their impacts on alpine biodiversity. International Journal of Wildland Fire 17, 793–808.
| Large fires in Australian alpine landscapes: their part in the historical fire regime and their impacts on alpine biodiversity.Crossref | GoogleScholarGoogle Scholar |
Wood TG (1970) Decomposition of plant litter in montane and alpine soils on Mt Kosciusko, Australia. Nature 226, 561–562.
| Decomposition of plant litter in montane and alpine soils on Mt Kosciusko, Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2MzpslGgtA%3D%3D&md5=c26361e7ee1378151e69c730cbbb46eaCAS | 16057384PubMed |
Wood TG (1974) The distribution of earthworms (Megascolecidae) in relation to soils, vegetation and altitude on the slopes of Mt Kosciusko, Australia. Journal of Animal Ecology 43, 87–106.
| The distribution of earthworms (Megascolecidae) in relation to soils, vegetation and altitude on the slopes of Mt Kosciusko, Australia.Crossref | GoogleScholarGoogle Scholar |
Yang H, Yuan Y, Zhang Q, Tang J, Liu Y, Chen X (2011) Changes in soil organic carbon, total nitrogen, and abundance of arbuscular mycorrhizal fungi along a large-scale aridity gradient. Catena 87, 70–77.
| Changes in soil organic carbon, total nitrogen, and abundance of arbuscular mycorrhizal fungi along a large-scale aridity gradient.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXoslCksbk%3D&md5=c3d59c012389b12de8a9b271b9c4c223CAS |
Zarcinas BA, McLaughlin MJ, Smart MK (1996) The effect of acid digestion technique on the performance of nebulization systems used in inductively coupled plasma spectrometry. Communications in Soil Science and Plant Analysis 27, 1331–1354.
| The effect of acid digestion technique on the performance of nebulization systems used in inductively coupled plasma spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XivVKltrg%3D&md5=9832c8220ec09d588e11280dd4ee540cCAS |
Zech W, Alt HG, Haumaier L, Blasek R (1987) Characterization of phosphorus fractions in mountain soils of the Bavarian Alps by 31P NMR spectroscopy. Zeitschrift für Pflanzenernährung und Bodenkunde 150, 119–123.
| Characterization of phosphorus fractions in mountain soils of the Bavarian Alps by 31P NMR spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXkt1amtr8%3D&md5=04d1b3ef64eb423aeb6fce59b3d3e4dcCAS |