A quantitative assessment of shoot flammability for 60 tree and shrub species supports rankings based on expert opinion
Sarah V. Wyse A B G , George L. W. Perry A C , Dean M. O’Connell D , Phillip S. Holland D , Monique J. Wright D , Catherine L. Hosted D E , Samuel L. Whitelock D , Ian J. Geary D F , Kévin J. L. Maurin D and Timothy J. Curran DA School of Environment, University of Auckland, Private Bag 92019 Auckland 1142, New Zealand.
B Royal Botanic Gardens Kew, Wakehurst Place, RH17 6TN, UK.
C School of Biological Sciences, University of Auckland, Private Bag 92019 Auckland 1142, New Zealand.
D Ecology Department, Lincoln University, PO Box 85084, Lincoln 7647, Canterbury, New Zealand.
E Wai-Ora Forest Landscapes Ltd, 48 Watsons Road, Harewood 8051, Christchurch, New Zealand.
F Department of Geology, University of Otago, PO Box 56 Dunedin 9054, New Zealand.
G Corresponding author. Email: S.Wyse@kew.org
International Journal of Wildland Fire 25(4) 466-477 https://doi.org/10.1071/WF15047
Submitted: 13 February 2015 Accepted: 8 December 2015 Published: 25 February 2016
Journal Compilation © IAWF 2016
Abstract
Fire is an important ecological disturbance in vegetated ecosystems across the globe, and also has considerable impacts on human infrastructure. Vegetation flammability is a key bottom-up control on fire regimes and on the nature of individual fires. Although New Zealand (NZ) historically had low fire frequencies, anthropogenic fires have considerably impacted indigenous vegetation as humans used fire extensively to clear forests. Few studies of vegetation flammability have been undertaken in NZ and only one has compared the flammability of indigenous plants; this was a qualitative assessment derived from expert opinion. We addressed this knowledge gap by measuring the flammability of terminal shoots from a range of trees and shrubs found in NZ. We quantified shoot flammability of 60 indigenous and exotic species, and compared our experimentally derived ranking with expert opinion. The most flammable species was the invasive exotic shrub Gorse (Ulex europaeus), followed by Manna Gum (Eucalyptus viminalis), Kūmarahou (Pomaderris kumeraho), Rimu (Dacrydium cupressinum) and Silver Beech (Lophozonia menziesii). Our experimentally derived ranking was strongly correlated with expert opinion, lending support to both methods. Our results are useful to ecologists seeking to understand how fires have and will influence NZ’s ecosystems, and for fire managers identifying high-risk landscapes, and low flammability species for ‘green firebreaks’.
Introduction
As an ecological disturbance fire shapes community structure and ecosystem processes around the world (Bond 2005; Bowman et al. 2009). An ecosystem’s fire regime emerges from interactions among climate, landscape and the characteristics of the available fuel (Whitlock et al. 2010). The flammability of the vegetation combusted in a fire is an important bottom-up control on the nature of individual fires and on the overall fire regime in an ecosystem or landscape (Bond and Midgley 1995; Fogarty 2001; Bond 2005). Additionally, the composition and arrangement of the vegetative fuel in a landscape can be altered by human activities, such as agricultural and forestry practices, the introductions of invasive plant species and the use of ‘green firebreaks’ (areas of vegetation comprised of low flammability species and that may be irrigated, which act as barriers to help reduce fire spread: Johnson 1975; White and Zipperer 2010; Keeley et al. 2012). Planting decisions in both urban and rural areas, including the provision of green firebreaks, can be used to modify the vegetative fuel and hence landscape flammability, and so minimise fire risk in inhabited areas. Most authors broadly define flammability as the capacity of a material to ignite and sustain a fire, but the components measured to provide an assessment of flammability vary across authors and disciplines (e.g. Anderson 1970; Martin et al. 1994; Liodakis et al. 2002; Gill and Zylstra 2005; White and Zipperer 2010; Jaureguiberry et al. 2011; Madrigal et al. 2011; Pausas and Moreira 2012).
As a plant trait flammability consists of four main components, which are often strongly correlated: ignitability (how easily a plant ignites), combustibility (the speed or intensity at which a plant burns), sustainability (the length of time a plant continues to burn once ignited) and consumability (how much of a sample is burnt) (Anderson 1970; Martin et al. 1994). Measurements of plant flammability may quantify all or some of these components and have been undertaken using a variety of methodologies (e.g. Anderson 1970; Martin et al. 1994; Liodakis et al. 2002; Etlinger and Beall 2004; Gill and Zylstra 2005; Weise et al. 2005; White and Zipperer 2010; Jaureguiberry et al. 2011). Plant flammability is controlled by two main factors: tissue type or quality (including moisture content), and the structure and architecture of the plant (Perez-Harguindeguy et al. 2013). However, most measurements of flammability traits involve small plant components, typically leaves or needles, small twigs, or litter (e.g. Owens et al. 1998; Dimitrakopoulos and Papaioannou 2001; Kane et al. 2008; Cornwell et al. 2015). Such measurements accurately characterise the flammability of the chosen plant tissues and provide important information on surface fuels, but do not incorporate plant architecture, providing less ecologically meaningful results for canopy fuels than methods that use whole shoots or entire plants (Etlinger and Beall 2004; Jaureguiberry et al. 2011; Schwilk 2015). The most recent plant functional trait ‘handbook’ (Perez-Harguindeguy et al. 2013) advocates a shoot-level approach (following Jaureguiberry et al. 2011) as a standardised method of assessing plant flammability. This method preserves much of the architecture of the plant, particularly the fine fuels, and has recently been suggested as a suitable way to measure the flammability of samples from the plant canopy for a wide range of species, albeit with less precision than some laboratory-based approaches (Schwilk 2015).
New Zealand (NZ) has historically had low fire frequencies across its three main islands. Although charcoal has been found in sediment samples of all ages (Perry et al. 2014), fire recurrence times in NZ during the pre-human Holocene were in the order of centuries or even millennia (Ogden et al. 1998). Those fires that did occur were predominantly in tree-clad wetlands, forested ecosystems following (rare) volcanic eruptions and, to some extent, in drier areas of forest during the late Holocene (Perry et al. 2014). The infrequency and unpredictability of fire in time and space may explain the lack of specific fire adaptations in the NZ flora. The few NZ species (e.g. the serotinous Myrtaceous shrub Mānuka (Leptospermum scoparium)) that do possess obvious fire adaptations are relatively recent arrivals (Pliocene or later) that are closely related to eastern Australian species (McGlone et al. 2001). Despite the low fire frequencies in primeval NZ, the history of human colonisation shows that NZ forests were highly susceptible to fire. The first arrival of humans in NZ ~1280 AD (Wilmshurst et al. 2008) resulted in considerable changes in fire regimes. The initial burning period that followed Polynesian settlement caused rapid and extensive forest loss (likely >40%) and the transition from closed-canopy forests to early-successional Bracken (Pteridium esculentum), shrub and grassland communities (McWethy et al. 2009; McWethy et al. 2014; Perry et al. 2014). Further anthropogenic fire converted forest and secondary shrubland to pasture following European settlement ~1840 AD (McWethy et al. 2009; Perry et al. 2014).
Despite palaeoecological and historical evidence that the NZ flora burns readily, there is a lack of quantitative assessment of the flammability of NZ plants. Studies based in other parts of the world have assessed the flammability of some species that occur in NZ. These include the invasive weeds Gorse (Ulex europaeus) (Núñez-Regueira et al. 1996; Madrigal et al. 2012) and Radiata Pine (Pinus radiata) (Fonda 2001), other exotic species (e.g. Manna Gum (Eucalyptus viminalis)) and some indigenous NZ species (Mānuka, Akeake (Dodonea viscosa) and Bracken) that occur elsewhere, such as Tasmania (Dickinson and Kirkpatrick 1985). In NZ, multiple studies have assessed the flammability of Gorse (Anderson and Anderson 2009, 2010), whereas the only published comparative assessment of indigenous trees and shrubs is qualitative and derived from expert opinion (Fogarty 2001). Forty two indigenous species were ranked by Fogarty (2001) in terms of their average flammability score based on a survey of fire managers. In that survey, experts were asked to assign species to flammability categories based on their observations of the species during and after prescribed burns and wildfires under different fire danger conditions. The majority of species (28/42; 66.7%) were assigned to low or moderately low flammability categories, with only six species considered to be moderately high or high flammability (Fogarty 2001). While that study represented an important and useful first attempt at ranking the flammability of NZ species, and has been used to provide planting guidelines for green firebreaks, Fogarty (2001) recognised that his rankings lacked empirical testing. This testing has not been undertaken until now. It is imperative to quantify the flammability of NZ species and empirically test Fogarty’s (2001) widely used ranking because climate change scenarios suggest increasing summer water deficits for much of NZ (Mullan et al. 2005), with conditions potentially conducive to higher fire risk (Pearce et al. 2010). Additionally, examination of the flammability of a flora that has evolved in the absence of frequent and significant fires makes a useful addition to the fire ecology literature, which is dominated by studies from fire-prone environments.
In this study we measured the shoot-level flammability of 60 plant species that occur in a range of forest and scrub habitats in NZ. We aimed to: 1) quantify the shoot-level flammability of a range of indigenous and common exotic species in NZ and 2) compare experimentally derived rankings using the whole-shoot flammability method (Jaureguiberry et al. 2011; Perez-Harguindeguy et al. 2013) with those determined from expert opinion (Fogarty 2001). This second aim represents a novel contribution to the literature; such qualitative flammability data have not previously been tested against quantitative rankings.
Methods
Sample collection
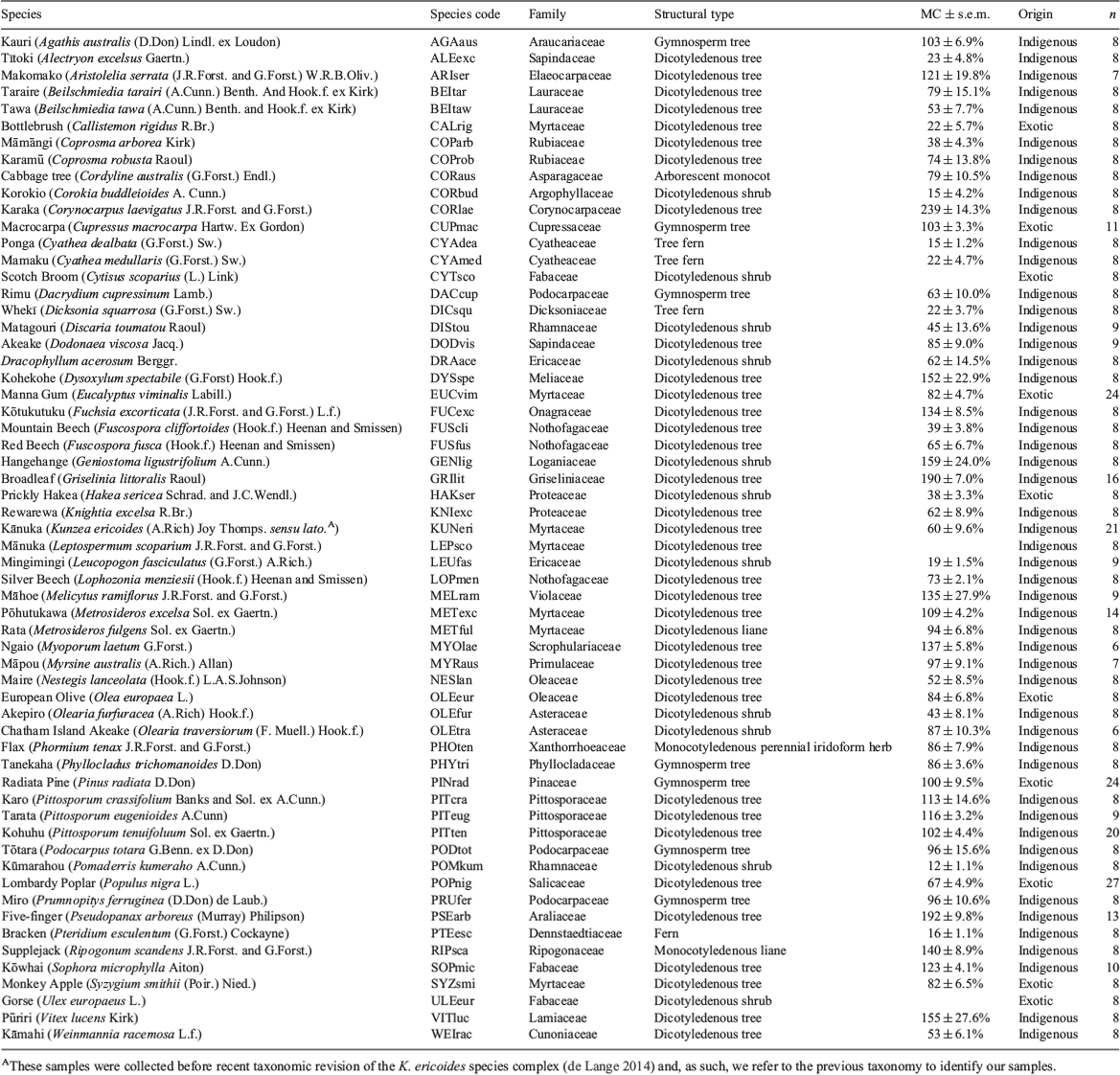
We collected samples from 50 NZ indigenous and 10 exotic plant species, across 37 families (Table 1). The indigenous species encompassed three monocotyledons (one liana, one iridoform perennial herb and one arborescent monocot), four ferns (including three tree ferns), five gymnosperm trees, one dicotyledonous liana, and 37 dicotyledonous trees and shrubs. The species chosen occur across a broad range of habitats in both the North and South Islands of NZ (see Fig. S2 in the Supplementary material, available online only, for collection locations). The indigenous species that we assessed are all abundant in different areas of the country, both in native forest and as garden plants, and were chosen to span a range of taxonomic groups. The exotic species are all commonly planted (n = 7) or abundant weeds (n = 8) in NZ, and included two gymnosperms and eight dicotyledonous trees and shrubs. Many of these exotic species were chosen because they are known to be flammable in other locations.
|
One sample was taken per individual from a minimum of six individuals per species (Table 1). The sampled individuals were all healthy, not visibly water-stressed at the time of sampling, and, for heteroblastic species, possessed a mature growth-form. Wherever possible we sampled sun-exposed branches (Perez-Harguindeguy et al. 2013). Following Jaureguiberry et al. (2011) for trees, shrubs and lianas, samples consisted of 70 cm-long-terminal branches, with lianas sampled in the canopies of their host trees where they produce mature, leafy branches that are sun exposed. All fern samples consisted of a 70 cm terminal section of a single frond. A different sampling technique was employed for Flax (Phormium tenax), as per herbaceous species sampled by Jaureguiberry et al. (2011). To preserve the shoot architecture of this iridoform species, a sample consisted of a single clump cut at ground level and trimmed from the leaf tips to 70 cm in length. All samples were stored for less than one week in sealed plastic bags at 4–8°C before burning. Experimental burns were conducted primarily in spring or summer, with some evergreen species burnt at the end of winter.
Flammability measurements
To measure shoot flammability we largely followed the methods described by Jaureguiberry et al. (2011) and Burger and Bond (2015), who used a custom-built portable device constructed from an 85 × 60 cm barrel cut in half and arranged horizontally on four metal legs. Our device was built following the specifications of Jaureguiberry et al. (2011) and adjusted to meet NZ safety standards; see ‘Device specifications’ in Supplementary material for further details.
When conducting flammability tests it is important to match the ignition source to the moisture content of the material being tested (White and Zipperer 2010). Pilot burning trials showed that only the most flammable species (e.g. Eucalyptus spp.) consistently ignited on our device when burned immediately after collection, which meant that we could only measure relative differences in flammability for those few most flammable species. Thus, all samples were air-dried at room temperature for 24 h before burning to enable a wider range of species to be ignited by the blowtorch, and so allowing a representative assessment of their comparative flammability. The methodology we employed to burn our samples followed that specified by Jaureguiberry et al. (2011). The burner was turned on until the grill reached a temperature of ~150°C and left on for the duration of the experiment. Grill temperature was measured before each sample. Unlike Jaureguiberry et al. (2011), who attempted to maintain the grill at 150°C throughout their experiments, we burned samples with grill temperatures ranging between 100–160°C. Regressions showed no relationships between grill temperature and the three measured flammability variables or our overall flammability index (P > 0.4, r2 <0.002 in all cases).
Samples were horizontally placed on the grill and preheated for two minutes, following Jaureguiberry et al. (2011). The blowtorch was then turned on for 10 s to ignite the samples. Jaureguiberry et al. (2011) determined this protocol on the basis of the literature (Stephens et al. 1994; Dimitrakopoulos and Papaioannou 2001) and their own trials. Measurements started immediately after the blowtorch was turned off. We recorded the length of time the sample burned (sustainability), the maximum temperature reached by the burning sample (combustibility) and, once burning was complete, as per Burger and Bond (2015), we visually estimated the percentage of the original biomass that had burnt (consumability). Visual estimations of burnt biomass were made by two people to minimise observer error. Samples that failed to maintain flaming ignition once the blowtorch was turned off were considered to not have ignited and given values of zero for all three variables. Frequency of ignition was recorded as a measure of ignitability, and was defined as the percentage of each species samples that had achieved ignition and burnt once the blowtorch was turned off.
Sub-samples were taken from each sample and weighed to determine their fresh mass (FM) at the time of burning. These sub-samples were oven-dried at 65°C for 48 h to determine dry mass (DM). Moisture content (MC) on a dry mass basis of the sub-samples at the time of burning was calculated using Eqn 1 (Behm et al. 2004).
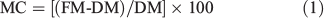
Statistical analyses
All statistical analyses were conducted using R version 3.1.2 (R Development Core Team 2014). Using the princomp() function from the stats package, we performed a principal components analysis (PCA) to investigate flammability patterns across species using burn time, maximum temperature and burnt biomass for every sample. Because the variables were on different scales, we performed the PCA using the correlation matrix of the data. A second PCA (see Fig S3) was undertaken using the mean values per species for the three measured flammability variables, as well as the percentage of each species samples that had achieved ignition and burnt once the blowtorch was turned off (i.e. frequency of ignition). Given that all flammability traits were negatively correlated with the first axis of this second PCA (and which explained nearly 80% of the variation in the data), we used position on this axis to provide an index of flammability for the species tested; species with the lowest axis one scores had the highest values for the four flammability traits and therefore the highest overall flammability. We used this index of flammability and K-means clustering (Hartigan and Wong 1979) to divide the species into flammability categories following Fogarty (2001): high, moderate/high, moderate, low/moderate and low, with an additional ‘very high’ category for Gorse. We compared the flammability ranking of the 27 species common to our study and that of Fogarty (2001) via a Spearman’s rank correlation. We then computed the probability of obtaining the resultant Spearman’s correlation co-efficient by chance alone by randomly shuffling the ranks of the 27 species and computing the Spearman’s rank correlation between the random ranking and the Fogarty (2001) ranking 10 000 times. Finally, we investigated the relationships among the four flammability components for the species burned in this study via Pearson’s product-moment correlations on all combinations of the flammability variables. Correlations were performed using the mean values per species of the three measured flammability variables and also the ignition frequency per species.
Results
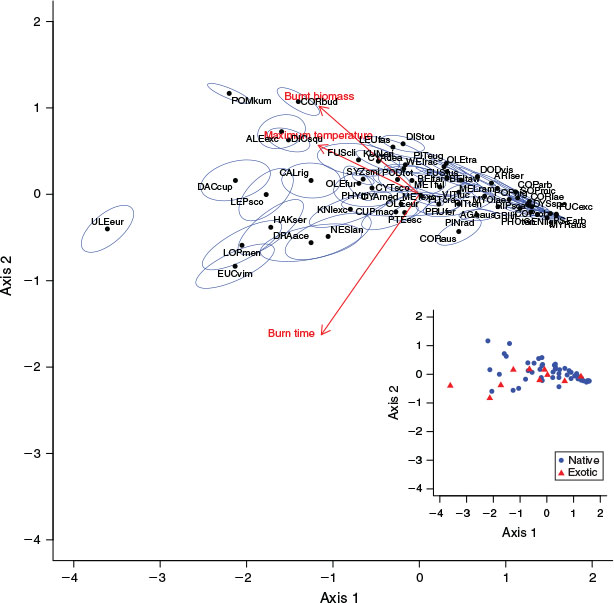
The 60 species we assessed varied widely in their flammability traits (Fig. 1). The first PCA axis explained 78% of the variation in the data and was negatively associated with all three flammability variables. The loadings of the three flammability variables were approximately equal on axis one (maximum temperature: –0.59, burn time: –0.57, burnt biomass: –0.58). The second PCA axis explained 12% of the variation and was negatively correlated with burn time (loading = –0.81) and positively correlated with maximum temperature and burnt biomass (loadings = 0.28 and 0.51). Variation on the second PCA axis was negatively related to position along axis one, as axis two variation increased with decreasing axis one scores (increasing flammability); high flammability species (low PCA axis one values) ranged from–0.83–1.17 in axis two position, whereas low flammability (high axis one values) species all scored similar values for axis two, clustering around –0.1 on this axis. Many low flammability species samples failed to ignite after 10 s with the blowtorch on, while those that did ignite only reached comparatively low values for all measured variables. See Table S1 for summary data of the measured flammability traits for each species.
|
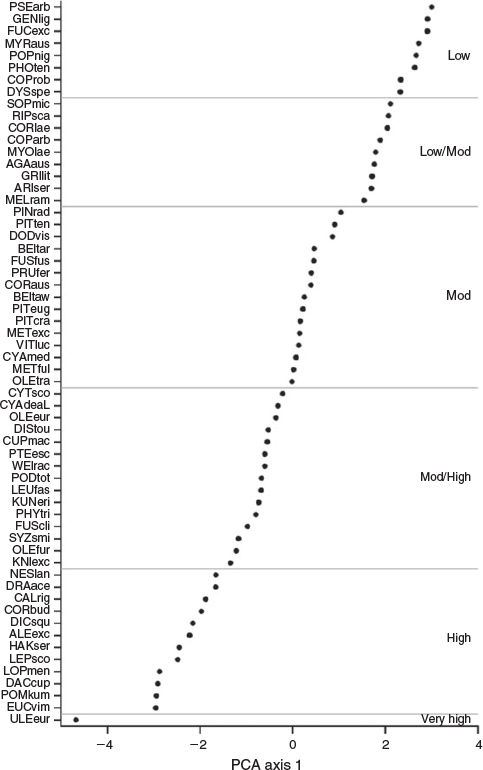
With the exception of the exotic Gorse, our PCA revealed few differences in flammability between NZ indigenous and exotic species (Fig. 1). Excluding Gorse, the lower limits of PCA axis one (i.e. highest flammabilities) were similar between indigenous (–2.20) and the remaining exotic species (–2.13). This finding includes Manna Gum, a naturalised species that was selected for this study in part because it is known to burn readily in its native Australia. Compared with the lower limits of axis one, there were greater differences in the upper axis one limits (i.e. the low flammability end of the spectrum) between indigenous (1.58) and exotic (1.28) species. Nevertheless, exotic species occurred in all flammability categories (as determined by K-means clustering of the flammability index), including the lowest flammability category (Fig. 2). According to our flammability index, the most flammable species were the European shrub Gorse, the indigneous heathland shrub Kūmarahou (Pomaderris kumeraho), the indigenous trees Rimu (Dacrydium cupressinum), Silver Beech (Lophozonia menziesii) and Mānuka (Leptospermum scoparium) and the Australian shrub Prickly Hakea (Hakea sericea) (Fig. 2). We found similarities in species flammability within genera such as Beilschmiedia, Coprosma, Cyathea and Metrosideros, but considerable differences were observed within genera such as Fuscospora and, to a lesser extent, Olearia (Fig. 2).
|
Our experimentally derived ranking was strongly correlated with the ranking produced from expert opinion for the 27 species common to both (Spearman’s ρ = 0.638, P < 0.001; Fig. 3). The probability of obtaining a Spearman’s correlation coefficient of 0.638 or higher was 0.0003, suggesting the strong correlation between our experimentally derived ranking and that of Fogarty (2001) was extremely unlikely to result from random chance. The species differing most between our assessment and that of Fogarty (2001) were Kauri (Agathis australis), Rimu, Akeake, Rewarewa (Knightia excelsa), Silver Beech and Flax (Fig. 3). Kauri, Akeake and Flax were less flammable than suggested by expert opinion, while Rimu, Rewarewa and Silver Beech were found to be more flammable by our study. Almost half (13) of the species common to these two studies were assigned to the same flammability category in each study. Nine species differed by one category, four species differed by two categories and a single species (Silver Beech) differed by three categories. Of these five species that differed by two or more categories, Flax was assigned to a lower flammability category in our study, whereas Rimu, Rewarewa, Silver Beech and Kāmahi (Weinmannia recemosa) were assigned to higher flammability categories in our study.
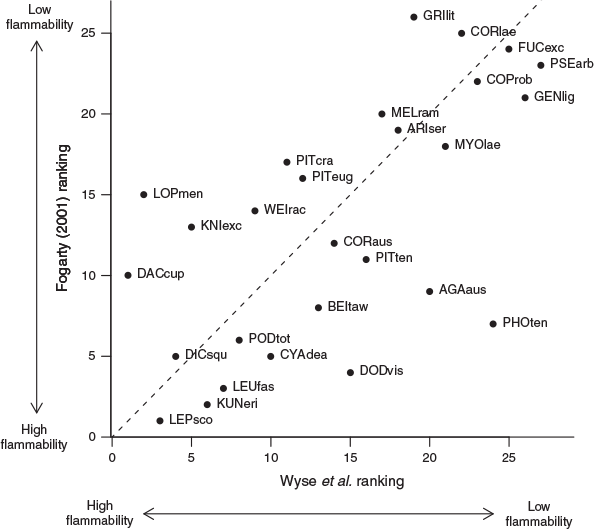
|
Intermediate to strong positive correlations were found among species means for the four flammability components (r > 0.58, P < 0.0001 in all instances; Table 2). The strongest correlations were found when the maximum temperature (combustibility) reached by a sample was one of the variables assessed. The weakest correlations were between the percentage of samples ignited (ignitability) and both burn time (sustainability) and burnt biomass (consumability).
Moisture content of the samples ranged from 12–239% (Table 1). The species with the highest moisture contents were (in descending order): Karaka (Corynocarpus laevigatus), Five-finger (Pseudopanax arboreus), Broadleaf (Griselinia littoralis), Hangehange (Geniostoma ligustrifolium), Pūriri (Vitex lucens) and Kohekohe (Dysoxylum spectabile), with mean moisture contents ranging from 152–239% on a dry mass basis. These high moisture content species all displayed low levels of flammability. The species with the lowest moisture contents were (in ascending order): Kūmarahou, Ponga (Cyathea dealbata), Korokio (Corokia buddleioides), Bracken and Mingimingi (Leucopogon fasciculatus). Moisture contents for these species ranged from 12–19%.
Discussion
Flammability of New Zealand trees and shrubs
The first aim of our study was to quantify the shoot flammability of several NZ plants. Flammability levels varied across the species investigated, with some burning at high temperatures (up to 861°C) and for relatively long time periods (up to 173 s), while others consistently failed to maintain flaming ignition once the blowtorch was turned off (see Table S1). The three flammability variables measured per sample, maximum temperature, burn time and burnt biomass, were all negatively correlated with the first PCA axis. However, the spread of samples on the second PCA axis suggested that there were a range of variable combinations that defined a high flammability species. At the flammable end of the spectrum (i.e. negative values on PCA axis 1), species fell on a continuum from those with high biomass consumption and maximum temperatures, but comparatively low burn time, to those with low biomass consumption and maximum temperatures but high burn time. The most flammable species that we assessed, Gorse, had high values for three parameters measured, while the least flammable species displayed consistently low values for all. The dichotomy in flammability types between species with high consumability (burnt biomass) and combustibility (maximum temperature) versus those with high sustainability (burn time) has been noted previously in comparisons of the flammability of litter types (a different fuel type to that burned here), albeit with a different measure of combustibility (flame height) to that used here (de Magalhães and Schwilk 2012). Ignitability, a trait recorded only at the species level rather than per sample, was strongly correlated with the maximum temperature at which the samples burned. Correlations among the flammability components were expected, as these measures of flammability are not orthogonal (White and Zipperer 2010). Given that ignitability is the key component determining whether a sample actually burns (Martin et al. 1994), we expected ignitability to be the component explaining most of the variability in the other three flammability components. However, we found weak correlations between this component and both burn time and burnt biomass, while maximum temperature (combustibility) was most strongly correlated with the other three components.
The NZ indigenous species we studied were all from habitats where fire has not been a major selective pressure, being neither a frequent nor predictable cause of disturbance (Perry et al. 2014). Conversely, several of the exotic species we included (e.g. Bottlebrush (Callistemon rigidus), Manna Gum, Prickly Hakea and Gorse) were selected because they are native to fire-prone environments and are known to burn readily. The Mutch (1970) hypothesis suggests that plants from fire-dependent communities have evolved characteristics that increase their propensity to burn, though this has since been much debated in the literature (e.g. Snyder 1984; Bond and Midgley 1995; Schwilk 2003; Bowman et al. 2014). Bowman et al. (2014) showed that fire-enhancing plant traits can exist in plant communities rarely exposed to fire, and proposed that such traits have therefore evolved independently of landscape fire as the result of other selective pressures, although some may be exaptations. Despite fire not being a selective pressure in the evolutionary history of the indigenous NZ species (with the possible exception of some shrubs such as Mānuka and some non-woody wetland taxa: Perry et al. 2014), we found several indigenous species to be at least as flammable as exotic species from fire-prone environments, although none were as flammable as Gorse. Interestingly, the most fire-adapted indigenous species burned here, Mānuka, was not the most flammable of the indigenous species. Although our study was not designed to test their hypothesis, these findings support Bowman et al. (2014) as we demonstrate high levels of flammability in species from communities that are rarely burnt. High shoot-level flammability was also recently reported for species that occur in the fire-free forest biome in South Africa (Calitz et al. 2015). For species such as Rimu and Silver Beech, the traits causing them to have relatively high flammability have evolved without fire as a selective force and likely have been the result of other evolutionary pressures, or are ancestral characters that have been retained since the species arrived in NZ. Indeed, where fire is rare in a community, traits that enhance flammability but confer other selective benefits such as drought or herbivore tolerance may arise due to a lack of selective pressure against them. This may explain why Rimu and Silver Beech, species from comparatively wet habitats, were two of the most flammable species tested here. Future research should focus on identifying the physical traits influencing the flammability of New Zealand plant species and their evolutionary significance (Murray et al. 2013; Burger and Bond 2015).
We found indigenous early successional forest and scrub species such as Kūmarahou, Mānuka and, to a lesser extent, Akepiro (Olearia furfuracea) and Kānuka (Kunzea ericoides), to be among the most flammable species, along with the mature forest species Silver Beech and Rimu. Conversely, species found to be of the lowest flammability in this study included common lowland forest species such as Karamū (Coprosma robusta), Karaka, Kohekohe, Hangehange and Five-finger. We recommend the use of this latter group of species, along with Kōtukutuku (Fuchsia excorticat) and Hangehange, in green fire breaks. These species were placed in the low flammability categories in our study and by Fogarty (2001). Species such as Karamū and Hangehange, in particular, have broad environmental tolerances and are commonly planted throughout the country, while others such as Karaka and Kohekohe are more appropriate for areas or habitats with milder climates. Macrocarpa (Cupressus macrocarpa) and Lombardy Poplar (Populus nigra) are two common species planted as shelterbelts on NZ farms. Based on the relative flammability measured here we recommend the use of Lombardy Poplar in this context, as it is likely to have a lower fire risk than Macrocarpa.
Of the 10 exotic species examined in this study, Scotch Broom (Cytisus scoparius), Prickly Hakea and Gorse are abundant invasive weeds of farmland and early successional communities in NZ (Williams 2011). Our results suggest that the canopy foliage and shoots of Scotch Broom presents less of a fire risk than Prickly Hakea or Gorse. However, these latter two species could increase the flammability of the early successional communities that they invade, as has occurred with other woody invasions (Berry et al. 2011; Mandle et al. 2011). Perry et al. (2014) hypothesised that this may then slow post-fire succession in NZ as communities spend longer in flammable, early successional states. The potential combined flammability of mixtures of indigenous early successional species and these high flammability invasive species is unknown for NZ species and ecosystems, and warrants further investigation (e.g. following Mola et al. 2014). However, it is evident that unchecked spread of these flammable species, particularly Gorse in farmland across most of the country, should be a priority for control. In Spain, Madrigal et al. (2012) advocated intensive management of Gorse shrubland to prevent plants exceeding five years of age. At this stage rapid physiological and structural changes considerably increase plant flammability (Madrigal et al. 2012), most likely through retention of dead biomass (Dent, Lustig, Buckley and Curran unpubl. data).
Shoot-level flammability assessment in comparison to expert opinion
Vegetation flammability is a much-debated subject, both in terms of its usefulness as a concept and the most appropriate methods for its assessment (e.g. White and Zipperer 2010; Fernandes and Cruz 2012; Pausas and Moreira 2012). White and Zipperer (2010) provide a review of different quantitative methods for assessing plant flammability, evaluating the methodologies and advocating for the standardisation of flammability measures among studies. White and Zipperer (2010) also compared species’ relative flammability rankings as determined by several methodologies, while Dimitrakopoulos (2001) compared expected relative flammability based on species physical and chemical properties to laboratory measures of ignitability. In their respective studies, White and Zipperer (2010) found reasonable agreement among some, but not all, of the methodologies investigated, and Dimitrakopoulos (2001) found good agreement between the expected flammability rankings and empirical results. To our knowledge, however, flammability rankings based on expert opinion have not previously been evaluated against quantitative experimental measures, so our comparison represents a novel contribution to this debate. Long et al. (2006) used expert opinion to identify a suite of species to test empirically, but did not attempt to compare the outcomes of the two approaches.
Our experimental assessments broadly agreed with the qualitative rankings of Fogarty (2001). However, there were also some notable discrepancies between the two approaches. First, our results illustrated that despite their common physiognomy different tree fern taxa vary in their flammability (in comparison to Fogarty 2001). Additional discrepancies between the two studies, such as in the rankings of Rimu, Rewarewa and Silver Beech, may result from the types of data used to generate the assessments. While a fire manager is likely to integrate observations of the flammability of a species with knowledge of the environmental conditions that species inhabits, we compared species flammability traits under common (controlled) environmental conditions in an experimental context. Fogarty’s (2001) methodology likely, therefore, integrates flammability with the opportunity to burn in a given environment, whereas we have assessed flammability given the presence of an ignition source. Due to these differences, the results of our study may provide a more accurate depiction of species’ relative flammability in a common environment; although caution must be applied when extrapolating from our laboratory setting to field conditions (Pausas and Moreira 2012). In contrast, the assessments of Fogarty (2001) may better reflect the relative likelihood of species burning in their natural environments. Between these two studies we are starting to gain a robust view of the comparative flammability of many trees and shrubs occurring in the NZ landscape. However, future work involving whole branches and shrubs, and ideally large-scale experimental burns in the field that account for the structure of the live and dead fuel, are necessary to gain an understanding of NZ plant flammability across a range of scales.
Suitability of the methodology
Because they preserve much of the architecture of the plant shoots, the methods we used provide a more realistic comparison of relative canopy flammability among species than methods that use smaller plant components (Perez-Harguindeguy et al. 2013). Our methods can be used to provide relative indices of flammability among species at the shoot-level. However, like all laboratory methods, caution must be applied when scaling beyond the experimental setting, as the flammability of species in field conditions will be influenced not only by the characteristics of the species itself but also by its environment (White and Zipperer 2010; Fernandes and Cruz 2012). In this study we have standardised our experimental units across species as single, 70 cm-long-terminal sections. However, this may not accurately reflect the characteristics of the fuel as it is on the plant for some species.
There are two particular circumstances where methodological improvements are needed in terms of the definition of a sampling unit. The first situation that warrants improvement is where a species has sparsely placed leaves (or pinnae) along a single branch (or frond), but where in the field branches would typically overlap on the whole plant and increase bulk density and fuel continuity. When such samples burn the individual leaves may burn readily, but the flame does not carry along the sample and is quickly extinguished having burnt little of the biomass. For example, Bracken is regarded as highly flammable (McGlone et al. 2005) and we found that individual pinnae burn readily. However, only one or two pinnae would be ignited by the blowtorch and, owing to the sparse placement of pinnae along the frond, the flame would not carry along the sample. In the field, bracken fronds grow in a very dense, intertwined fashion and fire would spread easily through a plant.
The second methodological issue we identified was with species that retain dead material, particularly around their trunks. This is the case for some tree fern species and also Cabbage Tree (Cordyline australis). The retention of dead leaf material increases plant flammability (Bowman et al. 2014), and preliminary tests found dead tree fern and Cabbage Tree fronds were more flammable than the corresponding live material. For such species the flammability of the dead material will likely be a more important driver of plant flammability than that of a live shoot, yet the present method only measures this where dead material is part of a terminal shoot section. This issue may be one that is best resolved by burning entire plants or large branches.
Conclusions
We have developed the first empirical ranking of flammability across a range of indigenous and exotic New Zealand plant species. Our results indicate that invasive exotic species such as Gorse and Prickily hakea are highly flammable and therefore have the potential to increase fire risk in the communities that they invade. We also determined that many indigenous plant species – notably Rimu, Silver Beech and Kūmarahou – burn readily and were among the most flammable species evaluated in this study. We found our shoot-level flammability assessments to largely concur with a previous qualitative assessment based on expert opinion (Fogarty 2001), providing support for both methods and progressing towards a better understanding of plant flammability in New Zealand. These close correlations between rankings derived from our shoot-level experiments and those gleaned from expert opinion support Schwilk's (2015) suggestion that further measurements of canopy-held fuels are warranted and that these could be measured at the shoot scale; for instance, with the device designed by Jaureguiberry et al. (2011). Future research should examine the plant traits that give rise to the flammability patterns measured here, while larger-scale and field-based studies should be employed to further our understanding of links between plant flammability and fire behaviour.
The dataset used for this study is available online as Supplementary material (Table S3).
Acknowledgements
We thank the University of Auckland (UoA) and Lincoln University (LU) for funding for this research. UoA provided funding through a Faculty of Science Research Development Grant awarded to GLWP; LU provided funding via Early Career Researcher and LU Research Fund grants to TJC, Summer Scholarships to PSH, MJW and IJG, and an internship to KJLM. We also thank UoA, LU, Landcare Research Lincoln, Christchurch City Council, the Auckland Botanic Gardens and several private landholders for allowing sampling on their properties.
References
Allan HH (1961) ‘Flora of New Zealand. Volume I. Indigenous Tracheophyta – Psilopsida, Lycopsida, Filicopsida, Gymnospermae, Dicotyledons.’ (P.D. Hasselberg, Govt. Printer: Wellington)Anderson HE (1970) Forest fuel ignitibility. Fire Technology 6, 312–319.
| Forest fuel ignitibility.Crossref | GoogleScholarGoogle Scholar |
Anderson SAJ, Anderson WR (2009) Predicting the elevated dead fine fuel moisture content in gorse (Ulex europaeus L.) shrub fuels. Canadian Journal of Forest Research 39, 2355–2368.
| Predicting the elevated dead fine fuel moisture content in gorse (Ulex europaeus L.) shrub fuels.Crossref | GoogleScholarGoogle Scholar |
Anderson SAJ, Anderson WR (2010) Ignition and fire spread thresholds in gorse (Ulex europaeus). International Journal of Wildland Fire 19, 589–598.
| Ignition and fire spread thresholds in gorse (Ulex europaeus).Crossref | GoogleScholarGoogle Scholar |
Behm AL, Duryea ML, Long AJ, Zipperer WC (2004) Flammability of native understory species in pine flatwood and hardwood hammock ecosystems and implications for the wildland-urban interface. International Journal of Wildland Fire 13, 355–365.
| Flammability of native understory species in pine flatwood and hardwood hammock ecosystems and implications for the wildland-urban interface.Crossref | GoogleScholarGoogle Scholar |
Berry ZC, Wevill K, Curran TJ (2011) The invasive weed Lantana camara increases fire risk in dry rainforest by altering fuel beds. Weed Research 51, 525–533.
| The invasive weed Lantana camara increases fire risk in dry rainforest by altering fuel beds.Crossref | GoogleScholarGoogle Scholar |
Bond WJ (2005) Large parts of the world are brown or black: a different view on the ‘Green World’ hypothesis. Journal of Vegetation Science 16, 261–266.
Bond WJ, Midgley JJ (1995) Kill thy neighbour: an individualistic argument for the evolution of flammability. Oikos 73, 79–85.
| Kill thy neighbour: an individualistic argument for the evolution of flammability.Crossref | GoogleScholarGoogle Scholar |
Bowman DMJS, Balch JK, Artaxo P, Bond WJ, Carlson JM, Cochrane MA, D’Antonio CM, DeFries RS, Doyle JC, Harrison SP, Johnston FH, Keeley JE, Krawchuk MA, Kull CA, Marston JB, Moritz MA, Prentice IC, Roos CI, Scott AC, Swetnam TW, van der Werf GR, Pyne SJ (2009) Fire in the Earth system. Science 324, 481–484.
| Fire in the Earth system.Crossref | GoogleScholarGoogle Scholar |
Bowman DMJS, French BJ, Prior LD (2014) Have plants evolved to self-immolate? Frontiers in Plant Science 5, 590
| Have plants evolved to self-immolate?Crossref | GoogleScholarGoogle Scholar |
Burger N, Bond WJ (2015) Flammability traits of Cape shrubland species with different post-fire recruitment strategies. South African Journal of Botany
| Flammability traits of Cape shrubland species with different post-fire recruitment strategies.Crossref | GoogleScholarGoogle Scholar |
Calitz W, Potts AJ, Cowling RM (2015) Investigating species-level flammability across five biomes in the Eastern Cape, South Africa. South African Journal of Botany
| Investigating species-level flammability across five biomes in the Eastern Cape, South Africa.Crossref | GoogleScholarGoogle Scholar |
Cornwell WK, Elvira A, van Kempen L, van Logtestijn RSP, Aptroot A, Cornelissen JHC (2015) Flammability across the gymnosperm phylogeny: the importance of litter particle size. New Phytologist 206, 672–681.
| Flammability across the gymnosperm phylogeny: the importance of litter particle size.Crossref | GoogleScholarGoogle Scholar |
de Lange PJ (2014) A revision of the New Zealand Kunzea ericoides (Myrtaceae) complex. PhytoKeys 40, 1–185.
| A revision of the New Zealand Kunzea ericoides (Myrtaceae) complex.Crossref | GoogleScholarGoogle Scholar |
de Magalhães RMQ, Schwilk DW (2012) Leaf traits and litter flammability: evidence for non-additive mixture effects in a temperate forest. Journal of Ecology 100, 1153–1163.
| Leaf traits and litter flammability: evidence for non-additive mixture effects in a temperate forest.Crossref | GoogleScholarGoogle Scholar |
Dickinson KJM, Kirkpatrick JB (1985) The flammability and energy content of some important plant species and fuel components in the forests of southeastern Tasmania. Journal of Biogeography 12, 121–134.
| The flammability and energy content of some important plant species and fuel components in the forests of southeastern Tasmania.Crossref | GoogleScholarGoogle Scholar |
Dimitrakopoulos AP (2001) A statistical classification of Mediterranean species based on their flammability components. International Journal of Wildland Fire 10, 113–118.
| A statistical classification of Mediterranean species based on their flammability components.Crossref | GoogleScholarGoogle Scholar |
Dimitrakopoulos AP, Papaioannou KK (2001) Flammability assessment of Mediterranean forest fuels. Fire Technology 37, 143–152.
| Flammability assessment of Mediterranean forest fuels.Crossref | GoogleScholarGoogle Scholar |
Etlinger MG, Beall FC (2004) Development of a laboratory protocol for fire performance of landscape plants. International Journal of Wildland Fire 13, 479–488.
| Development of a laboratory protocol for fire performance of landscape plants.Crossref | GoogleScholarGoogle Scholar |
Fernandes RM, Cruz MG (2012) Plant flammability experiments offer limited insight into vegetation-fire dynamics interactions. New Phytologist 194, 606–609.
| Plant flammability experiments offer limited insight into vegetation-fire dynamics interactions.Crossref | GoogleScholarGoogle Scholar |
Fogarty LG (2001) A flammability guide for some common New Zealand native tree and shrub species. Forest Research Bulletin 143, Forest and Rural Fire Scientific and Technical Series Report 6. Forest Research Institute in association with the New Zealand Fire Service Commission and National Rural Fire Authority, Rotorua, Wellington.
Fonda RW (2001) Burning characteristics of needles from eight pine species. Forest Science 47, 390–396.
Gill AM, Zylstra P (2005) Flammability of Australian forests. Australian Forestry 68, 87–93.
| Flammability of Australian forests.Crossref | GoogleScholarGoogle Scholar |
Hartigan JA, Wong MA (1979) A K-means clustering algorithm. Applied Statistics 28, 100–108.
| A K-means clustering algorithm.Crossref | GoogleScholarGoogle Scholar |
Jaureguiberry P, Bertone G, Diaz S (2011) Device for the standard measurement of shoot flammability in the field. Austral Ecology 36, 821–829.
| Device for the standard measurement of shoot flammability in the field.Crossref | GoogleScholarGoogle Scholar |
Johnson VJ (1975) Hardwood fuel-breaks for north eastern United States. Journal of Forestry 73, 588–589.
Kane JM, Varner JM, Hiers JK (2008) The burning characteristics of southeastern oaks: discriminating fire facilitators from fire impeders. Forest Ecology and Management 256, 2039–2045.
| The burning characteristics of southeastern oaks: discriminating fire facilitators from fire impeders.Crossref | GoogleScholarGoogle Scholar |
Keeley JE, Bond WJ, Bradstock RA, Rundel PW (2012) ‘Fire in Mediterranean ecosystems: ecology, evolution and management.’ (Cambridge University Press: New York)
Liodakis S, Bakirtzis D, Lois E (2002) TG and autoignition studies on forest fuels. Journal of Thermal Analysis and Calorimetry 69, 519–528.
| TG and autoignition studies on forest fuels.Crossref | GoogleScholarGoogle Scholar |
Long AJ, Behm A, Zipperer WC, Hermansen A, Maranghides A, Mell W (2006) Quantifying and ranking the flammability of ornamental shrubs in the southern United States. In ‘2006 Fire ecology and management conference proceedings (DVD).’ (The Association for Fire Ecology/Washington State University Extension: San Diego, CA)
Madrigal J, Guijarro M, Hernando C, Diez C, Marino E (2011) Estimation of peak heat release rate of a forest fuel bed in outdoor laboratory conditions. Journal of Fire Sciences 29, 53–70.
| Estimation of peak heat release rate of a forest fuel bed in outdoor laboratory conditions.Crossref | GoogleScholarGoogle Scholar |
Madrigal J, Marino E, Guijarro M, Hernando C, Diez C (2012) Evaluation of the flammability of gorse (Ulex europaeus L.) managed by prescribed burning. Annals of Forest Science 69, 387–397.
| Evaluation of the flammability of gorse (Ulex europaeus L.) managed by prescribed burning.Crossref | GoogleScholarGoogle Scholar |
Mandle L, Bufford JL, Schmidt IB, Daehler CC (2011) Woody exotic plant invasions and fire: reciprocal impacts and consequences for native ecosystems. Biological Invasions 13, 1815–1827.
| Woody exotic plant invasions and fire: reciprocal impacts and consequences for native ecosystems.Crossref | GoogleScholarGoogle Scholar |
Martin RE, Gordon DA, Gutierrez MA, Lee DS, Molina DM, Schroeder RA, Sapsis DB, Stephens SL, Chambers M (1994) Assessing the flammability of domestic and wildland vegetation. In ‘Proceedings of the 12th conference on fire and forest meteorology.’ pp. 130–137. (Society of American Foresters: Bethesda, Maryland)
McGlone MS, Duncan RP, Heenan PB (2001) Endemism, species selection and the origin and distribution of the vascular plant flora of New Zealand. Journal of Biogeography 28, 199–216.
| Endemism, species selection and the origin and distribution of the vascular plant flora of New Zealand.Crossref | GoogleScholarGoogle Scholar |
McGlone MS, Wilmshurst JM, Leach HM (2005) An ecological and historical review of bracken (Pteridium esculentum) in New Zealand, and its cultural significance. New Zealand Journal of Ecology 29, 165–184.
McWethy DB, Whitlock C, Wilmshurst JM, McGlone MS, Li X (2009) Rapid deforestation of South Island, New Zealand, by early Polynesian fires. The Holocene 19, 883–897.
| Rapid deforestation of South Island, New Zealand, by early Polynesian fires.Crossref | GoogleScholarGoogle Scholar |
McWethy DB, Wilmshurst JM, Whitlock C, Wood JR, McGlone MS (2014) A high-resolution chronology of rapid forest transitions following Polynesian arrival in New Zealand. PLoS One 9, e111328
| A high-resolution chronology of rapid forest transitions following Polynesian arrival in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Mola JM, Varner JM, Jules ES, Spector T (2014) Altered community flammability in Florida’s Apalachicola Ravines and implications for the persistence of the endangered conifer Torreya taxifolia. PLoS One 9, e103933
| Altered community flammability in Florida’s Apalachicola Ravines and implications for the persistence of the endangered conifer Torreya taxifolia.Crossref | GoogleScholarGoogle Scholar |
Moore LB, Edgar E (1976) ‘Flora of New Zealand. Volume II. Indigenous tracheophyta – monocotyledons except graminae.’ (A.R. Shearer, Govt. Printer: Wellington)
Mullan B, Porteous A, Wratt D, Holls M (2005) Changes in drought risk with climate change. Prepared for Ministry for the Environment (NZ Climate Change Office) and Ministry of Agriculture and Forestry. NIWA Client Report: WLG2005–23. (National Institute of Water and Atmospheric Research, Wellington).
Murray BR, Hardstaff LK, Phillips ML (2013) Differences in leaf flammability, leaf traits and flammability–trait relationships between native and exotic plant species of dry sclerophyll forest. PLoS One 8, e79205
| Differences in leaf flammability, leaf traits and flammability–trait relationships between native and exotic plant species of dry sclerophyll forest.Crossref | GoogleScholarGoogle Scholar |
Mutch RW (1970) Wildfires and ecosystems – a hypothesis. Ecology 51, 1046–1051.
| Wildfires and ecosystems – a hypothesis.Crossref | GoogleScholarGoogle Scholar |
Núñez-Regueira L, Rodriguez JA, Castineiras JP (1996) Calorific values and flammability of forest species in Galicia coastal and hillside zones. Bioresource Technology 57, 283–289.
| Calorific values and flammability of forest species in Galicia coastal and hillside zones.Crossref | GoogleScholarGoogle Scholar |
Ogden J, Basher L, McGlone M (1998) Fire, forest regeneration and links with early human habitation: evidence from New Zealand. Annals of Botany 81, 687–696.
| Fire, forest regeneration and links with early human habitation: evidence from New Zealand.Crossref | GoogleScholarGoogle Scholar |
Owens MK, Lin C-D, Taylor CAJ, Whisenant SG (1998) Seasonal patterns of plant flammability and monoterpenoid content in Juniperus ashei. Journal of Chemical Ecology 24, 2115–2129.
| Seasonal patterns of plant flammability and monoterpenoid content in Juniperus ashei.Crossref | GoogleScholarGoogle Scholar |
Pausas JG, Moreira B (2012) Flammability as a biological concept. New Phytologist 194, 610–613.
| Flammability as a biological concept.Crossref | GoogleScholarGoogle Scholar |
Pearce HG, Kerr J, Clark A, Mullan B, Ackerley D, Carey-Smith T, Yang E (2010). Improved estimates of the effect of climate change on NZ fire danger. Scion Client Report No. 18087. (Scion, Rural Fire Research Group, Christchurch).
Perez-Harguindeguy N, Diaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, de Vos AC, Buchmann N, Funes G, Quetier F, Hodgson JG, Thompson K, Morgan HD, ter Steege H, van der Heijden MGA, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2013) New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61, 167–234.
| New handbook for standardised measurement of plant functional traits worldwide.Crossref | GoogleScholarGoogle Scholar |
Perry GLW, Wilmshurst JM, McGlone MS (2014) Ecology and long-term history of fire in New Zealand. New Zealand Journal of Ecology 38, 157–176.
R Development Core Team (2014) ‘R: A language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna)
Schwilk DW (2003) Flammability is a niche construction trait: canopy architecture affects fire intensity. American Naturalist 162, 725–733.
| Flammability is a niche construction trait: canopy architecture affects fire intensity.Crossref | GoogleScholarGoogle Scholar |
Schwilk DW (2015) Dimensions of plant flammability. New Phytologist 206, 486–488.
| Dimensions of plant flammability.Crossref | GoogleScholarGoogle Scholar |
Snyder JR (1984) The role of fire: Mutch ado about nothing? Oikos 43, 404–405.
| The role of fire: Mutch ado about nothing?Crossref | GoogleScholarGoogle Scholar |
Stephens SL, Gordon DA, Martin RE (1994) Combustibility of selected domestic vegetation subjected to desiccation. In ‘Proceedings of the 12th Conference on Fire and Forest Meteorology.’ pp. 565–571. (Society of American Foresters: Bethesda, Maryland)
Webb CJ, Sykes WR, Garnock-Jones PJ (1988) ‘Flora of New Zealand. Volume IV. Naturalised pteridophytes, gymnosperms, dicotyledons.’ (Botany Division, D.S.I.R.: Christchurch)
Weise DR, White RH, Beall FC, Etlinger M (2005) Use of the cone calorimeter to detect seasonal differences in selected combustion characteristics of ornamental vegetation. International Journal of Wildland Fire 14, 321–338.
| Use of the cone calorimeter to detect seasonal differences in selected combustion characteristics of ornamental vegetation.Crossref | GoogleScholarGoogle Scholar |
White RH, Zipperer WC (2010) Testing and classification of individual plants for fire behaviour: plant selection for the wildland-urban interface. International Journal of Wildland Fire 19, 213–227.
| Testing and classification of individual plants for fire behaviour: plant selection for the wildland-urban interface.Crossref | GoogleScholarGoogle Scholar |
Whitlock C, Higuera PE, McWethy DB, Briles CE (2010) Paleoecological perspectives on fire ecology: revisiting the fire-regime concept. The Open Ecology Journal 3, 6–23.
| Paleoecological perspectives on fire ecology: revisiting the fire-regime concept.Crossref | GoogleScholarGoogle Scholar |
Williams PA (2011) Secondary succession through non-native dicotyledonous woody plants in New Zealand. New Zealand Natural Sciences 36, 73–91.
Wilmshurst JM, Anderson AJ, Higham TFG, Worthy TH (2008) Dating the late prehistoric dispersal of Polynesiams to New Zealand using the commensal Pacific rat. Proceedings of the National Academy of Sciences of the United States of America 105, 7676–7680.
| Dating the late prehistoric dispersal of Polynesiams to New Zealand using the commensal Pacific rat.Crossref | GoogleScholarGoogle Scholar |



