The formation of charcoal reflectance and its potential use in post-fire assessments
Claire M. Belcher A B and Victoria A. Hudspith AA wildFIRE Lab, Hatherly Laboratories, University of Exeter, Prince of Wales Road, Exeter, Devon, EX4 4PS, UK.
B Corresponding author. Email: c.belcher@exeter.ac.uk
International Journal of Wildland Fire 25(7) 775-779 https://doi.org/10.1071/WF15185
Submitted: 21 October 2015 Accepted: 5 April 2016 Published: 11 May 2016
Journal Compilation © IAWF 2016
Abstract
Charcoal has an exceptional ability to reflect light when viewed using reflectance microscopy. The amount of light reflected is variable depending on the differential ordering of graphite-like phases within the charcoal itself. It has been suggested that this relates to the temperature of formation, whereby higher formation temperatures result in high charcoal reflectance. However, this explanation is derived from oven-based chars that do not well represent the natural combustion process. Here, we have experimentally created charcoals using a cone calorimeter, in order to explore the development of charcoal reflectance during pre-ignition heating and peak heat-release rate, through to the end of flaming and the transition to char oxidation. We find that maximum charcoal reflectance is reached at the transition between pyrolysis and char oxidation, before its conversion to mineral ash, and indicates that our existing understanding of reflectance is in error. We suggest that charcoal reflectance warrants additional study as it may provide a useful quantitative addition to ground-based fire severity surveys, because it may allow exploration of surface heating after the main fire front has passed and the fire transitions to smouldering phases.
Additional keywords: calorimetry, fire severity, pyrogenic carbon, reflectance microscopy, wildfire.
Introduction
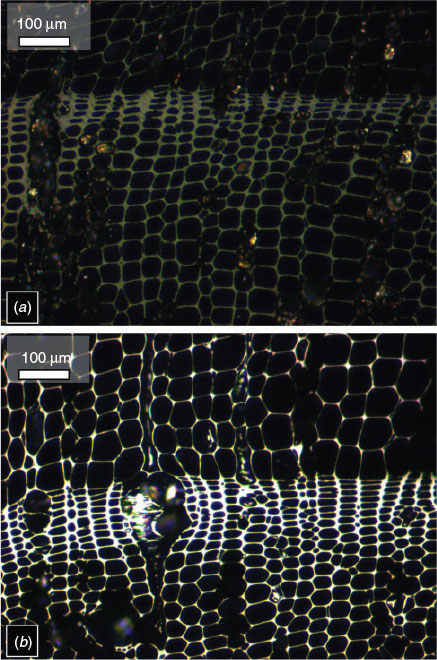
Qualitative assessments of fire severity have long been used to determine ecosystem recovery and to inform fire management strategies (Morgan et al. 2014) because building an understanding of the impact of a wildfire is critical to the management of ecosystems. In particular, aspects of fire severity such as duration of surface heating have even been shown to relate to the survival of rhizomes or seedbanks (Keeley 2009), as well as post-fire vegetation recovery (Gagnon et al. 2015). Yet there are no quantitative measurements that assess heat distributions in current post-burn ground-based fire severity assessments; instead, these are predominantly qualitative visual assessments of organic matter loss, and as such can be subjective and variable between ecosystems. It has been suggested that charcoal retains information about the nature of the fire in which it was created (Jones et al. 1991) and one physical property of charcoal that can be measured post-fire is its ability to reflect light when studied under oil using reflectance microscopy (Fig. 1). The amount of light reflected back from the charcoal is thought to be due to alterations in the extent of graphite-phases within the molecular structure of charcoal that increase as temperature rises (Cohen-Ofri et al. 2006). Thus, this ordering of the charcoal structure translates to a predictable increase in measurable light reflected from the sample (Scott 2010). These observations suggest that charcoal, which is left on the ground in abundance following wildfires, may be a potential source of quantitative information that could be coupled with existing qualitative ground-based fire severity assessments.
|
Our existing understanding of charcoal reflectance has been established using oven-created charcoals formed across a range of temperatures that have been used to generate calibration curves (e.g. McParland et al. 2009; Ascough et al. 2010; Hudspith et al. 2015), from which reflectance values of charcoal produced at unknown temperatures can be extrapolated (see Fig. S1 in the online supplementary material). This relationship suggests that by taking measurements of the reflectance of charcoals collected from a site directly following a fire, temperature distributions across a burned area may be quantifiable. However, this understanding has been derived from oven-based charring experiments, which do not well replicate the conditions in a wildfire and critically fail to capture the full combustion process. The temperature field in a wildfire is constantly changing in time and space (Alexander 1982; Finney et al. 2015); therefore, a singular heat flux or temperature (like that of an oven) does not represent the complex energy exchange that occurs. In a wildfire, charcoal is produced during the pyrolysis stage of combustion, which is the stage at which the fuel is thermally decomposed to produce flammable volatile gases (pyrolysate) that are consumed by the flames. During flaming combustion, oxidation reactions occur in the gas phase above the fuel, causing the flame itself to limit the diffusion of oxygen to the fuel surface (Tran and White 1992). Charcoals produced in an oven were previously assumed to replicate this process, with the oxygen-depleted conditions in an oven representing the effect of a flame on the surface of the fuel. However, in a wildfire, as flaming ceases, both pyrolysis and oxidation of the solid fuel can co-occur as the flaming phase of the fire transitions to a smouldering fire (Rein 2013). This phase of combustion is therefore neither captured in oven-based experiments, nor in the reflectance of charcoals produced by this method.
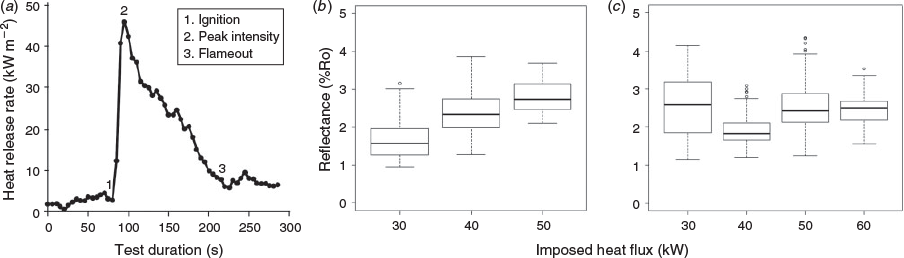
In order to assess the use of reflectance as a tool in post-fire assessments, we need to investigate the reflectance of charcoals formed in more realistic conditions. In this study, we present the first reflectance measurements of charcoals formed using oxygen-depletion calorimetry. These test conditions allow combustion to proceed and critically allow the quantification of the heat release rate from the fire. During the experiments, the sample was exposed to a predetermined heat flux to generate pyrolysis gases, which could then be ignited by a spark igniter (similar to a firebrand or flame in an actual wildfire). Throughout combustion, the heat release rate from the burning samples was measured (Fig. 2a) and the char created removed at different phases of the heat-release profile in order to investigate at what stage maximum reflectance becomes imprinted in the charcoal. In addition, we explored other factors such as fuel moisture, species and fuel density, all of which are also known to affect combustion characteristics and therefore potentially charcoal reflectance. Here, we present the results of these experiments to assess the validity of existing understanding of charcoal reflectance, in order to work towards considering the utility of charcoal reflectance in post-fire assessments.
Materials and methods
We undertook four sets of experiments that explored the relationship between combustion and the formation of charcoal reflectance. In the first set of experiments, we took the same wood samples used in the oven-based experiments of Hudspith et al. (2015) (see Fig. S1) and exposed them to heat fluxes ranging from 30 to 50 kWm2 for 300 s, using an iCone calorimeter (Fire Testing Technology, East Grinstead, UK). The species were Populus tremuloides, Betula papyrifera, Picea mariana and Picea glauca (hereafter collectively termed boreal samples) and were 15 × 15 × 15-mm branch pieces with bark intact, laboratory-cured to 7–8.5% moisture (oven-dry basis). The samples were simply exposed to the incident heat flux and were not allowed to ignite, mimicking charcoal formation in an oven. Our aim here was to consider how reflectance varied according to a range of known heat fluxes, as opposed to oven temperatures, in order to allow direct comparison with the oven-produced charcoals of Hudspith et al. (2015) (Fig. S1). In order to expose the samples to a more realistic fire scenario, another set of the same-sized boreal wood samples was exposed to the same range of incident heat fluxes but allowed to ignite. Here, a spark igniter positioned above the sample during heating caused ignition of the pyrolysis gases, leading to flaming. Once the samples ceased flaming, they were collected and the charcoal reflectance analysed.
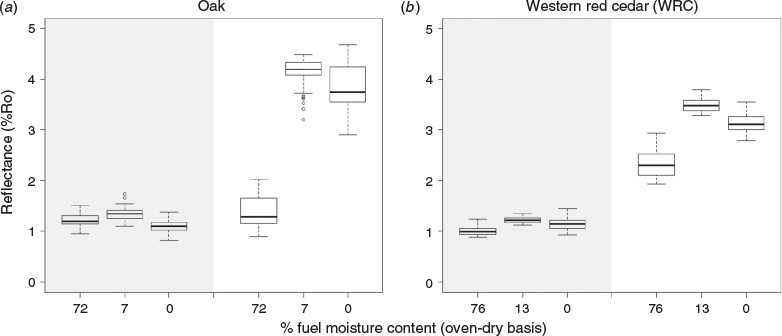
In a third set of charring experiments, larger pieces of wood were tested that allowed us to extract the charcoal generated (1) shortly after the peak heat-release rate of the fire was reached (i.e. at maximum fire intensity), and (2) at the end of flaming combustion (see Fig. 2a); here, the samples were extracted when no flame, however small, could be seen on the samples surface. These larger wood samples were of oak and western red cedar (WRC) measuring 90 × 90 × 35 mm. The oak and WRC samples were exposed to 50 kW m–2 in the iCone calorimeter, following ISO 5660–1/ASTM E1354 (International Organization for Standardization (ISO) 2015/ASTM International 2016), which mimicked the heat from an approaching fire front and began to decompose the wood sample, generating pyrolysis gases (pyrolysate) as the surface began to char (pyrolyse). Once the volume of pyrolysate released was sufficient, a spark igniter ignited the pyrolysate, leading to flaming ignition. During combustion, the iCone calorimeter measured the rate of heat release throughout the burn (e.g. see Fig. 2a) quantifying heat release rate (HRR), peak heat release rate (pHRR) and total heat release (THR) (e.g. see Table S1) during the flaming phase of the fire. In Fig. 2a, the rate of heat release can be seen to rapidly rise following ignition, and then decay gradually as the volume of pyrolysate dwindles. Our aim with these experiments was to explore at what point in the evolution of the fire the maximum charcoal reflectance value is set.
Fuel moisture is a major determinant of flammability; therefore, to investigate whether fuel moisture content influenced charcoal reflectance, the oak and WRC were prepared to a range of fuel moistures (0–78% oven-dry basis), which allowed testing of end members in moisture from green to oven-dried. Green moisture contents were 71.9% for oak and 75.7% for WRC (oven-dry basis). Laboratory-conditioned samples were 7.04% for oak, 13.2% for WRC and were created by leaving the samples at 21°C and 55% humidity until reaching equilibrium with their environment. Finally, oven-dried samples had 0% free moisture, having been dried at 50°C until a constant weight was attained. Each set of fuel moistures was tested in triplicate for each species. The oak and WRC samples also allowed consideration of hard- and softwoods, as variation in density is known to impact charring rate (Tran and White 1992; Zhao et al. 2014). Mean densities for dry samples were 769 kg m–3 for oak and 392 kg m–3 for WRC.
Finally, a fourth set of experiments was undertaken to explore the difference in heat release from pyrolysis of virgin fuel and subsequent char oxidation of the oak and WRC samples. Here, we separated the processes of pyrolysis and oxidation using the Federal Aviation Administration (FAA) microcalorimeter (Fire Testing Technology). Equally sized pieces of wood (10–20 mg) were heated at a ramp rate of 3°C per minute, in the microcalorimeter according to test Method A of ASTM D7309 (ASTM International 2013), which allowed controlled thermal decomposition of the samples, and the heat released from the volatile component of the specimen (pyrolysate) to be quantified. The resulting char (weighing ~1.5 mg) was then returned to the microcalorimeter and heated according to test Method B of ASTM D7309 (ASTM International 2013), which allows controlled thermal oxidative decomposition and the heat released from the oxidation of the char to be quantified. Both methods are fully detailed in the international testing standard method ASTM D7309 (ASTM International 2013).
Charcoals resulting from experiments 1–3 were embedded in polyester resin, then ground and polished using a Buehler MetaServ 250 grinder–polisher (Buehler, Neckar, Germany). First, the top surface of the resin was ground down using a silicon carbide disc (50-μm grain size) until the surface of the charcoal was exposed. This surface was then re-impregnated with resin and placed in a vacuum oven to ensure that resin was pulled into the cells of the char. Once cured, the top surface was ground down again, and then polished using a Kemet synthetic silk polishing pad with Kemet 3-μm diamond suspension (Kemet International, Maidstone, UK), to remove any scratches. The polished samples were analysed under oil (RI 1.514) at 23°C, using a Zeiss Axio-Scope A1 optical microscope, with a TIDAS-MSP 200 microspectrometer (SMCS Ltd, Baldock, UK). Samples were studied using a ×50 objective (with ×32 eyepiece magnification), and reflectance measurements were obtained manually using MSP200 v 3.47 software. The system was calibrated with three synthetic reflectance standards, strontium titanite (5.41% reflectance in oil (Ro)), gadolinium gallium garnet (GGG) (1.719% Ro) and spinel (0.42% Ro). Manual reflectance measurements were taken at cell-wall junctions across the polished surface of the charcoal. The polishing removed some of the surface of the charcoal; therefore, to ensure that our reflectance measurements represented as best as possible the fuel surface, we assessed changes in the reflectance with depth down the profile. These profiles are included in Fig. S2 and show the expected decline in reflectance down the depth transect, indicating that our polishing retained as much of the top surface of the fuel as possible. Subsequently, 50 reflectance measurements were taken from the surface of each oak and WRC sample, and 30 measurements for the boreal wood samples.
Results and discussion
Oven-formed charcoals have been shown to record a strong positive correlation between temperature and reflectance (Fig. S1). The boreal wood samples that were exposed to incrementally increasing heat fluxes in ambient air, without ignition, also revealed a positive trend between charcoal reflectance and heat flux (Fig. 2b). This makes sense because pyrolysis is endothermic and requires an external supply of heat to continue, such that when exposed to a given heat flux, the temperature of non-ignited wood remains constant (Boonmee and Quintiere 2005). This also explains the positive correlation between reflectance and temperature in oven-created chars.
When the boreal wood samples were allowed to ignite, the correlation between heat flux and charcoal reflectance was lost (Fig. 2c), indicating that the dynamics of combustion are able to overprint the original reflectance as subsequent pyrolysis and oxidation reactions proceed. This was further confirmed by the experiments that compared char extracted at pHRR and at the end of flaming combustion (Fig. 3a, b). Both the oak and the WRC showed an increase in charcoal reflectance as combustion proceeded, with the samples extracted at pHRR consistently having a lower reflectance than those extracted at the end of flaming combustion (Fig. 3). This was true for all fuel moistures and fuel states tested. It was observed that as flaming subsided, the flames became smaller in size and were restricted to fissures in the wood, allowing char oxidation to begin to occur across parts of the solid-fuel surface. During the main phase of flaming, pyrolysis dominates the surface of fuel while oxidation is focussed in the gas phase. However, as the volume of pyrolysate declines, in situ oxidation of the solid fuel also begins to occur. Oxidation is an exothermic reaction, meaning that it releases heat; therefore, once the regime switches to and includes char oxidation, the solid fuel should experience the greatest amount of heating. This is clearly demonstrated in our microcalorimeter experiments in which the THR from char oxidation was found to be ~3 times greater than that from pyrolysis alone. Mean THR for pyrolysis of oak was 11.0 kJ g–1 compared with mean char oxidation for oak of 30.2 kJ g–1, while mean THR for pyrolysis of WRC was 11.2 kJ g–1 and the mean for char oxidation of WRC was 35.4 kJ g–1. As such, the most energetic phase experienced by the solid fuel is when it undergoes oxidation and not during pyrolysis. Oxidation of the solid fuel must provide an additional heat flux to the solid capable of causing further chemical transitions in the char, which is therefore a critical stage not captured by producing charcoal in an oven.
Our experiments indicate that the existing understanding of charcoal reflectance is based solely on pyrolysis and does not explore the formation of charcoal reflectance throughout the phases of a natural fire. Oven charring fails to capture the progressive transitions in reflectance as combustion proceeds, shifting from pyrolysis and oxidation in the gas phase, to oxidation of the solid fuel. As such, based on our novel experiments, we are able to conclude that maximum char reflectance is neither set at the onset of pyrolysis, nor at the pHRR of the fire, and reveal that it continually evolves until the combustion process is complete. We therefore assume that maximum measureable reflectance of charcoals will be observed at the end of flaming combustion, during the transitioning between pyrolysis and char oxidation, before the char itself is ultimately consumed and turned to mineral ash.
Conclusions
Our preliminary findings suggest that charcoal reflectance is unable to provide information about aspects of fire behaviour such as fire intensity (pHRR), fire temperatures or flame temperatures, and therefore that existing reflectance–temperature calibrations cannot be used in post-fire assessments to consider wildfire temperature distributions (e.g. McParland et al. 2009; Ascough et al. 2010; Scott 2010; Hudspith et al. 2015). However, charcoal reflectance may still have utility in bringing semiquantitative measurements to fire severity surveys, particularly where surface fires are an important part of the fire regime. Our results imply that charcoal reflectance will continue to increase during additional heating at the transition to smouldering phases of combustion that occurs behind the flaming front. Current quantitative descriptors of fire behaviour tend to focus on the dynamics of the flaming phase rather than the smouldering phase, when it is the latter that likely has a larger impact on subsequent surface heating (Hollis et al. 2011; Gagnon et al. 2015). Therefore, we suggest that the analysis of charcoal reflectance from charcoals collected directly following a wildfire across a burned area may be useful in assessing the possible heat transfer associated with the transition to smouldering phases and may in time provide an indicator of fire severity. However, further work is needed to explore whether the pattern of reflectance observed in our laboratory experiments is detectable in wildfire-derived chars and therefore the full range of reflectance distributions produced during wildfire events should be considered. As such, in future work we aim to explore these relationships further by coupling heat-transfer measurements with charcoal reflectance from a range of natural and prescribed fires.
Acknowledgements
This research was funded by a European Research Council Starter Grant ERC-2013-StG-335891-ECOFLAM (awarded to CMB). We thank Tavistock Woodland Sawmill for providing the cut WRC and oak samples; the Morton Arboretum, Illinois, USA, and D. Marin for providing branch boreal wood samples; and Mark Grosvenor for laboratory assistance at the University of Exeter.
References
Alexander ME (1982) Calculating and interpreting forest-fire intensities. Canadian Journal of Botany 60, 349–357.| Calculating and interpreting forest-fire intensities.Crossref | GoogleScholarGoogle Scholar |
Ascough PL, Bird MI, Scott AC, Collinson ME, Cohen-Ofri I, Snape CE, Le Manquais K (2010) Charcoal reflectance measurements: implications for structural characterization and assessment of diagenetic alteration. Journal of Archaeological Science 37, 1590–1599.
| Charcoal reflectance measurements: implications for structural characterization and assessment of diagenetic alteration.Crossref | GoogleScholarGoogle Scholar |
ASTM International (2013) ASTM D7309-13: Standard test method for determining flammability characteristics of plastics and other solid materials using microscale combustion calorimetry. ASTM International, West Conshohocken, PA. Available at http://www.astm.org/Standards/D7309 [Verified 7 April 2016]
ASTM International (2016) E1354: Standard test method for heat and visible smoke release rates for materials and products using an Oxygen Consumption Calorimeter. ASTM International, West Conshohocken, PA. Available at http://www.astm.org/Standards/E1354.htm [Verified 26 April 2016]
Boonmee N, Quintiere JG (2005) Glowing ignition of wood: the onset of surface combustion. Proceedings of the Combustion Institute 30, 2303–2310.
| Glowing ignition of wood: the onset of surface combustion.Crossref | GoogleScholarGoogle Scholar |
Cohen-Ofri I, Weiner L, Boaretto E, Mintz G, Weiner S (2006) Modern and fossil charcoal: aspects of structure and diagenesis. Journal of Archaeological Science 33, 428–439.
| Modern and fossil charcoal: aspects of structure and diagenesis.Crossref | GoogleScholarGoogle Scholar |
Finney MA, Cohen JD, Forthofer JM, McAllister SS, Gollner MJ, Gorham DJ, Saito K, Adafuah NK, Adam BA, English JD (2015) Role of buoyant flame dynamics in wildfire spread. Proceedings of the National Academy of Sciences 112, 9833–9838.
| Role of buoyant flame dynamics in wildfire spread.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtFOksbnM&md5=8bce922e49fb2496b7c2fdbca1e16d7fCAS |
Gagnon PR, Passmore HA, Slocum M, Myers JA, Harms KE, Platt EJ, Paine CET (2015) Fuels and fires influence vegetation via above- and belowground pathways in a high-diversity plant community. Journal of Ecology 103, 1009–1019.
| Fuels and fires influence vegetation via above- and belowground pathways in a high-diversity plant community.Crossref | GoogleScholarGoogle Scholar |
Hollis JJ, Anderson WR, McCaw WL, Cruz MG, Burrows ND, Ward B, Tolhurst KG, Gould JS (2011) The effect of fireline intensity on woody fuel consumption in southern Australian eucalypt forest fires. Australian Forestry 74, 81–96.
| The effect of fireline intensity on woody fuel consumption in southern Australian eucalypt forest fires.Crossref | GoogleScholarGoogle Scholar |
Hudspith VA, Belcher CM, Kelly R, Hu FS (2015) Charcoal reflectance reveals early Holocene boreal deciduous forests burned at high intensities. PLoS One 10, e0120835
| Charcoal reflectance reveals early Holocene boreal deciduous forests burned at high intensities.Crossref | GoogleScholarGoogle Scholar | 25853712PubMed |
International Organization for Standardization (2015) ISO 5660–1: Reaction-to-fire tests – Heat release, smoke production and mass loss rate – Part 1: Heat release rate (cone calorimeter method) and smoke production rate (dynamic measurement). International Organization for Standardization, Geneva, Switzerland. Available at https://www.iso.org/obp/ui/#iso:std:iso:5660:-1:ed-3:v1:en [Verified 7 April 2016]
Jones T, Scott AC, Cope M (1991) Reflectance measurements against temperature of formation for modern charcoals and their implications for the study of fusain. Bulletin de la Société Géologique de France 162, 193–200.
Keeley JE (2009) Fire intensity, fire severity and burn severity: a brief review and suggested usage. International Journal of Wildland Fire 18, 116–126.
| Fire intensity, fire severity and burn severity: a brief review and suggested usage.Crossref | GoogleScholarGoogle Scholar |
McParland LC, Collinson ME, Scott AC, Campbell G (2009) The use of reflectance values for the interpretation of natural and anthropogenic charcoal assemblages. Archaeological and Anthropological Sciences 1, 249–261.
| The use of reflectance values for the interpretation of natural and anthropogenic charcoal assemblages.Crossref | GoogleScholarGoogle Scholar |
Morgan P, Keane RE, Dillon GK, Jain TB, Hudak AT, Karau EC, Sikkink PG, Holden ZA, Strand EK (2014) Challenges of assessing fire and burn severity using field measures, remote sensing and modelling. International Journal of Wildland Fire 23, 1045–1060.
| Challenges of assessing fire and burn severity using field measures, remote sensing and modelling.Crossref | GoogleScholarGoogle Scholar |
Rein G (2013) Smouldering fires and natural fuels. In ‘Fire phenomena and the Earth system: an interdisciplinary guide to fire science’. (Ed. CM Belcher) pp. 15–33 (John Wiley & Sons: Oxford, UK)
Scott AC (2010) Charcoal recognition, taphonomy and uses in palaeoenvironmental analysis. Palaeogeography, Palaeoclimatology, Palaeoecology 291, 11–39.
| Charcoal recognition, taphonomy and uses in palaeoenvironmental analysis.Crossref | GoogleScholarGoogle Scholar |
Tran HC, White RH (1992) Burning rate of solid wood measured in a heat release rate calorimeter. Fire and Materials 16, 197–206.
| Burning rate of solid wood measured in a heat release rate calorimeter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXkslCrs7c%3D&md5=e7f0041175ba3a4591dc7e5a42c7c843CAS |
Zhao W, Blauw LG, van Logtestijn RSP, Cornwell WK, Cornelissen JHC (2014) Interactions between fine wood decomposition and flammability. Forests 5, 827–846.
| Interactions between fine wood decomposition and flammability.Crossref | GoogleScholarGoogle Scholar |