Variance component analysis of body mass in a wild population of deer (Odocoileus virginianus): results from two decades of research
Stephen L. Webb A D , Kenneth L. Gee A , Randy W. DeYoung B and Seth M. Harju CA The Samuel Roberts Noble Foundation, 2510 Sam Noble Parkway, Ardmore, OK 73401, USA.
B Caesar Kleberg Wildlife Research Institute, Texas A&M University-Kingsville, MSC 218, Kingsville, TX 78363, USA.
C Heron Ecological, LLC, PO Box 235, Kingston, ID 83839, USA.
D Corresponding author. Email: slwebb@noble.org
Wildlife Research 40(7) 588-598 https://doi.org/10.1071/WR12224
Submitted: 21 December 2012 Accepted: 18 December 2013 Published: 30 January 2014
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
Context: Long-term studies of large, vertebrate mammals using capture–recapture data are scarce, even though long-term ecological studies are requisite to understanding quantitative genetics and evolutionary processes that can be applied as part of management programs.
Aims: Objectives were to (1) partition components of variation in body mass to understand the differential effects of environmental variation on the sexes during ontogeny, to better prescribe habitat-improvement projects, and (2) estimate repeatability to assess potential for selection on body mass.
Methods: We used a 23-year dataset (1983–2005) of capture–recapture records of wild white-tailed deer (Odocoileus virginianus) to estimate components of variance and repeatability of body mass. We used an animal-model approach that employed the use of general linear mixed models and restricted maximum likelihood to adjust for the effects of age (i.e. fixed effect), and to partition the total phenotypic variance into among-individual (i.e. the deer), permanent environmental (i.e. year of birth) and temporary environmental (i.e. year of measurement and residual) effects (all modelled as random effects).
Key results: We found that body mass increased with age in both sexes, repeatability of body mass was 0.595 for females and 0.716 for males, and among-individual variation was more influential on body mass than were permanent and temporary environmental effects combined. Year of birth was more important in males than females, but changed during the course of ontogeny for both sexes. Year of measurement did not influence post-rut body mass in males, but did contribute to variation in body mass of females.
Conclusions: These long-term data offer insights into the sources of variation that influence body mass of deer, which can be used to understand how environmental sources of variation influence phenotypic traits, and for developing management plans and making selection decisions.
Implications: Knowledge of repeatability (as an upper limit to heritability) can be used to make management decisions related to selection, culling and breeding, whereas understanding environmental effects can lead to better management recommendations (e.g. habitat-improvement projects).
Additional keywords: age, environment, individual variation, mixed model, phenotype, repeatability, white-tailed deer.
Introduction
Most species of ungulates have a K-selected life-history strategy, characterised by comparatively slow maturity and large investment of resources in few offspring (DeYoung 2011). Short-term studies have provided useful insights into the general ecology, behaviour and applied management of ungulate populations (Peters 2010). However, some aspects of ungulate ecology, population dynamics and evolutionary biology require long-term datasets to realise (Clutton-Brock and Sheldon 2010). Long-term studies have played a critical role in our understanding of how ungulate populations function and their effects on the ecosystem. For instance, some populations grow in a density-dependent manner, as population density affects the quality and quantity of forage (McCullough 1979). Furthermore, differential investment in growth and reproduction between males and females results in sex- and age-specific responses to resource availability (Clutton-Brock et al. 1982).
Recently, ecologists have begun to understand and quantify the effects of the environment on populations of ungulates. Ungulates are long-lived, and populations may be affected by both annual and long-term variations in the environment that affect forage and habitat quality. Long-term climatic fluctuations, such as the North Atlantic Oscillation and El Niño Southern Oscillation, affect ungulate forage resources through altered patterns of temperature and precipitation (Langvatn et al. 1996; Marshal et al. 2002; Stenseth et al. 2003). Populations in variable environments may not display density-dependent population dynamics because resource availability varies dramatically independent of population density (McCullough 1999; Owen-Smith 2010; DeYoung 2011). For example, soil and habitat quality can influence the physical development of populations throughout entire regions (Strickland and Demarais 2000), which underscores the importance of environmental variation on phenotypes throughout an individual’s life.
We have gained a better understanding of environmental effects at the population level, but the nature and extent of environmental variation among individuals remains poorly documented. Individual phenotypes are influenced by environmental variation (Falconer and Mackay 1996; Clutton-Brock and Sheldon 2010), which can either mask the underlying genetic potential for a trait or make it difficult to untangle the interaction between genetics and environment. Individual-based research (i.e. following unique individuals over time) allows estimation of environmental effects on phenotype, the evolutionary potential of phenotypic traits, and the estimation of heritability (Clutton-Brock and Sheldon 2010). Recently, the application of quantitative genetic analyses, including the ‘animal model’ (Wilson et al. 2010), to individual-based field data has expanded our knowledge of quantitative-trait variation in wild ungulate populations (e.g. Kruuk et al. 2002).
The collection of longitudinal or age-specific measurements in long-lived animals is difficult (Réale et al. 1999), resulting in most early studies on quantitative genetics (e.g. to estimate heritability of phenotypic traits) being conducted on captive populations in which pedigrees were available and breeding was controlled (Williams et al. 1994; Lukefahr and Jacobson 1998; Lockwood et al. 2007). Although long-term datasets on wild populations continue to accumulate (e.g. Jorgenson et al. 1997; Milner et al. 1999; Slate et al. 2000; Coulson et al. 2001; Kruuk et al. 2002; Koerth and Kroll 2008; Foley et al. 2012; Webb and Gee 2014), cost and logistics limit the number and type of populations that can be examined. We assembled a large dataset of wild, white-tailed deer (Odocoileus virginianus) recaptures during a 23-year period that allowed collection of age- and sex-specific phenotypic data on individuals. Our goal was to quantify the effect of environmental sources of variation on a quantitative trait, namely body mass, for use as part of management programs targeted at improving deer body condition and/or development through habitat-improvement projects. Specific objectives were to (1) partition components of variation in body mass to understand the differential effects of environmental variation on the sexes during ontogeny, to better prescribe habitat improvement projects, and (2) estimate repeatability to assess potential for selection on body mass.
Materials and methods
Study area
We conducted the study on the Samuel Roberts Noble Foundation Wildlife Unit located in southern Oklahoma, USA, in Coal, Hughes, and Pontotoc Counties. The area was ~1214 ha in size and located within the Cross Timbers and Prairies ecoregion (Gee et al. 2011). The study area was ~60% wooded and 40% open, with a high degree of interspersion (Gee et al. 2011). Habitat management involved rotational grazing of livestock, prescribed fire and selective control of woody plants via single-stem herbicide treatments. Water was readily available, with one permanent water source for every 33 ha. Total annual precipitation averaged 109.8 cm from 1985 to 2005 (Ada, Pontotoc County, Oklahoma; Oklahoma Mesonet, https://climate.mesonet.org, verified 23 May 2013) (Appendix 1). Estimates of deer density based on nocturnal spotlight surveys and infrared-camera sightings ranged from 19.0 to 5.9 ha deer–1 (Appendix 1). Potential predators to deer (primarily neonates) on the study area were bobcats (Lynx rufus) and coyotes (Canis latrans).
The study area experienced changes in harvest intensity and control of the deer population during the course of the study (Webb and Gee 2014). Deer were free-ranging from 1983 to 1987. A 2.44-m, 15-strand high-tensile electric fence was constructed in 2.4–4.8-km segments per year during 1987–92. Once completed in 1992, the fence was not a complete barrier to dispersal, but did restrict deer movements (Webb et al. 2009a, 2010a). Harvest was permitted, and intensified during 1985–87, as part of a food habits study (Gee et al. 2011). Harvest was allowed on both sexes until 2000, restricted to females during 2001–02, and restricted all together after the 2002 hunting season.
Capture and handling
We captured deer annually by using a drop-net (Ramsey 1968) baited with corn from 1983 to 2005. We sedated deer using xylazine (3–6 mg kg–1, Phoenix Scientific, St Joseph, Missouri, USA) or a mixture of Telazol and xylazine (4.4 mg kg–1 Telazol, Fort Dodge Animal Health, Fort Dodge, Iowa, USA, plus 2.2 mg kg–1 xylazine). We used yohimbine (Abbott Laboratories, North Chicago, Illinois, USA) at 0.125 mg kg–1, or tolazine at 0.4 mg kg–1, as an antagonist to the xylazine. We aged deer according to tooth replacement and wear (Severinghaus 1949). However, the accuracy of the tooth replacement and wear method declines with the age of the deer and one cannot reliably place deer aged ≥2 years old into annual cohorts (Gee et al. 2002; Lewis 2010). Therefore, we analysed only known-age individuals for the present paper; otherwise, the number of age-specific groupings would be reduced if deer with estimated ages were included into the analyses. Deer were classified as known-age if they were captured first as a fawn (6–9 months) or yearling (18–21 months) because these age classes can be aged definitively on the basis of tooth-replacement patterns (Gee et al. 2002).
Beginning in 1986, we used a spring-loaded scale to record whole body mass of deer to the nearest pound; mass was converted from pounds to kilograms for analysis. Capture occurred during a 2-month window, February–March, which occurred after the rut and when females were entering their second trimester of gestation. Trap sites were dispersed throughout the property, monitored continuously for 2–4 weeks, and all ages and both sexes were captured. Therefore, we assumed that captured deer were a representative sample of the population. We chose not to include body mass at the time of harvest into the dataset because (1) harvest occurred during October–November, ≥8 months after capture, (2) the sample size was relatively small compared with the capture dataset of known-aged individuals (harvest: n = 48; total: n = 391) and (3) harvested animals may be a biased subset because of hunter selection (Simard et al. 2008). However, all data before the harvest were included into analyses.
All capture, handling and marking procedures were consistent with the guidelines of the American Society of Mammalogists (Gannon et al. 2007) and were approved by permit from the Oklahoma Department of Wildlife Conservation (Permit nos 2243, 2374, 2701, 046, 195, 493, 627, 796, 1000, 1224, 1432, 1654, 1912, 2141, 2424, 2517, 2723, 2941, 3065, 3174, 3239, 3364, 3591, 3726).
Variance-component analysis
We estimated variance components of body mass using fixed and random effects (MIXED procedure) implemented in general linear mixed models (GLMM) with restricted maximum likelihood (REML) estimators (Littell et al. 2006) in the computer program SAS 9.2 and 9.3 (SAS Institute, Inc., Cary, NC, USA). These analyses are analogous to the ‘animal model’ approach in quantitative genetic analyses, except that we did not have pedigreed individuals. Therefore, we were unable to estimate ‘true’ genetic parameters; using deer identity as a random effect accounts for among-individual variation (VI). Our goal was to quantify and partition environmental sources of variance, with the understanding that some components of variance also contain genetic variation. Specifically, we partitioned phenotypic variance (VP) into among-individual (VI), temporary environmental (ET) and permanent environmental (EP) sources, and the residual variance. The among-individual variance component includes both genetic and environmental effects (Wilson et al. 2010). We included year of body mass measurement (i.e. year of capture) as a source of ET and birth year as a source of EP, to further partition environmental sources of variation from VI. Depending on the particular model, the residual variance included either within-individual variation (sex-specific models) or both among- and within-individual variation (age- and sex-specific models).
Known-aged females and males were analysed separately to quantify sex-specific differences in variance components. We treated age as a categorical fixed effect because all levels of the factor for which we were testing were found in the data, and to determine the effect of each component of variance on the mean for each factor level (Wilson et al. 2010). This approach allows variation in body mass to be modelled relative to the population average (Wilson et al. 2005). We pooled all data for deer ≥3 years of age because sample sizes were limited for older age classes.
We also incorporated random effects into sex-specific analyses to further partition phenotypic variance and to make inferences about individual and environmental components of variance to the larger population. The phenotypic variance was partitioned into among-individual variation (VI) by fitting individual identity as a random effect (Wilson et al. 2010). We included year of birth as a categorical random effect because shared environmental conditions (e.g. year of birth) can have as much of an effect on similarity of traits as heritable genetic effects (Kruuk and Hadfield 2007; Rodriguez-Hidalgo et al. 2010). Therefore, year of birth is a potential source of permanent environmental variance (EP). We included year of measurement as a categorical random effect to account for year-to-year differences that can occur for phenotypic traits such as body mass; these differences arise from variable availability of resources (e.g. forage quality and quantity) or changes in population density (Kruuk et al. 2002; Simard et al. 2008). Thus, the year effect is a source of temporary environmental variance (ET). Finally, for the sex-specific analysis where deer enter the dataset more than once, we included a term for residual variation, which includes non-additive genetic and environmental variation unique to each individual within each year (Falconer and Mackay 1996; Wilson et al. 2010).
For sex-specific models, we used the Kenward–Rogers denominator degrees of freedom adjustment (Kenward and Roger 1997) for the fixed-effects component (i.e. age) of the model, to account for unbalanced data and multiple random effects (Littell et al. 2006). We also output the best linear unbiased predictions (BLUPs) of the random effects for year of measurement (ET) and year of birth (EP), to examine variation across years. The BLUPs are normally distributed with mean of zero, and provide an indication of temporary sources of environmental variation (ET) and carry-over effects from year of birth (EP) on body mass. The contribution of each variance component was expressed as a percentage (%) of the total phenotypic variance (VP) of body mass.
In addition to a full model with all ages, we also conducted sex-specific variance-component analyses of body mass for each of the four age classes. These sex- and age-specific models allow for changes in phenotypic variance of body mass by age (i.e. ontogenetic changes; van den Berg and Garrick 1997; Wilson et al. 2005). We compared ontogenetic changes in variance components for both sexes at 1-year intervals from 0 to ≥3 years of age. We used a fully random effects model because age was no longer included as a fixed effect. However, deer identity was not modelled as a random effect because most deer entered the dataset only once, except for the ≥3-year category. Additionally, year of measurement (ET) was excluded because of non-independence with year of birth (EP) (Cramér’s V ≥ 0.857, P ≤ 0.001), which was tested using Cramér’s V for association between two nominal variables (i.e. ET and EP; Cramér 1999). Therefore, the residual variance primarily will include among-individual variation, but also other sources of unaccounted variation that were not specified within the model (e.g. temporary environmental effects).
We used a null-model likelihood-ratio test to determine whether our global sex-specific models fit the data better than a null model calculated as χ2 = [−2(LLnm)] – [−2(LLfm)], with q–1 degrees of freedom, where −2 LL is −2 times the log-likelihood, nm is the null model, fm is the fitted model, and q is the difference in the number of covariance parameters between nm and fm. The global sex-specific models for females (χ2 = 658.2, d.f. = 4, P < 0.001) and males (χ2 = 525.7, d.f. = 4, P < 0.001) fitted the data better than did a null model.
Finally, we estimated repeatability of body mass for females and males separately (Falconer and Mackay 1996). The basic premises of repeatability are as follows: (1) environmental variance is partitioned, not the genetic variance, (2) the within-individual component of variance is entirely environmental in origin, caused by temporary differences in environmental conditions from one measurement to the next, (3) the among-individual component of variance is both environmental and genetic in origin, and (4) temporary environmental variance is partitioned from the rest of the components. Repeatability (r) is the proportion of the total phenotypic variance (VP) that is due to permanent environmental (EP) and among-individual effects (VI), and is expressed as
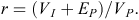
Repeatability defines the upper limit to the broad-sense heritability, the extent that phenotypes are determined by genotypes, and the narrow-sense heritability, the degree that phenotypes are determined from genes contributed by the parents (Falconer and Mackay 1996; Lynch and Walsh 1998). Values near 0 indicate that variation is completely within individuals (i.e. strong environmental variation), whereas values near 1 indicate that variation is among individuals (i.e. strong genetic variation; Lynch and Walsh 1998).
Results
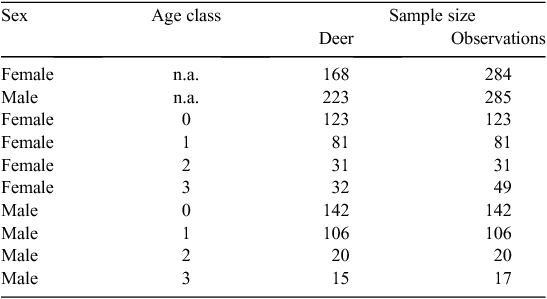
We captured 522 deer, 391 known-age deer and 131 deer of estimated age. However, we analysed data only for the 391 known-age deer, which comprised 168 females and 223 males (Table 1). For the known-age group, we analysed data from 569 observations, including recaptures, for 284 and 285 observations of females and males, respectively (Table 1). A summary of the raw data and parameter estimates of the GLMM (parameter estimates reported in parentheses) showed that, as expected, body mass increased with age for both sexes (Fig. 1). Mean ± s.e. body mass of known-age females was 30.2 kg ± 0.3 (30.0 kg ± 0.5), 45.7 kg ± 0.4 (45.7 kg ± 0.5), 52.3 kg ± 0.8 (51.4 kg ± 0.6) and 54.1 kg ± 0.6 (53.6 kg ± 0.6) for fawns, yearlings, 2-year olds, and ≥3-year olds, respectively. Body mass of known-age males averaged 33.9 kg ± 0.3 (34.2 kg ± 0.5) for fawns, 49.4 kg ± 0.5 (49.5 kg ± 0.5) for yearlings, 59.2 kg ± 1.3 (59.2 kg ± 0.9) for 2-year olds, and 64.9 kg ± 1.8 (65.4 kg ± 0.9) for ≥3-year olds.
|
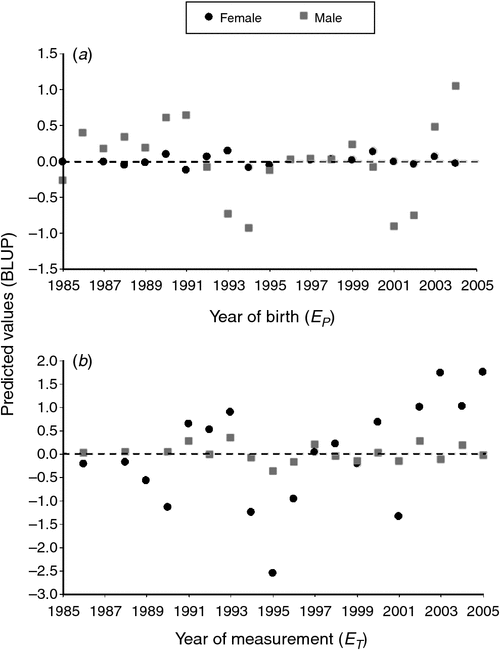
In sex-specific analyses, age was modelled as a categorical fixed effect and had a significant influence on body mass in both females (F3,149 = 1073, P < 0.001) and males (F3,101 = 604, P < 0.001). Most (58.89%) variation in female body mass was in the among-individual component (VI), followed by residual (27.87%), year of measurement (ET, 12.66%) and year of birth (EP, 0.59%; Table 2). Year of birth had less contribution to female than male body mass, as indexed by trends in BLUPs (Fig. 2a). However, year of measurement (ET) contributed more to variation in female body mass than male body mass, and the latter years of the study had greater predicted values for body mass (Fig. 2b). On the basis of the variance-component analysis, repeatability of body mass in females was 0.595
|
For males, the among-individual component also contributed most to the variation in body mass (67.66%), followed by residual variance (27.44%; Table 2). Year of birth accounted for 3.95% of the variation in body mass of males, which was 6.7 times greater than in females; the larger influence of year of birth on male body mass was also revealed in greater variation among year-specific BLUPs (Fig. 2a). Year of measurement (0.95%) contributed little to the variation observed in body mass for males (Table 2), which was 1/13 of that in females (Fig. 2b). Repeatability of body mass in males was 0.716.
Residual variation effects, which primarily include among-individual variation, on female body mass were greatest (>88%) as fawns and yearlings, and then progressively declined to 72.5% at 2-year- and 67.2% at ≥3-year-old animals (Table 3). Year of birth explained little variation in body mass for female fawns (11.4%) and yearlings (1.1%), but increased at 2 years (27.5%) and ≥3 years of age (32.8%; Table 3).
In age-specific analyses of males, residual variation and among-individual effects on body mass were ≥78.4% at all ages, but were greatest at 1 (96.8%) and 2 years of age (100%, and smallest as fawns (78.4%; Table 3). As expected, the influence of year-of-birth effects on body mass declined from fawns (21.6%) to yearlings (3.2%) and again at 2 years of age (0%). However, permanent environmental effects appeared again when deer were ≥3 years of age (17.9%; Table 3).
Discussion
Among-individual variation (VI) had the single greatest influence on the variation of body mass in both sexes. However, the source and magnitude of variation in body mass differed between the sexes and during ontogeny. For females, permanent sources of environmental variation became more important later in life. For instance, the variation in female body mass attributed to birth year (EP) was 11.4% for fawns and generally increased from the yearling (1.1%) age class through 2 (27.5%) and ≥3 (32.8%) years of age. In contrast, variation in body mass attributable to birth year decreased in males from 21.6% in fawns to zero at 2 years, but reappeared when ≥3 years of age (17.9%). Year-of-measurement effects (ET) were greater in females (12.7%) than in males, which were virtually non-existent (0.95%). For females, the variation attributed to among-individual components (estimated in residual variance) was greatest as fawns and yearlings, which may depend on whether or not a fawn or yearling conceived during the rut, thereby influencing body-mass measurements in February or March. In contrast, adult females were likely to have greater body reserves because asymptotic body mass is nearly reached at 2 years of age, which can help buffer against rapid loss of body mass at the time of capture. Among-individual (residual) variance decreased when ≥2 years of age when permanent environmental effects began to influence body mass. Generally, among-individual differences, predominantly comprising the residual variance, were high (>78%) for males at all ages, and greatest from 1 through to 2 years of age.
Some of the sex-specific differences in environmental components of variation stem from ontogeny of body development where females attain physical maturity earlier than do male deer. Also, the differences in timing of investment in reproduction or permanent environmental effects influence the sexes differently. The increase in variation attributed to year of birth in adult females might reflect the environmental conditions in utero or during early life. Year of birth was modelled as a permanent environmental effect owing to the fact that environmental conditions during, and preceding, the year of birth in white-tailed deer can have long-lasting effects on both phenotypic and life-history traits (Knox et al. 1991; Shea et al. 1992; Gray et al. 2002) because of variable nutritional conditions (Kirkpatrick and Lande 1989; Mech et al. 1991). The amount and timing of precipitation influences the quality and quantity of vegetation, and hiding cover for fawns, which affects nutrition of the dam (and correspondingly the offspring) as well as birthweight and mass gain of fawns (Knox et al. 1991; Shea et al. 1992; Clutton-Brock et al. 1996). However, it is not clear why permanent environmental effects were minimal until females attained ≥2 years of age, but could partially be explained by the fact that females have attained most of their maximum adult body mass by this age. We could not conclusively answer this question because we could not account for litter size, skeletal growth, or other factors that might cause permanent effects. Nonetheless, a year-of-birth effect in adult (≥2 years of age) females is plausible because of the strong correlation between early nutrition and recruitment (McCullough 1979; Clutton-Brock et al. 1989), meaning that permanent effects are manifested as adult females expend greater resources toward reproduction. In addition, first-time mothers rarely recruited offspring in this population, and most fawns (>80%) were raised by females >2 years old (K. L. Gee and R. W. DeYoung, unpubl. data). The finding of greater variation in body mass of females as a result of permanent environmental effects indicates large carry-over effects later in life, resulting from conditions experienced early in life, which potentially could influence reproduction.
In males, we observed a decrease in variation attributed to birth year from fawn to adult age classes similar to maternal effects, which tend to disappear later in life (Lukefahr and Jacobson 1998). The decrease in EP for male deer from fawns to 2 years of age may reflect compensatory growth or diminishing permanent environmental effects over time. However, we can only speculate as to why EP would reappear for male deer ≥3 years of age. The most likely effect may be due to compensatory growth. Male deer could exhibit compensatory development after being disadvantaged by a bad start early in life (e.g. BLUPs less than zero). However, for EP to reappear could indicate differential mortality, whereby deer born either during good or bad years (on the basis of BLUPs) had a tendency to die before reaching 3 years of age. One of two scenarios could occur. First, deer born during suboptimal years died at younger ages because they were less fit from being disadvantaged early in life. Or second, deer born during better years could be predisposed to increased mortality at older ages because they are usually larger, which may increase their participation in rut-related activities (e.g. intraspecific combat, greater movement, increased energy expenditure and breeding). Numerous studies have reported that mortality related to rutting activities is a primary cause of mortality in adult male deer (Gavin et al. 1984; Ditchkoff et al. 2001; Webb et al. 2007).
The reason for a relatively small year effect in adult males may also be related to participation in the rut (i.e. the mating-effort hypothesis). For males, most of the cost of reproduction occurs during the rut, as males increase movements (Webb et al. 2009b, 2010b) and activity, and forego eating to search for mates (Mysterud et al. 2008; Hewitt 2011). Adult males may lose up to 30% of their mass during rut, and most natural mortality of adult males occurs during the post-rut period (Gavin et al. 1984; Ditchkoff et al. 2001; Webb et al. 2007). If adult males adjust mating effort on the basis of their pre-rut condition, most males may end the rut in a relatively poor condition each year, even if pre-rut condition varies (e.g. the mating-effort hypothesis; Ditchkoff et al. 2001). Pre-rut condition of males probably varies according to other unmeasured sources of environmental variation (which would be captured by the residual variance) that affects nutrition and body development (e.g. rainfall or population density); these same variations affect annual recruitment of offspring for females. Therefore, at the time of measurement, we would expect less variation in body mass that could be attributable to annual or temporary environmental conditions.
Although we were able to quantify sources of environmental variation in body mass, other important sources of variation remain unestimated (e.g. seasonal and true genetic effects). For instance, we did not adjust body mass for date of capture because we assumed mass would vary little during winter and the relatively short time interval (4–8 weeks) over which capture occurred. If body mass changed within season, we would have introduced greater variation into the analyses for the year-of-measurement (ET) component of variance. Other effects comprise the among-individual variance component (VI), including both genetic and environmental effects, such as additive variance, permanent environmental variance, the variance as a result of maternal effects and the maternal environment, and dominance variance (Wilson et al. 2010). Variation in body mass among deer may become greater at older age classes because some deer are better able to acquire nutrients, expend less energy, or select resources that provide quality habitat and forage (Ditchkoff 2011). Body mass is moderately heritable (Williams et al. 1994; van den Berg and Garrick 1997). However, the genetic contribution may be influenced or masked by environmental variation (Nussey et al. 2005; Wilson et al. 2006) or plasticity in the expression of quantitative traits (Nussey et al. 2007).
Best linear unbiased predictions revealed that body mass in females may have been increasing over time (Fig. 2b). This predicted increase in body mass coincided with intensity of land management over the course of the study period that was intended to improve habitat quality and subsequently deer condition. Together, these patterns suggested that female body mass was influenced positively by the cumulative effects of management. Although our modelled metric for year of body-mass measurement was not as important to male deer, there still would be temporary environmental effects at play that could be captured in the residual variance. Additionally, the improvement in body condition of females as the study progressed would contribute to the variance observed in body mass of males through permanent environmental effects as well as maternal effects, meaning that animals born during later years of the study most likely benefited from management intervention. Therefore, management targeted at improving deer condition is likely to have reduced natural environmental variation that is typical in wild populations (Schmidt and Hoi 2002). For example, in a semiarid environment in southern Texas, management practices include supplementing wild populations with enhanced nutrition, which has been found to dampen environmental variation that contributes to antler development in white-tailed deer (Foley et al. 2012). Animals in captivity typically are under greater control because food, shelter, water and various other factors are provided ad libitum, unlike for free-ranging wildlife populations (Foley et al. 2012). The end result in captive populations is less environmental variation, resulting in increased heritability and potential for making phenotypic improvements through selection (Webb et al. 2012).
By using the aforementioned line of reasoning, we may have observed relatively high repeatability for most sex- and age-specific combinations because this population of deer was managed throughout the course of the study. This means that through management, some of the environmental variation was moderated, which would result in greater among-individual variation and repeatability. In this population of deer, repeatability was generally high (0.595 for females, 0.716 for males) and comparable to other ungulate species, in which repeatability was ≥0.5 in most instances (12 of 15, 80%) and averaged 0.59 across studies (Table 4). Repeatability often is used for setting the upper limit of heritability, and for making selection decisions. When a phenotypic character is highly repeatable, then a large proportion of the variation is available to be exploited for selection (van den Berg and Garrick 1997). Put another way, repeatability is the correlation between prior measurements; thus, lower repeatability values imply less predictability in the future (Foley et al. 2012). Given the amount of environmental variation in free-ranging populations, and thus, lower repeatability, the efficacy of selective harvest to improve phenotypic traits in free-ranging populations is questionable (Webb et al. 2012).
The present study found that among-individual variation in body mass, which contributes most to repeatability, was generally high for most sex- and age-specific groupings. However, the sources and magnitude of environmental variation differed depending on the sex. Through intensive site-specific management practices (e.g. prescribed fire, water improvement projects, rotational grazing and selective plant removal), it appears that body mass of females improved during the course of the study (Fig. 2b), which also could influence male body mass through permanent environmental and maternal effects. Because harvest restrictions often are applied on the basis of phenotypic traits such as antlers in white-tailed deer (Demarais and Strickland 2011), examining how body mass and antler traits co-vary in relation to each other could facilitate management recommendations, specifically selective harvest decisions. When environmental variation is large, selection for greater body mass may not culminate in improved body mass because environmental variation can mask genetic potential (Kruuk et al. 2002). However, some deer may exhibit above-average phenotypes even under unfavourable environmental conditions (Lockwood et al. 2007). If individuals exhibiting preferred phenotypic traits during periods of increased environmental variation can be identified, then response to selection may have a place as part of management programs.
Acknowledgements
We thank J. Holman for his numerous contributions to the project; all interns and volunteers that assisted during deer captures; M. Newell for early discussions of variance component analyses; and A. Taylor and several anonymous reviewers for improving earlier drafts of this manuscript. Funding was provided by The Samuel Roberts Noble Foundation.
References
Clutton-Brock, T. H., and Sheldon, B. C. (2010). Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends in Ecology & Evolution 25, 562–573.| Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology.Crossref | GoogleScholarGoogle Scholar |
Clutton-Brock, T. H., Guinness, F. E., and Albon, S. D. (1982). ‘Red Deer: Behavior and Ecology of Two Sexes.’ (University of Chicago Press: Chicago, IL.)
Clutton-Brock, T. H., Albon, S. D., and Guinness, F. E. (1989). Fitness costs of gestation and lactation in wild mammals. Nature 337, 260–262.
| Fitness costs of gestation and lactation in wild mammals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1M%2FpslaksA%3D%3D&md5=dfe01360424ce00118a25b1e1c0eeab7CAS | 2911365PubMed |
Clutton-Brock, T. H., Stevenson, I. R., Marrow, P., MacColl, A. D., Houston, A. I., and McNamara, J. M. (1996). Population fluctuations, reproductive costs and life-history tactics in female soay sheep. Journal of Animal Ecology 65, 675–689.
| Population fluctuations, reproductive costs and life-history tactics in female soay sheep.Crossref | GoogleScholarGoogle Scholar |
Coulson, T., Catchpole, E. A., Albon, S. D., Morgan, B. J. T., Pemberton, J. M., Clutton-Brock, T. H., Crawley, M. J., and Grenfell, B. T. (2001). Age, sex, density, winter weather, and population crashes in soay sheep. Science 292, 1528–1531.
| Age, sex, density, winter weather, and population crashes in soay sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXktVyqsrw%3D&md5=7de5192bca742997a64eb65ad9b27250CAS | 11375487PubMed |
Cramér, H. (1999). ‘Mathematical Methods of Statistics.’ (Princeton University Press: Princeton, NJ.)
Demarais, S., and Strickland, B. K. (2011). Antlers. In ‘Biology and Management of White-tailed Deer’. (Ed. D. G. Hewitt.) pp. 107–145. (CRC Press: Boca Raton, FL.)
DeYoung, C. A. (2011). Population dynamics. In ‘Biology and Management of White-tailed Deer’. (Ed. D. G. Hewitt.) pp. 147–180. (CRC Press: Boca Raton, FL.)
Ditchkoff, S. S. (2011). Anatomy and physiology. In ‘Biology and Management of White-tailed Deer’. (Ed. D. G. Hewitt.) pp. 43–73. (CRC Press: Boca Raton, FL.)
Ditchkoff, S. S., Welch, E. R., Lochmiller, R. L., Masters, R. E., and Starry, W. R. (2001). Age-specific causes of mortality among white-tailed deer support mate-competition theory. The Journal of Wildlife Management 65, 552–559.
| Age-specific causes of mortality among white-tailed deer support mate-competition theory.Crossref | GoogleScholarGoogle Scholar |
Falconer, D. S., and Mackay, T. F. C. (1996). ‘Introduction to Quantitative Genetics.’ 4th edn. (Pearson Prentice Hall: Harlow, UK.)
Foley, A. M., DeYoung, R. W., Lukefahr, S. D., Lewis, J. S., Hewitt, D. G., Hellickson, M. W., Draeger, D. A., and DeYoung, C. A. (2012). Repeatability of antler characteristics in mature white-tailed deer in south Texas: consequences of environmental effects. Journal of Mammalogy 93, 1149–1157.
| Repeatability of antler characteristics in mature white-tailed deer in south Texas: consequences of environmental effects.Crossref | GoogleScholarGoogle Scholar |
Gannon, W. L., Sikes, R. S., Animal Care and Use Committee of the American Society of Mammalogists (2007). Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy 88, 809–823.
| Guidelines of the American Society of Mammalogists for the use of wild mammals in research.Crossref | GoogleScholarGoogle Scholar |
Gavin, T. A., Suring, L. H., Vohs, P. A., and Meslow, E. C. (1984). Population characteristics, spatial organization, and natural mortality in the Columbian white-tailed deer. Wildlife Monographs 91, 1–41.
Gee, K. L., Holman, J. H., Causey, M. K., Rossi, A. N., and Armstrong, J. B. (2002). Aging white-tailed deer by tooth replacement and wear: a critical evaluation of a time-honored technique. Wildlife Society Bulletin 30, 387–393.
Gee, K. L., Porter, M. D., Demarais, S., and Bryant, F. C. (2011). ‘White-tailed Deer: their Foods and Management in the Cross Timbers.’ 3rd edn. Publication NF-WF-11-02. (The Samuel Roberts Noble Foundation: Ardmore, OK.)
Gray, W. N., Ditchkoff, S. S., Causey, M. K., and Cook, C. W. (2002). The yearling disadvantage in Alabama deer: effect of birth date on development. Proceedings of the Southeastern Association of Fish and Wildlife Agencies 56, 255–264.
Hewitt, D. G. (2011). Nutrition. In ‘Biology and Management of White-tailed Deer’. (Ed. D. G. Hewitt.) pp. 75–105. (CRC Press: Boca Raton, FL.)
Jorgenson, J. T., Festa-Bianchet, M., Gaillard, J.-M., and Wishart, W. D. (1997). Effects of age, sex, disease, and density on survival of bighorn sheep. Ecology 78, 1019–1032.
| Effects of age, sex, disease, and density on survival of bighorn sheep.Crossref | GoogleScholarGoogle Scholar |
Kenward, M. G., and Roger, J. H. (1997). Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53, 983–997.
| Small sample inference for fixed effects from restricted maximum likelihood.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2svntVGitw%3D%3D&md5=f987916e210c59f5c5f06958d83ee18cCAS | 9333350PubMed |
Kirkpatrick, M., and Lande, R. (1989). The evolution of maternal characters. Evolution 43, 485–503.
| The evolution of maternal characters.Crossref | GoogleScholarGoogle Scholar |
Knox, W. M., Bara, M. O., and Miller, K. V. (1991). Effects of fawning date on physical development in yearling male white-tailed deer. Proceedings of the Southeastern Association of Fish and Wildlife Agencies 45, 30–36.
Koerth, B. H., and Kroll, J. C. (2008). Juvenile-to-adult antler development in white-tailed deer in south Texas. The Journal of Wildlife Management 72, 1109–1113.
| Juvenile-to-adult antler development in white-tailed deer in south Texas.Crossref | GoogleScholarGoogle Scholar |
Kruuk, L. E. B., and Hadfield, J. D. (2007). How to separate genetic and environmental causes of similarity between relatives. Journal of Evolutionary Biology 20, 1890–1903.
| How to separate genetic and environmental causes of similarity between relatives.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2svpsFGmuw%3D%3D&md5=1bbded8bd409f365df430e53c33563d7CAS |
Kruuk, L. E. B., Slate, J., Pemberton, J. M., Brotherstone, S., Guinness, F., and Clutton-Brock, T. H. (2002). Antler size in red deer: heritability and selection but no evolution. Evolution 56, 1683–1695.
| 1:STN:280:DC%2BD38vpt1Cqsg%3D%3D&md5=625743a66fbc006ef418268be620ce4fCAS |
Langvatn, R., Albon, S. D., Burkey, T., and Clutton-Brock, T. H. (1996). Climate, plant phenology and variation in age of first reproduction in a temperate herbivore. Journal of Animal Ecology 65, 653–670.
| Climate, plant phenology and variation in age of first reproduction in a temperate herbivore.Crossref | GoogleScholarGoogle Scholar |
Lewis, J. S. (2010). Factors influencing antler size in free-ranging white-tailed deer and mark/recapture estimates of demographic traits. Ph.D. Dissertation. Texas A&M University – Kingsville: Kingsville, TX.
Littell, R. C., Milliken, G. A., Stroup, W. W., Wolfinger, R. D., and Schabenberger, O. (2006). ‘SAS for Mixed Models.’ 2nd edn. (SAS Institute: Cary, NC.)
Lockwood, M. A., Frels, D. B., Armstrong, W. E., Fuchs, E., and Harmel, D. E. (2007). Genetic and environmental interaction in white-tailed deer. The Journal of Wildlife Management 71, 2732–2735.
| Genetic and environmental interaction in white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Lukefahr, S. D., and Jacobson, H. A. (1998). Variance component analysis and heritability of antler traits in white-tailed deer. The Journal of Wildlife Management 62, 262–268.
| Variance component analysis and heritability of antler traits in white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Lynch, M., and Walsh, B. (1998). ‘Genetics and Analysis of Quantitative Traits.’ (Sinauer Associates: Sunderland, MA.)
Marshal, J. P., Krausman, P. R., Bleich, V. C., Ballard, W. B., and McKeever, J. S. (2002). Rainfall, El Nino, and dynamics of mule deer in the Sonoran Desert, California. The Journal of Wildlife Management 66, 1283–1289.
| Rainfall, El Nino, and dynamics of mule deer in the Sonoran Desert, California.Crossref | GoogleScholarGoogle Scholar |
McCullough, D. R. (1979). ‘The George Reserve Deer Herd: Population Ecology of a K-selected Species.’ (University of Michigan Press: Ann Arbor, MI.)
McCullough, D. R. (1999). Density dependence and life-history strategies of ungulates. Journal of Mammalogy 80, 1130–1146.
| Density dependence and life-history strategies of ungulates.Crossref | GoogleScholarGoogle Scholar |
McManus, C. (1993). Within-farm estimates of genetic and phenotypic parameters for growth and reproductive traits for red deer. Animal Production 57, 153–159.
| Within-farm estimates of genetic and phenotypic parameters for growth and reproductive traits for red deer.Crossref | GoogleScholarGoogle Scholar |
Mech, L. D., Nelson, M. E., and McRoberts, R. E. (1991). Effects of maternal and grandmaternal nutrition on deer mass and vulnerability to wolf predation. Journal of Mammalogy 72, 146–151.
| Effects of maternal and grandmaternal nutrition on deer mass and vulnerability to wolf predation.Crossref | GoogleScholarGoogle Scholar |
Milner, J. M., Albon, S. D., Illius, A. W., Pemberton, J. M., and Clutton-Brock, T. H. (1999). Repeated selection of morphometric traits in the soay sheep on St Kilda. Journal of Animal Ecology 68, 472–488.
| Repeated selection of morphometric traits in the soay sheep on St Kilda.Crossref | GoogleScholarGoogle Scholar |
Milner, J. M., Pemberton, J. M., Brotherstone, S., and Albon, S. D. (2000). Estimating variance components and heritabilities in the wild: a case study using the ‘animal model’ approach. Journal of Evolutionary Biology 13, 804–813.
| Estimating variance components and heritabilities in the wild: a case study using the ‘animal model’ approach.Crossref | GoogleScholarGoogle Scholar |
Morris, C. A., McCall, J. E., Baker, R. L., and Southey, B. R. (1992). Genetic parameters for live weights in fallow deer (Dama dama L.). Proceedings of the New Zealand Society of Animal Production 52, 133–135.
Mysterud, A., Bonenfant, C., Loe, L. E., Langvatn, R., Yoccoz, N. G., and Stenseth, N. C. (2008). Age-specific feeding cessation in male red deer during rut. Journal of Zoology 275, 407–412.
| Age-specific feeding cessation in male red deer during rut.Crossref | GoogleScholarGoogle Scholar |
Nussey, D. H., Clutton-Brock, T. H., Albon, S. D., Pemberton, J., and Kruuk, L. E. B. (2005). Constraints on plastic responses to climate variation in red deer. Biology Letters 1, 457–460.
| Constraints on plastic responses to climate variation in red deer.Crossref | GoogleScholarGoogle Scholar | 17148232PubMed |
Nussey, D. H., Wilson, A. J., and Brommer, J. E. (2007). The evolutionary ecology of individual phenotypic plasticity in wild populations. European Society for Evolutionary Biology 20, 831–844.
| 1:STN:280:DC%2BD2s3lvVansQ%3D%3D&md5=29f90d44d9742c859e4de2bd1accd6ebCAS |
Owen-Smith, N. (Ed.) (2010). ‘Dynamics of Large Herbivore Populations in Changing Environments: Toward Appropriate Models.’ (Wiley-Blackwell: Oxford, UK.)
Pelletier, F., Réale, D., Garant, D., Coltman, D. W., and Festa-Bianchet, M. (2007). Selection on heritable seasonal phenotypic plasticity of body mass. Evolution 61, 1969–1979.
| Selection on heritable seasonal phenotypic plasticity of body mass.Crossref | GoogleScholarGoogle Scholar | 17683438PubMed |
Peters, D. P. C. (2010). Accessible ecology: synthesis of the long, deep, and broad. Trends in Ecology & Evolution 25, 592–601.
| Accessible ecology: synthesis of the long, deep, and broad.Crossref | GoogleScholarGoogle Scholar |
Ramsey, C. W. (1968). A drop-net deer trap. The Journal of Wildlife Management 32, 187–190.
| A drop-net deer trap.Crossref | GoogleScholarGoogle Scholar |
Réale, D., Festa-Bianchet, M., and Jorgenson, J. T. (1999). Heritability of body mass varies with age and season in wild bighorn sheep. Heredity 83, 526–532.
| Heritability of body mass varies with age and season in wild bighorn sheep.Crossref | GoogleScholarGoogle Scholar | 10620024PubMed |
Rodriguez-Hidalgo, P., Gortazar, C., Tortosa, F. S., Rodriquez-Vigal, C., Fierro, Y., and Vicente, J. (2010). Effects of density, climate, and supplementary forage on body mass and pregnancy rates of female red deer in Spain. Oecologia 164, 389–398.
| Effects of density, climate, and supplementary forage on body mass and pregnancy rates of female red deer in Spain.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cfit1Glug%3D%3D&md5=5de3a6662efbfe2c32d7483dcf23151bCAS | 20508950PubMed |
Schmidt, K. T., and Hoi, H. (2002). Supplemental feeding reduces natural selection in juvenile red deer. Ecography 25, 265–272.
| Supplemental feeding reduces natural selection in juvenile red deer.Crossref | GoogleScholarGoogle Scholar |
Severinghaus, C. W. (1949). Tooth development and wear as criteria of age in white-tailed deer. The Journal of Wildlife Management 13, 195–216.
| Tooth development and wear as criteria of age in white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Shea, S. M., Breault, T. A., and Richardson, M. L. (1992). Relationship of birth date and physical development of yearling white-tailed deer in Florida. Proceedings of the Southeastern Association of Fish and Wildlife Agencies 46, 159–166.
Simard, M. A., Côté, S. D., Weladji, R. B., and Huot, J. (2008). Feedback effects of chronic browsing on life-history traits of a large herbivore. Journal of Animal Ecology 77, 678–686.
| Feedback effects of chronic browsing on life-history traits of a large herbivore.Crossref | GoogleScholarGoogle Scholar |
Slate, J., Kruuk, L. E. B., Marshall, T. C., Pemberton, J. M., and Clutton-Brock, T. H. (2000). Inbreeding depression influences lifetime breeding success in a wild population of red deer (Cervus elaphus). Proceedings. Biological Sciences 267, 1657–1662.
| Inbreeding depression influences lifetime breeding success in a wild population of red deer (Cervus elaphus).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MvhvFOmtQ%3D%3D&md5=d1515d924bf89d2ac158041c9e7ce6d5CAS |
Stenseth, N. C., Ottersen, G., Hurrell, J. W., Mysterud, A., Lima, M., Chan, K., Yoccoz, N. G., and Adlandsvik, B. (2003). Studying climate effects on ecology through the use of climate indices: the North Atlantic Oscillation, El Nino Southern Oscillation and beyond. Proceedings. Biological Sciences 270, 2087–2096.
| Studying climate effects on ecology through the use of climate indices: the North Atlantic Oscillation, El Nino Southern Oscillation and beyond.Crossref | GoogleScholarGoogle Scholar |
Strickland, B. K., and Demarais, S. (2000). Age and regional differences in antlers and mass of white-tailed deer. The Journal of Wildlife Management 64, 903–911.
| Age and regional differences in antlers and mass of white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
van den Berg, G. H. J., and Garrick, D. J. (1997). Inheritance of adult velvet antler weights and live weights in farmed red deer. Livestock Production Science 49, 287–295.
| Inheritance of adult velvet antler weights and live weights in farmed red deer.Crossref | GoogleScholarGoogle Scholar |
Webb, S. L., and Gee, K. L. (2014). Annual survival and site fidelity of free-ranging white-tailed deer (Odocoileus virginianus Zimmermann): comparative demography before (1983–1992) and after (1993–2005) spatial confinement. Integrative Zoology 9, 14–23.
| Annual survival and site fidelity of free-ranging white-tailed deer (Odocoileus virginianus Zimmermann): comparative demography before (1983–1992) and after (1993–2005) spatial confinement.Crossref | GoogleScholarGoogle Scholar |
Webb, S. L., Hewitt, D. G., and Hellickson, M. W. (2007). Survival and cause-specific mortality of mature male white-tailed deer. The Journal of Wildlife Management 71, 555–558.
| Survival and cause-specific mortality of mature male white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Webb, S. L., Gee, K. L., Demarais, S., Strickland, B. K., and DeYoung, R. W. (2009a). Efficacy of a 15-strand high-tensile electric fence to control white-tailed deer movements. Wildlife Biology in Practice 5, 45–57.
| Efficacy of a 15-strand high-tensile electric fence to control white-tailed deer movements.Crossref | GoogleScholarGoogle Scholar |
Webb, S. L., Riffell, S. K., Gee, K. L., and Demarais, S. (2009b). Using fractal analyses to characterize movement paths in white-tailed deer and response to spatial scale. Journal of Mammalogy 90, 1210–1217.
| Using fractal analyses to characterize movement paths in white-tailed deer and response to spatial scale.Crossref | GoogleScholarGoogle Scholar |
Webb, S. L., Gee, K. L., and Wang, G. (2010a). Survival and fidelity of an enclosed white-tailed deer population using capture–recapture-reporting data. Population Ecology 52, 81–88.
| Survival and fidelity of an enclosed white-tailed deer population using capture–recapture-reporting data.Crossref | GoogleScholarGoogle Scholar |
Webb, S. L., Gee, K. L., Strickland, B. K., Demarais, S., and DeYoung, R. W. (2010b). Measuring fine-scale white-tailed deer movements and environmental influences using GPS collars. International Journal of Ecology 2010, 1–12.
| Measuring fine-scale white-tailed deer movements and environmental influences using GPS collars.Crossref | GoogleScholarGoogle Scholar |
Webb, S. L., Demarais, S., Strickland, B. K., DeYoung, R. W., Kinghorn, B. P., and Gee, K. L. (2012). Effects of selective harvest on antler size in white-tailed deer: a modeling approach. The Journal of Wildlife Management 76, 48–56.
| Effects of selective harvest on antler size in white-tailed deer: a modeling approach.Crossref | GoogleScholarGoogle Scholar |
Williams, J. D., Krueger, W. F., and Harmel, D. H. (1994). Heritabilities for antler characteristics and body weight in yearling white-tailed deer. Heredity 73, 78–83.
| Heritabilities for antler characteristics and body weight in yearling white-tailed deer.Crossref | GoogleScholarGoogle Scholar | 8077113PubMed |
Wilson, A. J., Kruuk, L. E. B., and Coltman, D. W. (2005). Ontogenetic patterns in heritable variation for body size: using random regression models in a wild ungulate population. American Naturalist 166, E177–E192.
| Ontogenetic patterns in heritable variation for body size: using random regression models in a wild ungulate population.Crossref | GoogleScholarGoogle Scholar | 16475080PubMed |
Wilson, A. J., Pemberton, J. M., Pilkington, J. G., Coltman, D. W., Mifsud, D. V., Clutton-Brock, T. H., and Kruuk, L. E. B. (2006). Environmental coupling of selection and heritability limits evolution. PLoS Biology 4, 1270–1275.
| Environmental coupling of selection and heritability limits evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntlCjsrg%3D&md5=102be90473704e6eca64afa8c5968c82CAS |
Wilson, A. J., Réale, D., Clements, M. N., Morrissey, M. M., Postma, E., Walling, C. A., Kruuk, L. E. B., and Nussey, D. H. (2010). An ecologist’s guide to the animal model. Journal of Animal Ecology 79, 13–26.
| An ecologist’s guide to the animal model.Crossref | GoogleScholarGoogle Scholar | 20409158PubMed |






