Landscapes without boundaries: wildlife and their environments in northern Australia
J. C. Z. Woinarski A B , R. J. Williams B C , O. Price A B and B. Rankmore A DA Parks and Wildlife Commission of the Northern Territory, PO Box 496, Palmerston, NT 0831, Australia.
B Tropical Savannas Cooperative Research Centre, Northern Territory University, Darwin, NT 0909, Australia.
C CSIRO Sustainable Ecosystems, PMB 44 Winnellie, NT 0821, Australia.
D Key Centre for Tropical Wildlife Management, Northern Territory University, Darwin, NT 0909, Australia.
Wildlife Research 32(5) 377-388 https://doi.org/10.1071/WR03008
Submitted: 23 January 2003 Accepted: 23 December 2003 Published: 8 August 2005
Abstract
This paper provides an introduction to the ecological fabric of northern Australia, described here as being a land characterised by extreme climatic seasonality and largely devoid of marked topographic features. Largely as a result of the latter trait, many species have extensive geographic ranges, and the spatial turnover in species composition is extremely limited. Somewhat counter-intuitively, these two traits can be accommodated by organisms only through reliance on critical, but often subtle, landscape variation. We present some preliminary models for Gouldian finch (Erythrura gouldiae) and black-footed tree-rat (Mesembriomys gouldii) to illustrate patterns of variation in their resource availability, and the consequences of such variation. We discuss briefly some studies that have attempted to integrate, or at least consider, these elements.
Introduction
This paper presents an introduction to some of the features of northern Australia that underpin its ecology. It collates examples of some works in progress on disparate themes, and reflects briefly on the more detailed studies of individual species or groups of related species described in subsequent papers in this symposium. The core argument of this paper is that the ecology and conservation management of northern Australia is dependent upon the nurturing of complex and often subtle interactions between biota and their environment.
Review of wildlife ecology in northern Australia is hampered by the (understandably) short-term or localised nature of most studies. These provide a poor foundation for the prediction or understanding of dynamic patterns over the longer term, especially for the discernment of response to northern Australia’s substantial climatic variability. They provide a poor foundation for extrapolating to the vast landscapes of northern Australia. And they provide a poor foundation for evaluating response to major or even subtle changes in land use, management or the incursion of new factors into the environment.
Previously, this inadequacy has been tolerated, or not noticed, because there has been widespread acceptance of the notion that our lands are so extensive and so little disturbed. This is the troublesome nub of conservation planning in northern Australia: the value of any given area is difficult to circumscribe. There are vast areas that are ostensibly similar, suggesting that it is relatively easy to represent an environment within a reserve system or, conversely, that the loss of any local area may be inconsequential for an environment that is far more widespread. However, many of the ecological processes underpinning the environment also must operate over very extensive landscape scales, such that even large reserves may not be immune to the treatment of their surrounds, and the loss of a small part may have regional repercussions.
In this paper, we describe in more detail the features of this landscape, addressing the theme of a land without sharp boundaries, and emphasising the importance of subtle variations within an ostensible uniformity. We then describe examples of the problems of measuring habitat quality in such a fluid landscape and illustrate some studies that have best approached those problems. Our focus is on the monsoonal tropics of the Northern Territory, but much of the argument applies equally to northern Australia as a whole.
Characteristics of the landscape
Spatial variation in environments
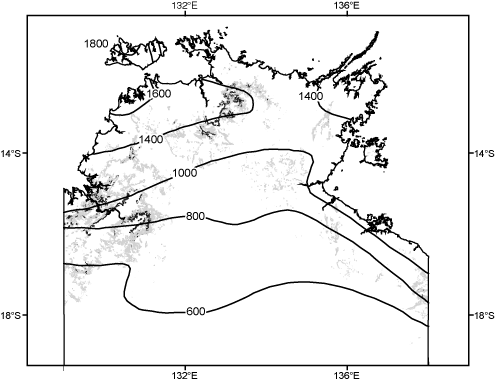
Spatial variation in climate in northern Australia is remarkably simple. There is very minor variation along the broad axis of 2500 km from the Kimberley to mid-Queensland, but a well defined latitudinal gradient in rainfall from the northern coast inland (Fig. 1). Topographic variation is also relatively simple (Fig. 1), with but a few areas of isolated uplands (particularly in the north Kimberley and western Arnhem Land). Soils are slightly more intricately patterned, but infertile sandy-loams are by far the main elements in the matrix, interspersed most markedly by relatively localised areas of younger clay floodplains around the lower reaches of some of the main rivers, and more extensive clay plains of the Barkly Tableland and western Queensland.
|
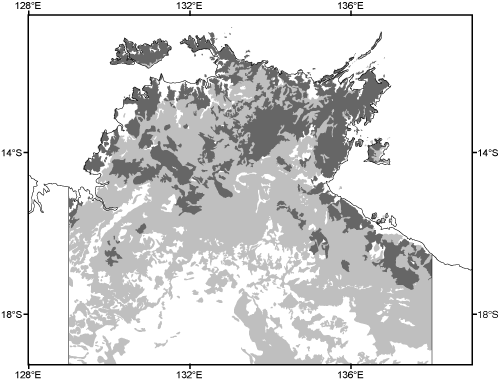
The relative invariance of these features dictates a comparably simple patterning of vegetation, again marked by minor variation over very large areas. For example, open forests codominated by the closely related Eucalyptus miniata and/or E. tetrodonta extend over more than 180 000 km2 of the Northern Territory alone (Fig. 2), and many of the constituent species in this vegetation type are shared with the even larger areas of open forests and woodlands dominated by other eucalypts.
|
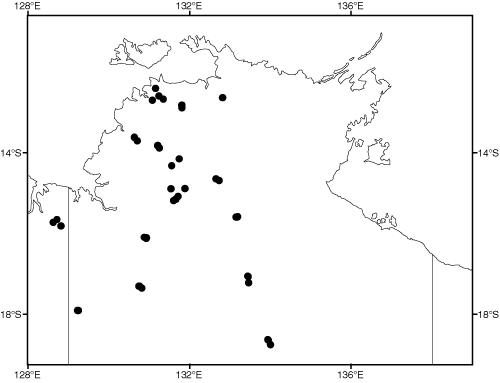
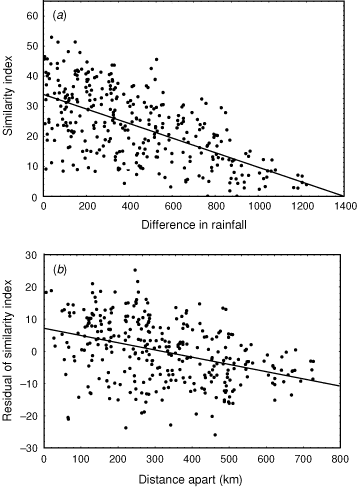
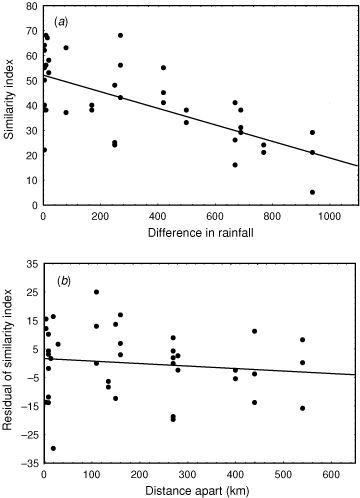
We can quantify some of the pattern of this variation. As part of a study examining broad-scale variation in vegetation patterning, we have recorded all plant species present in 45 quadrats (Fig. 3) situated across the rainfall gradient and to sample the three main soil types of clay, loam and sand. We calculated similarity in the species composition between all pairs of these quadrats, using the Bray–Curtis index, which varies from 100 if all plant species are shared between sites to zero if no species are shared. This similarity decreases as the difference in average annual rainfall between quadrats increases (Fig. 4a: r = –0.61), but, of more interest to the context of this paper, the scatter about the regression line, the residuals, can be related to the distance between quadrats (Fig. 4b). The slope of this regression is remarkably shallow. For two sites with the same rainfall, similarity in plant species composition decreases by only 10 units for every 400 km of geographic separation, or, to express it in different terms, it would require ~4000 km separation for a complete turnover of plant species. A comparable pattern applies for spatial change in faunal composition. For example, Fig. 5 depicts similarity in bird species composition between pairs of quadrats (based on samples of 30 quadrats described in Woinarski et al. (1999), and which were coincident with most of the plant quadrats described above). Again, there is a reasonably steady faunal change along a rainfall gradient, but the residuals about this regression imply only a very minor compositional change with spatial separation between pairs of sites, with a 10% change in composition taking an average of ~900 km separation.
|
|
|
Thus, at a coarse scale, the region can be categorised as having relative uniformity in species composition over large areas. But these graphs also suggest another feature. Although rainfall and distance explain much of the variation in floristic similarity between pairs of plots, there is still much variation unexplained (distance explains only 15% of the variation in the residuals from the rainfall regression). Combining the two graphs suggests that two coincident quadrats will have similarity in plant species composition of only ~41. This suggests that fine-scale features other than the broad rainfall patterns or broad soil typology also very strongly influence plant species composition. Thus, we have relative uniformity over large areas, but may also have some substantial local variation associated with more subtle factors, possibly including fire history, slope, rockiness or geological variation.
This feature – broad-scale homogeneity interrupted by localised variation – is a recurring motif in the ecology of northern Australia. Diversity in any area is built up largely in the cracks and interstices between slabs of extensive near-uniform environments. For example, the 16 500 small patches of monsoon rainforests comprise only 0.4% of the land area of the Kimberley and northern half of the Northern Territory (Russell-Smith et al. 1992), yet, in the Northern Territory, these provide the primary habitat for 585 native plant species (Liddle et al. 1994) from a total Northern Territory flora of ~4550 species (I. Cowie, personal communication). Likewise, the narrow riparian strips along the major watercourses of northern Australia support an unrepresentatively high proportion of the biodiversity: for example, Woinarski et al. (2000) noted that the area within 2 km of mapped watercourses comprised ~5.6% of the land area of the north of the Northern Territory but included 19.5% of all records of birds in a major distributional database, and that many species were largely restricted to these narrow strips. But environmental variation need not be so conspicuous as these examples to be significant: in the Mitchell grasslands of the Barkly Tableland, Fisher (2001) noted that animal and plant species richness in 100-km2 paddocks was increased by 25–50% if those paddocks contained small areas (<5% of the paddock extent) of inconspicuous gravel rises, elevated by barely 1 m above the surrounds. In all these cases, the spatially restricted environments harboured specialist endemic species, but also were used for parts of the year by species more typically associated with the extensive environments in which they were embedded.
Climatic seasonality and resource fluctuation
Rainfall in northern Australia is starkly seasonal, with a long winter drought and shorter summer wet season. Recent intercontinental comparisons suggest that the seasonality here is even more extreme than that found in the monsoonal tropics elsewhere (Cook and Mordelet 1997). The climatic seasonality dictates a massive annual fluctuation in resources available to the northern Australian biota, inevitably delivering times of glut and times of hardship (e.g. Woinarski and Tidemann 1991; Woinarski et al. 2000). Consumer species respond to this variation in several ways, including:
-
Migration out of the area during the lean times. Few species use this option, but seasonal intercontinental migrants include the dollarbird (Eurystomus orientalis), channel-billed cuckoo (Scythrops novaehollandiae), common koel (Eudynamys scolopacea), oriental cuckoo (Cuculus saturatus) and oriental pratincole (Glareola maldivarum) (Barrett et al. 2002).
-
Broad-scale dispersal around northern Australia (e.g. waterfowl, honeyeaters and flying foxes: Whitehead et al. 1992 ; Palmer and Woinarski 1999; Palmer et al. 2000; Vardon et al. 2001; Woinarski et al. 2000).
-
More local-scale (tens of kilometres) movements, including habitat shifting (e.g. Gouldian finch (Erythrura gouldiae), water python (Liasis fuscus), dusky rat (Rattus colletti): Madsen and Shine 1996; Dostine et al. 2001).
-
Diet shifting (e.g. many opportunistically nectarivorous birds: Franklin 1999).
-
Changes in activity rates (including aestivation by a range of frogs and reptiles including freshwater turtles and crocodiles: Grigg et al. 1986; Kennett and Christian 1993), although no birds or mammals become torpid.
-
Food storage or caching (e.g. harvester ants (Meranoplus spp.): Andersen et al. 2000), although no vertebrates in this system make major use of such behaviours, in contrast to the squirrels of boreal Europe and North America.
For biota, the problem of resource fluctuation is exacerbated by the lack of pronounced spatial environmental variation in this landscape. In an extensive, flat and relatively featureless environment, resource shortage at one site is likely to be repeated endlessly across the landscape. At such times, any discontinuity or subtle difference in the landscape may become critical for survival because it may provide different resources, or resources that fluctuate slightly out of phase with the rest of the landscape.
To some extent, we can add or subtract from intrinsic environmental heterogeneity, and hence alter options for survival through the lean times. Recent broad-scale decline of the savanna fauna has been linked to our deliberate or inadvertent reduction in fine-scale heterogeneity, either through change from intricate to coarse-grained fire regimes or through the landscape-wide impacts of grazing by domestic and feral stock (Fisher 2001; Woinarski et al. 2001; Yibarbuk et al. 2001; Pardon et al. 2003).
The most obvious, simple and effective mechanism for the manipulation of landscape heterogeneity is through the imposition of fire. Fire affects resource production and access in diverse ways. In the short term, fire in savanna environments will clear the dense grass layer, allowing easier access to many resources (such as fallen seeds: Woinarski 1990) but also increasing predation risks for some species and, conversely, hunting efficiency for some predators (Oakwood 2000). Fire will affect phenology, typically reducing the production, and delaying the onset, of flowers, fruits and seeds (Setterfield 1997; Crowley and Garnett 1999; Williams et al. 1999). Depending upon the timing and the intensity of fire, it may also abort or destroy that production. Fire may trigger the germination and growth of some plants, notably leading to fresh vegetative growth in some grasses (‘green pick’). It may also destroy the whole cohort of annual species, if fires occur during the wet season between germination and seed production (Lane and Williams 1997). Over longer periods, different fire regimes will lead to divergence in vegetation structure and floristics (Bowman et al. 1988; Russell-Smith et al. 2003), and thus change in habitat suitability for many wildlife species.
As an example of how management may exert a strong influence over the limited ‘natural’ variation, we calculated the similarity in floristic species composition between a set of quadrats within a Eucalyptus miniata–E. tetrodonta open forest that had been protected from fire for 23 years, and a neighbouring (<1 km distant) set of quadrats in a comparable setting subjected to annual fires (Woinarski et al. 2004). The average similarity in plant species composition between pairs of annually burnt and fire-protected quadrats was 17, whereas that between pairs of annually burnt quadrats was 48. If these values are compared against the data illustrated in Fig. 4, then the level of difference between the annually burnt and the nearby fire-protected quadrats is equivalent to that between sites with more than 900 mm difference in annual rainfall, or sites separated by more than 1600 km.
The extent to which fire may lead to increased diversity of available resources is dependent upon the scale of fire pattern relative to the dispersal distance of any individual consumer. For any individual, fire will not increase the heterogeneity of resource availability if the entire home range is burnt (or if the entire home range is unburnt). Home ranges may vary from a few square metres for many invertebrates and small skinks, through 1–10 ha for many rodents, bandicoots, small bush birds and possums, tens of square kilometres for some macropods, and some birds such as finches, parrots and pigeons, to thousands of square kilometres for cockatoos and some raptors. For individuals of those species that have smaller home ranges, useful heterogeneity can be provided by fire if and only if that fire is applied intricately, with fire patch sizes of the same order as home-range size (that is, ~1–10 ha for most small mammals and birds). Such a fine-scaled fire regime seems to have been that which was applied over tens of thousands of years by Aboriginal land managers, to the advantage of much of the savanna biota (Yibarbuk et al. 2001; Price et al. 2005).
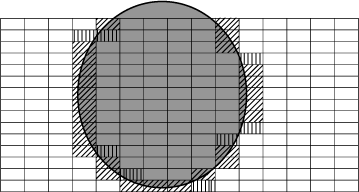
If we scale up from individuals to populations, persistence of a species may be achieved even where the scale of fire pattern mismatches that of home ranges. Fig. 6 presents an example: here a large area is subdivided by a nominal species into 225 territories. The area is subjected to just one fire during the year, which obviously burns at a scale far greater than that of the individual home range for this species. In this case, individuals in the 120 completely unburnt and the 70 completely burnt territories will have to try to eke out a living from a largely featureless environment, whereas individuals in the 35 partly burnt territories at the fire perimeter (especially the 27 territories where burnt and unburnt areas are relatively similar in size) have the advantage of an additional imposed variation, with diverse resource availability and asynchronous pulsing across their territory. On a landscape scale these latter areas may act as ‘sources’ in this year, whereas the others will act as ‘sinks’, with little or no reproduction and possibly no persistence. Depending upon rates of reproductive output, dispersability, rates to maturation and the ratio of partly burnt to fully burnt and fully unburnt territories, the source territories may each year replenish the populations across the rest of the landscape. Note that the ‘sources’ will vary in location kaleidoscopically every year, a far more dynamic system than that originally applied to the source–sink concept (Pulliam 1988).
|
The same argument can be applied when fire histories are extended over more than one year. Individuals are likely to have access to a more diverse resource base (with different phasing for resource nadirs) if parts of their lands have varied fire histories (e.g. part last burnt ten years ago, part last burnt three years ago, and part burnt last year). In much of northern Australia, access to such a varied menu of times since fire has been curtailed, as long-unburnt pockets become disappearingly rare (Williams et al. 2003; Woinarski 2004).
Exploring impacts of, and response to, resource fluctuation and spatial variability
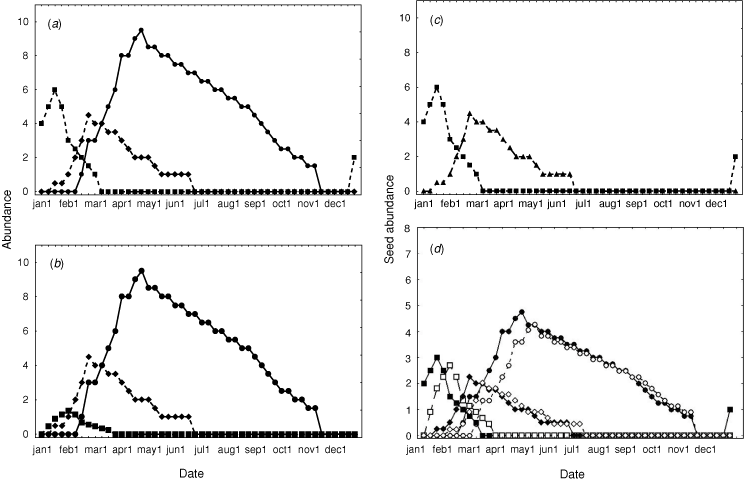
For illustration, we explore two examples of how resource fluctuation challenges the north Australian biota. The first is loosely drawn for a seed-eating bird, the Gouldian finch, at the Yinberrie Hills near Katherine, with resource availability grossly simplified, but based loosely on known patterns (Woinarski and Tidemann 1991; Dostine et al. 2001). For this example (Fig. 7a), seed is available from three grass species: Sorghum sp., Alloteropsis semialata and Chrysopogon fallax. Sorghum is by far the dominant species, and provides seed for much of the year, partly because its large seed generally persists on the soil surface throughout the dry season. The seeds of the other two species are far less persistent. The ecological pivot in this system is the first substantial rain in the wet season (here between the second and third week of November). This triggers germination of all seeds, suddenly emptying the landscape of the carpet of fallen seed that had provided the staple resource for many months. At any site, this germination heralds a resource nadir for seed-eating animals, extending in this example to nil resources for five weeks, until the first seeds are produced on Alloteropsis, a species that produces seed far earlier than the others, due to more rapid growth and maturation, and because smaller rainfall events may trigger germination. There may then be another (less pronounced) resource nadir between the end of seed-production in Alloteropsis and the start of seed production in the next grass species. The main resource dearth (late November–December) is an ecological disaster to species restricted to this site. Without the ability to fast or store seed, a sedentary granivore will not survive this period. Instead, species such as the Gouldian finch persist only by playing the environmental grain. In this case, the first rainfall events may be insufficient to trigger landscape-wide germination, but the moisture from these events may be channeled into drainage lines and depressions, where it accumulates sufficiently to locally exceed the moisture germination threshold for some populations of Alloteropsis, which may reach maturity even before the first large rainfall that triggers landscape-wide germination of the staple Sorghum seed. The first storms are also characteristically highly localised, with patches of heavy rainfall restricted to a few square kilometres, and no rainfall elsewhere in the landscape. This allows dispersive consumers to shop around, exploiting spatially asynchronous variation in the resource cycle. It is all a knife-edge persistence, with the difference between survival and mortality dictated by a capricious and quite variable timing of the onset of the wet season and characteristics of the early storms (Garnett and Crowley 1994, 1995).
Again, management can exacerbate or ameliorate the hazard in this system. Alloteropsis is highly fancied by cattle, and it responds to grazing by declining in abundance and lagging in its seed production (Crowley and Garnett 2001). Such a response leads to substantial stretching of the main resource nadir for seed-eaters, thereby increasing the likelihood of their local extinction (Fig. 7b). In a system that still possesses a dense grass sward, we may not notice a delayed reproduction in, or reduction in the abundance of, one relatively insignificant looking grass species, and hence be puzzled by the loss of finches or rodents. This system is also substantially affected by fire. If the site is burnt in the wet season between germination and seed production, annual sorghums may be completely eliminated, removing the resource that is the mainstay during the dry season (Fig. 7c) (although in the real case, most of the Sorghum at Yinberrie Hills happens to be a perennial species, and not so readily removed). Burning half of the site in the dry season produces a shadowing effect in resource availability, with the burnt half of the site having a slightly reduced seedset pulsing slightly staggered from that of the unburnt sections of the site, as if there were six species present rather than three (Fig. 7d). In this case, the burning does not lengthen or shorten the main (November–December) nadir, but it does close the gap in the second (February) nadir. More tailored burning (including of parts of the drainage lines within the site) would help to bridge the first principal nadir.
Next, we explore a more complex example, nominally focused on a more generalist small mammal that eats some seed and some fruit, such as the black-footed tree-rat, with model parameters used here developed from information in Friend and Taylor (1985), Friend (1987) and the unpublished studies of BR. For this example, we have developed a substantially expanded phenological dataset for 14 woody plant species (Terminalia ferdinandiana, Planchonia careya, Buchanania obovata, Pandanus spiralis, Livistona humilis, Persoonia falcata, Cycas armstrongii, Ampellocissus acetosa, Gardenia megasperma, Ficus scobina, Exocarpos latifolius, Petalostigma quadriloculare, Syzygium suborbiculare and Xanthostemon paradoxus) and three grass species (Alloteropsis semialata, Sorghum sp. and Heteropogon triticeus), based largely on Williams et al. (1999), and our own unpublished observations. For the sake of the modelling, this phenology varies, for each species, from 0 (nil availability of resource) to 1 (peak production of resource), for each week of the year. For each plant species, this score is multiplied by an additional variable (here termed α) that provides a measure of the ‘amount’ of the resource provided at the resource peak, with some consideration of the persistence of the seed or fruit following production. This value varies among species, depending upon our estimate of their typical abundance in the landscape and their relative seed/fruit production (Williams et al. 1999; Woinarski et al. 2004). Our model focuses on a 100-cell square (a ‘territory’: for the sake of argument, with each cell being 20 m × 20 m). We assume that there is a slight variation in floristics across cells: for each plant species, we give α a normal distribution across cells with a standard error equal to 20% of the mean (with this inter-cell variation fixed across cells for each plant species for the year). Thus, any cell in any one week will hold, on average, food units to the value of ∑ (phenology indexa) × αa, where ‘a’ represents one of the plant species included.
Next, we assume that our rat can visit any, and any number, of the 100 cells in its territory on any night, except that it will not forage in cells where the total resource available is less than some cell-visit threshold. Arbitrarily, we give this cell-visit threshold an initial value of 0.4 food-units. Next, we assume that our poor rat dies if it has to go three nights in succession where the total food available in cells it can visit is less than a total territory threshold. Again, we arbitrarily give an initial value of this territory threshold as 4 food-units. Our arbitrariness in this initial parameterisation was actually designed to give realistic ballpark values for the range of mortality patterns likely to be observed.
We can next ask how our rat fares when exposed to different forms of management, and different patterns of resource variation. The questions (and associated model parameterisation) are:
-
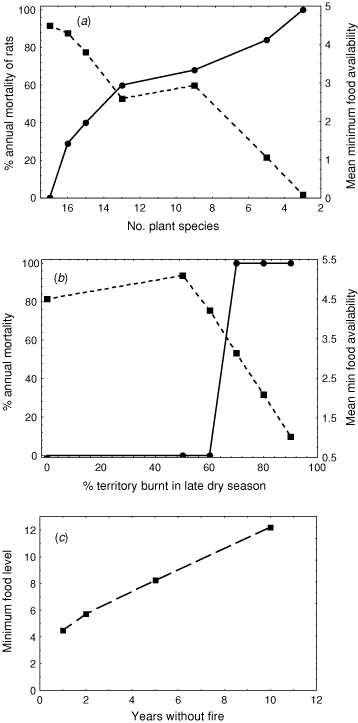
What is the impact of changing plant species richness? This is addressed by running the model with random selections of 3, 5, 9, 13, 15 and 16 of the 17 plant species, and examining impacts on rat survival and the resource nadir (the lowest daily amount of rat-food for the territory).
-
What is the short-term impact of different fire regimes? In this case, we examine the change in food resources and annual survival with an early dry-season fire that burns 40% of cells, and assume that, for each burnt cell, phenology is delayed by 4 weeks and production reduced by 20%. We also consider the impacts of late dry-season fire (burning 50, 60, 70, 80 and 90% of the total number of cells), with phenology delayed by 6 weeks and production reduced by 40% in burnt cells.
-
What is the long-term impact of fire? Here, we assume that each year of fire exclusion leads to a 1% increase in α for woody plants and a 1% reduction in α for grasses. We model for 2, 5 and 10 years of fire exclusion.
In all cases, models were run with at least 25 simulations.
The impacts of changing the number of plant species present were dramatic. In every case where one of the plant species randomly removed was Cycas armstrongii, our rat died. The rat also died in most cases where any of Planchonia careya, Exocarpos latifolius, Petalostigma quadriloculare and/or Xanthostemon paradoxus was removed, and there was a strong positive relationship between the probability of rat survival and the number of plant species present (Fig. 8a). In general, the more plant species present in a territory the better, but some plant species in particular were critical (in this case, Cycas, which seeded at a time of year when other resources were scant). This result is consistent with field studies that have demonstrated a correlation between the abundance of black-footed tree-rats and (woody) plant species diversity (Friend and Taylor 1985; Friend 1987; Woinarski et al. 2004).
|
The single early dry-season fire made no difference to the 100% rat survival, and increased the resource nadir from 4.50 rat food-units in the unburnt territory scenario to 5.53 rat food-units. In contrast, the late fire scenarios all reduced the resource nadir, and led to mortality in all simulations where at least 70% of the cells were burnt. In this case, this was a threshold value, with no mortality associated with 50% or 60% of cells burnt in the late dry season (Fig. 8b).
Fire exclusion led to substantial amelioration of the resource nadir and no change from the 100% survival rate of the base model (Fig. 8c). In this case, the leanest time of year for these rats was the mid to late dry season, and increasing the abundance or size of woody plants (through fire exclusion) results in a substantial bolster of food resources at that time.
These are crude heuristic models, but they serve to illustrate how managers can readily – and, often to human eyes, imperceptibly – change the pattern of resource availability, and hence habitat suitability, to the advantage or detriment of the savanna biota.
Examples of some studies describing habitat associations and models for individual species
The above sections provide an introduction to some of the critical ecological characteristics of northern Australia, and some partly hypothetical explorations of the way that these features dictate the fate of some of its biota. From that consideration, we can draw out some of the ingredients that must be considered in any study attempting to consider habitat suitability for any species in this region. These are:
-
Variation in resource availability needs to be measured across a range of scales, throughout the year, and between years.
-
The spatial patterning of the variation in environmental features relevant to the organism needs to be understood and mapped.
-
The dispersal response of the organism to this variation in environments and resources needs to be assessed, through monitoring of movement patterns (of individuals or populations).
-
The fate (survivorship, reproductive success, etc.) of individuals needs to be monitored, with individuals selected to represent a range of landscape configurations.
-
The impacts of a range of land-use and management factors (e.g. different fire regimes, or levels of grazing intensity) needs to be assessed, in terms of the consequences for resource availability pattern and of the fate of individuals.
-
Ideally, this information set should then be encased in modelling to allow prediction and amelioration of impacts of a range of management scenarios
This is a challenging set of objectives, requiring either long-term or multidisciplinary studies, and preferably both. It is probably the case that no study, or set of studies, has successfully addressed all these facets for any single species. However, there are some notable examples that consider many of these issues. Below we review very briefly some of these studies.
One of the most substantial and intriguing long-term ecological studies anywhere in the world is that considering the interactions between the water python and dusky rat. This is an ostensibly remarkably simple system – one main predator and one main prey species, in a reasonably tightly circumscribed and not particularly diverse habitat, the floodplains of the lower Adelaide River, ~30 km east of Darwin. The study has extended over 15 years, and involved detailed assessment of the fate, reproductive success and dispersal of marked individuals, and of the temporal and spatial variation in prey abundance. Madsen and Shine (1999a) found that the demographic characteristics (abundance, condition, and rates of survival, growth and reproduction) of dusky rats showed marked spatial and temporal variation, determined by annual variation in the timing and intensity of the monsoonal rains, mediated by minor spatial variation in elevation (and hence the period of inundation) across the floodplains. Because of high rates of reproduction, small rain-induced differences in the duration of their reproductive season can cause massive differences in subsequent rat density. For the water pythons, the interannual differences in rat abundance led to marked differences in body condition and reproductive output (Shine and Madsen 1997; Madsen and Shine 1999b) and long-term growth rates and survivorship of offspring (Madsen and Shine 2000). Within years, the local distribution of rats changed markedly to higher topographic positions during the wet season, and pythons responded by migrating up to at least 12 km to follow the rat populations (Madsen and Shine 1996). The pythons also showed substantial spatial variation in reproductive output, associated with subtle differences in microtopography (Madsen and Shine 1999c). The system is extraordinary in that its plentitude (exceptional abundance and biomass of pythons and rats) hinges on so few species, and on the remarkable subtleties of, and sensitivities to, spatial and temporal variation in resources.
Magpie geese (Anseranas semipalmata) share the same environment and respond in related ways to such variation in rainfall, resources and environmental patterning. Nesting success varies markedly between years depending upon the timing and amount of rainfall. Spatial variation in this rainfall pattern may also lead to changes between years in nesting location among and within catchments. The suitability of any site for breeding depends upon the juxtaposition of different components of the landscape, with contrasting habitat requirements for nest siting and for feeding areas for fledged young. In contrast to the pythons, seasonal dispersal may be over a large scale, with movements of hundreds of kilometres to track different resources as they ebb and flow through the year (Whitehead et al. 1992). In contrast to the python research, the magpie geese studies have also included some consideration of the impacts of a range of different management scenarios, most notably indicating that invading exotic pasture grasses can dominate and simplify the preferred nesting habitats of magpie geese, in the process eliminating the environmental variation on which their ecology hinges (Whitehead 1999; Whitehead and Dawson 2000).
A more complex example is that of highly mobile frugivorous vertebrates (such as flying-foxes and fruit-pigeons) that disperse between rainforest patches in association with spatial asynchrony in fruit availability. This system was examined in a multidisciplinary study involving assessment of phenological patterning across a network of rainforest patches (Bach 2002), of temporal variation in the abundance of frugivorous birds and bats in those same patches over the same period (Palmer and Woinarski 1999; Palmer et al. 2000), of the movement patterns of these species among patches and into the broader non-rainforest landscape (Price et al. 1999; Price 2004), and of the dispersal pattern of seeds of rainforest plants that used these vertebrates as dispersal agents (Shapcott 1998, 1999, 2000). Although each rainforest patch was coarsely similar in structure and floristics, the patterning and amount of the fruit resources of each patch varied idiosyncratically (such that frugivores had to track the landscape-wide system of patches) and the variability among patches served to muffle the general more-deterministic pattern of seasonal highs and lows. The study included modelling of management actions, notably concluding that there were so many interdependent linkages that loss of any patch (such as through land-clearing) would reverberate across the network as a whole, and that very large areas of intact vegetation, including the rainforest patches and the broader landscape in which these were embedded, were required for the persistence of any of the frugivorous species (Price et al. 1999).
Discussion and direction
This paper attempts to provide some conceptual framework for the studies reported elsewhere in this issue. The dominant characteristics of the north Australian landscapes are simple: extreme climatic seasonality dictates a major fluctuation in resources across the year, and a relatively uniform landscape offers little pronounced spatial variation. These features are so marked and overriding that it is almost quixotic that the ecology, indeed persistence, of so many organisms depends upon nuances of variation, in topography, landscape features, timing of rainfall and phenological pattern. Much of this vital subtlety is mercurial, changing in place from month to month or year to year. Much of the variation, and its significance, depends on landscape context, the pattern of juxtaposition of different landscape elements. Much of the ecology of individual species is played out at different scales, and, in these extensive unfragmented landscapes, at least some of these scales may be vast. These features present a profound challenge to ecologists and wildlife managers, and conspire to subvert the simple application of habitat-suitability models translocated from more fixed and more distinctively demarcated environments elsewhere.
The examples considered in the section above indicate that these research and management challenges can be overcome, that we can understand at least some components of this extraordinarily dynamic land. But that understanding may require substantial investment in research effort, placed either over many years or through the collaboration of researchers from a range of disciplines. The examples considered also indicate the extent to which land-management actions can, wittingly or unwittingly, alter that landscape in a way that may remove or may bolster elements on which any species may depend in at least some part of the year. For such an apparently robust land, the foundations for the ecology of any species may be extremely tenuous.
Acknowledgments
We appreciate the interest of Peter Whitehead and Jeremy Russell-Smith in inviting this contribution. The ideas discussed here owe much to a long collaboration involving them and many other Darwin-based ecologists. For information from unpublished studies, we are grateful to Garry Cook, Alaric Fisher and Christine Bach. Maps were produced by Greg Connors and Craig Hempel. Comments from Stephen Garnett and an anonymous referee were most helpful.
Andersen, A. N. , Azcarate, F. M. , and Cowie, I. D. (2000). Seed selection by an exceptionally rich community of harvester ants in the Australian seasonal tropics. Journal of Animal Ecology 69, 975–984.
| Crossref | GoogleScholarGoogle Scholar |
Bowman, D. M. J. S. , Wilson, B. A. , and Hooper, R. J. (1988). Response of Eucalyptus forest and woodland to four fire regimes at Munmarlary, Northern Territory Australian Journal of Ecology 76, 215–232.
Crowley, G. , and Garnett, S. (1999). Seeds of the annual grasses Schizachyrium spp. as a food resource for tropical granivorous birds. Australian Journal of Ecology 24, 208–220.
| Crossref | GoogleScholarGoogle Scholar |
Franklin, D. C. (1999). Opportunistic nectarivory: an annual dry season phenomenon among birds in monsoonal northern Australia. Emu 99, 135–141.
Madsen, T. , and Shine, R. (1996). Seasonal migration of predators and prey – a study of pythons and rats in tropical Australia. Ecology 77, 149–156.
Russell-Smith, J. , Whitehead, P. J. , Cook, G. D. , and Hoare, J. L. (2003). Response of Eucalyptus-dominated savanna to frequent fires: lessons from Munmarlary, 1973–1996. Ecological Monographs 73, 349–375.
Williams, R. J. , Myers, B. A. , Eamus, D. , and Duff, G. A. (1999). Reproductive phenology of woody species in a north Australian tropical savanna. Biotropica 31, 626–636.
Woinarski, J. C. Z. (1990). Effects of fire on bird communities of tropical woodlands and open forests in northern Australia. Australian Journal of Ecology 15, 1–22.
Woinarski, J. C. Z. , and Tidemann, S. C. (1991). The bird fauna of a deciduous woodland in the wet–dry tropics of northern Australia. Wildlife Research 18, 479–500.
| Crossref | GoogleScholarGoogle Scholar |

Woinarski, J. C. Z. , Fisher, A. , and Milne, D. (1999). Distribution patterns of vertebrates in relation to an extensive rainfall gradient and soil variation in the tropical savannas of the Northern Territory, Australia. Journal of Tropical Ecology 15, 381–398.
| Crossref | GoogleScholarGoogle Scholar |

Woinarski, J. C. Z. , Franklin, D. , and Connors, G. (2000). Thinking honeyeater: nectar maps for the Northern Territory, Australia, showing spatial and temporal variation in nectar availability. Pacific Conservation Biology 6, 61–80.

Woinarski, J. C. Z. , Milne, D. J. , and Wanganeen, G. (2001). Changes in mammal populations in relatively intact landscapes of Kakadu National Park, Northern Territory, Australia. Austral Ecology 26, 360–370.
| Crossref | GoogleScholarGoogle Scholar |

Woinarski, J. C. Z. , Risler, J. , and Kean, L. (2004). The response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia. Austral Ecology 29, 156–176.
| Crossref | GoogleScholarGoogle Scholar |

Yibarbuk, D. , Whitehead, P. J. , Russell-Smith, J. , Jackson, D. , Godjuwa, C. , Fisher, A. , Cooke, P. , Choquenot, D. , and Bowman, D. M. J. S. (2001). Fire ecology and Aboriginal land management in central Arnhem Land, northern Australia: a tradition of ecosystem management. Journal of Biogeography 28, 325–343.
| Crossref | GoogleScholarGoogle Scholar |