Effect of prefeeding, sowing rate and sowing pattern on efficacy of aerial 1080 poisoning of small-mammal pests in New Zealand
Graham Nugent A B , Bruce Warburton A , Caroline Thomson A , Peter Sweetapple A and Wendy A. Ruscoe AA Landcare Research, PO Box 40, Lincoln 7640, New Zealand.
B Corresponding author. Email: nugentg@landcareresearch.co.nz
Wildlife Research 38(3) 249-259 https://doi.org/10.1071/WR10198
Submitted: 29 October 2010 Accepted: 26 April 2011 Published: 13 July 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Context: Aerial poisoning using sodium fluoroacetate (1080) is an important but controversial technique used for large-scale control of brushtail possums (Trichosurus vulpecula) and other pests in New Zealand. The technique reliably produces near total kills of possums and rats, provided that many tens of baits (and therefore many tens of individually lethal doses) are sown for each target animal present.
Aim: The aim of this study was to further refine aerial 1080 poisoning by determining the effect of prefeeding, sowing rate, and sowing pattern on effectiveness.
Methods: Eighteen experimental treatments comprising all possible combinations of three sowing rates (1, 2, and 5 kg/ha of bait), three frequencies of non-toxic prefeed (0, 1, and 2) and two sowing patterns (parallel and cross-hatched) were applied to each of two forested areas. Treatment effectiveness was assessed from changes in the rate of interference recorded on baited cards for three species: possum, ship rat (Rattus rattus) and mouse (Mus musculus).
Key results: Outcomes were highly variable, ranging from increases in pest activity to near total reductions. Possum reductions were highest where one or two prefeeds were used, and at the higher sowing rates (2 or 5 kg/ha), but with some interactions between these factors. For rats, two prefeeds resulted in the highest reductions but sowing rate had no effect. For mice, post-poisoning indices were often high, indicating low effectiveness.
Conclusions: Some treatments were highly effective so poor kills were unlikely to have resulted from pests not encountering bait, or the bait being unpalatable. Rather they appeared to reflect sub-lethal poisoning either as a result of low acceptance (as a result of a lack of familiarity and/or satiation) or bait fragmentation. We infer that for possum and rats prefeeding helps reduce this risk of sub-lethal poisoning not only by increasing familiarity, but also (in conjunction with high sowing rates) by increasing the bait encounter rate, particularly for possums.
Implications: There is scope to further reduce the amount of toxic bait sown and the cost of poisoning, without compromising efficacy, by fine-tuning the balance between prefeeding and sowing rate based on which species are being targeted and, for possums, reducing bait fragmentation.
Additional keywords: bait fragmentation, brushtail possums, mice, Mus musculus,Rattus rattus, sodium fluoroacetate, ship rats, Trichosurus vulpecula.
Introduction
Aerial poisoning using carrot or cereal baits containing sodium fluoroacetate (1080) is an important but highly controversial tool used for large-scale control of introduced brushtail possums (Trichosurus vulpecula) and other pests in New Zealand (Philp 2009). The aim is usually to protect native plants and animals and/or to help reduce the incidence of bovine tuberculosis in livestock. A recent official assessment of 1080 (ERMA 2007) concluded that, despite public antipathy to the tool, its cost-effectiveness outweighed its real and perceived adverse impacts. However, continued concerns about safety (e.g. Weaver 2006) mean that the ongoing use of 1080 will be increasingly constrained (ERMA 2007). This drives a need to decrease unwanted side effects and to increase benefits and cost-effectiveness to ensure ongoing availability of this important control method.
Current practice for aerial poisoning has evolved out of 50 years of operational trial-and-error and research (Morgan 2004). The technique can now reliably produce kills of >95% (Morgan et al. 2006). However, failures still occur occasionally, such as the 60% possum kill reported by Coleman et al. (2007). In addition, aerial 1080 poisoning is being used increasingly for rodent control during periodic irruptions of rats (Rattus rattus) and/or mice (Mus musculus), as well as to control the ensuing stoat (Mustela erminea) population surge that follows these irruptions (Elliott and Suggate 2007). Such irruptions can critically threaten several highly endangered bird species (O’Donnell 1996). While Innes et al. (1995) documented some high rat kills during aerial 1080 operations, they also report some failures, and there has been little research into the efficacy of the tool against mice.
Despite historical gains in efficiency, toxic bait is usually sown in excess, at least in terms of the number of lethal doses sown relative to the number of pests to be killed. Rat and possum numbers rarely exceed 10 per ha, yet many hundreds of baits (most containing at least one lethal dose) are usually sown. Optimising baiting thus provides potential cost efficiencies as well as reducing non-target risks and minimising environmental contamination. The aim of this study was to further refine aerial poisoning as a multi-pest control tool, by determining the relative effect of three factors (prefeeding, sowing rate, and sowing pattern) that were perceived as important determinants of cost and effectiveness by a group of aerial poisoning experts at a workshop in 2005 by the two senior authors (GN, BW) (Nugent et al. 2005).
Prefeeding with non-toxic bait is now standard practice in aerial poisoning of possums, as it increases efficacy (Henderson et al. 1999a, 1999b; Brown and Urlich 2005; Coleman et al. 2007). The prefeed is usually sown on only a single occasion about a week before the toxic bait is sown, although two prefeeds may be more effective than one (Coleman et al. 2007). However, each additional prefeed increases cost substantially, so we asked whether the extra cost produces a worthwhile increase in efficacy. Likewise, sowing rate (the amount of bait sown per hectare) is an obvious driver of cost. Sowing rates have reduced over time, with an average of 3–4 kg/ha of toxic carrot or cereal bait used in poisoning operations on conservation land since 2000 (Table 3, Veltman and Westbrooke 2011) compared to rates of 15–45 kg/ha used in the 1970s (Morgan et al. 1997). However, there are no data on the trade-off between prefeeding and sowing rate, so we aimed to determine whether the increased cost of prefeeding could be offset by reducing sowing rates.
Use of GPS-guided helicopters with sowing buckets able to broadcast laterally from parallel flight paths was adopted to ensure complete bait coverage (Morgan 1994). However, temporary blockages in the sowing bucket occasionally result in large gaps in bait coverage within which the resident possums may not encounter any toxic bait. The third aim was therefore to determine whether covering an area twice (dual sowing) would eliminate any significant gaps in coverage and so increase efficacy and/or reduce risk of operational failure.
Materials and methods
Study area
The study sites, Whirinaki (NZMG 2827540E 6269700N) and Mokaihaha (NZMG 2779315E 6330560N), were hilly forested areas in the central North Island. The mixed broadleaved–conifer forests in both areas were dominated by tawa (Beilschmiedia tawa), an important food source for possums, up to ~800 m a.s.l., with beech (Nothofagus spp.)-dominated forest above that in small ridge-top sites at Whirinaki only. In winter 2006, we established 18 1-km-square blocks in each area. These were grouped into two sets of nine contiguous blocks: Mokaihaha East (blocks E1–E9) and West (W1–W9), and Whirinaki North (N1–N9) and South (S1–S9).
In July 2005, a 12 500-ha area around (but not including) the Whirinaki site was aerially poisoned with a single prefeed of non-toxic carrot bait (3 kg/ha) followed a week later by 5 kg/ha of 0.08% w/w1080 carrot bait. The operation was considered a failure (and was partially repeated as a result) because post-poison monitoring using the Residual Trap Catch Index method based on leg-hold trapping (RTCI; NPCA 2004) indicated an RTCI of 11.2%, well above the target of 2%. RTCIs exceeded 10% on 6 of the 16 monitoring traplines (maximum = 70%), all in the low-altitude tawa forest.
The Mokaihaha site was not part of any other control operation, but the western half of the site had been poisoned several times until 2 years previously using brodifacoum in bait stations. However, pre-trial pilot trapping indicated possum numbers there had increased to moderate levels (~10% RTCI, or ~2 possums/ha; Ramsey et al. 2005).
Aerial poisoning trials
The three factors under investigation were varied systematically between experimental treatments, with three levels of sowing rate (1, 2, and 5 kg/ha of bait), three levels of prefeeding (0, 1, and 2 prefeeds) and two sowing patterns (parallel, which is normal practice, and cross-hatched). Each of the 18 possible treatments was assigned randomly to one of the blocks at both Whirinaki and Mokaihaha.
The same sowing rates were used for the prefeed (where applied) and the toxic sowings. There was an interval of 5–10 days between sowings depending on weather. For the parallel sowing pattern, toxic bait was sown in a single pass along parallel flight paths, while for the cross-hatched pattern, toxic bait was sown in two halves 5 days apart, with the flight paths for the second sowing at right angles to those for the first.
The prefeeds for the cross-hatched treatments were not cross-hatched, except where two prefeeds were sown, so the spatial coverage of prefeed differed between the single- and dual-prefeed treatments. With single prefeeds, the flight paths used to sow toxic bait were aligned to those used for prefeeding. With dual prefeeds, the flight paths used to sow toxic bait were aligned with those of the first prefeed. Recent trials suggest that this may be counterproductive. Likewise, delaying for 5 days the sowing of the second half of the toxic bait in the cross-hatched blocks (with the aim of ‘mopping up’ pests that had not been exposed to toxic bait in the first sowing) may have simply had the effect of halving the sowing rate. These design complexities mean the experimental cross-hatched treatments differed from the conventional parallel treatments in more than just sowing pattern, so they were excluded from some analyses.
All blocks were aerially poisoned by Epro, an experienced possum-control contractor. Toxic bait was sown in August (Whirinaki) and September 2006 (Mokaihaha). A GPS-guided helicopter using an under-slung bucket was used to broadcast non-toxic or toxic carrot bait along predetermined parallel flight paths spaced 100 m apart. The carrot bait was chopped to an average size of ~8–10 g per bait, and 1080 concentrate was sprayed on to produce the desired loading of ~0.15% w/w 1080.
To monitor poisoning effectiveness, we used ChewTrack Card Indices (CTCIs). ChewTrack Cards (CTCs) were developed to provide an affordable tool for simultaneously indexing the level of interference activity caused by possums, rats, and mice, based on the rate at which they chewed or left tracks on the baited cards (Sweetapple and Nugent 2011). We assume that the interference activity measured by the percentage of cards bitten or tracked by a species is positively related to the abundance of that species. The equivalent assumption has been validated for leg-hold trapping of possums (Forsyth et al. 2005) and for other bite-interference or tracking-rate indices (Brown et al. 1996; Thomas et al. 2003; Warburton et al. 2004; Ogilvie et al. 2006). We used a single monitoring device because the cost of using leg-hold trapping for possums and separate trapping or tracking devices for rodents would have been prohibitive. An unforeseen consequence was that indices were affected directly and indirectly by inter-species interactions (see Results), which complicated analysis and interpretation.
About 3 weeks before poisoning, four 1-km-long transects were established ~200 m apart within each 100-ha block. CTCs were placed at 25-m intervals along each transect, and then checked 11–13 days later. New CTCs were placed at different locations (within 5 m) along the same transects ~2 weeks after poisoning and again checked 11–14 days later.
During the pre-poison surveys, multiple 1.14-m plots were searched to determine the presence or absence of possum, pig, and deer faecal pellets (Baddeley 1985). For possums, these pellet frequencies (the percentage of plots on which pellets were detected) provided an independent measure of possum abundance. There was a positive correlation between the possum CTCIs and pellet frequencies recorded in each of the 32 blocks (R2 = 0.82, P < 0.001; Sweetapple and Nugent 2011) providing some support for the assumed link between CTCIs and pest abundance. The pellet surveys also helped qualitatively assess the overall abundance of other potential consumers of bait. Deer and pig abundance was moderate at Whirinaki (16.8% and 2.3% plots with pellets present, respectively, n = 2784 plots) and low at Mokaihaha (4.8% and 0.5%, respectively, n = 2880 plots), so bait consumption by these large non-target species may have been about three times higher at Whirinaki.
Post-control possum CTCIs tended to be higher at the edges of blocks, suggesting some post-control use of the fringes of blocks by unpoisoned possums from adjacent areas. The analyses for all species were therefore restricted to cards 200 m from the edge of each block (i.e. to the central 0.6-km-square core of each 100-ha block).
The monitoring data are expressed as presence/absence percentages; i.e. the CTCI index (%CTCI) is the percentage of cards on which a species was detected by either bite marks and/or tracks. The CTCIs are not corrected for the variation (11–14 days) in how long cards were left out in the field, as unpublished data from other trials show that there are few further-detections after about a week. These indices were Poisson-transformed (Caughley 1977; Hone 1988) to reduce the effect of index saturation at high interference rates.
In some blocks, especially for mice, there were increases in CTCIs immediately after poisoning that cannot have resulted from actual increases in pest abundance (see Results). The relative change in activity between blocks was therefore indexed by expressing the post-control CTCI for each line as a percentage of the total CTC sites on that line that had either pre- or post-interference, or both. This ‘relative reduction in activity’ index (RRAI) has the biologically sensible property of never being negative; i.e. it did not allow the spurious inference of an increase in pest numbers as a result of increased activity in the few weeks following poisoning.
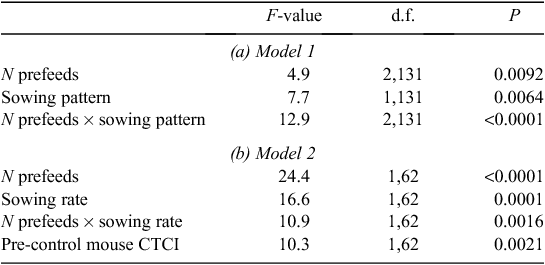
Analysis of the determinants of RRAI was carried out separately for each species using the Linear Mixed Effects (‘lme’) procedure in the statistical computing environment ‘R’ (version 2.9.2). Lines, in blocks within areas, were treated as random effects, while prefeed, sowing rate, and sowing pattern were treated as the fixed main effects that are the focus of this paper, and the pre-control CTCIs for the other two species were included as covariates. We make no effort to interpret the differences between the two main sites, so the random ‘area’ effects (line/block/site) are not reported. The statistical significance or otherwise (α = 0.05) of interactions and main effects was determined using F-tests. Non-significant effects were dropped and the model refitted. This procedure was repeated until all terms remaining in the model were significant. The analysis was repeated for the parallel sowing treatments alone, because in the cross-hatched treatment the number of prefeeds and their spatial application were potentially confounded (see above).
Bait consumption in the field
To help interpret the outcomes of the experiment above, we assessed in two of the 100-ha blocks (W3 and E7) at Mokaihaha how much of the bait sown was encountered and removed by the animals present. We measured the density of baits on plots, bait size, and bait disappearance rates along four 1-km-long parallel transects in each block during both the prefeeding and toxic phases. On the days prefeed bait was sown possum leg-hold traps lured with a flour/icing sugar mix were set at 66-m intervals along transects (60 traps per block), and 2-m-radius plots spaced 20 m apart were searched for baits. Each bait found was marked using a small metal spoke and its maximum length (in cm) was recorded. In addition, a single unsown bait was placed at the centre of all plots. The traps were checked and the plot searches were repeated on the following 2–3 days to determine the rate of bait disappearance of marked baits. The stomachs of trapped possums were analysed using the layer separation method (Sweetapple and Nugent 1998) to determine the wet weight of total stomach contents, carrot bait, and tawa fruit (the main food at that time). Long mean food retention times in possums (Wellard and Hume 1981) suggest slow turnover of stomach contents, so we presume most of the carrot bait eaten the previous night would have still been in the stomach when the possums were killed.
The plot searches were repeated on the day, and for the 3–4 days after, toxic bait was sown. Again a single unsown toxic bait was placed at the plot centre of each plot. We also searched the areas around the transects for poisoned possums, and analysed their stomach contents as above.
Controllability and bait acceptance of survivors
In February 2007, possums in two separate 100-ha Whirinaki blocks (N6 and the contiguous halves of S2 and S3) were intensively controlled to reduce possums for a concurrent study. There were few rats left in these blocks after poisoning (CTCIs 1–8%; Appendix 1, which is available as an Accessory Publication on the Wildlife Research website). We used this operation to assess bait acceptance and possum controllability. A 1-mL extrusion of non-toxic prefeed paste (Ferafeed®) was placed at sites 20 m apart along nine or ten parallel 1-km-long transects spaced 100 m apart. At the same time, single non-toxic ~10-g carrot and 12-g Wanganui #7 cereal baits (Animal Control Products, Wanganui, New Zealand) were alternated at 50-m intervals. The aim was to assess whether survivors had developed an aversion to carrot but would still accept a different bait. The baits were nailed to tree trunks to prevent easy removal by rodents, using different trees to those used for the Ferafeed® paste. Baits were checked 2 days later, and encapsulated cyanide tablets (Feratox) were placed at the Ferafeed® sites and left in place for 4 days. Two days after the Feratox was deployed, traps were set at 100-m intervals along these transects for the final 2 nights of poisoning. After that the traps were moved forward 50 m and laterally 50 m and set for 2 further nights, to determine how many possums survived in the areas furthest from the cyanide and traps sites.
Supplementary data
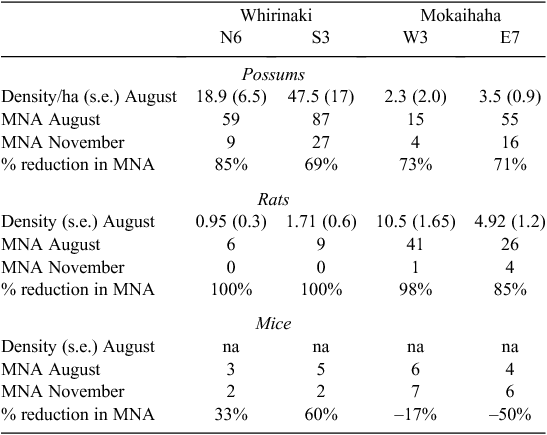
Additional data on pest abundance and diet were obtained from a concurrent project. Estimates of actual numbers of possums, rats, and mice were obtained in August 2006 and November 2006 on trapping grids located in four of the 100-ha blocks (N6 and S3 at Whirinaki; E7 and W3 Mokaihaha). Possums were trapped around the perimeter of a 270 × 270-m grid, with 36 live-capture traps set at 30-m intervals. Nested within that, rats were trapped on a 240 × 240-m grid with 64 traps spaced 30 m apart, and mice were trapped on a 120 × 120-m grid with 64 traps spaced at 15-m intervals. Traps were set for 5 days. Animals were individually tagged and released, and the minimum numbers alive per site were calculated for each trapping session. Density estimates were calculated from the recapture data using recapture distances to calculate effective trapping area by maximum likelihood estimation (Borchers and Efford 2008) in Program DENSITY (Efford 2007).
In a parallel investigation of possum diet, four sets of possum stomachs were obtained before poisoning in August 2006. Each sample of 9 or 10 stomachs was randomly selected from possums killed along four more or less circular transects 4–5 km long, with each transect spanning one of the four main sets or groups of nine blocks. The layer separation method (Sweetapple and Nugent 1998) was used to analyse stomach contents.
Results
Pest densities
Before poisoning, the indices of abundance for each species varied widely between blocks (Appendix 1). Density estimation indicated high possum densities in two Whirinaki blocks, but low densities in two western Mokaihaha blocks, whereas the reverse was true for rats (Table 1). The minimum numbers of mice alive were lower than for the other species (Table 1).
|
Overall effects of control
In the four trapped blocks, the number of animals known to be alive decreased after aerial poisoning by 69–85% for possums and by 85–100% for rats, but by less than 60% for mice (with increases for mice in two blocks) (Table 1).
Core-area CTCIs for mice (and, far less so, possums) were sometimes substantially higher 2–4 weeks after control than before (Appendix 1), particularly at Mokaihaha where pre-control rat interference was virtually universal (mean CTCI = 96%). The increases were partly an artefact of the reduction in interference by other species, although the percentage of the 104 core-area cards from both areas combined (i.e. those that were not used by rats or possums before or after control and those that were used by mice) increased significantly from 48% to 70% (Fisher’s exact test, P < 0.001). Likewise, at Mokaihaha, there were 24 core-area cards that were not used by either rats or mice before or after control, and on these the percentage used by possums increased significantly from 8% to 38% (Fisher’s exact test, P < 0.015). These increases indicate increased mouse and possum CTC activity (rather than abundance) after the removal of rats. Overall, there were only a few large reductions in activity of any species, indicating that substantial numbers of all species survived. Post-control possum and rat CTCIs below 5% were recorded in only six blocks each (Appendix 1).
Effects on possums
At Mokaihaha, core-area possum CTCIs decreased significantly (Fisher’s exact test, P < 0.05) in only two of the 18 blocks after control, compared with 10 out of 18 at Whirinaki. Importantly, however, there were large (>90%) reductions in four blocks (Appendix 1). These indicate that the bait was accepted by nearly all possums in those blocks. High survival in the other blocks was therefore not a consequence of either poor bait acceptability or some innate possum aversion to carrot.
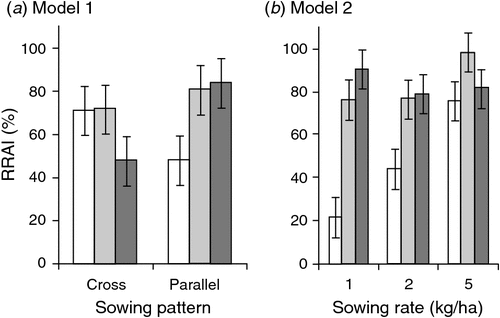
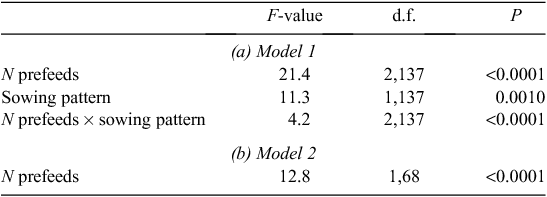
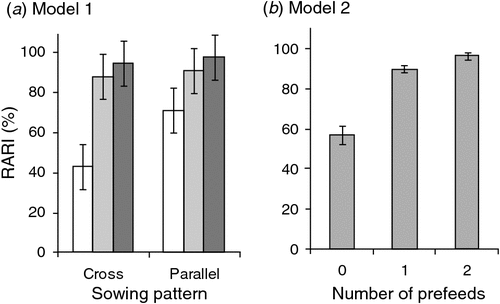
The first statistical model (Model 1) included all three experimental main effects. It showed no significant effect of pre-control rat or mouse CTCI on the RRAI for possums. However, mean RRAI for possums was significantly lower with cross-hatched sowing (63%) than for parallel sowing (70%), and there was a significant prefeeding effect that differed with sowing pattern (Table 2a, Fig. 1a). Prefeeding was associated with increased RRAI in the case of parallel sowing, but with decreased RRAI in the case of cross-hatched sowing. The inconsistencies in how prefeed was applied make interpretation of that result difficult. However, where no prefeed was used, the cross-hatched treatment produced greater reductions in possum abundance, even though the second half of the toxic bait was applied 5 days after the first.
|
|
The second statistical model (Model 2) only included data from the parallel sowing pattern. There was a significant interaction between the effect of prefeeding and sowing rate on RRAI (Table 2b). At the lowest sowing rate, 1 kg/ha, prefeeding appeared to be required to obtain a high RRAI (Fig. 1b). At the intermediate and highest sowing rate, however, high RRAIs were obtained without prefeeding, and prefeeding had no consistent effect (Fig. 1b). Prefeeding is treated as a binary variable (prefed or not prefed) in this model because inclusion of three prefeed levels (zero, once, twice) did not increase explanatory power (Akaike’s Information Criterion (AIC) values of 3.03 and 3.88 respectively). Possum RRAI tended to be higher when mouse pre-control abundance was high (Table 2b) largely reflecting the generally lower mouse CTCI and higher RRAIs in the Whirinaki blocks. Pre-control mouse CTCIs were inversely correlated with pre-control rat CTCIs (r = –0.2796, n = 144, P < 0.001), so this mouse effect could be viewed as representing an overall ‘rodent’ effect.
Effects on rats
Rat CTCI activity decreased by 65% at Whirinaki and 60% at Mokaihaha. In one Whirinaki block (two prefeeds, parallel-sown, at 2 kg/ha) no rats were detected after control despite a 70% pre-control CTCI in that block, indicating that the low reductions in some blocks were not attributable to poor bait.
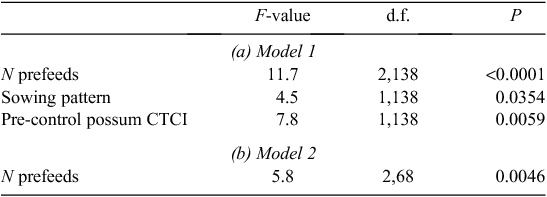
In the all-factor model for rats, there was no significant effect of sowing rate, nor any significant possum- or rat-related effects. However, mean rat RRAI was significantly lower with cross-hatched sowing than parallel sowing, and there was a significant prefeeding effect that differed with sowing pattern (Table 3a, Fig. 2a).
|
|
In the two-factor model, only prefeeding had a significant effect on rat RRAI (Table 3b). Prefeeding twice resulted in higher rat RRAI than prefeeding once, as inclusion of three prefeed levels (zero, once, twice) increased explanatory power (AIC = −88.5 compared with AIC = −77.1 for prefeed as a binary variable) (Fig. 2b).
Effects on mice
Overall, there was a 10-fold increase in mouse CTCIs after poisoning at Mokaihaha, with increases in every block (Appendix 1). At Whirinaki mouse activity also increased slightly overall, but with some significant decreases, the greatest being 82%, recorded in the unprefed 1-kg/ha cross-hatched block. At Mokaihaha, the three smallest increases were also in cross-hatched sown, unprefed treatments.
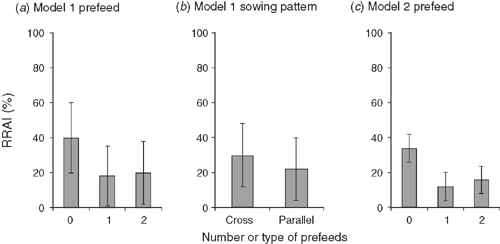
In the all-factor model for mice, there was no significant effect of sowing rate, no significant interactions, and no rat effect, but a significant possum effect (Table 4a). However, mean mouse RRAI was significantly higher with cross-hatched sowing than for parallel sowing, and there was a significant negative prefeeding effect that differed with sowing pattern (Table 4a, Fig. 3a, b).
|
|
In the two-factor model, only prefeeding had a significant effect on mouse RRAI (Table 4b), with lower RRAIs in prefed blocks (Fig. 3c).
Bait consumption in the field
The density of prefeed baits after sowing was highly variable, with the mean per transect ranging from 0 to 477 baits/ha (overall mean per transect of 275 ± 130 (95% CL)) across E7 and W3. However, there was no evidence of large (>100 m) gaps, as the transects with low or zero bait density were always within 50 m of a transect with high bait density. The prefeed sowing rate for these blocks was nominally 2 kg/ha (~200 baits/ha). The densities of toxic bait were also variable, with a mean per transect of 159 ± 159 (range 17–230) for E7 (nominally sown at 2 kg/ha (~200 baits/ha)) and 136 ± 67 (range 96–180) for W3 (nominally 1 kg/ha (~100 baits/ha)).
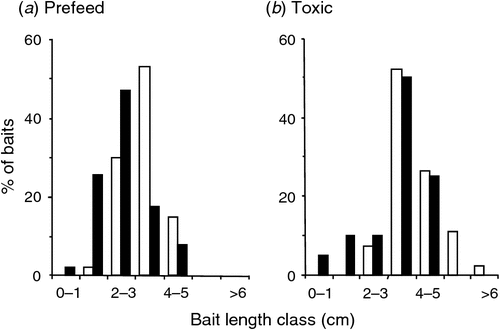
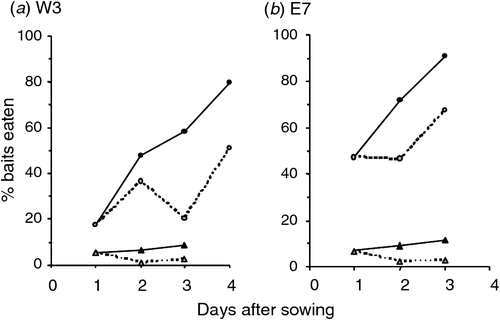
In both the prefeeding and toxic phases, the sown baits were on average shorter than the unsown baits placed by hand (χ2 = 35.0, d.f. = 3, P < 0.001 for prefeed, Fig. 4a; χ2 = 6.8, d.f. = 2, P = 0.033 for toxic, Fig. 4b), indicating the likelihood that some bait had shattered during the sowing process. Most of the prefeed disappeared within 3–4 days (Fig. 5). The percentage of remaining bait eaten each night increased significantly from 25% on the first night to 47% and 49% on the second and third nights respectively (χ2 = 44, d.f. = 2, P < 0.001), even though there was less bait available on each successive night. This suggests increased search effort by the animals. Only 9% (W3) and 12% (E7) of the toxic baits disappeared over 3 nights (Fig. 5). Of the 40 toxic baits removed (both blocks combined) 58% disappeared on the first night, 28% on the second night and 15% on the third.
|
|
During the prefeeding phase in W3, the possum trap-catch rate was positively correlated with the number of nights that prefeed had been previously available (0.0%, 3.3%, 8.3% and 11.7% (n = 60 traps) on nights 1–4 respectively; R2 = 0.99, d.f. = 2, P = 0.003). In E7, trapping effort for the first night was not accurately recorded, so no parallel or pooled analysis was possible.
Of the 25 possums trapped during prefeeding, carrot bait was detected in only 14, and half of those contained <5 g of bait. It appeared that most of these 14 possums had eaten only 1–3 baits before being trapped, although that will be a substantial underestimate because possums will often have been trapped well before they had finished feeding for the night. For the 16 possums found dead and presumed poisoned, no carrot was found in three, over half had less than 5 g of bait, and only three had >20 g (26.6, 48.1, and 52.6 g respectively).
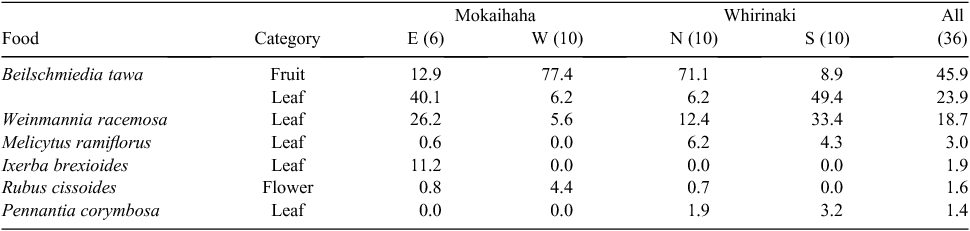
One factor potentially influencing bait take was naturally occurring foods. Tawa fruit was the predominant food being eaten by possums immediately before poisoning in winter 2006 (Table 5). We assume that the tawa fruit was eaten in the canopy because the stomach content layers in which it was found were not contaminated with litter or mixed with ‘ground’ foods such as ferns and herbs. The percentage of tawa fruit in the diet in the four groups of blocks was correlated with an index assumed to reflect the level of possum activity at ground level (the pre-control CTCI/ pellet-frequency ratio; R2 = 0.92, d.f. = 2, P = 0.043).
Controllability and bait acceptance by survivors
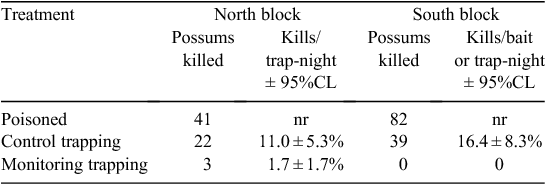
Possums that survived the August 2006 carrot 1080 poisoning operation were able to be trapped or poisoned in February 2007, as low-intensity cyanide poisoning (4 baits/ha for 4 nights) and trapping (1 trap/ha for 2 or 3 nights) reduced the numbers of trappable possums at points midway between the poison lines to near-zero levels within 4–5 days (Table 6).
|
The surviving pests mostly avoided the 200 carrot baits placed in these two blocks before the February 2007 poisoning. Only 2.5% of the carrot baits were completely consumed, 3.5% were partly eaten by possums, and a further 3.5% were bitten by possums but not eaten. In contrast 52.5% of the cereal baits were eaten and a further 2% were partly eaten. The differences in the numbers of whole baits eaten were significant (North block χ2 = 80.1, d.f. = 1, P < 0.001; South block χ2 = 36.6, d.f. = 1, P < 0.001).
Discussion
For possums, at least, current best-practice prefed aerial broadcast 1080 poisoning usually results in very low residual pest densities (Morgan et al. 2006). In this trial, however, some of the poisoning treatments appeared to have little or no effect on pest abundance, particularly at Mokaihaha. While disappointing, these failures have nonetheless provided insight into the factors that may determine poisoning success. We suggest that there are three interdependent factors or probabilities that ultimately determine whether an aerial 1080 poisoning operation succeeds in killing most of the target pests; the probability a pest will encounter toxic bait, the probability that that bait will be acceptable and the probability that individual bait is, by itself, be lethal. It is clear that for many of our experimental treatments at least one of these probabilities was too low.
It is obviously crucial that the probability of a pest encountering bait be very high. A total kill can be achieved only if every target animal has some toxic bait sown into its home range. The low amount of bait found on some W3 and E7 transects indicated that bait density was very low in some places. However, there was always a much higher density of bait found on the nearest parallel transect nominally 50 m away so there will have been bait within that 50-m distance between the transects. That suggests that all possums, rats, and mice will have been exposed, as their home-range sizes are typically more than 0.5 ha (Cowan 2005; Innes 2005; Ruscoe and Murphy 2005). For possums, Morgan (1990) showed that even when the distance between flight paths was 200 m, 95% of possums found and consumed dyed bait within 3 days. Likewise, the near-total reductions in possum and rat activity achieved in some blocks (Appendix 1) indicate coverage must have been adequate for these species in those blocks. Further, it is unlikely under our application regime (flight paths 100 m apart with GPS guidance) that the control failures were caused by gaps in bait coverage, since an examination of the GPS-based track logs of the helicopter flight paths indicated no difference in uniformity of coverage between high and low reduction blocks.
It is also essential that all animals encounter bait within their home range before it detoxifies or becomes unpalatable. In these trials most of the toxic bait was not eaten (Fig. 5). Apparently undecayed carrot was still common at least 5 weeks afterward (fieldworker observations), and 1080 does not rapidly leach out of carrot bait (Bowen et al. 1995). The undecayed bait would still have been toxic, so the pests should have had time to encounter a lethal amount of edible bait. However, this might not have occurred if they spent little time on the ground. Ward (1978) found possums in his study spent an average of only 15% of each night on the ground. In this study, we sometimes recorded moderately high faecal pellet frequencies yet very low CTCIs before control (e.g. N6; Appendix 1b). This suggests the possibility that possums were present, but spending most of their time in the canopy, and therefore were not being detected as often on the CTCs at ground level. If so, the rate at which they encountered toxic bait is likely to have been low in these blocks. Arguably, the better but still modest reductions in the unprefed cross-hatch blocks than in the equivalent parallel blocks (Fig. 1) are consistent with this. The low acceptance of carrot baits compared with cereal bait 6 months after poisoning (Table 4) indicates most survivors were carrot shy (at least in the two blocks tested). That implies that in all blocks possums must have encountered some bait but that in blocks with poor kills they did not eat enough to kill them. High levels of bait shyness appear to be the norm for survivors of aerial 1080 operations (O’Connor and Matthews 1999; Ross et al. 2000). We conclude that few possums, if any, failed to encounter toxic bait, but hypothesise that for some (the survivors) the rate at which they did so was too low to kill them.
The probability the pests accept (i.e. eat) toxic bait when they encounter it must also be high to achieve a high kill. The large reductions in possum and rat CTCIs in some of the blocks (e.g. S1, S5, S8; Appendix 1b) indicate the bait used in these trials was acceptable and palatable to the great majority of individuals of those two species in those blocks. The failures in other blocks therefore suggest the possibility that acceptability may not have been adequate. This might occur if the pests were unfamiliar with the novel food or if they had access to more acceptable and/or palatable foods and had fed extensively on them before encountering toxic bait.
The role of prefeeding in mitigating the risk of lack-of-familiarity is well documented for possums (Moss et al. 1998; Ross et al. 2000; Nugent et al. 2009) and it seems to be a key factor for rats (Fig. 2). In contrast, the risk of low acceptance because the animals are satiated with another food is less well researched. We suggest this may explain the minimal possum reductions in two of the unprefed parallel-sown blocks at Whirinaki and the possum failures at Mokaihaha. Possibly, possums had little interest in carrot bait because they were satiated with tawa fruit. Tawa fruit is carried high in the canopy for 18–20 months, but is not eaten by possums until it begins to ripen. It is apparently not much eaten by rats (Knowles and Beveridge 1982). The fruits’ 6–12-g drupes are in some respects similar to carrot bait. Tawa trees were abundant in all blocks, so the low consumption of tawa fruit by possums in August 2006 in the southern blocks at Whirinaki (Table 5) probably reflects depletion of that food by the high density of possums there, making them easier to poison than in the western blocks at Mokaihaha where tawa fruit comprised 77% of stomach contents (Table 5).
We may also have oversupplied prefeed in some blocks, especially those prefed twice at 5 kg/ha. The low total biomass of possums and rodents in some blocks makes it unlikely that they could have eaten all the non-toxic carrot by the time toxic bait was sown. With cross-hatched sowing, in particular (where only half the toxic bait was present for the first 5 days after poisoning), uneaten prefeed, if present, would have increased the mean number of baits required to kill a possum. That may explain why, at the 5-kg/ha sowing rate, possum reductions appeared to be lower with two prefeeds than with one, especially for cross- hatched sowing (Fig. 1). We suggest the absence of this hypothesised ‘dilution’ effect on rat reductions (Fig. 4) reflects the greater likelihood that a single bait contained enough 1080 to kill a rat, because of their much smaller size.
The probability of a single bait being lethal is clearly also important. It is obviously best if each toxic bait contains sufficient toxin to kill the largest and most-toxin-tolerant individual. If some baits do not, then at least some animals have to find two or more baits, and do so before the onset of toxicosis stops them from searching for more bait. For 1080, that period is often less than an hour (Henderson et al. 1999a). In recognition of this there has been a shift to large (12 g) 1080 cereal baits for aerial poisoning of possums (Frampton et al. 1999), and toxic carrot bait is screened to remove the smallest fragments (Fig. 4). However, there is evidence that fragmentation of bait occurs during aerial 1080 poisoning, both for carrot (Fig. 4.) and for the cereal bait types that are often used instead of carrot (Nugent et al. 2011). The smallest baits that result from fragmentation are unlikely to contain a lethal dose of 1080, particularly for possums. The presence of sub-lethal doses, and a relatively short pre-toxicosis window, makes the rate at which baits are subsequently encountered (rather than encounter per se) crucial for an individual whose first encounter is with a sub-lethal bait or bait fragment.
Increasing sowing rate increases bait density and consequently bait encounter rate, shortening the time required to find the first and subsequent baits. In line with that, the reductions in possum activity increased markedly with sowing rate in the unprefed parallel-sown blocks (Fig. 1b). There was no evidence of an equivalent sowing rate effect with rats (Table 3) presumably because the proportion of baits containing sublethal doses was much lower for rats than for possums.
Prefeeding may also increase encounter rate by changing foraging behaviour. This effect is suggested by the significant increase in trap-catch rate over 4 days during prefeeding in E7, the contrary pattern to trapping in the absence of prefeeding. Prefeeding is known to markedly change where possums spend their time (Warburton et al. 2009). We suggest prefeeding teaches animals that if they encounter bait they are likely to find another bait on the ground nearby (rather than, for example, up the nearest tree). For rats (Fig. 2b), and possibly also for possums at the lower sowing rates in parallel-sown blocks (Fig. 1b), there was some evidence that two prefeeds resulted in greater reductions than one. A single exposure to prefeed appears sufficient to familiarise captive possums with a novel food (Nugent et al. 2009), so we infer that at least part of the prefeeding effect reflects a change in foraging behaviour (encounter rate) as well as a change in feeding behaviour (bait familiarity and acceptance).
Sub-lethal doses can also be created after sowing as a result of partial consumption of a bait. Likewise when two animals encounter a toxic bait at the same time (as when female possums are accompanied by near-independent young in spring, and when males consort with females in autumn) one or both of them is likely to ingest a sub-lethal portion of that bait. That may partly explain why aerial 1080 poisoning of possums tends to be less effective in spring and autumn (Henderson et al. 1999b).
There was a significant positive effect of pre-control mouse CTCI on possum reductions. Although this is difficult to explain as a stand-alone effect, an intuitive possibility is that it simply reflects the strong negative correlation between rat and mouse interference rates, so that the apparent positive mouse affect actually reflects a more plausible negative effect of high rat numbers on possum kill, at least in part. Such an effect could be mediated via rapid depletion (either by consumption or by caching) of prefeed and/or toxic bait by rats, or partial consumption of baits by rats decreasing their lethality.
Overall, for possums, we speculate that the availability of tawa fruit in the canopy was a major contributing factor to the poor kills, keeping some possums away from bait and/or minimising the amount of bait eaten, leading to sub-lethal poisoning. We note that the larger operation within which these trials were embedded also (and unusually for the operator) failed to achieve the control target (see Methods). The rarity of such failures (R. Lorigan, Epro, pers. comm.) may reflect both the relative rarity of tawa forest nationally, and coincidence of this control operation with a year when a large fruit crop was produced. Intuitively, the quantity of bait ingested is likely to follow some continuous distribution, with at least some of the survivors inevitably ingesting doses only marginally lower than needed to kill them. An observed correlation between temperature and the effectiveness of aerial 1080 poisoning of possums (Veltman and Pinder 2001) may reflect possums being able to survive slightly higher marginal doses when conditions are warmer. Overall we suggest that fragmentation of bait is one of the major potential risks in aerial poisoning of possums. We hypothesise that current best-practice of sowing far more toxic bait than is eaten by the possums (Fig. 5) and/or prefeeding reduces that risk by increasing bait density and teaching possums to search on the ground for bait. Use of prefeeding appears to substantially reduce the sowing rate needed to achieve a high kill (Fig. 1b; Coleman et al. 2007).
For rats, sowing rate had no significant effect, suggesting sufficient baits were available even at the lowest sowing rate and that most baits and bait fragments were potentially lethal to rats. Cross-hatching appeared to be counterproductive (Fig. 5), suggesting bait distribution was not a major problem. In contrast prefeeding was crucial for rats and two prefeeds were better than one (Fig. 4). Prefeeding twice and poisoning, all with 1 kg/ha, produced, on average, similar percent reductions (90.1%, n = 4) to prefeeding once and poisoning with 5 kg/ha (90.9%, n = 4) and used 60% less non-toxic bait and 80% less toxic bait.
Mice CTCIs usually increased substantially after poisoning, even on cards not bitten by possums or rats. This phenomenon was first observed in the 1990s but there has been uncertainty as to whether it is a behavioural response or a demographic one (Innes et al. 1995), as mice numbers can increase quickly after rat removal (Witmer et al. 2007). Here the size and speed of the increases leave little doubt it is a behavioural response, with mice ranging more freely and widely at ground level once rats and possums had been removed. The effect appears to be mainly a rat effect, as possum and mouse detections sometimes increased in tandem (e.g. E8, E9, W8; Appendix 1a). Despite the difficulty of separating the effects of prefeeding, sowing rate and sowing pattern on mouse kill because of the apparent change in mouse behaviour in response to rat removal coupled with the variation in post-control rat abundance, it is clear that most experimental treatments were ineffective in controlling mice.
In summary, we believe that in these trials we exposed most if not all target pests to toxic bait but often failed to kill them for a combination of three interacting reasons – bait acceptability and palatability, competing food sources, and bait fragmentation – with the importance of each differing between species. Cross-hatched sowing (as we implemented it) did not appear to help increase kill, indicating that broadcasting baiting along flight paths 100 m part provides adequate coverage for possums and rats, with no strong evidence one way or the other for mice. Prefeeding and, to a lesser degree, sowing rate appear to increase possum kill. One of several speculative inferences from this trial, and from subsequent demonstration that fragmentation also occurs in operations using cereal bait (Nugent et al. 2011), is that bait fragmentation was a major factor in the poor possum kills achieved, and that prefeeding and ‘overbaiting’ (sowing much more toxic bait than is used by the pests) are effective but unrecognised solutions to the fragmentation problem. We argue both achieve this by increasing the rate at which possums encounter bait. Prefeeding appeared to be essential for good rat kills. As bait quantity appeared to be less important than prefeeding, at least for rats, this trial suggests that where both possums and rats are targeted high efficacy may be achieved by increasing the number of prefeeds and reducing the sowing rate. We speculate that for possums and rats, there remains scope to further reduce the amount of toxic bait sown without compromising efficacy. This speculation has since been validated by subsequent field trials in 2010 which showed near total reductions in possum, rat and mouse numbers with as little as 0.17 kg/ha of cereal 1080 bait (GN, unpubl. data). We also speculate that the availability of abundant high quality natural foods, and/or high rodent numbers, may sometimes adversely affect possum kill.
Acknowledgments
We thank the many people who helped in the fieldwork for this study, particularly those who suffered through a mid-winter trip to Whirinaki after a major wind and snow storm had created difficult field conditions. We also thank Epro, especially Roger Lorigan and Ian Roberts, for input and guidance on the choice of experimental treatments, and Kane Stafford for supervising the complex implementation of the aerial baiting treatments. The research was contracted by the Animal Health Board as part of Project R-10688 (Local elimination: A new strategic approach to large-scale control of small mammal pests), and co-funded by the New Zealand Foundation for Research, Science and Technology (Programme C09X0507 Multispecies pest control). Comments on early drafts by Phil Cowan and an anonymous reviewer helped improve this manuscript.
References
Baddeley, C. J. (1985). Assessment of wild animal abundance. Forest Research Institute Bulletin No. 106. (Forest Research Institute: Christchurch, New Zealand.)Borchers, D. L., and Efford, M. G. (2008). Spatially explicit maximum likelihood methods for capture–recapture studies. Biometrics 64, 377–385.
| Spatially explicit maximum likelihood methods for capture–recapture studies.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1czhvVWlsA%3D%3D&md5=2537b38915e9c82ccc2f7d58b80b970aCAS | 17970815PubMed |
Bowen, L. H., Morgan, D. R., and Eason, C. T. (1995). Persistence of sodium monofluoroacetate (1080) in baits under simulated rainfall. New Zealand Journal of Agricultural Research 38, 529–531.
| Persistence of sodium monofluoroacetate (1080) in baits under simulated rainfall.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhvFWjt7w%3D&md5=de6e06d2b641224a84b02b610b66dc86CAS |
Brown, K. P., and Urlich, S. C. (2005). Aerial 1080 operations to maximise biodiversity protection. DOC Research & Development Series 216. (Department of Conservation: Wellington, New Zealand.)
Brown, K. P., Moller, H., Innes, J., and Alterio, N. (1996). Calibration of tunnel tracking rates to estimate relative abundance of ship rats (Rattus rattus) and mice (Mus musculus) in a New Zealand forest. New Zealand Journal of Ecology 20, 271–275.
Caughley, G. (1977). ‘Analysis of Vertebrate Populations.’ (John Wiley: London.)
Coleman, J. D., Fraser, K. W., and Nugent, G. (2007). Costs and benefits of pre-feeding for possum control. New Zealand Journal of Zoology 34, 185–193.
| Costs and benefits of pre-feeding for possum control.Crossref | GoogleScholarGoogle Scholar |
Cowan, P. E. (2005). Brushtail possum. In ‘The handbook of New Zealand mammals’. 2nd edn. (Ed. C. M. King.) pp. 56–80. (Oxford University Press: Melbourne.)
Efford, M. G. (2007). ‘DENSITY 4.1: Software for Spatially-explicit Capture–Recapture.’ (Department of Zoology, University of Otago: Dunedin, New Zealand.) Available at http://www.otago.ac.nz/density [accessed 31 May 2009].
Elliott, G., and Suggate, R. (2007). ‘Operation Ark – Three Year Progress Report.’ (Department of Conservation: Wellington, New Zealand.)
ERMA (2007). ‘Environmental Risk Management Authority Decision 1080.’ (Environmental Risk Management Authority: Wellington, New Zealand.)
Forsyth, D. M., Link, W. A., Webster, R., Nugent, G., and Warburton, B. (2005). Nonlinearity and seasonal bias in an index of brushtail possum abundance. Journal of Wildlife Management 60, 976–984.
| Nonlinearity and seasonal bias in an index of brushtail possum abundance.Crossref | GoogleScholarGoogle Scholar |
Frampton, C. M., Warburton, B., Henderson, R., and Morgan, D. R. (1999). Optimising bait size and 1080 concentration (sodium monofluoroacetate) for the control of brushtail possums (Trichosurus vulpecula). Wildlife Research 26, 53–59.
| Optimising bait size and 1080 concentration (sodium monofluoroacetate) for the control of brushtail possums (Trichosurus vulpecula).Crossref | GoogleScholarGoogle Scholar |
Henderson, R. J., Frampton, C. F., Morgan, D. R., and Hickling, G. J. (1999a). The efficacy of baits containing sodium monofluoroacetate (1080) to captive brushtail possums (Trichosurus vulpecula). The Journal of Wildlife Management 63, 1138–1151.
| The efficacy of baits containing sodium monofluoroacetate (1080) to captive brushtail possums (Trichosurus vulpecula).Crossref | GoogleScholarGoogle Scholar |
Henderson, R. J., Morgan, D. R., and Frampton, C. M. (1999b) Avoiding bait-shyness in possums by improving bait standards. Report LC9899/060. (Landcare Research: Lincoln, New Zealand.)
Hone, J. (1988). Feral pig rooting in a mountain forest and woodland: distribution, abundance and relationships with environmental variables. Australian Journal of Ecology 13, 393–400.
| Feral pig rooting in a mountain forest and woodland: distribution, abundance and relationships with environmental variables.Crossref | GoogleScholarGoogle Scholar |
Innes, J. G. (2005). Ship rat. In ‘The handbook of New Zealand mammals’. 2nd edn. (Ed. C. M. King.) pp. 187–203. (Oxford University Press: Melbourne.)
Innes, J., Warburton, B., Williams, D., Speed, H., and Bradfield, P. (1995). Large-scale poisoning of ship rats (Rattus rattus) in indigenous forests of the North Island, New Zealand. New Zealand Journal of Ecology 19, 5–17.
Knowles, B., and Beveridge, A. E. (1982). Biological flora of New Zealand. 9. Beilschmiedia tawa (A. Cunn) Benth et Hook. f. ex Kirk (Lauraceae) tawa. New Zealand Journal of Botany 20, 37–54.
Morgan, D. R. (1990). Behavioral response of brushtail possums, Trichosurus vulpecula, to baits used in pest control. Australian Wildlife Research 17, 601–613.
Morgan, D. R. (1994). Improved cost-effectiveness and safety of sodium monofluoroacetate (1080) possum control operations. Miscellaneous Series No. 28. pp. 144–150. (The Royal Society of New Zealand: Wellington, New Zealand.)
Morgan, D. R., Thomas, M. D., Meenken, D., and Nelson, P. C. (1997). Less 1080 bait usage in aerial operations to control possums. Proceedings of the New Zealand Plant Protection Society Conference 50, 391–396.
Morgan, D. R. (2004). Maximising the effectiveness of aerial 1080 control of possums (Trichosurus vulpecula). Ph.D. Thesis, Lincoln University, Lincoln, New Zealand.
Morgan, D. R., Nugent, G., and Warburton, B. (2006). Benefits and feasibility of local elimination of possum populations. Wildlife Research 33, 605–614.
| Benefits and feasibility of local elimination of possum populations.Crossref | GoogleScholarGoogle Scholar |
Moss, Z. N., O’Connor, C. E., and Hickling, G. J. (1998). Implications of prefeeding for the development of bait aversions in brushtail possums (Trichosurus vulpecula). Wildlife Research 25, 133–138.
| Implications of prefeeding for the development of bait aversions in brushtail possums (Trichosurus vulpecula).Crossref | GoogleScholarGoogle Scholar |
NPCA (2004). ‘Possum Population Monitoring Using the Trap-catch Method.’ (National Possum Control Agencies: Wellington, New Zealand.)
Nugent, G., Warburton, B., and Morgan, D. (2005). Local elimination of pests: can we achieve it? What research is most needed? Landcare Research-convened workshop on Local Elimination of Pests, Taupo, 3 August 2005.
Nugent, G., Turner, J., and Warburton, B. (2009). Sustained recall of bait acceptability in captive brushtail possums (Trichosurus vulpecula). New Zealand Journal of Zoology 36, 473–478.
| Sustained recall of bait acceptability in captive brushtail possums (Trichosurus vulpecula).Crossref | GoogleScholarGoogle Scholar |
Nugent, G., Clayton, R., Morgan, D., and Warburton, B. (2011). Improving the efficacy of aerial poisoning of brushtail possums (Trichosurus vulpecula) through reduced fragmentation of bait. International Journal of Pest Management 57, 51–59.
O’Connor, C. E., and Matthews, L. R. (1999). 1080-induced bait aversions in wild possums: influence of bait characteristics and prevalence. Wildlife Research 26, 375–381.
| 1080-induced bait aversions in wild possums: influence of bait characteristics and prevalence.Crossref | GoogleScholarGoogle Scholar |
O’Donnell, C. F. J. (1996). Predators and the decline of New Zealand forest birds: an introduction to the hole-nesting bird and predator programme. New Zealand Journal of Zoology 23, 213–219.
| Predators and the decline of New Zealand forest birds: an introduction to the hole-nesting bird and predator programme.Crossref | GoogleScholarGoogle Scholar |
Ogilvie, S. C., Paterson, A. M., Ross, J. G., and Thomas, M. D. (2006). Improving techniques for the WaxTag® possum (Trichosurus vulpecula) monitoring index. New Zealand Plant Protection 59, 28–33.
Philp, M. (2009). A toxic debate. North and South 276, 57–65.
Ramsey, D., Efford, M., Ball, S., and Nugent, G. (2005). The evaluation of indices of animal abundance using spatial simulation of animal trapping. Wildlife Research 32, 229–237.
| The evaluation of indices of animal abundance using spatial simulation of animal trapping.Crossref | GoogleScholarGoogle Scholar |
Ross, J. G., Hickling, G. J., Morgan, D. R., and Eason, C. T. (2000). The role of non-toxic prefeed and postfeed in the development and maintenance of 1080 bait shyness in captive brushtail possums. Wildlife Research 27, 69–74.
| The role of non-toxic prefeed and postfeed in the development and maintenance of 1080 bait shyness in captive brushtail possums.Crossref | GoogleScholarGoogle Scholar |
Ruscoe, W. A., and Murphy, E. C. (2005) House mouse. In ‘The Handbook of New Zealand Mammals’. 2nd edn. (Ed. C. M. King.) pp. 204–221. (Oxford University Press: Melbourne.)
Sweetapple, P. J., and Nugent, G. (1998). Comparison of two techniques for assessing possum (Trichosurus vulpecula Kerr) diet from stomach contents. New Zealand Journal of Ecology 22, 181–188.
Sweetapple, P. J., and Nugent, G. (2011). Chew-track-cards: a multiple-species small mammal detection device. New Zealand Journal of Ecology 35, 153–162.
Thomas, M. D., Brown, J. A., Maddigan, F. W., and Sessions, L. A. (2003). Comparison of trap-catch and bait interference methods for estimating possum densities. New Zealand Plant Protection 56, 81–85.
Veltman, C. J., and Pinder, D. N. (2001). Brushtail possum mortality and ambient temperatures following aerial poisoning using 1080. The Journal of Wildlife Management 65, 476–481.
| Brushtail possum mortality and ambient temperatures following aerial poisoning using 1080.Crossref | GoogleScholarGoogle Scholar |
Veltman, C. J., and Westbrooke, I. M. (2011). Forest bird mortality and baiting practices in New Zealand aerial 1080 operations from 1986 to 2009. New Zealand Journal of Ecology 35, 21–29.
Warburton, B., Barker, R., and Coleman, M. (2004). Evaluation of two relative-abundance indices to monitor brushtail possums in New Zealand. Wildlife Research 31, 397–401.
| Evaluation of two relative-abundance indices to monitor brushtail possums in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Warburton, B., Clayton, R., Nugent, G., Graham, G., and Forrester, G. (2009). Effect of prefeeding on foraging patterns of brushtail possums (Trichosurus vulpecula) about prefeed transects. Wildlife Research 36, 659–665.
| Effect of prefeeding on foraging patterns of brushtail possums (Trichosurus vulpecula) about prefeed transects.Crossref | GoogleScholarGoogle Scholar |
Ward, G. D. (1978). Habitat use and home range of radio- tagged opossums Trichosurus vulpecula (Kerr) in New Zealand lowland forest. In ‘The Ecology of Arboreal Folivores. Proceedings of the National Zoological Park Symposium No. 1’. (Ed. G. G. Montgomery.) pp. 267–287. (Smithsonian Institution Press: Washington, DC.)
Weaver, S. A. (2006). Chronic toxicity of 1080 and its implications for conservation management: a New Zealand case study. Journal of Agricultural & Environmental Ethics 19, 367–389.
| Chronic toxicity of 1080 and its implications for conservation management: a New Zealand case study.Crossref | GoogleScholarGoogle Scholar |
Wellard, G. A., and Hume, I. D. (1981). Digestion and digesta passage in the brushtail possum, Trichosurus vulpecula (Kerr). Australian Journal of Zoology 29, 157–166.
| Digestion and digesta passage in the brushtail possum, Trichosurus vulpecula (Kerr).Crossref | GoogleScholarGoogle Scholar |
Witmer, G. W., Boyd, F., and Hillis-Star, Z. (2007). The successful eradication of introduced roof rats (Rattus rattus) from Buck Island using diphacinone, followed by an irruption of house mice (Mus musculus). Wildlife Research 34, 108–115.
| The successful eradication of introduced roof rats (Rattus rattus) from Buck Island using diphacinone, followed by an irruption of house mice (Mus musculus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXksFOlu78%3D&md5=05e8ae5f21988c8c94c54784e8d3cec0CAS |