Survival of translocated sharp-tailed grouse: temporal threshold and age effects
Steven R. Mathews A B C , Peter S. Coates A and David J. Delehanty BA US Geological Survey, Western Ecological Research Center, Dixon Field Station, 800 Business Park Drive, Suite B, Dixon, CA 95620, USA.
B Department of Biological Sciences, Idaho State University, Pocatello, ID 83201, USA.
C Corresponding author. Email: mathstev@isu.edu
Wildlife Research 43(3) 220-227 https://doi.org/10.1071/WR15158
Submitted: 5 August 2015 Accepted: 22 March 2016 Published: 12 May 2016
Journal Compilation © The authors 2016
Abstract
Context: The Columbian sharp-tailed grouse (Tympanuchus phasianellus columbianus) is a subspecies of conservation concern in the western United States, currently occupying ≤10% of its historic range. Land and management agencies are employing translocation techniques to restore Columbian sharp-tailed grouse (CSTG) populations. However, establishing self-sustaining populations by translocating grouse often is unsuccessful, owing, in part, to low survivorship of translocated grouse following release.
Aims: We measured and modelled patterns of CSTG mortality for 150 days following translocation into historic range, to better understand patterns and causes of success or failure in conservation efforts to re-establish grouse populations.
Methods: We conducted two independent multi-year translocations and evaluated individual and temporal factors associated with CSTG survival up to 150 days following their release. Both translocations were reintroduction attempts in Nevada, USA, to establish viable populations of CSTG into their historic range.
Key results: We observed a clear temporal threshold in survival probability, with CSTG mortality substantially higher during the first 50 days following release than during the subsequent 100 days. Additionally, translocated yearling grouse exhibited higher overall survival (0.669 ± 0.062) than did adults (0.420 ± 0.052) across the 150-day period and higher survival than adults both before and after the 50-day temporal threshold.
Conclusions: Translocated CSTG are especially vulnerable to mortality for 50 days following release, whereas translocated yearling grouse are more resistant to mortality than are adult grouse. On the basis of the likelihood of survival, yearling CSTG are better candidates for population restoration through translocation than are adult grouse.
Implications: Management actions that ameliorate mortality factors for 50 days following translocation and translocations that employ yearling grouse will increase the likelihood of population establishment.
Additional keywords: Columbian sharp-tailed grouse, game bird conservation, re-establishment, restoration, survival threshold, translocation, Tympanuchus phasianellus columbianus.
Introduction
The Columbian sharp-tailed grouse (Tympanuchus phasianellus columbianus, hereafter CSTG) is one of seven described subspecies (T. p. hueyi, extinct) of sharp-tailed grouse, a widespread North American prairie grouse. Historic range of CSTG lies within the Columbian drainage of the Pacific North-west and northern portions of the Great Basin of the Intermountain West of the United States. Like other sharp-tailed grouse subspecies, and North American prairie grouse in general, the CSTG employs a lek mating system in which males congregate at display areas and females assess displaying males in the process of choosing their mating partners (Selous 1906–1907; Beehler and Foster 1988). Similar to other North American prairie grouse (Storch 2007), CSTG populations have declined substantially (Gregg and Niemuth 2000; Drummer et al. 2011), now occupying less than 10% of the subspecies’ historic range (Miller and Graul 1980; Giesen and Connelly 1993). Conversion of native plant communities to agricultural crop production and habitat degradation as a result of livestock grazing are hypothesised to be important factors in CSTG population declines (Connelly et al. 1998).
The complex and dramatic lek mating behaviour of CSTG and other prairie grouse and their importance as a game animal (Hoffman and Thomas 2007) result in high scientific (e.g. Connelly et al. 1998) and social value (e.g. NDOW 2008) as well economic value (e.g. IAFWA 2002). The preservation and expansion of remaining CSTG populations in the northern Great Basin region of North America is an important wildlife management goal for the US state wildlife agencies of Idaho, Utah and Nevada, and these agencies seek to employ population-restoration actions to achieve these goals (NDOW 2008).
Translocation, the movement and intentional release of animals to the wild, often is used to establish, re-establish or augment populations (Griffith et al. 1989). However, translocated animals face challenges that can act to prevent population establishment, including physiological stress, social disruption, unfamiliarity with physical and ecological features of the release location, and the effects of small population size (Armstrong and Seddon 2008; Dickens et al. 2009a; Dickens et al. 2010). The success rate in establishing self-sustaining populations through translocation is low (Toepfer et al. 1990, Snyder et al. 1999). Greater sage-grouse (Centrocercus phasianellus), for example, are difficult to re-establish via translocation (Reese and Connelly 1997), likely because, in part, disruption of their complex mating behaviour involving established leks, a social feature that is absent in unoccupied habitat. Toepfer et al. (1990) estimated the success rate of translocation in establishing self-sustaining populations of prairie grouse to be 32% across >52 translocation efforts since 1900.
Failure of translocations to produce self-sustaining populations of prairie grouse has been attributed to many factors. Behavioural factors include dispersal from the release area (Patterson 1952; Jacobs 1959; Toepfer et al. 1990), increased vulnerability to predators (Toepfer 1988), and a lack of reproduction by translocated individuals (Toepfer et al. 1990; Coates et al. 2006). A lack of suitable habitat at the release area (Griffith et al. 1989; Toepfer et al. 1990), too few individuals or too few years of releases (Griffith et al. 1989; Snyder et al. 1999), also are hypothesised to be important factors. High post-release mortality (Toepfer et al. 1990; Snyder et al. 1999), especially during the ‘establishment phase’ (Armstrong and Seddon 2008; Dickens et al. 2009a), exacerbates the effects of small population size.
Most factors hypothesised to explain translocation failure or success directly or indirectly relate to the performance of individual birds following release. For example, in the United States, translocations involving pen-raised grouse have never succeeded in establishing a self-sustaining population (Storch 2000), and pen-raised grouse perish rapidly following release into the wild (Toepfer et al. 1990). Successful restoration of prairie grouse populations by translocation employed the capture, translocation and release of large numbers of wild grouse across several seasons (Snyder et al. 1999), overcoming presumed high rate of post-release mortality.
High mortality rate immediately following release (i.e. ≤150 days post-release for grouse) diminishes initial population size and increases the probability of population failure through stochastic effects, founding effects, and other deleterious aspects of small population size (Armstrong and Seddon 2008). Physiological stress, particularly a condition known as chronic stress, experienced by translocated individuals is hypothesised to be an important factor in the failure to establish populations (Letty et al. 2000; Dickens et al. 2009a) by diminishing survival probability during the establishment phase and influencing individual movement (Dickens et al. 2009b). Birds in a state of chronic stress exhibit an impaired ability to mount adaptive endocrinological stress responses to challenges (Dickens et al. 2009a). Prevailing evidence based on experimental chukar (Alectoris chukar) translocations in the wild indicates that the process of capturing, holding, moving and releasing birds to a novel site induces a state of chronic stress (Dickens et al. 2009a, 2010) that persists for weeks despite no further handling. Grouse translocation success rate might be improved not only by identifying high-quality release sites and minimising translocation stressors, but also by identifying age and sex classes of grouse best able to survive the physiological and ecological challenges of the establishment phase.
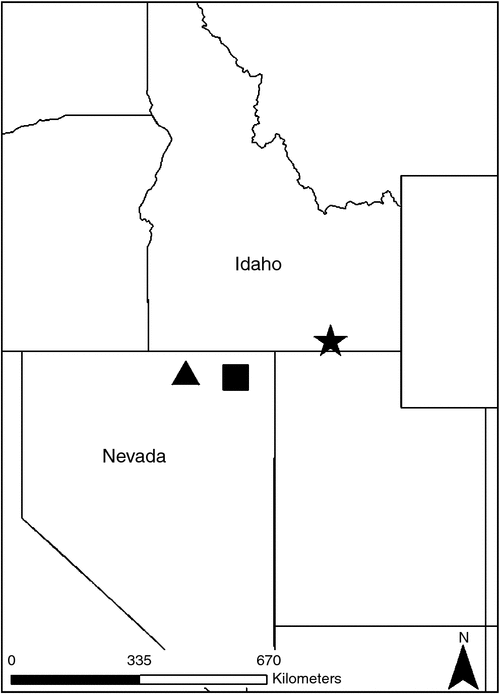
We evaluated survival probability of CSTG translocated from south-eastern Idaho, USA, to north-eastern Nevada, USA (Fig. 1), hypothesising that survival probability is not constant through time following their release, because this non-uniform probability relates to an establishment phase. We also hypothesised that survival probability differs across age and sex classes on the basis of life-history differences between sexes and behavioural differences among ages. If true, then certain age or sex classes of grouse may be superior candidates for future translocations designed to increase survival rate during the establishment phase, i.e. a key period of vulnerability. This information could improve the effectiveness of CSTG restoration to the northern Great Basin of North America.
|
Materials and methods
Use of grouse in the present study, including the capture, handling and monitoring of grouse at source and release sites was approved by the Idaho State University Institutional Animal Care and Use Committee Protocols ARP0302438 and 717.
Study area
We translocated CSTG from south-eastern Idaho, USA, to two sites in north-eastern Nevada, USA (Fig. 1), in the process of conducting two distinct reintroduction projects separated by several years. We used the same source populations of CSTG for both translocations, a robust breeding population described previously (Coates et al. 2006, 2011) in Bannock, Oneida, and Franklin counties of south-eastern Idaho, USA (elevation ~1600 m).
The first release site was located in the Snake Mountains of Elko County, NV, USA (elevation 1600–2500 m), ~49 km south of Jackpot, NV, USA, and 13 km west of US Route 93. Grouse were released between Dry Creek and Dead Bull Creek within the Thousand Springs Creek drainage, a site selected because of its physiographic similarity to the source location as well as the presence of year-round water resources (Coates et al. 2006). Translocations to the Snake Mountains occurred from 1999 to 2002; however, here we evaluate only those grouse translocated during the first 2 years of releases, so as to compare findings with the 2 years of translocations conducted at our second reintroduction site.
The second reintroduction site was located in the Bull Run Basin of the northern Independence Mountains of Elko County, NV, USA (elevation 1800–2500 m), where 92 grouse were translocated from 2013 to 2014. The release area is ~90 km north of Elko, NV, USA, 38 km south of Owyhee, NV, USA, 29 km south-west of Mountain City, NV, USA, and is located along State route NV 11a. The Bull Run Basin release site was selected on the basis of its physiographic similarity, as grass/shrub steppe, to CSTG nesting habitat in south-eastern Idaho (Coates et al. 2011), and was geographically separated from the first release site by a distance of ~100 km. We captured grouse on leks in April via walk-in funnel traps (Schroeder and Braun 1991) and nighttime spot-lighting (Wakkinen et al. 1992). We trapped only at those leks that consisted of ≥15 displaying males. Once captured, we placed each grouse into an individual opaque cardboard box for holding before measuring, marking and examining the grouse. We measured age and sex of grouse and banded grouse with individual aluminum leg bands. We classified grouse as yearling (<1 year old when translocated) or adult (≥1 year old when translocated) on the basis of the wear patterns of primary remiges 9 and 10 (Ammann 1944). We classified sex on the basis of presence (male) or absence (female) of a well developed supraorbital comb, presence (male) or absence (female) of a non-intromittant phallus, and by evaluation of feather pigmentation patterns of the crown and ventral aspect of the rectrices (Johnsgard 2008). Most grouse were outfitted with a necklace-style VHF radio-transmitter (16 g, <3% bodyweight; Advanced Telemetry Systems Inc., Isanti, MN, USA). Radio-transmitters were equipped with mortality signals that pulsed on ≥8 h of grouse inactivity. After processing, grouse were placed into wooden transport boxes with an individual compartment for each bird. To minimise further disturbances, transport boxes also served as release boxes. Once a grouse was placed into a transport box, it was not handled again. Grouse were transported to the release sites where, in the first year, grouse were released via a soft-release method (Rodgers 1992) at dawn of the morning following capture. In subsequent years, grouse were released at nascent leks forming at the release sites. We reduced or ceased using grouse silhouettes and audio playback of lekking calls when living males were present and displaying. From capture to release, grouse were confined for ~24 h.
We tracked 167 (Snake Mountains n = 82; Bull Run Basin n = 85) of 211 released translocated grouse by using hand-held radio receivers (Advanced Telemetry Systems Inc.). Locations for each grouse were obtained every 3–10 days. To minimise disturbance, we circled grouse at a radius of ~50 m to obtain an accurate position and then recorded the UTM coordinates. We retrieved radio-transmitters that emitted the mortality signal within 48 h of first hearing the signal. Mortality was immediately confirmed by conducting intensive searches for any grouse remains or other signs of fatality (e.g. bite marks on the transmitter). In all years, we tracked radio-marked grouse from early April to early September and we evaluated survival for 150 days following their release.
Statistical analyses
We estimated the survival probability of CSTG across the first 150 days following translocation, and evaluated factors that explain variation in post-release survival. To avoid excessive disturbance of newly released grouse, we did not seek to relocate grouse daily. Also, CSTG are capable of long-distance movement and some grouse left the study area, but returned to it days or weeks later. To accommodate these constraints, we divided the 150-day monitoring period into a series of 10-day sampling intervals (n = 15 intervals) and sought to relocate each radio-marked grouse at least once during every interval. We used a known-fate analysis (White and Burnham 1999) with 15 equal 10-day time intervals for each individual grouse, starting on the day that each grouse was released and ending 150 days later. Grouse were released within 24 h of capture from the source population and released successively across days or weeks during the 4 years of translocations. Therefore, calendar dates encompassing the 150-day post-release survival period varied among grouse. Grouse known to be alive throughout an interval or known to have perished during an interval were classified accordingly. Grouse of unknown survival status that were confirmed to be dead in a later time interval were censored from analysis for those intervals for which their status was unknown, but included as mortalities in the interval for which death was confirmed. Once a grouse was confirmed to be dead, it was censored from all subsequent time intervals. We computed parameter estimates using R statistical software (R Core Team 2013) with the RMark package (Laake 2013) that implements MARK (White and Burnham 1999).
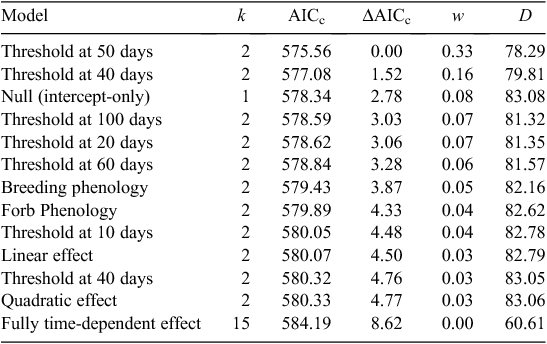
To identify temporal and individual factors that influence survival, we evaluated multiple models that consisted of temporal and individual covariates, using a two-step approach. We first modelled temporal effects on grouse survival (step one, below) and then modelled effects of grouse age, sex, release location, and year (step two, below) on grouse survival within the context of temporal effects. In other words, we sought to assess whether age, sex, location and year attributes explained grouse survival, while accounting for temporal effects. We compared grouse-survival models using the Akaike Information Criterion (Akaike 1973) with second-order bias correction (AICc; Burnham and Anderson 2002) against null models. Models with a smaller AICc score than that of the null model or ≤2 ΔAICc were considered to be explanatory.
Step one – temporal effects
The objective of step one was to evaluate evidence for temporal effects in survival probability during the first 150 days following release. We hypothesised that mortality rate would be greatest immediately following release and that it would progressively diminish, and that change in mortality rate need not be constant across time. Our approach was exploratory and our a priori hypotheses led to the development of 13 temporal survival models. For example, we developed multiple models where the survival estimate was constrained to be constant before and after prospective temporal thresholds. We then evaluated evidence for these prospective temporal thresholds. For example, our first model evaluated a prospective threshold in survival probability at 10 days post-release. The model consisted of two covariates, namely, estimated survival probability during the first 10-day time interval (Days 1–10) and the estimated survival probability for the remaining 14 10-day intervals combined (Days 11–150). Similarly, we modelled a prospective temporal threshold in survival to occur at 50 days following release (model covariates: estimated survival for Intervals 1–5 (Days 1–50) and estimated survival for Intervals 6–15 (Days 51–150)). We then created a set of five additional models that systematically evaluated prospective temporal thresholds in estimated survival rates across the 150-day analysis period. Creating thresholds at two-interval iterations, we evaluated prospective temporal thresholds occurring at 20, 40, 60, 80 and 100 days following release to the translocation site.
We also created temporal models where prospective thresholds in survival probability were based on phenological events. The first phenological model used CSTG breeding behaviour. We subdivided the 150-day post-release period into three new intervals: nesting (Days 1–50), brood-rearing (Days 51–100) and summer (Days 101–150) periods, and constrained the survival probability across each of these intervals. In the second phenological model, we created three intervals based on local forb growth: growing and flowering of large forbs such as mule’s ear (Wyethia mollis), lupine (Lupinus spp.) and arrowleaf balsamroot (Balsamorhiza sagittata) (Days 1–40); greatest forb presence (Days 41–80); and forb desiccation (Days 81–150). The phenological models each employed three groups representing the three phenological time periods, respectively. We also included models with linear and quadratic functions of time as covariates. We then used the best temporal model from step one as a baseline model for step two.
Step two – individual covariate effects
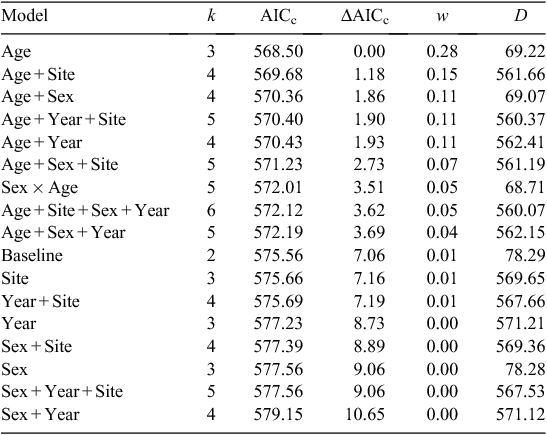
In step two, we evaluated the extent to which covariates categorising individual grouse explained mortality. Our objective was to evaluate evidence for two individual covariates, namely grouse age and sex, a site-level covariate that represented different release sites, and a release-year covariate to account for variation between years. We developed and compared a suite of additive models against the baseline model to assess whether grouse attributes further explained survival probability. We explored the explanatory strength of these covariates on survival, using a set of 17 models. Sixteen models tested all of the additive combinations of the baseline and the four covariates (age, sex, site and year), and one model evaluated an a priori hypothesis of an age × sex interaction on the basis of survival patterns for translocated mountain quail (Oreortyx pictus – Troy et al. 2013).
Results
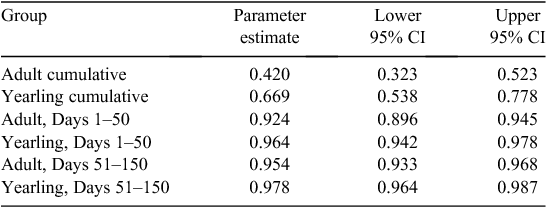
Among the 13 temporal models evaluated in step one, two models had greater support than the null model (intercept-only; Table 1). The model expressing a temporal threshold in daily survival rate at Day 50 (Intervals 1–5 vs 6–15) was the most parsimonious (Table 1). The estimated survival probability differed distinctly across before versus after a temporal threshold of 50 days (Interval 1–5 = 0.941 ± 0.009; Interval 6–15 = 0.965 ± 0.006; Fig. 2). A model expressing a temporal threshold in survival probability at 40 days (Interval 1–4 vs 5–15) also had greater support than the null model, but less support than the 50-day threshold model (Table 1). Grouse attributes further explained estimated survival probabilities. Nine of seventeen models had greater support than the baseline model (50-day temporal covariate). A model that consisted of the additive effect of grouse age had significant explanatory power (Table 2). In other words, the age of released grouse added meaningful explanatory power to survival probabilities, while accounting for variation explained by a temporal threshold occurring at 50 days. Overall, yearling grouse survived the 150-day post-release period at a higher rate than did adult grouse, both before and after the 50-day temporal threshold. The overall probability of a yearling grouse surviving for 150 days following release was 0.669 ± 0.062. For adults, the probability was 0.420 ± 0.052 (Table 3). The estimated probability of a yearling grouse surviving the first 50 days was 0.964 ± 0.009, compared with the adult rate of 0.924 ± 0.012 (Table 3). After the 50-day temporal threshold, the estimated probability for yearling grouse survival increased to 0.978 ± 0.006, compared with an adult increase to 0.954 ± 0.009 (Table 3).
|
|
|
Discussion
Using two distinct translocation sites and 4 years of post-release measurements, we observed a temporal threshold in survival rate occurring at ~50 days following release among CSTG translocated from south-eastern Idaho, USA, to unoccupied historic range in north-eastern Nevada, USA (Fig. 1). Grouse that survived the first 50 days following release subsequently survived at a higher rate during the following 100 days. We also observed an important age effect. Yearling CSTG survived at a higher rate than adult grouse, both before and after the 50-day temporal threshold in overall survival rates (Table 3, Fig. 3). These results indicated a period of elevated vulnerability during the first 50 days following translocation and that translocated yearling grouse are less vulnerable to mortality factors than adult grouse. Adjusting to these aspects of CSTG translocation dynamics could improve the effectiveness of efforts to restore CSTG to historic range within the northern Great Basin of North America.
There are multiple reasons to expect elevated mortality rates following translocation. For example, relocated individuals are unfamiliar with the risks and resources of their new location, increasing their vulnerability to mortality factors such as predation and exposure, while decreasing the efficiency of self-maintenance activities such as foraging. To gain familiarity, individuals probably engage in a relatively increased amount of risky exploratory behaviour (Coates et al. 2006; Dickens et al. 2009b). Also, some individuals attempt to return to their former, distant home range (Dickens et al. 2009b) and perish in the process. Social cohesion within social subsets of the source population is likely lost when disparate individuals are captured and collectively translocated, resulting in grouse individuals at the release site that are unfamiliar with one another. And the translocation process appears to place translocated birds into a physiological state of chronic stress (Dickens et al. 2009a) that diminishes their physiological capacity to manage environmental insults for weeks or months following release (Dickens et al. 2010). Assuming that individuals vary in their inherent capacity to manage the challenges of translocation, the most vulnerable individuals will tend to perish rapidly on release, with such selective ‘culling’ resulting in a temporal threshold in survival.
The 50-day temporal threshold in post-release survival should be viewed as an approximation, not a rigid demarcation. In each year of our study, some individuals could not be located during the first 30 days following release and were later located as mortalities. For example, some grouse rapidly moved long distances from the release area and eventually were located as mortalities. These individuals were censored from analysis during early time intervals when they already may have been dead, and this would cause us to overestimate somewhat the time elapsed to the temporal threshold in survival. Nevertheless, we observed distinguishable phases in survival probability, culminating at 50 days post-release, after which the probability of survival increased substantially. A better understanding of avian recovery time from chronic stress resulting from the translocation process would be especially valuable in understanding the post-release threshold in survival probability. For example, it may require ~50 days for grouse to return to basal levels of corticosterone and regain their endocrinological capacity to mount adaptive stress responses. Also, identifying age or sex classes of grouse that are especially prone to attempt to return to the source location would be helpful insofar as identifying poor candidates for translocation. By 50 days, individuals that remain in the release area may have gained familiarity with their surroundings and with conspecifics.
We do not know the reasons for higher yearling survival, but several explanations are possible. Although age of dispersal for CSTG remains an unsettled question, during spring, when our translocations were conducted, yearling grouse still may be in a dispersal phase of life. If so, yearlings would likely be predisposed to acclimate to a new location and new social interactions, making translocation relatively less disruptive for yearlings than for adults. Moreover, adult prairie grouse may possess a strong affiliation with a lek that continues across years (Dunn and Braun 1985; Hoffman and Thomas 2007). Because leks are absent or small at the release location during the first 2 years of reintroduction, adult grouse may be especially vulnerable if they are searching for a familiar lek or a robust lek. However, yearlings have never bred and have not experienced an alternative to the release location, perhaps reducing any behavioural resistance to prevailing circumstances at the release location. Alternatively, because of their developmental stage, yearlings may be less prone to translocation-induced chronic stress or better able to recover from it.
Grouse age need not be the sole covariate influencing survival. Nine of the grouse covariate models with additive effects were competitive (≤ Baseline AICc), but lacked evidence from the data because the AICc value for those models was not lower than that for a model that consisted of only age and the 50-day temporal effect (Table 2). Furthermore, grouse age was the only covariate supported in all top models, and the model that consisted of the age covariate additive with a 50-day time covariate had the lowest AICc score of any model evaluated. In the present study, grouse age was both parsimonious and explanatory for post-release survival of CSTG.
During our study, the CSTG source population of south-eastern Idaho, USA, was stable and represented 50–75% of the remaining subspecies population (USFWS 2006; Gillette 2014). In south-eastern Idaho, CSTG survival rate of females during the breeding season was 58–62%, with no significant difference in the probability of survival observed between adult and yearlings (Gillette 2014). These survival dynamics in the source population suggest that the greater rate of survival among translocated yearlings than adult grouse (Fig. 3) is associated with the yearling’s capacity to adjust to the translocation event rather than an inherent age difference in breeding-season survival.
We do not discount causes of translocation failure previously identified in the literature, such as dispersal away from the release area (Patterson 1952; Jacobs 1959; Toepfer et al. 1990), low reproductive success by translocated individuals (Toepfer et al. 1990; Coates and Delehanty 2006) and a lack of suitable habitat at the release area (Griffith et al. 1989; Toepfer et al. 1990) and, by itself, the translocation of yearling CSTG may not lead to population establishment. But our results indicated that yearlings may be especially good candidates for translocations intended to restore breeding populations of CSTG.
Our findings indicate that translocated CSTG are especially vulnerable to mortality during the first 50 days following release, probably for many ecological and social reasons as well as from physiological impairment resulting from chronic stress following capture and handling. It also follows that minimising translocation stressors and releasing grouse into high-quality habitat with conspecifics (Coates et al. 2006) are reasonable and intuitive preventative measures. Yearling CSTG grouse appear to be better candidate than adult CSTG for overcoming the challenges of translocation. We base this on higher yearling than adult CSTG survival probability before and after the critical 50-day post-release threshold in survival probability. Wildlife managers should consider targeting yearling CSTG over adult CSTG when capturing grouse during spring lekking and translocating them to a restoration site. Yearling grouse are first-time breeders that are at or near the natural breeding dispersal phase of their life and may not yet have formed a strong lek affiliation. In practice, when females are trapped at the source population, yearling females could be preferentially selected for translocation, whereas adult females are preferentially retained in the source population for local reproduction. To the extent that yearling females make first visits to leks later in the lekking period than do adult females, trapping later during the lekking period would act to increase the proportion of yearlings among captured grouse and be consistent with previous management recommendations that advocated later trapping to increase the probability of capturing females that already have been inseminated (Coates and Delehanty 2006). Trapping later in the lekking period has the added benefit of leaving in place high-quality adult female breeders that have formed a strong affiliation with a lek within the source population but that are relatively poorer candidates for survival if translocated than yearling CSTG. This study may help refine management actions to reflect breeding behaviour of grouse and the apparent capacity of young grouse to adjust to the challenges of being captured and then released into a new location.
Acknowledgements
The Nevada Department of Wildlife (NDOW), the Nevada Chukar Foundation, the Carson Valley Chukar Club, and Nevada Bighorns Unlimited funded this work directly and through grants to the University of Nevada, Reno and Idaho State University. The Idaho Department of Fish and Game (IDFG) provided essential logistic support. We expressly thank M. Wackenhut, Z. Lockyer, B. Gullett, D. Rose, J. Knetter of IDFG and S. Stiver (ret.), S. Espinosa, K. Gray, and M. Jeffress of NDOW for their critical support. We thank the many technicians from both agencies for their tireless on-the-ground assistance. We thank M. Casazza of the US Geological Survey WERC office for integral logistic support and B. Brussee for help with statistical analyses. We also thank K. Andrle and K. Howe who provided important feedback during manuscript preparation. Any use of trade, product, website, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the USA Government.
References
Akaike, H. (1973). Information theory and an extension of the maximum likelihood principle. In ‘Proceedings of the Second International Symposium on Information Theory’. (Eds B. N. Petrov and S. Caski.) pp. 27–281. (Akademiai Kaido: Budapest.)Ammann, G. A. (1944). Determining the age of pinnated and sharp-tailed grouse. The Journal of Wildlife Management 8, 170–171.
| Determining the age of pinnated and sharp-tailed grouse.Crossref | GoogleScholarGoogle Scholar |
Armstrong, D. P., and Seddon, P. J. (2008). Directions in reintroduction biology. Trends in Ecology & Evolution 23, 20–25.
| Directions in reintroduction biology.Crossref | GoogleScholarGoogle Scholar |
Beehler, B. M., and Foster, M. S. (1988). Hotshots, hotspots, and female preference in the organization of lek mating systems. American Naturalist 131, 203–219.
| Hotshots, hotspots, and female preference in the organization of lek mating systems.Crossref | GoogleScholarGoogle Scholar |
Burnham, K. P., and Anderson, D. R. (2002). ‘Model Selection and Multimodel Inference. A Practical Information-theoretic Approach.’ 2nd edn. (Springer: New York.)
Coates, P. S., and Delehanty, D. J. (2006). Effect of capture date on nest-attempt rate of translocated sharp-tailed grouse Tympanuchus phasianellus. Wildlife Biology 12, 277–283.
| Effect of capture date on nest-attempt rate of translocated sharp-tailed grouse Tympanuchus phasianellus.Crossref | GoogleScholarGoogle Scholar |
Coates, P. S., Stiver, S. J., and Delehanty, D. J. (2006). Using sharp-tailed grouse movement patterns to guide release site selection. Wildlife Society Bulletin 34, 1376–1382.
| Using sharp-tailed grouse movement patterns to guide release site selection.Crossref | GoogleScholarGoogle Scholar |
Coates, P. S., Dudko, J. E., Delehanty, D. J., and Casazza, M. L. (2011). Data summary of a Columbian sharp-tailed grouse habitat suitability examination between Idaho and Nevada: United States Geological Survey Data Summary. US Geological Survey, Dixon, CA.
Connelly, J. W., Gratson, M. W., and Reese, K. P. (1998). Sharp-tailed grouse (Tympanuchus phasianellus). Account 354. In ‘The Birds of North America’. (Eds A. Pool and F. Gill.) (Academy of Natural Sciences: Philadelphia, PA and American Ornithologists’ Union: Washington, DC)
Dickens, M. J., Delehanty, D. J., and Romero, L. M. (2009a). Stress and translocations: alterations of the stress physiology of translocated birds. Proceedings. Biological Sciences 276, 2051–2056.
| Stress and translocations: alterations of the stress physiology of translocated birds.Crossref | GoogleScholarGoogle Scholar |
Dickens, M. J., Delehanty, D. J., Reed, J. M., and Romero, L. M. (2009b). What happens to translocated game birds that ‘disappear’? Animal Conservation 12, 418–425.
| What happens to translocated game birds that ‘disappear’?Crossref | GoogleScholarGoogle Scholar |
Dickens, M. J., Delehanty, D. J., and Romero, L. M. (2010). Stress: an inevitable component of animal translocation. Biological Conservation 143, 1329–1341.
| Stress: an inevitable component of animal translocation.Crossref | GoogleScholarGoogle Scholar |
Drummer, T. D., Coraceiii, R. G., and Sjogren, S. J. (2011). Sharp-tailed grouse lek attendance and fidelity in upper Michigan. The Journal of Wildlife Management 75, 311–318.
| Sharp-tailed grouse lek attendance and fidelity in upper Michigan.Crossref | GoogleScholarGoogle Scholar |
Dunn, P. O., and Braun, C. E. (1985). Natal dispersal and lek fidelity of sage grouse. The Auk 102, 621–627.
Giesen, K. M., and Connelly, J. W. (1993). Guidelines for management of Columbian sharp-tailed grouse habitats. Wildlife Society Bulletin 21, 325–333.
Gillette, G. L. (2014). Ecology and management of Columbian sharp-tailed grouse in southern Idaho: evaluating infrared technology, the conservation reserve program, statistical population reconstruction, and the olfactory concealment theory. Ph.D. Dissertation, University of Idaho, Moscow, ID.
Gregg, L. E., and Niemuth, N. D. (2000). History, status, and management of sharp-tailed grouse in Wisconsin. Passenger Pigeon 62, 159–164.
Griffith, B., Scott, J. M., Carpenter, J. W., and Reed, C. (1989). Translocation as a species conservation tool: status and strategy. Science 245, 477–480.
| Translocation as a species conservation tool: status and strategy.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cvitlKqsA%3D%3D&md5=de6fb9f4f9b24852ce51ebaf19069fcaCAS | 17750257PubMed |
Hoffman, R. W., and Thomas, A. E. (2007). ‘Columbian Sharp-tailed Grouse (Tympanuchus phasianellus columbianus): a Technical Conservation Assessment.’ (USDA Forest Service, Rocky Mountain Region: Golden, CO.)
International Association of Fish and Wildlife Agencies (IAFWA) (2002). ‘Economic Importance of Hunting in America.’ (IAFWA: Washington, DC.) Available at http://www.fishwildlife.org/files/Hunting_Economic_Impact.pdf [Accessed November 2014]
Jacobs, K. F. (1959). ‘Restoration of the Greater Prairie Chicken.’ (Oklahoma Department of Wildlife Conservation, Division of Federal Aid: Oklahoma City, OK.)
Johnsgard, P. A. (2008). ‘Grouse and Quails of North America.’ (University of Nebraska-Lincoln: Lincoln, NE.)
Laake, J. L. (2013). RMark: an R interface for analysis of capture–recapture data with MARK. AFSC processed report 2013-01, NOAA, Seattle, WA.
Letty, J., Marchandeau, S., and Aubineau, J. (2000). Improving translocation success: an experimental study of anti-stress treatment and release method for wild rabbits. Animal Conservation 3, 211–219.
| Improving translocation success: an experimental study of anti-stress treatment and release method for wild rabbits.Crossref | GoogleScholarGoogle Scholar |
Miller, G. C., and Graul, W. D. (1980). Status of sharp-tailed grouse in North America. In ‘Proceedings of the Prairie Grouse Symposium’. pp. 18–28. (Oklahoma State University: Stillwater, OK.)
Nevada Department of Wildlife (NDOW) (2008). ‘Nevada Upland Game Species Management Plan. Game Division Internal Document.’ (Nevada Department of Wildlife Headquarters: Reno, NV.)
Patterson, R. L. (1952). ‘The Sage Grouse of Wyoming.’ (Sage Books Inc.: Denver, CO.)
R Core Team (2013). ‘R: a Language and Environment for Statistical Computing.’ (R Foundation for Statistical Computing: Vienna.)
Reese, K. P., and Connelly, J. W. (1997). Translocations of sage grouse Centrocercus urophasianus in North America. Wildlife Biology 3, 235–241.
Rodgers, R. D. (1992). A technique for establishing sharp-tailed grouse in unoccupied range. Wildlife Society Bulletin 20, 101–106.
Schroeder, M. A., and Braun, C. E. (1991). Walk-in traps for capturing greater prairie-chickens on leks. Journal of Field Ornithology 62, 378–385.
Selous, E. (1906–1907). Observations tending to throw light on the question of sexual selection in birds, including a day-to-day diary on the breeding habits of the ruff (Machetes pugnax). Zoologist 4, 201–381.
Snyder, J. W., Pelren, E. C., and Crawford, J. A. (1999). Translocation histories of prairie grouse in the United States. Wildlife Society Bulletin 27, 428–432.
Storch, I. (2000). Status survey and conservation action plan 2000–2004: grouse. IUCN, Gland, Switzerland and World Pheasant Association, Reading, UK.
Storch I. 2007 Conservation status of grouse worldwide: and update. Wildlife Biology 13 Suppl. 1 5 12
Toepfer, J. E. (1988). Ecology of the prairie-chicken as related to reintroductions. Ph.D. Dissertation, Montana State University, Bozeman, MT.
Toepfer, J. E., Eng, R. L., and Anderson, R. K. (1990). Translocating prairie grouse: what have we learned? Transactions of the North American Wildlife and Natural Resources Conference 55, 569–579.
Troy, R. J., Coates, P. S., Connelly, J. W., Gillette, G., and Delehanty, D. J. (2013). Survival of mountain quail translocated from two distinct source populations. The Journal of Wildlife Management 77, 1031–1037.
| Survival of mountain quail translocated from two distinct source populations.Crossref | GoogleScholarGoogle Scholar |
United States Fish and Wildlife Service (USFWS) (2006). Endangered and threatened wildlife and plants: 90-day finding on a petition to list the Columbian sharp-tailed grouse as threatened. FR 71(224), pp. 67 318–67 325.
Wakkinen, W. L., Reese, K. P., Connelly, J. W., and Fischer, R. A. (1992). An improved spotlighting technique for capturing sage grouse. Wildlife Society Bulletin 20, 425–426.
White, G. C., and Burnham, K. P. (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S138.
| Program MARK: survival estimation from populations of marked animals.Crossref | GoogleScholarGoogle Scholar |




