Reversed sexual dimorphism and altered prey base: the effect on sooty owl (Tyto tenebricosa tenebricosa) diet
Rohan J. Bilney A , John G. White A and Raylene Cooke A BA School of Life and Environmental Sciences, Deakin University, 221 Burwood Highway, Burwood, Vic. 3125, Australia.
B Corresponding author. Email: raylene.cooke@deakin.edu.au
Australian Journal of Zoology 59(5) 302-311 https://doi.org/10.1071/ZO11101
Submitted: 15 December 2011 Accepted: 13 April 2012 Published: 11 May 2012
Abstract
The ecology and function of many Australian predators has likely been disrupted following major changes in prey base due to declines in distribution and abundance of small mammals following European settlement. This study investigated various aspects of the dietary ecology of sooty owls (Tyto tenebricosa tenebricosa), including sexual variation as they potentially exhibit the greatest degree of reversed sexual dimorphism of any owl species worldwide. Sooty owls are highly opportunistic predators of non-volant small mammals, consuming most species known to exist in the region, so their diet fluctuates seasonally and spatially due to varying prey availability, and is particularly influenced by the breeding cycles of prey. Significant intersexual dietary differences existed with female sooty owls predominantly consuming much larger prey items than males, with dietary overlap at 0.62. The current reliance on relatively few native mammalian species is of conservation concern, especially when mammal declines are unlikely to have ceased as many threatening processes still persist in the landscape. Sooty owl conservation appears inextricably linked with small mammal conservation. Conservation efforts should be focussed towards improving prey densities and prey habitat, primarily by implementing control programs for feral predators and preventing the loss of hollow-bearing trees throughout the landscape.
Additional keywords: Australia, biomass, hollow dependant, predator, seasonal.
Introduction
Predators can perform important ecosystem functions by influencing and shaping prey communities through top-down processes (McLaren and Peterson 1994; Henke and Bryant 1999; Schmitz et al. 2000; Terborgh et al. 2001; Duffy 2002). Bottom-up processes can, however, significantly influence predator ecology (Hunter and Price 1992; Suarez and Case 2002; Palkovacs and Post 2008), with modification to the prey base impacting upon ecological aspects, including home-range size, habitat usage, breeding success, population density and distribution (Newton 1979, 2002; Carey et al. 1992; Ward et al. 1998; Sergio et al. 2004).
In Australia, many small mammal species have suffered extensive declines in distribution and abundance since European settlement (Burbidge and McKenzie 1989; Short and Smith 1994; Burbidge et al. 2008; Bilney et al. 2010), resulting in significant ecological impacts on many native predators (Peake et al. 1993; Bilney et al. 2006). It is therefore essential to understand how predators have adapted to changes in their prey base.
One predator for which there is knowledge of prehistoric diet, and diet change since European settlement, is the sooty owl (Tyto tenebricosa tenebricosa) (Morris et al. 1997; Bilney et al. 2006; Hollands 2008). The sooty owl predominantly consumes non-volant mammalian prey, incorporating a wide-range of arboreal and terrestrial species ranging from 10 to 1300 g in body weight (Kavanagh 2002a; Bilney et al. 2011a). Although diet is the most examined ecological attribute of the sooty owl, most dietary studies have focussed on single or few localities with limited collection of prey items (Smith 1984; Loyn et al. 1986; Lundie-Jenkins 1993; Holmes 1994; Hollands 2008). Few studies have developed a broader understanding of their feeding ecology, such as temporal and geographical variation (Kavanagh 1997, 2002a; Bilney et al. 2006, 2007). There are considerable knowledge gaps associated with the dietary ecology of this species, which limit the ability to develop holistic conservation strategies.
The sooty owl is a naturally uncommon, hollow-dependent, strongly territorial, threatened species that primarily occupies large home-ranges (500–3500 ha) in tall wet forests in south-eastern Australia (Kavanagh 1997; Higgins 1999; Bilney et al. 2011b). It potentially exhibits the greatest degree of reversed sexual dimorphism of any owl species in the world (Mooney 1993; Krüger 2005), with females weighing 1000–1200 g, and males being significantly smaller, weighing 550–700 g (Higgins 1999; Hollands 2008). With such pronounced reversed sexual dimorphism, it is likely that intersexual niche partitioning occurs, especially in relation to diet (Selander 1966; Storer 1966; Earhart and Johnson 1970). Sexual differences relating to home-range size and roosting preferences have also been detected in the sooty owl (Bilney et al. 2011a, 2011b). It is therefore important for long-term sooty owl conservation that intersexual differences are understood, so that conservation strategies cater for each sex appropriately.
As changes in prey availability are likely to have severely influenced ecological attributes of the sooty owl, it is essential that ecological attributes such as dietary ecology are well understood, not only to better understand the species, but also to improve conservation strategies for the species and its prey. The aim of this study was to examine a range of dietary characteristics of the sooty owl with specific aims being to determine: (1) whether there are intersexual dietary differences, (2) the extent of any geographic and seasonal variation in the diet, and (3) the influence of prey availability on the diet.
Materials and methods
Study area
The study area was 80 km long, comprising contiguous foothill and coastal forests less than 500 m in elevation, ranging from the Mitchell River in the west, through to the Snowy River in East Gippsland, Victoria, Australia. The western edge of the study area was ~200 km due east of Melbourne, Victoria. Dietary remains of the sooty owl were collected from 45 sites, comprising 30 foothill forest sites and 15 coastal forest sites.
Locating roosting sites and collection of pellets
Sooty owls are known to have a strong affinity for rainforest (Higgins 1999), which generally occurs in geographically restricted and isolated pockets within the study region (Peel 1999). Maps of Ecological Vegetation Classes were used to locate patches of rainforest, while sheltered gorges and cliffs potentially containing cave/rock roosting sites were located using 1 : 50 000 contour maps. Once potential sites were identified each site was surveyed by walking the entire stretch of the potential habitat searching for whitewash (faeces), regurgitated pellets and roosting birds.
Prey remains of the sooty owl were determined through identification of skeletal remains within regurgitated pellets. Most regurgitated pellets collected during this study were collected under foliage or cave/rock roosting sites between September 2002 and March 2008. Individual pellets were collected separately, however, on occasions where bones could not be allocated to a single pellet, all prey remains were collected together. All pellets located within one area (usually from the same gully) were regarded as belonging to a single site.
To determine seasonal and sexual variations in the diet, regular pellet collection was undertaken at each site. Prey items used in this research came from regularly used, long-term roosting sites that were inspected during each survey. From each site, all prey items from a given season were combined for seasonal dietary analysis, as sample sizes were often too small to compare annual seasonal variation. At 14 sites, sooty owls of identifiable sex were regularly observed at a particular roosting site where they roosted alone, therefore allowing regurgitated pellets to be assigned to each sex with confidence. The pellets from females were collected from cave and foliage roosting sites in the non-breeding period, at a time when the female was likely to be capturing predominantly her own prey.
Analysis of pellets
Regurgitated pellets were dried before their contents were analysed. Once dried, pellets were dissected and all major skeletal elements (femora, humeri, mandibles and skulls) were removed to allow for identification of prey remains and for calculating the minimum number of individuals. Identification of skeletal material was undertaken by comparing to reference material held at Museum Victoria and the Australian National Wildlife Collection. Cranial material was used to distinguish between agile antechinus (Antechinus agilis) and white-footed dunnart (Sminthopsis leucopus) and between bush rats (Rattus fuscipes) and black rats (Rattus rattus), while cranial material and humeri were used to distinguish between bandicoot species. All Trichosurus remains were identified to genus. Bird and invertebrate species were not identified in this study. Determining the minimum number of individuals involved counting the most numerous left or right skeletal element of each species contained within a particular sample unit (e.g. single pellet, bag of pellets/bones).
Calculating prey size and biomass
The body weights of individual prey items detected in regurgitated pellets were estimated on the basis of regression analysis equations between body weight and a particular skeletal element length (Table 1). The regression equations were derived from measurements (using calipers) to the nearest 0.01 mm of the femur, humerus and mandible from skeletal specimens with known body weight held at Museum Victoria, the Australian National Wildlife Collection and the Australian Museum. The femur and humerus were measured at maximum length from the proximal to the distal ends, while the mandibles were measured from the condyloid process to the tip of the incisors. Regression equations were calculated only for the main dietary prey species greater than 100 g in adult body weight. Measurements of skeletal remains were undertaken only from a subset of regurgitated pellets.
Estimating percentage prey biomass involved determining the average body weight of each species detected from the sooty owl diet, and multiplying this figure by the average frequency of consumption for that species. The total biomass for each species was calculated and converted to a percentage of the diet. As regression equations were not calculated for several species, the average body size of those species was estimated in order to calculate biomass. For species with adult body weights less than 50 g, including dusky antechinus (Antechinus swainsonii), the average body weight was calculated as two-thirds the adult body weight (based on average adult body weights given by Menkhorst and Knight (2001)), and half adult body weight for the yellow-bellied glider (Petaurus australis), while species such as rabbits (Oryctolagus cuniculus) and Trichosurus spp. were assigned arbitrary estimates of 500 g. Insufficient museum specimens representing a range of body weights were available for these three mammal species, so regression equations could not be determined. The body weights of birds were arbitrarily estimated to be 100 g.
Mammal surveys
Live trapping and spotlight surveys were undertaken to determine small mammal presence and relative abundance in the two main study regions, i.e. the foothill forest and coastal forest. Trapping surveys involved using Elliot traps and cage traps baited with a standard mixture of rolled oats, honey and peanut butter, with linseed oil. Traps were set during early winter 2006 and 2007 within areas where a large number of sooty owl pellets had been collected. A total of 15 trapping transects were undertaken in both the coastal and foothill forests, with 28 traps (25 Elliot traps, and 3 cage traps) in each transect. Each trap was separated by 15 m. Each mammal trap was set for five consecutive nights and checked each morning. Each small mammal captured was ear clipped to calculate abundance. Trap success was estimated from the number of new individuals captured from the total trap-nights per site.
Spotlighting involved walking and/or driving to survey for arboreal mammals. Surveys commenced no earlier than 1 h after sunset, and were undertaken for 1 h per night per site. Estimating the area surveyed by spotlighting involved estimating an average distance that was visible from the transect (e.g. 50 m), multiplied by the distance traversed and converted into hectares. The average number of arboreal mammals observed was recorded per hectare. All surveys were conducted less than 2 km from sites where sooty owl pellets had been collected.
Statistical analysis
Multidimensional scaling (MDS), SIMPER and ANOSIM were used to compare differences in seasonal, sexual and geographical diets based on percentage composition. Data used for these analyses were computed in the statistical software package Primer ver. 6.0 (Clarke and Warwick 1994). Additional statistical procedures including Chi-square tests, Students t-tests, bivariate analysis, and Mann–Whitney U tests were conducted using SPSS (ver. 16). Further to the MDS analysis, bivariate analysis was used to determine the prey species that contributed to significant dietary differences between samples. The results of this analysis are shown on the axis of the MDS plot, revealing the direction of the differences.
Dietary breadth was calculated using the formula provided by Levins (1968). Dietary overlap between the sexes of sooty owls was calculated for both relative frequency of prey items and biomass using the formula of Pianka (1973). Values of this index range between 0 (no overlap) and 1 (complete overlap).
Results
Total diet
A total of 5300 prey items was identified from recently regurgitated pellets and skeletal material collected below sooty owl roosts from 45 sites. Mammals represented 96.6% of total prey remains, while birds and invertebrates accounted for 3.35% and 0.05% respectively. A total of 18 mammalian species were detected, of which 14 species were native and four introduced (Table 2). Only five species were considered major dietary items, constituting 90.8% of the total diet. These five species were the sugar glider (Petaurus breviceps), the greater glider (Petauroides volans), the bush rat, the common ringtail possum (Pseudocheirus peregrinus) and the agile antechinus. The additional 13 mammalian species collectively represented 5.8% of the total diet. True arboreal mammals dominated the diet at every site where dietary remains were collected, with sugar gliders being the most frequently consumed species at 27 of 32 sites where more than 25 prey items were collected (Table 2).

|
Sexual variation
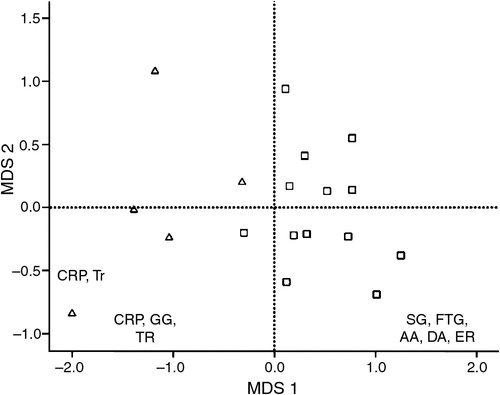
Of the 5300 prey items identified, 2003 could be allocated to a specific sex of the sooty owl, these being 1722 prey items from male sooty owls across 13 sites and 281 prey items from females across five sites. In terms of the relative frequency of prey items consumed, there were significant differences between the diets of male and female sooty owls (ANOSIM), with three of 10 000 random permutations exceeding the global R statistic (0.741, P < 0.001) (Fig. 1). Three species, the sugar glider, greater glider and common ringtail possum, were the main prey species in the diet of the female, accounting for 91.9% of the similarity, while these three species and the bush rat accounted for 92.5% of the similarity in the diet of the male (Table 3). Sugar gliders were the main prey item of the male, while greater gliders were the main prey item of the female, therefore these prey species were also the greatest contributing species to the dissimilarity between the diets. Overall, dietary overlap between the total relative frequency of the diets of males and females was 0.62, indicating some degree of difference between the sexes. Dietary breadth was not significantly different (t = 0.708, d.f. = 16, P = 0.489) between females (mean ± s.d. = 2.9 ± 0.6) and males (2.7 ± 0.7).
|

|
The diets of the male and female were projected on an MDS plot, and variables were then correlated against the main MDS axes (Fig. 1). Variables that were positively associated with MDS1 were mostly prey species with adult body size less than 200 g (r = 0.964, P < 0.001), such as the sugar glider, bush rat and agile antechinus, while variables negatively associated with MDS1 included prey species with adult body weights greater than 200 g (r = –0.961, P < 0.001), including the greater glider, common ringtail possum and Trichosurus spp., indicating that body size of prey was related to dietary differences.
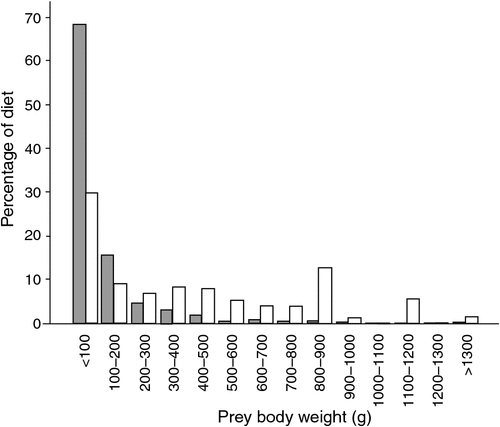
There were significant differences between female and male sooty owls regarding prey size selection of the same species (Table 4) and overall prey selection (χ2 = 158.325, d.f. = 11, P < 0.001) (Fig. 2). Female sooty owls generally consumed much larger prey, readily up to and over 1000 g, while males mainly consumed prey less than 500 g.

|
|
Due to the significant differences in the frequency of consumption rates, along with prey size differences, dietary biomass varied considerably between males and females (χ2 = 64.93, d.f. = 4, P < 0.001). For females, the main dietary items that contributed to biomass included greater gliders (58%), common ringtail possums (34%), sugar gliders (4%) and bush rats (2%). For males, the main dietary species contributing to biomass included sugar gliders (37%), greater gliders (24%), common ringtail possums (21%) and bush rats (9%). Biomass dietary overlap between males and females was 0.64. Greater gliders and sugar gliders were again the main drivers of dissimilarity between the male and female diets regarding biomass.
Geographical variation
A total of 2849 prey items was detected from 30 sites distributed throughout foothill forests, while a further 2451 prey items were detected from 15 sites within coastal forests (Table 2). No mammal species was exclusively consumed in the foothill forests, while six species were detected only from coastal forests, these being the southern brown bandicoot (Isoodon obesulus), long-nosed bandicoot (Perameles nasuta), yellow-bellied glider, eastern bent-winged bat (Miniopterus schreibersii), house mouse (Mus musculus) and an unidentified placental mammal.
For comparisons between the sooty owl diet from foothill forest and coastal forest, only the diet of the male sooty owl was used, to eliminate trends that may be distorted by sexual differences. A total of 887 prey items from six sites in foothill forest was compared with 835 prey items identified from seven sites in coastal forest. There were significant differences in the male sooty owl diet between the foothill forests and coastal forests (ANOSIM), with seven of 10 000 random permutations exceeding the global R statistic (0.327, P = 0.004). Three mammal species, the sugar glider, greater glider and bush rat accounted for 93.2% of the similarity between foothill forest sites, while these three species plus the common ringtail possum and agile antechinus accounted for 94.4% of the similarity between coastal forest sites. The main species contributing to the dissimilarity between foothill and coastal forests were the sugar glider and greater glider, which were detected in higher quantities in foothill forests, and the common ringtail possum, bush rat, agile antechinus and dusky antechinus, which were all detected at higher quantities in coastal forests.
Average mammal species richness in the diet per site was significantly higher in coastal forests compared with foothill forests (t = 2.24, d.f. = 11, P = 0.047), averaging 8.43 (±0.98) and 6.83 (±1.83) respectively. Dietary breadth was therefore also significantly different between coastal forests and foothill forests (t = 2.762, d.f. = 11, P = 0.018), and averaged 3.06 (±0.67) and 2.18 (±0.38) respectively. True arboreal mammals were detected in significantly higher quantities in foothill forests than in coastal forests (t = 5.124, d.f. = 11, P < 0.001) while both scansorial mammals (t = 2.71, d.f. = 11, P = 0.020) and true terrestrial mammals (t = 2.456, d.f. = 11, P = 0.032) were detected in significantly higher quantities in coastal forests.
Seasonal diet
Detailed analysis of seasonal variation was conducted for only four sites from coastal forests, where prey remains were predominantly male, with no known female dietary remains included. There were significant differences between the sooty owl diet from each season (ANOSIM) with none of the 10 000 random permutations exceeding the global R statistic (0.483, P = 0.001). Consumption rates of all major dietary items fluctuated seasonally, and dietary composition varied significantly between most seasons except between January–March and April–June, and between January–March and October–December (Table 5). Major changes in diet include high rates of consumption of greater gliders during October–December, which declined over the rest of the year. Consumption rates of dusky antechinus increased dramatically in July–September and remained low throughout the rest of the year. Bush rat consumption was highest during January–March, and lowest during the cooler months of April–September.
Mammal surveys
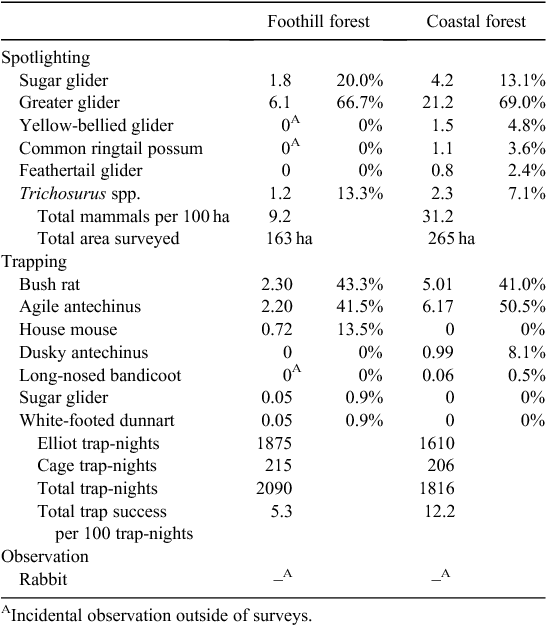
A total of 13 (Trichosurus spp. combined) non-volant mammal species were either trapped or observed during nocturnal surveys in this study (Table 6). All species detected by conventional survey techniques were also detected in the sooty owl diet, while three small mammal species, the southern brown bandicoot, eastern pygmy possum (Cercartetus nanus), and black rat were detected in the sooty owl diet but were undetected by conventional survey techniques. At particular localities however, some species (yellow-bellied glider, white-footed dunnart, house mouse, Trichosurus spp.) were detected (all at low densities) by conventional survey techniques but were undetected in the sooty owl diet.
|
Spotlight surveys did not detect a significant difference in the mammal community composition between foothill forests and coastal forests (χ2 = 3.000, d.f. = 5, P = 0.700). However, there were significant differences detected by trapping (χ2 = 44.856, d.f. = 6, P < 0.001). Overall, all major prey items were detected at much higher abundances in the coastal forests than in the foothill forests (Table 6). However, statistically significant differences were detected only for the agile antechinus (t = 3.3631, d.f. = 28, P = 0.001), bush rat (t = 2.616, d.f. = 28, P = 0.014) and greater glider (U = 224, P = 0.015).
Despite apparent difference in detection rate of sugar gliders between coastal and foothill forests, they were not significantly different (U = 300, P = 0.185), due to their overall low detection rate. The white-footed dunnart and house mouse were exclusively detected in the foothill forests, while dusky antechinus, long-nosed bandicoots and feathertail gliders (Acrobates pygmaeus) were exclusively detected in coastal forests from surveys. Although the yellow-bellied glider, common ringtail possum and long-nosed bandicoots were not recorded during conventional surveys from the foothill forests, they were incidentally recorded at low abundances in these areas outside of surveys. Overall, the most common small mammal species detected by survey techniques were also the main dietary items of sooty owls throughout the study region.
Discussion
The sooty owl is a highly opportunistic predator of small mammals, capable of incorporating virtually all non-volant small mammal species less than 1300 g that exist in an area into its diet. Its diet, therefore, varies significantly spatially and temporally (Kavanagh 1997; Bilney et al. 2006) due to differing prey availability and the susceptibility of the prey to predation (Braithwaite et al. 1988; Bennett et al. 1991; Catling and Burt 1995; Kavanagh et al. 1995; Menkhorst 1995). This was highlighted by the marked seasonal dietary changes that often corresponded to breeding cycles of some main prey species, indicating increased susceptibility to predation during these times. This included heightened consumption rates during mating (suggesting behavioural susceptibility to predation: agile antechinus and bush rats), after mating (associated only with male die-off in agile antechinus and dusky antechinus) and of juveniles, especially when they first become available (greater gliders, bush rats, long-nosed bandicoots) (Tyndale-Biscoe and Smith 1969; Robinson 1987; Lazenby-Cohen and Cockburn 1993; Menkhorst 1995). There was a strong link with high consumption rates of substandard individuals, especially juveniles and unhealthy or dying individuals, which has been observed with other predators (e.g. Temple 1987).
Overall, the sooty owl’s dietary flexibility provides great resilience to changing prey availability, and, combined with intersexual dietary differences, offers substantial ecological advantages to a pair. This enables a pair to exploit available resources more comprehensively while minimising intersexual competition (Selander 1966; Earhart and Johnson 1970; Newton 1979). Despite whatever factors are involved in the evolutionary development of reversed sexual dimorphism (e.g. Amadon 1975; Snyder and Wiley 1976; Andersson and Norberg 1981; Wheeler and Greenwood 1983; Lundberg 1986; Mueller 1986; Krüger 2005), perhaps the most crucial issue arising from studies investigating intersexual niche utilisation differences in raptors is often overlooked, and that relates to the conservation implications arising from the niche utilisation differences. Understanding intersexual niche differences and the differing resource demands and requirements should enable the development of a more comprehensive conservation strategy for the species, rather than focusing on the species as a single entity.
Although it is of conservation concern that an opportunistic predator is currently consuming predominantly only two prey species (sugar glider and greater glider), what is of further concern is that these two prey species are hollow dependent. The main threatening processes to hollow-dependent mammals is the loss of hollow-bearing trees, which is exacerbated by land management practices such as clear-fell logging and frequent fire regimes (Lunney 1989; Catling 1991; Kavanagh et al. 1995; Lindenmayer et al. 1997; Kavanagh and Webb 1998; Kavanagh 2000; Gibbons and Lindenmayer 2002; Garnett et al. 2003). The density of numerous arboreal mammals is typically limited by the number of hollow-bearing trees in the landscape (Smith and Lindenmayer 1988; Lindenmayer et al. 1990), often with the lowest populations remaining in heavily logged areas (Kavanagh and Bamkin 1995; Kavanagh and Webb 1998; Kavanagh 2000). It is therefore highly likely that populations of hollow-dependent mammals will decline significantly in wood-production forests over the long term (Lindenmayer et al. 1997).
The results of this study therefore suggest that clear-fell logging is likely to be detrimental to populations of sooty owls where hollow-dependent mammals dominate the small mammal community. Previous studies that have investigated the impacts of logging regimes on large forest owls have focussed in regions where non-hollow-dependent small mammals species such as common ringtail possums and bush rats are abundant (Kavanagh and Bamkin 1995; Kavanagh et al. 1995; Kavanagh 2002b) and therefore dominate the sooty owl diet (Kavanagh 2002a). Although studies have shown that sooty owls can be considerably resilient to logging practices (Kavanagh 1997; DEC 2006) it appears that this pattern is dictated by high prey densities. It is therefore paramount that future research investigates the impact that land management practices such as logging and fire regimes have on owl populations, especially where hollow-dependant mammals dominate the mammal community.
Although the sooty owl has shown considerable resilience to changes in prey base following European settlement, its diet is constrained by the limited number of species that currently remain common and widespread (Bilney et al. 2006, 2010). Sooty owls, like many other native predators, may now be close to a tipping point, due to the limited alternative prey available and may not be able to tolerate further mammal declines. It is likely that the maintenance or enhancement of a high diversity of small mammals is the most important conservation issue for sooty owls, and it is likely that the resilience of sooty owls to particular land management practices and stochastic events will be primarily dictated by prey availability.
Predation by feral predators is a major factor responsible for suppressing small mammal population densities (Sinclair et al. 1998; Kinnear et al. 2002; Dexter and Murray 2009). Long-term predator-control programs should greatly benefit sooty owls and other native predators by enhancing the diversity of critical-weight-range terrestrial mammals (Sinclair et al. 1998; Dexter and Murray 2009). This should also provide suitable conditions for the reintroduction of locally extinct species, many of which historically were important prey items for sooty owls (Bilney et al. 2006, 2010). Increasing prey densities should also alleviate both intersexual and interspecific competitive interactions for food (Bilney et al. 2011a).
It is important that all land management practices ensure that healthy mammal populations are retained, and ultimately enhanced. There should be greater efforts towards retaining high densities of hollow-bearing trees in the landscape, and it also should be ensured that a heterogeneous age structure and species diversity is maintained within commercial forests (Scotts 1991; Gibbons and Lindenmayer 2002). Fire regimes should be compatible with ecological requirements (Catling 1991; SAC 2001, 2003; Clarke 2008). These programs will not only improve long-term small mammal conservation, but also native predator conservation, while also improving ecosystem function and health in the process.
Acknowledgements
Roger and Carolyn Bilney are thanked for their endless support during this study. We are particularly grateful to Tony Mitchell for collecting a large sample of sooty owl pellets from Nargun Cave which were analysed in this study. Particular thanks to Bob, Julie and Emily Hollingsworth for considerable fieldwork assistance. Thanks also to Frank Bird, John Burns, Cliff Cunningham, Rob Grant, Fiona Hogan, David Hollands, Felicity L’Hotellier, Clare McCutcheon, and Ken Pickering for help in the field. Thanks to Les and Darrilyn Goldsmith and Josie Jackobi, for allowing access onto their property. Funding was provided by Holsworth Wildlife Research Endowment, Stuart Leslie Bird Research Fund, Museum Victoria 1854 Student Scholarship and Deakin University. Research was undertaken using Department of Sustainability and Environment research permits 10003725 and 10004023, with ethics approval A23/2006 from Deakin University Animal Welfare Committee.
References
Amadon, D. (1975). Why are female birds of prey larger than males? The Journal of Raptor Research 9, 1–11.Andersson, M., and Norberg, R. A. (1981). Evolution of reversed sexual size dimorphism and role partitioning among predatory birds, with a size scaling of flight performance. Biological Journal of the Linnean Society. Linnean Society of London 15, 105–130.
| Evolution of reversed sexual size dimorphism and role partitioning among predatory birds, with a size scaling of flight performance.Crossref | GoogleScholarGoogle Scholar |
Bennett, A. F., Lumsden, L. F., Alexander, J. S. A., Duncan, P. E., Johnson, P. G., Robertson, P., and Silveira, C. E. (1991). Habitat use by arboreal mammals along an environmental gradient in north-eastern Victoria. Wildlife Research 18, 125–146.
| Habitat use by arboreal mammals along an environmental gradient in north-eastern Victoria.Crossref | GoogleScholarGoogle Scholar |
Bilney, R. J., Cooke, R., and White, J. (2006). Change in the diet of sooty owls (Tyto tenebricosa) since European settlement: from terrestrial to arboreal prey and increased overlap with powerful owls. Wildlife Research 33, 17–24.
| Change in the diet of sooty owls (Tyto tenebricosa) since European settlement: from terrestrial to arboreal prey and increased overlap with powerful owls.Crossref | GoogleScholarGoogle Scholar |
Bilney, R. J., Kavanagh, R. P., and Harris, J. M. (2007). Further observations on the diet of the sooty owl (Tyto tenebricosa) in the Royal National Park, Sydney. Australian Field Ornithology 24, 64–69.
Bilney, R. J., Cooke, R., and White, J. G. (2010). Underestimated and severe: small mammal decline from the forests of south-eastern Australia since European settlement, as revealed by a top order predator. Biological Conservation 143, 52–59.
| Underestimated and severe: small mammal decline from the forests of south-eastern Australia since European settlement, as revealed by a top order predator.Crossref | GoogleScholarGoogle Scholar |
Bilney, R. J., Cooke, R., and White, J. G. (2011a). Potential competition between two top-order predators following a dramatic contraction in the diversity of their prey base. Animal Biology 61, 29–47.
| Potential competition between two top-order predators following a dramatic contraction in the diversity of their prey base.Crossref | GoogleScholarGoogle Scholar |
Bilney, R. J., White, J. G., L’Hotellier, F. A., and Cooke, R. (2011b). Spatial ecology of sooty owls in south-eastern Australian coastal forests: implications for forest management and reserve design. Emu 111, 92–99.
| Spatial ecology of sooty owls in south-eastern Australian coastal forests: implications for forest management and reserve design.Crossref | GoogleScholarGoogle Scholar |
Braithwaite, L. W., Binns, D. L., and Nowlan, R. D. (1988). The distribution of arboreal marsupials in relation to Eucalypt forest types in the Eden (N.S.W.) woodchip concession area. Australian Wildlife Research 15, 363–373.
| The distribution of arboreal marsupials in relation to Eucalypt forest types in the Eden (N.S.W.) woodchip concession area.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. A., and McKenzie, N. L. (1989). Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications. Biological Conservation 50, 143–198.
| Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. A., McKenzie, N. L., Brennan, K. E. C., Woinarski, J. C. Z., Dickman, C. R., Baynes, A., Gordon, G., Menkhorst, P. W., and Robinson, A. C. (2008). Conservation status and biogeography of Australia’s terrestrial mammals. Australian Journal of Zoology 56, 411–422.
| Conservation status and biogeography of Australia’s terrestrial mammals.Crossref | GoogleScholarGoogle Scholar |
Carey, A. B., Horton, S. P., and Biswell, B. L. (1992). Northern spotted owls: influence of prey base and landscape character. Ecological Monographs 62, 223–250.
| Northern spotted owls: influence of prey base and landscape character.Crossref | GoogleScholarGoogle Scholar |
Catling, P. C. (1991). Ecological effects of prescribed burning practices on mammals of south eastern Australia. In ‘Conservation of Australia’s Forest Fauna’. (Ed. D. Lunney.) pp. 353–363. (Royal Zoological Society of New South Wales: Sydney.)
Catling, P. C., and Burt, R. J. (1995). Studies of the ground-dwelling mammals of eucalypt forests in south-eastern New South Wales: the effect of habitat variables on distribution and abundance. Wildlife Research 22, 271–288.
| Studies of the ground-dwelling mammals of eucalypt forests in south-eastern New South Wales: the effect of habitat variables on distribution and abundance.Crossref | GoogleScholarGoogle Scholar |
Clarke, K. R., and Warwick, R. M. (1994). ‘Changes in Marine Communities: an Approach to Statistical Analysis and Interpretation.’ (Plymouth Marine Laboratory: Plymouth, UK.)
Clarke, M. F. (2008). Catering for the needs of fauna in fire management: science or just wishful thinking? Wildlife Research 35, 385–394.
| Catering for the needs of fauna in fire management: science or just wishful thinking?Crossref | GoogleScholarGoogle Scholar |
DEC (2006). NSW Recovery Plan for the large forest owls: powerful owl (Ninox strenua), sooty owl (Tyto tenebricosa) and masked owl (Tyto novaehollandiae). Department of Environment and Conservation, Sydney.
Dexter, N., and Murray, A. J. (2009). The impact of fox control on the relative abundance of forest mammals in East Gippsland, Victoria. Wildlife Research 36, 252–261.
| The impact of fox control on the relative abundance of forest mammals in East Gippsland, Victoria.Crossref | GoogleScholarGoogle Scholar |
Duffy, J. E. (2002). Biodiversity and ecosystem function: the consumer connection. Oikos 99, 201–219.
| Biodiversity and ecosystem function: the consumer connection.Crossref | GoogleScholarGoogle Scholar |
Earhart, C. M., and Johnson, N. K. (1970). Size dimorphism and food habits of North American owls. The Condor 72, 251–264.
| Size dimorphism and food habits of North American owls.Crossref | GoogleScholarGoogle Scholar |
Garnett, S. T., Loyn, R. H., and Lowe, K. (2003). Loss of hollow-bearing trees from Victorian native forests and woodlands. Action Statement No. 192. Department of Sustainability and Environment, Melbourne.
Gibbons, P., and Lindenmayer, D. (2002). ‘Tree Hollows and Wildlife Conservation in Australia.’ (CSIRO Publishing: Melbourne.)
Henke, S. E., and Bryant, F. C. (1999). Effects of coyote removal on the faunal community in western Texas. Journal of Wildlife Management 63, 1066–1081.
| Effects of coyote removal on the faunal community in western Texas.Crossref | GoogleScholarGoogle Scholar |
Higgins, P. (1999). ‘Handbook of Australian, New Zealand and Antarctic birds. Volume 4: Parrots to Dollarbird.’ (Oxford University Press: Melbourne.)
Hollands, D. (2008). ‘Owls, Frogmouths and Nightjars of Australia.’ (Bloomings Books: Melbourne.)
Holmes, G. (1994). Prey of the sooty owl in subtropical Australia. Sunbird 24, 25–27.
Hunter, M. D., and Price, P. W. (1992). Playing chutes and ladders: heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 73, 724–732.
Kavanagh, R. P. (1997). Ecology and management of large forest owls in south-eastern Australia. Ph.D. Thesis, University of Sydney.
Kavanagh, R. P. (2000). Effects of variable-intensity logging and the influence of habitat variables on the distribution of the greater glider (Petauroides volans) in montane forest, southeastern New South Wales. Pacific Conservation Biology 6, 18–30.
Kavanagh, R. P. (2002a). Comparative diets of the powerful owl (Ninox strenua), sooty owl (Tyto tenebricosa) and masked owl (Tyto novaehollandiae) in southeastern Australia. In ‘Ecology and Conservation of Owls’. (Eds I. Newton, R. P. Kavanagh, J. Olsen and I. Taylor.) pp. 175–191. (CSIRO Publishing: Melbourne.)
Kavanagh, R. P. (2002b). Conservation and management of large forest owls in southeastern Australia. In ‘Ecology and Conservation of Owls.’ (Eds I. Newton, R. P. Kavanagh, J. Olsen, and I. Taylor.) pp. 201–219. (CSIRO Publishing: Melbourne.)
Kavanagh, R. P., and Bamkin, K. L. (1995). Distribution of nocturnal forest birds and mammals in relation to the logging mosaic in south-eastern New South Wales, Australia. Biological Conservation 71, 41–53.
| Distribution of nocturnal forest birds and mammals in relation to the logging mosaic in south-eastern New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Kavanagh, R. P., and Webb, G. A. (1998). Effects of variable-intensity logging on mammals, reptiles and amphibians at Waratah Creek, southeastern New South Wales. Pacific Conservation Biology 4, 326–347.
Kavanagh, R. P., Debus, S., Tweedie, T., and Webster, T. (1995). Distribution of nocturnal forest birds and mammals in north-eastern New South Wales: relationships with environmental variables and management history. Wildlife Research 22, 359–377.
| Distribution of nocturnal forest birds and mammals in north-eastern New South Wales: relationships with environmental variables and management history.Crossref | GoogleScholarGoogle Scholar |
Kinnear, J. E., Sumner, N. R., and Onus, M. L. (2002). The red fox in Australia: an exotic predator turned biocontrol agent. Biological Conservation 108, 335–359.
| The red fox in Australia: an exotic predator turned biocontrol agent.Crossref | GoogleScholarGoogle Scholar |
Krüger, O. (2005). The evolution of reversed sexual size dimorphism in hawks, falcons and owls: a comparative study. Evolutionary Ecology 19, 467–486.
| The evolution of reversed sexual size dimorphism in hawks, falcons and owls: a comparative study.Crossref | GoogleScholarGoogle Scholar |
Lazenby-Cohen, K. A., and Cockburn, A. (1993). Intense predation by owls on lekking brown antechinus Antechinus stuartii. In ‘Australian Raptor Studies’. (Ed. P. Olsen.) pp. 175–180. (RAOU: Melbourne.)
Levins, R. (1968). ‘Evolution in Changing Environments.’ (Princeton University Press: Princeton.)
Lindenmayer, D. B., Cunningham, R. B., Tanton, M. T., Smith, A. P., and Nix, H. A. (1990). The conservation of arboreal marsupials in the montane ash forests of the central highlands of Victoria, southeast Australia. 1. Factors influencing the occupancy of trees with hollows. Biological Conservation 54, 111–131.
| The conservation of arboreal marsupials in the montane ash forests of the central highlands of Victoria, southeast Australia. 1. Factors influencing the occupancy of trees with hollows.Crossref | GoogleScholarGoogle Scholar |
Lindenmayer, D. B., Cunningham, R. B., and Donnelly, C. F. (1997). Decay and collapse of trees with hollows in eastern Australian forests: impacts on arboreal marsupials. Ecological Applications 7, 625–641.
| Decay and collapse of trees with hollows in eastern Australian forests: impacts on arboreal marsupials.Crossref | GoogleScholarGoogle Scholar |
Loyn, R. H., Traill, B. J., and Triggs, B. E. (1986). Prey of the sooty owl in East Gippsland, Victoria, Australia before and after fire. The Victorian Naturalist 103, 147–149.
Lundberg, A. (1986). Adaptive advantages of reversed sexual size dimorphism in European owls. Ornis Scandinavica 17, 133–140.
| Adaptive advantages of reversed sexual size dimorphism in European owls.Crossref | GoogleScholarGoogle Scholar |
Lundie-Jenkins, G. (1993). The diet of the sooty owl (Tyto tenebricosa) in the Blue Mountains, NSW. Emu 93, 124–127.
| The diet of the sooty owl (Tyto tenebricosa) in the Blue Mountains, NSW.Crossref | GoogleScholarGoogle Scholar |
Lunney, D. (1989). Effects of logging, fire and drought on possums and gliders in the coastal forests near Bega, NSW. Australian Wildlife Research 16, 207–215.
| Effects of logging, fire and drought on possums and gliders in the coastal forests near Bega, NSW.Crossref | GoogleScholarGoogle Scholar |
McLaren, B. E., and Peterson, R. O. (1994). Wolves, moose, and tree rings on Isle Royale. Science 266, 1555–1558.
| Wolves, moose, and tree rings on Isle Royale.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXisVGlsr8%3D&md5=17dc6b30e37c131e961fca8850326587CAS |
Menkhorst, P. W. (1995). ‘Mammals of Victoria: Distribution, Ecology and Conservation.’ (Oxford University Press: Melbourne.)
Menkhorst, P. W., and Knight, F. (2001). ‘A Field Guide to the Mammals of Australia.’ (Oxford University Press: Melbourne.)
Mooney, N. (1993). Diet of the masked owl in Tasmania: past and present. In ‘Australian Raptor Studies’. (Ed. P. Olsen.) pp. 160–174. (RAOU: Melbourne.)
Morris, D. A., Augee, M. L., Gillieson, D., and Head, J. (1997). Analysis of a late quaternary deposit and small mammal fauna from Nettle Cave, Jenolan, New South Wales. Proceedings of the Linnean Society of New South Wales 117, 135–161.
Mueller, H. C. (1986). The evolution of reversed sexual dimorphism in owls: an empirical analysis of possible selective factors. The Wilson Bulletin 98, 387–406.
Newton, I. (1979). ‘Population Ecology of Raptors.’ (Poyser: Berkhamsted.)
Newton, I. (2002). Population limitation in Holarctic owls. In ‘Ecology and Conservation of Owls’. (Eds I. Newton, R. P. Kavanagh, J. Olsen. and I. Taylor.) pp. 3–29. (CSIRO Publishing: Melbourne.)
Palkovacs, E. P., and Post, D. M. (2008). Eco-evolutionary interactions between predators and prey: can predator-induced changes to prey communities feed back to shape predator foraging traits? Evolutionary Ecology Research 10, 699–720.
Peake, P., Conole, L. E., Debus, S. J. S., McIntyre, A., and Bramwell, M. (1993). The masked owl in Victoria. Australian Bird Watcher 15, 124–136.
Peel, B. (1999). ‘Rainforests and Cool Temperate Mixed Forests of Victoria.’ (Department of Natural Resources and Environment: Melbourne.)
Pianka, E. R. (1973). The structure of lizard communities. Annual Review of Ecology and Systematics 4, 53–74.
| The structure of lizard communities.Crossref | GoogleScholarGoogle Scholar |
Robinson, A. C. (1987). The ecology of the bush rat, Rattus fuscipes (Rodentia: Muridae) in Sherbrooke Forest, Victoria. Australian Mammalogy 11, 35–49.
SAC (2001). Final recommendation on a nomination for listing: high frequency fire resulting in disruption of life cycle processes in plants and animals and loss of vegetation structure and composition (Potentially Threatening Process) (Nomination No. 565). Scientific Advisory Committee, Flora and Fauna Guarantee. Department of Natural Resources and Environment, Melbourne.
SAC (2003). Final Recommendation on a nomination for listing: inappropriate fire regimes causing disruption to sustainable ecosystem processes and resultant loss of biodiversity (Potentially Threatening Process) (Nomination No. 664) Scientific Advisory Committee, Flora and Fauna Guarantee. Department of Sustainability and Environment, Melbourne.
Schmitz, O. J., Hamback, P. A., and Beckerman, A. P. (2000). Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. American Naturalist 155, 141–153.
| Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants.Crossref | GoogleScholarGoogle Scholar |
Scotts, D. J. (1991). Old-growth forests: their ecological characteristics and value to forest-dependant vertebrate fauna of south-east Australia. In ‘Conservation of Australia’s Forest Fauna’. (Ed. D. Lunney.) pp. 147–159. (Royal Zoological Society of New South Wales: Sydney.)
Selander, R. K. (1966). Sexual dimorphism and differential niche utilization in birds. The Condor 68, 113–151.
| Sexual dimorphism and differential niche utilization in birds.Crossref | GoogleScholarGoogle Scholar |
Sergio, F., Marchesi, L., and Pedrini, P. (2004). Integrating individual habitat choices and regional distribution of a biodiversity indicator and top predator. Journal of Biogeography 31, 619–628.
| Integrating individual habitat choices and regional distribution of a biodiversity indicator and top predator.Crossref | GoogleScholarGoogle Scholar |
Short, J., and Smith, A. (1994). Mammal decline and recovery in Australia. Journal of Mammalogy 75, 288–297.
| Mammal decline and recovery in Australia.Crossref | GoogleScholarGoogle Scholar |
Sinclair, A. R. E., Pech, R. P., Dickman, C. R., Hik, D., Mahon, P., and Newsome, A. E. (1998). Predicting effects of predation on conservation of endangered prey. Conservation Biology 12, 564–575.
| Predicting effects of predation on conservation of endangered prey.Crossref | GoogleScholarGoogle Scholar |
Smith, P. (1984). Prey items of the sooty owl and barn owl at Bega, New South Wales. Corella 8, 71–72.
Smith, A. P., and Lindenmayer, D. (1988). Tree hollow requirements of Leadbeater’s possum and other possums and gliders in timber production ash forests of the Victorian central highlands. Australian Wildlife Research 15, 347–362.
| Tree hollow requirements of Leadbeater’s possum and other possums and gliders in timber production ash forests of the Victorian central highlands.Crossref | GoogleScholarGoogle Scholar |
Snyder, N. R., and Wiley, W. S. (1976). Sexual size dimorphism in hawks and owls of North America. Ornithological Monographs 20, 1–96.
Storer, R. W. (1966). Sexual dimorphism and food habits in three North American accipiters. The Auk 83, 423–436.
Suarez, A. V., and Case, T. J. (2002). Bottom-up effects on persistence of a specialist predator: ant invasions and horned lizards. Ecological Applications 12, 291–298.
| Bottom-up effects on persistence of a specialist predator: ant invasions and horned lizards.Crossref | GoogleScholarGoogle Scholar |
Temple, S. A. (1987). Do predators always capture substandard individuals disproportionately from prey populations? Ecology 68, 669–674.
| Do predators always capture substandard individuals disproportionately from prey populations?Crossref | GoogleScholarGoogle Scholar |
Terborgh, J., Lopez, L., Nunez, P., Rao, M., Shahabuddin, G., Orihuela, G., Riveros, M., Ascanio, R., Adler, G. H., Lambert, T. D., and Balbas, L. (2001). Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926.
| Ecological meltdown in predator-free forest fragments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXovFWrtbc%3D&md5=50e72469f209b0f041afdee087bdbce8CAS |
Tyndale-Biscoe, C. H., and Smith, R. F. C. (1969). Studies on the marsupial glider, Schoinobates volans (Kerr). II. Population structure and regulatory mechanisms. Journal of Animal Ecology 38, 637–650.
| Studies on the marsupial glider, Schoinobates volans (Kerr). II. Population structure and regulatory mechanisms.Crossref | GoogleScholarGoogle Scholar |
Ward, J. P., Gutierrez, R. J., and Noon, B. R. (1998). Habitat selection by northern spotted owls: the consequences of prey selection and distribution. The Condor 100, 79–92.
| Habitat selection by northern spotted owls: the consequences of prey selection and distribution.Crossref | GoogleScholarGoogle Scholar |
Wheeler, P., and Greenwood, P. J. (1983). The evolution of reversed sexual dimorphism in birds of prey. Oikos 40, 145–149.
| The evolution of reversed sexual dimorphism in birds of prey.Crossref | GoogleScholarGoogle Scholar |




