Physalopterine nematodes in Australian reptiles: interactions and patterns of infection
Hugh I. JonesMicrobiology and Immunology, M502, School of Pathology and Laboratory Medicine, University of Western Australia, Nedlands, WA 6009, Australia. Email: hugh.jones@uwa.edu.au
Australian Journal of Zoology 62(2) 180-194 https://doi.org/10.1071/ZO13033
Submitted: 27 April 2013 Accepted: 31 March 2014 Published: 21 May 2014
Abstract
Spirurid nematodes (family Physalopteridae) are widespread as adults or as encysted larvae in many species of Australian reptiles. Fifteen species of physalopterine nematodes (subfamily Physalopterinae) in the genera Kreisiella, Abbreviata and Skrjabinoptera infect more than 40 species of reptile in the five families Agamidae, Varanidae, Gekkonidae, Scincidae and Elapidae. Four species of nematode are host-species specific, six are host-family specific to varanid lizards, and three to agamid lizards. Larger species of reptile support a higher prevalence and abundance of nematodes, and often support multiple infections with more than one species, with the potential for interspecific competition. Geographic distribution of nematodes is partially limited by host distribution, and by climatic factors, mainly precipitation and temperature. There are strong positive and negative associations between several pairs of nematodes. Two species of nematode with the most pronounced muscular development at the anterior end, Abbreviata tumidocapitis and Abbreviata glebopalmae, only occur concurrently, and in low numbers, with species of nematode without these morphological features, suggesting differences in feeding in the hosts’ stomachs. A combination of host specificity, geographic distribution and habitat, climatic factors and feeding organ morphology are factors that probably reduce the potential for interspecific competition. There is no evidence that concurrent infections affect either prevalence or abundance of nematodes, or cause discernible pathological changes to their hosts.
Introduction
Spirurid nematodes in the subfamily Physalopterinae are the predominant gastric nematodes in the Australian reptile families Varanidae (monitor lizards), Agamidae (dragon lizards), Scincidae (skinks), Gekkonidae (geckos) and Elapidae (venomous snakes). Adult nematodes in the genera Abbreviata and Skrjabinoptera occur primarily in larger reptiles, in particular in drier and hotter areas of Australia, and nematodes in the genus Kreisiella occur in smaller skinks and agamids (Jones 1983a, 1983b, 1985b, 1986, 1988, 2005, 2007b). The anterior ends of physalopterine nematodes possess two lateral lips that border the dorso-ventrally elongate mouth, each lip bearing two sessile papillae and an amphid on the external surface, and denticles on the medial surface of each lip, whose number, size and disposal are important features in identification (Fig. 1). In four species, the anterior end of the muscular oesophagus is enlarged, with the development of accessory musculature. Physalopterine nematodes require an arthropod intermediate host (Anderson 2000), and in Australia one species of Abbreviata (A. antarctica) has been shown to develop in the tropical cricket, Teleogryllus oceanicus (King et al. 2013). Many smaller species of reptile, especially of skinks and geckos, act as paratenic hosts to physalopterine larvae (Jones 1995c, 2010). At least 15 species of adult nematode (12 species of Abbreviata, two of Kreisiella and one Skrjabinoptera) occur commonly within these reptile families. Infection prevalence in the larger species of lizards may be close to 100% (e.g. in Varanus giganteus, V. tristis and V. rosenbergi: Jones 1985b, 1995b, 2005), with intensity of several hundred worms (including larvae), and there may be significant concurrent infection with more than one species. These nematodes vary in their geographical range across the continent, as well as in their prevalence and abundance in different host species and in different habitats and parts of their ranges. Varanid lizards are active predatory species, feeding principally on a range of arthropods, especially orthopterans (James et al. 1992); larger species may consume many species of smaller lizards (Pianka 1994). Agamid lizards, on the other hand, primarily use ‘sit and wait’ tactics, and their diet also comprises arthropods, and small lizards (Pianka 1986). Little is known of life expectancy in the wild; V. rosenbergi may live to more than six years, and in captivity V. gouldii may live for at least seven years (King and Green 1999). Physignathus lesueurii has been recorded to live for 14 years (Hay 1972), and for Tiliqua rugosa an age of 20 years has been estimated (Holmes and Light 1983).
Interactions between nematodes in the gastrointestinal organs of their vertebrate hosts have been extensively studied (Quinnell et al. 1990; Hatcher and Dunn 2011), revealing a wide range of host–parasite relationships, and there is unlikely to be a single explanation for all forms of parasite interactions within a host. Broader patterns of infection are not evident from studies focussed on a single genus or species of reptile host. In the present study, therefore, I bring together data from many studies, cited above and subsequently, to search for patterns in the populations of gastric nematodes. As physalopterine nematodes are abundant in these reptile hosts, and concurrent infections, and infections with multiple species in different habitats or regions, are widespread, a key question is whether competition has a role in shaping these communities. I address this question by examining various parameters, in particular host specificity, geographical distribution, host habitat and methods of feeding deduced from their mouthparts, which may mitigate interspecific competition. From this, I deduce the relevance of competition in the dynamics of these nematode populations.
Materials and methods
Material examined
I dissected a total of 2390 preserved lizards and snakes of 65 species held in The Australian Museum, Northern Territory Museum, Queensland Museum, Tasmanian Museum in Hobart, the Queen Victoria Museum in Launceston, Museum Victoria, the Western Australian Museum, and in collections held by CSIRO in Canberra. All reptiles had been collected in the field in their natural habitats, over a period of many years. In addition, I identified nematodes collected by other workers, in particular the late Dennis King, in the course of their own research, and examined more than 3000 stomachs from 45 species of lizard from the Great Victoria Desert collected E. R. Pianka. These have been published in a series of papers. Data collected and not published have been used (e.g. concurrent infections, host size), and I include previously unpublished data from another 40 Varanus gouldii and 10 V. panoptes, mostly from Victoria and Queensland, and 76 V. acanthurus. I also examined specimens of Abbreviata in the Australian Helminth Collection held in the South Australian Museum, Adelaide. The published papers and these previously unpublished data form the basis for the present study. In total, reptiles from the following host families were examined: Acrochordidae, Agamidae, Colubridae, Elapidae, Gekkonidae, Pythonidae, Scincidae, Typhlopidae, and Varanidae.
Methods
Helminths were removed, cleaned and identified. The number of hosts examined was compared with the number of species of nematode recovered from each host species. Host specimens were measured in millimetres, and their food residues noted. All snout–vent lengths (SVL) are those of the reptiles examined. Histological sections of nematodes attached to host stomach wall and cut at 5 or 6 µm, were prepared and stained with haematoxylin and eosin in order to determine host reactions.
Statistical methods
Regression analyses were run on StatPlus for Macintosh (AnalystSoft) and were used to investigate relationships between prevalence, abundance and host size across species. Chi-square tests (2 × 2) were run on GraphPad QuickCalcs online calculator, and were used to analyse pair-wise associations between nematodes across hosts.
Ecological terms
These follow the definitions of Bush et al. (1997). Prevalence refers to the number of hosts infected with one or more individuals of a particular parasite, expressed as a percentage. Intensity refers to the number of individuals of a particular parasite species in a single infected host. Abundance refers to the number of individuals of a particular parasite present in a single infected host, expressed as a mean across all specimens of that host species. For those host species with more than one nematode species present, the combined prevalence was calculated as the percentage of specimens of that species that were infected with one or more species of physalopterine nematode. Likewise, the combined abundance mean was calculated by the total number of physalopterine worms in specimens of that species, divided by the number of host specimens surveyed.
Examples of all nematode species in this study have been re-examined to confirm previous descriptions. Not infrequently, reptiles infected by a dominant species also harboured one or two adult or immature nematodes of related species. Spurious infections can also occur from nematodes ingested in their prey, and for these reasons data from hosts with few worms and low prevalence (generally <3%) have not been considered.
Results
Physalopterine nematodes recovered
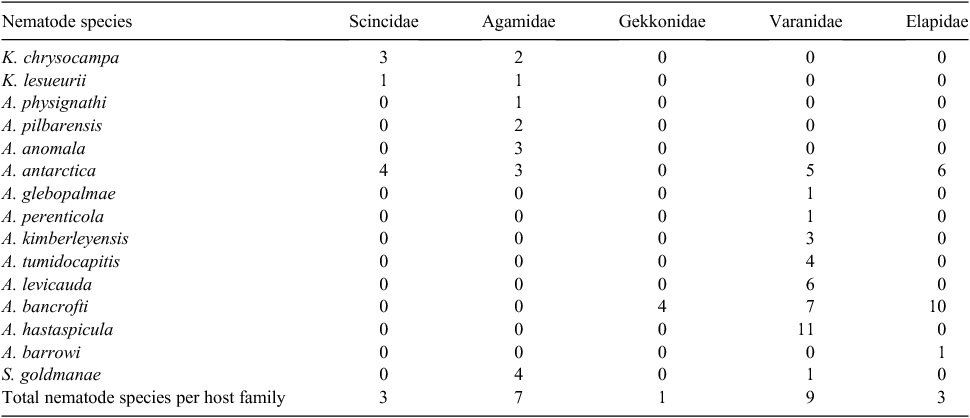
Fifteen species of nematode belonging to three genera in the subfamily Physalopterinae were recovered: Kreisiella chrysocampa, K. lesueurii, Abbreviata anomala, A. antarctica, A. bancrofti (of which A. confusa is a junior synonym: Jones 2013b), A. barrowi, A. glebopalmae, A. hastaspicula, A. kimberleyensis, A. levicauda, A. perenticola, A. physignathi, A. pilbarensis, A. tumidocapitis, and Skrjabinoptera goldmanae. These infected 45 species of lizard in the families Scincidae, Gekkonidae, Agamidae and Varanidae (subgenera Varanus and Odatria), and 10 species of snakes in the family Elapidae.

|
Specificity varied from one to 21 host species. Ten species of nematode were host-family specific, of which four (A. physignathi, A. glebopalmae, A. perenticola and A. barrowi) were host-species-specific. The two species of Kreisiella were recovered only from smaller skinks and agamids, and S. goldmanae predominantly from agamid lizards.
Infection patterns: single-species infections (Table 2)
Thirty species of reptile, principally smaller species, supported infections with a single species of gastric nematode. Within Scincidae, the four smallest lizard species with adult nematode infection supported only Kreisiella spp., and in the four larger skink species, only A. antarctica was recorded. Within Gekkonidae four species of leaf-tailed geckos (Saltuarius cornutus, S. swaini, S.moritzi and Phyllurus platurus) supported infection with Abbreviata bancrofti only. Six species of Agamidae also supported a single nematode species: Kreisiella species occurred in the three smallest agamid lizards, A. antarctica in two species of Pogona, and the host-species-specific A. physignathi in the water dragon Physignathus lesueurii. Five species of smaller Varanidae (subgenus Odatria) supported single-species infections of A. hastaspicula (three host species), and A. levicauda (two host species). The larger V. (O.) scalaris supported A. bancrofti only. Abbreviata antarctica occurred in all host families except Gekkonidae, A. hastaspicula occurred in several species of Varanidae, and A. bancrofti in Varanidae, Gekkonidae and Elapidae. With the exception of V. giganteus, which supported only the host-specific A. perenticola, single-species infections occurred predominantly in smaller lizards, usually at low mean abundance and prevalence and, except in the largest hosts, at low prevalence. Nematodes in the two largest hosts to support a single species infection, A. perenticola in V. giganteus and A. physignathi in P. lesueurii, were both host-species specific. Five species of snake in the family Elapidae were host to A. antarctica only.
Multiple-species infections
Three species of agamid lizard, eleven species of varanid lizard, and one elapid snake supported infections with 2–6 species of physalopterine nematode. Within Varanidae, species supporting more than a single nematode species were larger (mean SVL 125–474 mm) than those with single-species infections (mean SVL 93–140), apart from V. (O.) scalaris (mean SVL 183 mm) and V. giganteus (mean SVL 379 mm). Larger lizards supported a higher prevalence and abundance of nematodes; prevalence of A. kimberleyensis in V. glebopalma and A. antarctica in V. rosenbergi were higher than 90% (Table 3). Abundance of adult Abbeviata spp. in V. rosenbergi was 59 per host, and 109 in V. gouldii. There was no obvious relationship between number of nematode species infecting one host species, and host specificity or habitat, nor were there discernible relationships between prevalence, intensity, and concurrent infections (Jones 1983b, 2005).

|
Infection and host size, single and multiple infections
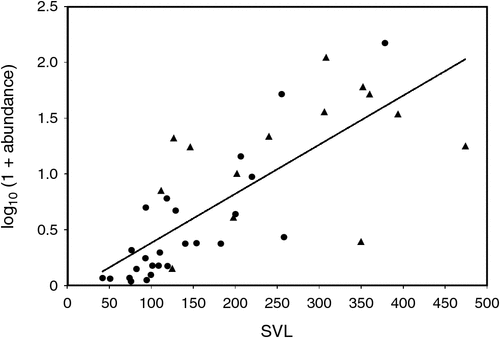
Prevalence (r = 0.5945, P < 0.001) and abundance (r = 0.7606, P < 0.0005) (Fig. 3) were both positively associated with increasing host size across species. Within species, there were positive associations between increasing host size and prevalence of infection with A.hastaspicula in V. gouldii (r = 0.35; P < 0.05) and in V. panoptes (r = 0.76; P < 0.01).
|
There was a positive relationship between host size, and multiple infections (r = 0.0399; P < 0.05). The sample size for each host species, and the number of species of nematode recovered from each host species were also related (r = 0.505; P < 0.001).
|
|
|
|
|
|
|
|
|
|
|
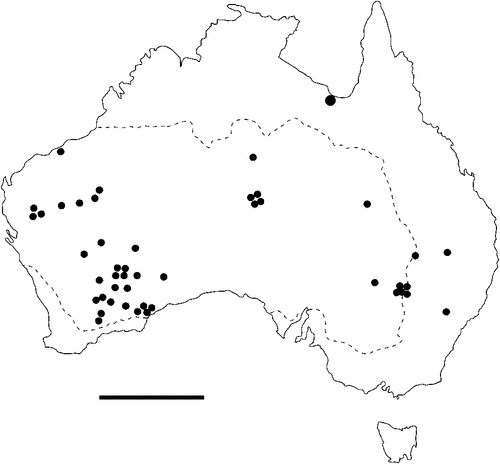
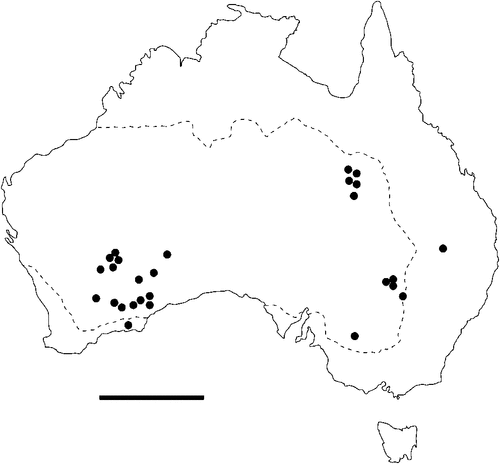
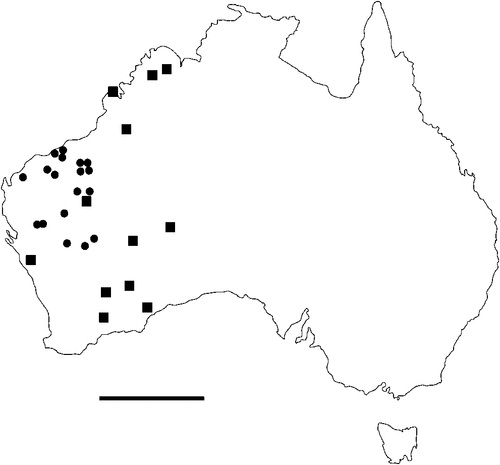
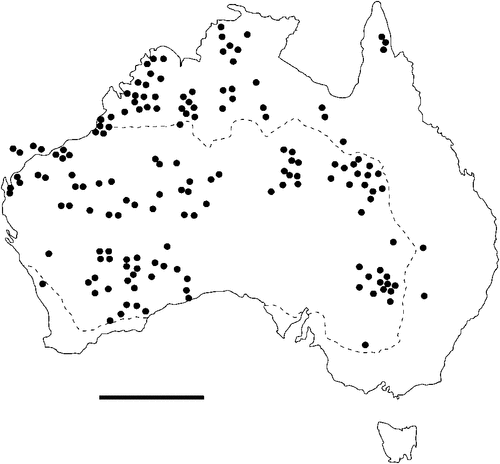
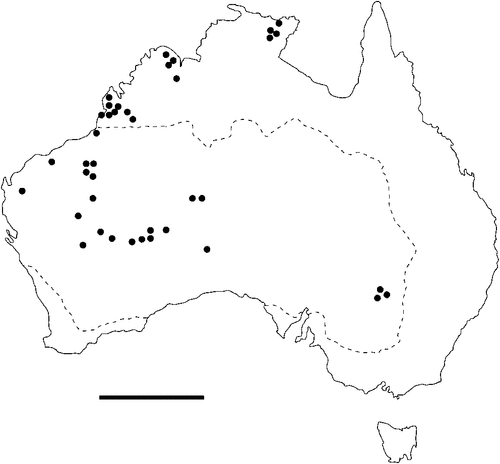
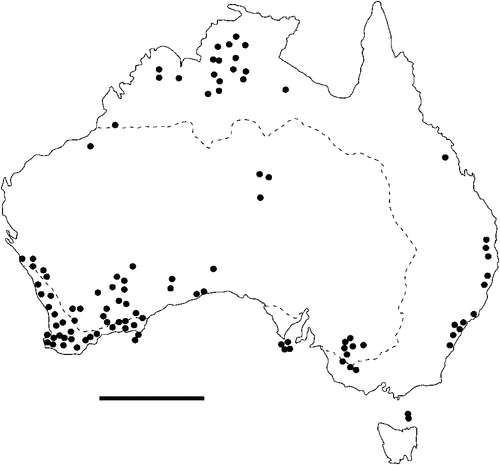
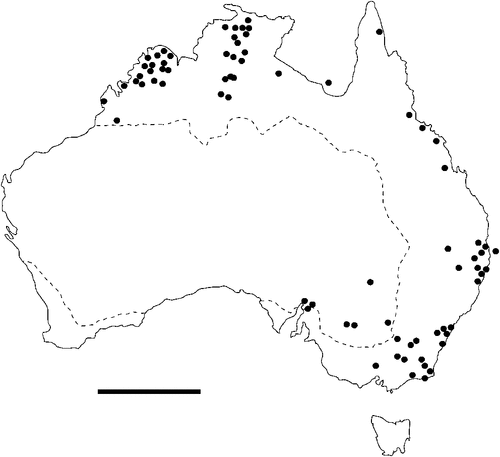
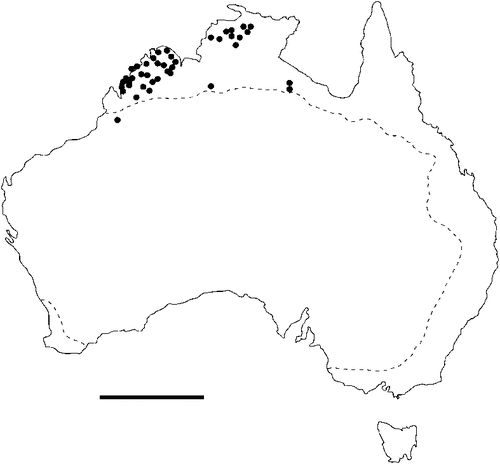
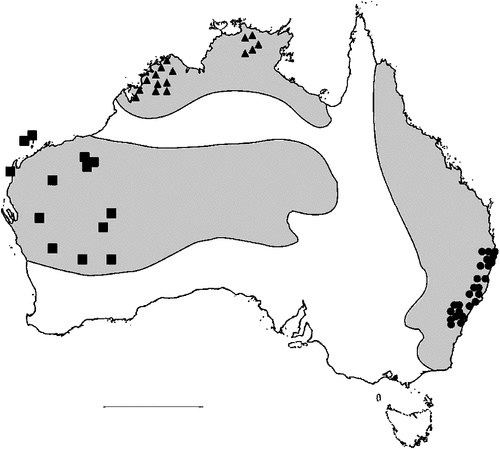
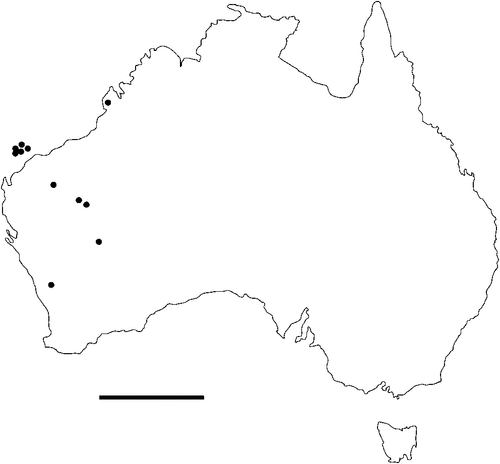
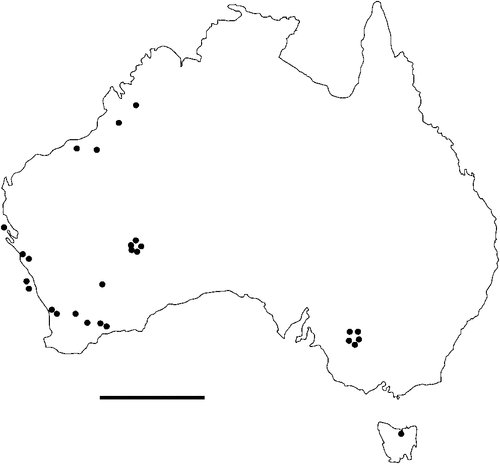
The geographical distribution of nematodes is limited to the geographical range of their hosts, and falls into several patterns. Abbreviata levicauda, A. tumidocapitis, A. pilbarensis (Figs 4–6) and A. perenticola (Fig. 12) occur almost exclusively in drier central regions where the annual average rainfall is less than 400 mm. Abbreviata anomala, A. hastaspicula, and S. goldmanae (Figs 6–8) also occur in central drier areas and extend into the northern tropics with a mean annual temperature of >18°C. Abbreviata bancrofti (Fig. 10) is found predominantly in coastal, south and east, and northern tropical areas with higher rainfall. Abbreviata antarctica (Fig. 9) occurs across a wider temperature range than any other species, and is the only species of Abbreviata found south of the continent, on Flinders Island, and, at generally lower prevalence and intensity, in the tropical north in the lizards V. acanthurus and V. glebopalma (Jones 1988). Abbreviata kimberleyensis and A. glebopalmae are confined to the localised distribution of their hosts V. glebopalma and V. glauerti in the Kimberley and tropical Northern Territory with a mean precipitation >600 mm and mean annual temperatures of 21–33°C (Figs 11, 12). Abbreviata physignathi in Physignathus lesueurii occurs near the east coast (Fig. 12), and A. barrowi occurs in western areas of Western Australia (Fig. 13). Kreisiella chrysocampa and K. lesueurii appear to have a wide distribution, from Tasmania to the Great Victoria Desert and the tropical north (Fig. 14); owing to their presence as immature worms, or their poor condition, the two species could not always be differentiated from one another.
However, several species of lizards in the families Varanidae and Agamidae, and the snake Pseudechis australis, have wide ranges across the continent; and thus there are potentially wide areas of sympatry of their nematodes: V. gouldii, V. rosenbergi, V. tristis and P. minor are host to five or six species of nematode. By contrast, hosts with a more restricted range (V. pilbarensis in the Pilbara and V. glauerti in the Kimberley) are host to one and two nematode species respectively.
Concurrent infections
There were both strong positive and negative associations between pairs of nematode species. The presence of Abbreviata levicauda and A. hastaspicula were positively associated (P < 0.0001) and both these species were strongly positively associated with A. tumidocapitis (P < 0.0001). Skrjabinoptera goldmanae and A. pilbarensis were also positively associated (P = 0.0238), and all infections with A. glebopalmae occurred with A. kimberleyensis. There were strong negative associations between A. antarctica and A. bancrofti (P = 0.0129), A. antarctica and A. levicauda (P < 0.0001) and A. antarctica and A. hastaspicula (P < 0.0001), and between A. bancrofti and A. levicauda (P < 0.0001), and A. bancrofti and A. hastaspicula (P = 0.0101), and between A. hastaspicula and A. kimberleyensis (P < 0.0001). There were no significant associations between S. goldmanae and A. anomala, A. antarctica and A. kimberleyensis, A. bancrofti and A. kimberleyensis or A. antarctica and A. tumidocapitis.
Principal host species (Table 3)
Each species of nematode predominated in one or two host species, at prevalences between 12.5% and 98% (mean 55%). Principal host species occupied a wide range of habitats across the continent. Abbreviata levicauda occurred predominantly in V. tristis and V. pilbarensis, which occupy different habitats (V. tristis being largely arboreal and V. pilbarensis being rock-dwelling in the Pilbara). Abbreviata bancrofti occurred predominantly in V. glauerti and V. varius, which also occupy different habitats, and which are allopatric. Abbreviata hastaspicula, A. levicauda, A. kimberleyensis, A. perenticola, A. glebopalmae and A. tumidocapitis were recovered only from varanid lizards; the latter two species always occurred at considerably lower prevalence and intensity than co-occurring species of parasite. Both A. bancrofti and A. antarctica have been recovered from many species of reptile in the three and four families respectively of the five families under consideration. Abbreviata anomala, A. pilbarensis and A. physignathi occurred exclusively in agamid lizards, and Kreisiella species occurred in skinks and the smaller agamids. Abbreviata barrowi was found in elapid snakes, and S. goldmanae occurred principally, but not exclusively, in agamid lizards.
Morphology of the anterior end of nematodes (Fig. 1)
The morphology of the anterior end of A. antarctica, A. bancrofti, A. levicauda, A. hastaspicula and A. perenticola conforms to the basic pattern for this genus, with a large single externolateral and a smaller (usually bifid) internolateral apical tooth, and doubled submedian teeth at the dorsal and ventral lip margin. Mouth corner denticles are present in A. antarctica, A. bancrofti, and A. kimberleyensis, are inconsistent in A. hastaspicula, and absent in A. levicauda. Abbreviata kimberleyensis bears in addition a row of very fine denticles along the lip margin. Abbreviata anomala, A. pilbarensis and A. physignathi bear a row of denticles along the lip margin; the doubled denticles at the dorsal and ventral lip margins are distinguishable from this row, which are small in the first two, and large in A. physignathi. In K. chrysocampa and K. lesueurii the relatively narrow mouth dorso-ventrally is surrounded by a row of small even teeth from which apical denticles cannot be distinguished. The four remaining species are characterised by various degrees of anterior end enlargement. This is least pronounced in A. barrowi, is more pronounced in A. glebopalmae and A. tumidocapitis, and is most extreme in S. goldmanae. None of the four species with cephalic muscular enlargement possess mouth corner denticles.
Discussion
Arid-zone Australia has a richer reptile fauna than any other comparable region of the world (Pianka 1986), and this study reveals patterns in the specificity, morphology, and distribution of their gastric nematodes.
Reptile families infected
Adult physalopterine nematodes in the three genera Kreisiella, Abbreviata and Skrjabinoptera are the predominant or exclusive gastric nematodes of reptiles in the families Scincidae (Jones 1985a, 1992a; Jones and Watharow 2010), Varanidae (Johnston and Mawson 1947; Jones 1983a, 1983b, 1985b, 1988, 1992b, 1995b, 2005, 2007b, 2010, 2013c), Agamidae (Baylis 1924; Jones 1986, 1994; 1995a, 2007a), Gekkonidae (Jones 2013a, 2013b), and Elapidae (Johnston and Mawson 1942a, 1942b, 1951; Jones 1978a) (Tables 2, 3). All large species in the four families of lizards that have been studied are host to adult nematodes in one or more of these genera. Adult physalopterine nematodes are infrequent or absent in Typhlopidae (10 Rhamphotyphlops pinguis examined) Acrochordidae (12 Acrocordus spp. examined), and Colubridae (eight species, 62 individuals examined) (Jones 1978b and unpublished). However, A. bancrofti has recently been identified from the colubrid snakes Stegonotus cucullatus and Tropidonophis mairii in the Northern Territory (D. Barton, unpublished). Python spilotes was recorded as host to species of Abbreviata from various sites, mainly in southern and eastern Australia, but I cannot ascertain whether reports of Abbreviata spp. from seven species of pythons (Johnston and Mawson 1948; Thomas 1959; Jones 1979, 1980) were genuine infections or spurious infections from ingested prey.
Three other species of gastric nematodes may occur in significant numbers in some of these five families of Australian reptiles. Tanqua anomala (Spirurida : Gnathostomatoidea) is found in aquatic snakes and in species of Varanus occupying aquatic or coastal habitats in northern Australia (Johnston and Mawson 1948; Jones 1988, 2004; Dewi et al. 2008). Physalopteroides filicauda (Spirurida : Thubunaeinae) occurs almost exclusively in smaller lizards, principally skinks (often concurrently with Kreisiella spp.) and geckos (Jones 1985a, 1995b; Goldberg and Bursey 2012), in which species of Abbreviata occur as encysted third-stage larvae (Jones 1995b). Ophidascaris pyrrhus (Ascaridida) occurs in many species of elapid snakes (Johnston and Mawson 1942a, 1948; Jones 1980; Sprent 1988).
Host-specificity
There is a wide range in the host-specificity of physalopterine nematodes. The two species of Kreisiella – K. chrysocampa and K. lesueurii –occur only in skinks and agamids, and they are found infrequently in the same hosts as adult Abbreviata. Nematodes in the genus Abbreviata in the subfamily Physalopterinae are closely related (Chabaud 1975), and more than one nematode species may occur in more than one host species. Six species of Abbreviata are specific to Varanidae, and have not been recorded outside Australia. Two of these species (A. glebopalmae and A. perenticola) are host-specific. (I reviewed the nematodes from V. giganteus identified as A. confusa by Johnston and Mawson (1947) and identified them as A. perenticola (Jones, unpublished)). Three nematode species (A. anomala, A. pilbarensis and A. physignathi) are found only in the family Agamidae. Abbreviata antarctica occurs in at least 18 species in four host families, and A. bancrofti in 21 species in three host families, including several species of elapid snakes (Johnston and Mawson 1942a, 1942b, 1948, 1951), Varanidae and Gekkonidae (Johnston and Mawson 1947; Jones 2013a, 2013b). Finally, A. barrowi has been found only in the elapid snake Pseudechis australis (Jones 1978a and unpublished).
Goldberg and Bursey (2012), in a wide-ranging study of helminth parasites of Australian reptiles in the families Agamidae, Gekkonidae and Scincidae, and a review of Australian lizard helminths, concluded that their parasites are generalist, with negligible host-specificity. However, the present study indicates that several species of physalopterine nematodes exhibit strong host-species or host-family specificity. The significance of this specificity in reducing competition between species of nematode cannot yet be conclusively demonstrated.
Infections and host size
Larger lizards support a higher prevalence and abundance of nematodes, and more multiple species infections. Many large lizards (Varanus, Tiliqua, Physignathus) are relatively long lived (Hay 1972; Holmes and Light 1983; King and Green 1999), and often range widely (Pianka 1994), and thus have more opportunities to ingest larval stages in their prey. The association between host size, and prevalence and abundance of infection across species may therefore be a function both of longevity and of behaviour. The number of nematode species infecting a host species was related to sample size for each host. However, multiple infections are also positively related to host size. Though mean sample sizes were smaller for single-species infections (mean = 28 hosts) than for multiple-species infection (mean = 74 hosts), most infections in smaller reptiles were at lower intensity than in larger lizards. This suggests that smaller lizards acquire infections less frequently than larger lizards, the latter therefore having the potential to become infected with more than one species of nematode. The findings presented here are therefore unlikely to be invalidated by the sample sizes.
Species of Kreisiella are found only in smaller skinks and agamids with a mean SVL of 42–110 mm. Apart from the small varanids V. brevicauda, V. kingii and V. storri (subgenus Odatria), which have evolved dwarfism (Pianka and King 2004), the smallest lizards found infected with species of adult Abbreviata had an SVL of >90 mm; it is striking that the host size threshold below which species of Abbreviata do not mature is similar in all four lizard families that support infection with these nematodes. The smaller species of lizard in all four lizard families above this size support a single nematode species, usually at low intensity. Adult Abbreviata species that occur as single-species infections are (with the exception of the host-specific A. physignathi and A. perenticola) also the most widespread species, with lowest host-specificity – A. antarctica, A. bancrofti and A. hastaspicula, the latter being confined to several species of Varanus.
Many species of skink and gecko below ~90 mm SVL act as paratenic hosts to physalopterid nematodes, with third-stage larvae usually encysted in the abdominal viscera (Jones 1995b, 1995c, 2010). These lizards ingest third-stage larvae in their arthropod prey. Density-dependent effects may regulate and stabilise nematode populations (Paterson and Viney 2002), their numbers being constrained by the host immune response (Viney et al. 2006), but whether this mechanism limits the development and size of parasitic nematodes and prevents the encysted larvae developing into adults has not been investigated in these species. The gecko Phyllurus platurus and the aquatic V. mitchelli are the only species so far examined that may support both encysted larvae and adult Abbreviata species (Jones 1988, 2013b).
Geographical distribution of nematodes and host habitat
The number of nematode species to which a reptile is susceptible has been shown to increase with host geographical range in several species (Aho 1990), thus potentially encompassing the distribution of more than one nematode species. Species of Kreisiella have a wider geographical range than any species of Abbreviata so far studied in Australia, having been recorded in Tasmania (Jones 2003), and in New Britain, Papua New Guinea (as Physaloptera heterocephala: Kreis 1940). Of the various environmental parameters examined, annual precipitation and mean annual temperature most closely define distributions of nematodes. These climatic factors determine both vegetation, and, in many species, host distribution. Abbreviata antarctica and A. bancrofti occur principally in areas with an annual precipitation above 400 mm (Jones 2005, 2007b); two species of snake (Austrelaps superbus and Notechis ater) were infected with A. antarctica on Flinders Island; neither Abbreviata spp. nor Skrjabinoptera sp. have been recorded from Tasmania itself (Jones 2003). On the other hand, A. levicauda and A. tumidocapitis occur predominantly in drier areas with an annual precipitation below 400 mm (Jones 1983b). Abbreviata hastaspicula and S. goldmanae occur in both hot drier areas and in the humid tropics (Mawson 1970; Jones 1983b, 1988, 1995b). Apart from the widespread A. antarctica and the species-specific A. physignathi, the two other species of Abbreviata that inhabit agamid lizards (A. anomala and A. pilbarensis) occur predominantly in hotter and often drier parts of the continent; neither of these two species have been recorded in areas with annual precipitation >400 mm (Jones 1986). Skrjabinoptera goldmanae is mainly specific to the Agamidae, though I have recorded this species in two species of Varanus in the Great Victoria Desert (Jones 1995b). The distribution of these species of nematode is probably a function of either the distribution of suitable arthropod intermediate hosts, or the ability of the eggs to survive and remain viable in the external environment. The range of intermediate host species is not known; one effective arthropod intermediate host for A. antarctica is the native tropical cricket Teleogryllus oceanicus (King et al. 2013); other species of arthropod hosts remain to be determined. Seasonal changes in food type and availability may expose lizards to different potential intermediate hosts, and these factors may, in part, account for the infrequency with which similar species occur together. Thus the geographical range of nematodes, partly resulting from climatic factors, acting either directly on their eggs in the external environment, or indirectly by affecting the range of suitable arthropod intermediate hosts, may be a factor in reducing competition between species.
There are, however, wide areas of sympatry between many nematode species, which share distributions but not necessarily specific habitats. Host habitat may further limit exposure of potentially suitable hosts to infection. Shine (1986) has shown that four sympatric species of Varanus may have major interspecific differences in habitat use and diet. Varanus tristis occurs sympatrically with V. gouldii over large areas of Australia (Pianka 1994); both species represent species complexes (Pianka and King 2004). The complexity of host–parasite associations is underscored by the fact that the two principal hosts of A. levicauda, V. tristis and V. pilbarensis, occupy different habitats, the former being arboreal (Pianka 1994) and the latter rock-inhabiting (King 2004), and the principal hosts of A. bancrofti, V. glauerti and V. varius, are allopatric. These differences in habitat and range of two principal host species, and variations in nematode morphology, particularly in A. antarctica (Irwin-Smith 1922; Johnston and Mawson 1941; Jones 1978a) and in A. hastaspicula (Jones, unpublished) suggest that molecular studies may reveal these morphospecies to be species complexes.
Cephalic morphology and feeding
The morphology of the cephalic ends of nematodes reflects their methods of feeding. Cephalic dentition with a conspicuous external apical tooth, a small bifid internal apical tooth, and a small bifid tooth on the dorsal and ventral margin of each lip (Chabaud 1956) prevails in the three species that have lowest host specificity and widest geographical range: A. antarctica, A. bancrofti, and A. hastaspicula. The high number of host species in which these three nematodes occur, particularly the first two (Fig. 2), their high prevalence (up to 100%), intensity (to >400 adult worms per host), and large size of worms (females to 38 mm); (Irwin-Smith 1922; Johnston and Mawson 1942b; Jones 1983b, 1988, 1995b, 2005), as well as their ability to feed both from the host tissues, and from ingested prey, suggests that this is an efficient feeding structure. Four species (A. hastaspicula, A. levicauda, A. kimberleyensis, A. perenticola) have similar mouthparts, mainly differing in the presence or absence of mouth-corner denticles. From the data available, I cannot ascertain whether this is due to convergence, or to a common line of descent. Both A. hastaspicula and A. levicauda may attach loosely to the host stomach wall and ingest blood (Jones 1983b). However, when there is ample prey in the host stomach, nematodes are deeply embedded in the prey items, especially in tissues such as hind limbs and head capsules of Orthoptera (Jones 1983b), indicating that they are feeding. Abbreviata tumidocapitis, with strongly hypertrophied musculature at the anterior end, attaches firmly to the stomach wall of the hosts, though its food has not been investigated (whether A. glebopalmae also attaches to the host stomach wall was not noted: Jones 1988). The anterior ends of S. goldmanae are buried in the host stomach wall for a distance of up to 5 mm, but no blood was seen in the oesophagus or intestine, and possibly these worms feed on inflammatory exudate from the host (Jones 1994). In becoming embedded in host tissues, both the adult female S. goldmanae themselves, and the eggs in the uteri, become destroyed by host immune responses (Jones 1994). In the three species of nematode occurring only in Agamidae, A. anomala, A. pilbarensis and A. physignathi, morphological similarities include well defined lines of denticles around the mouth (Jones 1986), particularly well developed in A. physignathi (Baylis 1924), and reduced bifid dorsal and ventral denticles bordering the mouth.
Cephalic morphology and concurrent infections
Both A. levicauda and A. hastaspicula occur in hotter areas of the country, the former being principally a parasite of V. tristis, in which A. hastaspicula is uncommon. There is little difference in the mouthparts of these two species, mouth corner denticles being absent in A. levicauda and inconsistent in A. hastaspicula. On the basis of their morphology there is thus the potential for competition between these two species, though I could find no evidence for this, as there was a strong positive correlation between the presence of these two species of nematode. The negative associations between the sympatric A. antarctica and A. bancrofti, whose mouthparts are similar, may be due to host specificity (the former being the dominant nematode in V. rosenbergi and the latter in V. varius), or to as-yet unidentified aspects of their biology. Though they may occur in the same host species, it is uncommon to find both species in the same host individual (Jones 2005, 2007b). The negative associations between A. bancrofti and A. levicauda probably result from their environmental preferences, A. bancrofti occurring in more humid regions and A. levicauda in drier areas. Abbreviata hastaspicula, however, occurs in the tropical north where A. bancrofti is also present, but few lizards harboured both species. The musculature development at the anterior end of A. tumidocapitis and A. glebopalmae suggests different feeding strategies from those of concurrent species, thus reducing the potential for competition.
More studies are needed to ascertain whether these observed associations between geographical distributions of nematodes, host specificities, and differences in their mouthparts, are significant factors in reducing interspecific competition.
Despite this, larger host species may harbour significant numbers of more than one nematode species concurrently. In these instances, to what extent is there competition for food? Several observations show that direct competition for resources among these nematodes is limited; where one lizard species is infected with large numbers of nematodes of more than one species, e.g. V. gouldii with A. antarctica and with A. hastaspicula in the Goldfields area of Western Australia (Jones 1983b), and with V. rosenbergi in southern Australia (Jones 2005), there is no detectable diminution in numbers, size or fecundity of parasites compared with single-species infections from the same area. Indeed, in V. rosenbergi the mean intensity of the predominant nematode, A. antarctica, is higher when occurring concurrently with up to four other species (Jones 2005). In V. gouldii, intensity of A. hastaspicula is similar in single-species infection to that when there were concurrent infections with A. levicauda. Possibly the differences in their mouthparts, though slight, effectively reduce the potential for competition in feeding (Jones 1983b). Similarly, the prevalence of A. kimberleyensis in V. glauerti was higher when A. glebopalmae was also present (Jones 1988). These examples evidently reflect optimal conditions for transmission of these species of nematodes, and illustrate the complexity of the host–prey–parasite–environment dynamics, and are further evidence that, at least in some biotopes, interspecific competition is not significant. Exceptions are A. tumidocapitis and A. glebopalmae, which only occur concurrently with more numerous species (A. hastaspicula and A. levicauda, and A. kimberleyensis respectively), and have developed accessory muscles at the anterior end (Jones 1983b, 1988) that enable them to exploit different sources of food. The association between S. goldmanae and A. pilbarensis in agamid lizards is less marked than in the two examples above, as A. pilbarensis rarely occurs in the core host of S. goldmanae, C. kingii (Jones 1994).
Cephalic morphology and host phylogeny
The feeding structures of the nematodes discussed in this paper display a spectrum of development, from Kreisiella with numerous undifferentiated labial teeth, through to Skrjabinoptera in which there is only a single apical tooth (Fig. 1). Host-family specificity, in particular to the Varanidae and Agamidae, occurs in many nematodes in the genus Abbreviata. Varanoid lizards occupy a range of habitats in Australia and despite great differences in size between species, are monophyletic (Baverstock et al. 1993; Ast 2001; Fitch et al. 2006) and morphologically similar (Pianka and King 2004). A phylogeny of Australian agamid lizards, which have radiated widely in Australian deserts, has been established by Hugall et al. (2008) using nuclear and mitochondrial genes. However, the morphological forms displayed by their physalopterine nematodes may be adaptive, and do not necessarily reflect evolutionary relationships, and I was not able to relate these forms to host phylogenies. Molecular studies of this group of nematodes would be fruitful in this regard.
Intraspecific competition
Evidence for intraspecific competition comes from the large number of larval physalopterids (assumed to be species of Abbreviata: Jones 2010) associated with adult A. hastaspicula in V. gouldii, from >700 to >1500 larvae (Jones 1983b, 1988, 1995b). These numbers are far in excess of recorded numbers of adults, and the presence of large numbers of adult nematodes may suppress the development of larvae. The presence of species of Abbreviata in the intestine may have been due to wandering after the death of the host, but in V. gouldii, specimens of A. antarctica were found only in the intestine in lizards harbouring more than 55 adult worms (Jones 1983b), suggesting the effects of crowding in the stomach.
Effects of host activity
The seasonal activity of the hosts may also affect the population of infecting nematodes. Activity in C. kingii is highly seasonal (Shine and Lambeck 1989), and the field metabolic rate in the dry season is about one-quarter of that in the wet season, affecting its food intake (Christian and Green 1994). As there is little concurrent infection with other species of nematode in C. kingii, the muscular development of S. goldmanae in this host suggests that the feeding regime of the host, rather than competition, may be a crucial factor in determining their feeding strategy, and hence anatomy. Similarly A. barrowi from the snake Pseudechis australis was recorded concurrently with A. antarctica in only one individual snake, and it thus has little competition from congeners in its only recorded host. Snakes, especially larger species, may only feed at long intervals of several months, and may undergo long periods of dormancy. However, other large reptile species are also inactive for several months during the year (Pianka 1970, 1971, 1994; Christian et al. 1995), and this diminution or lack of food intake for a prolonged period is likely to have an impact on the feeding regime, and hence potential for competition, among infecting nematodes. At such times the metabolism of their nematodes may adapt to conserve energy, as is known to occur in some free-living nematodes (Lant and Storey 2010). Life expectancy in nematodes is very variable (Gems 2000); that of species of spirurid nematode dwelling in the gastrointestinal tract of poikilotherms is not known.
Effect of parasite load on host
I could find no evidence that high prevalence and intensity of nematodes affects the health of their hosts. Despite often having heavy worm burdens, infected lizards appeared to be in good health, which in turn is conducive to the health and survival of the parasites, and is further evidence of a long evolutionary association. However, whether high numbers affect other aspects of their biology, such as breeding success or longevity, could not be determined, and deserves study.
In conclusion, interspecific competition when two or more species are present is not evident from intensity, maturity or size of worms, and a range of biological, evolutionary and nematode anatomical factors enable host reptiles to support large populations of nematodes. Host specificity, host and nematode distribution, host habitat, host size, and nematode feeding organ morphology are the principal factors I have identified that may limit the potential for interspecific competition. Further, an evident abundance of available food (at least during seasons when the reptiles are active) probably contributes to the success of physalopterine nematodes in these families of Australian reptiles. Thus a long evolutionary association in an environment conducive to the maintenance of infection (high reptile population and diversity, apparent absence of adverse effects on the host, an abundance of actual and potential arthropod hosts, and a climate favourable to ectotherms) has provided a stimulus for the maintenance of a rich spirurid fauna. These factors allow large populations of nematodes to coexist. Studies on further identification of arthropod intermediate hosts, stresses put on nematodes during prolonged periods when the hosts do not feed, molecular studies to investigate the robustness of identifications based on morphology alone, and whether reptiles derive any benefit from supporting an abundance of nematodes, would add considerably to understanding the ecology and evolution of this successful group of nematodes.
Acknowledgements
I am grateful to the many Curators of Reptiles, acknowledged by name in previous publications, in Australian Museums in which I have worked; they have invariably been helpful and supportive. Russ Hobbs provided invaluable statistical assistance, and both he and Dave Spratt read and made many constructive suggestions on improving this manuscript. I am also grateful to two anonymous referees, whose comments and suggestions have further strengthened this paper.
References
Aho, J. M. (1990). Helminth communities of amphibians and reptiles: comparative approaches to understanding patterns and processes. In ‘Parasite Communities: Patterns and Processes’. (Eds G. Esch, A Bush and J. Aho.) pp. 157–195. (Chapman and Hall: London and New York.)Anderson, R. C. (2000). ‘Nematode Parasites of Vertebrates.’ 2nd edn. (CABI Publishing: Wallingford, UK.)
Ast, J. C. (2001). Mitochondrial DNA evidence and evolution in Varanoidea (Squamata). Cladistics 17, 211–226.
| Mitochondrial DNA evidence and evolution in Varanoidea (Squamata).Crossref | GoogleScholarGoogle Scholar |
Baverstock, P. R., King, D., King, M., Birrell, J., and Krieg, M. (1993). The evolution of species of the Varanidae: microcomplement fixation analysis of serum albumins. Australian Journal of Zoology 41, 621–638.
| The evolution of species of the Varanidae: microcomplement fixation analysis of serum albumins.Crossref | GoogleScholarGoogle Scholar |
Baylis, H. A. (1924). A new species of Physaloptera (Nematoda) from an Australian lizard. Annals & Magazine of Natural History 13, 309–311.
Bush, A. O., Lafferty, K. D., and Shostak, A. W. (1997). Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology 83, 575–583.
| Parasitology meets ecology on its own terms: Margolis et al. revisited.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2svht1OmsA%3D%3D&md5=db67b8832bb604e6e07d120c25b39539CAS | 9267395PubMed |
Chabaud, A. G. (1956). Essai de révision des physaloptères parasites de reptiles. Annales de Parasitologie Humaine et Comparee 31, 29–52.
| 1:STN:280:DyaG28%2FptlWgsA%3D%3D&md5=519290d08bcca5935243fd99def31136CAS | 13340427PubMed |
Chabaud, A. G. (1975). ‘CIH Keys to the nematode parasites of vertebrates. No. 3. Keys to the order Spirurida. Part 1. Camallanoidea, Dracunculoidea, Gnathostomatoidea, Physalopteroidea, Rictularioidea and Thelazioidea.’ (Eds R. C. Anderson, A. G. Chabaud and S. Willmott.) (Commonwealth Agricultural Bureaux, Farnham Royal: Buckinghamshire, UK.)
Christian, K. A., and Green, B. (1994). Seasonal energetic and water turnover of the frillneck lizard, Chlamydosaurus kingii, in the wet–dry tropics of Australia. Herpetologica 50, 274–281.
Christian, K. A., Griffiths, A. D., and Bedford, G. (1995). Physiological ecology of frill-neck lizards in a seasonal tropical environment. Oecologia 106, 49–56.
Cogger, H. G. (2014). ‘Reptiles and Amphibians of Australia.’ 7th edn. (CSIRO Publishing: Melbourne.)
Dewi, K., Jones, H. I., and Hamidy, A. (2008). The status of Tanqua anomala (von Linstow, 1904) (Nematoda: Gnathostomatoidea). Transactions of the Royal Society of South Australia 132, 7–13.
Fitch, A. J., Goodman, A. E., and Donnellan, S. C. (2006). A molecular phylogeny of the Australian monitor lizards (Squamata: Varanidae) inferred from mitochondrial DNA sequences. Australian Journal of Zoology 54, 253–269.
| A molecular phylogeny of the Australian monitor lizards (Squamata: Varanidae) inferred from mitochondrial DNA sequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XotV2ru7Y%3D&md5=42071d55225d331747eaf50d6af5853dCAS |
Gems, D. (2000). Longevity and ageing in parasitic and free-living nematodes. Biogerontology 1, 289–307.
| Longevity and ageing in parasitic and free-living nematodes.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3Mnmt1yhsw%3D%3D&md5=e471609cfaeeeda057a0c88db8e77305CAS | 11708211PubMed |
Goldberg, S. R., and Bursey, C. R. (2012). Intestinal helminths in nine species of endemic Australian lizards, Lophognathus longirostris (Agamidae), Heteronotia binoei and Lucasium stenodactylum (Gekkonidae), Ctenotus grandis, Ctenotus helenae, Cyclodomorphus branchialis, Egernia depressa, Eremiascincus richardsonii, Morethia butleri and Morethia lineoocellata (Scincidae), with a review of Australian lizard helminths. Comparative Parasitology 79, 247–268.
| Intestinal helminths in nine species of endemic Australian lizards, Lophognathus longirostris (Agamidae), Heteronotia binoei and Lucasium stenodactylum (Gekkonidae), Ctenotus grandis, Ctenotus helenae, Cyclodomorphus branchialis, Egernia depressa, Eremiascincus richardsonii, Morethia butleri and Morethia lineoocellata (Scincidae), with a review of Australian lizard helminths.Crossref | GoogleScholarGoogle Scholar |
Hatcher, M. J., and Dunn, A. M. (2011). ‘Parasites in Ecological Communities.’ (Cambridge University Press: Cambridge.)
Hay, M. (1972). The breeding of Physignathus lesueurii L. in captivity. Herpetofauna 5, 2–3.
Holmes, R., and Light, A. (1983). A serendipitous age estimation of a lizard, Tiliqua rugosa (Lacertilia: Scincidae). Western Australian Naturalist 15, 159–160.
Hugall, A. F., Foster, R., Hutchinson, M., and Lee, M. S. (2008). Phylogeny of Australasian agamid lizards based on nuclear and mitochondrial genes: implications for morphological evolution and biogeography. Biological Journal of the Linnean Society 93, 343–358.
| Phylogeny of Australasian agamid lizards based on nuclear and mitochondrial genes: implications for morphological evolution and biogeography.Crossref | GoogleScholarGoogle Scholar |
Irwin-Smith, V. (1922). Notes on nematodes in the genus Physaloptera. Part III. The Physaloptera of Australian lizards. Proceedings of the Linnean Society of New South Wales 46, 232–244.
James, C. D., Losos, J. B., and King, D. R. (1992). Reproductive biology and diets of goannas (Reptilia: Varanidae) from Australia. Journal of Herpetology 26, 128–136.
| Reproductive biology and diets of goannas (Reptilia: Varanidae) from Australia.Crossref | GoogleScholarGoogle Scholar |
Johnston, T. H., and Mawson, P. M. (1941). Some parasitic nematodes in the collection of the Australian Museum. Records of the Australian Museum 21, 9–16.
| Some parasitic nematodes in the collection of the Australian Museum.Crossref | GoogleScholarGoogle Scholar |
Johnston, T. H., and Mawson, P. M. (1942a). The Gallard collection of parasitic nematodes in the Australian Museum. Records of the Australian Museum 21, 110–115.
| The Gallard collection of parasitic nematodes in the Australian Museum.Crossref | GoogleScholarGoogle Scholar |
Johnston, T. H., and Mawson, P. M. (1942b). Some new and known Australian parasitic nematodes. Proceedings of the Linnean Society of New South Wales 67, 90–94.
Johnston, T. H., and Mawson, P. M. (1947). Some nematodes from Australian lizards. Transactions of the Royal Society of South Australia 71, 22–27.
Johnston, T. H., and Mawson, P. M. (1948). Some new records of nematodes from Australian snakes. Records of the South Australian Museum 9, 101–106.
Johnston, T. H., and Mawson, P. M. (1951). Report on some parasitic nematodes from the Australian Museum. Records of the Australian Museum 22, 289–297.
| Report on some parasitic nematodes from the Australian Museum.Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (1978a). Abbreviata (Nematoda: Physalopteroidea) from Western Australian snakes. Australian Journal of Zoology 26, 789–807.
| Abbreviata (Nematoda: Physalopteroidea) from Western Australian snakes.Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (1978b). Gastrointestinal nematodes from aquatic Australian snakes. Memoirs of the Queensland Museum 118, 243–254.
Jones, H. I. (1979). Gastrointestinal nematodes, including three new species, from Australian and Papua New Guinean pythons. Proceedings of the Helminthological Society of Washington 46, 1–14.
Jones, H. I. (1980). Observations on nematodes from west and central Australian snakes. Australian Journal of Zoology 28, 423–433.
| Observations on nematodes from west and central Australian snakes.Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (1983a). Prevalence and intensity of Abbreviata Travassos (Nematoda: Physalopteridae) in the ridge-tailed monitor Varanus acanthurus Boulenger in northern Australia. Records of the Western Australian Museum 11, 1–9.
Jones, H. I. (1983b). Abbreviata (Nematoda: Physalopteridae) in lizards of the Varanus gouldii complex (Varanidae) in Western Australia. Australian Journal of Zoology 31, 285–298.
| Abbreviata (Nematoda: Physalopteridae) in lizards of the Varanus gouldii complex (Varanidae) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (1985a). Two new species of nematode (Spirurida: Physalopteridae) from Australian lizards (Reptilia: Scincidae: Gekkonidae). Journal of Natural History 19, 1231–1237.
| Two new species of nematode (Spirurida: Physalopteridae) from Australian lizards (Reptilia: Scincidae: Gekkonidae).Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (1985b). Gastrointestinal nematodes of the perentie, Varanus giganteus (Grey), in Western Australia, with description of a new species of Abbreviata Travassos (Nematoda: Physalopteridae). Records of the Western Australian Museum 12, 379–387.
Jones, H. I. (1986). Gastrointestinal nematodes in the lizard genus Pogona Storr, 1982 (Agamidae) in Western Australia. Australian Journal of Zoology 34, 689–705.
| Gastrointestinal nematodes in the lizard genus Pogona Storr, 1982 (Agamidae) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (1988). Nematodes from nine species of Varanus (Reptilia) from tropical Northern Australia, with particular reference to the genus Abbreviata (Physalopteridae). Australian Journal of Zoology 36, 691–708.
| Nematodes from nine species of Varanus (Reptilia) from tropical Northern Australia, with particular reference to the genus Abbreviata (Physalopteridae).Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (1992a). Gastrointestinal nematodes in the lizard genera Tiliqua and Cyclodomorphus (Scincidae) in Western Australia. Australian Journal of Zoology 40, 115–126.
| Gastrointestinal nematodes in the lizard genera Tiliqua and Cyclodomorphus (Scincidae) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (1992b). Gastrointestinal nematodes of Varanus caudolineatus (Reptilia: Varanidae) in Western Australia. Records of the Western Australian Museum 16, 109–112.
Jones, H. I. (1994). Gastrointestinal nematodes of the frillneck lizard, Chlamydosaurus kingii (Agamidae) with particular reference to Skrjabinoptera goldmanae (Spirurida: Physalopteridae). Australian Journal of Zoology 42, 371–377.
| Gastrointestinal nematodes of the frillneck lizard, Chlamydosaurus kingii (Agamidae) with particular reference to Skrjabinoptera goldmanae (Spirurida: Physalopteridae).Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (1995a). The functional anatomy of the anterior end of Skrjabinoptera goldmanae from Australian agamid lizards. Journal of Helminthology 69, 33–37.
| The functional anatomy of the anterior end of Skrjabinoptera goldmanae from Australian agamid lizards.Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (1995b). Gastric nematode communities in lizards from the Great Victoria Desert, and an hypothesis for their evolution. Australian Journal of Zoology 43, 141–164.
| Gastric nematode communities in lizards from the Great Victoria Desert, and an hypothesis for their evolution.Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (1995c). Pathology associated with physalopterid larvae (Nematoda: Spirurida) in the gastric tissues of Australian reptiles. Journal of Wildlife Diseases 31, 299–306.
| Pathology associated with physalopterid larvae (Nematoda: Spirurida) in the gastric tissues of Australian reptiles.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK287ntlGguw%3D%3D&md5=b83a002797fc17bf4c4144927434cfddCAS | 8592348PubMed |
Jones, H. I. (2003). Parasitic worms of reptiles from Tasmania and the islands of Bass Strait. Papers and Proceedings of the Royal Society of Tasmania 137, 7–12.
Jones, H. I. (2004). Gastric nematodes, including a new species of Abbreviata (Spirurida: Physalopteridae) from the mangrove monitor, Varanus indicus (Reptilia: Varanidae). Transactions of the Royal Society of South Australia 128, 53–59.
Jones, H. I. (2005). The gastrointestinal nematodes of Varanus rosenbergi (Reptilia: Varanidae) and the effects of habitat change in southern Australia, with particular reference to the genus Abbreviata (Physalopteroidea). Records of the Western Australian Museum 22, 259–263.
Jones, H. I. (2007a). Nematodes of the water dragon, Physignathus lesueurii (Reptilia: Agamidae) in Australia, with a description of Spinicauda fluviatica sp. nov. (Nematoda: Heterakoidea). Australian Journal of Zoology 55, 161–168.
| Nematodes of the water dragon, Physignathus lesueurii (Reptilia: Agamidae) in Australia, with a description of Spinicauda fluviatica sp. nov. (Nematoda: Heterakoidea).Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (2007b). Helminth parasites of the lace monitor, Varanus varius (Reptilia: Varanidae) in eastern Australia. Transactions of the Royal Society of South Australia 131, 162–168.
Jones, H. I. (2010). Dwarf monitor lizards (Varanidae: Varanus, Odatria s. gen) as definitive and paratenic hosts for physalopteran nematodes. Australian Journal of Zoology 58, 69–75.
| Dwarf monitor lizards (Varanidae: Varanus, Odatria s. gen) as definitive and paratenic hosts for physalopteran nematodes.Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (2013a). Gastrointestinal nematodes from three species of Australian leaf-tailed gecko (Reptilia: Saltuarius spp), with descriptions of new species of Skrjabinodon (Oxyuroidea: Pharyngodonidae) and Hedruris (Habronematoidea: Hedruridae). Comparative Parasitology 80, 47–59.
| Gastrointestinal nematodes from three species of Australian leaf-tailed gecko (Reptilia: Saltuarius spp), with descriptions of new species of Skrjabinodon (Oxyuroidea: Pharyngodonidae) and Hedruris (Habronematoidea: Hedruridae).Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (2013b). Gastrointestinal nematodes of the Australian leaf-tailed gecko Phyllurus platurus (Reptilia: Gekkonidae), with a redescription of Abbreviata bancrofti (Irwin-Smith, 1922), and observations on physalopterid larvae. Comparative Parasitology 80, 217–224.
| Gastrointestinal nematodes of the Australian leaf-tailed gecko Phyllurus platurus (Reptilia: Gekkonidae), with a redescription of Abbreviata bancrofti (Irwin-Smith, 1922), and observations on physalopterid larvae.Crossref | GoogleScholarGoogle Scholar |
Jones, H. I. (2013c). Gastric nematodes from the plains goanna, Varanus spenceri (Reptilia: Varanidae), from central Queensland. Memoirs of the Queensland Museum 56, 457–460.
Jones, H. I., and Watharow, S. (2010). Gastrointestinal helminths in two allopatric sibling species of swamp skink, Lissolepis coventryi and Lissolepis luctuosus (Reptilia: Scincidae), from south-eastern and south-western Australia, with descriptions of three new species of nematode. Comparative Parasitology 77, 37–51.
| Gastrointestinal helminths in two allopatric sibling species of swamp skink, Lissolepis coventryi and Lissolepis luctuosus (Reptilia: Scincidae), from south-eastern and south-western Australia, with descriptions of three new species of nematode.Crossref | GoogleScholarGoogle Scholar |
King, D. (2004). Varanus pilbarensis. In ‘Varanoid Lizards of the World’. (Eds E. R. Pianka, D. R. King and R. A. King.) pp. 430–433. (Indiana University Press: Bloomington and Indianapolis.)
King, D., and Green, B. (1999). ‘Goannas. The Biology of Varanid Lizards.’ (University of New South Wales Press: Sydney.)
King, C., Jones, H. I., and Tay, A. (2013). Arthropod intermediate hosts of Abbreviata antarctica (Nematoda: Physalopteridae) in Australia. The Journal of Parasitology 99, 708–711.
| Arthropod intermediate hosts of Abbreviata antarctica (Nematoda: Physalopteridae) in Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3szjsV2gtg%3D%3D&md5=03b869714c9db5aac85d00366029b8e6CAS | 23360383PubMed |
Kreis, H. A. (1940). Beiträge zur Kenntnis parasitischer Nematoden. IX. Parasitische nematoden aus dem Naturhistorischen Museum Basel. Zentralblatt für Bakteriologie. Abt.1. Originale 145, 163–208.
Lant, B., and Storey, K. B. (2010). An overview of stress response and hypometabolic strategies in Caenorhabditis elegans. Conserved and contrasting signals with the mammalian system. International Journal of Biological Sciences 6, 9–50.
| An overview of stress response and hypometabolic strategies in Caenorhabditis elegans. Conserved and contrasting signals with the mammalian system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXntV2jtA%3D%3D&md5=d2e61c675fcd3218100ac0a5378052d0CAS | 20087441PubMed |
Mawson, P. M. (1970). Skrjabinoptera goldmanae n. sp. (Nematoda: Physalopteridae) from an Australian agamid lizard. Transactions of the Royal Society of South Australia 94, 223–225.
Paterson, S., and Viney, M. E. (2002). Host immune responses are necessary for density dependence in nematode infections. Parasitology 125, 283–292.
| Host immune responses are necessary for density dependence in nematode infections.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38vpslSktg%3D%3D&md5=4885ffe388fa3d58677ce3d58a60bfe4CAS | 12358425PubMed |
Pianka, E. R. (1970). Notes on the biology of Varanus gouldii. Western Australian Naturalist 11, 141–144.
Pianka, E. R. (1971). Notes on the biology of Varanus tristis. Western Australian Naturalist 11, 180–183.
Pianka, E. R. (1986). ‘Ecology and Natural History of Desert Lizards.’ (Princeton University Press: New Jersey.)
Pianka, E. R. (1994). Comparative ecology of Varanus in the Great Victoria Desert. Australian Journal of Ecology 19, 395–408.
| Comparative ecology of Varanus in the Great Victoria Desert.Crossref | GoogleScholarGoogle Scholar |
Pianka, E. R., and King, D. R. (2004). Introduction. In ‘Varanoid Lizards of the World’. (Eds E. R. Pianka, D. R. King, and R. A. King.) pp. 1–8. (Indiana University Press: Bloomington and Indianapolis.)
Quinnell, R. J., Medley, G. F., and Keymer, A. E. (1990). The regulation of gastrointestinal helminth populations. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences 330, 191–201.
| The regulation of gastrointestinal helminth populations.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M7msVCjsA%3D%3D&md5=8ca369e6d4b39fe8d66bd66be2efb6a6CAS |
Shine, R. (1986). Food habits, habitats and reproductive biology of four sympatric species of varanid lizards in tropical Australia. Herpetologica 42, 346–360.
Shine, R., and Lambeck, R. (1989). Ecology of frillneck lizards, Chlamydosaurus kingii (Agamidae), in tropical Australia. Australian Wildlife Research 16, 491–500.
| Ecology of frillneck lizards, Chlamydosaurus kingii (Agamidae), in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Sprent, J. F. A. (1988). Ascaridoid nematodes of amphibians and reptiles: Ophidascaris Baylis, 1920. Systematic Parasitology 11, 165–213.
| Ascaridoid nematodes of amphibians and reptiles: Ophidascaris Baylis, 1920.Crossref | GoogleScholarGoogle Scholar |
Thomas, P. M. (1959). Some nematode parasites from Australian hosts. Transactions of the Royal Society of South Australia 82, 151–162.
Viney, M. E., Steer, M. D., and Wilkes, C. P. (2006). The reversibility of constraints on size and fecundity in the parasitic nematode Strongyloides ratti. Parasitology 133, 477–483.
| The reversibility of constraints on size and fecundity in the parasitic nematode Strongyloides ratti.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28rjsFGltA%3D%3D&md5=c183ba2b438705a6a00546e2751b94fdCAS | 16817996PubMed |
Watharow, S., and Jones, H. I. (2009). Gastric nematodes of snakes and lizards from the Victorian mallee. Herpetofauna 39, 21–27.