A molecular and morphological investigation of species boundaries and phylogenetic relationships in Australian free-tailed bats Mormopterus (Chiroptera : Molossidae)
T. B. Reardon A B F , N. L. McKenzie C , S. J. B. Cooper A B , B. Appleton D , S. Carthew E and M. Adams A BA School of Earth and Environmental Sciences and Australian Centre for Evolutionary Biology and Biodiversity, University of Adelaide, Adelaide, SA 5005, Australia.
B Evolutionary Biology Unit, South Australian Museum, North Terrace, Adelaide, SA 5000, Australia.
C Department of Parks and Wildlife, PO Box 51, Wanneroo, WA 6846, Australia.
D School of Life and Environmental Sciences, Deakin University, Waurn Ponds, Vic. 3216, Australia.
E Research Institute for Environment and Livelihoods, Charles Darwin University, Darwin, NT 0909, Australia.
F Corresponding author. Email: terry.reardon@samuseum.sa.gov.au
Australian Journal of Zoology 62(2) 109-136 https://doi.org/10.1071/ZO13082
Submitted: 10 October 2013 Accepted: 2 February 2014 Published: 19 June 2014
Abstract
The taxonomic uncertainty surrounding several prominent genera of Australian microbat has been a long-standing impediment to research and conservation efforts on these groups. The free-tail bat genus Mormopterus is perhaps the most significant example, with a long history of acknowledged species-level confusion. This study uses a combined molecular and morphological approach to conduct a comprehensive assessment of species and subgeneric boundaries, between-species phylogenetic affinities and within-species phylogeographic structure in Australian members of Mormopterus. Phylogenetic analyses based on 759 base pairs of the NADH Dehydrogenase subunit 2 mitochondrial gene were concordant with species boundaries delineated using an expanded allozyme dataset and by phallic morphology, and also revealed strong phylogeographic structure within two species. The levels of divergence evident in the molecular and morphological analyses led us to recognise three subgenera within Australia: Micronomus, Setirostris subgen. nov. and Ozimops subgen. nov. Within Ozimops we recognise seven Australian species, three of which are new, and none are conspecific with Indo-Papuan species. The family Molossidae now comprises eleven species across three subgenera in Australia, making it the continent’s second most speciose family of bats.
Additional keywords: cryptic species, morphometrics, mtDNA barcoding, subgenera.
Introduction
The order Chiroptera (bats) is highly diverse worldwide and contains about one fifth of all mammalian species, with ~1200 species now described (Simmons 2005). Bats are also a significant component of Australia’s terrestrial mammalian fauna (roughly 23%), where they are currently represented by 70 extant species in 25 genera, spread across eight of the world’s 19 bat families (Churchill 2008). Despite this prominence, several key taxonomic issues remain unresolved, including the need for formal recognition of known cryptic species, delineation of suspected cryptic taxa, clarification of the taxonomic affinities of Australian and extralimital populations of what are apparently the same species, and elucidation of higher-level systematic relationships. A rigorous systematic framework for Australian bats is both overdue and necessary to underpin research and conservation, especially given that over 10% of species are currently listed nationally as threatened (http://www.environment.gov.au/cgi-bin/sprat/public/publicthreatenedlist.pl?wanted = fauna August 2013).
The family Molossidae is currently represented in Australia by three genera, the polytypic Mormopterus Peters, and the two monotypic genera Austronomus Troughton and Chaerephon Dobson. Mormopterus, as currently defined in Simmons (2005), is represented worldwide by three species groups, one in Madagascar, Mascarene Islands and Sumatra (four species in total), another in the Neotropics (three species), and the third group comprising an unresolved complex in the Indo-Australian region. These three groups are likely to be polyphyletic (Lamb et al. 2011) and the Indo-Australian group has previously been recognised as a separate genus, namely Micronomus (Troughton 1944). Australian Mormopterus comprises a distinctive group of small, free-tailed bats found throughout the Australian mainland, with representatives in most major habitat types (Van Dyck and Strahan 2008). Mormopterus taxa occur in moderate abundance over much of the continent and are an often surveyed component of the microbat fauna. However, the genus is widely acknowledged to be in taxonomic disarray (Adams et al. 1988; Churchill 2008; Van Dyck and Strahan 2008).
The taxonomic history of Australian Mormopterus until 1989 is thoroughly reviewed in Mahoney and Walton (1988) and in Allison (1989). Both reviews concluded by tentatively recognising three species in Australia, M. norfolkensis, M. planiceps, and M. beccarii, and both made it clear that this was an interim arrangement and that cryptic species were likely to be present. Significant reviews or comments on the species-level taxonomy of Australian Mormopterus before this time include those of Dobson (1876), Troughton (1926, 1941), Iredale and Troughton (1934), Tate (1941, 1952), Hill (1961, 1983), Felten (1964), Ride (1970), Hall and Richards (1979), Freeman (1981), Koopman (1984), Legendre (1984) and Peterson (1985).
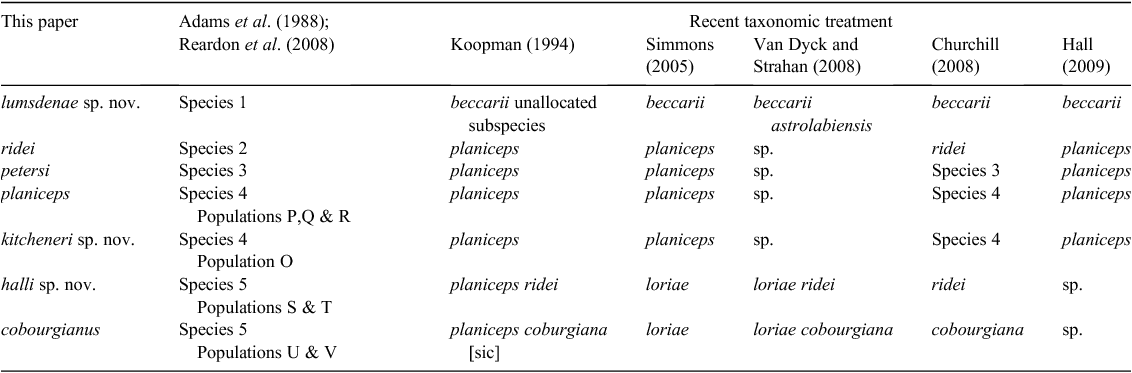
Around the time of the 1988/89 reviews an allozyme study by Adams et al. (1988) on Australian Molossidae was published, and it provided a new taxonomic hypothesis that recognised a minimum of six Mormopterus species (referred to in the paper as Species 1 through Species 6; for convenience, we hereinafter use this nomenclature without citing Adams et al. (1988) on each occasion). In addition, two of the proposed species (Species 4 and Species 5), each comprised two genetically distinct, eastern and western allopatric populations of uncertain taxonomic status. Adams et al. (1988) declined to equate any of their candidate species to existing named forms other than to say that Species 3 and Species 4 together ‘… appear to correspond to planiceps’. Importantly, tissues from typical M. norfolkensis specimens were not available to the Adams et al. (1988) study, implying the likely presence of a seventh candidate species in the genus. The taxa delineated by Adams et al. (1988) were quickly and widely adopted as operational taxonomic units by Australian bat researchers and were thereafter referred to under a range of formal and informal names. This remained the de facto situation for Australian Mormopterus until Reardon et al. (2008) clarified the taxonomic status of Mormopterus norfolkensis (Gray, 1839) in Australia and described a new species M. eleryi Reardon & McKenzie 2008 (equivalent to Species 6). The taxonomic resolution of the remaining group Species 1–5, referred to as the ‘planiceps–beccarii–loriae complex’ in Reardon et al. (2008) and hereinafter as the ‘planiceps complex’, is the subject of this paper.
Herein we present a molecular and morphological assessment of the ‘planiceps complex’. This study has the 3-fold aims of (1) formally stabilising species boundaries in this complex, (2) providing a solid phylogenetic framework for the genus as a null hypothesis for future investigations, and (3) documenting cases of strong phylogeographic structure within each species. Although this study has focussed on the resolution of the systematics of Australian members of Mormopterus, we are mindful that this group is clearly allied to taxa in the Indo-Papuan region. Having examined many of the available New Guinean and Indonesian specimens, including type material, it is clear to us that the taxonomic situation in that region is complex and a satisfactory resolution will require further assessment of additional key specimens. We do show, however, that the named forms from that region (namely, beccarii, astrolabiensis and loriae) are taxonomically distinct from the Australian species described herein.
Materials and methods
Allozyme analyses
An allozyme study of 65 previously uncharacterised tissues, representing the key morphotypes and geographic regions covered by all new vouchers or specimens collected since 1988, were genotyped at a suite of 41 putative allozyme loci. The loci in question were the 40 loci surveyed by Reardon et al. (2008) plus the single locus encoded by the enzyme Glutathione peroxidase (GPX; E.C. number 1.11.1.9). Included in this study were 12 previously genotyped tissues from the original study of Adams et al. (1988), which served as reference profiles for Species 1–6 and therefore permitted us to combine all three studies into a single allozyme dataset. As for the previous allozyme studies, the Australian molossids Austronomus australis and Chaerephon jobensis were included as outgroup species. All tissues were obtained from the Australian Biological Tissues Collection (ABTC), based at the South Australian Museum. Details of all specimens included in the combined allozyme dataset are provided in the Supplementary Material.
Our laboratory methods used for allozyme electrophoresis of liver homogenates follow those detailed previously (Adams et al. 1988; Reardon et al. 2008). The data were initially analysed using the multivariate clustering technique of Principal Coordinates Analysis (PCO), which has been found previously to be ideal for identifying cohesive genetic groupings among individuals, independent of any a priori expectations as to taxon (Horner and Adams 2007). Stepwise PCO was undertaken on the overall pair-wise matrix of Roger’s genetic distance among individuals, and on various subsets of these individuals, as fully detailed in Horner and Adams (2007). Subsequent labelling of individuals on these various PCOs according to morphotype and/or mtDNA lineage permitted us to define taxa based on concordant, diagnostic differences across all datasets examined. For the allozyme comparisons, we used the presence of multiple fixed differences (as defined in Reardon et al. 2008) among taxa as the best measure of taxon diagnosability. Thereafter, a Neighbour Joining (NJ) tree, including bootstrap values from 1000 pseudoreplicates, was constructed from a pair-wise matrix of unbiased Nei’s Distances, according to the methodologies presented in Adams et al. (2013).
Mitochondrial DNA sequencing and analysis
In total, 91 tissue samples, either as frozen or alcohol-preserved liver, kidney or wing tissue, were obtained either from museum collections, or from specimens specifically collected for the project and subsequently released in the field. Tissues were selected to cover the available geographic spread in Australia of each of Species 1–5, and where available included specimens also represented in one or more of the allozyme and morphological datasets (Supplementary Material). Tissues of M. eleryi and M. norfolkensis as well as M. loriae and M. beccarii astrolabiensis from Papua New Guinea and Mormopterus cf. beccarii from Ceram, Indonesia, were also sequenced, as were reference specimens of the three molossid outgroup taxa Cheiromeles torquatus, Chaerephon jobensis, and Austronomus australis. The recent phylogenetic study of world molossid genera (Ammerman et al. 2012) showed Cheiromeles as a basal lineage to all molossids and it was initially used as an outgroup to show that Austronomus represented a sister lineage to the Australian Mormopterus.
Total genomic DNA was extracted from these tissues using the Qiagen DNeasy kit and following the manufacturer’s protocol. An ~760-bp region of the mitochondrial gene NADH dehydrogenase subunit 2 (ND2) gene was amplified by polymerase chain reaction (PCR) using the primers Forward 5′-CTAATTAAGCTATCGGGCCCATAC-3′ and Reverse 5′-TTCTTGGATRATTAKTCATTTKGG-3′. These primers were modified from L-5208 and H-6315 respectively from Kirchman et al. (2001). Each 50-μL reaction contained 0.8 μm of each primer, 0.25 mm dNTP, 1U of HotMaster Taq DNA polymerase (Eppendorf) or Taq DNA polymerase (Promega), 1 × corresponding PCR buffer (2 mm MgSO4), and ~50–100 ng template DNA. The amplification protocol comprised denaturation at 94°C for 2 min, followed by 35 cycles of denaturing for 45 s at 92°C, 45 s of annealing at 50−55°C, and 60 s extension at 72°C. The reaction ended with one longer extension phase at 72°C for 5 min. PCR clean-up and sequencing was carried out by Macrogen (Seoul, S. Korea – http://dna.macrogen.com), using the same ND2 primers for sequencing.
PCR amplifications with these ND2 primers failed to amplify DNA from some key specimens where the only available tissue was highly degraded (Roper River, Northern Territory; Kandanji village, Papua New Guinea; Solea village, Ceram, Indonesia). We designed the following internal primers: forward 5′-GAGTCCCAGAAGTAACCCAAGG-3′, 5′-ACAAGCTCACAYTGAC-3′ and 5′-CAGGCCAATGAACTGTCA-3′ and reverse 5′-GTTTGGTTTAGTCCTCCTCA-3′, 5′-GTGGTTGTGGTGATTAGTG-3′ and 5′-GGATTCCTTGGGTTACTTC-3′ and, using the same PCR conditions above, we were able to amplify and assemble a composite sequence of ~450–680 bp for each of these species.
Forward and reverse sequences were assembled using BioEdit ver. 7.1.11 (Hall 1999) and aligned by eye. Each sequence was translated to an amino acid sequence using MEGA ver. 5 (Tamura et al. 2011) and checked for the presence of stop or nonsense codons or frameshifts indicative of the presence of nuclear copies of mitochondrial DNA in the dataset (Zhang and Hewitt 1996).
Phylogenetic analyses were conducted using Bayesian inference (BI) and implemented using MrBayes 3.1.2 (Ronquist and Huelsenbeck 2003). We used MrModeltest 2.2 (Nylander 2004) to identify the best-fit model of sequence evolution using the Akaike Information Criterion. This produced a general time-reversible (GTR) model (Tavaré 1986) with a proportion of invariant sites (I) and gamma distributed among-site rate variation (Yang 1996) (i.e. GTR + I + G). We ran the analysis for five million generations, sampling every 1000 generations. The average standard deviation of split frequencies reached 0.008. We used Tracer ver. 1.5 (Rambaut and Drummond 2007) to test whether the analyses had sufficient effective sample sizes (>200) for each parameter. The first one million generations were discarded as burn-in.
A matrix of uncorrected pair-wise distances (p-distance) among species and among key phylogeographic lineages within species was generated using the program MEGA ver. 5 (Tamura et al. 2011).
Morphological analyses
In total, 479 specimens of Australian Mormopterus and 51 specimens from New Guinea and Indonesia formed the basis for the mensurative component of the first part of the study to establish species boundaries and nomenclature. An additional 648 museum and field specimens were subsequently identified to species using the morphological criteria defined in the first part of the project, and these were used to supplement the distribution maps. Specimens were drawn from existing museum collections or collected specifically for this study under permit. Relevant details for all specimens used in this study are given in the Supplementary Material. Cranial photographs were taken with a Canon 450D camera mounted on a Stackshot step driver (Cognisys Inc.) and each final photograph is the combined stack of 10 images using Zerene Stacker ver. 1.04 (Zerene Systems).
Cranial and external measurements
The following 12 external and 11 cranial measures, with abbreviations in parentheses, were taken to the nearest 0.01 mm with digital calipers in combination with a binocular microscope: forearm length (FA), metacarpal digit 3 length (D3M), proximal phalanx length digit 3 (D3PP), middle phalanx digit 3 length (D3MP), metacarpal digit 4 length (D4M), proximal phalanx length digit 4 (D4PP), middle phalanx digit 4 length (D4MP), metacarpal digit 5 length (D5M), proximal phalanx length digit 5 (D5PP), middle phalanx digit 5 length (D5MP), tibia length (TL), pes length (PL), greatest skull length (GSL), condylobasal length (CBL), mastoid breadth of the skull (MB), postzygomatic breadth (PZB), postpalatal length (PPL), palate length (PaL), interorbital breadth (IOB), length from outer margins of upper canine to third molar (C1-M3), width across outer margins of upper third molars (M3-M3), width between outer margins of upper canines (C1-C1). The final cranial measure, skull height (SH) was measured using calipers that were modified by having a notch ground into one of the tangs, so that this side could be laid transversely across the pterygoid processes at the postpalatal margin while the other tang was placed at the top of skull. This measure would not be accessible with unmodified calipers without causing damage to the zygomatic arch. All univariate statistics, bivariate plots, Principal Components Analyses (PCA) and Discriminant Function Analyses (DFA) were generated using Statistica ver. 12 (StatSoft Inc.).
Phallic morphology
The glans penis is defined here as in Ryan (1991), being that portion of the penile shaft distal to the point where the inner prepuce (foreskin) adheres to the shaft; this junction is usually about one-third the length of the penis from where it joins the body. All species have a prepuce (foreskin) that covers the glans penis when the penis is flaccid, so it was necessary to manipulate the prepuce back along the penile shaft to fully expose the glans penis. This manipulation is not difficult in live animals, but is often difficult in preserved specimens. We therefore made a single cut along the prepuce on the side of the penis to expose the glans penis for examination in some specimens. We noted that in most preserved specimens, the shape of the glans penis was compromised, typically with marked dorso-ventral flattening of the glans body as well as distortion due to dehydration. Since our descriptions were based mainly on preserved specimens, they may vary a little from those that might be observed on a live animal. The descriptions and illustrations in this study were derived from observations made under a light microscope, which placed limits on our ability to resolve some of the finer detail of the surface structures. Glans penis photographs were taken with a Canon 450D camera with a Photar microscope lens; the camera was mounted on a Stackshot step driver (Cognisys Inc.) and each final photograph is the combined stack of 50 images using Zerene Stacker ver. 1.04 (Zerene Systems).
Institution and collection acronyms
ABTC: Australian Biological Tissue Collection, South Australian Museum, Adelaide, Australia; AMS: Australian Museum, Sydney, Australia; ANWC: Australian National Wildlife Collection, Canberra, Australia; BMNH: The Natural History Museum, London, United Kingdom; DECWA: Department of Environment and Conservation, Perth, Western Australia; MSNG: Museo Civico di Storia Naturale di Genova ‘Giacomo Doria’, Genova, Italy; NMV: National Museum of Victoria, Melbourne, Australia; NRM: Naturhistoriska Rijkmuseet, Stockholm, Sweden; NTM: Museum and Art Gallery of the Northern Territory, Darwin, Australia; QM: Queensland Museum, Brisbane, Australia; ROM: Royal Ontario Museum, Toronto, Canada; SAMA: South Australia Museum, Adelaide, Australia; SMF: Forschungsinstitut und Natur-Museum Senckenberg, Frankfurt-am-Main 1, Germany; UNIMAS: Universiti Malaysia Sarawak, Kota Samarahan, Sarawak; UPNG: University of Papua New Guinea Port Moresby, Papua New Guinea; USNM: National Museum of Natural History, Washington, DC, USA; WAM: Western Australia Museum, Perth, Western Australia; ZMB: Universität Humboldt, Zoologisches Museum, Berlin, Germany.
Supplementary material
We have included several tables and figures in the Supplementary Material and where these are referred to in the text, the table and figure number is prefixed by ‘S’.
Results
Nomenclatural framework for this paper
Given the wide range of informal taxon names used to designate taxa in the past, and the completely new arrangement of formal names presented in this paper, we have opted for clarity by employing our final nomenclature for the new subgeneric arrangement and all seven species in the ‘planiceps complex’ from this point forward. The relationship between our new taxonomic nomenclature and the names applied to each in the recent literature is detailed in Table 1. All species in this complex belong to the new subgenus Ozimops erected herein. The justification for our application of the names to delineated species is described in the Systematics section. Newly described species have been identified using the qualifier ‘sp. nov.’ It is also important to note that some of the species retaining existing valid names have been rediagnosed herein, and are not directly equivalent to species thus labelled in previous studies.
|
Allozyme analyses
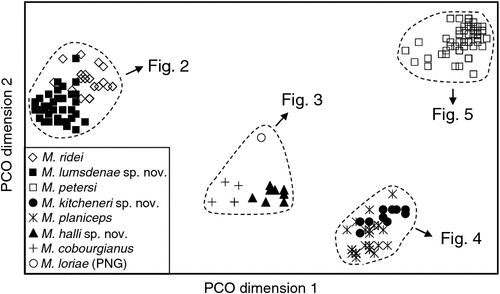
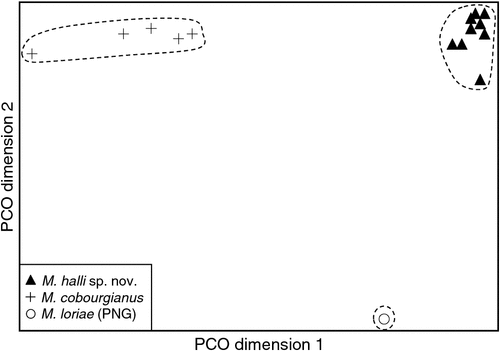
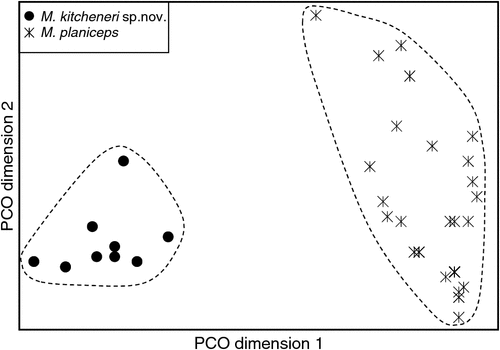
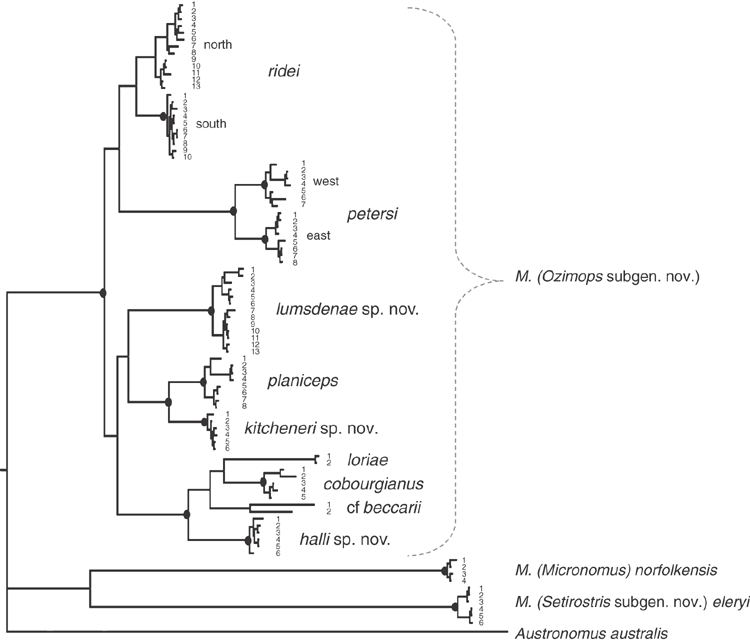
The final allozyme dataset, which combined the current study with the two previous datasets generated in the same laboratory, consisted of 230 individuals (65 from the present study and 9 from Reardon et al. (2008) (Table S1), and 156 from Adams et al. (1988)) genotyped at 35–41 allozyme loci. An initial PCO on the 193 ‘planiceps complex’ individuals revealed four primary genetic groups (Fig. 1), corresponding to (1) M. ridei/M. lumsdenae sp. nov., (2) M. halli sp. nov./M. cobourgianus/M. loriae (the latter a single tissue from PNG), (3) M. kitcheneri sp. nov./M. planiceps, and (4) M. petersi. Follow-up PCOs on each of these four groups (Figs 2–5) confirmed that all species found to overlap in the first two dimensions of the initial PCO were genuinely diagnosable from one another in deeper PCO dimensions. Most importantly, all species within the ‘planiceps complex’ displayed multiple fixed differences from one another (minimum two fixed differences between M. kitcheneri sp. nov. and M. planiceps, maximum 11 fixed differences between M. petersi and M. cobourgianus: Table S2). The allele frequencies for all molossid species surveyed and the key diagnostic loci for the seven ‘planiceps complex’ species are presented in Table S3.
|
|
|
|
|
The results of the NJ analysis of allozyme data from all 12 molossid species are presented in Fig. 6. As it is not possible to use either or both outgroups to root the NJ tree such that all species currently assigned to Mormopterus are monophyletic, the results are shown as an unrooted network. The analysis demonstrates moderate support (bootstrap = 70%) for the monophyly of all seven species within the ‘planiceps complex’ and suggests that M. eleryi and M. norfolkensis, the two other species currently assigned to the genus, are only distantly related to these other seven species and to each other. Importantly, the pair-wise genetic distance values among these three basal lineages of Australian Mormopterus are roughly double the maximum values found within the ‘planiceps complex’, being instead more comparable to those that distinguish Mormopterus from the two outgroup genera Austronomus and Chaerephon (Table S2).
|
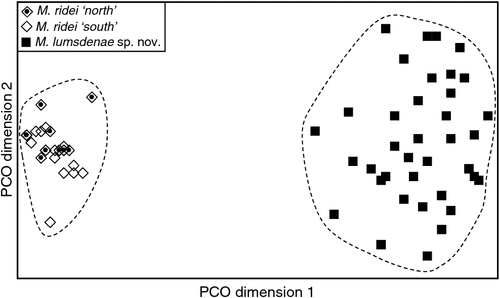
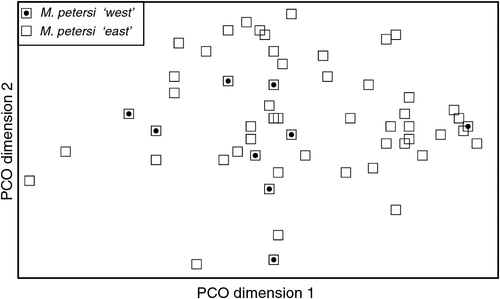
With respect to documenting phylogeographic structure within species, neither the follow-up PCOs (Figs 2, 5) nor an examination of the raw genotypes provided any evidence that the distinct mtDNA lineages observed within two species (‘north’ and ‘south’ in M. ridei; ‘east’ and ‘west’ in M. petersi; see below and Fig. 7), displayed concordant differences in allozyme profile.
|
Phylogenetic analyses of the mtDNA sequence data
The final mtDNA dataset comprised 91 Mormopterus individuals, plus three outgroup sequences for 759 bp of ND2 (Table S1). Sequences for all taxa have been deposited in GenBank as accession numbers KJ588277 – KJ588367. Initial phylogenetic analysis (Bayesian Inference) was conducted using all three outgroup genera, and then subsequent analyses iteratively dropped the most distant outgroup taxon, namely Cheiromeles and then Chaerephon, to arrive at the closest outgroup to maximise the number of phylogenetically informative sites for the ingroup. Each of these analyses consistently show 100% support for the monophyly of the Australian Mormopterus species and its closer relationship to Austronomus. These analyses are not presented here, but will feature in a subsequent paper evaluating the phylogenetic affinities of all Australian molossids. The final analysis presented included the single outgroup Austronomus australis.
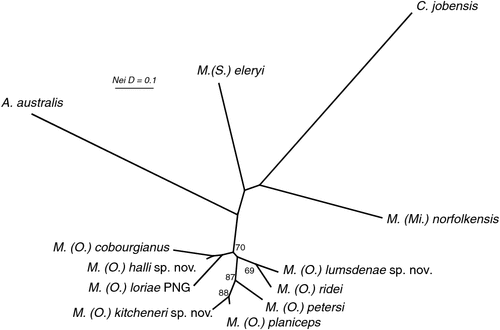
The BI phylogenetic tree is presented in Fig. 7 and includes posterior probabilities from BI analyses for key nodes. Genetic distances (p-distances) between and within major clades are shown in Table S4. The deep structure in the tree defines two major lineages: one clade comprising all members of the Australian (and New Guinean) ‘planiceps complex’ (supported by a posterior probability (pp) of 0.93) and a further lineage comprising M. eleryi and M. norfolkensis (supported by a pp of 0.95). The tree shows that both M. norfolkensis and M. eleryi are distant relatives of each other and of the ‘planiceps complex’, differing from each other at an average p-distance of 14.1%, and from the ‘planiceps complex’ at an average of 14.1%, a value only marginally lower than those that characterise the ingroup versus outgroup genera (Table S4).
Within the ‘planiceps complex’ the seven Australian taxa delineated by Adams et al. (1988) each form well supported clades, generally with high BI posterior probabilities (pp > 0.97). The only exception was the monophyletic group comprising two clades referred to as M. ridei, which was supported by a pp of 0.61.
A key feature of the tree is a strongly supported clade (pp = 1) that includes the Indo-Papuan species M. loriae and M. beccarii and the Australian species M. cobourgianus and M. halli sp. nov. The tree also shows divergent clades within both M. petersi and M. ridei. Within M. petersi there are two strongly supported clades (pp = 0.97 and 0.98) that reflect eastern and western samples and these differ on average at 3.6% of ND2 sites. Within M. ridei there are two clades (pp = 0.98 and 0.87) that represent northern and southern samples that differ on average at 3.1% of ND2 sites.
Overall, there is an average genetic divergence between the seven ‘planiceps complex’ taxa of 8.1% (range = 4.5–10.1%) with low intrataxon distances of 0.2–2.6%; however, the phylogenetic relationships among the species in the ‘planiceps complex’ are generally poorly resolved by the mtDNA data.
Phallic morphology
Amongst the Australian ‘planiceps complex’ male specimens available for the study, seven different forms of phallic morphologies were evident and these correspond to the seven groups defined by the allozyme analyses of Adams et al. (1988). The morphology of the glans penis for each species is illustrated in Fig. 8 and diagnostic features for each species are described in detail in the Systematics section below. Key features of the penis are illustrated in Fig. 9. Amongst the seven glans penis types, two are more similar in form, but these two and the remaining five show remarkable diversity in morphology, especially for a group of species that are otherwise morphologically conservative. The length of the penis varies between species and is itself diagnostic for some species. In most species there is a small gland on the distodorsal surface of the prepuce and it is usually accompanied by several long hairs. This feature seems to vary in degree of development within species and between species (and is therefore possibly diagnostic) although the development of this gland among species in the ‘planiceps complex’ almost never exceeds the proportions of those found in M. eleryi and M. norfolkensis (Reardon et al. 2008).
In all species the glans penis comprises a body and head. The body is roughly cylindrical at the proximal end, but exhibits variation distally among species. In all species the body is covered in basally directed epithelial spines that vary in extent of coverage and size, depending on the species. The fine structure of the head of the glans is difficult to observe under the microscope, but it appears to be constructed of two parts, the distal bacular mound that varies in shape, and the inner head, which for some species is contained mostly within the end of the glans body. The head is smooth and without spines. The urinary meatus is a longitudinal slit on the ventral surface of the head. The head is readily observed and its relative shape, position and size provide distinguishing features. A ventral transverse opening (lip) is present in some species. Although there is considerable variation in morphology of the glans penis amongst the species of the ‘planiceps complex’, this variation does not extend to including the remarkable structures such as lateral serrations in M. eleryi and the distal claw in M. norfolkensis.
Summary
Species boundaries and nomenclature
There is strong concordant evidence from allozymes, mtDNA and penis morphology to confidently show that a minimum of seven species occur within the Australian ‘planiceps complex’. In addition, the combinations of allozyme, mtDNA data and morphology (elaborated in the Systematics section below) support the species-level distinction between all Australian forms and M. loriae from southern Papua New Guinea, and M. beccarii beccarii from the type locality of Ambon, Indonesia, and specimens referable to M. beccarii astrolabiensis from northern Papua New Guinea. The justification and process for matching available species names to our delineated species is detailed in the Systematics section.
Subgeneric boundaries
Although the generic status and species composition of Mormopterus have both been historically unsettled, Mormopterus has been treated as a genus in its own right by most authors since the revision of Freeman (1981). The genus, as currently recognised, comprises the following valid species: jugularis (Peters, 1865) (the type species), acetabulosus (Hermann, 1804), norfolkensis (Gray, 1839), planiceps (Peters, 1866), beccarii Peters, 1881, kalinowskii (Thomas, 1893), loriae (Thomas, 1897), minutus (Miller, 1899), doriae Andersen, 1907, phrudus (Handley, 1956), eleryi Reardon & McKenzie, 2008, and francoismoutoui Goodman et al., 2008 (Simmons 2005; Goodman et al. 2008).
Some authors have recognised ‘species groups’ within this suite of species that largely correspond to geographic regions, namely, the acetabulosus group (jugularis (Madagascar), acetabulosus and francoismoutoui (Mascarene Islands), and doriae (Sumatra)), the kalinowskii group (kalinowskii and phrudus (western South America), and minutus (Cuba)) and the norfolkensis group (beccarii, loriae, norfolkensis, planiceps (Indo-Australia)) (Koopman 1994; Simmons 2005). More formal taxonomic distinctions for these groups have been previously proposed. Miller (1907), for the species recognised at that time and that he was able to examine, included jugularis, acetabulosus, kalinowskii and minutus in the genus Mormopterus, and loriae and norfolkensis in Nyctinomus. Iredale and Troughton (1934) considered that Australian forms were generically distinct from true Mormopterus and proposed (albeit invalidly) the name Micronomus to accommodate them. Troughton (1944) is the first valid publication of the name Micronomus (see note below regarding the date of publication). Churchill (2008) recognised Micronomus norfolkensis but used Mormopterus for the remaining Australian species.
Other authors have recognised Mormopterus and Micronomus as subgenera, with the former including both the acetabulosus and kalinowskii groups and the latter the norfolkensis group (Tate 1952; Laurie and Hill 1954; Legendre 1984; Nowak 1994; Hand et al. 1999). We agree that the norfolkensis group is, at the least, distinct from true Mormopterus (= the acetabulosus group) at the subgeneric level, a view also supported by our unpublished sequencing data. The systematic placement of the kalinowskii group is not fully clear but the molecular evidence of Lamb et al. (2011) and the inference of Goodman et al. (2008) suggest that this group’s affinities (subgeneric or generic) are likely to lie elsewhere other than with either true Mormopterus or its Australian exemplars.
Within the Australian Mormopterus, the magnitude of the genetic distances exhibited by allozymes and mtDNA between the ‘planiceps complex’ and M. eleryi and M. norfolkensis, and those between M. eleryi and M. norfolkensis, are close to those shown between these three groups and other molossid outgroup genera. This together with the corresponding cranial and dental differences evident between these three groups, lead us to conclude that these three groups each warrant subgeneric status.
Systematics
Family Molossidae Gervais, 1855
Genus Mormopterus Peters, 1865
Subgenus Micronomus Troughton, 1944
Revised diagnosis
Differs from the subgenus Mormopterus (jugularis, acetabulosus, francoismoutoui and doriae) by the following characters: possesses two rather than three lower incisors; has two upper premolars rather than one; lacks a gular sac that is well developed in Mormopterus.
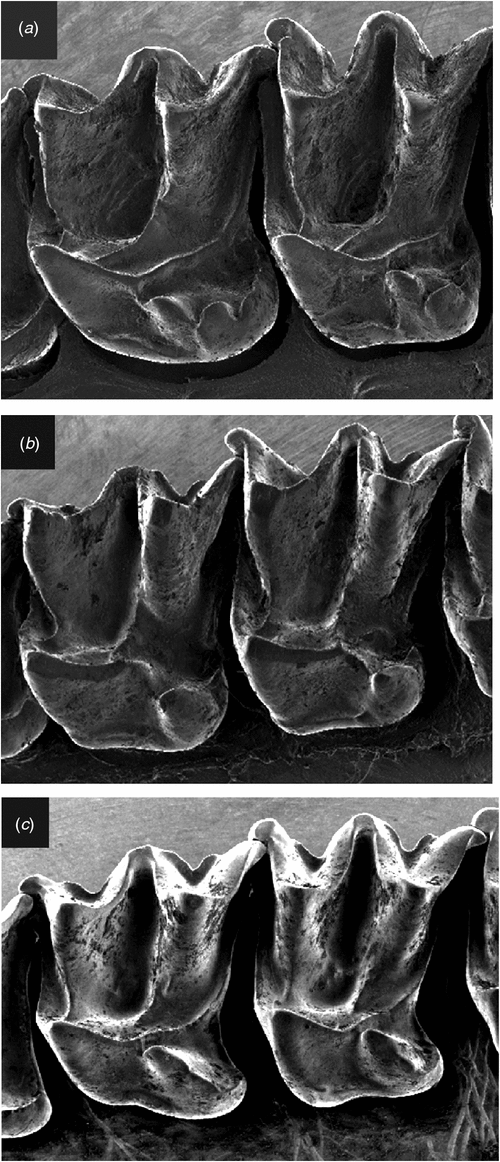
Differs from Setirostris subgen. nov. as follows: the upper first and second molars with scallop-shaped lobes extending from the posterolingual margin of the heel with the most anterior lobe in the position typical of a hypocone (Fig. 10a), as opposed to a single crochet-hook shaped ‘hypocone’ extending from the disto-lingual edge of the heel (Fig. 10b); is larger in most cranial and external characters; lacks strongly developed facial bristles; has radically different glans penis structure with distal claw and no lateral serrated edge; exhibits fixed allelic differences at 20 independent allozyme loci (Tables S2, S3); mtDNA haplotypes highly divergent (average ND2 sequence divergence >14%: Table S4).
|
Differs from Ozimops subgen. nov. as follows: skull profile more domed rather than straight; the upper first and second molars with scallop-shaped lobes extending from the posterolingual margin of the heel with the most anterior lobe in the position typical of a hypocone (Fig. 10a) as opposed to a semi-isolated well developed cone- or blade-shaped hypocone arising from the central body of the heel (Fig. 10c), and with the tip of the hypocone terminating close to the metaconule but separated via a short saddle; has radically different glans penis structure with distal claw; exhibits fixed allelic differences at a minimum of 17 independent allozyme loci (Tables S2, S3); highly divergent mtDNA haplotypes (average ND2 sequence divergence >14%: Table S4). A detailed description of this monotypic subgenus is given in Reardon et al. (2008).
Note on the publication date
The years 1943 and 1944 have variously been attributed as the date of publication of the second edition of Troughton’s ‘Furred Animals of Australia’ in which the first valid use of Micronomus appears. The year of publication printed in the book itself is 1943. We have followed Mahoney and Walton (1988) in accepting 1944 as the official date of publication based on confirmation by letter from the publisher to Walton.
Subgenus Setirostris Reardon, McKenzie & Adams, subgen. nov.
Diagnosis
Differs from the subgenus Mormopterus (jugularis, acetabulosus, francoismoutoui and doriae) by the following characters: possesses two rather than three lower incisors; has two upper premolars rather than one; lacks a gular sac that is well developed in Mormopterus.
Differs from the subgenus Micronomus as described above. Differs from Ozimops subgen. nov. by the following characters: skull profile more domed rather than flatter profile; upper first and second molars with a single crochet-hook-shaped ‘hypocone’ extending from the disto-lingual edge of the heel (Fig. 10b) as opposed to a semi-isolated well developed cone-shaped hypocone arising from the central body of the heel, and with the tip of the hypocone terminating close to the metaconule but separated via a short saddle (Fig. 10c); has radically different glans penis structure with serrated edge; fixed allelic differences at a minimum of 15 independent allozyme loci (Tables S2, S3); reciprocal monophyly (Fig. 7) for highly divergent mtDNA haplotypes (average ND2 sequence divergence = 14%: Table S4). A detailed description of this monotypic subgenus is given in Reardon et al. (2008).
Etymology
From seta L. = bristle + rostrum L. = beak or snout, referring to the characteristic stout bristles on the face. Gender of name is feminine.
Subgenus Ozimops Reardon, McKenzie & Adams, subgen. nov.
Content
Indo-Papuan forms: M. beccarii, M. b. astrolabiensis and M. loriae, and seven Australian species described below.
Diagnosis
Differs from the subgenus Mormopterus by the following characters: possesses two rather than three lower incisors; has two upper premolars rather than one; lacks a gular sac that is well developed in Mormopterus. Differs from the subgenera Micronomus and Setirostris by the characters described above.
General description
A group of small stout free-tailed bats (forearm length 29–41 mm and live mass 6–18 g) with dental formula 1/2, 1/1, 2/2, 3/3 = 30 although in some species, especially in M. halli sp. nov. the upper anterior premolar on one or both sides is occasionally lost in older animals; upper incisors convergent, simple with a small posterolingual cusp close to the cingulum; upper canine weak with cingulum roughly triangular in occlusal outline; the very small upper anterior premolar varies in shape and cingulum development, and is sometimes isolated but often compressed between the cingulum of the canine and that of the posterior premolar and sometimes pushed well out to the labial margin of the tooth row when the cingulum of the canine and of the posterior premolar are in contact; the two upper anterior molars have a tall protocone with the postprotocrista linked by a short crest to the metacingulum, although this crest may be lost with wear, the heel is moderately developed, although this is more reduced in some Indo-Papuan forms, the hypercone sometimes strongly developed as a semi-isolated cone or as a blade-like point and linked by a crest to the postprotocrista such that the crest sometimes forms a valley or is in line with the tip of hypocone and postprotocrista (Fig. 10c); the posterior upper molar is reduced but with a well developed paracone and protocone and with a well developed metacone; the lower incisors with flared and bifurcated tips; the lower premolars have a well developed cingulum, and the relative widths of these two teeth to one another varies between species. In general, the dental morphology (excluding size) is similar amongst the species of Ozimops and any differences appear to be confounded by substantial intraspecific variation. Skulls with relatively straight profile, sagittal crests weakly developed, lambdoidal crests usually well developed and form a high lambda; the mandible with a high coronoid process (higher than the tip of the canine).
Ears triangular with tips rounded, not joined together but separated by 2–4 mm; ears held laterally at rest but upright when the animal is active; tragus broad at the base narrowing to a thicker rounded tip; antitragus varies from being scarcely indicated to a semicircular lobe and composed of thicker skin than the rest of the ear. Muzzle broad, convex, but tapering anteriorly; upper part of the rostrum generally furred with very short fine hairs and some longer vibrissae, the sides of the muzzle ranging from a sparser covering of short hairs to almost naked with some tubercles displaying vibrissae and a few short bristles, but overall the rostrum never has long thick bristles as in M. eleryi. The upper lip wrinkled and with short dense fringe of hairs that overhang the bottom lip. Nostrils are slightly procumbent with crenulations on the outer margins and centre line. Body fur generally short not woolly, usually uniform in colour on the head and dorsum, and lighter underneath, colour ranging from darker brown to lighter grey-brown and sometimes with yellowish tinge and typically lighter ventrally. Albino forms have been reported. Wings long and narrow, with some New Guinean forms having the skin pigment bleached. Legs short, stout. Tail emerges from posterior margin of the uropatagium.
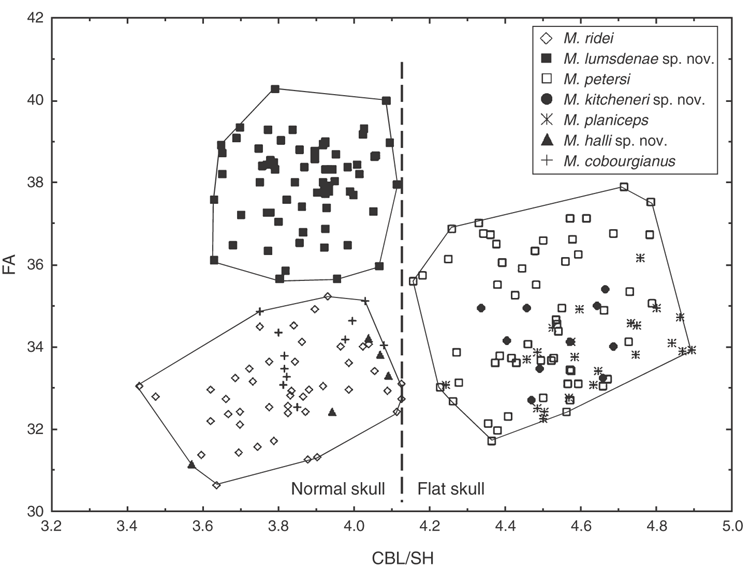
Sexual dimorphism is not strongly developed in the Australian species (with some character exceptions), but we cannot comment on Indo-Papuan species. For the cranial measures taken, sexual dimorphism as analysed by t-tests is evident only in M. lumsdenae sp. nov., in which all measures except M3-M3 and CBL/SH are significant between sexes (P < 0.05); for M. ridei only C1-C1 has a P < 0.05 and M. petersi IOB and CC; our sample size was too small to test M. halli sp. nov. For 17 external characters measured, the following species and characters (in parentheses) have P < 0.05: M. petersi (D3DP, D5M), M. kitcheneri sp. nov. (FA, D3M, D3PP, D4M, D5M), M. lumsdenae sp. nov. (D3DP, D5PP) and M. ridei (D5MP, D5PP).
Etymology
Ozi = colloquialism for Australia (as the centre of diversity of this group) + mops = suffix for many molossid genera. Gender of name is masculine.
Mormopterus planiceps (Peters, 1866)
South-eastern free-tailed bat
|
|
Synopsis of taxonomic history
Dobson (1876) considered planiceps as a junior synonym of norfolcensis (= norfolkensis), which Peters (1881) himself appears to have agreed with by omitting reference to planiceps in his description of M. beccarii. Thomas (1906) reinstated the specific status of planiceps and it has remained since as a valid species.
Referred specimens
See Tables S1, S5, S6.
Diagnosis
Distinguishable from all other species in the subgenus by having an exceptionally long penis that is greater than 7.5 mm in length. Differs from all species except kitcheneri sp. nov. and petersi by having an unusually flattened skull (Fig. 11). Otherwise similar in appearance to all other species with mostly overlapping external and cranial measurements other than with lumsdenae sp. nov., from which it differs by being smaller in most measures (Tables S7, S8). Distinguished from all Australian forms and loriae by having a unique combination of allozyme alleles (Table S3) and with the number of fixed differences from other species ranging from 2 to 10 (Table S2); also forms a monophyletic assemblage of ND2 haplotypes and differs from other species in average unweighted pairwise genetic distances of 0.045–0.095 (Table S4).
Revised description and contrasts with sympatric species
Sympatric species: M. petersi, M. ridei and possibly M. lumsdenae sp. nov.
External. A small species with a forearm range of 32.2–36.1 mm and mass of 6.5–12.0 g with features typical of the description for the subgenus. Head and dorsal fur uniform in colour, but varies between individuals from grey-brown to light brown. Ventral fur is lighter, usually pale brown (hairs white based, light brown mid-shaft and lighter tips). Similar in appearance to petersi but usually darker in fur colour, and with slightly smaller width across the nostrils. DFA using wing bone measures as described earlier may allow confident discrimination for some individuals. Similar to lighter-fur-coloured ridei, but with larger width across the nostrils, and slightly longer ears, and longer rostrum length. Is smaller than lumsdenae sp. nov. on most external measures.
The phallus is by far the longest in the genus (9–11 mm) and is described in detail in Krutzsch and Crichton (1987). The glans penis averages nearly 9 mm and is highly distinctive in shape (Fig. 8). The glans is long and slender and divided into two parts roughly equal in length. Krutzsch and Crichton (1987) describe these two parts as the primary glans (dorsal) and secondary glans (ventral) and these are demarcated by a large lateral opening on the ventral side. The head of the glans (bacular mound) is broad and rounded posteriorly but quickly tapers to a blunt point that is ventrally inflected. The glans body has longitudinal fissures and ridges extend along its length, some originating lateroventrally and stretching to the dorsal base. The glans body has proximally directed epithelial spines. The great length of the phallus would immediately allow unequivocal identification of this species.
Cranial (Fig. 13a). We have not examined the lectotype but have Felten’s (1964) description and skull drawing of it, as well as a series of photographs of the skull and dentary kindly provided by the Senckenberg Museum. The lectotype skull observations fit well within the range of variation we observe in our sample, but with the benefit of now possessing a large series of identified specimens, we are able to observe more variation in some of the characters than Felten observed. While the skull profile is indeed almost straight, many specimens have small depressions and inflations. In most specimens the lambdoidal crests are well developed, arising from near the postoccipital process and continue in the same size till converging together and with the sagittal crest forming elevated raised triangular-shaped raised lambda. However in some specimens the lambdoidal crests fade near their confluence and in place of a raised lambda, that part of the skull is simply a rounded continuum of the supraoccipital onto the parietals. The sagittal crest is absent but for where it forms the lambda. The orbital rim varies in the degree of strength in development, with some specimens showing a pronounced ridge while in others there is no ridge. The sphenorbital pits are shallow, but often scarcely indicated. Teeth generally as described for the subgenus.
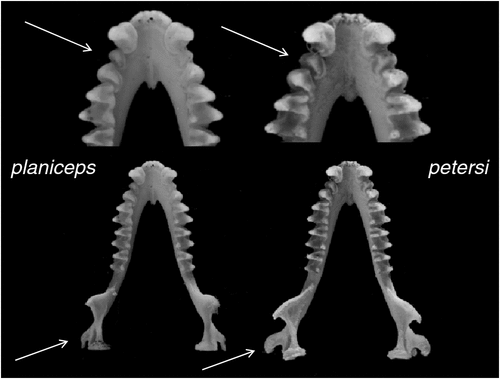
Similar to petersi but distinguishable by a less outward rotated angular process and a smaller ratio of the widths of the lower anterior to posterior premolar. Similar to ridei with GSL and CBL averaging slightly larger but with strong overlap in range, but distinguished by much larger CBL/SH. Differs from lumsdenae sp. nov. by being overall less robust and by being smaller on most cranial measures but larger CBL/SH (Table S8).
Distribution and habitat
The distribution map (Fig. 14) is based solely on specimens that we have examined and identified from morphology and/or from molecular methods. From these records, planiceps has a distribution that follows along the western slopes of the Great Dividing Range in New South Wales to most of Victoria and into southern South Australia to the Flinders Ranges and the Gawler Ranges in a band that has 300–700 mm annual rainfall. We have no museum records from Queensland or from east of the Great Dividing Range although there are reports of occurrences from there. This species is known to roost in tree hollows and thus far is not recorded from caves. It is also often reported roosting in the roofs and walls of buildings. Across its distribution area it occurs in mallee, mulga, box iron bark, river red gum.
Remarks
The name wilcoxii (Krefft 1873) appears to be a valid and available name in the context of this study. The species name first appeared in Krefft (1864) as Molossus wilcoxii and was subsequently used in Krefft (1871), but both were invalid acts because neither was accompanied by a description. Krefft (1873) is the first valid use of the name because it is accompanied by a brief description. Dobson (1876) synonymised wilcoxii (and planiceps) with norfolcensis (= norfolkensis), citing Krefft (1871) and it has remained a junior synonym since. The history of wilcoxii is partly discussed in Allison (1989), although curiously Allison considered wilcoxii a nomen nudum despite citing Krefft (1873), perhaps because he was influenced by Hill (1961), who only referred to Krefft (1871) and therefore rightly treated the name wilcoxii as a nomen nudum. Mahoney and Walton (1988) imply that wilcoxii Krefft (1873) is a valid name by listing it as a synonym of norfolkensis (ignoring Felten 1964) but agreeing with Hill (1961). Further, they note: ‘syntypes, not found in the AM [= Australian Museum], whereabouts unknown’.
The valid description in 1873 does not designate a type or specific museum specimens and thus we must consider that all the specimens upon which Krefft based his description, that is, those referred to in his described distribution ‘first discovered.on the Clarence River and occurs as far as Rockhampton’ comprise the syntypes. However, we do not know which specimens, or how many, but, given the designated localities, it is likely that the series contains more than one species.
Although no candidate syntypes specimens are yet known from Australian collections, we do know of two Mormopterus specimens currently held in European collections and donated by Krefft, one in the BMNH (BM 64.8.14.1) and one in the ZMB (ZMB 3521). Significantly, the BMNH specimen was catalogued into the collection in 1864 as indicated by the registration number and therefore is most likely a member of the original series upon which Krefft named wilcoxii. This is also the specimen that Dobson referred to in synonymising wilcoxii with norfolkensis. Subsequently, this specimen was identified as a planiceps by Felten (1964), with Thomas (1906) having earlier resurrected planiceps from norfolkensis. Felten (1964) suggested that this could be the original wilcoxii specimen. This may be true although it is a female and Krefft’s first invalid use of wilcoxii in 1864 is based on a male from the Clarence River, New South Wales.
Felten (1964) describes BMNH 64.8.14.1 as flat-skulled, which, in eastern Australia and under our species concept, ensures that it must either represent planiceps or petersi. We have not personally examined this specimen, but using Dobson’s (1878) measures for FA, D3M, D3PP, D4M, D4PP, D4MP, D5M and D5PP, the a posteriori classification using DFA places it in planiceps with a 98.1% probability (Wilks’ Lambda = 0.381, approximate F81,16 = 23.583, P < 0.0000). Paula Jenkins of the BMNH also kindly examined the specimen and confirmed that the specimen matches Fig. 12. with respect to the radial displacement of the angular process and the relative widths of the anterior versus posterior premolars.
In view of the potential for other specimens to be discovered that might be considered syntypes of wilcoxii, and the potential for those to comprise more than one species, one of which could displace a proposed junior synonym, it seems prudent for us to take action on this matter in the interest of future stability of the taxonomy presented in this study. We consider BMNH 64.8.14.1 as a genuine syntype of wilcoxii and hereby designate it as the lectotype, and in consequence, we treat wilcoxii (Krefft 1873) as a junior synonym of M. planiceps. We have not examined the Berlin Museum specimen and its identity remains to be determined.
Mormopterus petersi (Leche, 1884)
Inland free-tailed bat
Synopsis of taxonomic history
Thomas (1906) considered petersi as a junior synonym of planiceps and it has been treated as such to the present but for brief periods when it was recognised as a valid species by Wood Jones (1925) and later Peterson (1985).
Referred specimens
See Table S1, S5, S6.
Diagnosis
Distinguishable from all other species in the subgenus except lumsdenae sp. nov. by having a penis that is cylindrical for its entire length with a small bacular mound emanating from within the anterior rim of the glans body. Differs from all species except planiceps and kitcheneri sp. nov. by having an unusually flattened skull. Otherwise similar in appearance to all other species with mostly overlapping external and cranial measurements other than with lumsdenae sp. nov., from which it differs by being smaller in most measures (Tables S7, S8). Distinguished from all Australian forms and loriae by having a unique combination of allozyme alleles (Table S3) and with the number of fixed differences from other species ranging from 4 to 11 (Table S2); also forms a monophyletic assemblage of ND2 haplotypes and differs from other species in average unweighted pairwise genetic distances from 0.075 to 0.094 (Table S4).
Revised description and contrasts with sympatric species
Sympatric species: M. planiceps, M. kitcheneri sp. nov., M. ridei, and possibly M. lumsdenae sp. nov.
External. A small species with forearm range 31.9–38.2 mm and mass 7.5–11.8 g. There is a clinal increase in size of most wing (and cranial) measures from south to north. The external appearance is as described for planiceps except that the fur is sometimes shorter and the colour lighter brown than in planiceps, although inconsistently so. Not easily distinguished from kitcheneri sp. nov., although DFA on wing element measures generally distinguish petersi from kitcheneri sp. nov. and planiceps. The smaller southern forms of petersi that co-occur with the lighter forms of ridei are similar in appearance. The large Northern Territory forms of petersi approach the size of lumsdenae sp. nov. but in this region they do not appear to occur together. In south-eastern Queensland these two species may occur together but are separable by the smaller external measures of petersi.
The phallus is short, averaging around 5 mm, with the glans penis averaging 2.4 mm in length. The preputial gland is a small bump accompanied by some short hairs. The glans is cylindrical along its length in the live animal but typically there is dorsoventral flattening in many of the preserved specimens. The anterior rim of the glans body is almost circular surrounding the bacular mound, although in some specimens that ventral extremity of the rim extends slightly further than the dorsal extremity. The bacular mound is round and its anterior extent hardly protrudes beyond the anterior rim. The bacular mound is not attached to the very edge of the rim, but is instead attached via an inner head within the glans body. The glans body has a series of longitudinal ridges and fissures running from the anterior rim to near the junction of the prepuce and penis; these ridges are covered with small epithelial spines. There is a small depression in the mid body of the dorsal surface of the glans body.
The glans penis of petersi is most similar in size and form to that of lumsdenae sp. nov., but lumsdenae sp. nov. has a considerably larger bacular mound that protrudes much further than it does in petersi. In lumsdenae sp. nov. there is a single central dorsal ridge that bifurcates slightly just before the rim, but this ridge extends back along the length of the glans body. In petersi there is a fissure on the centre line of the dorsal glans body, and the ridges either side diverge from one another at about a glans width’s length back from the rim and surround a central mound or depression.
Cranial (Fig. 13b). The skull is as described for planiceps but with the following differences: the lower part of the ramus of the mandible is outwardly displaced such that the inner margin of the angular process sits well outside the line of the outer edge of the condyle when viewed from above (Fig. 12). In addition, the relative width of the first to the second lower premolar is greater than 0.75.
Distribution and habitat
The distribution map (Fig. 15) is based solely on specimens that we have examined and identified from morphology and/or from molecular methods. Distribution is largely arid to semiarid regions in the southern half of the continent. There appears to be a genuine disjunction in distribution between Western Australian records and those in central and eastern Australia. Natural roosts are in tree hollows but this species is sometimes found in buildings (Richards et al. 2008).
Remarks
The mtDNA data also show a marked difference between eastern and western populations, with the 3.6% average sequence divergence approaching a magnitude that might indicate species-level differences. At present these two populations appear to be allopatric and we can find no concordant morphological or allozymic support for recognising these populations at a higher taxonomic level. However, we flag this as a possibility for future investigation, especially if additional specimens can be obtained from the geographic zone of potential overlap.
Mormopterus kitcheneri McKenzie, Reardon & Adams, sp. nov.
South-western free-tailed bat
Referred specimens
See Tables S1, S5, S6.
Diagnosis
Distinguishable from all other species in the subgenus by having a penis that is shorter than 7.5 mm but with a pointed bacular mound (Fig. 8). Differs from all species except planiceps and petersi by having an unusually flattened skull (Fig. 11). Otherwise similar in appearance to all other species with mostly overlapping external and cranial measurements, other than with lumsdenae sp. nov., from which it differs by being smaller in most measures (Tables S7, S8). Distinguished from all Australian forms and loriae by having a unique combination of allozyme alleles (Table S3) and with the number of fixed differences from other species ranging from 2 to 10 (Table S2); also forms a monophyletic assemblage of ND2 haplotypes and differs from other species in average unweighted pair-wise genetic distances from 0.045 to 0.094 (Table S4).
Description and contrasts with sympatric species
Sympatric species: M. petersi.
External. A small species with forearm length 32.6–35.4 mm and body mass 7.5–10.5 g, and appearance as described for planiceps. The fur colour tends to be grey-brown, dorsally and lighter ventrally, but variable in length. DFA using wing measurements should distinguish kitcheneri and Western Australian petersi.
The phallus is ~6 mm in length and the glans averages 5 mm and is mostly cylindrical in shape, with a slight tapering at the distal end. The bacular mound is distinctive by being sharply tapered to a dorsally inflected point. The dorsal surface of the bacular mound is joined as a continuous extension of the upper central glans body. The ventral surface of the mound emerges from within the cavity of the glans body and the ventral anterior rim of the body forms a lip. The body has longitudinal ridges and fissures running the length of the entire glans body. The ridges have rows of proximally directed epithelial spines. The length of the penis and the pointed shape of the glans will enable easy identification from the sympatric petersi.
Cranial (Fig. 13e). As described in planiceps, but averaging slightly larger in all measures (Tables S7, S8). The angular process of the mandible is as described for planiceps (Fig. 12), therefore differs from petersi in the same manner.
Distribution and habitat
The distribution map (Fig. 16) is based solely on specimens that we have examined and identified from morphology and/or from molecular methods. Sympatric with petersi in Avon and Coolgardie regions of Western Australia. Found in mesic and semiarid woodlands throughout south-western Australia, and forages in semiopen and open air spaces. Roosts in tree hollows.
Etymology
This species is named in honour of Dr Darrell Kitchener for his prolific contribution to elucidating the systematics of Indo-Australian mammals, especially bats.
Mormopterus lumsdenae Reardon, McKenzie & Adams, sp. nov.
Northern free-tailed bat
|
Referred specimens
See Tables S1, S5, S6.
Diagnosis
Distinguishable from all other species in the subgenus by having a penis that is cylindrical along its entire length but with a large bulbous bacular mound (Fig. 8). Is the largest species in the subgenus and averages larger in most external and cranial measurements (Tables S7, S8). Distinguished from all Australian forms and loriae by having unique combination of allozyme alleles (Table S3) and with the number of fixed differences from other species ranging from 3 to 9 (Table S2); also forms a monophyletic assemblage of ND2 haplotypes and differs from other species in average unweighted pairwise genetic distances from 0.067 to 0.102 (Table S4). Differs from beccarii as described in the Remarks section.
Description and contrasts with sympatric species
Sympatric species: M. ridei, M. cobourgianus, M. halli sp. nov. and possibly M. petersi.
External. The largest species of the subgenus (forearm 35.2–40.4 mm, mass 11–19.5 g). Robust looking, with all external measures except D5MP averaging much greater than for other species; in contrast, D5MP is larger in cobourgianus and halli sp. nov. Females have a long clitoral projection. Dorsal fur rich brown and lighter ventrally.
The phallus is short, averaging near 4 mm, and often has a well developed elongated preputial gland. The glans penis averages 2.6 mm in length and is cylindrical for its length; the distal rim with the dorsal extremity reaching less distance than the ventral rim. The bacular mound is bulbous and usually extends well beyond the dorsal margin of the distal rim of the glans body and at least to the ventral margin of the rim. As in the case of petersi, the bacular mound is not contiguous with the distal dorsal surface of the glans body but emanates from slightly within the glans body and is attached as described for petersi. The lateral ridges with epithelial spines and fissures extend from the distal rim to just before the junction of the glans and inner preputial skin. The glans is most similar to that of petersi but the bacular mound is much larger.
Cranial (Fig. 13f). Robust skull with most cranial measures averaging, and sometimes absolutely, much greater than all other Australian Ozimops species. The skull has a low sagittal crest running from the lambda to the interorbital constriction. The lambdoidal crests are well developed and the lambda itself rises as a large prominence onto the skull. The preorbital ridge is moderately enlarged. The skull is readily distinguished from that of petersi by a much smaller CBL/SH. Most skull dimensions are absolutely larger than for cobourgianus and, on average, from halli sp. nov. (Table S8).
Etymology
This species is named in honour of Dr Lindy Lumsden for her contribution to the study of Australian bat ecology, for her mentoring of students and for her advocacy for conservation of bats through public engagement.
Distribution and habitat
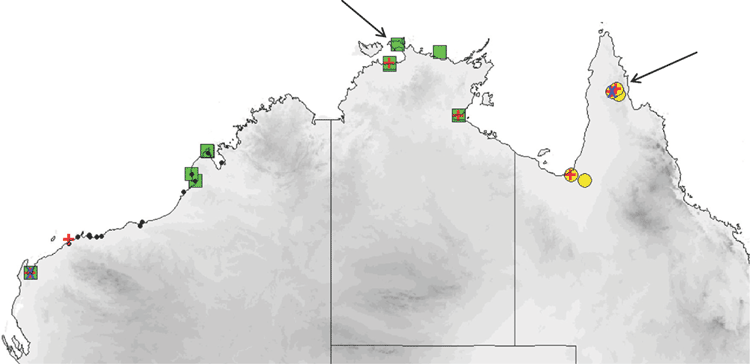
The distribution map (Fig. 17) is based on specimens that we have examined and identified from morphology and/or from molecular methods. The species is confined to the northern half of the continent within 600 km of the coast, encompassing annual rainfalls from 200 mm to over 1500 mm. The southern Northern Territory distribution given in Milne and Pavey (2011) is based on previously misidentified museum specimens that have been reidentified in this study as the very large form of petersi. Across this distribution it is found in drier shrublands and grasslands to rainforest. It occurs in arid and semiarid areas of Western Australia north of 26°S, where it favours woodland patches and riparian zones (McKenzie and Bullen 2009). Roosts in tree hollows and buildings.
Remarks
The first application of the name beccarii Peters, 1881 to this large northern Australian species appears in Winter and Allison (1980) and has been in subsequent use till now. Hill (1983) ascribed Australian forms to the subspecies astrolabiensis Meyer, 1899, but suggested that a new subspecies might be erected to account for the size difference he observed between Papuan and Australian forms. Peterson (1985) suggested that the Australian large Mormopterus was distantly related to beccarii but closer to astrolabiensis, which he considered a full species. Koopman (1994) also suggested that the Australian form had not been allocated yet to a subspecies of beccarii.
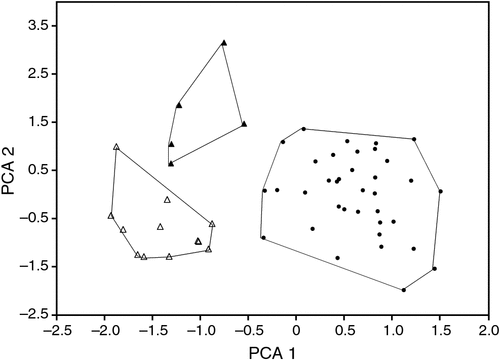
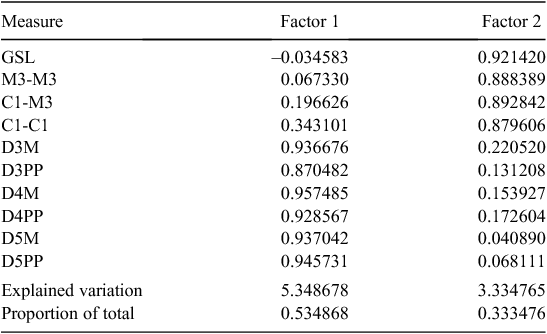
The type locality of beccarii is Ambon Island in Indonesia, and of astrolabiensis is Bongu, Papua New Guinea. The holotype of astrolabiensis was lost during allied bombing during World War II. We have examined both the holotype of beccarii (MCG CE44447) and have measures for a large series from the type locality (kindly provided by Judith Eger, ROM). We have also measured a series of specimens from northern Papua New Guinea from the putative distribution of beccarii astrolabiensis, including a specimen collected 30 km from the type locality. The glans penis morphology of beccarii beccarii and beccarii astrolabiensis differs radically from that of lumsdenae. Both beccarii and astrolabiensis have a long tapering glans penis that is clothed with large broad-based epithelial spines, and both have a ventral transverse lip, whereas the glans penis of lumsdenae is shorter and not tapered, the spines are fine and there is no ventral transverse lip. We also conducted a PCA analysis based on four external and seven cranial measures for available specimens from Ambon, northern Papua New Guinea, and lumsdenae (Fig. 18), which shows clear delineation between the three taxa. The factor loadings for the 10 measures are presented in Table 2. No frozen tissues of beccarii beccarii or beccarii astrolabiensis were available for inclusion in the allozyme study, but our ND2 sequencing of a specimen of astrolabiensis and a specimen from near the type locality of beccarii beccarii showed that they belonged to clades distant from lumsdenae. While the species-level systematics of Indo-Papuan Mormopterus remains unclear, it is certain that lumsdenae is not allied with either beccarii or astrolabiensis. lumsdenae is readily differentiated from loriae from Papua New Guinea by its greater size in most measures, allozyme and ND2 divergence and glans penis morphology.
|
Mormopterus ridei (Felten, 1964)
Eastern free-tailed bat
|
|
Synopsis of taxonomic history
Felten’s concept of ridei as a subspecies has persisted for most of its short history although it has been treated as a subspecies of planiceps rather than loriae by some authors (Koopman 1984; Mahoney and Walton 1988). In an opinion that has been mostly ignored, Peterson (1985) considered all small Australian species including ridei to be synonymous with, or races of, petersi. Churchill (2008) recognised ridei as a species in its own right.
Referred specimens
See Tables S1, S5, S6.
Diagnosis
Distinguishable from all other species in the subgenus by having a glans penis that is short, cylindrical and tapered at the tip, with dorsal glans body contiguous with the bacular mound and the glans body covered in small epithelial spines (Fig. 8). Distinguished from all Australian forms and M. loriae by having a unique combination of allozyme alleles (Table S3) and with the number of fixed differences from other species ranging from 3 to 10 (Table S2); also forms a monophyletic assemblage of ND2 haplotypes and differs from other species in average unweighted pair-wise genetic distances from 0.061 to 0.088 (Table S4).
Redescription and contrasts with sympatric species
Sympatric species: M. planiceps, M. petersi, M. lumsdenae, and probably M. halli sp. nov.
External. A small, compact-looking species with forearm 30–35 mm and mass 5–11.2 g. Averages slightly smaller on several external characters from sympatric species, but typically with considerable overlap with most other species except with lumsdenae, which is much larger. Fur colour quite variable, with some forms being lighter brown-grey, similar to planiceps and petersi, but typically darker brown dorsally and lighter underneath (although these differences in colour are not correlated with the two intraspecific mtDNA subclades) (Fig. 7). Where it occurs together with petersi and planiceps it is difficult to distinguish on external characters. The ears and the length of the rostrum are slightly smaller than in planiceps and petersi. Average values for external characters much smaller than for halli sp. nov.
The penis length averages ~4 mm. The glans body is cylindrical for its length but tapers at the tip. The glans body has a series of ridges with small epithelial spines and fissures that run the length of the body. The bacular mound is round and small and dorsally is contiguous with the central part of the dorsal glans body (thus differing from petersi and lumsdenae, in which the bacular mound emanates from within the body of the glans). The anterior edge of the ventral glans body is not attached to the bacular mound but forms an opening to the body of the glans body. The ventral part of the bacular mound emanates from within the cavity of the glans body. The glans is distinctive in that it has a bullet-shaped tip compared with the blunted shape of petersi and should be easily distinguished on live animals.
Cranial (Fig. 13c). Skull averages shorter than in planiceps and petersi and differs clearly by the much smaller CBL/SH. Weak to no sagittal crest, well developed lambdoidal crests, although the lambda varies considerably, with some specimens having almost no visible indication through to those with prominent triangular-shaped elevation. The skull measures average much smaller than in lumsdenae but absolutely so in GSL and CBL (Table S8). Averages much smaller than in halli sp. nov. but reliably distinguished on DFA using all cranial measures. Also differs from halli sp. nov. by having a less crowded upper tooth-row with the small upper premolar always present and in line with the tooth-row.
We tested both external and cranial measures for correlation to latitude and hence the north versus south mtDNA boundary, but failed to find support for any correlation. Based on our few sequenced samples in south-eastern Queensland, the current boundaries of the north and south mtDNA clades (Wrattens State Forest and Cherbourg State Forest) are only 45 km apart and at about the same latitude.
Distribution and habitat
The distribution map (Fig. 19) is based on specimens that we have examined and identified from morphology and/or from molecular methods. M. ridei occurs in eastern Australia in the higher-than-500-mm annual rainfall zone, but also occurs in the lower-rainfall areas of South Australia, Victoria and southern central New South Wales but always associated with rivers and swamps. In South Australia it occurs along most of the length of the Murray River but has not yet been recorded very far from the river or its associated streams. Habitat ranges from wet tropical forest to swamplands, river red gum–lined streams and open woodland. Roosts in eucalypt hollows and in buildings (Hoye et al. 2008).
Remarks
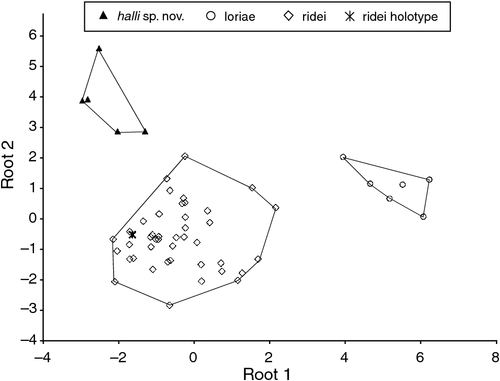
Felten (1964) described ridei as a subspecies of loriae and for most of its short history it has been treated as such. Since Adams et al. (1988), the name ridei has usually been associated with Species 5 populations ‘S & T’ from far northern Queensland. However, the type locality for ridei is Cairns, which falls better within the distribution of Species 2, rather than Species 5. Had Felten (1964) chosen a male for the holotype, it would have been a simple task to associate ridei with Species 2 on the basis of glans penis morphology. To test which of the three possible candidate species, Species 2 (our ridei), Species 5 populations S & T (halli sp. nov.) and also loriae from Papua New Guinea, matched the ridei holotype, we ran a DFA using CBL, MB, C1-C1, FA, D3M, D3PP, D3MP, D4M, D4PP, D4MP, D5M, D5PP. There was very strong discrimination between these three taxa (Wilks’ Lambda = 0.069, approximate F22,80 = 10.16, P < 0.0000) and there was a 100% posterior probability of the holotype belonging to ridei (Fig. 20).
Mormopterus cobourgianus (Johnson, 1959)
North-western free-tailed bat
Synopsis of taxonomic history
Johnson’s concept of cobourgiana as a subspecies has persisted for most of its short history, although it has been treated as a subspecies of planiceps rather than of loriae by some authors (Koopman 1984; Mahoney and Walton 1988). In an opinion that has been mostly ignored, Peterson (1985) considered all small Australian species including cobourgiana as synonymous with, or races of, petersi. Churchill (2008) recognised cobourgiana as a species in its own right.
Referred specimens
See Tables S1, S5, S6.
Diagnosis
Distinguishable from all other species in the subgenus by having a glans penis with large epithelial spines covering the glans body from the base of the glans to the bacular mound (Fig. 8). Distinguished from all Australian forms and loriae by having a unique combination of allozyme alleles (Table S3) and with the number of fixed differences from other species ranging from 4 to 11 (Table S2); also forms a monophyletic assemblage of ND2 haplotypes and differs from other species in average unweighted pair-wise genetic distances from 0.065 to 0.102 (Table S4).
Redescription and contrasts with sympatric species
Marginally sympatric with M. lumsdenae.
External. A small species with forearm 32.0–35.1 mm and live mass 6.8–10.5 g. Head and dorsal fur cream based with light orange brown and sometimes frosting of grey brown. Ventral fur cream, sometimes yellowy cream. The ventral fur extends well onto the wing to a line extending from the mid humerus to the knee. Upper lip fringe is light creamy yellow. Northern Territory forms (n = 3) are slightly darker above and below. Sympatric only with lumsdenae, from which it is readily distinguished by being much smaller on most external measures. Ears triangular with relatively straight leading edge and rounded tips. The bottom of the ear ends in a semicircular antitragus. An unusual feature of this species (and of halli sp. nov.) is the relatively long D5MP, even though the other wing elements are of similar length to, or smaller than, those of lumsdenae.
The penis is sparsely covered by long hairs and a small preputial gland with 10 or so long hairs. The glans penis has a cylindrical body but with a tapering end where it joins the glans head. A broad transverse lip is present on the ventral side close the head. The entire glans body back from the margin with the glans head is covered with large epithelial spines. There is a central longitudinal fissure both ventrally and dorsally. The head has no spines and comprises two sections, the more distal globular bacular mound and the inner head. The glans penis morphology easily distinguishes cobourgianus from other Australian species.
Cranial (Fig. 13d). Small slender skull with no sagittal crest. Upper anterior premolar, while small and compressed between the canine and posterior premolar, is taller than the cingulum of the canine and readily visible. The width of the lower first premolar is ~80% the size of the second premolar. Readily distinguished from lumsdenae by smaller averages on all cranial measures and by some measures being always smaller (Table S8).
Distribution and habitat
The distribution map (Fig. 21) is based on specimens that we have examined and identified from morphology and/or from molecular methods. M. cobourgianus occurs coastally from Exmouth to western Gulf of Carpentaria. However, the Western Australian and Northern Territory populations appear disjunct, with no records yet from the northern and eastern Kimberley coast or islands, despite this area being well surveyed (McKenzie and Bullen 2012). Associated with mangrove communities in Western Australia (McKenzie and Bullen 2009, 2012) but also woodland in the Northern Territory (Milne et al. 2008). Roosts in tree hollows. Although we have found no evidence yet that the allopatric populations in Western Australia and Northern Territory are taxonomically distinct, a more detailed examination might be worthwhile as there is some divergence in ND2 haplotypes. Only six specimens are currently known from the Northern Territory.
Mormopterus halli Reardon, McKenzie & Adams, sp. nov.
Cape York free-tailed bat
Diagnosis
Distinguishable from all other species in the subgenus by having a short tapering glans penis with a ventral transverse lip at about half the length of the glans body, and with large epithelial spines that cover the glans body dorsally from the head to the base and ventrally from the lip to the base (Fig. 8) Distinguished from all Australian forms and loriae by having a unique combination of allozyme alleles (Table S3) and with the number of fixed differences from other species ranging from 4 to 7 (Table S2); also forms a monophyletic assemblage of ND2 haplotypes and differs from other species in average unweighted pair-wise genetic distances from 0.067 to 0.101 (Table S4).
Description and contrasts with sympatric species
Sympatric species: M. lumsdenae and probably M. ridei.
External. A small stout species with forearm length 31–35 mm and live mass ~9 g. Head, body and wings as described for the subgenus but with the following additional observations: dorsal fur variable from rich brown to orange brown and only weakly contrasting with lighter ventral fur; yellowish tinge to the fur on the side of the neck. Skin on ears, wings, and muzzle very dark brown; ears with half round antitragus. Similar in appearance and colouration to lumsdenae but averages smaller on most wing element measures, and absolutely smaller on FA and D3M lengths (Table S7). Likely to be sympatric with ridei and, apart from glans penis morphology, may be difficult to distinguish on external characters. However C1-C1 is easy to measure on a live animal and is close to unequivocally distinguishing these two species (Table S8).
Penis with sparse short hairs and preputial gland is short with five or so long hairs. Glans penis body ~5 mm in length. The glans penis is cylindrical but tapering a little distally to a relatively long head. The dorsal body is covered with large epithelial spines extending to its junction with globular shaped head. The ventral body is composed of two sections of approximately equal lengths, and divided by a broad transverse lip, the ends of which reach half way up the sides of the glans body. The proximal section of the ventral body is covered with large epithelial spines but the section distal to the lip has no spines and is characterised by a series of transverse folds until it ultimately joins the glans head. A longitudinal slot marking the urinary meatus is situated ventrally in the glans head, just proximally from the tip of the bacular mound.
Cranial (Fig. 13g). The skull is large and robust, averaging larger than all species other than lumsdenae. Lambdoidal crest strongly developed but weak sagittal crest. The mandibular body is tall and thick at the anterior end. Tooth-row crowded and the upper anterior premolar is very small and, when present, is often pushed out the line of the row between the cingulum of the second premolar and the canine. In some specimens either one or both little upper premolars are missing. Differs from both ridei and lumsdenae as outlined in the accounts of those species.
Distribution and habitat
The distribution map (Fig. 21) is based on specimens that we have examined and identified from morphology and/or from molecular methods. Thus far halli is known from only 11 specimens and has been collected from two disparate locations: central Cape York Peninsula and near Normanton in the Gulf Country. All specimens have been captured by mist nets and all over, or very near to, fresh water. The small number of specimens especially on Cape York Peninsula probably does not reflect that the species is uncommon or geographically restricted, but more that previous survey effort in that region has focussed on rainforest and riparian habitats along major river courses rather than in the woodland habitat of halli (Reardon et al. 2010). Survey efficiency for this species will be improved when its echolocation call is characterised. Natural roosts are unknown although likely to be tree hollows.
Etymology
This species is named in honour of Dr Leslie Hall for his life-long contribution to the conservation of Australasian bats through his research, mentoring of students and public engagement.
Discussion
This revision provides the first solid working hypothesis for the species boundaries, nomenclature and higher taxonomic structure in Australian Mormopterus. After years of confusion with extralimital species, we have shown that Australia does not share Mormopterus species with the Indo-Papuan region and therefore Australia is home to nine endemic species. These nine species are readily referrable to three distinctive basal groups, which we have conservatively designated herein as subgenera.
Despite the molecular and morphological scope of this study, several knowledge gaps remain in Australian Mormopterus. In particular, the geographic distribution limits for most of the Australian species of the new subgenus Ozimops are far from fully understood. halli and Northern Territory cobourgianus are currently known from only a handful of localities, and whether these are truly rare species or just rarely collected remains to be determined. Given that our maps are based only on records unequivocally identified from glans penis morphology or molecular methods, we expect that knowledge gaps regarding the distribution and abundance of each species are likely to be gradually filled as the species boundaries outlined herein become understood by field workers.
Traditional biogeographic barriers are consistent with the apparent allopatry of closely related pairs of species and intraspecies clades, namely the Gulf of Carpentaria for cobourgianus and halli, and the Nullarbor for the closely related species planiceps and kitcheneri plus the east and west clades of petersi. In all three cases it is possible that further collecting will demonstrate geographic adjacency or overlap between the pairs of species/clades. Interestingly, the distributional boundaries of the north and south ND2 clades of ridei, based on our limited sampling, meet in the Macleay–McPherson Overlap (Burbidge 1960).
Significance of mtDNA clades within species
The discovery of significantly divergent mtDNA clades within both ridei and petersi (and to a lesser degree between Western Australian and Northern Territory clades of cobourgianus) warrant more detailed genetic and morphological investigation, particularly to resolve whether these clades should reflect taxonomic distinction. The lack of supportive allozyme or morphological differences for these clades is somewhat surprising, given that the magnitude of these ND2 divergences approaches that between other allozymically distinctive species (e.g. the p-distance between kitcheneri and planiceps is 0.045 versus 0.042 for the east and west clades of petersi). Nevertheless, mtDNA gene trees are commonly discordant with their underlying population/species trees (Funk and Omland 2003), and numerous possible explanations have been proposed to account for such discrepancies (Toews and Brelsford 2012).
The species kitcheneri and planiceps are sister species on the basis of the mtDNA phylogeny (Fig. 7), with the former species distributed in south-west Western Australia and the latter from the Eyre Peninsular to eastern Australia, with each species apparently absent from the Nullarbor plain. These two species are also distinguished on the basis of two fixed differences at allozyme loci and major differences in glans penis morphology. This result contrasts with that obtained for the east and west clades of petersi, which show no clear allozyme and morphological distinction. These two petersi clades are similarly found on either side of the Nullarbor plain, though the distribution of petersi also extends into parts of central Australia, suggesting that it may be more arid-adapted than kitcheneri or planiceps. Nevertheless, the similar geographic and matrilineal genetic pattern displayed by the two species/populations on either side of the Nullarbor suggests that their differentiation may have resulted from similar evolutionary/environmental forces. Interestingly, a similarly high level of ND2 divergence (p-distance = 0.049) was found among populations of the bird species Melithreptus lunatus located on either side of the Nullarbor plain, and this was estimated to equate to a population fragmentation time of between 594 000 and 3.4 million years ago (Dolman and Joseph 2012). This period coincides with a period of increasing aridity on the Australian continent that commenced during the Plio-Pleistocene and intensified during Milankovitch cycles of the Pleistocene, when the Nullarbor plain is likely to have become a significant barrier to gene flow of mesic-adapted animals in open forest and woodland habitats (Fujioka et al. 2005; Byrne et al. 2008; Dolman and Joseph 2012). Currently, our data are too limited to provide robust date estimates for the divergence of the bat populations, but additional comparative phylogeographic studies of bat species distributed across southern Australia would be of considerable interest, and may potentially reveal similar patterns of population differentiation.
The lack of supporting morphological differences associated with the ND2 clade divergence in petersi may also reflect the general conservative nature of the morphology among the species, and perhaps the limits of examining finer features of the genitalia under the microscope alone. The absence of nuclear gene divergence within petersi, in contrast to the mtDNA divergence, could alternatively reflect features of the biology of the species, such as sex-biased dispersal and/or female philopatry. For example, male-biased dispersal was suggested as an explanation for the incongruence between patterns of gene flow inferred from mitochondrial and nuclear DNA markers in the ghost bat Macroderma gigas (Worthington-Wilmer et al. 1999). However, this finding is not strictly analogous, first because microsatellite nuclear markers (likely more sensitive than allozymes for detecting sex-biased dispersal) were used in the latter study, and second because ghost bat mtDNA clades were correlated with defined distributions around maternity caves, whereas Ozimops species are not cave dwellers and are not obviously confined by roosting opportunity.
Comparisons of the new taxonomy with fossils
Fossil remains of molossid bats in Australia are rare, with only four sets of material thus far described (Hand 1990; Hand et al. 1997, 1999; Martinez 2010). While various preliminary taxonomic assessments have been made of this material, a key impediment to identifying and interpreting this fossil material has been the lack of a solid taxonomic framework for extant Australian Mormopterus (Martinez 2010). Our taxonomic revision of the extant taxa now provides a strong basis for developing a more robust understanding of intra- and interspecies variation in dental and cranial morphology.
Field identification
The shape features and size of the glans penis varies considerably between species and is both one of the clearest field diagnostic characters to identify species from a live animal, as well as being easily observed under a hand lens or microscope with minimal retraction of the prepuce. However, identification of female Mormopterus remains difficult for some sets of sympatric species. We are hoping that with a more robust taxonomic framework in place, field biologists will uncover reliable diagnostic characters, especially those more easily and consistently observed in live animals rather than preserved specimens. This situation is similar to that in another Australian bat genus, Vespadelus, in which the male genitalia are highly distinctive but diagnostic characters in females for distinguishing some species are elusive (Churchill 2008). Until diagnostic characters are available for distinguishing these species, we recommend that skin biopsies be collected to enable confirmation of the species using genetic analyses.
Acknowledgements
We are grateful to the following people who provided measurements for some key specimens including the type material, or provided information or images: Dieter Kock, Katrin Krohmann, Don Wilson, Ibnu Maryanto. Judith Eger, Steven van der Mije, Paulina Jenkins, Gabor Csorba and Bruce Thomson. We thank the following for the loan of specimens and access to collections, or provision of tissues: Mohd Tajuddin Abdullah, Bo Fernholm, Sven Kullander, Sandy Ingleby, Wayne Longmore, Heather Janetzki, Robert Palmer, Judith Chupasko, Giulliano Doria, Gavin Dally, David Stemmer, Claire Stevenson, Norah Cooper, Maria Adams, Frieder Mayer, Teresa Kearney, Steve Hamilton, Bruce Thomson, Carrie Bies, Jenny Maclean, Trish Wimberley, Anna McConville, and Michael Pennay. Several people provided a contribution to the project with field assistance: Michael Pennay, Luke Hogan, Annette Scanlon, April Reside and Bob Bullen. Others have provided helpful advice: Harry Parnaby, Suzanne Hand, Stephen Jackson, Damian Milne, John Weyland and Denis Brothers. Jennifer Thurmer prepared the glans penis illustrations. We thank the reviewers for their helpful comments. Field work and the collections of specimens were conducted under state permit numbers SF6113 (WA) and WITK03991306 (Qld), and animal ethics licence AEC S-026-2006. We are grateful to the Traditional Owners and land managers who allowed access to their land for fieldwork. This study is submitted as part of the Ph.D. program of the first author, who is grateful to the University of Adelaide and the South Australian Museum for a Ph.D. scholarship. This work was partly funded by grant number 206-47 to Mark Adams and Terry Reardon from the Australian Biological Resources Study.
References
Adams, M., Reardon, T. R., Baverstock, P. R., and Watts, C. H. S. (1988). Electrophoretic resolution of species boundaries in Australian Microchiroptera. 4. The Molossidae (Chiroptera). Australian Journal of Biological Sciences 41, 315–326.| 1:CAS:528:DyaL1MXhtFags7k%3D&md5=6142cd56c2076e0b507771e5e7f5d2a5CAS |
Adams, M., Page, T. J., Hurwood, D. A., and Hughes, J. M. (2013). A molecular assessment of species boundaries and phylogenetic affinities in Mogurnda (Eleotridae): a case study of cryptic biodiversity in the Australian freshwater fishes. Marine and Freshwater Research 64, 920–931.
| A molecular assessment of species boundaries and phylogenetic affinities in Mogurnda (Eleotridae): a case study of cryptic biodiversity in the Australian freshwater fishes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFGrsLjO&md5=8ac013f523b727f9b9de5bd7ffac2e9bCAS |
Allison, F. R. (1989). Molossidae. In ‘Fauna of Australia. 1B. Mammalia’. (Eds D. W. Walton, and B. J. Richardson.) pp. 21–88. (Australian Government Publishing Service: Canberra.)
Ammerman, L. K., Lee, D. N., and Tipps, T. M. (2012). First molecular phylogenetic insights into the evolution of free-tailed bats in the subfamily Molossinae (Molossidae, Chiroptera). Journal of Mammalogy 93, 12–28.
| First molecular phylogenetic insights into the evolution of free-tailed bats in the subfamily Molossinae (Molossidae, Chiroptera).Crossref | GoogleScholarGoogle Scholar |
Burbidge, N. T. (1960). The phytogeography of the Australian region. Australian Journal of Botany 8, 75–211.
| The phytogeography of the Australian region.Crossref | GoogleScholarGoogle Scholar |
Byrne, M., Yeates, D. K., Joseph, L., Kearney, M., Bowler, J., Williams, M. A., Cooper, S. J. B., Donnellan, S. C., Keogh, S., Leijs, R., Melville, J., Murphy, D., Porch, N., and Wyrwoll, K.-H. (2008). Birth of a biome: synthesizing environmental and molecular studies of the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398–4417.
| Birth of a biome: synthesizing environmental and molecular studies of the assembly and maintenance of the Australian arid zone biota.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cjhvFGruw%3D%3D&md5=be4ed4521bf1145ad0d36ac29682e04eCAS | 18761619PubMed |
Churchill, S. (2008). ‘Australian Bats.’ 2nd edn. (Allen and Unwin: Sydney.)
Dobson, G. E. (1876). A monograph of the group Molossi. Proceedings of the Zoological Society of London 1876, 701–735.
Dobson, G. E. (1878). ‘Catalogue of the Chiroptera in the Collection of the British Museum.’ (British Museum: London.)
Dolman, G., and Joseph, L. (2012). A species assemblage approach to comparative phylogeography of birds in southern Australia. Ecology and Evolution 2, 354–369.
| A species assemblage approach to comparative phylogeography of birds in southern Australia.Crossref | GoogleScholarGoogle Scholar | 22423329PubMed |
Felten, H. (1964). Zur Taxonomie indo-australischer Fledermäuse der Gattung Tadarida (Mammalia, Chiroptera). Senckenbergiana Biologica 45, 1–13.
Freeman, P.W. (1981). A multivariate study of the Family Molossidae (Mammalia, Chiroptera): morphology, ecology, evolution. Fieldiana Zoology 7, 1–173.
Fujioka, T., Chappell, J., Honda, M., Yatsevich, I., Fifield, K., and Fabel, D. (2005). Global cooling initiated stony deserts in central Australia 2–4 Ma, dated by cosmogenic Ne-21-Be-10. Geology 33, 993–996.
| Global cooling initiated stony deserts in central Australia 2–4 Ma, dated by cosmogenic Ne-21-Be-10.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlCit7nO&md5=161ad6a6aad507a0b15a749b5abb22f1CAS |
Funk, D. J., and Omland, K. E. (2003). Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annual Review of Ecology, Evolution and Systematics 34, 397–423.
| Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA.Crossref | GoogleScholarGoogle Scholar |
Goodman, S. M., van Vuuren, B. J., Ratrimomanarivo, F., Probst, J.-M., and Bowie, R. C. K. (2008). Specific status of populations in the Mascarene Islands referred to Mormopterus acetabulosus (Chiroptera: Molossidae), with description of a new species. Journal of Mammalogy 89, 1316–1327.
| Specific status of populations in the Mascarene Islands referred to Mormopterus acetabulosus (Chiroptera: Molossidae), with description of a new species.Crossref | GoogleScholarGoogle Scholar |
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41, 95–98.
| 1:CAS:528:DC%2BD3cXhtVyjs7Y%3D&md5=1bc8594ada2830f08a4bc74ad945df91CAS |
Hall, L. S., and Richards, G. C. (1979). ‘Bats of Eastern Australia.’ Booklet 12. (Queensland Museum: Brisbane.)
Hand, S. J. (1990). First Tertiary molossid (Microchiroptera: Molossidae) from Australia: its phylogenetic and biogeographic implications. Memoirs of the Queensland Museum 28, 175–192.
Hand, S. J., Archer, M., and Godthelp, H. (1997). First record of Hydromops (Microchiroptera: Molossidae) from Australia: its biocorrelative significance. In ‘Actes du Congrès BiochroM’97’. (Eds J.-P. Aguilar, S. Legendre and J. Michaux.) pp. 153–162. Mémoires et Travaux del’E.P.H.E., Institut de Montpellier 21
Hand, S. J., Mackness, B., Wilkinson, C., and Wilkinson, D. (1999). First Australian Pliocene molossid bat: Micronomus sp. from the Chinchilla Local Fauna, southeastern Queensland. Records of the Western Australian Museum 57, 291–298.
Hill, J. E. (1961). Indo-Australian bats of the genus Tadarida. Mammalia 25, 29–56.
| Indo-Australian bats of the genus Tadarida.Crossref | GoogleScholarGoogle Scholar |
Hill, J. E. (1983). Bats (Mammalia: Chiroptera) from Indo-Australia. Bulletin of the British Museum (Natural History), Zoology Series 45, 103–208.
Horner, P., and Adams, M. (2007). A molecular-systematic assessment of species boundaries in Australian Cryptoblepharus (Reptilia: Squamata: Scincidae) – a case study for the combined use of allozymes and morphology to explore cryptic biodiversity. The Beagle: Records of the Museums and Art Galleries of the Northern Territory 3, 1–19.
Hoye, G. A., Law, B. S., and Lumsden, L. F. (2008). Eastern free-tailed bat. In ‘The Mammals of Australia’. 3rd edn. (Eds S. van Dyck and R. Strahan.) pp. 493–494. (Reed New Holland: Australia.)
Iredale, T., and Troughton, E. (1934). A checklist of mammals recorded from Australia. Memoirs of the Australian Museum 6, 1–122.
| A checklist of mammals recorded from Australia.Crossref | GoogleScholarGoogle Scholar |
Johnson, D. H. (1959). Four new mammals from the Northern Territory of Australia. Proceedings of the Biological Society of Washington 72, 183–187.
Kirchman, J. J., Hackett, S. J., Goodman, S. M., and Bates, J. M. (2001). Phylogeny and systematics of ground rollers (Brachypteraciidae) of Madagascar. The Auk 118, 849–863.
| Phylogeny and systematics of ground rollers (Brachypteraciidae) of Madagascar.Crossref | GoogleScholarGoogle Scholar |
Kohlstedt, S. G. (1980). century. Historical Records of Australian Science 5, 1–29.
| century.Crossref | GoogleScholarGoogle Scholar |
Koopman, K. F. (1984). Taxonomic and distributional notes on tropical Australian bats. American Museum Novitates 2778, 1–48.
Koopman, K. F. (1994). ‘Chiroptera: Systematics.’ Handbook of Zoology Band/Volume VIII. Mammalia. (Walter de Gruyter: Berlin.)
Krefft, G. (1864). ‘Catalogue of Mammalia in the collection of the Australian Museum.’ p. 6. (Trustees of the Australian Museum: Sydney.)
Krefft, G. (1871). ‘The Mammals of Australia.’ (Thomas Richards, Government Printer: Sydney.)
Krefft, G. (1873). The bat tribe (continued). The Sydney Mail and New South Wales Advertiser. Volume 16. No. 700. p. 694, cols 1–2.
Krutzsch, P. H., and Crichton, E. G. (1987). Reproductive biology of the male little mastiff bat Mormopterus planiceps Chiroptera Molossidae in southeast Australia. The American Journal of Anatomy 178, 352–368.
| Reproductive biology of the male little mastiff bat Mormopterus planiceps Chiroptera Molossidae in southeast Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s3mslelsw%3D%3D&md5=fbc46f8edf6ff763e3b4b76422d5e50fCAS | 3604955PubMed |
Lamb, J. M., Ralph, T. M. C., Naidoo, T., Taylor, P. J., Ratrimomanarivo, F., Stanley, W. T., and Goodman, S. M. (2011). Toward a molecular phylogeny for the Molossidae (Chiroptera) of the Afro-Malagasy region. Acta Chiropterologica 13, 1–16.
| Toward a molecular phylogeny for the Molossidae (Chiroptera) of the Afro-Malagasy region.Crossref | GoogleScholarGoogle Scholar |
Laurie, E. M. O., and Hill, J. E. (1954). ‘List of Land Mammals of New Guinea, Celebes and Adjacent Islands 1758–1952.’ (British Museum: London.)
Leche, W. (1884). On some species of Chiroptera from Australia. Proceedings of the Zoological Society of London 1884, 49–54.
Legendre, S. (1984). Étude odontologíque des représentants actuels du groupe Tadarida (Chiroptera, Molossidae). Implications phylogéniques, systématiques et zoogéographiques. Revue Suisse de Zoologie 91, 399–442.
Mahoney, J. A., and Walton, D. W. (1988). Molossidae. In ‘Zoological Catalogue of Australia. Mammalia’. (Ed. D. W. Walton.) pp. 146–150. (Australian Government Publishing Service: Canberra.)
Martinez, S. (2010). Palaeoecology of the Mount Etna Bat Fauna, coastal eastern Queensland. Ph.D. Thesis, Queensland University of Technology, Brisbane.
McKenzie, N. L., and Bullen, R. D. (2009). The echolocation calls, habitat relationships, foraging niches and communities of Pilbara microbats. Records of the Western Australian Museum 78, 121–153.
McKenzie, N. L., and Bullen, R. D. (2012). An acoustic survey of zoophagic bats on islands in the Kimberley, Western Australia, including data on the echolocation ecology, organisation and habitat relationships of regional communities. Records of the Western Australian Museum 81, 67–108.
Mertens, R. (1925). Verzeichnis der Säugetier-Typen des Senckenbergischen Museums. Senckenbergiana Biologica 7, 18–37.
Miller, G. S. (1907). The families and genera of bats. Bulletin of the United States National Museum 57, 1–282.
Milne, D. J., and Pavey, C. R. (2011). The status and conservation of bats in the Northern Territory. In ‘The Biology and Conservation of Australasian Bats’. (Eds B. Law, P. Eby, D. Lunney and L. Lumsden.) pp. 208–225. (Royal Zoological Society of New South Wales: Sydney.)
Milne, D. J., Armstrong, K. N., and McKenzie, N. L. (2008). Western little free-tailed bat. In ‘The Mammals of Australia’. 3rd edn. (Eds S. van Dyck and R. Strahan.) pp. 488–489. (Reed New Holland: Australia.)
Nowak, R. M. (1994). ‘Walker’s Bats of the World.’ (The John Hopkins University Press: Baltimore.)
Nylander, J. A. A. (2004). MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University.
Peters, W. (1866). Über einige neue oder weniger bekannte Flederthiere. Monatsberichte der Königlichen Preussische Akademie der Wissenschaften zu Berlin 1866, 16–24.
Peters, W. (1881). Über die Chiropterengattung Mormopterus und die dahin gehörigen Arten. Monatsberichte Preussische Akademie der Wissenschaften zu Berlin 1881, 482–485.
Peterson, R. L. (1985). A systematic review of the molossid bats allied with the genus Mormopterus (Chiroptera: Molossidae). Acta Zoologica Fennica 170, 205–208.
Rambaut, A., and Drummond, A. J. (2007). Tracer v1.4. Available from: http://beast.bio.ed.ac.uk/Tracer.
Reardon, T. B., Adams, M., McKenzie, N., and Jenkins, P. (2008). A new species of Australian freetail bat Mormopterus eleryi sp. nov. (Chiroptera: Molossidae) and a taxonomic reappraisal of M. norfolkensis (Gray). Zootaxa 1875, 1–31.
Reardon, T. B., Robson, S. K. A., Parsons, J. G. M., and Inkster, T. (2010). Review of the threatened status of microchiropteran bat species on Cape York Peninsula. Report to Natural Resources Management, Queensland Section, Australian Government Land and Coasts.
Richards, G. C., Ford, G. I., and Pennay, M. (2008). Inland free-tailed bat. In ‘The Mammals of Australia’. 3rd edn. (Eds S. van Dyck and R. Strahan.) pp. 494–495. (Reed New Holland: Australia.)
Ride, W. D. L. (1970). ‘A Guide to the Native Mammals of Australia.’ (Oxford University Press: Melbourne.)
Ronquist, F., and Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574.
| MrBayes 3: Bayesian phylogenetic inference under mixed models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXntlKms7k%3D&md5=544b296d48ad75d42c45092f433e09b6CAS | 12912839PubMed |
Ryan, J. M. (1991). Morphology of the glans penis in four genera of molossid bats (Chiroptera: Molossidae). Journal of Mammalogy 72, 658–668.
| Morphology of the glans penis in four genera of molossid bats (Chiroptera: Molossidae).Crossref | GoogleScholarGoogle Scholar |
Simmons, N. B. (2005). Order Chiroptera. In ‘Mammal Species of the World: A Taxonomic and Geographic Reference’. 3rd edn. (Eds D. E. Wilson and D. M. Reeder.) pp. 312–529. (Johns Hopkins University Press: Baltimore.)
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28, 2731–2739.
| MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1eiu73K&md5=e841dcb65bf5743a3261692bb5310fe6CAS | 21546353PubMed |
Tate, G. H. H. (1941). Results of the Archbold Expeditions. No. 38. Molossid bats of the Archbold collections. American Museum Novitates 1142, 1–4.
Tate, G. H. H. (1952). Results of the Archbold Expedions. No. 66. Mammals of Cape York Peninsula, with notes on the occurrence of rain forest in Queensland. Bulletin of the American Museum of Natural History 98, 567–616.
Tavaré, S. (1986). Some probabilistic and statistical problems in the analysis of DNA sequences. Lectures on Mathematics in the Life Sciences 17, 57–86.
Thomas, O. (1906). On mammals collected in south-west Australia for Mr W. E. Balston. Proceedings of the Zoological Society of London 76, 468–478.
Toews, D. P. L., and Brelsford, A. (2012). The biogeography of mitochondrial and nuclear discordance in animals. Molecular Ecology 21, 3907–3930.
| The biogeography of mitochondrial and nuclear discordance in animals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1Cgt7rE&md5=1fdd53b58d420337620da2b89b5cbe3fCAS |
Troughton, E. le G. (1926). The bats of Australia and New Guinea. In ‘The Wild Animals of Australasia’. (Eds A. S. Le Souef and H. Burrell.) pp. 21–88. (George G. Harrap: London.)
Troughton, E. G. (1941). ‘Furred Animals of Australia.’ 1st edn. (Angus and Robertson: Sydney.)
Troughton, E. G. (1944). ‘Furred Animals of Australia.’ 2nd edn. (Angus and Robertson: Sydney.)
Turni, H., and Kock, D. (2008). Type specimens of bats (Chiroptera: Mammalia) in the collections of the Museum fur Naturkunde, Berlin. Zootaxa 1869, 1–82.
Van Dyck, S., and Strahan, R. (2008). ‘The Mammals of Australia.’ 3rd edn. (Reed New Holland: Sydney.)
Winter, J. W., and Allison, F. R. (1980). The native mammals of Cape York Peninsula – changes in status since the Archbold Expeditions. In ‘Contemporary Cape York Peninsula’. (Eds N. C. Stevens and A. Bailey.) pp. 31–44. (Royal Society of Queensland: Brisbane.)
Wood Jones, F. (1925). The Monodelphia. In ‘The Mammals of South Australia. Part III’. (Government Printer: Adelaide.)
Worthington Wilmer, J., Hall, L., Barratt, E., and Moritz, C. (1999). Genetic structure and male-mediated gene flow in the ghost bat (Macroderma gigas). Evolution 53, 1582–1591.
| Genetic structure and male-mediated gene flow in the ghost bat (Macroderma gigas).Crossref | GoogleScholarGoogle Scholar |
Yang, Z. (1996). Among-site rate variation and its impact on phylogenetic analyses. Trends in Ecology & Evolution 11, 367–372.
| Among-site rate variation and its impact on phylogenetic analyses.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7itFGjtw%3D%3D&md5=2a7c32049a02122782d3750d6a5f8141CAS |
Zhang, D.-X., and Hewitt, G. M. (1996). Nuclear integrations: challenges for mitochondrial DNA markers. Trends in Ecology & Evolution 11, 247–251.
| Nuclear integrations: challenges for mitochondrial DNA markers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7itFCmsw%3D%3D&md5=e1d1cc99225097032bb6f47180929071CAS |


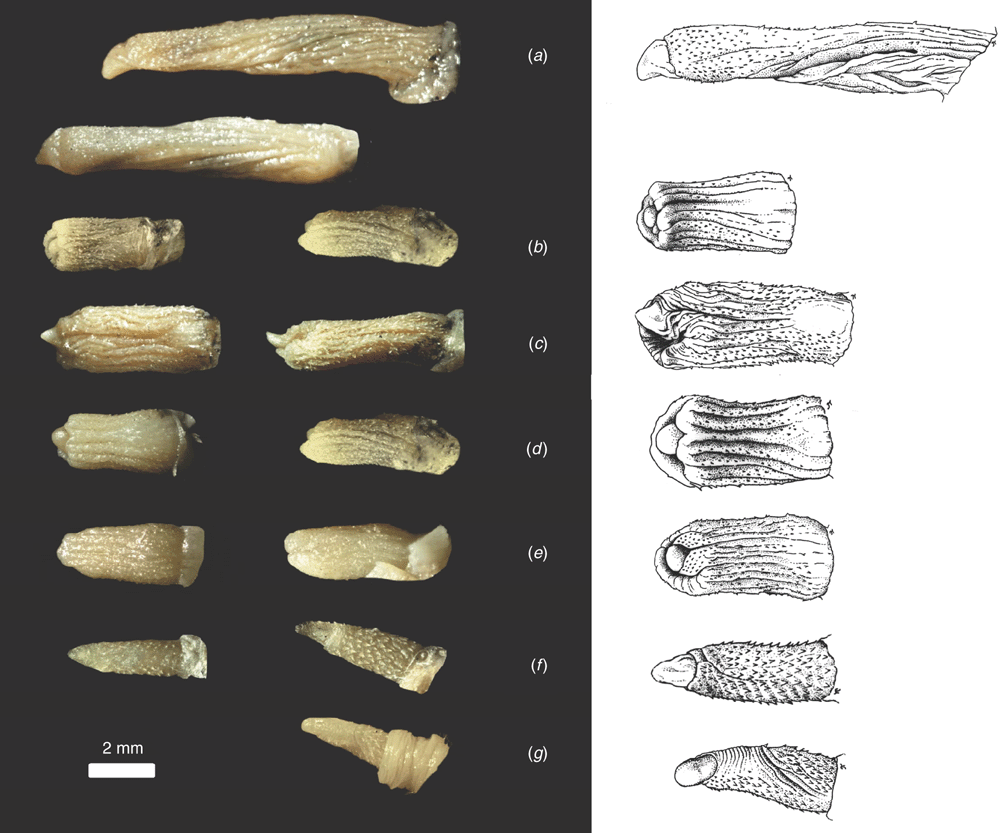
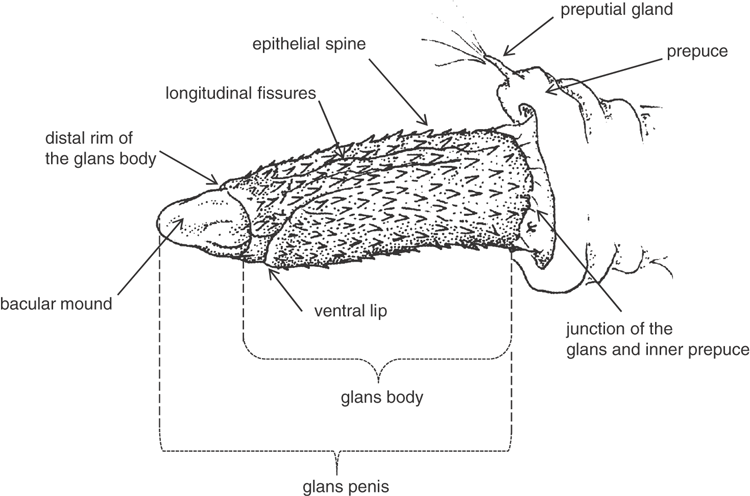
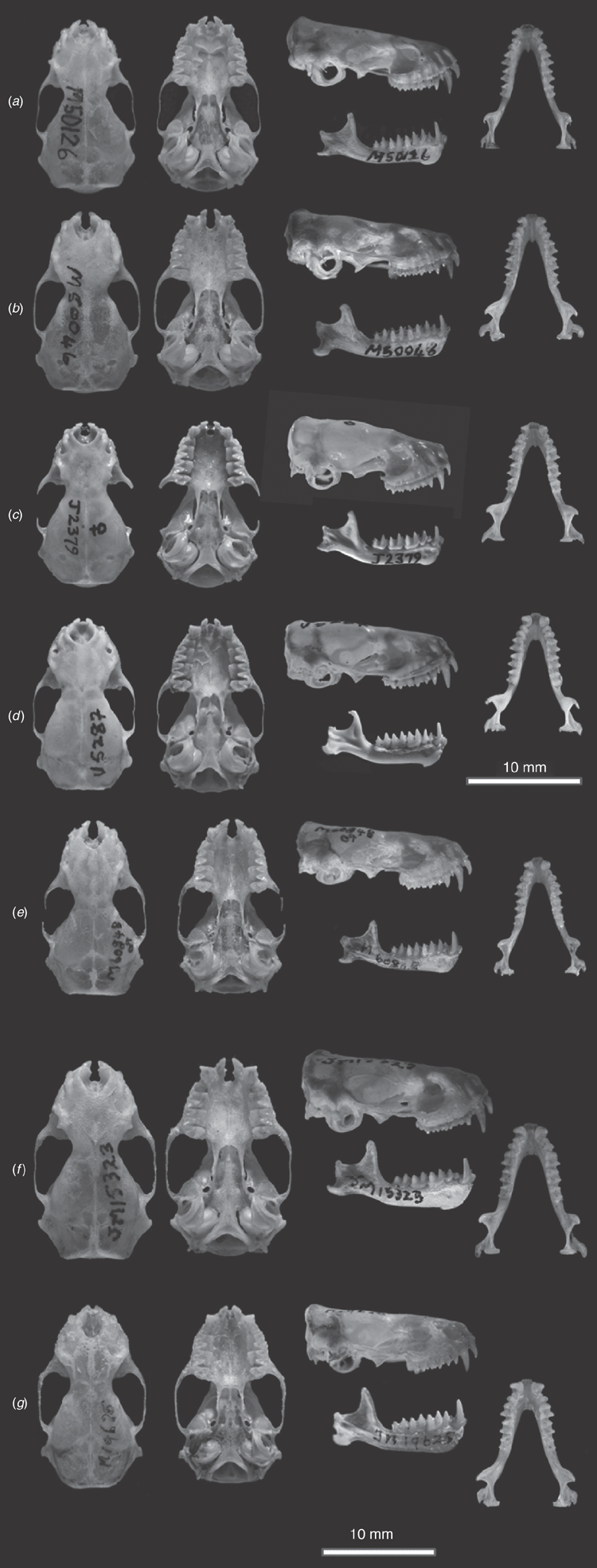
 ; mtDNA =
; mtDNA =  ; allozymes =
; allozymes =  ; other museum specimens identified on phallic morphology or previous allozyme studies = ●. Type locality (arrowed) given as ‘near Sydney?’
; other museum specimens identified on phallic morphology or previous allozyme studies = ●. Type locality (arrowed) given as ‘near Sydney?’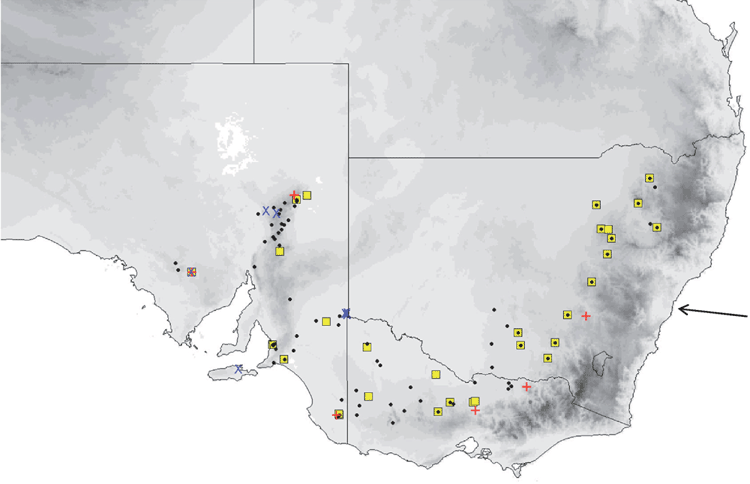
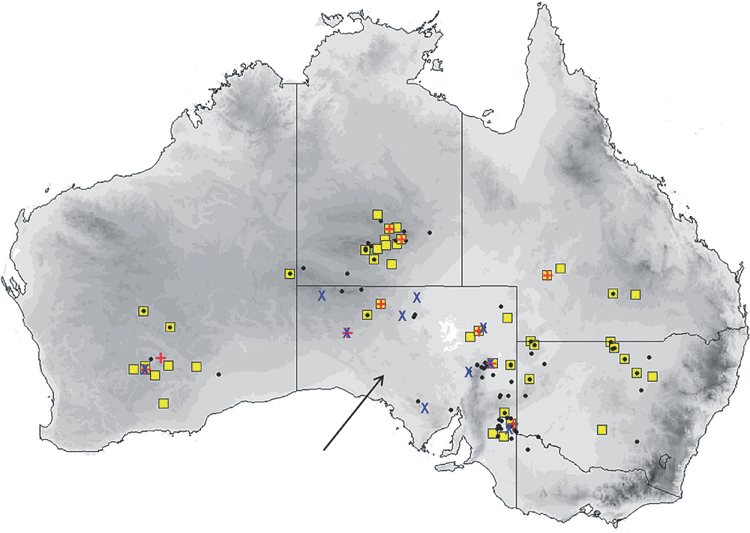
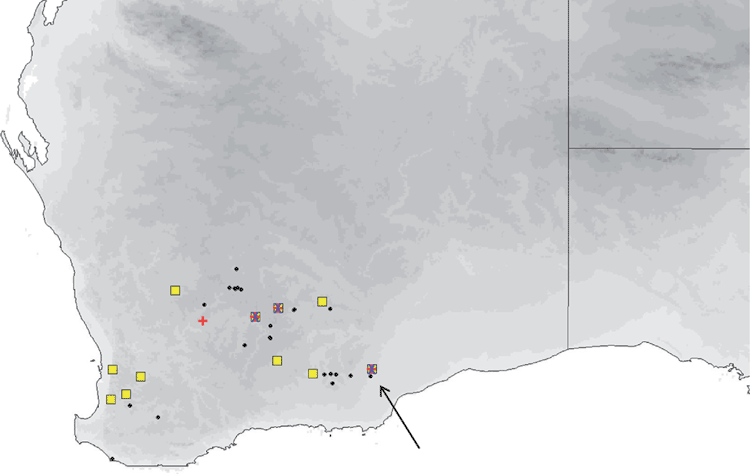
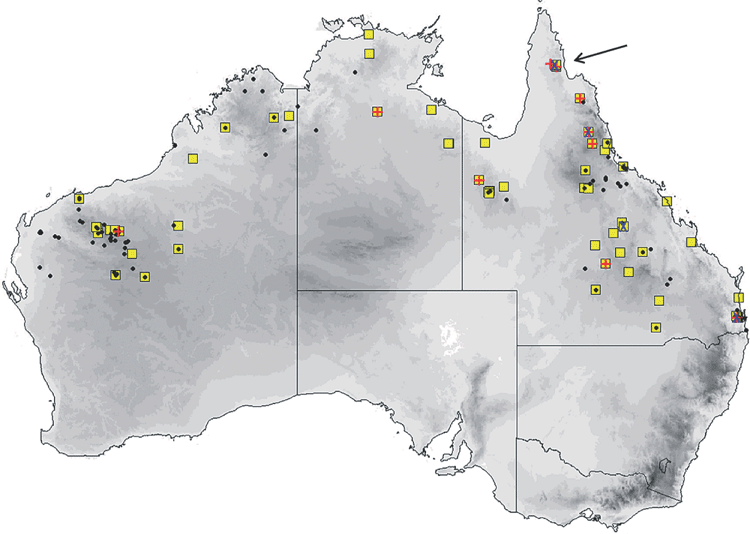
 (cobourgianus) and
(cobourgianus) and  (halli); mtDNA =
(halli); mtDNA =