Molecular evidence for mid-Pleistocene divergence of populations of three freshwater amphipod species (Talitroidea : Chiltoniidae) on Kangaroo Island, South Australia, with a new spring-associated genus and species
Rachael A. King A B D and Remko Leys A B CA South Australian Museum, North Terrace, Adelaide, SA 5000, Australia.
B Australian Centre for Evolutionary Biology and Biodiversity, School of Earth and Environmental Sciences, The University of Adelaide, Adelaide, SA 5005, Australia.
C School of Biological Sciences, Flinders University of South Australia, GPO Box 2100, Adelaide, SA 5001, Australia.
D Corresponding author. Email: Rachael.King@samuseum.sa.gov.au
Australian Journal of Zoology 62(2) 137-156 https://doi.org/10.1071/ZO13099
Submitted: 21 November 2013 Accepted: 3 February 2014 Published: 19 June 2014
Abstract
Recent molecular and morphological analyses have shown that chiltoniid amphipods, once thought to be a relictual group, are a diverse and speciose family of Australian freshwater amphipods. As part of a larger examination of the family, chiltoniids from Kangaroo Island in South Australia were collected and analysed using molecular (COI and 28S) and morphological methods in order to understand species distributional patterns and relationships. Kartachiltonia moodyi gen. nov., sp. nov., a spring-associated species endemic to the island, was discovered and populations of three additional mainland species (Austrochiltonia australis, A. dalhousiensis and A. subtenuis) were examined. The island populations of A. australis, A. dalhousiensis and A. subtenuis were found to form natural groups with differing haplotype coalescence times dating from the Early to Mid-Pleistocene. Numerous cycles of regional climate change throughout the Pleistocene are likely to have driven speciation in chiltoniid amphipods in southern Australia and the presence of multiple chiltoniid species at Kangaroo Island indicates that it exists at a likely convergence of species distribution patterns. Three possible hypotheses to explain the evolution and diversity of chiltoniids in southern Australia are discussed as are evidence for potential introduction and long-distance dispersal events.
Additional keywords: Amphipoda, australis, Austrochiltonia, biogeography, dalhousiensis, dispersal, groundwater, Kartachiltonia, morphology, mtDNA, subtenuis.
Introduction
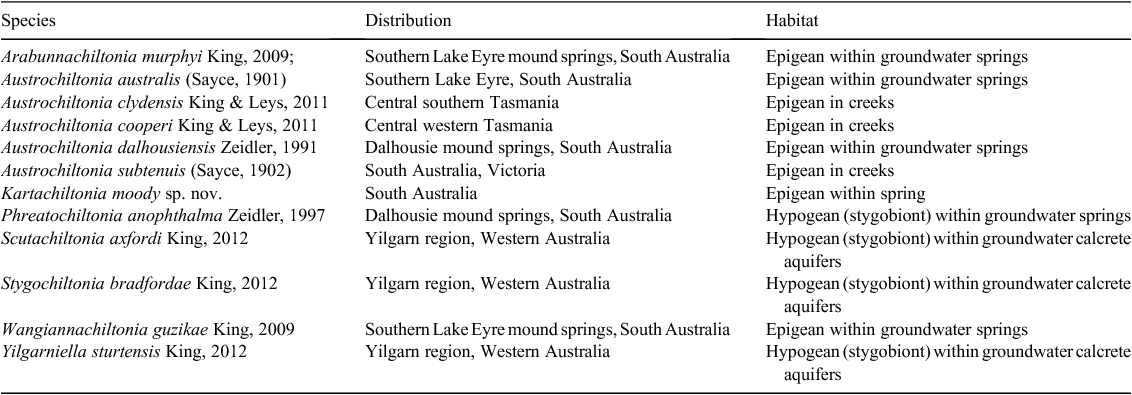
The Chiltoniidae (Amphipoda : Talitroidea) has long been regarded as a species-poor, relictual group of freshwater amphipods, with a Gondwanan distribution across southern Australia, South Africa and New Zealand (Barnard and Barnard 1983). However, recent exploration of diverse Australian groundwater-associated habitats has revealed chiltoniid amphipods to be a dominant and highly diverse member of the southern Australian subterranean fauna (Cooper et al. 2007; King 2009; Murphy et al. 2009, 2012; King and Leys 2011; King et al. 2012). As part of a larger research project to examine and define chiltoniid diversity, 11 species from seven Australian genera have now been described (Table 1). It is estimated that a further nine genera and ~40 species are still to be described from Western Australia and South Australia (R. A. King, unpubl. data); furthermore, a diverse chiltoniid fauna is reported from epigean and groundwater-associated habitats in eastern Australia (Victoria, New South Wales and Queensland) that has not yet been formally assessed (Fensham et al. 2010; R. A. King, unpubl. data). Much of this recent research has involved analyses of molecular data, which have provided a framework for morphological examination. This has allowed for detailed examination and confirmation of extensive morphological variability within Australian chiltoniid species (King and Leys 2011; King et al. 2012), which confounded earlier attempts to delineate species (see Williams 1962; Zeidler 1988).
|
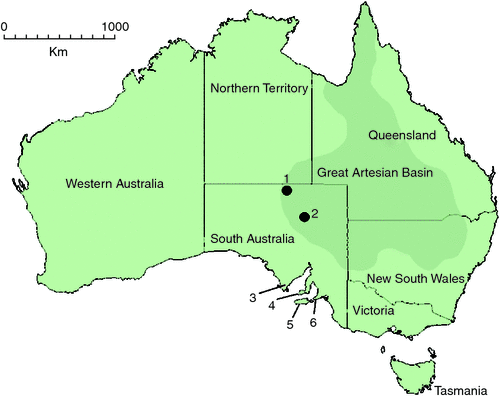
Molecular data also provide opportunities for dating lineages, to hypothesise the divergence of species or other taxa over geological time. In southern Australia, it is likely that significant climatic events have been responsible for driving freshwater habitat and biodiversity changes in the region (Byrne et al. 2008). Multiple successive oscillations as a result of contraction and expansion of Antarctic ice during the Pliocene (6–2.5 million years ago) (Williams et al. 1988) and between glacial and interglacial climates occurring during the Pleistocene (2.5 million years ago to 400 000 years ago) are believed to be responsible for causing global sea level and habitat biome changes (Williams et al. 1988; Lambeck and Chappell 2001; Raymo and Nisancioglu 2003; Byrne et al. 2008; Davis et al. 2013). In Australia, these climatic shifts are thought to have further driven the aridification of inland Australia that began in the late Miocene, with long periods of substantial cooling and decreased precipitation creating stony deserts and sand dune systems in formerly warm forest areas that would have had permanent lake and river systems (Markgraf et al. 1995; Byrne et al. 2008; Davis et al. 2013). Within this changeable landscape southern Australian freshwater habitats, and their associated aquatic invertebrate fauna, became fragmented and aquatic refugia were formed: karstification events created caves and subterranean aquifers, waterholes formed in dry riverine systems, and localised and artesian springs became regional sources of fresh water (Fensham et al. 2011; Davis et al. 2013). Within South Australia, groundwater-fed spring systems (‘mound springs’) in the Lake Eyre Basin, fed by uplifted water along the south-western edge of the subterranean Great Artesian Basin (Fig. 1), are important locally as permanent habitats for freshwater invertebrates (Prescott and Habermehl 2008; Fensham et al. 2011).
|
Two epigean chiltoniid species (Austrochiltonia australis (Sayce, 1901) and Austrochiltonia subtenuis (Sayce, 1902)) and four Great Artesian Basin mound spring–associated chiltoniid species (Arabunnachiltonia murphyi King, 2009; Austrochiltonia dalhousiensis Zeidler, 1997; Phreatochiltonia anophthalma Zeidler, 1991; Wangiannachiltonia guzikae King, 2009) have been recorded from South Australia. P. anophthalma and A. dalhousiensis are known from the Dalhousie Springs complex in Witjira National Park in northern South Australia (Zeidler 1991, 1997) (Fig. 1). A. murphyi and W. guzikae are distributed within the southern Lake Eyre mound springs in northern South Australia (Murphy et al. 2009; King 2009; Murphy et al. 2012) (Fig. 1). King and Leys (2011) recently confirmed the epigean A. australis and A. subtenuis as divergent species, using molecular and morphological methods, and redefined their distributions across southern Australia (A. subtenuis with a broad Murray–Darling basin distribution and A. australis with a more confined, mostly coastal catchment distribution across Victoria and parts of South Australia).
During recent wide-ranging chiltoniid collecting, samples were obtained from Kangaroo Island, South Australia, that indicated that at least three epigean species of chiltoniid amphipods were present across the island, whilst there was one (A. subtenuis) dominant chiltoniid amphipod on the adjacent mainland. Importantly, A. dalhousiensis, formerly known only from the inland Dalhousie Springs complex, was tentatively identified on the island along with A. subtenuis and A. australis.
Kangaroo Island is Australia’s third largest island at 150 km long and with an area of ~4400 km2. It is situated at the entrance to the Gulf St Vincent, separated from the mainland by ~13 km at its closest point (Cape Jervis) and adjacent to the Fleurieu and Yorke Peninsulas of South Australia (Fig. 1). The island was connected to mainland Australia during the last glacial period, until at least 9800 years ago (Belperio and Flint 1999). There are few published accounts of the freshwater invertebrates of Kangaroo Island, with most concentrating on freshwater-associated insects (Gross et al. 1979; Suter 1986; Suter and Bishop 1990), as well as crayfish introductions to the island (Zeidler 1982, 2000). Freshwater faunal diversity is thought to be similar to that of the nearby Fleurieu Peninsula on the mainland, with diverse larval insects, crustaceans (Copepoda, Ostracoda, Amphipoda), water mites, annelids and molluscs being some of the larger represented groups (Austin et al. 2002). There have been no formal taxonomic treatments of the Amphipoda on Kangaroo Island; however, A. australis has been reported as ‘commonly distributed’ (Austin et al. 2002).
With preliminary evidence of several diverse chiltoniid species on Kangaroo Island, a more comprehensive survey of the island was deemed necessary. The overall aims were to collect chiltoniid amphipods extensively across available habitats and to analyse their molecular and morphological diversity. Specific questions were whether natural groups of species were occurring on the island, or if anthropogenic introductions could be detected; whether relationships and distributional patterns between species on the island were similar to those documented on the mainland; whether analyses of the ages of island and mainland groups would prove informative in hypothesising the evolution and distribution of chiltoniid amphipod species across southern Australia.
Materials and methods
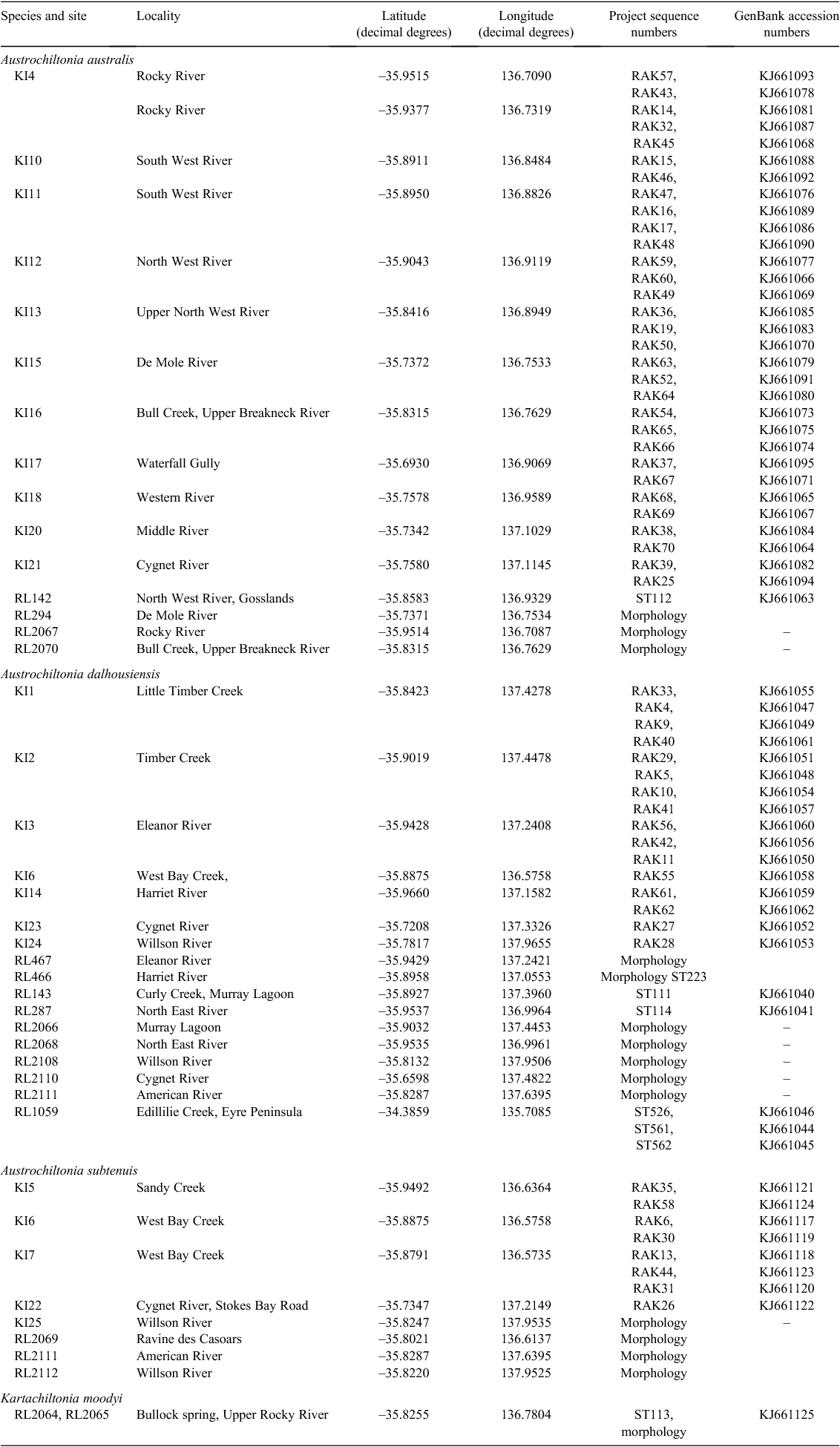
During December 2009 and January 2010, 27 freshwater sites across Kangaroo Island were sampled and chiltoniid amphipods were collected at 24 sites. Material from 18 additional sites, sampled in 2003, 2005, 2011 and 2012, stored in 100% EtOH as well as from the frozen tissue collection of the South Australian Museum, was also included in the analysis (Table 2).
Many of the sites on Kangaroo Island where chiltoniids were successfully collected were small creeks or edges of small rivers with lower water flow and vegetation/or root structure apparent. Several additional attempts to sample amphipods from groundwater in bore-holes at two sites in Rocky River and from alluvial sediments in Sandy Creek were unsuccessful.
Dip nets were used to sample amphipods from around vegetation/root structures. Specimens were hand sorted in the field to minimise bycatch, placed in 100% EtOH, and kept cold (4°C or less) to maximise tissue preservation for DNA analysis.
In addition to the Kangaroo Island material, chiltoniid specimens tentatively identified as A. dalhousiensis collected from the Eyre Peninsula, South Australia (Fig. 1) were added to the analyses (Table 2). Specimens and sequence data of A. australis, A. subtenuis and A. dalhousiensis from previous research efforts (Murphy et al. 2009; King and Leys 2011) were included for more robust comparative analyses.
Abbreviations: SAMA – South Australian Museum collection numbers; KI and RL – prefix to individual site (locality) numbers; RAK and ST – prefix to individual DNA sequence numbers.
Molecular methods
Genomic DNA was extracted from 2–3 dissected pereopods or from the entire body of small specimens using the Gentra (puregene) method or using DNAzol (Molecular Research Center, Cincinnati, Ohio) (Chomczynski et al. 1997) with some modifications. Before extraction, ethanol-preserved material was completely desiccated, and before centrifugation the homogenate was incubated at room temperature for 2 h with proteinase K (400 μg mL–1; Sigma, St Louis, MO) after which DNA was precipitated overnight at –20°C with 100% ethanol. PCR amplification and sequencing were performed as described in Cooper et al. (2007) with the addition that, for specimens collected after 2006, 50 μL PCR products were sent to Macrogen, South Korea, for purification and sequencing.
A 1506-bp region of the mitochondrial Cytochrome oxidase subunit 1 (CO1) gene was amplified using a combination of universal primers: M414 (forward, 5′-GGT CAA CAA ATC ATA AAG ATA TTG G-3′, alias LCO1490: Folmer et al. 1994), M423 (reverse, 5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′, alias LCO2198: Folmer et al. 1994), M202 (forward, 5′-CAA CAT TTA TTT TGA TTT TTT GG-3′, alias Jerry: Simon et al. 1994) and M493 (reverse, 5′-TCA CAT TTT CTG TCA CTT T-3′, developed in our laboratory specifically for chiltoniid amphipods; this primer replaces TL-2-N-3014 (alias Pat) of Simon et al. 1994). In addition, primers specifically developed for chiltoniid amphipods were used to amplify ~900 bp in cases where the former primer pairs did not result in the required products: M735 (forward, 5′-CGT ATA AAT AAY ATA AGA TTT TG-3′) and M737 (reverse, 5′-CAA CAA GAA AAA GAA TCA GG-3′) (Murphy et al. 2009). An ~696-bp region of the nuclear RNA gene 28S was amplified using primers G1768 (forward, 5′-GCT CAG AGT ATA AGC CGA TGG CGC-3′) and G1769 (reverse, 5′-CGC CTC GGT TTT GTG AGA CCC-3′) developed for chiltoniid amphipods (Murphy et al. 2009). This fragment of the nuclear gene 28S was sequenced for a small number of individuals of each species in order to get improved resolution of the phylogenetic relationships among the currently named chiltoniid species.
SeqEd ver. 1.0.3 (Applied Biosystems) and ChromasPro ver. 1.34 (Technelysium Pty Ltd) were used to edit chromatogram files, to determine a consensus sequence from both strands, and to align sequences. ClustalX (Thompson et al. 1997) was used to prealign 28S rRNA sequences and adjustment to the alignment was done by eye using a secondary structure model of Apis mellifera (Gillespie et al. 2006).
Molecular analyses
Phylogenetic analyses of aligned sequence data of Kangaroo Island specimens as well as from previous work (King and Leys 2011) were carried out using the programs PAUP* ver. 4.0b8 (Swofford 2001) and BEAST ver. 1.7.2 (Drummond and Rambaut 2007). PAUP* was used for generating and editing data matrices as well as error proofing using neighbour-joining runs, and for analyses of uncorrected sequence divergence. BEAST was used to apply relaxed molecular clock methods. As fossils are not known for chiltoniid amphipods, a mean rate of 0.0115 substitutions per site per million years (Brower 1994) was used as the prior in analyses with an uncorrelated log-linear molecular clock. We are aware of the restrictions of using this clock rate but, because it is the average of several independent rate calibrations, for the moment we consider it as a best guess. A Yule process of speciation was used for the chiltoniid analyses of genera and a coalescence model with constant population size for analyses of the individual species. The analyses were performed by first applying unlinked data partitions for each of the COI gene codons for the analyses of the chiltoniid genera, and then two data partitions (first + second, third codons) for analyses of A. australis, A. subtenuis and A. dalhousiensis. A general time-reversible model of sequence evolution with invariable sites and gamma distributed rates across sites (GTR + I + G) was used. Tracer ver. 1.5 (Rambaut and Drummond 2007) was used to make sure that the effective sample size of the parameters during the BEAST runs were larger than 100.
Likelihood ratio tests (Huelsenbeck and Bull 1996) were used to investigate whether phylogenetic structure reflects drainage patterns. This was done by comparing the likelihood of the unconstrained analysis with the likelihood of the analysis where specimens collected from the same catchments were forced in the same clades (the constrained analysis).
Morphological methods
The molecular phylogeny was used as framework for focussed morphological study. Specimens from divergent lineages were examined and morphological diversity explored in an effort to discover and analyse characters that would reliably delineate species. For the taxonomic work, specimens were dissected along the left side, and illustrations produced with a drawing tube attachment to a Nikon Eclipse 80i microscope. All type material has been lodged with the South Australian Museum (SAMA prefix).
Results
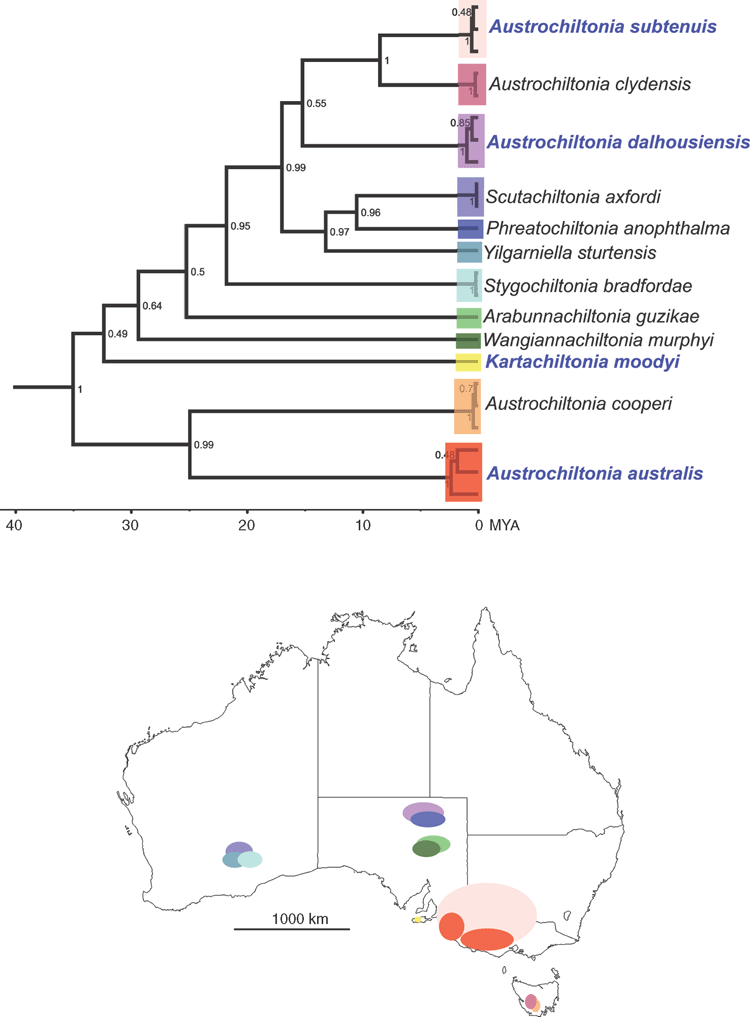
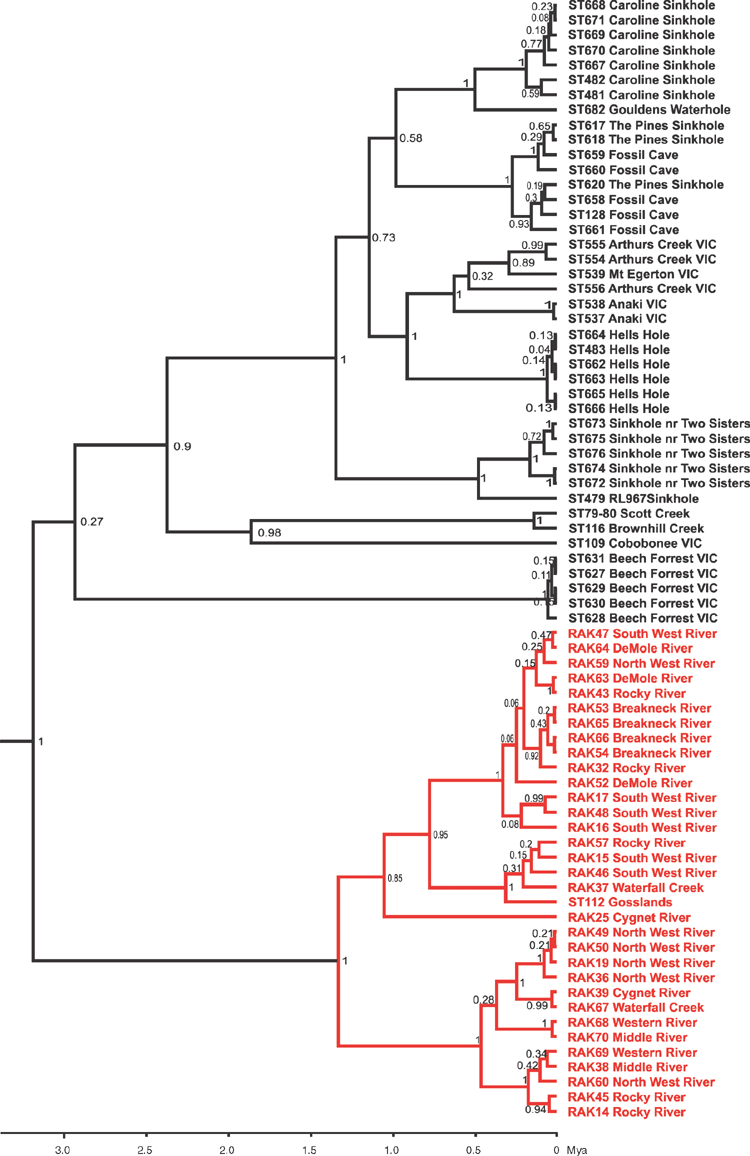
Phylogenetic analysis of CO1 and 28S molecular data and subsequent morphological examination of Kangaroo Island chiltoniid amphipods revealed a new endemic spring-associated species (Kartachiltonia moodyi gen. nov., sp. nov.) and confirmed the presence of three species that also occur on mainland South Australia (A. australis, A. dalhousiensis and A. subtenuis). A preliminary phylogeny of the currently described Australian chiltoniid species (Fig. 2) illustrates their relationships with the Kangaroo Island lineages.
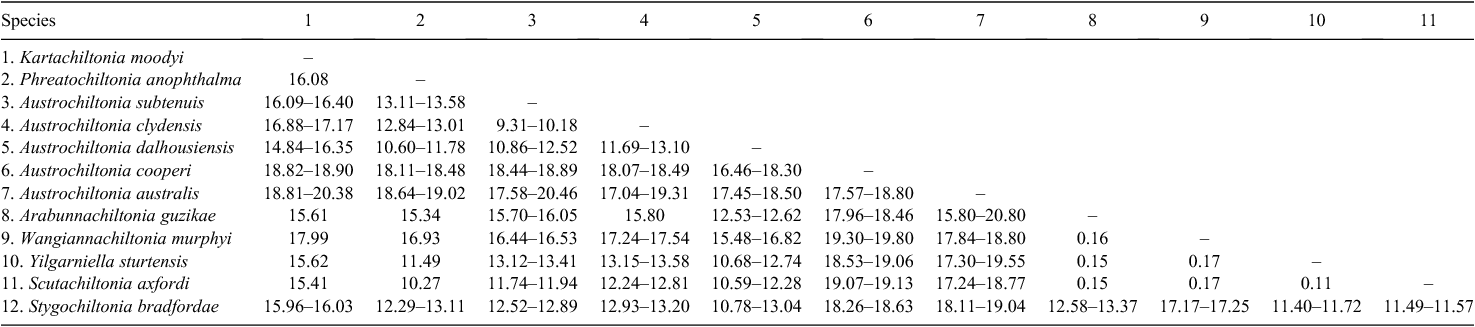
Kartachiltonia moodyi gen. nov., sp. nov. was discovered from a spring (Bullock spring) near the headwaters of the western tributary of the Rocky River in Flinders Chase National Park and is morphologically significant by its possession of sternal gills on pereonites 2–7 and slender coxal gills on pereonites 2–6. Neither sternal gills nor slender coxal gills have been recorded in Australian species but are present in at least two New Zealand species subsequently examined during the analyses (Chiltonia rivertonensis Hurley, 1954; and Chiltonia mihiwaka (Chilton, 1898) (King, pers. obs.)). Phylogenetic analyses placed Kartachiltonia at or near the base of all other Australian taxa, although the current data of CO1 and 28S do not completely resolve that part of the tree (Fig. 2). Given the highly divergent nature of the molecular lineage of this species (15–20% COI uncorrected pairwise sequence divergences with other species: Table 3) indicating ancient lineage separation, its isolation within a groundwater-associated spring habitat, and the presence of sternal gills, it was deemed necessary to erect a new genus for the species.
|
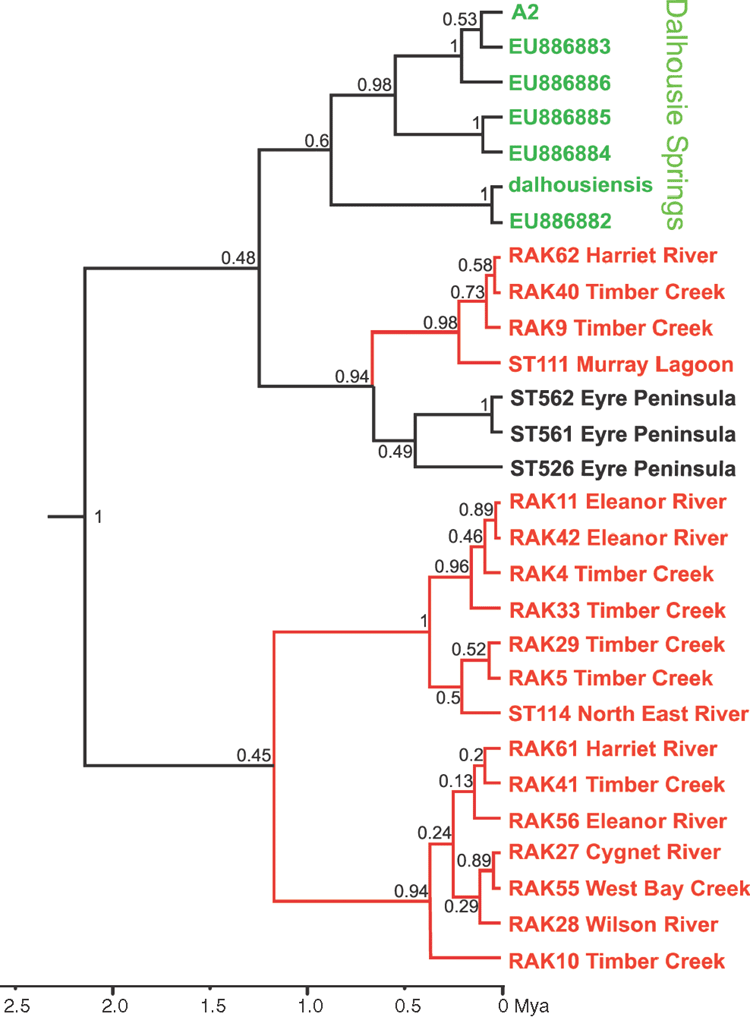
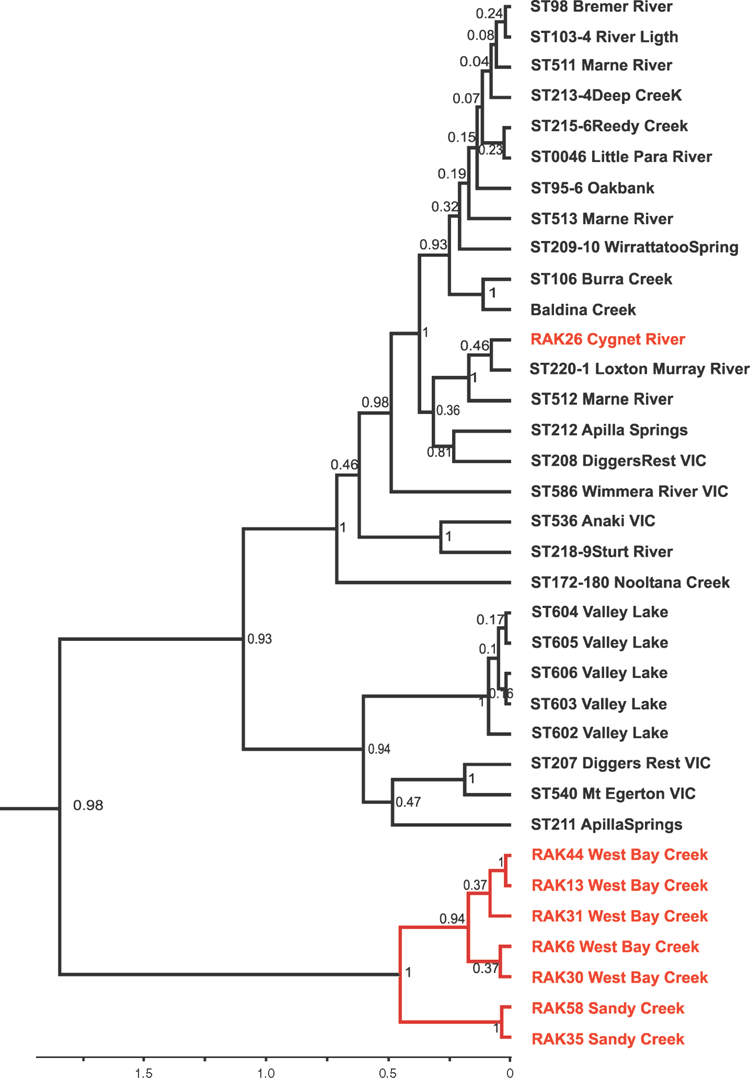
Phylogenetic analyses using CO1 (Figs 3–5) indicate that the three mainland species, A. australis, A. dalhousiensis and A. subtenuis, form natural groups on Kangaroo Island, as divergence times from mainland lineages (with a single exception of one lineage of A. subtenuis, see Discussion) largely predate that of human occupation of Australia. For each species, the coalescence time of the haplotype divergences of the Kangaroo Island lineages occurred at different times. In A. australis there is a single lineage that diverged around 1.33 million years ago (Fig. 3); A dalhousiensis has two independent haplotype lineages that diverged 1.16 million and 0.22 million years ago respectively (Fig. 4); and A. subtenuis has a single lineage that dates back to ~0.45 million years ago (Fig. 5). Molecular data also confirmed that additional chiltoniid specimens from Eyre Peninsula, used for comparative purposes, were likely a natural population of A. dalhousiensis with ~1.5–2.5% CO1 uncorrected pairwise sequence divergences with populations from Kangaroo Island and Dalhousie Springs.
|
|
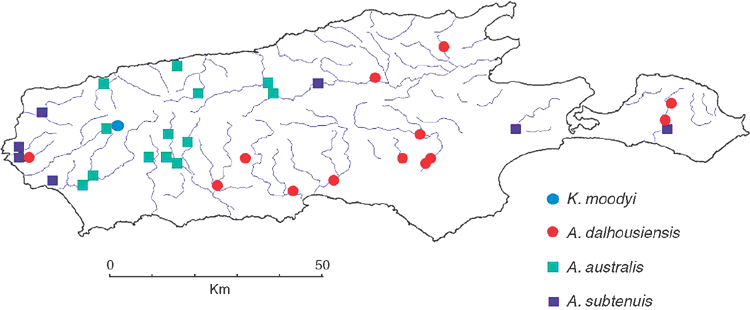
The four chiltoniid species are not uniformly distributed on Kangaroo Island (Fig. 6), but appear to be correlated with salinity levels of the surface waters. A. australis is limited to the upper parts of the river catchments on the western half of the island with salinity levels not exceeding 1500 mg L–1. The distribution of A. dalhousiensis is largely restricted to the eastern half of the island in the lower parts of the river catchments where salinity levels are usually brackish (1500–5000 mg L–1) to saline (>5000 mg L–1). A similar range of salinity values has been measured at sites where A. dalhousiensis was collected on Eyre Peninsula (1180–17 640 (+28 300 outlier) mg L–1, medium 6450 mg L–1, n = 24 localities: data from South Australian Environmental Protection Authority) and at Dalhousie Springs (~700–9700 mg L–1) (Smith 1989). A. subtenuis is the least recorded species on the island, in direct contrast to adjacent mainland distributions of chiltoniid species where it is the dominant species. On the island it has a disjunct distribution mainly in lower parts of catchments in the east and west (Fig. 6). K. moodyi is restricted to a single locality, a spring with fresh water (salinity <500 mg L–1). Low levels of sympatry (at only three localities of the 41 sites studied did A. subtenuis and A. dalhousiensis co-occur (American River, Willson River and West Bay Creek); also, all three species occur in different parts of the large Cygnet River system), may further indicate that each species has specific ecological preferences. It may also indicate that competition and/or precedence within habitats is an issue (Waters et al. 2013).
The Kangaroo Island lineages within A. australis (Fig. 3) show no obvious distributional patterns related to catchments. Analysis of the phylogenetic structure of A. australis on Kangaroo Island, using both unconstrained and drainage system–constrained analyses indicates that the relationships within the unconstrained phylogeny do not reflect current drainage systems. The unconstrained analysis resulted in a significantly higher likelihood compared with the drainage-constrained analysis (likelihood ratio test: 2lnΔ = 426, P << 0.01 (ln = –2148 versus –2361)), indicating that there is or recently has been gene flow across drainages. The analyses could not be completed for A. subtenuis and A. dalhousiensis due to smaller sample sizes. However, A. dalhousiensis (Fig. 4) shows similar patterns to A. australis, with individuals from widely separate catchments (e.g. Cygnet River, West Bay Creek and Wilson River) appearing to be more closely related than individuals from neighbouring catchments.
Morphological variability
Substantial morphological variability within populations has previously been reported in chiltoniid amphipods (Zeidler 1991; King 2009; King and Leys 2011) and results from this study largely reflect that high variability. Within the Kangaroo Island populations, A. subtenuis and A. australis are similar in morphology to mainland populations in South Australia. A. subtenuis continues to be defined by a short male gnathopod 2 propodus, uropod 3 of one article, and a broad basis of pereopod 7. A. australis remains distinguished by male gnathopod 2 elongate, uropod 3 of two articles, and a narrow basis of pereopod 7. A. dalhousiensis populations on Kangaroo Island do show some differences from those from the type locality at Dalhousie Springs: most adult specimens are larger (5 mm compared with ~4 mm average at Dalhousie Springs) and in Kangaroo Island populations the morphology of uropod 1 is robust in the male (and not seen in Dalhousie Springs populations); gnathopod, pereopod and epimera shape is indistinguishable between populations at each location. The robust (possibly late-stage) male uropod 1 morphology has been noted in almost all epigean chiltoniid species but never in groundwater-associated populations, including Dalhousie Springs populations and Kartachiltonia (R. A. King, unpubl. data).
It should be noted that a defining character of previous chiltoniid keys (setation of the uropod 1 rami) (King and Leys 2011) was found to be variable within the Kangaroo Island species studied and is likely to be so in mainland populations as more are studied: the distinction between ‘robust setae along entire lengths’ and ‘1–3 scattered at mid length’ was not obvious. Instead, epimera and the shape of male gnathopod 1 was used to reliably distinguish between the species. A. australis is quite easily distinguished by the epimera 1–3 heavily projecting into spines and the gnathopod 1 palm shorter than propodus length, whilst A. subtenuis and A. dalhousiensis are distinguished by rounded epimera 1–3 and gnathopod 1 palm as long as propodus length. Further distinction between A. subtenuis and A. dalhousiensis is subtle: in A. subtenuis epimera 1–3 usually project into very small spines whilst in A. dalhousiensis epimera 1–3 are rounded and rarely project into small spines. A. dalhousiensis is further distinguished from A. subtenuis by the pereopod 7 basis (narrow rather than as broad as long), and uropod 3 of two articles. Additional Eyre Peninsula populations of A. dalhousiensis, confirmed here via molecular data, are morphologically indistinguishable from the Kangaroo Island populations.
Systematics
Infraorder Talitrida Rafinesque, 1815
Superfamily Talitroidea Rafinesque, 1815
Family Chiltoniidae Barnard, 1972
Austrochiltonia dalhousiensis Zeidler, 1997
Material examined
Distribution
South Australia: Dalhousie Springs (Witjira National Park), southern Eyre Peninsula, Kangaroo Island.
Remarks
New locality data for A. dalhousiensis show that the species’ geographical distribution, apart from its type locality at Dalhousie Springs in the far north of South Australia, now also includes Kangaroo Island and Eyre Peninsula.
Kartachiltonia King & Leys, gen. nov.
Diagnosis
Antenna 1 no more than 1/3 length of body, slightly longer than antenna 2. Coxae 1–3 short (no more than 1.5 times as long as wide); coxae 2–3 with small defined proximal corner; coxa 4 as long as broad, with defined proximal corner. Coxal gills narrow. Sternal gills present on pereonites 2–7. Pereopod 5–7 bases with small postero-distal lobe (not reaching past ischium). Epimera 1–3 with postero-distal corners defined with a blunt spine.
Remarks
Molecular evidence shows that the Bullock springs specimens form a unique, clearly divergent lineage (15–18% COI pairwise sequence divergences) and with morphological evidence including the presence of sternal gills on pereonites 2–7, narrow coxal gills (compared with the broad coxal gills in all other Australian species), and coxae 2–3 with developed proximal corners, a new genus was necessary for this species.
Etymology
‘Karta’ is an Aboriginal name for Kangaroo Island, meaning ‘island of the spirits/dead’, together with ‘chiltonia’ for its placement within the Chiltoniidae.
Kartachiltonia moodyi, sp. nov.
|
|
Diagnosis
Antenna 1 around 1/3 body length, flagellum twice as long as the peduncle; antenna 2 around 1/4 body length, flagellum similar size to peduncle; gnathopod 2 propodus (in males) around 1.6 times as long as broad; pereopod 7 basis about as broad as long; uropod 3 biarticulate.
Material examined
Distribution
Known only from the type locality: ‘Bullock Springs’, Flinders Chase National Park, Kangaroo Island, South Australia.
Description
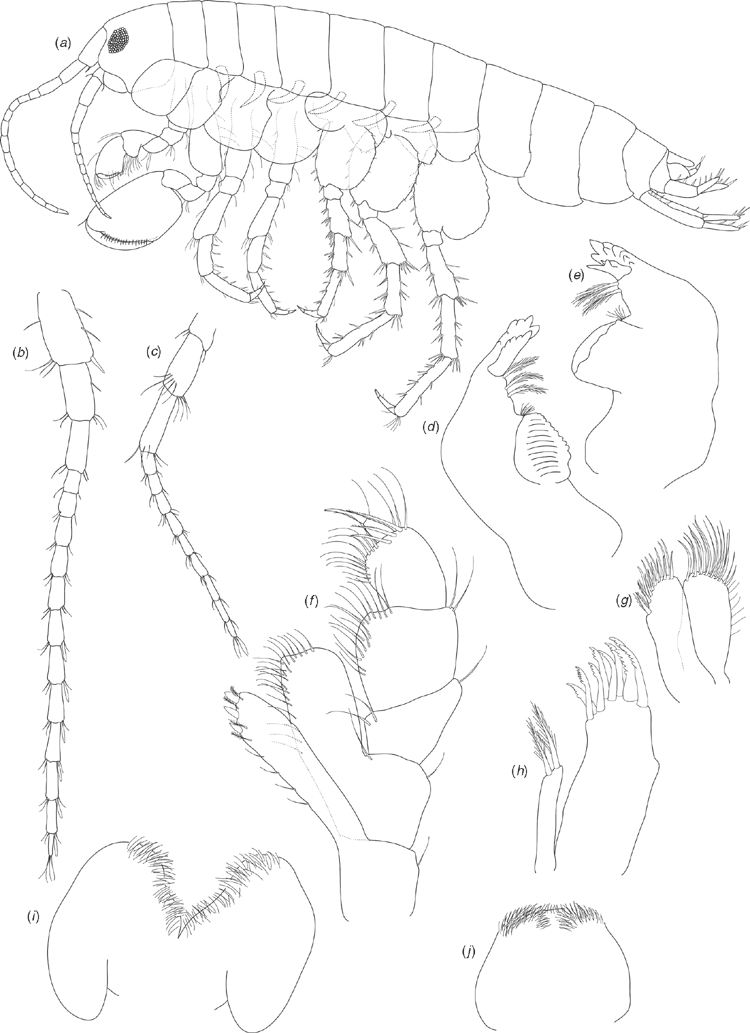
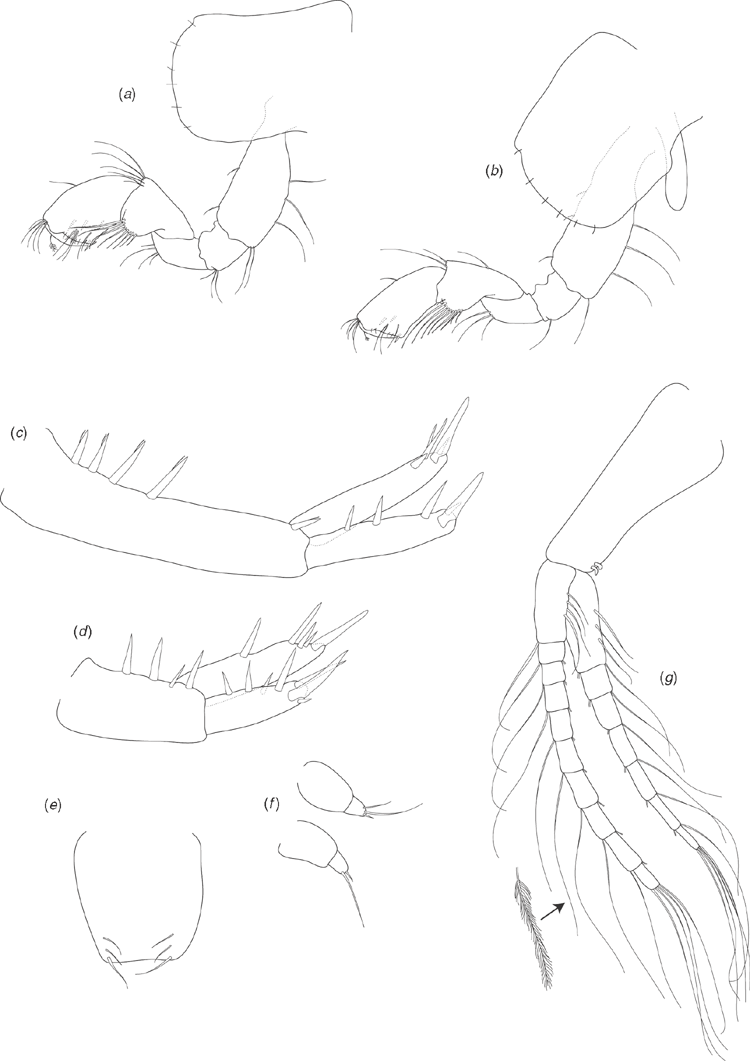
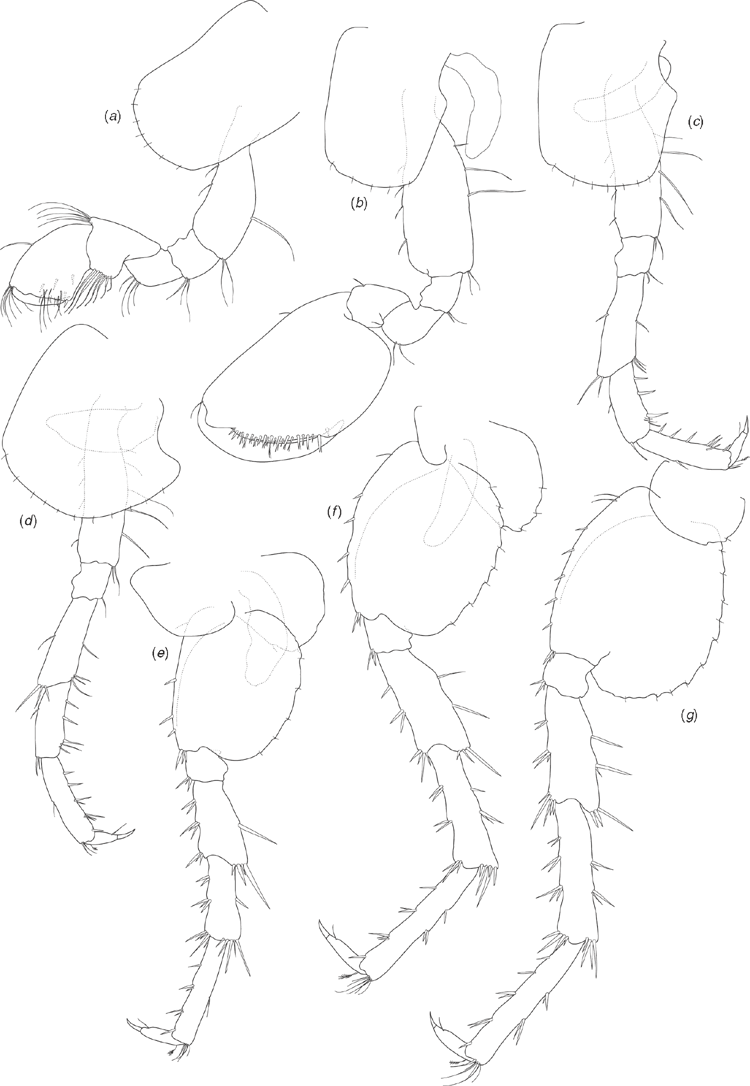
Holotype male (based on SAMA C7884). Length: 3.78 mm. Head about as long as deep (Fig. 7a). Antenna 1 (Fig. 7b) 0.4 times body length, peduncular article 1 twice as long as broad, inner lateral margin with two robust setae, distoventral margin with single robust seta; peduncular article 2 shorter than article 1 (0.75 times as long), twice as long as broad; peduncular article 3 similar length to article 2, three times as long as broad; flagellum two times longer than peduncle, of 12 articles, with ventral aesthetascs on the proximal margins of the seven distal articles. Antenna 2 (Fig. 7c) ~0.6 times length of antenna 1; peduncular article 3 broader than long, inner-distal margin with one robust seta; peduncular article 4 longer than article 3, 2.2 times longer than broad; peduncular article 5 longer than article 4, four times as long as broad; flagellum longer than peduncle (1.4 times), of nine articles.
Upper lip (Fig. 7j) slightly broader than long, apically bluntly rounded, with numerous short setae along apical margin. Lower lip (Fig. 7i) with rounded lateral lobes, apical margins rounded, apical and inner margins with numerous short setae. Left mandible (Fig. 7d) with incisor of six teeth, lacinia mobilis of four teeth, spine row of three plumose setae and triturative molar. Right mandible (Fig. 7e) with incisor of seven teeth, lacinia mobilis of three teeth, spine row of two plumose setae and triturative molar (long plumose seta not recorded). Maxilla 1 (Fig. 7h) outer plate with nine setulate robust setae; inner plate with two long apical plumose setae. Maxilla 2 (Fig. 7g) outer plate with apical and oblique rows of seven to eight simple setae; inner plate with apical and oblique rows of six to seven simple setae, with two plumose setae on the inner lateral margin below the apical row. Maxilliped (Fig. 7f) inner plate apical margin with three short spine-like robust setae, with short simple and plumose seta along apical and inner lateral margins; outer plate with numerous simple setae along apical and inner lateral margins; palp articles 1 and 2 similar width, palp article 2 with numerous simple setae on inner lateral margin; palp article 3 not as broad as articles 1 and 2, with numerous simple setae on inner lateral and distal margins, posterior margin with three long robust setae with setules; palp article 4 short, less than half as broad as article 3, with unguis and seta on distal margin. Gnathopod 1 (Fig. 8a) coxa 1.5 times as long as broad, distal margin with seven short simple setae; basis dorsal and ventral margins with scattered long simple setae, distoventral corner with cluster of simple setae; ischium, and merus distoventral corners with clusters of setae; carpus with ventral-lateral lobe and row of seven setulate setae becoming longer distally, distodorsal margin with long setae; propodus 1.5 times as long as broad, subchelate, palm transverse, distoventral corner with one robust seta at corner of palm, medial palm margin with short robust and long simple setae, distodorsal margin with long simple setae, inner face with three robust plumose setae; dactylus curved, fitting against palm, with proximal plumose seta. Gnathopod 2 (Fig. 8b) coxa 1.3 times as long as broad, distal margin with five short simple setae; basis dorsal and ventral margins with scattered simple setae; ischium and merus with scattered setae on ventral margins; propodus 1.5 times as long as broad, subchelate, with proximal lobe covering distal margin of carpus, palm transverse, distoventral corner marked by dactylar socket, palm margin with numerous robust setae with subterminal spines. Pereopod 3 (Fig. 8c) coxa 1.2 times as long as broad, distal margin with seven short simple setae; basis dorsal and ventral margins with scattered simple setae, distoventral corner with clusters of setae; ischium distoventral corner with clusters of setae; merus with distinct distodorsal lobe, ventral margin with scattered simple setae, distoventral corner with cluster of setae; carpus ventral margin with robust setae and scattered simple setae; propodus dorsal margin with one cluster of simple setae; ventral margin with five clusters of robust and simple setae; dactylus dorsal margin with plumose seta, ventral margin with simple seta, unguis present. Pereopod 4 (Fig. 8d) coxa with distinct proximal excavation, distal margin with 11 short simple setae; basis dorsal and ventral margins with scattered simple setae, distoventral corner with cluster of simple setae; ischium distoventral corner with cluster of setae; merus dorsal margin with distinct distal lobe, ventral margin with scattered simple setae, distoventral corner with cluster of setae; carpus ventral margin with scattered robust and simple setae; propodus ventral margin with five clusters of robust and simple setae; dactylus dorsal margin with proximal plumose seta, ventral margin with simple seta, unguis present. Pereopod 5 (Fig. 8e) coxa posterior margin with three short setae along margin; basis 1.3 times as long as broad, distal lobe weak (not reaching to mid length of ischium), dorsal margin with a single proximal simple seta and three robust setae along length, distodorsal margin with three robust setae, ventral margin subtly crenulated and with five short simple setae along length; ischium distodorsal margin with distal robust setae; merus with weak postero-distal lobe, dorsal margin with robust setae in three clusters, ventral margin with robust setae in two clusters; carpus as long as merus, dorsal margin with robust setae in three clusters, ventral margin with robust setae in two clusters; propodus longer than merus, dorsal margin with four clusters of robust setae, ventral margin with two clusters of simple setae; dactylus with plumose seta on ventral margin, unguis present. Pereopod 6 (Fig. 8f) coxa posterior margin with three setae along margin; basis 1.2 times as long as broad, dorsal margin with a single proximal simple seta and four robust setae along length, distal end of dorsal margin with cluster of robust setae, ventral margin distinctly crenulated and with eight short simple setae along length; ischium dorsal margin with distal robust setae; merus with weak postero-distal lobe, dorsal margin with robust setae in three clusters, ventral margin with robust setae in three clusters; carpus as long as merus, dorsal margin with robust setae in three clusters, ventral margin with robust setae in three clusters; propodus longer than merus, dorsal margin with four clusters of robust setae, ventral margin with two clusters of robust setae and cluster of simple setae distally; dactylus with plumose seta on ventral margin, unguis present. Pereopod 7 (Fig. 8g) coxa ventral margin with one short simple seta; basis 1.2 times as long as broad, dorsal margin with two proximal simple setae and five robust setae along length, distal end of dorsal margin with three robust setae, ventral margin distinctly crenulated and with 10 short simple setae along length; ischium dorsal margin with distal cluster of robust setae; merus with weak postero-distal lobe, dorsal margin with robust setae in three clusters, ventral margin with robust setae in three clusters; carpus as long as merus, dorsal margin with robust setae in four clusters, ventral margin with robust setae in three clusters; propodus longer than merus, dorsal margin with five clusters of robust setae, ventral margin with two clusters of robust setae and cluster of simple setae distally; dactylus with plumose seta on ventral margin, unguis present.
Pleopods 1–3 (Fig. 9g) unmodified (compared with Chiltonia), peduncle inner margins with two distal retinacula (coupling hooks), inner ramus of seven articles, outer ramus of 10 articles.
Uropod 1 (Fig. 9c) peduncle distinctly longer than rami, dorsal margin with five robust setae along the length of the outer margin; outer ramus with distal cluster of three robust setae and a row of two robust setae along length; inner ramus with distal cluster of five robust setae and no setae along length. Uropod 2 (Fig. 9d) peduncle similar length to inner ramus, dorsal margin with four robust setae; outer ramus distinctly smaller than inner ramus, with distal cluster of four robust setae, with a row of three robust setae along length; inner ramus with distal cluster of four robust setae, with one robust seta along length. Uropod 3 (Fig. 9f) biarticulate, article 2 distal margin with one short robust seta and one or two long simple setae apically.
Telson (Fig. 9e) entire, as long as broad, apically truncate, with three pairs of simple setae around each distodorsal corner.
Allotype female (SAM C7885). Length: 3.69 mm. Similar morphology to male except for the following: Gnathopod 1 (Fig. 9a) coxa distal margin with six short simple setae; carpus with ventral-lateral lobe and row of eight setulate setae becoming longer distally; propodus ~1.8 times as long as broad. Gnathopod 2 (Fig. 9b) similar to gnathopod 1 except propodus 2.2 times as long as broad.
Oostegites present on coxae 2–5 to form the marsupium, margins with scattered curved hooks.
Remarks
The type locality of this species is a small spring at the rim of a swampy area along the western tributary of the Rocky River. During the winter season (2011) from this spring we recorded ~15 L of clear water emerging per minute. However, during the subsequent summer season the water flow ceased completely and no surface water was apparent. We believe that the amphipods survive these fluctuations by retracting with the water and oversummer in subterranean conditions.
Etymology
Named for Ian Moody, for his valued support with chiltoniid field sampling.
Discussion
A new and unique species
The discovery of a new Australian spring-associated chiltoniid amphipod (Kartachiltonia moodyi sp. nov.) with diverse molecular and morphological characteristics, in comparison to the existing Australian species, was not an anticipated result of this study. Its discovery highlights the importance of surveying and sampling spring sources (vents) and headwaters for potentially elusive groundwater-associated fauna. The phylogenetic results for the known species presented here (Fig. 2) place Kartachiltonia within a basal position to a clade with taxa from diverse Australian areas that include subterranean and epigean species. This basal divergence is clearly shown morphologically in Kartachiltonia by the presence of curved anterior-facing sternal gills and slender coxal gills. Sternal gills are a character not previously noted in Australian chiltoniid species and were thought to be specific to New Zealand species. In contrast, in Kartachiltonia the male pleopod 1 resembles that of other Australian species and is not modified into a ‘whip-like’ structure, as in all described New Zealand species. Further analyses and the inclusion of New Zealand specimens in a molecular phylogeny of Australian taxa (in preparation) will provide answers to questions of broader relationships.
Towards explaining chiltoniid diversity and distributions in southern Australia
Kangaroo Island habitats possess a diverse assemblage of chiltoniid amphipods that, according to our phylogenetic analyses (Figs 3–5), date from Early to Mid-Pleistocene. As such, they provide valuable data that may be used to hypothesise processes that have led to the current distributions of chiltoniids across south-eastern Australia. The diversity and distribution of Australian freshwater amphipod taxa such as the Melitidae and Bogidiellidae may well be explained by historical marine inundation and regression events in the Cretaceous, particularly when their distributions are closely allied to ancient shorelines (Humphreys 2012). The freshwater Melitidae (particularly Nedsia Barnard & Williams, 1995 and Norcapensis Bradbury & Williams, 1997 from the Pilbara in Western Australia, Nurina Bradbury & Eberhard, 2000 from the Nullarbor and Brachina Barnard & Williams, 1995 from the Flinders Ranges in South Australia) exist within a large (~45 genera) cosmopolitan and predominantly marine family and are thought to provide such evidence of marine to freshwater amphipod colonisation events within Australia (Humphreys 2012). The Chiltoniidae are classified within the superfamily Talitroidea, a worldwide group of marine, estuarine, freshwater and terrestrial amphipods (Serejo 2004; Lowry and Myers 2013). On the basis of morphological cladistics, Serejo (2004) suggested that the Chiltoniidae had a marine ancestor that colonised freshwater environments. While we do not herein attempt to provide definitive answers as to whether the Australian Chiltoniidae are derived from ancestral marine colonisers of fresh water, we present several alternative hypotheses that might help in explaining the current distributions of the species.
‘Terrestrial ancestor’ hypothesis
Within the Talitroidea the family Talitridae, which is presented as the sister group to the remaining families in the superfamily, consists of species that live in estuarine, intertidal and terrestrial habitats (Serejo 2004; Lowry and Myers 2013), and it seems possible that the ancestors of this group colonised terrestrial habitats from estuarine and seashore habitats. Terrestrial species within this group inhabit the leaf litter of wet forests of eastern and southern Australia (Peart and Lowry 2006). A terrestrial ancestor for the current species in the Chiltoniidae would require that that species secondarily made transitions to freshwater habitats in various locations in Australia, likely triggered by aridification. Evidence for this hypothesis includes the current species distributions, which are consistent with freshwater drainages that may have served as refugia (i.e. A. subtenuis distributed in the Murray River catchment), and that in the more temperate areas of Australia some species are distributed along several coastal catchments (i.e. A. australis and a still undescribed epigean species from south-west Western Australia (Leys, pers. obs.)). Where surface water completely disappeared terrestrial species could have colonised groundwater habitats (i.e. Yilgarn stygobitic chiltoniid species (King et al. 2012) in a similar process to that proposed for Haloniscus isopods (Cooper et al. 2008). That there are no extant terrestrial chiltoniid species known counts against this hypothesis, as it is likely that if it were true they would be inhabiting leaf litter environments in temperate rainforests of south-eastern Australia.
‘Single marine ancestor’ hypothesis
As proposed by Serejo (2004), the Chiltoniidae could have had a widespread marine ancestor that colonised freshwater habitats of different southern continents (South Africa, Australia and New Zealand) once. Although this suggestion was based on cladistic analyses of morphology and a concise number of taxa, our analyses using molecular data of New Zealand and Australian species show different branching times for the New Zealand and Australian lineages (unpublished). In addition, some well supported nodes in the species phylogeny (Fig. 2) branch off at different times, (i.e. the splits between the mainland and Tasmanian sister-species groups A. cooperi–A. australis and A. subtenuis–A. clydensis) and thus do not support this hypothesis.
‘Multiple marine ancestors’ hypothesis
Australian chiltoniid taxa may have originated from marine or estuarine ancestors that independently colonised freshwater habitats at different places and times during the past. This hypothesis is supported by observations that currently known species distributions are restricted to catchments; that sister species from well separated areas have different node ages, i.e. above-mentioned mainland and Tasmanian species and Scutachiltonia (Yilgarn, WA) – Phreatochiltonia (Dalhousie Springs, SA) (Fig. 2); as well, some areas (the mound springs from the Great Artesian Basin and calcrete aquifers from the Yilgarn) contain multiple old lineages of Chiltoniidae (King et al. 2012; Murphy et al. 2012). Multiple colonisations of freshwater habitats would seem to require that a marine lineage would have been present for some time. It is important to note, however, that currently no closely related marine ancestors or extant marine species are known within the Chiltoniidae.
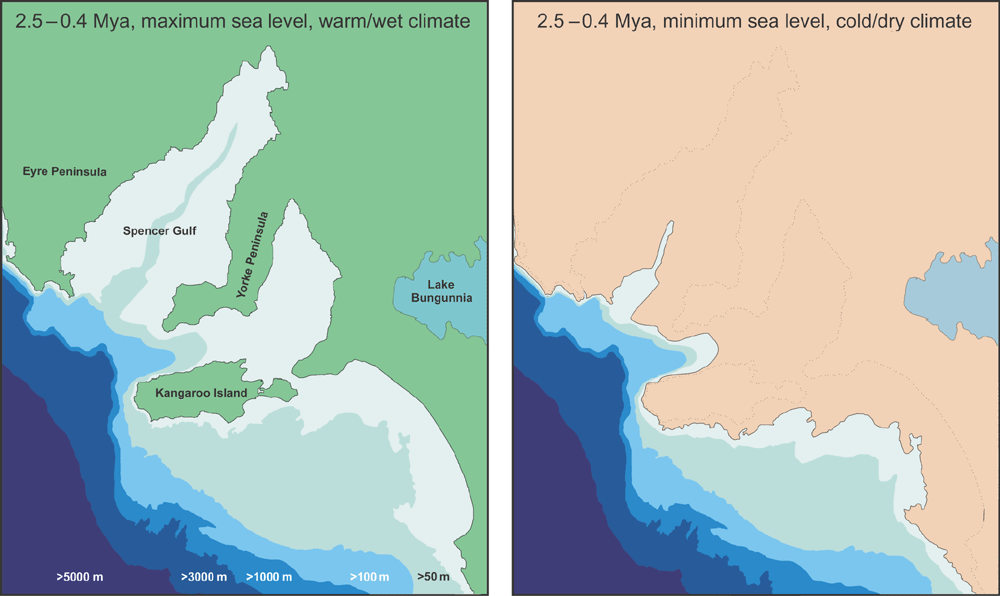
Differentiating between these hypotheses using the available data on species distribution patterns, phylogeny and node ages is a challenge, given that the expected pattern for both the terrestrial and multiple marine ancestor hypotheses are very similar. However, it is clear from the phylogenies of the three species that isolation of the Kangaroo Island lineages took place during the Early to Mid-Pleistocene (Figs 3–5). During this period, sea levels around Kangaroo Island fluctuated to ~50 m below the current level and the climate oscillated between warm and wet in the interglacials to cold and dry during glacial maxima (Fig. 10) (Williams et al. 1988; Byrne 2008). It is possible that through one or more of these climatic oscillation events, chiltoniid amphipods were stranded in disparate remnant freshwater areas – perhaps as marine/estuarine species that adapted to freshwater environments or as freshwater or terrestrial species adapting to contraction and fragmentation of freshwater habitats. Similar Pliocene–Pleistocene divergences have been recorded for southern Australian frogs and froglets, freshwater fish species, and invertebrates such as the isopod Phreatomerus latipes (Chilton 1922) and the freshwater shrimp Paratya australiensis (Kemp 1917) (Cook et al. 2006; Edwards et al. 2007; Symula et al. 2008; Faulks et al. 2010; Guzik et al. 2012).
Austrochiltonia dalhousiensis, previously known only from the Dalhousie Springs area, is seemingly tolerant of habitats of varied salinity with the potential to make it more adaptable to distribution through changeable aquatic habitats formed during climatic shifts. A. dalhousiensis is likely to have been more widely distributed along the Spencer Gulf; with the current potentially remnant distributions at the southern part of Eyre Peninsula and Kangaroo Island testament to this. It is possible that A. dalhousiensis would have also occurred on Yorke Peninsula during the wet interglacial periods. However, it might not have survived the cooler and much dryer glacial periods and currently there is no permanent surface water on Yorke Peninsula that would support populations of chiltoniid amphipods.
Populations of A. australis are distributed in low-salinity coastal catchments mainly along the south-western coast of Victoria to Kangaroo Island as well as in numerous isolated sinkholes in the Mount Gambier area of south-eastern South Australia (King and Leys 2011). Past distributions of A. australis across southern Australia may have been limited by a clear preference to stream headwaters and low-salinity systems, in contrast to A. subtenuis and A. dalhousiensis, which are more tolerant of varied salinities. It is clear from the phylogeny of A. australis (Fig. 3) that isolation in the sinkholes of south-eastern South Australia started around 1.3 million years ago, similar to the divergence of the Kangaroo island lineages. It is not clear why A. australis is abundant on Kangaroo Island and only sporadically distributed on the adjacent mainland, although it is possible that the steep ravine habitats on western Kangaroo Island, where A. australis dominates, are complex and the native landscape better preserved (within National parks and conservation areas) compared with the adjacent mainland. Whilst the Kangaroo Island specimens studied here form a monophyletic group, it is also possible that colonisation of the island catchments took place from the same genetic stock at different episodes.
Current records indicate that A. subtenuis, a species able to tolerate habitats of varied salinity, is the dominant freshwater amphipod in the southern lowland habitats of South Australia (mainly the Murray River catchment and Fleurieu Peninsula, as well as catchments on the western side of the Lofty Ranges). The main distribution of A. subtenuis is the Murray River catchment and the approximate node age of the species (Fig. 5) indicates that it may have been present in the shallow inland sea of the western Murray Basin that later became Lake Bungunnia (Stephenson 1986). The relatively recent divergence of the Kangaroo Island lineage (of less than 0.5 million years ago: Fig. 5) may indicate that the island was colonised by descendents of A. subtenuis inhabiting the Murray Basin, after the draining of Lake Bungunnia and the forming of the lower Murray River, which happened sometime after 0.7 million years ago (Stephenson 1986; McLaren et al. 2011).
The occurrence of multiple species of Austrochiltonia on Kangaroo Island may be best explained by the position of Kangaroo Island, being at the cross roads of individual species distributions, as well as the numerous regional climate change cycles that enhanced the colonisation of the island creeks.
Introductions and long-distance dispersals
Despite most of the individuals sampled falling within one of four naturally occurring species on the island, recent and possibly anthropogenic introductions of amphipods onto Kangaroo Island cannot be excluded. One specimen of A. subtenuis (sequence RAK26, site KI22 (Cygnet River): Fig. 5, Table 2) was found to be most closely related to an individual from the Murray River near Loxton, South Australia (~400 km away). Human-mediated dispersal has been proposed for A. subtenuis before by King and Leys (2011) and may be a more common feature for the species. In additional to the example on Kangaroo Island, three other closely related specimen pairs from widely separate localities exist within the phylogeny (Fig. 5: e.g. Diggers Rest, Vic (sequence ST207) versus Appila Springs, SA (sequence ST211): ~900 km away) (Table 2).
The discovery of new localities on Kangaroo Island and Eyre Peninsula for A. dalhousiensis is interesting as the disjunct distribution of the populations (between Dalhousie Springs (the type locality) and Eyre Peninsula/Kangaroo Island) exceeds 900 km. Phylogenetic analysis of A. dalhousiensis (Fig. 4) shows an approximate age of the divergences within the species of ~2 million years, which, if A. dalhousiensis was primarily a coastal species, may be too recent to be the result of natural dispersal along permanent water bodies connecting the Spencer Gulf region and associated creeks with Lake Eyre, Lake Torrens and Dalhousie Springs, which may have existed only until the Late Miocene or early Pliocene (Sandiford et al. 2009). Alternately, it is possible that the formation of the Dalhousie Springs ~1–2 million years ago, amid the aridification of surrounding wetlands, created the necessary isolation by distance barrier that led to a distinct Dalhousie lineage within the species, with populations in between Dalhousie springs and current coastal populations going extinct (similar to the phylogenetic history proposed for Phreatomerus latipes in the Lake Eyre mound springs by Guzik et al. 2012). However, this hypothesis requires habitat connectivity and exchange between Dalhousie Springs and coastal populations to be in place up to 2 million years ago, while some evidence suggests that freshwater connectivity between the two regions may have ceased as early as the Late Miocene as a result of uplift of the north-west Flinders Ranges area (Sandiford et al. 2009).
An often proposed natural mechanism for long-distance dispersal of aquatic invertebrates is hitch-hiking on the legs or plumage of, or via endozoochorous dispersal through, water birds (Charalambidou and Santamaría 2002; Green et al. 2002; Frisch et al. 2007; Rachalewski et al. 2013). Experimental work by Rachalewski et al. (2013) presented the possibility of Crangonyx amphipods hitchhiking on the legs or plumage of mallards; however, this was only for very restricted periods (less than 6 min) and a maximum flight distance of approximately 7 km. For chiltoniid amphipods this mechanism is considered extremely unlikely as individuals would quickly die of desiccation once out of the water, especially considering that bridging distances in the Australian landscape are likely to be in the order of hundreds or thousands of kilometres. Moreover, chiltoniids do not have protected life stages such as ‘cysts’ or ephippia that can survive periods of environmental stress (such as desiccation or passing through host intestines). Human-mediated dispersal remains a possibility (i.e. A. subtenuis in the phylogeny), most likely occurring alongside historical and ongoing translocations of freshwater crayfish into pastoral dams and creeks (Cherax sp.) by farmers. The phylogeny (Fig. 4) shows an age of ~0.8 million years for the clade containing the Dalhousie Springs specimens of A. dalhousiensis, which would not fit a recent-introduction scenario; however, the sister group of the clade contains only three specimens from Eyre Peninsula, all from the same locality, Edillilie Creek. It is possible that the overall genetic divergence on Eyre Peninsula may be much larger than reported here. Further specimens from additional localities on Eyre Peninsula are therefore needed to be incorporated into the analyses in order to test whether the Dalhousie Springs specimens are monophyletic and form a natural group, or have been transported by humans on one or more occasions.
Acknowledgements
The morphological work presented was supported by the Australian Biological Resources Study (ABRS) grant No. 208-61. Specimen collections on Kangaroo Island were subject to Permit U25782 1, Department of Environment, Water and Natural Resources, South Australia. Thanks to Michelle Guzik and Ian Moody for field assistance. We are grateful to Ranger Deb Davis (DEWNR, Flinders Ranges National Park) for information and assistance during Kangaroo Island collections. Peter Goonan (South Australian Environmental Protection Authority) is thanked for making Eyre Peninsula samples available, along with valuable environmental data. We express our gratitude to Steve Cooper for valuable comments on the manuscript.
References
Austin, A. D., Fisher, R. H., Harvey, M. S., Hirst, D. B., Locket, N. A., Madden, C. P., Reay, F., and Zeidler, W. (2002). Terrestrial and freshwater invertebrates. In ‘Natural History of Kangaroo Island’. 2nd edn. (Eds M. Davies, C. R. Twidale and M. J. Tyler.) pp. 123–141. (Royal Society of South Australia: Adelaide.)Barnard, J. L. (1972). The marine fauna of New Zealand: algae-living littoral Gammaridea (Crustacea Amphipoda). Memoirs of the New Zealand Oceanographic Institute 62, 1–216.
Barnard, J. L., and Barnard, C. M. (1983). ‘Freshwater Amphipoda of the World. I. Evolutionary Patterns. II. Handbook and Bibliography.’ (Hayfield Associates: Mt Vernon, VA.)
Barnard, J. L., and Williams, W. D. (1995). The taxonomy of Amphipoda (Crustacea) from Australian fresh waters: Part 2. Records of the Australian Museum 47, 161–201.
| The taxonomy of Amphipoda (Crustacea) from Australian fresh waters: Part 2.Crossref | GoogleScholarGoogle Scholar |
Belperio, A. P., and Flint, R. B. (1999). Geomorphology and geology. In ‘A Biological Survey of Kangaroo Island South Australia’. (Eds A. C. Robinson and D. M. Armstrong.) pp. 19–31. (Endeavour Press.)
Bradbury, J. H., and Eberhard, S. (2000). A new stygobiont melitid amphipod from the Nullarbor Plain. Records of the Western Australian Museum 20, 39–50.
Bradbury, J. H., and Williams, W. D. (1997). The amphipod (Crustacea) stygofauna of Australia: description of new taxa (Melitidae, Neoniphargidae, Paramelitidae), and a synopsis of known species. Records of the Australian Museum 49, 249–341.
| The amphipod (Crustacea) stygofauna of Australia: description of new taxa (Melitidae, Neoniphargidae, Paramelitidae), and a synopsis of known species.Crossref | GoogleScholarGoogle Scholar |
Brower, A. (1994). Rapid morphological radiation and convergence among races of the butterfly Heliconius erato inferred from patterns of mitochondrial DNA evolution. Proceedings of the National Academy of Sciences of the United States of America 91, 6491–6495.
| Rapid morphological radiation and convergence among races of the butterfly Heliconius erato inferred from patterns of mitochondrial DNA evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmt1KrsLw%3D&md5=070f2740ec407cc79ca603b62aff29abCAS | 8022810PubMed |
Byrne, M. (2008). Evidence for multiple refugia at different timescales during Pleistocene climatic oscillations in southern Australia inferred from phylogeography. Quaternary Science Reviews 27, 2576–2585.
| Evidence for multiple refugia at different timescales during Pleistocene climatic oscillations in southern Australia inferred from phylogeography.Crossref | GoogleScholarGoogle Scholar |
Byrne, M., Yeates, D. K., Joseph, L., Kearney, M., Bowler, J., Williams, M. A. J., Cooper, S. J. B., Donnellan, S. C., Keogh, J. S., Leys, R., Melville, J., Murphy, D. J., Porch, N., and Wyrwoll, K.-H. (2008). Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17, 4398–4417.
| Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cjhvFGruw%3D%3D&md5=be4ed4521bf1145ad0d36ac29682e04eCAS | 18761619PubMed |
Charalambidou, I., and Santamaría, L. (2002). Waterbirds as endozoochorous dispersers of aquatic organisms: a review of experimental evidence. Acta Oecologica 23, 165–176.
| Waterbirds as endozoochorous dispersers of aquatic organisms: a review of experimental evidence.Crossref | GoogleScholarGoogle Scholar |
Chilton, C. (1898). A new freshwater amphipod from New Zealand. Annals and Magazine of Natural History: Series 7 1, 423–426.
| A new freshwater amphipod from New Zealand.Crossref | GoogleScholarGoogle Scholar |
Chilton, C. (1922). A new isopod from central Australia belonging to the Phreatoicidae. Transactions of the Royal Society of South Australia 46, 23–33.
Chomczynski, P., Mackey, K., Drews, R., and Wilfinger, W. (1997). DNAzol: a reagent for the rapid isolation of genomic DNA. BioTechniques 22, 550–553.
| 1:CAS:528:DyaK2sXhvVajs78%3D&md5=92c172807a396ddfb26b23d49dea735aCAS | 9067036PubMed |
Cook, B. D., Baker, A. M., Page, T. J., Grant, S. C., Fawcett, J. H., Hurwood, D. A., and Hughes, J. M. (2006). Biogeographic history of an Australian freshwater shrimp, Paratya australiensis (Atyidae): the role life history transition in phylogeographic diversification. Molecular Ecology 15, 1083–1093.
| Biogeographic history of an Australian freshwater shrimp, Paratya australiensis (Atyidae): the role life history transition in phylogeographic diversification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjslWms74%3D&md5=ab1c19943f830c8dbaa6e264e9faa24bCAS | 16599968PubMed |
Cooper, S. J. B., Bradbury, J. H., Saint, K. M., Leys, R., Austin, A. D., and Humphreys, W.F. (2007). Subterranean archipelago in the Australian arid zone: mitochondrial DNA phylogeography of amphipods from central Western Australia. Molecular Ecology 16, 1533–1544.
| Subterranean archipelago in the Australian arid zone: mitochondrial DNA phylogeography of amphipods from central Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlt1Gmu7w%3D&md5=e6fc0ee5aa3d4494098bd39ca811a3f1CAS |
Cooper, S. J. B., Saint, K. M., Taiti, S., Austin, A. D., and Humphreys, W. F. (2008). Subterranean archipelago: mitochondrial DNA phylogeography of stygobitic isopods (Oniscidea: Haloniscus) from the Yilgarn region of Western Australia. Invertebrate Systematics 22, 195–203.
| Subterranean archipelago: mitochondrial DNA phylogeography of stygobitic isopods (Oniscidea: Haloniscus) from the Yilgarn region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Davis, J., Pavlova, A., Thompson, R., and Sunnucks, P. (2013). Evolutionary refugia and ecological refuges: key concepts for conserving Australian arid zone freshwater biodiversity under climate change. Global Change Biology 19, 1970–1984.
| Evolutionary refugia and ecological refuges: key concepts for conserving Australian arid zone freshwater biodiversity under climate change.Crossref | GoogleScholarGoogle Scholar | 23526791PubMed |
Drummond, A. J., and Rambaut, A. (2007). BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7, 214.
| BEAST: Bayesian evolutionary analysis by sampling trees.Crossref | GoogleScholarGoogle Scholar | 17996036PubMed |
Edwards, D. L., Roberts, J. D., and Keogh, J. S. (2007). Impact of Plio-Pleistocene arid cycling on the population history of a southwestern Australian frog. Molecular Ecology 16, 2782–2796.
| Impact of Plio-Pleistocene arid cycling on the population history of a southwestern Australian frog.Crossref | GoogleScholarGoogle Scholar | 17594447PubMed |
Faulks, L. K., Gilligan, D. M., and Beheregaray, L. B. (2010). Evolution and maintenance of divergent lineages in an endangered freshwater fish, Macquaria australasica. Conservation Genetics 11, 921–934.
| Evolution and maintenance of divergent lineages in an endangered freshwater fish, Macquaria australasica.Crossref | GoogleScholarGoogle Scholar |
Fensham, R. J., Ponder, W. F., and Fairfax, R. J. (2010). Recovery plan for the community of native species dependent on natural discharge of groundwater from the Great Artesian Basin. Report to Department of the Environment, Water, Heritage and the Arts, Canberra. Queensland Department of Environment and Resource Management, Brisbane.
Fensham, R. J., Silcock, J. L., Kerezsy, A., and Ponder, W. (2011). Four desert waters: setting arid zone wetland conservation priorities through understanding patterns of endemism. Biological Conservation 144, 2459–2467.
| Four desert waters: setting arid zone wetland conservation priorities through understanding patterns of endemism.Crossref | GoogleScholarGoogle Scholar |
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R. (1994). DNA primers for the amplification of mitochondrial cytochrome c oxidase subunit I from metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294–299.
| 1:CAS:528:DyaK2MXjt12gtLs%3D&md5=64874205a2734610b194abfa9da78e6dCAS | 7881515PubMed |
Frisch, D., Green, A. J., and Figuerola, J. (2007). High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds. Aquatic Sciences 69, 568–574.
| High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds.Crossref | GoogleScholarGoogle Scholar |
Gillespie, J. J., Johnson, J. S., Cannone, J. J., and Gutell, R. R. (2006). Characteristics of the nuclear (18S, 5.8S, 28S and 5S) and mitochondrial (12S and 16S) rRNA genes of Apis mellifera (Insecta: Hymenoptera): structure, organization and retrotransposable elements. Insect Molecular Biology 15, 657–686.
| Characteristics of the nuclear (18S, 5.8S, 28S and 5S) and mitochondrial (12S and 16S) rRNA genes of Apis mellifera (Insecta: Hymenoptera): structure, organization and retrotransposable elements.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1yqsLvL&md5=8ed7b5629d2c137bb928fdfeb085528dCAS | 17069639PubMed |
Green, A. J., Figuerola, J., and Sánchez, M. (2002). Implications of waterbird ecology for the dispersal of aquatic organisms. Acta Oecologica 23, 177–189.
| Implications of waterbird ecology for the dispersal of aquatic organisms.Crossref | GoogleScholarGoogle Scholar |
Gross, G. F., Lee, D. C., and Zeidler, W. (1979). Invertebrates. In ‘Natural History of Kangaroo Island’. (Eds M. J. Tyler, C. R. Twidale and J. K. Ling.) pp. 129–137. (Royal Society of South Australia: Adelaide.)
Guzik, M. T., Adams, M. A., Murphy, N. P., Cooper, S. J. B., and Austin, A. D. (2012). Desert springs: deep phylogeographic structure in an ancient endemic crustacean (Phreatomerus latipes). PLoS ONE 7, e37642.
| Desert springs: deep phylogeographic structure in an ancient endemic crustacean (Phreatomerus latipes).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVyrs7%2FF&md5=7815efea7a941d4b41b9381635fd6d34CAS | 22815684PubMed |
Hill, P. J., De Deckker, P., von der Borch, C., and Murray-Wallace, C. V. (2009). Ancestral Murray River on the Lacepede Shelf, southern Australia: Late Quaternary migrations of a major river outlet and strandline development. Australian Journal of Earth Sciences 56, 135–157.
| Ancestral Murray River on the Lacepede Shelf, southern Australia: Late Quaternary migrations of a major river outlet and strandline development.Crossref | GoogleScholarGoogle Scholar |
Huelsenbeck, J. P., and Bull, J. J. (1996). A likelihood ratio test to detect conflicting phylogenetic signal. Systematic Biology 45, 92–98.
| A likelihood ratio test to detect conflicting phylogenetic signal.Crossref | GoogleScholarGoogle Scholar |
Humphreys, W. F. (2012). Diversity patterns in Australia. In ‘Encyclopedia of Caves’. (Eds W. B. White and D. C. Culver.) pp. 203–219. (Academic Press.)
Hurley, D. E. (1954). Studies on the New Zealand amphipodan fauna. No. 2. The family Talitridae: the freshwater genus Chiltonia Stebbing. Transactions of the Royal Society of New Zealand 81, 563–577.
Kemp, S. (1917). XVII. Notes on Crustacea Decapoda in the Indian Museum. XI. Atyidae of the genus Paratya (= Xiphocaridina). Records of the Indian Museum 13, 293–306.
King, R. A. (2009). Two new genera and species of chiltoniid amphipods (Crustacea: Amphipoda: Talitroidea) from freshwater mound springs in South Australia. Zootaxa 2293, 35–52.
King, R. A., and Leys, R. (2011). The Australian freshwater amphipods Austrochiltonia australis and Austrochiltonia subtenuis (Amphipoda: Talitroidea: Chiltoniidae) confirmed and two new cryptic Tasmanian species revealed using a combined molecular and morphological approach. Invertebrate Systematics 25, 171–196.
| The Australian freshwater amphipods Austrochiltonia australis and Austrochiltonia subtenuis (Amphipoda: Talitroidea: Chiltoniidae) confirmed and two new cryptic Tasmanian species revealed using a combined molecular and morphological approach.Crossref | GoogleScholarGoogle Scholar |
King, R. A., Bradford, T., Austin, A. D., Humphreys, W. F., and Cooper, S. J. B. (2012). Divergent molecular lineages and not-so-cryptic species: the first descriptions of stygobitic chiltoniid amphipods from Western Australia. Journal of Crustacean Biology 32, 465–488.
| Divergent molecular lineages and not-so-cryptic species: the first descriptions of stygobitic chiltoniid amphipods from Western Australia.Crossref | GoogleScholarGoogle Scholar |
Lambeck, K., and Chappell, J. (2001). Sea-level change through the last glacial cycle. Science 292, 679–686.
| Sea-level change through the last glacial cycle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjt1els74%3D&md5=d57e90352b257ea850f0fc83df4c5ab0CAS | 11326090PubMed |
Lowry, J. K., and Myers, A. A. (2013). A phylogeny and classification of the Senticaudata subord. nov. (Crustacea: Amphipoda). Zootaxa 3610, 1–80.
| A phylogeny and classification of the Senticaudata subord. nov. (Crustacea: Amphipoda).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2cngtleltg%3D%3D&md5=f13dc47833036b4dd68c57ba6dfa7cdaCAS | 24699701PubMed |
Markgraf, V., McGlone, M., and Hope, G. (1995). Neogene paleoenvironmental and paleoclimatic change in southern temperate ecosystems – a southern perspective. Trends in Ecology & Evolution 10, 143–147.
| Neogene paleoenvironmental and paleoclimatic change in southern temperate ecosystems – a southern perspective.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7itFajug%3D%3D&md5=7cbc6e19584d2e5909a001427fedf330CAS |
McLaren, S., Wallace, M. W., Gallagher, S. J., Miranda, J. A., Holdgate, G. R., Gow, L. J., Snowball, I., and Sandgren, P. (2011). Palaeogeographic, climatic and tectonic change in southeastern Australia: the Late Neogene evolution of the Murray Basin. Quaternary Science Reviews 30, 1086–1111.
| Palaeogeographic, climatic and tectonic change in southeastern Australia: the Late Neogene evolution of the Murray Basin.Crossref | GoogleScholarGoogle Scholar |
Murphy, N. P., Adams, M., and Austin, A. D. (2009). Independent colonization and extensive cryptic speciation of freshwater amphipods in the isolated groundwater springs of Australia’s Great Artesian Basin. Molecular Ecology 18, 109–122.
| 1:CAS:528:DC%2BD1MXit1Grsr0%3D&md5=728464eeecaf9ed0b2cb796df57ef811CAS | 19140968PubMed |
Murphy, N. P., Breed, M. F., Guzik, M. T., Cooper, S. J. B., and Austin, A. D. (2012). Trapped in desert springs: phylogeography of Australian desert spring snails. Journal of Biogeography 39, 1573–1582.
| Trapped in desert springs: phylogeography of Australian desert spring snails.Crossref | GoogleScholarGoogle Scholar |
Peart, R., and Lowry, J. K. (2006). The amphipod genus Arcitalitrus (Crustacea: Amphipoda: Talitridae) of New South Wales forests, with descriptions of six new species. Records of the Australian Museum 58, 97–118.
| The amphipod genus Arcitalitrus (Crustacea: Amphipoda: Talitridae) of New South Wales forests, with descriptions of six new species.Crossref | GoogleScholarGoogle Scholar |
Prescott, J. R., and Habermehl, M. (2008). Luminescence dating of spring mound deposits in the southwestern Great Artesian Basin, northern South Australia. Australian Journal of Earth Sciences 55, 167–181.
| Luminescence dating of spring mound deposits in the southwestern Great Artesian Basin, northern South Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXms1Sksbg%3D&md5=7cba6c0c3f22b1dfeec99b787b202d15CAS |
Rachalewski, M, Banha, F, Grabowski, M, and Anastácio, P. M. (2013). Ectozoochory as a possible vector enhancing the spread of an alien amphipod Crangonyx pseudogracilis. Hydrobiologia 717, 109–117.
| Ectozoochory as a possible vector enhancing the spread of an alien amphipod Crangonyx pseudogracilis.Crossref | GoogleScholarGoogle Scholar |
Rafinesque, C. S. (1815). ‘Analyse de la Nature ou Tableau de l’Univers et des Corps Organisés.’ (L’Imprime’rie de Jean Barravecchia: Palermo.)
Rambaut, A., and Drummond, A. J. (2007). Tracer v1.4. Available at: http://beast.bio.ed.ac.uk/Tracer [verified November 2013]
Raymo, M. E., and Nisancioglu, K. (2003). The 41 kyr world: Milankovitch’s other unsolved mystery. Paleoceanography 18, 1011.
| The 41 kyr world: Milankovitch’s other unsolved mystery.Crossref | GoogleScholarGoogle Scholar |
Richardson, L., Mathews, E., and Heap, A. (2005). Geomorphology and sedimentology of the South Western Planning Area of Australia: review and synthesis of relevant literature in support of Regional Marine Planning. Geoscience Australia, Record 2005/17. 124pp.
Sandiford, M., Quigley, M., de Broekert, P., and Jakica, S. (2009). Tectonic framework for the Cainozoic cratonic basins of Australia. Australian Journal of Earth Sciences 56, S5–S18.
| Tectonic framework for the Cainozoic cratonic basins of Australia.Crossref | GoogleScholarGoogle Scholar |
Sayce, O. A. (1901). Description of some new Victorian freshwater Amphipoda. Proceedings of the Royal Society of Victoria 13, 225–242.
Sayce, O. A. (1902). Description of some new Victorian freshwater Amphipoda. No. 2. Proceedings of the Royal Society of Victoria 15, 47–58.
Serejo, C. S. (2004). Cladistic revision of talitroidean amphipods (Crustacea, Gammaridea), with a proposal of a new classification. Zoologica Scripta 33, 551–586.
| Cladistic revision of talitroidean amphipods (Crustacea, Gammaridea), with a proposal of a new classification.Crossref | GoogleScholarGoogle Scholar |
Simon, C., Frati, F., Beckenbach, A., Crespi, B., Liu, H., and Flook, P. (1994). Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America 87, 651–701.
| 1:CAS:528:DyaK2MXis1Wiu7g%3D&md5=ae59658adb8f61bd8b5026da9ff3e5a9CAS |
Smith, P. C. (1989). Hydrology. In ‘The Natural History of Dalhousie Springs’. (Eds W. Zeidler and W. F. Ponder.) pp. 27–39. (South Australian Museum: Adelaide.)
Stephenson, A. E. (1986). Lake Bungunnia, a Plio-Pleistocene megalake in southern Australia. Palaeogeography, Palaeoclimatology, Palaeoecology 57, 137–156.
| Lake Bungunnia, a Plio-Pleistocene megalake in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Suter, P. J. (1986). The Ephemeroptera (mayflies) of South Australia. Records of the South Australian Museum 19, 339–397.
Suter, P. J., and Bishop, J. E. (1990). Stoneflies (Plecoptera) of South Australia. In ‘Mayflies and Stoneflies: Life Histories and Biology’. (Ed. I. C. Campbell.) pp. 189–207. (Kluger Academic Publishers.)
Swofford, D. L. (2001). ‘PAUP*: Phylogenetic Analysis Using Parsimony (* and other methods). Version 4.0b8.’ (Sinauer: Sunderland, MA.)
Symula, R., Keogh, J. S., and Cannatella, D. C. (2008). Ancient phylogeographic divergence in southeastern Australia among populations of the widespread common froglet, Crinia signifera. Molecular Phylogenetics and Evolution 47, 569–580.
| Ancient phylogeographic divergence in southeastern Australia among populations of the widespread common froglet, Crinia signifera.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlslKlu7c%3D&md5=9d769fbb2d2868266a033223c5badcabCAS | 18348908PubMed |
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., and Higgins, D. G. (1997). The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25, 4876–4882.
| The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXntFyntQ%3D%3D&md5=969320e63a3cd35c223b824f2f314d8bCAS | 9396791PubMed |
Waters, J. M., Frase, C. I., and Hewitt, G. M. (2013). Founder takes all: density-dependent processes structure biodiversity. Trends in Ecology & Evolution 28, 78–85.
| Founder takes all: density-dependent processes structure biodiversity.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (1962). The Australian freshwater amphipods. Australian Journal of Marine and Freshwater Research 13, 198–216.
| The Australian freshwater amphipods.Crossref | GoogleScholarGoogle Scholar |
Williams, D. F., Thunell, R. C., Tappa, E., Domenico, R., and Raffi, I. (1988). Chronology of the Pleistocene oxygen isotope record: 0–1.88 m.y. B.P. Palaeogeography, Palaeoclimatology, Palaeoecology 64, 221–240.
| Chronology of the Pleistocene oxygen isotope record: 0–1.88 m.y. B.P.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXksVWitrw%3D&md5=6964a906b586a5450b0fa6b40621648eCAS |
Zeidler, W. (1982). South Australian freshwater crayfish. South Australian Naturalist 56, 36–42.
Zeidler, W. (1988). A redescription of Afrochiltonia capensis (K.H. Barnard, 1916) with a review of the genera of the family Ceinidae (Crustacea, Amphipoda). Annals of the South African Museum 98, 105–119.
Zeidler, W. (1991). A new genus and species of phreatic amphipod (Crustacea: Amphipoda) belonging in the “chiltonia” generic group, from Dalhousie Springs, South Australia. Transactions of the Royal Society of South Australia 115, 177–187.
Zeidler, W. (1997). A new species of freshwater amphipod, Austrochiltonia dalhousiensis sp. nov. (Crustacea: Amphipoda: Hyalellidae) from Dalhousie Springs, South Australia. Transactions of the Royal Society of South Australia 121, 29–42.
Zeidler, W. (2000). Note on the origin of freshwater crayfish occurring on Kangaroo Island, South Australia. Records of the South Australian Museum 33, 71–72.