Morphology and histology of the uropygial gland in Antarctic birds: relationship with their contact with the aquatic environment?
María Cecilia Chiale A B , Patricia E. Fernández C , Eduardo J. Gimeno B C , Claudio Barbeito B C D and Diego Montalti A B EA División Zoología Vertebrados, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Paseo del Bosque s/n, B1900FWA-La Plata, Argentina.
B Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), C1033AAJ-Buenos Aires, Argentina.
C Cátedra de Patología General, Facultad de Ciencias Veterinarias, Universidad Nacional de La Plata, Calle 60 and 118, B1900-La Plata, Argentina.
D Cátedra de Histología y Embriología, Facultad de Ciencias Veterinarias, Universidad Nacional de La Plata, Calle 60 and 118, B1900-La Plata, Argentina.
E Corresponding author. Email: dmontalti@fcnym.unlp.edu.ar
Australian Journal of Zoology 62(2) 157-165 https://doi.org/10.1071/ZO13103
Submitted: 23 May 2013 Accepted: 18 March 2014 Published: 28 April 2014
Abstract
The uropygial gland is morphologically different in diverse bird species. This gland was macroscopically and microscopically examined in penguins, storm petrels and skuas. In all the studied species, the gland showed a connective tissue capsule and one papilla. A negative relationship was observed between the relative glandular mass and the body mass, being highest in petrels (small glands) and lowest in penguins (large glands). Birds that spend much time in water (penguins) have gland characteristics related to a continuous, but not stored, secretion, such as straight adenomers, the presence of abundant elastic fibres in the connective tissue and the absence of a primary storage chamber. Instead, birds that have less contact with water (storm petrels) have a gland with much more tortuous adenomers and a small primary storage chamber. The secretory cells showed a positive PAS reaction in all the glandular zones. Therefore, no differences could be seen between the sebaceous and glucogenic zones, as proposed in other birds. These results allow the conclusion that, in aquatic birds, there is no connection between the relative mass of the uropygial gland and the time in contact with water, though the differences found in the histological structure could be related to a different contact with the aquatic environment.
Additional keywords: avian gland, preen gland, seabird.
Introduction
The uropygial gland is a bilobed sebaceous organ of birds, located dorsally to the last caudal vertebrae (Jacob and Ziswiler 1982). The histology, secretion, chemistry, and possible functions of the uropygial gland have also been widely described (Elder 1954; Lucas and Stettenheim 1972; Jacob and Ziswiler 1982; Gutiérrez et al. 1998; Montalti et al. 2001, 2005; Reneerkens et al. 2002; Moyer et al. 2003). Its secretion confers water-repellent properties to the feather coat and also maintains the suppleness of the feathers. Other physiological roles of the secretion of the uropygial gland may be associated with pheromone production, control of plumage hygiene, thermal insulation and defence against bacteria and predators (Bandyopadhyay and Bhattacharyya 1996, 1999; Montalti et al. 2006; Salibián and Montalti 2009; Møller et al. 2010; Soler et al. 2012; Vincze et al. 2013).
The uropygial gland is of variable size and morphology in different species. Generally, it has two lobes and one papilla with cylindrical, conic or alveolar morphology on its caudal extreme, which has external ducts (Jacob and Ziswiler 1982). The inner part of each lobe is composed of tubular or tubule-alveolar adenomers, where the secretion is synthesised, as well as chambers, where the secretion is stored. The adenomers have a stratified epithelium with four cell layers, named the germinative, intermediate, secretory and degenerative layers. Three zones have been described: Zone I includes the peripheral area with a well developed germinative layer; Zone II comprises the medial portion of the tubule, characterised by fully developed intermediate and degenerative layers; and Zone III corresponds to the inner part of an adenomer. In Zone III the adenomers converge to form collecting ducts that drain into secondary chambers and finally flow into a primary chamber. Furthermore, differences in the arrangement of the adenomers allow them to be classified into: straight course, convoluted course, intensively branched and slightly branched (Jacob and Ziswiler 1982).
The gland papilla is classified into several types on the basis of its internal structure: (1) In the compact type, the dense connective tissue of the interlobular septum continues in the papilla and surrounds the excretory pores, with an epidermic-like epithelium. Also, there are zones of slack connective tissue and longitudinal and circular smooth muscle layers. (2) The delicate type has wide ducts separated from each other by slight connective tissue septa. Smooth muscle fibres can be observed in the external wall and the apex of the papilla. And (3) the wart type, common in Passeriformes, has a specialised valvular system, formed by two lamellae with collagen and elastic fibres, which avoids the reflux of the secretion to the lobules (Jacob and Ziswiler 1982). The entire gland is surrounded by a capsule of dense connective tissue from which a septum departs and supports the lobes.
The histology of the uropygial gland has been studied in different bird species and some dissimilar characteristics have been described (Hsu 1936; Bo 1953; Bhattacharyya 1972; Bride 1975; Menon et al. 1981; Kamiya et al. 1986; Suzuki 1994; Montalti et al. 2001; Sandilands et al. 2004; Sawad 2006; Harem et al. 2010). However, no studies have linked the histological structure of the gland with the environment. Based on the hypothesis that birds have uropygial glands with different morphological characteristics (number of secretory layer cells, branched adenomers and ducts, and the presence of a reservoir for secretion storage) related to their degree of contact with the aquatic environment, the aim of our study was to describe and comparatively analyse the uropygial gland in seabirds with different degrees of water contact and relationship with their natural habitat.
Materials and methods
Adelie (Pygoscelis adeliae) and Gentoo (P. papua) penguins feed exclusively by diving at sea, they stay in the water for long periods and their diet consists principally of crustaceans and fish (Williams 1995). Wilson (Oceanites oceanicus) and black-bellied (Fregetta tropica) storm petrels fly mostly in pelagic or offshore marine waters. They feed on small crustaceans by flying low over the water and plunging their heads beneath the water (Warham 1990). Brown (Stercorarius antarcticus) and south polar (S. maccormicki) skuas are coastal and pelagic seabirds. They are scavengers, and feed on fish (in shallow waters, near the surface submerging only their heads) or prey on eggs and chicks of other bird species (Furnes 1996; Acosta Hospitaleche et al. 2009; Graña Grilli and Montalti 2012).
Three adults males of each species were captured on Laurie Island (60°44′S, 44°37′W), South Orkney Islands and Hope Bay (63°24′S, 57°0′W) (from SCAR-MarBIN Portal at http://www.scarmarbin.be/SearchGazetteer.php), Antarctic Peninsula, Antarctica, during the austral summer. The animals were then euthanased after being deeply anaesthetised. The Program of Environmental Management and Tourism (Instituto Antártico Argentino, Ministerio de Relaciones Exteriores, Comercio Internacional y Culto) gave Dr D. Montalti the approval and the protocol of ethical conditions under which this work was carried out.
The body mass of each bird was recorded with a spring balance (accuracy ±5 g) and the gland was dissected following the protocol described in Montalti et al. (1998) and weighed (accuracy ±0.001 g). These values were used to calculate the gland mass as a percentage of body mass in each bird:
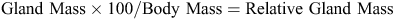
A regression analysis was performed using Statistica 7.0 software.
The glands were fixed in 10% formalin and processed for their inclusion in paraffin. Transversally and longitudinally cut samples of the different parts of the gland were included. Samples were then cut in series (3 μm) and stained either with haematoxylin–eosin for general histological description, orcein for elastic fibres, Masson trichrome for collagen fibres and Gomori for reticular fibres. In addition, other slices were processed using the histochemical PAS method.
The classification of the papillae, partition of the secretory area and characterisation of the different layers of the glandular epithelium were carried out according to Jacob and Ziswiler (1982).
Results
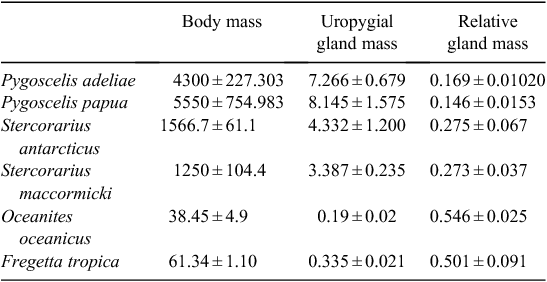
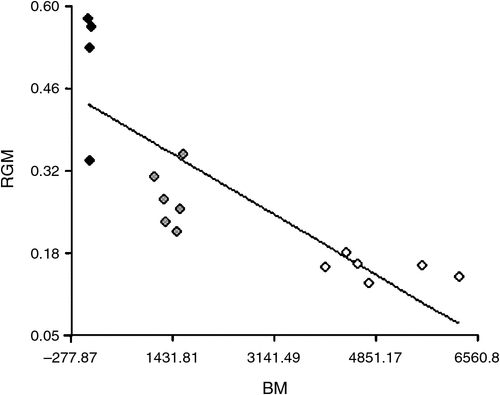
Table 1 details the body mass, gland mass and relative gland mass of the birds studied. The regression analysis (Fig. 1) relating body mass and relative gland mass was significantly different (P < 0.0001) in the distribution pattern of the three bird families.
|
|
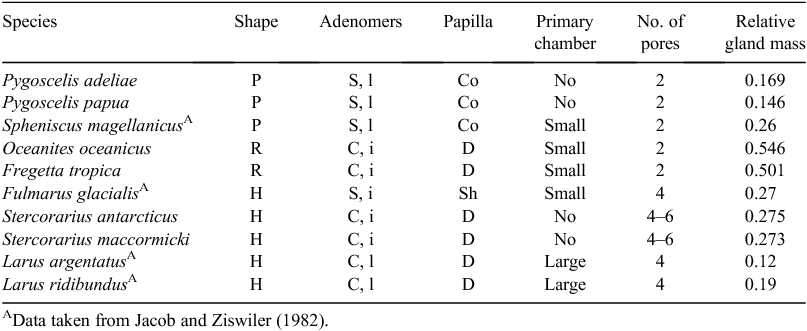
Table 2 shows the macroscopic and microscopic results in all studied species, in comparison with others of the same order. The uropygial gland of the seabird species comprised a capsule of connective tissue with two lobes and a papilla, with a feather tuft surrounding the excretory openings. In all species the lobes were separated by a septum of connective tissue, going from the covering capsule to the inner part of each lobe. The lobes were composed of tubule-alveolar adenomers. Three well defined zones were identified from the outside to the centre, taking into account the epithelium height and the secretion flow to storage chambers or duct systems. These zones had many similarities and some differences characterising each of the species studied. Invariably, the adenomers showed a stratified epithelium that included sebaceous-like cells (Fig. 2).

|
In Zone I of the adenomers, the basal epithelium comprised two cell layers (except in petrels, which had a monolayer) and some mitotic figures; the intermediate epithelium comprised two cell layers in penguins and petrels and up to five layers in skuas; and the secretory epithelium was composed of five or six layers in penguins, four to eight layers in petrels and only three layers in skuas. The lobule lumen was small and occasionally filled with degenerative cells (Fig. 3a). PAS positive reaction was significant in the basal epithelial cells in petrels and in the basal and intermediate epithelial cells in skuas, but in general it decreased towards the epithelial surface (Fig. 3b).
In Zone II, the basal epithelium comprised two layers in penguins, one in petrels and one or two in skuas; the intermediate epithelium, with polyhedral cells and scarce lipid content, comprised two layers in penguins and one or two in petrels and skuas; and the secretory epithelium, rich in lipids, comprised one to three layers in penguins and petrels and two to four in skuas, and one to three layers of flat cells in penguins and one or two in petrels and skuas, with picnotic or absent nuclei, and eventually giving rise to a degenerative layer (Fig. 3c).
In Zone III, the lobule lumen is wider and showed PAS positivity both in the cells and in the secretion. In penguins, the adenomers had three to five PAS-positive cell layers with scarce or undetectable lipid content, corresponding to the basal and intermediate layers, and one to three flat and cornified cells, losing the nuclei towards the centre. Storm petrels and skuas had one layer of basal cells, one layer of intermediate epithelium, one or two layers of secretory cells and one or two layers of degenerative cells; however, in some areas, this number varied more extensively (Fig. 3d).
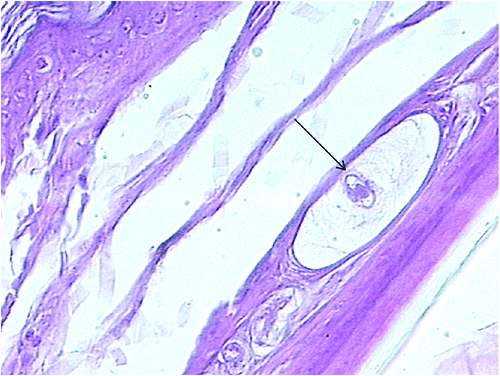
Adenomers were surrounded by a PAS-positive basal lamina, a stratum of reticular fibres and peripheral fibroblasts. In the gland outlet, we observed some feather follicles, as well as nerves and Herbst corpuscles (Fig. 4).
|
Penguins
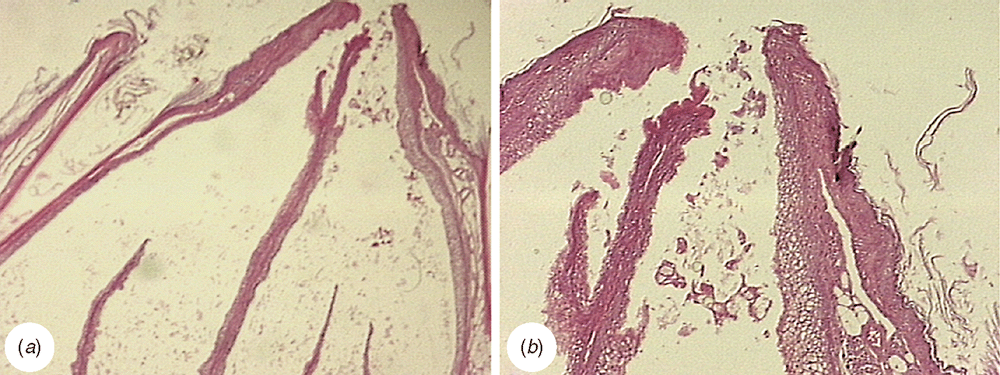
In penguins, the lobes of the uropygial gland are torpedo-shaped (Fig. 5) and are long and cylindrical, well differentiated from the papilla. The papilla is compact, with abundant connective tissue, more developed in P. papua (Fig. 6a). The capsule has scarce muscle tissue and contains elastic fibres (Fig. 6b), whereas no muscle fibres were observed among the adenomers. The interstitial tissue is thicker in the region of the centre of the adenomers. The tubuloalveolar adenomers and their ducts are straight-coursed and slightly branched. The terminal portion of these ducts ends in secondary chambers (larger in the Gentoo penguin) and is covered by a flat stratified epithelium surrounded by sparse connective tissue (Fig. 6c). Some ducts originate from this point, which is full of connective tissue rich in adipocytes, blood vessels, nerves and muscle fibres nearby (Fig. 6d). These ducts converge to be connected towards the end of the tract where each is surrounded by slight connective tissue, close to the epithelium, and dense connective tissue with muscle fibres towards the outside. These large ducts are encircled by connective tissue, blood and lymphatic vessels. There are two excretory ducts with a flat stratified epithelium that increases its cornification up to the end of the tract, with an epidermis-like structure (Fig. 6a). In the papilla, the feather follicles surround the pores of the ducts end. Adipose tissue exists between the terminal ducts and disappears in the tract ends, where abundant muscle fibres can be observed between and surrounding the ducts. In this region, there are numerous Herbst corpuscles.
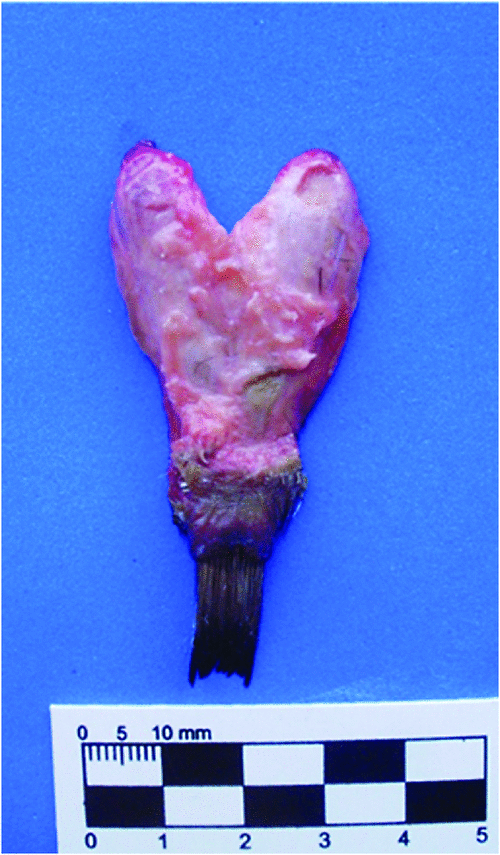
|
Storm petrels
In these species, the gland is round, the papilla is short and cylindrical and the lobes cannot be macroscopically identified (Fig. 7).
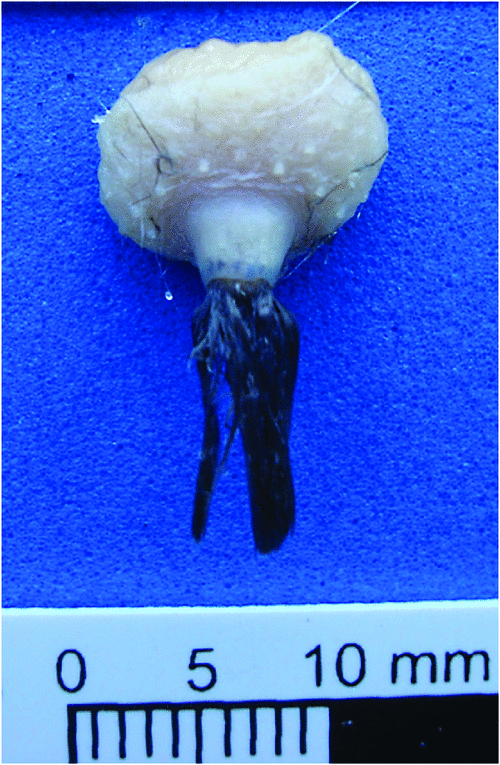
|
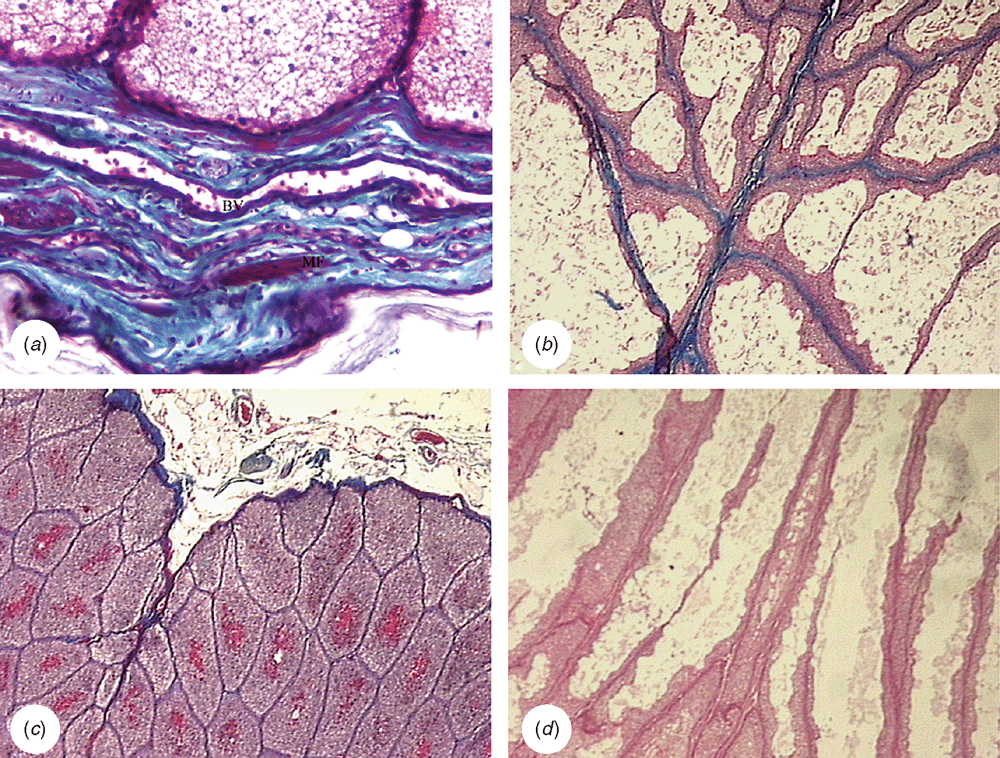
The histological structure was identical in both storm petrel species. The capsule is thin and the connective tissue is scarce, not only in the interlobular septum but also among the adenomers. The central septum is slim and incomplete. Muscle fibres are rare in the capsule (Fig. 8a), and absent in the medium septum and interalveolar tissue (Fig. 8b, c). The adenomers are tubule-alveolar, of convoluted course type and intensively branched only in their initial tract. The epithelium height decreases towards the centre. Furthermore, there is a system of branched collecting ducts with a wide lumen on the terminal tract. Nevertheless, no storage chambers were apparent, except close to the principal duct, where a small cavity was observed. The adenomers continue in ducts containing sebaceous-like cells without epithelial cornification in their starting zone (Fig. 8d). These ducts flow into a small storage chamber, and then a short duct emerges for each lobe (Fig. 9a, b). In the black-bellied storm petrel, pigmented cells can be observed in the epithelium near the end of the ducts. The papilla is delicate, with scanty connective tissue and some muscle fibres among the ducts. In both species, the feather follicles form a line encircling the terminal zone of the ducts.
Skuas
In these species, the uropygial gland is heart-shaped, with two well defined lobes and a very short and poorly developed papilla (Fig. 10).
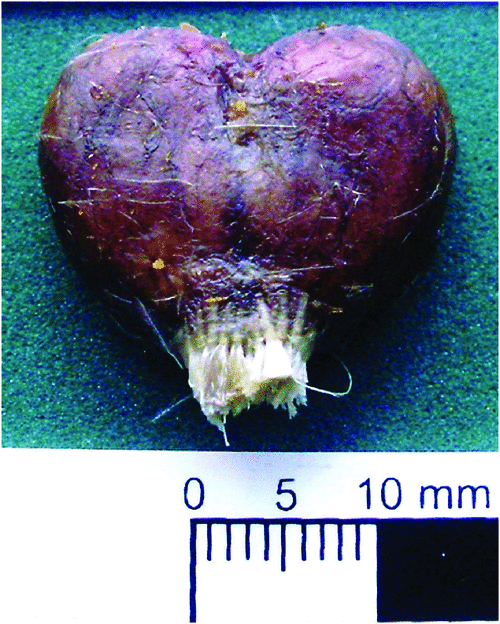
|
The capsule is formed by dense connective tissue (Fig. 11a) with some smooth muscle fibres and elastic fibres. Thin branches of connective tissue start from the capsule and medium septum and completely divide the lobes into secretory units (Fig. 11a, b) while the interadenomer connective tissue becomes thicker towards the intertubular septum. With respect to the parenchyma, the convoluted and intensively branched tubule-alveolar secretory units are dilated distally. The epithelium height decreases towards the central zone, where the units converge to form larger ducts (Fig. 11c, d), and no storage chambers were found. The terminal portion of the ducts shows secretory-like cells and a flat epidermis-like epithelium (Fig. 11c, d). The ducts join and end up in four to six ducts per lobe. There is no connective tissue between the ends of the ducts. Nevertheless, there is a connective septum separating the ducts from both lobes. Both species have an intermediate-type papilla with more connective tissue than in the delicate type. In this region, we observed abundant adipose tissue and a crown of feather follicles surrounding the ducts (Fig. 11c, d). Other follicles were found in the connective tissue between these ducts. Feather follicles are less abundant in the south polar skua than in the brown skua.
Discussion
The uropygial gland is dissimilar morphologically and in size in different bird taxa (Jacob and Ziswiler 1982; Johnston 1988; Montalti and Salibián 2000). The morphological and cellular structure detailed above in penguins, storm petrels and skuas allowed a comparative analysis not only among the studied species but also between them and other species belonging to sister taxa, whose characteristics have been reported previously (Jacob and Ziswiler 1982).
The shape of the gland in the species included in our study showed remarkable similarities to those of the same families analysed by Jacob and Ziswiler (1982) and Johnston (1988), a finding that may be attributed to their phylogenetic proximities.
By comparing aquatic and terrestrial birds, Montalti and Salibián (2000) hypothesised the absence of a direct relationship between these ecological groups and their relative gland mass. In the present study, by comparing aquatic species that differ with respect to the kind of contact with water, we confirmed the previous hypothesis.
With regard to the relative mass of the uropygial gland, a probable negative allometry between the glandular and the body mass (Galván et al. 2008) should be taken into account, because smaller birds do have a relatively larger gland.
Some histological characteristics of the uropygial gland could be linked to the degree of contact that the bird has with water. According to Jacob and Ziswiler (1982), the tortuosity of the tubules limits the speed of secretion, because the more winding the tract the longer it takes for the secretion to be transported. Penguins have long-lasting periods of aquatic activity, a fact that demands greater feather moisture and the immediate availability of abundant secretion. This may explain the presence of straight and minimally branched adenomers and the absence of a primary storage chamber in species of Pygoscelis. The presence of elastic fibres may also facilitate an easier and continuous gland secretion in these species.
On the other hand, storm petrels, have a complex duct system and a storage chamber, without elastic fibres. These features could be related to the fact that these species store secretion and use it randomly.
The uropygial gland of skuas has some characteristics of the glands of birds that have a close contact with water, like penguins, and of those that have a lesser contact with water, like storm petrels.
The secretory epithelium of the six species included in our study was composed of the same epithelial layers as those of other species (Jacob and Ziswiler 1982; Montalti et al. 2001; Sawad 2006; Harem et al. 2010; Kozlu et al. 2011; Sadoon 2011). The lobes showed a PAS positive reaction, as demonstrated in the rock pigeon (Columba livia) (Montalti et al. 2001). This could explain the fact that both lipids and glycoproteins are secreted along the adenomer. Some of these glycoproteins may have functions related to protection from pathogenic agents (Soler et al. 2012). In marine mammals, it has been demonstrated that some cutaneous glands produce glycoproteins with microbicidal action (Meyer et al. 2003).
The uropygial gland of most birds has only one excretory pore per glandular lobe. Nevertheless, this number is very variable (Jacob and Ziswiler 1982). Therefore, this feature could not be associated with the bird habitat.
In Pygoscelis, the papilla is similar to that in the Magellanic penguin (Jacob and Ziswiler 1982). On the other hand, in storm petrels, it is delicate but has feather follicles, a fact that has not been reported in other groups with this type of papilla, such as Columbiformes. In Fulmarus glacialis, a member of the same order but of larger size, another type of papilla (the ‘short type’) has been described (Jacob and Ziswiler 1982). Thus, the size of the bird and the type of papilla seem to be related. In skuas, the papilla is of an intermediate type, different from that of other members of the suborder (Jacob and Ziswiler 1982).
Also, the glandular mass and the quantity of connective tissue are directly related, a fact that could be associated with the structural support function. There is no typical muscular sphincter in the species studied. Nevertheless, in penguins we found muscle fibres surrounding the ducts, as described in other species with the same type of papillae (Jacob and Ziswiler 1982).
In summary, although the uropygial gland of birds shows some characteristics in common, some variations do exist such as the relative gland mass, the type of papillae and the amount of connective tissue, which seem to be related to the size of the gland. On the other hand, other characteristics such as the tubular branching, its course and the presence of a small storage chamber, could be related to the bird’s habits and environment.
Acknowledgements
The authors thank Mr Rubén C. Mario for his skilled technical assistance. This work was partially supported by grants 11/V191 and 11/N583 from Universidad Nacional de La Plata, Argentina.
References
Acosta Hospitaleche, C., Montalti, D., and Marti, L. J. (2009). Skeletal morphoanatomy of the brown skua Stercorarius antarcticus lonnbergi and the south polar skua Stercorarius maccormicki. Polar Biology 32, 759–774.| Skeletal morphoanatomy of the brown skua Stercorarius antarcticus lonnbergi and the south polar skua Stercorarius maccormicki.Crossref | GoogleScholarGoogle Scholar |
Bandyopadhyay, A., and Bhattacharyya, S. P. (1996). Influence of fowl gland and its secretory lipid components on the growth of skin surface bacteria of fowl. Indian Journal of Experimental Biology 34, 48–52.
| 1:CAS:528:DyaK28XotVeisw%3D%3D&md5=2ef59973bc917e4b93f02bf887f90fa5CAS | 8698407PubMed |
Bandyopadhyay, A., and Bhattacharyya, S. P. (1999). Influence of fowl gland and its secretory components on the growth of skin surface fungi of fowl. Indian Journal of Experimental Biology 37, 1218–1222.
| 1:CAS:528:DC%2BD3cXjsVWhtQ%3D%3D&md5=de31ecc5a354cf69c7ed62b18c62ed03CAS | 10865889PubMed |
Bhattacharyya, S. P. (1972). A compartive study on the histology and histochemistry of glands. La Cellule 69, 113–126.
Bo, N. A. (1953). Observaciones sobre la glándula uropigia del biguá Phalacrocorax brasilianus brasilianus (Gmelin). Ciencia e Investigacion 9, 521–524.
Bride, J. (1975). Etude ultraestructurale de la morphogénése et de la différenciation de la glande uropygienne de canard (Anas platyrhynchos) Zoologie, Physiologie et Biologie Animale 12, 13–71.
Elder, W. H. (1954). The oil gland of birds. The Wilson Bulletin 66, 6–31.
Furnes, R. W. (1996). Family Stercorariidae (skuas). In ‘Handbook of the Birds of the World. Vol. 3’. del (Eds J. Hoyo, A. Elliot and J. Sargatal.). pp. 556–571. (Lynx Edicions: Barcelona.)
Galván, I., Barba, E., Piculo, R., Cantó, J. L., Cortés, V., Monrós, J. S., Atiénzar, F., and Proctor, H. (2008). Feather mites and birds: an interaction mediated by uropygial gland size? Journal of Evolutionary Biology 21, 133–145.
| 18028353PubMed |
Graña Grilli, M., and Montalti, D. (2012). Trophic interactions between brown and south polar skuas at Deception Island, Antarctica. Polar Biology 35, 299–304.
| Trophic interactions between brown and south polar skuas at Deception Island, Antarctica.Crossref | GoogleScholarGoogle Scholar |
Gutiérrez, A. M., Montalti, D., Reboredo, G. R., Salibián, A., and Catalá, A. (1998). Lindane distribution and fatty acid profile of gland and liver of Columba livia after pesticide treatment. Pesticide Biochemistry and Physiology 59, 137–141.
| Lindane distribution and fatty acid profile of gland and liver of Columba livia after pesticide treatment.Crossref | GoogleScholarGoogle Scholar |
Harem, I. S., Kocak-Harem, M., Turan-Kozlu, T., Akaydin-Bozkurt, Y., Karadag-Sari, E., and Altunay, H. (2010). Histologic structure of the gland of the osprey (Pandion haliaetus). Journal of Zoo and Wildlife Medicine 41, 148–151.
| Histologic structure of the gland of the osprey (Pandion haliaetus).Crossref | GoogleScholarGoogle Scholar | 20722270PubMed |
Hsu, W. S. (1936). Further cytological observations on the glands of birds. Cell and Tissue Research 24, 248–255.
Jacob, J., and Ziswiler, V. (1982). The uropygial gland. In ‘Avian Biology’. (Eds D. S. Farner, J. R. King, and K. C. Parkes.) pp. 199–324. (Academic Press: New York.)
Johnston, D. W. (1988). A morphological atlas of the avian gland. Bulletin of the British Museum of Natural History (Zoology) 54, 199–259.
Kamiya, S., Izumisawa, Y., Tsukushi, M., Amasaki, H., and Daigo, M. (1986). Histochemical studies on polysaccharides in the gland of ducks. Bulletin Nippon Veterinary Zootechnical College 35, 1–7.
| 1:CAS:528:DyaL2sXkvFert7o%3D&md5=fafef48b6511b8d644f92bbec68767e7CAS |
Kozlu, T., Akaydin Bozkurt, Y., and Ates, S. (2011). A macroanatomical and histological study of the gland in the white stork (Ciconia cicionia). International Journal of Morphology 29, 723–726.
| A macroanatomical and histological study of the gland in the white stork (Ciconia cicionia).Crossref | GoogleScholarGoogle Scholar |
Lucas, A. M., and Stettenheim, P. R. (1972). Uropygial gland. In ‘Avian Anatomy’. pp. 613–626. Agricultural Handbook, US Department of Agriculture. (US Government Printing Office: Washington, DC.)
Menon, G. K., Aggarwal, S. K., and Lucas, A. M. (1981). Evidence for the holocrine nature of lipoid secretion by avian epidermal cells: a histochemical and fine structural study of rictus and the gland. Journal of Morphology 167, 185–199.
| Evidence for the holocrine nature of lipoid secretion by avian epidermal cells: a histochemical and fine structural study of rictus and the gland.Crossref | GoogleScholarGoogle Scholar |
Meyer, W., Seegers, U., Herrmann, J., and Schnapper, A. (2003). Further aspects of the general antimicrobial properties of pinniped skin secretions. Diseases of Aquatic Organisms 53, 177–179.
| Further aspects of the general antimicrobial properties of pinniped skin secretions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3s7jsFGqug%3D%3D&md5=723dc99503d512c883deb06fdaab9052CAS | 12650250PubMed |
Møller, A. P., Erritzøe, J., and Nielsen, J. T. (2010). Predators and microorganisms of prey: goshawks prefer prey with small glands. Functional Ecology 24, 608–613.
| Predators and microorganisms of prey: goshawks prefer prey with small glands.Crossref | GoogleScholarGoogle Scholar |
Montalti, D., and Salibián, A. (2000). Gland size and avian habitat. Ornitologia Neotropical 110, 297–306.
Montalti, D., Gutiérrez, A. M., and Salibián, A. (1998). Técnica quirúrgica para la ablación de la glándula uropigia en la paloma casera Columba livia. Revista Brasileira de Biologia 58, 193–196.
Montalti, D., Quiroga, A., Massone, A., Idiart, J. R., and Salibián, A. (2001). Histochemical and lectinhistochemical studies on the gland of rock dove Columba livia. Brazilian Journal of Morphological Science 18, 33–39.
Montalti, D., Gutiérrez, A. M., Reboredo, G. R., and Salibián, A. (2005). The chemical composition of the gland secretion of rock dove. Comparative Biochemistry and Physiology 140, 275–279.
| The chemical composition of the gland secretion of rock dove.Crossref | GoogleScholarGoogle Scholar | 15792592PubMed |
Montalti, D., Gutiérrez, A. M., Reboredo, G. R., and Salibián, A. (2006). Removal urogygial gland does not affect serum lipids, cholesterol and calcium levels in the rock pigeon Columba livia. Acta Biologica Hungarica 57, 295–300.
| 1:STN:280:DC%2BD28nhs1agsA%3D%3D&md5=64c15bd33cb8bc7ee4e37d510e957231CAS | 17048693PubMed |
Moyer, B. R., Rock, A. N., and Clayton, D. H. (2003). Experimental test of the importance of preen oil in rock doves (Columba livia). The Auk 120, 490–496.
| Experimental test of the importance of preen oil in rock doves (Columba livia).Crossref | GoogleScholarGoogle Scholar |
Reneerkens, J., Piersma, T., and Sinninghe Damsté, J. S. (2002). Sandpipers (Scolopacidae) switch from monoester to diester preen waxes during courtship and incubation, but why? Proceedings of the Royal Society B: Biological Sciences 269, 2135–2139.
| Sandpipers (Scolopacidae) switch from monoester to diester preen waxes during courtship and incubation, but why?Crossref | GoogleScholarGoogle Scholar | 12396488PubMed |
Sadoon, A. H. (2011). Histological study of European starling gland (Sturnus vulgaris). International Journal of Poultry Science 10, 662–664.
| Histological study of European starling gland (Sturnus vulgaris).Crossref | GoogleScholarGoogle Scholar |
Salibián, A., and Montalti, D. (2009). Physiological and biochemical aspects of the avian gland. Brazilian Journal of Biology 69, 437–446.
| Physiological and biochemical aspects of the avian gland.Crossref | GoogleScholarGoogle Scholar |
Sandilands, V., Savory, J., and Powell, K. (2004). Preen gland function in layer fowls: affecting morphology and feather lipid levels. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 137, 217–225.
| Preen gland function in layer fowls: affecting morphology and feather lipid levels.Crossref | GoogleScholarGoogle Scholar |
Sawad, A. A. (2006). Morphological and histological study of gland in moorhen (G. gallinula c. choropus). International Journal of Poultry Science 50, 938–941.
Soler, J. J., Peralta-Sánchez, J. M., Martin-Platero, A. M., Martin-vivaldi, M., Martínez-Bueno, M., and Møller, A. P. (2012). The evolution of size of the uropygial gland: mutualistic feather mites and uropygial secretion reduce bacterial loads of eggshells and hatching failures of European birds. Journal of Evolutionary Biology , .
| The evolution of size of the uropygial gland: mutualistic feather mites and uropygial secretion reduce bacterial loads of eggshells and hatching failures of European birds.Crossref | GoogleScholarGoogle Scholar | 22805098PubMed |
Suzuki, T. (1994). Ultrastructural studies on the glands of quail. Japanese Poultry Science 31, 38–44.
| Ultrastructural studies on the glands of quail.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXisVSlsrs%3D&md5=32be419280abb922e1d1dc7ab72f2fdbCAS |
Vincze, O., Vágási, C. I., Kovács, I., Galván, I., and Pap, P. L. (2013). Sources of variation in uropygial gland size in European birds. Biological Journal of the Linnean Society 110, 543–563.
| Sources of variation in uropygial gland size in European birds.Crossref | GoogleScholarGoogle Scholar |
Warham, J. (1990). ‘The Petrels: Their Ecology and Breeding Systems.’ (Academic Press: London.)
Williams, T. D. (1995). ‘The Penguins.’ (Oxford University Press: New York.)