Survival from breast cancer: an analysis of Australian data by surgeon case load, treatment centre location, and health insurance status
David Roder A B I , Primali de Silva C , Helen M. Zorbas D , James Kollias C E , Peter L. Malycha F , Chris M. Pyke G and Ian D. Campbell HA Population Health, Cancer Australia, Locked Bag 3, Strawberry Hills, NSW 2012, Australia.
B Cancer Epidemiology, University of South Australia, GPO Box 2471, Adelaide, SA 5001, Australia.
C National Breast Cancer Audit Steering Committee, Royal Australasian College of Surgeons, 179-199 Ward St, North Adelaide, SA, Australia.
D Cancer Australia, Locked Bag 3, Strawberry Hills, NSW 2012, Australia.
E University of Adelaide, North Terrace, Adelaide, SA 5005, Australia.
F St Andrews Hospital, 350 South Terrace, Adelaide, SA 5000, Australia.
G University of Queensland Brisbane, Chancellors Place, St Lucia, QLD 4067, Australia.
H Waikato Clinical School, University of Auckland, Faculty of Medical and Health Sciences, Private Bag 92019, Auckland 1142, New Zealand.
I Corresponding author. Email: roder@internode.on.net
Australian Health Review 36(3) 342-348 https://doi.org/10.1071/AH11060
Submitted: 23 June 2011 Accepted: 13 December 2011 Published: 6 August 2012
Journal Compilation © AHHA 2012
Abstract
Objective. Early invasive breast cancer data from the Australian National Breast Cancer Audit were used to compare case fatality by surgeon case load, treatment centre location and health insurance status.
Method. Deaths were traced to 31 December 2007, for cancers diagnosed in 1998–2005. Risk of breast cancer death was compared using Cox proportional hazards regression.
Results. When adjustment was made for age and clinical risk factors: (i) the relative risk of breast cancer death (95% confidence limit) was lower when surgeons’ annual case loads exceeded 20 cases, at 0.87 (0.76, 0.995) for 21–100 cases and 0.83 (0.72, 0.97) for higher case loads. These relative risks were not statistically significant when also adjusting for treatment centre location (P ≥ 0.15); and (ii) compared with major city centres, inner regional centres had a relative risk of 1.32 (1.18, 1.48), but the risk was not elevated for more remote sites at 0.95 (0.74, 1.22). Risk of death was not related to private insurance status.
Conclusion. Higher breast cancer mortality in patients treated in inner regional than major city centres and in those treated by surgeons with lower case loads requires further study.
What is known about the topic? Studies in some countries show an association of poorer outcomes with lower case load and lack of private health insurance.
What does this paper add? Lower survivals apply in contemporary Australian environments where annual case loads are 20 or fewer and for patients treated in inner regional compared with major city centres. Poorer survivals for patients without private health insurance status are not statistically significant after adjusting for tumour size and other risk factors.
What are the implications for practitioners? Additional research is needed to determine why survivals are lower in Australian settings where case loads are low and when treatment is provided in inner regional centres. Meanwhile, it would be appropriate to target these settings in quality improvement programs.
Introduction
The National Breast Cancer Audit (NBCA) of the Royal Australasian College of Surgeons (RACS) has collected descriptive data on early breast cancer since 1998. The data support clinical audit by surgeons of their practices and are used for research into breast cancer and its management.1–5
The Audit’s main purpose is to strengthen quality improvement through the promotion of high clinical standards, but it is recognised that cancer outcomes would also be affected by structural features of the health system, potentially including governance structures, resource allocation, service coordination, workforce availability, and access and appropriateness of care.6–8 For example, poor survival from cancer among Aboriginal and Torres Strait Islander patients has been associated with geographic remoteness, cultural differences, socioeconomic and other social factors not adequately accommodated by the health system, rather than technical standards of care delivered.9
Increasingly, attention is being given around the world to health-system characteristics as determinants of inequalities in outcomes from breast and other cancers.6,7 This is exemplified by the Organisation for Economic Cooperation and Development (OECD) Health Quality Indicators project which includes cross-national analyses of survival in relation to health-system expenditure, workforce availability, healthcare infrastructure, pharmaceutical pricing, governance arrangements, and other health-system factors.6,7
Although the NBCA database includes only limited information on structural features of the health system, it allows assessment of surgeon case load, which would be affected by the distribution of healthcare settings and population size and dispersion. The data also allow classification of treatment location as major city, inner regional and more remote locations, where geographic access to adjuvant treatment services would vary. The data also include information on private insurance status, which is directly relevant to the OECD health quality indicator for expenditure.6,7
In this study, we use NBCA data to investigate associations of case load, treatment centre location and insurance status with breast cancer survival in contemporary NBCA settings and consider the policy and research implications. The data relate to cases diagnosed in 1998–2005, with a follow-up of survival to December 2007. The data have good face-value validity in that they show similar survivals to population-based data for early breast cancers from NSW and the USA (Surveillance Epidemiology and End Results (SEER) data).10–12 Differences in survival by conventional risk factors such as tumour size, grade, nodal status and oestrogen receptor status accord with expected differences, which add credibility to NBCA data.12–15
The objective of this study is to determine whether survival differences exist by case load, treatment location and insurance status, and if so, the extent to which differences are attributable to differences in age and clinical risk factors.16–18 Clinical factors investigated included tumour size, grade, nodal status, oestrogen receptor status, vascular invasion, histology type, and number of breast cancer foci detected.12–15
Other studies have reported differences in cancer survival by surgeon and hospital case load, with higher case loads generally being associated with higher survival.16,19–27 Often these were studies of other cancers, although higher survivals from breast cancer have been linked to higher case loads in North America, Europe, Asia and Western Australia.28–40 Results have generally been interpreted as reflecting higher skill development from managing higher case loads, although this interpretation has been questioned and alternative explanations suggested, including accompanying differences in degree of specialisation, access to multidisciplinary conferencing, and availability of specialist adjunctive services.22,33,41
In the USA, lack of private health insurance can be a barrier to appropriate breast and other cancer care for optimising survival.18,42 The extent to which this would apply in Australia is uncertain, given universal public health insurance, although differences in outcomes might still apply due to differences in socioeconomic and clinical risk factors or access to care.
NBCA surgeons have had evidence-based clinical guidelines distributed since the mid 1990s and RACS has promoted high standards of care through the NBCA and other means.2,43 The extent to which variations in case survival exist by case load, treatment centre location and health insurance status in this specialist environment is investigated and research and clinical policy implications considered. The study is novel in producing local evidence of direct relevance to local quality improvement initiatives and research.
Ethics approval for this research was obtained from the Australian Institute of Health and Welfare and RACS research ethics committees.
Methods
NBCA data for Australian women diagnosed with invasive cancer were linked to the National Death Index at the Australian Institute of Health and Welfare using the first three digits of surnames, dates of birth and jurisdiction of residence.12 A pilot investigation was undertaken in SA, in which data for 1179 women treated by South Australian surgeons were linked with official death records.12 The accuracy and completeness of the death data so obtained were compared with death information recorded on the South Australian Cancer Registry for the same women where full names were available for linkage purposes and resolution of doubtful links had been undertaken by Registry staff through active follow-up. The results showed a high accuracy of data linkage, with a sensitivity of breast cancer death detection of 93.1%, specificity of 99.9%, predictive value positive of 96.4%, and predictive value negative of 99.8%.12
Following the pilot, death data were obtained through the National Death Index for all women recorded on the NBCA database with cancer diagnosed since NBCA data collection commenced in 1998. The date of censoring of live cases for survival analysis was 31 December 2007. Analyses were limited to cancers diagnosed in 1998–2005 to allow enough follow-up time for survival assessment. A total of 31 493 cases were studied after excluding a small number where Australian residency status was uncertain.
Variables analysed as potential predictors of survival in three separate sets of analyses were surgeon case load, treatment centre location and health insurance status. The maximum number of cases recorded on the NBCA for each surgeon per annum was used to infer case load, which was classified as up to 20, 21–100, or over 100. This was similar to classifications in some other studies, although classification categories have not been consistent across studies.28–41 Treatment centre location was categorised as major city, inner regional or more remote, using the Australian Standard Geographical Classification.44 These categories are based on Census Collection Districts (CDs) categorised using the Accessibility/Remoteness Index for Australia (ARIA), which is a measure of the remoteness of a location from the services provided by large towns or cities.44
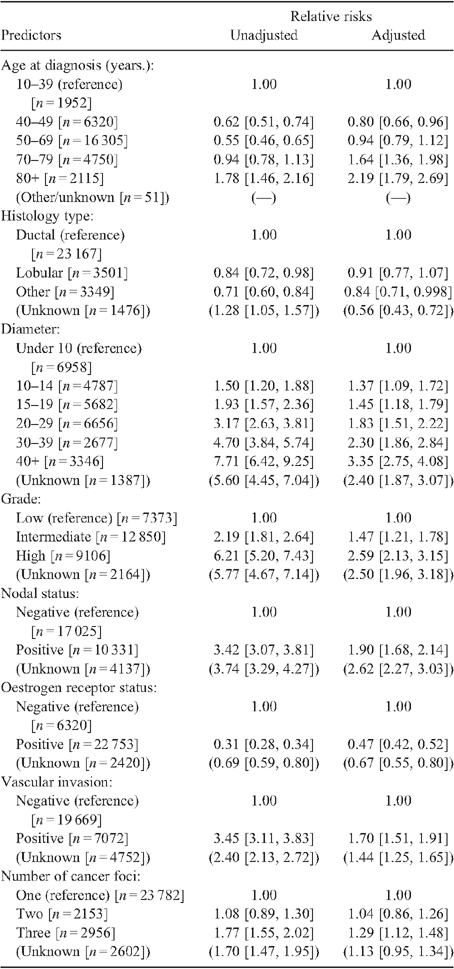
Co-variables used in the analyses and their categorisations are shown in Table 1. Disease-specific survivals from breast cancer were calculated, regarding as a breast cancer death those deaths where breast cancer was recorded as a cause on the death certificate.45 Disease-specific survival have been shown to correspond closely with relative survival in population-based studies in Australia. For example, breast cancer survivals in SA for 1977–2003 diagnoses were 80% at 5 years from diagnosis, both when using disease-specific and relative survivals, and 70% and 69% respectively at 10 years from diagnosis.46 Disease-specific survivals are often preferred in clinical studies where, due to referral practices, patients may not have risks of alternative causes of death that are equivalent to population norms (an assumption of relative survival).13,45,47
|
Survival times were calculated from diagnosis to 31 December 2007 or date of death, whichever occurred first. Relative risks of case fatality from breast cancer (i.e. hazards ratios) (95% confidence limits) were calculated by age and clinical characteristics, using Cox proportional hazards regression (unadjusted analysis).45 Characteristics were expressed as dummy variables using the categories in Table 1. In the second part of the analysis, all clinical variables were entered into the model with age and the principle variable of interest (i.e. case load, treatment centre location or private health insurance status) and backwards elimination used to test whether any could be omitted without significantly (P < 0.05) reducing model fit (adjusted analysis). Assumptions of proportionality and lack of co-linearity were checked and satisfied. In supplementary analyses, backward elimination was undertaken also including as a co-variable treatment centre location when investigating case load, case load when investigating treatment centre location, and case load and treatment centre location when investigating health insurance status. Health insurance status was recorded for a limited period in 2003–2005 only and was not available for adjustment on analyses of case load and treatment centre location for 1998–2005.
Results
Risk factors
Table 1 shows results of Cox proportional hazards regression analyses of age and clinical risk factors. Both unadjusted and adjusted analyses showed predictable increases in risks of breast cancer death with increasing tumour diameter, higher grade, positive nodal status, negative oestrogen receptor status, vascular invasion, and presence of three or more cancer foci. Differences in relative risks for clinical risk factors were generally less pronounced in the adjusted than unadjusted analyses. Lobular cancers had a lower relative risk than ductal histology types in the unadjusted analysis, but less so in the adjusted analysis where statistical significance was not achieved (P = 0.242). The pattern by age varied between adjusted and unadjusted analyses, in that 50–69 year olds no longer had a lower relative risk after adjustment than the reference category of women less than 40 years, whereas those aged 70–79 years and over had elevated relative risks. Women aged 40–49 years had a lower relative risk than women below 40 years in both analyses.
Surgeon case load
Unadjusted Cox analysis showed that, compared with the lowest annual case load of up to 20 cases, the relative risk of death (95% confidence limits) was 0.74 (0.65, 0.85) for women treated by surgeons with a case load in the 21–100 range, and 0.70 (0.60, 0.81) for those treated by surgeons with higher case loads (Table 2). The effect of adjusting for age and clinical risk factors was to increase the relative risk to 0.87 (0.76, 0.995) for the 21–100 case load range, and to 0.83 (0.72, 0.97) for higher case loads. When adjustment was also made for treatment centre location, the relative risks became higher at 0.90 (0.78, 1.04) and 0.93 (0.79, 1.09) and were no longer statistically significant (P ≥ 0.15) (Table 2).
Treatment centre location
Unadjusted Cox analysis showed that compared with major cities, the relative risk of death was elevated at 1.44 (1.29, 1.61) for inner regional locations. Although relative risks were also higher at 1.23 (0.96, 1.57) for more remote locations and 1.24 (0.83, 1.84) for unknown locations, these difference were not statistically significant (P ≥ 0.10) (Table 2). Adjusting for age and clinical risk factors reduced the relative risk to 1.32 (1.18, 1.48) for inner regional areas compared with major cities. There were not significant differences for more remote locations or unknown locations in the adjusted analysis, with relative risks of 0.95 (0.74, 1.22) and 1.26 (0.85, 1.88) respectively. When adjustment was also made for surgeon case load, little effect on risk estimates for treatment centre location was observed (Table 2).
Health insurance status
A total of 7208 cases were reported to have private health insurance and 4636 cases did not have this insurance during the period when this characteristic was recorded. Unadjusted Cox analysis indicated a higher risk of death for women without private health insurance at 1.41 (1.12, 1.78) (Table 2). After adjustment for age and the clinical risk factors in Table 1, the relative risk reduced to 1.20 (0.95, 1.52) which was not statistically significant (P = 0.13). When adjustment was also made for surgeon case load and treatment centre location, the relative risk reduced further to 1.14 (0.90, 1.44) (Table 2).
Discussion
NBCA cases included more localised (node negative) lesions than reported for all female breast cancers at a population level in NSW in 2004–08 (62% compared with 55%), likely reflecting the selection of early breast cancers for inclusion in the NBCA (note, data on extent of cancer at diagnosis are not routinely available from other Australian jurisdictions).48,49 When numbers of early breast cancers in Australia are estimated from population-based stage distributions observed in NSW, and the USA SEER program,10,11 it is evident from NBCA data that ~60% would have been covered by the NBCA in 1998–2005.
NBCA data are not population-based and the extent of selection bias for early breast cancers is not known. The extent of bias may not be large, in that survivals of NBCA cases are very similar to corresponding NSW and USA population-based survivals for early breast cancer, and their patterns of survival by conventional clinical risk factors accord with expected patterns.10,11,50–52 NBCA cases also appear to be broadly similar to all Australian female breast cancer cases in:
-
Age at diagnosis – e.g. the percentage aged less than 50, 50–69, and 70 years or more respectively equalled 26%, 52% and 22%, compared with the corresponding 24%, 51% and 25% reported by population registries for 200653
-
Grade – e.g. the percentage for low, intermediate and high grade respectively equalled 25%, 44% and 31%, compared with corresponding population-based data from SA for 1998–2005 of 26%, 43% and 31% (note, data on grade are not routinely available from other Australian jurisdictions).46
Furthermore, the proportion of NBCA lesions classified as lobular was 12%, equating with the 12% reported by population registries for 2006.53
The association between higher surgeon case load and higher survival found both in the unadjusted analysis and the analysis adjusted for age and clinical risk factors, accords with results of most studies in North America, Europe, Asia and Western Australia.16,28–40 The difference mostly occurred between 21–100 cases per annum and lower case loads, with little difference observed between the 21–100 and higher case loads. Other studies have shown a progressive association between higher case loads and survival, interpreted by some researchers as strengthening evidence of a causal relationship.28,37
The reasons for the association of survival with case load are not known. They may include effects of accompanying factors, such as degree of surgical specialisation, access to multidisciplinary conferencing, access to specialist adjunctive services, or other unspecified clinical factors.22 Also, there would be potential for confounding from differences in co-morbidity, but this could not be investigated with the data available.
Another possible explanation would be differences in primary course of care by case load. We consider investigation of effects of differences in treatment patterns is a complex undertaking beyond the scope of this initial study, warranting separate attention. Notably, the lower relative risks associated with higher case loads (>20 cases per annum) were less pronounced and no longer statistically significant after adjusting for treatment centre location, raising the possibility of confounding from treatment centre effects.
Minimum case loads have been proposed in the UK for performing breast cancer surgery as a quality safeguard.54 It is important that factors causing the lower relative risks observed in higher case load categories in this study be further investigated, including factors relating to treatment centre location.
Women treated in inner regional areas had lower survivals than those treated in major cities. This persisted after adjusting for age, clinical risk factors and surgeon case load. Supplementary analysis indicated that the percentage of patients receiving radiotherapy as part of the primary course of care was only slightly lower for inner regional than major city centres (i.e. 32% compared with 34% for mastectomy cases and 85% compared with 89% for cases having conservative breast surgery). Meanwhile, a similar proportion had systemic therapies (86% and 87% respectively). The reason for lower survivals in inner regional areas warrants further analysis. Although it would be expected that inner regional treatment centres may have less specialisation and lower access to multidisciplinary teams and specialist adjunctive services than centres in major cities, similar or greater differences might be expected in more remote areas, where adjusted survivals were similar to those for major cities.
Although lack of private health insurance can be a barrier to receiving appropriate breast and other cancer care in the USA,18,42 this disparity may not apply in Australia due to universal public insurance. The present results provide clear evidence of a lower unadjusted survival in breast cancer patients without private health insurance, but this difference was reduced, and was no longer statistically significant (P = 0.13), after adjusting for tumour size and other risk factors. Adjusting in addition for surgeon case load and treatment centre location further reduced differences in survival by private insurance status.
Conclusion
Although differences in risk of case fatality when case loads exceeded 20 per annum were reduced and not statistically significant after adjusting for treatment centre location, this difference warrants further investigation of causal factors, including possible differences in co-morbidity and centre location effects. In addition, the poorer outcome observed for women treated in inner regional centres compared with major city or remote centres requires further study.
Competing interests
The authors declare there are no competing interests.
Acknowledgements
The authors would like to acknowledge the National Breast Cancer Foundation for funding the study. National Breast and Ovarian Cancer Centre would like to acknowledge the input and advice from members of the Advisory Group established for the study. Members included Professor Dallas English and Ms Suellen Williams.
References
[1] Wang J, Boult M, Tyson S, Babidge W, Zorbas H, Kollias J, et al Trends in surgical treatment of younger patients with breast cancer in Australia and New Zealand. ANZ J Surg 2008; 78 665–9.| Trends in surgical treatment of younger patients with breast cancer in Australia and New Zealand.Crossref | GoogleScholarGoogle Scholar | 18796024PubMed |
[2] Wang J, Boult M, Roder D, Kollias J, Maddern G. Commentary: how surgical audits can be used to promote the update of surgical evidence. ANZ J Surg 2008; 78 437–8.
| Commentary: how surgical audits can be used to promote the update of surgical evidence.Crossref | GoogleScholarGoogle Scholar | 18522561PubMed |
[3] Marsh CJ, Boult M, Wang JX, Maddern GJ, Roder DM, Kollias J. National Breast Cancer Audit: the use of multidisciplinary care items by breast surgeons in Australia and New Zealand. Med J Aust 2008; 188 385–8.
| 18393739PubMed |
[4] Cuncins-Hearn A, Boult M, Babidge W, Zorbas H, Villanueva E, Evans A, et al National Breast Cancer Audit: ductal carcinoma in situ management in Australia and New Zealand. ANZ J Surg 2007; 77 64–8.
| National Breast Cancer Audit: ductal carcinoma in situ management in Australia and New Zealand.Crossref | GoogleScholarGoogle Scholar | 17295824PubMed |
[5] Cuncins-Hearn A, Boult M, Babidge W, Zorbas H, Villanueva E, Evans A, et al National Breast Cancer Audit: overview of invasive breast cancer management. ANZ J Surg 2006; 76 745–50.
| National Breast Cancer Audit: overview of invasive breast cancer management.Crossref | GoogleScholarGoogle Scholar | 16916399PubMed |
[6] Kelley E. Health, spending and the effort to improve quality in OECD countries: a review of the data. J R Soc Promot Health 2007; 127 64–71.
| Health, spending and the effort to improve quality in OECD countries: a review of the data.Crossref | GoogleScholarGoogle Scholar | 17402312PubMed |
[7] OECD Directorate for Employment, Labour and Social Affairs. OECD Health Care Quality Indicators and health data; 2009. Available at http://www.oecd.org/health/hcqi [verified June 2012].
[8] Reinhardt UE, Hussey PS, Anderson GF. Cross-national comparisons of health systems using OECD data, 1999. Health Aff (Millwood) 2002; 21 169–81.
| Cross-national comparisons of health systems using OECD data, 1999.Crossref | GoogleScholarGoogle Scholar |
[9] Miller J, Knott V, Wilson C, Cunningham J, Condon J, Roder D. Aboriginal and Torres Strait Islander cancer control research report. Canberra: Commonwealth of Australia; 2010.
[10] Tracey E, Baker D, Chen W, Stavrou E, Bishop J. Cancer in New South Wales: incidence, mortality and survival, 2005. Sydney: Cancer Institute NSW; 2007.
[11] Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ. et al. SEER cancer statistics review, 1975–2005. Bethesda: National Cancer Institute; 2008.
[12] Roder D, Wang JX, Zorbas H, Kollias J, Maddern G. Survival from breast cancers managed by surgeons participating in the National Breast Cancer Audit of the Royal Australasian College of Surgeons. ANZ J Surg 2010; 80 776–80.
| Survival from breast cancers managed by surgeons participating in the National Breast Cancer Audit of the Royal Australasian College of Surgeons.Crossref | GoogleScholarGoogle Scholar | 20969682PubMed |
[13] South ACR. Epidemiology of cancer in South Australia. Incidence, mortality and survival 1977 to 1999. Incidence and mortality 1999. Adelaide: Openbook Publishers; 2000.
[14] DeVita VT, Lawrence TS, Rosenberg SA, DePinho RA, Weinberg RA. Cancer: principles and practice of oncology. 8th ed. Philadelphia: Lippincott, Williams & Wilkins; 2008.
[15] Dabakuyo TS, Bonnetain F, Roignot P, Poillot ML, Chaplain G, Altwegg T, et al Population-based study of breast cancer survival in Cote d’Or (France): prognostic factors and relative survival. Ann Oncol 2008; 19 276–83.
| Population-based study of breast cancer survival in Cote d’Or (France): prognostic factors and relative survival.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1c%2FotlemsQ%3D%3D&md5=56a866bd6acdeeb424fc23a113c5c5dbCAS | 17962200PubMed |
[16] Ingram DM, McEvoy SP, Byrne MJ, Fritschi L, Joseph DJ, Jamrozik K. Surgical caseload for women with invasive breast cancer treated in Western Australia. Breast 2005; 14 11–7.
| Surgical caseload for women with invasive breast cancer treated in Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2M%2FmsFKktg%3D%3D&md5=771e78cb8eb0b7b4c62ff4792c6587b5CAS | 15695075PubMed |
[17] Twelves CJ, Thomson CS, Gould A, Dewar JA. Variation in the survival of women with breast cancer in Scotland. The Scottish Breast Cancer Focus Group and The Scottish Cancer Therapy Network. Br J Cancer 1998; 78 566–71.
| Variation in the survival of women with breast cancer in Scotland. The Scottish Breast Cancer Focus Group and The Scottish Cancer Therapy Network.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1cvhsFOnsw%3D%3D&md5=35f746c9c8d3f7f23515973e18ad89e4CAS | 9744492PubMed |
[18] Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med 1993; 329 326–31.
| The relation between health insurance coverage and clinical outcomes among women with breast cancer.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3szgvFGitg%3D%3D&md5=bf6b8658b79450ea68f075bd9a3e1802CAS | 8321261PubMed |
[19] Hölscher AH, Metzger R, Brabender J, Vallbohmer D, Bollschweiler E. High-volume centers – effect of case load on outcome in cancer surgery. Onkologie 2004; 27 412–6.
| High-volume centers – effect of case load on outcome in cancer surgery.Crossref | GoogleScholarGoogle Scholar | 15347901PubMed |
[20] Simons AJ, Ker R, Groshen S, Gee C, Anthone GJ, Ortega AE, et al Variations in treatment of rectal cancer: the influence of hospital type and caseload. Dis Colon Rectum 1997; 40 641–6.
| Variations in treatment of rectal cancer: the influence of hospital type and caseload.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2szjslOlsA%3D%3D&md5=6c43f29b9df6d88713053a88441a39a1CAS | 9194456PubMed |
[21] Kee F, Wilson RH, Harper C, Patterson CC, McCallion K, Houston RF, et al Influence of hospital and clinician workload on survival from colorectal cancer: cohort study. BMJ 1999; 318 1381–6.
| Influence of hospital and clinician workload on survival from colorectal cancer: cohort study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M3mslemtQ%3D%3D&md5=942626a8a424be77affd1f65200f5037CAS | 10334746PubMed |
[22] Gruen RL, Pitt V, Green S, Parkhill A, Campbell D, Jolley D. The effect of provider case volume on cancer mortality: systematic review and meta-analysis. CA Cancer J Clin 2009; 59 192–211.
| The effect of provider case volume on cancer mortality: systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 19414631PubMed |
[23] Schrag D, Panageas KS, Riedel E, Cramer LD, Guillem JG, Bach PB, et al Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg 2002; 236 583–92.
| Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection.Crossref | GoogleScholarGoogle Scholar | 12409664PubMed |
[24] Bilimoria KY, Bentrem DJ, Feinglass JM, Stewart AK, Winchester DP, Talamonti MS, et al Directing surgical quality improvement initiatives: comparison of perioperative mortality and long-term survival for cancer surgery. J Clin Oncol 2008; 26 4626–33.
| Directing surgical quality improvement initiatives: comparison of perioperative mortality and long-term survival for cancer surgery.Crossref | GoogleScholarGoogle Scholar | 18574159PubMed |
[25] Nathan H, Cameron JL, Choti MA, Schulick RD, Pawlik TM. The volume-outcomes effect in hepato-pancreato-biliary surgery: hospital versus surgeon contributions and specificity of the relationship. J Am Coll Surg 2009; 208 528–38.
| The volume-outcomes effect in hepato-pancreato-biliary surgery: hospital versus surgeon contributions and specificity of the relationship.Crossref | GoogleScholarGoogle Scholar | 19476786PubMed |
[26] Salz T, Sandler RS. The effect of hospital and surgeon volume on outcomes for rectal cancer surgery. Clin Gastroenterol Hepatol 2008; 6 1185–93.
| The effect of hospital and surgeon volume on outcomes for rectal cancer surgery.Crossref | GoogleScholarGoogle Scholar | 18829393PubMed |
[27] Hill JS, Zhou Z, Simons JP, Ng SC, McDade TP, Whalen GF, et al A simple risk score to predict in-hospital mortality after pancreatic resection for cancer. Ann Surg Oncol 2010; 17 1802–7.
| A simple risk score to predict in-hospital mortality after pancreatic resection for cancer.Crossref | GoogleScholarGoogle Scholar | 20155401PubMed |
[28] Roohan PJ, Bickell NA, Baptiste MS, Therriault GD, Ferrara EP, Siu AL. Hospital volume differences and five-year survival from breast cancer. Am J Public Health 1998; 88 454–7.
| Hospital volume differences and five-year survival from breast cancer.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c7nvFCrtA%3D%3D&md5=0928a071ec8d4aca243692fb59f3a533CAS | 9518982PubMed |
[29] Skinner KA, Helsper JT, Deapen D, Ye W, Sposto R. Breast cancer: do specialists make a difference? Ann Surg Oncol 2003; 10 606–15.
| Breast cancer: do specialists make a difference?Crossref | GoogleScholarGoogle Scholar | 12839844PubMed |
[30] Sainsbury R, Haward B, Rider L, Johnston C, Round C. Influence of clinician workload and patterns of treatment on survival from breast cancer. Lancet 1995; 345 1265–70.
| Influence of clinician workload and patterns of treatment on survival from breast cancer.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M3msVyitQ%3D%3D&md5=a745002b8624af45f81397fcf71871f9CAS | 7746056PubMed |
[31] Stefoski Mikeljevic J, Haward RA, Johnston C, Sainsbury R, Forman D. Surgeon workload and survival from breast cancer. Br J Cancer 2003; 89 487–91.
| Surgeon workload and survival from breast cancer.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3szotVeqsw%3D%3D&md5=d4b43fe741e1f14a8aabce17a182fca7CAS | 12888817PubMed |
[32] Hiotis K, Skinner KA. Volume-outcome relationships in breast cancer. Breast Cancer 2004; 7 8
| Volume-outcome relationships in breast cancer.Crossref | GoogleScholarGoogle Scholar |
[33] Scharl A, Gohring U-J. Does center volume correlate with survival from breast cancer? Breast Care 2009; 4 237–44.
| Does center volume correlate with survival from breast cancer?Crossref | GoogleScholarGoogle Scholar | 20877661PubMed |
[34] Bailie K, Dobie I, Kirk S, Donnelly M. Survival after breast cancer treatment: the impact of provider volume. J Eval Clin Pract 2007; 13 749–57.
| Survival after breast cancer treatment: the impact of provider volume.Crossref | GoogleScholarGoogle Scholar | 17824868PubMed |
[35] Wishart GC, Greenberg DC, Chou P, Brown CH, Duffy S, Purushotham AD. Treatment and survival in breast cancer in the Eastern Region of England. Ann Oncol 2010; 21 291–6.
| Treatment and survival in breast cancer in the Eastern Region of England.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c%2Fls1aiug%3D%3D&md5=e508cea8e2bc95117d5764ee011cd13eCAS | 19502647PubMed |
[36] Harcourt KF, Hicks K. Is there a relationship between case volume and survival in breast cancer? Am J Surg 2003; 185 407–10.
| Is there a relationship between case volume and survival in breast cancer?Crossref | GoogleScholarGoogle Scholar | 12727557PubMed |
[37] Oberaigner W, Stuhlinger W. Influence of department volume on cancer survival for gynaecological cancers – a population-based study in Tyrol, Austria. Gynecol Oncol 2006; 103 527–34.
| Influence of department volume on cancer survival for gynaecological cancers – a population-based study in Tyrol, Austria.Crossref | GoogleScholarGoogle Scholar | 16730055PubMed |
[38] Lee-Feldstein A, Anton-Culver H, Feldstein PJ. Treatment differences and other prognostic factors related to breast cancer survival. Delivery systems and medical outcomes. JAMA 1994; 271 1163–8.
| Treatment differences and other prognostic factors related to breast cancer survival. Delivery systems and medical outcomes.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2c3gt1Cjug%3D%3D&md5=8a9a7ac1617f8fe41773de1c98c86f61CAS | 8151873PubMed |
[39] Polednak AP. Hospital volume and survival of breast cancer patients in Connecticut. Am J Public Health 1999; 89 946–7.
| Hospital volume and survival of breast cancer patients in Connecticut.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M3os1OntA%3D%3D&md5=2d2ea16aab76c0c5d282a87fd208fa64CAS | 10358694PubMed |
[40] Chen CS, Liu TC, Lin HC, Lien YC. Does high surgeon and hospital surgical volume raise the five-year survival rate for breast cancer? A population-based study. Breast Cancer Res Treat 2008; 110 349–56.
| Does high surgeon and hospital surgical volume raise the five-year survival rate for breast cancer? A population-based study.Crossref | GoogleScholarGoogle Scholar | 17874183PubMed |
[41] Greene FL. Is case volume the only surrogate for oncological surgical quality? Ann Surg Oncol 2008; 15 14–5.
| Is case volume the only surrogate for oncological surgical quality?Crossref | GoogleScholarGoogle Scholar | 18004625PubMed |
[42] Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P, et al Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin 2008; 58 9–31.
| Association of insurance with cancer care utilization and outcomes.Crossref | GoogleScholarGoogle Scholar | 18096863PubMed |
[43] NHMRC National Breast Cancer Centre. Clinical practice guidelines for the management of early breast cancer. 1st ed. Canberra: NHMRC; 1995.
[44] National Breast and Ovarian Cancer Centre & Royal Australasian College of Surgeons. National Breast Cancer Audit. Public Health Monitoring Series, 2007 data. Sydney: National Breast and Ovarian Cancer Centre, Royal Australasian College of Surgeons & National Breast Cancer Foundation; 2009.
[45] Armitage P, Berry G. Statistical methods in medical research. Oxford: Blackwell Scientific Publications; 1987.
[46] South Australian Cancer Registry. Cancer in South Australia 2004 with incidence projections to 2007. Adelaide: South Australian Department of Health; 2007.
[47] South Australian Cancer Registry. Epidemiology of cancer in South Australia. Incidence, mortality and survival 1977 to 1996. Incidence and mortality 1996. Adelaide: Openbook Publishers; 1997.
[48] New South Wales Cancer Institute. NSW Cancer Registry statistical reporting, 2004–2008. Sydney: NSW Cancer Institute; 2010.
[49] Australian Institute of Health and Welfare & Australasian Association of Cancer Registries. Cancer in Australia: an overview, 2010. Cancer series no. 60. Cat. no. CAN 56. Canberra: Commonwealth of Australia; 2010.
[50] Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al., editors. SEER cancer statistics review, 1975–2006. Bethesda MD: National Cancer Institute; 2009.
[51] Office for National Statistics. Breast cancer survival, England and Wales, 1991–2003. London: Office for National Statistics; 2005.
[52] Berrino F, De Angelis R, Sant M, Rosso S, Bielska-Lasota M, Coebergh JW, et al Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–1999: results of the EUROCARE-4 study. Lancet Oncol 2007; 8 773–83.
| Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–1999: results of the EUROCARE-4 study.Crossref | GoogleScholarGoogle Scholar | 17714991PubMed |
[53] Australian Institute of Health and Welfare & National Breast and Ovarian Cancer Centre. Breast cancer in Australia: an overview, 2009. Cancer series no. 50. Cat. no. CAN 46. Canberra: Commonwealth of Australia; 2009.
[54] Cancer Guidance Sub-Group of the Clinical Outcomes Group. Guidance for Purchasers: Improving Outcomes in Breast Cancer – The Manual. London: Department of Health, HMSO; 1996.



