Costs of paying higher prices for equivalent effects on the Pharmaceutical Benefits Scheme
Jonathan Karnon A C , Laura Edney A and Michael Sorich BA School of Public Health, University of Adelaide, Level 7, 178 North Terrace, Adelaide, SA 5005, Australia. Email: laura.edney@adelaide.edu.au
B School of Medicine, Flinders University, GPO Box 2100, Adelaide, SA 5001, Australia. Email: michael.sorich@flinders.edu.au
C Corresponding author. Email: jonathan.karnon@adelaide.edu.au
Australian Health Review 41(1) 1-6 https://doi.org/10.1071/AH15122
Submitted: 26 June 2015 Accepted: 1 February 2016 Published: 9 March 2016
Journal Compilation © AHHA 2017 Open Access CC BY-NC-ND
Abstract
Objective The aims of the present study were to illustrate and discuss the effects of the non-maintenance of equivalent prices when the comparators of pharmaceuticals listed on the Pharmaceutical Benefits Schedule (PBS) on a cost-minimisation basis come off-patent and are subject to statutory price reductions, as well as further potential price reductions because of the effects of price disclosure.
Methods Service use, benefits paid, and price data were analysed for a selected sample of pharmaceuticals recommended for listing on a cost-minimisation basis between 2008 and 2011, and their comparators, to estimate the cost savings to the PBS of maintaining equivalent prices.
Results Potential cost savings for 12 pharmaceuticals, including alternative compounds and combination products across nine therapeutic groups, ranged from A$570 000 to A$40 million to April 2015. Potential savings increased significantly following recent amendments to the price disclosure process.
Conclusions Potential savings from maintaining equivalent prices for all pharmaceuticals listed on the PBS on a cost-minimisation basis could be over A$500 million per year. Actions to reduce these costs can be taken within existing policy frameworks, but legislative and political barriers may need to be addressed to minimise these costs, which are incurred by the taxpayer for no additional benefit.
What is known about the topic? Pharmaceuticals listed on the PBS must provide value for money. Many pharmaceuticals achieve this by demonstrating equal effectiveness to an already listed pharmaceutical and requesting the same price as this comparator; that is, listing on a cost-minimisation basis. When the comparator moves off-patent, the price of the still-patented pharmaceutical is protected, whereas the off-patent drug is subject to price disclosure and often steep price reductions.
What does this paper add? This paper adds to recent evidence on the costs to government of paying different prices for two or more pharmaceuticals that are equally effective. Between 2008 and 2011, the direct comparators for 68 pharmaceuticals listed on a cost-minimisation basis have moved onto the price disclosure list. Across 12 of these listings, the potential cost savings in the 10 months to April 2015 were A$73 million.
What are the implications for practitioners? The PBS costs the Australian government over A$9 billion per year. Annual savings over A$500 million per year could be achieved by maintaining cost-minimisation across equally effective pharmaceuticals. This would improve the efficiency of the PBS at no risk to patients. Legislation is required to remove the existing F1 and F2 categorisation of listed pharmaceuticals, but the proposed changes would remove the need for therapeutic group premiums and simplify the pricing of PBS items.
Introduction
The Pharmaceutical Benefits Schedule (PBS) in Australia lists all the medicines available to be dispensed to patients at a government-subsidised price. A requirement for the listing of a new pharmaceutical on the PBS is that the pharmaceutical provides value for money, relative to a comparator, that is the therapy likely to be most replaced by prescribers in practice. Sponsors of new pharmaceuticals make submissions to the Pharmaceutical Benefits Advisory Committee (PBAC), which reviews the evidence and makes a recommendation to the Minister for Health regarding the listing of proposed pharmaceuticals.
New pharmaceuticals can be listed on either a cost-minimisation or a cost-effectiveness basis. Pharmaceuticals listed on a cost-minimisation basis have demonstrated equivalence of effect with regard to a relevant comparator, and their price is generally set such that the costs are also equivalent between the listed pharmaceutical and its comparator. The prices of pharmaceuticals listed on the basis of cost-effectiveness are set such that demonstrated additional benefits against a relevant comparator are achieved at an acceptable additional cost to the government.
The PBS includes an F1 formulary for on-patent, single-brand pharmaceuticals and an F2 formulary for off-patent pharmaceuticals that have multiple brands. In most cases, comparators are listed on the F1 formulary when the value of a new pharmaceutical is being assessed, but the comparator will generally come off-patent and move onto the F2 formulary before the more recently listed pharmaceutical.
A statutory price reduction of 16% is applied to PBS-listed products when the first new brand or item that is bioequivalent or biosimilar and has the same manner of administration as an existing brand or item lists on the PBS. Moreover, off-patent pharmaceuticals are subject to competition from generic versions of the pharmaceutical, which can further reduce the listed price through price disclosure. Under price disclosure, pharmaceutical companies report the price at which medicines are supplied to pharmacies, and the PBS then funds the original pharmaceutical and its generic versions at the average price paid by pharmacies.
Prices are not referenced between these two categories; thus, a new pharmaceutical on the F1 formulary cannot be linked to the price of its comparator when this declines following recategorisation onto the F2 formulary. This increases the price difference between the newer pharmaceutical and its comparator, leading to the possibility that the newer pharmaceutical no longer provides value for money at the price at which it was originally listed.
This paper illustrates and discusses the effect of the non-maintenance of equivalent prices when the comparators of pharmaceuticals listed on the PBS on a cost-minimisation basis move onto the F2 formulary, and the cost savings that could have been achieved if the prices of those pharmaceuticals had declined at the same rate as their comparators.
Methods
Public summary documents describing all positive recommendations made by the PBAC in 2008, 2009, 2010 and 2011 (available from http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd, accessed 25 June 2015) were reviewed to identify pharmaceuticals that were recommended for listing on the PBS on a cost-minimisation basis. For each pharmaceutical identified, data describing the annual number of services and the annual costs for each relevant PBS code for the recommended and comparator pharmaceuticals were extracted from the PBS statistics website (http://medicarestatistics.humanservices.gov.au/statistics/pbs_item.jsp, accessed 25 June 2015). Data from July 1 2008 to April 30 2015 were extracted. The dispensed price for maximum quantity (DPMQ) in the first month of listing and on July 1 of each subsequent year was extracted from the PBS Publications Archive for each constituent PBS code for identified pharmaceutical and their comparators (http://www.pbs.gov.au/info/publication/schedule/archive, accessed 25 June 2015). Average annual prices for selected pharmaceuticals and their comparators were estimated as the price of each constituent PBS code in the first observed month of each financial year, weighted by the proportion of services for each code.
The price and volume data for the full set of pharmaceuticals identified were reviewed to select case study pharmaceuticals for which robust estimates of the potential cost savings of maintaining equivalent prices could be generated. The main exclusion criterion was difficulty defining the relevant PBS codes for the recommended or comparator pharmaceutical for the indication of interest because the pharmaceuticals were listed across many PBS codes for a range of indications.
For cases in which the comparator was an alternative compound, the annual average price for the comparator was estimated using the relativities recommended by the PBAC (http://www.pbs.gov.au/info/industry/pricing/pbs-items/therapeutic-relativity-sheets, accessed 25 June 2015). For both the recommended and comparator pharmaceuticals, a price index was estimated for each year subsequent to the initial listing as the ratio of the average price in each year to the price at the time of the initial listing.
Annual relative price differentials between the recommended and comparator pharmaceuticals were estimated as the indexed price of the comparator pharmaceutical divided by the indexed price of the recommended pharmaceutical. The annual potential cost savings from maintaining cost-minimisation were estimated using the following formula:
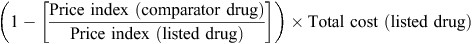
For combination products, the annual equivalent prices of the constituent components of each listed formulation of a combination product were estimated. The absolute differences in the prices of each listed combination product and the sum of its constituent components were estimated. The absolute price differences were multiplied by the annual numbers of services of the respective formulations for each combination product.
Results
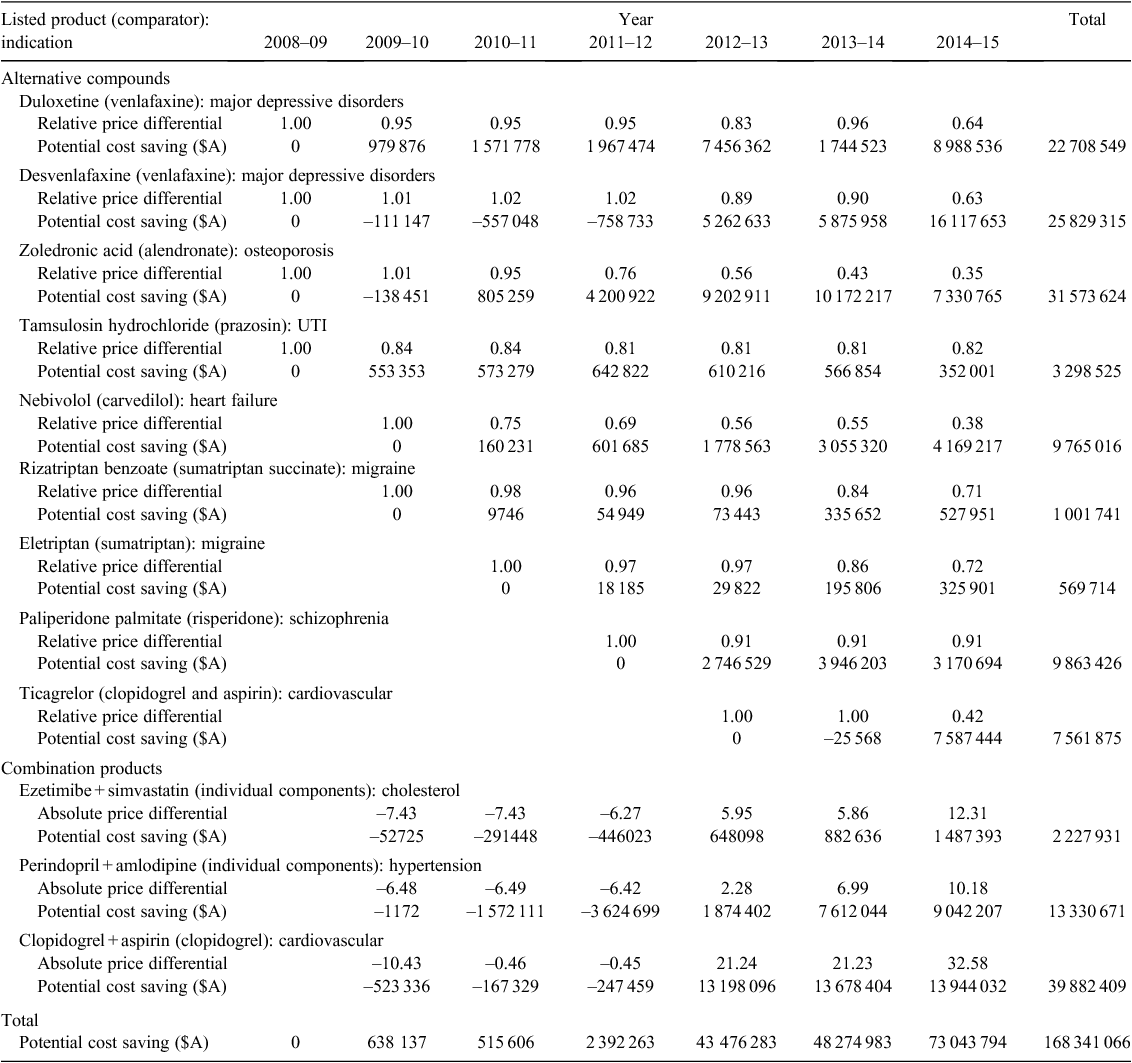
In the 4 years from 2008 to 2011, the direct comparator of 68 pharmaceutical products listed on the PBS on a cost-minimisation basis had moved onto the price disclosure list by April 2015. Twelve of the 68 pharmaceuticals were selected to illustrate the potential cost saving from maintaining cost-minimisation in the present study. Nine were compared with an alternative compound, whereas three involved the comparison of a combination product against the individual components of the product. The listings covered a range of therapeutic areas, although five were connected to some form of heart disease and three had a mental health-related indication.
Details of the potential cost saving across the 12 pharmaceuticals are given in Table 1, being A$168 million to April 2015, with the annual cost savings increasing from A$600 000 in 2009–10 to over A$73 million in the 10 months to April 2015. The largest individual potential cost saving was estimated for a combination product (clopidogrel with aspirin), which was initially listed at a lower price than its comparator (clopidogrel alone). However, by July 2014 the unit cost of the combination product was A$34 higher, and the potential cost saving in the incomplete 2014–15 financial year was almost A$14 million.
Among the listings involving an alternative compound, ongoing price equivalence for duloxetine, desvenlafaxine, and zoledronic acid could have saved the PBS over A$80 million over 5 years and A$32 million between July 2014 and April 2015 alone.
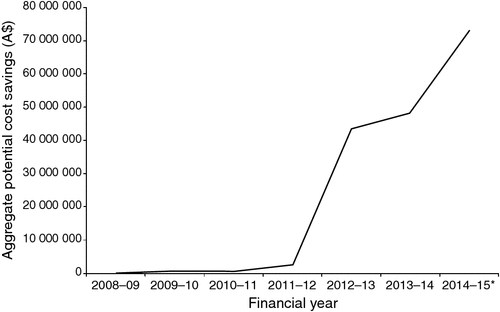
Figure 1 shows the growth in the aggregate annual potential savings, which increases rapidly as the comparator pharmaceuticals come off-patent.
|
Discussion
This study has estimated potential savings to the PBS, and the Australian Government, of maintaining equivalent prices for pharmaceuticals listed on a cost-minimisation basis and their comparators. Price differentials occur over time as comparator pharmaceuticals come off-patent and are subject to competition from generics and price disclosure, whereas the more recently listed pharmaceuticals remain on patent and are protected from price competition. If the price of the comparator pharmaceutical alone drops, then by definition the listed pharmaceutical has equal effects but is more costly, and so is no longer providing value for money, a criterion for being listed on the PBS.
This study has estimated the effects of price disclosure on the ongoing value for money of 12 of 68 pharmaceuticals listed on the PBS on a cost-minimisation basis between 2008 and 2011 for which the comparator has since moved onto the price disclosure list. The potential cost saving was A$168 million, of which A$73 million (43%) could have been saved between July 2014 and April 2015.
The potential savings are not restricted to patented pharmaceuticals following the movement of their comparators onto the price disclosure list. The case studies presented herein demonstrate the effects of the loss of cost-minimisation between combination products and their individual components, which is made possible by the marketing of multiple brands of the same combination product.1 The combination and individual component products are on the price disclosure list, but separate price reductions are estimated for each product. The price of the three combination products reported herein decreased at lower rates than at least one of their individual components, leading to potential cost savings of almost A$25 million in the incomplete financial year to April 2015. Combination products may potentially provide more value than their individual components (e.g. by improved adherence), but such claims were the not the basis for the proposed listing of the reviewed products on a cost-minimisation basis.
Similarly, cost-minimisation cannot be guaranteed for pharmaceuticals listed on a cost-minimisation basis against alternative compounds as they join their comparators on the price disclosure list. As an example, on 1 July 2014, duloxetine cost A$13 more than the equivalent service item of its comparator venlafaxine, resulting in potential cost savings of A$1 million per month.
Actual cost savings from maintaining cost-minimisation will be significantly higher than those reported herein. First, cost savings for the remaining 56 of the full set of 68 pharmaceuticals were not reported because of uncertainties around the estimation of equivalent prices; there would likely be significantly higher cost savings from maintaining cost-minimisation across the 68 pharmaceuticals. As an example, pharmaceuticals with significant cost offsets were not selected, but the potential cost savings for these listings is likely to be substantial. In the year following the listing of the powder-based chemotherapy nab-paclitaxel, the price of the solvent-based comparator decreased by 20% while the price of nab-paclitaxel remained constant. Over A$13 million was spent on nab-paclitaxel in that year.
Second, there are additional listings on a cost-minimisation basis for which the comparator has not moved onto the price disclosure list, but where the comparator to the comparator is now on the price disclosure list. An example is denosumab, for the treatment of osteoporosis, which was compared with zoledronic acid, which was compared with alendronate (Table 1). Applying the price indices for alendronate to denosumab (which has maintained its originally listed price), A$75 million of the A$115 million spent on denosumab between July 2014 and April 2015 could have been saved. This process of comparing new pharmaceuticals to listed products that are still on patent while older comparators are off-patent and subject to price disclosure has been particularly prevalent for diabetes medications. A new product (rosiglitazone) was listed on a cost-minimisation basis against insulin in 2003. While rosiglitazone remained on patent, subsequent submissions for diabetic medications used rosiglitazone as a comparator (e.g. for the listing of sitagliptin in 2008), but more recent submissions have cost minimised against products that cost minimised against rosiglitazone (e.g. sitagliptin was the comparator for the listing of linagliptin in 2011). This illustrates the potential for progressive cycles of fathering (using the comparator of a comparator), grandfathering (using the comparator of a comparator of a comparator), great grandfathering etc., in which new products are always cost minimised against an on-patent product.
Third, cost savings have been increasing over time (e.g. the cost savings reported herein increased by 52% between 2013 and 2014 and the incomplete financial year to April 2015). The larger effects are a result of the simplified price disclosure amendment, which reduced the time between price disclosure cycles, increasing the rate of decline in the prices of off-patent pharmaceuticals.2
Fourth, pharmaceuticals listed on a cost-minimisation basis, both before 2008 and after 2011, will have potential cost savings as their comparators move onto the price disclosure list.
Finally, this paper has focused on the value for money of pharmaceuticals listed on the PBS on a cost-minimisation basis. The value for money of pharmaceuticals listed on the basis of cost-effectiveness (i.e. pharmaceuticals that provide additional benefits at an acceptable additional cost) will also decline as comparator pharmaceuticals come off-patent. Other consequences include that pharmaceuticals that are subject to declining prices may become cost-effective for a broader population than the indication for which the pharmaceutical was originally listed on the PBS. There are also potential knock-on effects with regard to the value or cost-effectiveness of programs to improve uptake and adherence, which also become more cost-effective as drug prices decrease.
Potential policy solutions
The therapeutic group premium policy applies to therapeutic groups for which multiple pharmaceuticals of similar safety and health outcomes are listed on the PBS, such that the government subsidises up to the price of the lowest-priced drug in the group. If a more expensive drug is prescribed, the patient pays the therapeutic group premium, which is the price difference between the lowest-price drug and the drug prescribed. The policy excludes pharmaceuticals that are subject to price disclosure, and currently these premiums are applied to only two therapeutic groups.
A recent overlapping analysis of the therapeutic group premium policy estimated that the government could save A$320 million per year from the intended application of the policy to five cardiovascular therapeutic groups and to venlafaxine and its derivatives.3 Alternative methods were applied, but similar cost savings are estimated for common elements (e.g. venlafaxine and its derivatives), providing some cross-analysis validation. Neither analysis is exhaustive, but the non-common elements provide complementary data to reinforce the magnitude of the potential savings.
Duckett and Breadon propose maintaining the therapeutic group premium, but expanding the number of therapeutic groups so that pharmaceuticals that are on the price disclosure list are included and premiums are estimated based on dispensing data.3 Another option could focus on maintaining cost-minimisation between F1 and F2 formularies. Other than the first in class, all equally effective pharmaceuticals for which a therapeutic group may be defined are listed on a cost-minimisation basis. A commitment to maintain cost-minimisation as direct and indirect comparator pharmaceuticals move onto the price disclosure list is a more intuitive approach that does not negate the option of paying a premium for patients with a legitimate need for a more highly priced, equally effective (at a population level) pharmaceutical.
An alternative approach is to stop listing pharmaceuticals on the basis of cost-minimisation or to define indications that restrict the use of equally effective pharmaceuticals to patients with adverse reactions to the primary pharmaceutical within a therapeutic group. In New Zealand, the Pharmaceutical Management Agency (PHARMAC) funds far fewer ‘me too’ pharmaceuticals that are in an existing therapeutic class.4 This policy option would also reduce the sequential use of tolerated, equally effective pharmaceuticals funded by the PBS. The effectiveness of equally effective pharmaceuticals used in sequence is generally not known, but it may be reasonable to assume that a pharmaceutical will be less effective in patients for whom an equally effective therapy was not effective than in new patients. Pharmaceuticals listed on a cost-minimisation basis that are used sequentially are not likely to be providing value for money.
Another alternative is to rely on policies around the Quality Use of Medicines,5 as a component of the government’s National Medicines Policy, to promote the appropriate use of medicines. Rational, fully informed clinicians, whose treatment decisions reflect societal perspectives and opportunity costs, may refrain from prescribing higher-priced equally effective pharmaceuticals. In practice, these conditions are not being met and, as demonstrated in the present study, the taxpayer is often paying for the more expensive of multiple, equally effective treatments. This may be explained, in part, by producer incentives: manufacturers producing the higher-priced equally effective pharmaceutical have a greater incentive to promote the use of their product.
There are legislative and potential political barriers to price indexing pharmaceuticals listed on the PBS on a cost-minimisation basis. Legislation that effectively safeguarded the price of pharmaceuticals when their comparator goes off-patent was introduced with the National Health Amendment (Pharmaceuticals Benefits Scheme) Act (Cth) in 2007. The PBS formulary was split into F1 and F2 categories, comprising mostly patented and non-patented pharmaceuticals, respectively. Reference pricing between the two categories was precluded, which means that once a new pharmaceutical is listed on the PBS in the F1 category, its price cannot be linked to the price of its comparator if the comparator moves to the F2 category.6
Although the above amendments to the National Health Act 1953 (Cth) are not formally linked to the Australia–US Free Trade Agreement (AUSFTA), the AUSFTA reflects the principal political barrier to any legislative amendments to maintaining cost-minimisation between patented and non-patented equally effective pharmaceuticals. The patented pharmaceutical industry maintains a high profile and effective lobby in the US and Australia that focuses on the need to maintain high pharmaceutical prices to support future innovation.7 This is despite strong evidence that a price premium for pharmaceuticals does not improve health system efficiency in the short or long term.8
The Trans-Pacific Partnership Agreement has not been finalised, but although it appears that Australia has successfully opposed longer monopoly rights for pharmaceuticals, the agreement seems to lock in existing monopoly rights.
The patented pharmaceutical industry’s argument against ‘price controls and reference pricing’ is that they prevent valuing pharmaceutical innovation through the operation of ‘competitive markets’.9 In cases where two or more alternative pharmaceuticals have the same effect on the same condition in the same patients, the patent status of a pharmaceutical is not relevant. In a competitive market, the two or more pharmaceuticals with similar effects would compete for market share, driving down the price of both products. Price disclosure acts to uncover the value of pharmaceuticals in the competitive market for the supply of off-patent pharmaceuticals, but no corresponding approach is used to replicate the effects of competition on the price of an on-patent product when an equally effective comparator goes off-patent.
Currently, listings on the PBS are only reviewed if a new submission is made by the sponsor of a product, which is rare, and only occurs when the sponsor believes new data will support an increase in the price of a listed pharmaceutical. A formal review process of selected, high-impact drugs could incorporate changes in the relative costs of comparator pharmaceuticals, as well as incorporating any new clinical data to update estimates of cost-effectiveness for the original indication as well as new indications and programs.
In England, the National Institute for Health and Care Excellence (NICE) specifies a review date for evaluated technologies, to update decisions on the basis of new clinical and economic data. In the absence of new data, the review is expedited. If significant new data are available, the process of review is facilitated by independent technology appraisal groups. Similar groups exist in Australia that currently review submissions to PBAC, but could also undertake updated cost-effectiveness analyses of selected pharmaceuticals.
Conclusion
Pharmaceuticals listed on the PBS must demonstrate value for money. Pharmaceuticals listed on a cost-minimisation basis are determined to have the same effect as a pharmaceutical that is already listed on the PBS, and so they can charge an equivalent price. This study has illustrated the loss of value for pharmaceuticals listed on the PBS on a cost-minimisation basis as their comparators move onto the F2 formulary and are subject to statutory price reductions and price disclosure.
The present study, combined with other recent analyses of the effects of inefficient pricing on the PBS,3 suggests that the annual savings from addressing the issue of the non-maintenance of equivalent prices for all pharmaceuticals with equivalent effects could easily be over A$500 million.
Competing interests
JK and MS sit on the Economic Sub-Committee, which advises the Pharmaceutical Benefits Advisory Committee on the technical merits of submissions for the listing of pharmaceuticals on the Pharmaceutical Benefits Schedule.
References
[1] Clarke PM, Avery AB. Evaluating the costs and benefits of using combination therapies. Med J Aust 2014; 200 518–20.| Evaluating the costs and benefits of using combination therapies.Crossref | GoogleScholarGoogle Scholar | 24835707PubMed |
[2] Clarke P. Pharmaceuticals, pharmacists and profits: a health policy perspective. Aust Prescr 2014; 37 148–9.
| Pharmaceuticals, pharmacists and profits: a health policy perspective.Crossref | GoogleScholarGoogle Scholar |
[3] Duckett S, Breadon P. Premium policy? Fixing the policy for switching drugs. Melbourne: Grattan Institute; 2015.
[4] Babar Z, Vitry A. Differences in Australian and New Zealand medicines funding policies. Aust Prescr 2014; 37 150–1.
| Differences in Australian and New Zealand medicines funding policies.Crossref | GoogleScholarGoogle Scholar |
[5] Australian Government Department of Health Quality Use of Medicines (QUM). Available at: http://www.health.gov.au.proxy.library.adelaide.edu.au/internet/main/publishing.nsf/Content/nmp-quality.htm [verified 25 June 2015].
[6] Faunce T, Bai J, Nguyen D. Impact of the Australia–US Free Trade Agreement on Australian medicines regulation and prices. J Gene Med 2010; 7 18–29.
| Impact of the Australia–US Free Trade Agreement on Australian medicines regulation and prices.Crossref | GoogleScholarGoogle Scholar |
[7] Jena AB, Philipson TJ. Cost-effectiveness analysis and innovation. J Health Econ 2008; 27 1224–36.
| Cost-effectiveness analysis and innovation.Crossref | GoogleScholarGoogle Scholar | 18619695PubMed |
[8] Pekarsky BAK. The new drug reimbursement game: a regulator’s guide to playing and winning. Auckland: Springer; 2015.
[9] Faunce TA. Reference pricing for pharmaceuticals: is the Australia–United States Free Trade Agreement affecting Australia’s Pharmaceutical Benefits Scheme? Med J Aust 2007; 187 240–2.
| 17564579PubMed |