Plant-macrofossil assemblages during Pliocene uplift, South Island, New Zealand
Mike PoleCentre for Marine Studies, University of Queensland, St Lucia, Brisbane, Qld 4072, Australia. Email: mpole@marine.uq.edu.au
Australian Journal of Botany 55(2) 118-142 https://doi.org/10.1071/BT06055
Submitted: 21 March 2006 Accepted: 14 November 2006 Published: 16 March 2007
Abstract
Organically preserved plant macrofossils, which accumulated during the period of late Neogene tectonic uplift, were documented from four localities in the South Island. These include Arapito Road (near Karamea), Waitahu River (near Reefton), Tadmor (south of Nelson) and Grey River (north of Christchurch). The assemblages from these localities were species-poor compared with older Cenozoic assemblages, but included a range of conifers and angiosperms. Of note was the presence of Acmopyle (currently extinct in New Zealand) and Cupressaceae in all four localities. At least two new species of Acmopyle were present, with leaf shapes distinctly different from any currently known. One of them (A. kirrileeae sp. nov.) had unflattened, awl-like foliage, whereas the other (A. biformis sp. nov.) had dimorphic foliage, including very distinct bilaterally flattened leaves with a mucronate apex. Both of these were distinct from the flattened foliage, which predominates on extant Acmopyle. Other conifers included Araucaria, Dacrycarpus, Dacrydium, Phyllocladus and Libocedrus. Angiosperms included Beauprea (now extinct in New Zealand) Beilschmiedia, Knightia sp., Metrosideros, Nothofagus and probably Pseudowintera, Pseudopanax and Cunoniaceae. The assemblages suggest temperate conditions.
Acknowledgements
The support of the School of Life Sciences and the Centre for Microscopy and Microanalysis, University of Queensland, and the Queensland Herbarium and the specimens of Pseudowintera supplied by the Herbarium of the Botany Department, Otago University, are gratefully acknowledged.
Beu AG
(2001) Local stages to be used for the Wanganui Series (Pliocene–Pleistocene), and their means of definition. New Zealand Journal of Geology and Geophysics 44, 113–125.
described L. bidwillii Hook. f. as having leaves which are homomorphic and non-papillate at the base, whereas L. plumosa (D.Don) Sarg. has dimorphic leaves which are papillate at the base. Hill and Carpenter (1989) illustrated L. plumosa with ‘poor development’ of papillae.
Libocedrus from Grey River (Fig. 4C, D)
Reference specimen and locality: SL1097 (Grey-1).
Description
Leaf arrangement of opposite pairs of facial and lateral leaves. Leaves scale-like. Stomatal complexes monocyclic; encyclocytic with five or six subsidiary cells; networked, length 70–105 µm; width 33–48 µm; longitudinally oriented; also with a further one or two subdued papillae. Epidermal cells typically elongate; non-papillate.
Identification
The distinct lateral and facial leaves are more consistent with extant L. plumosa rather than L. bidwillii but in my opinion they are not distinct enough to place the specimen into the living species.
Reference specimen and locality: SL2799 (Waitahu-1).
Reference specimen and locality: SL1433 (Arapito-1).
Identification
The cuticle is consistent with both of the extant New Zealand species, Libocedrus bidwillii and L. plumosa. The small size of the leaves, absence of anything resembling margin leaves, and absence of papillate epidermal cells at the base of the leaves suggests the fossils are L. bidwillii (Florin and Boutelje 1954).
Podocarpaceae
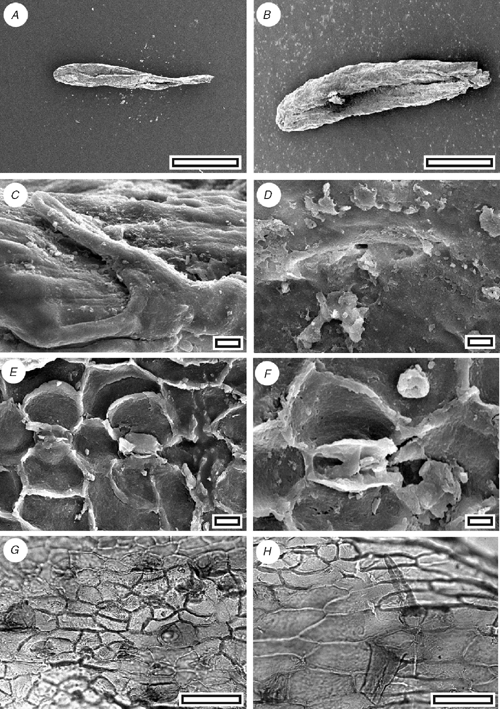
Acmopyle kirrileeae sp. nov. (Fig. 5)
|
Reference specimen and locality: SL1150 (Tadmor-1).
Diagnosis: an Acmopyle with unflattened, awl-like leaves.
Etymology: after Kirrilee O’Connor, who helped collect the specimens.
Description
Leaf arrangement unknown. Leaves linear and curved; not flattened, not keeled; 3–3.5 mm; 0.7 mm; apex rounded; margin smooth; single-veined. Stomata distributed in one broad zone about one-third to one-half the width of the leaf (7–8 stomata wide) and at least two further narrow zones; in rows and short chains, polar cells often shared; rows lateral subsidiary cells of adjacent stomata often touching. Stomatal complexes dicyclic; paratetracytic, typically with four subsidiary cells; angular; length 50–60 µm; width 35–45 µm; mainly longitudinally oriented, but with some slightly oblique; anticlinal walls not sloping obliquely away from guard cells; lateral subsidiary cells periclinal walls not thicker than normal epidermal cells. Epidermal cells typically short to isodiametric; straight-walled; unbuttressed. Unicellular trichomes present; discontinuously distributed, scattered throughout stomatal zone, among epidermal cells flanking stomatal zone, often common on adaxial margin near leaf base; length 50 to ~90 µm.
Identification
The presence of trichomes attached to epidermal cells and a stomatal form where the subsidiary cells are poorly differentiated from epidermal cells (there is a clear distinction between anticlinal and periclinal walls) are characters diagnostic of Acmopyle (Hill and Carpenter 1991) However, in contrast to all known species of Acmopyle, fossil and extant, there is little leaf flattening in this species.
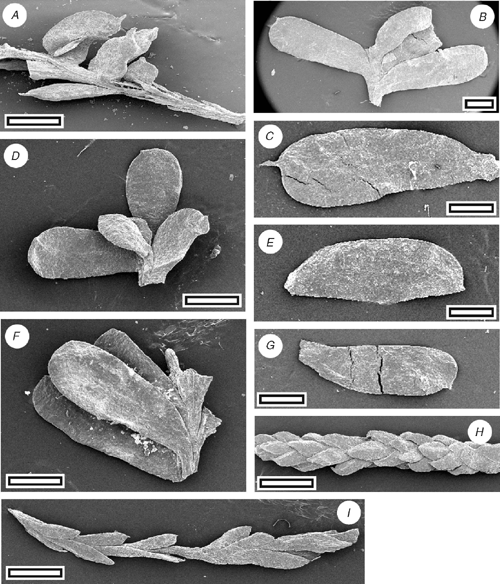
|
|
Reference specimen and locality: SL1108 (Grey-7).
Diagnosis: an Acmopyle with dimorphic foliage, including bilaterally flattened, obovate leaves, with bluntly rounded but prominently spinose apices.
Description
Leaf arrangement spiral, and dimorphic, from loosely imbricate to spreading. Leaves obovate, asymmetrical, expanded along the proximal margin; bilaterally flattened; length 2.3–5 mm; width 1–1.7 mm; apex bluntly rounded, except for a prominent, short ‘spike’ near the distal margin; margin smooth; single-veined. Stomata distributed in a single zone on one surface (with a possible indistinct gap over vein) and scattered on the other (very unequally amphistomatic); in short chains or isolated; rows isolated or sometimes overlapping. Or leaves scale-like, bifacially flattened. Length 0.6–1 mm; width 0.4 mm. Stomatal complexes dicyclic; paratetracytic with four to six subsidiary cells; length 40–70 µm; width 35–48 µm. Epidermal cells straight-walled; unbuttressed. Unicellular trichomes present; scattered on both leaf surfaces, more common on surface with less stomata, and especially common at base of leaf; length ~50 µm; base attached over one or two modified epidermal cells.
Identification
The presence of trichomes attached to epidermal cells and a stomatal form where the subsidiary cells are poorly differentiated from epidermal cells (there is a clear distinction between anticlinal and periclinal walls) are characters diagnostic of Acmopyle. The obovate-shaped, bilaterally flattened leaves with a spinose apex are unique within the genus.
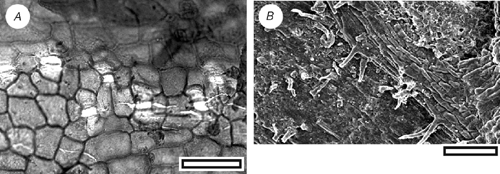
Acmopyle sp. (Fig. 8)
|
Description
Small cuticle fragments from Arapito (SL1452) and Waitahu (SL2817, SL2819) show the typical morphology of Acmopyle, but are too small for further identification.
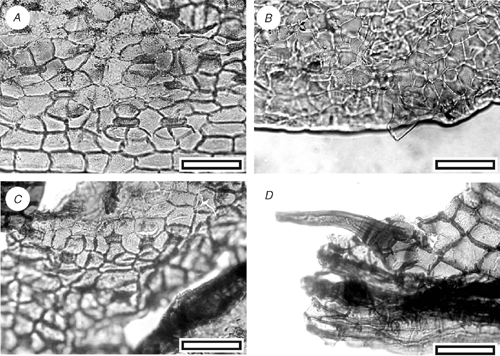
Dacrydium sp. (Fig. 9)
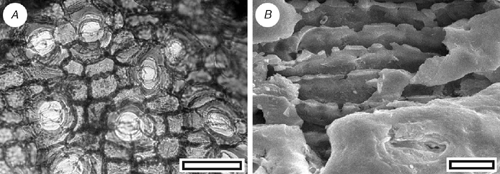
|
Reference specimen and locality: SL1155 (Tadmor-1).
Description
Leaf arrangement unknown. Leaves long and linear (awl-like) to short and scale-like; not flattened, not keeled; 2–3.5 mm; 0.5–0.6 mm; apex not incurved, or only slightly; margin smooth on the linear leaves, frilled with ctenoidally oriented epidermal cells on the scale-like leaves. Stomata distributed in two zones on each surface, extending to apex on adaxial surface, restricted to the basal part of the leaf on the abaxial surface; in rows and very long chains, polar subsidiary cells often shared. Stomatal complexes dicyclic; paratetracytic, typically with four subsidiary cells; length 110 µm; width 55 µm; longitudinally oriented. Epidermal cells typically elongate; straight-walled; buttressed and ‘beaded’, particularly away from the stomata.
Identification
The leaf and cuticular morphology is comparable with the extant New Zealand Dacrydium cupressinum Sol., but in the absence of more complete specimens, direct comparison is not made.
Phyllocladus sp. (Fig. 10A–D)
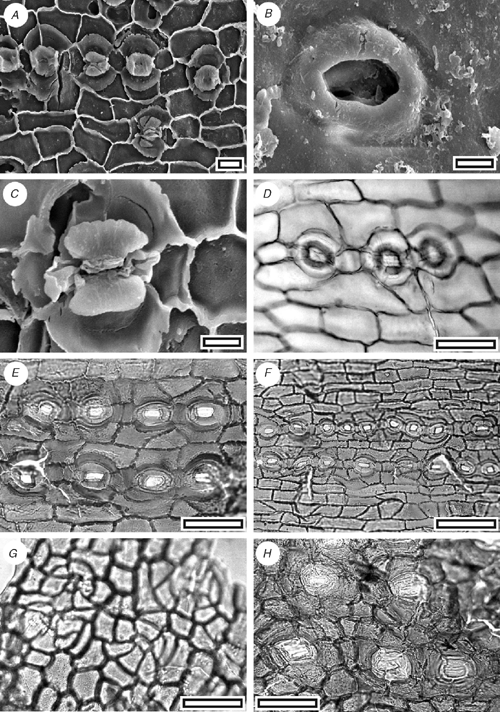
|
Reference specimen and locality: SL1459 (Tadmor-1).
Description
Phyllode arrangement unknown. Phyllode shape unknown (no complete specimens found); bifacially flattened; margin smooth on small fragments (too small to see if lobed); assumed multiveined. Stomata distributed in a single zone on one surface (assumed hypostomatic); in rows and short chains (although polar cells rarely shared); rows typically separated by two rows of epidermal cells; ~43 stomatal rows noted across 3 mm of lamina. Stomatal complexes dicyclic; paratetracytic, typically with four subsidiary cells; almost circular (polar cells often projecting slightly); length 55–60 µm; width 42–50 µm; longitudinally oriented; aperture circular to sub-rectangular; anticlinal walls sloping obliquely away from guard cells; lateral subsidiary cells periclinal, walls not thicker than normal epidermal cells; raised to form a Florin Ring. Epidermal cells typically elongate; straight-walled; unbuttressed; those on non-stomatal surface often isodiametric.
Identification
Relatively large cuticle fragments which have rounded, paratetracytic stomata with a distinct Florin Ring, which are ordered in rows (the largest fragment has at least 43 rows), but almost evenly spread (without distinction into zones), are suggestive of Phyllocladus (see illustrations in Hill 1989). I place these specimens into Phyllocladus, but with the provisio that better material may clarify their relationships.
Podocarpus
Podocarpus alpinus R.Br. ex Hook. f. (Fig. 10E, F)
Reference specimen and locality: SL1076 (Waitahu-1).
Description
Leaves lanceolate; bifacially flattened; 8 mm; 1.5–1.9 mm; single-veined. Stomata distributed in two zones on abaxial leaf surface (hypostomatic); in rows and very long chains, polar subsidiary cells often shared. Stomatal complexes dicyclic; paratetracytic with four or five subsidiary cells. Epidermal cells typically short to isodiametric; wavy-walled; buttressed; those on non-stomatal surface often isodiametric, slightly buttressed.
Identification
The long loose, and sometimes tight chains of paratetracytic stomata, and lanceolate leaves, are characteristic of Podocarpus. The stomatal outline and slight buttressing and frequently isodiametric shape of the adaxial epidermal cells suggests P. alpinus R.Br. ex Hook. f., a species currently restricted to Australia.
Conifer indet. sp. A. (Fig. 10G)
Reference specimen and locality: SL1453 (Arapito-1).
Description
Leaf arrangement unknown. Leaves scale-like; bifacially flattened; margin smooth; single-veined. Stomata distributed in an unclear manner; in a random array. Stomatal complexes dicyclic; encyclocytic with five or six subsidiary cells; angular; randomly oriented, irregular in shape and size. Epidermal cells typically short to isodiametric; straight-walled; unbuttressed.
Identification
The basic stomatal structure appears to be gymnosperm (the overall morphology is entirely distinct from any angiosperm I know of), with guard cells obscured by the subsidiary cells. There is no suggestion of cycad affinity and the most likely identification is conifer. In my opinion it is likely to be Podocarpaceae or Taxodiaceae s.l. but there are no obviously comparable taxa.
Conifer indet. sp. B. (Fig. 10H)
Reference specimen and locality: SL1428 (Arapito-1).
Description
Leaves scale-like. Stomata distributed in two zones on adaxial leaf surface (epistomatic); in a random array. Stomatal complexes encyclocytic with four or five subsidiary cells; mainly longitudinally oriented, but with some oblique. Epidermal cells typically short to isodiametric; straight-walled; unbuttressed.
Identification
The cyclocytic and longitudinally oriented stomatal complexes are comparable with Microstrobos and Microcachrys.
Angiosperms
Winteraceae
Pseudowintera sp.
CUT-Z-FAE (Fig. 11)
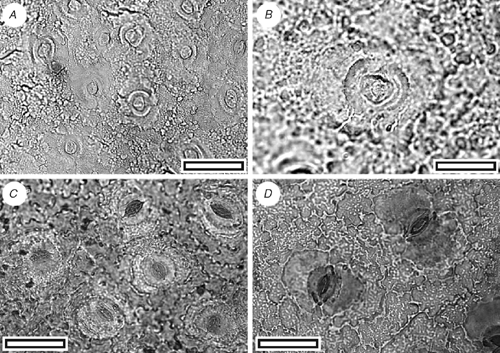
|
Reference specimen and locality: SL1439 (Arapito-1).
Description
Stomatal distribution over leaf surfaces unknown, evenly spread on stomatal surfaces; stomatal complexes isolated; randomly oriented; brachyparacytic; development unclear; size range unimodal and small; shape and size even; not distinct in thickness from normal epidermal cells; highly granular. Guard-cell pair outline circular; outlined by a well-defined anticlinal wall; length 18–28 µm; (medium); at the same level as subsidiary cells (exposed on surface); periclinal walls the same thickness as for normal epidermal cells; little polar development between guard cells (guard cells appear as continuous ring). Stomatal pore elliptical, narrowing slightly at the poles. Epidermal-cell flanges poorly visible because of granular texture; cells over veins not distinguished as ‘venal’; normal epidermal cells unclear; glabrous; unornamented. Epidermal-cell texture coarsely granular.
Identification
The distinctive combination of brachyparacytic stomatal complexes and very coarsely granular cuticle make this directly comparable with extant New Zealand Pseudowintera (Fig. 11C, D, and see Bongers 1973).
Lauraceae
Beilschmiedia sp.
CUT-L-FDI (Fig. 12)
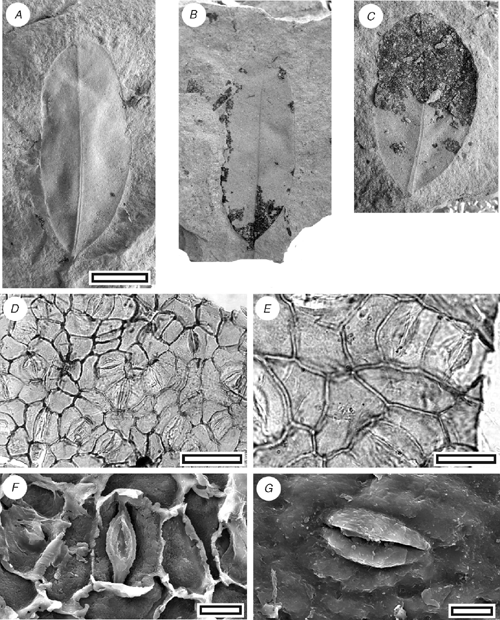
|
Reference specimen and locality: SL2614 (Waitahu-1).
Description
Cuticle. Stomatal distribution hypostomatic, in clear areoles between veins; isolated; randomly oriented; paracytic; stomatal complexes outline irregular, typically narrower than broad, with truncated polar regions and curved sides; length 20–30 µm long; (medium); size range unimodal and small; slightly sunken below level of normal epidermal cells. Subsidiary cell shape and size even; not distinct in thickness from normal epidermal cells; unornamented. Guard-cell pair outline elongate; overarched by subsidiary cells (cuticular scales present); periclinal walls thinner than normal epidermal cells. Stomatal pore slit-like. Cuticular scales narrow; very small. Epidermal cell flanges clearly visible under TLM; cells over fine venation distinguished (elongated) as ‘venal’; normal epidermal cells isodiametric; walls straight; with scars of trichome bases; unbuttressed; unornamented. Trichome bases common; homomorphic; inserted between epidermal cells; ~13 µm in diameter; epidermal cells around trichome base not clearly modified; number of foot cells six or seven. Trichomes deciduous (and therefore trichome type unknown).
Leaves. Leaf outline elliptical–slightly obovate, apex rounded, base obtuse, margin entire, length 30–40 mm, width 13–20 mm. Venation externodromous, primary vein prominent, finer venation much finer.
Identification
This cuticle exhibits the characteristic features of Lauraceae (Hill 1986). The morphology is rather bland, but it is similar enough with the extant New Zealand species of Beilschmiedia (B. tawa (A.Cunn.) Benth. et Hook. f. ex Kirk and B. tarairi (A.Cunn.) Benth. et Hook. f. ex Kirk, both of which have similar cuticle; M. Pole, pers. obs.) that it probably belongs in the same genus. The fossils are clearly different species on the basis of gross leaf architecture and size as well as subtle differences in stomatal complex size and prominence of the trichome bases. They clearly differ from Litsea calicaris (A.Cunn.) Kirk, the other species of New Zealand Lauraceae, which has a distinct circular collar around the stomatal complex. Christophel and Rowett (1996) noted that Australian Beilschmiedia are distinguishable by the buttressing of both abaxial and adaxial epidermal cells, although they did note an exception (B. recurva B.Hyland), and the extant New Zealand species are also distinct in this respect. The fossil cuticle is also distinct from all other Australian Lauraceae cuticle as illustrated by Christophel and Rowett (1996). The stomatal complexes are larger and have a more orthogonal outline than CUT-L-GBE, and the epidermal cells are larger and with relatively thinner anticlinal walls. The guard-cell ledges are clearly visible under TLM.
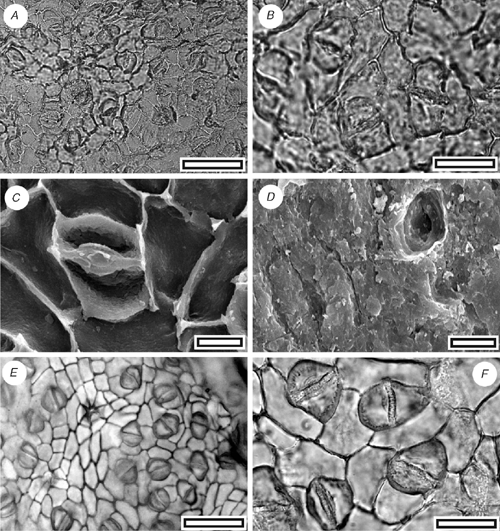
CUT-L-GBE (Fig. 13)
|
Type or reference specimen and locality: SL1475 (Tadmor-1).
Description
Stomatal distribution over leaf surfaces hypostomatic, stomatal complexes isolated; randomly oriented; paracytic; stomatal complexes outline typically with a flattened, rounded, often with a broader than long, diamond outline; size range unimodal and small, 15–21 µm long; (medium). Guard cells overlain by cuticle thicker than over normal epidermal cells, overarched by subsidiary cells (cuticular scales present); periclinal walls thinner than normal epidermal cells. Stomatal pore slit-like. Cuticular scales narrow; very small. Epidermal cell flanges clearly visible under TLM; unbuttressed. Trichome bases common; homomorphic; inserted between epidermal cells; 8–10 µm in diameter; epidermal cells around trichome base modified with thickened poral rim and radial walls; number of foot cells six or seven.
Identification
This cuticle also exhibits the characteristic features of Lauraceae (Hill 1986). However, there are no compelling similarities with Beilschmiedia and its generic affinities remain unclear. The stomatal complexes are smaller and have a more rounded, and sometimes more hexagonal outline than CUT-L-FDI (one specimen has almost diamond-shaped stomatal complexes (Fig. 13E, F), and the epidermal cells are smaller and with relatively thicker anticlinal walls. The guard-cell ledges are not clearly visible under TLM.
Nothofagaceae
Nothofagus sp. (Fig. 14)
|
Reference specimen and locality: SL1094 (Grey-7).
Description
A variety of fragments come from small leaves (probably ~12 mm long at most) with relatively large, irregular teeth. Repeated attempts to prepare cuticle were unsuccessful, the cuticle being much less resistant to chemicals than the rest of the leaf. Unprepared (for cuticle) leaf material was observed under SEM, but other than simple hair bases, no stomata could be located.
Identification
There is little doubt that these leaves are Nothofagus, at least morphologically similar to the extant N. menziesii (Hook. f.) Oerst.
Proteaceae
Beauprea sp.
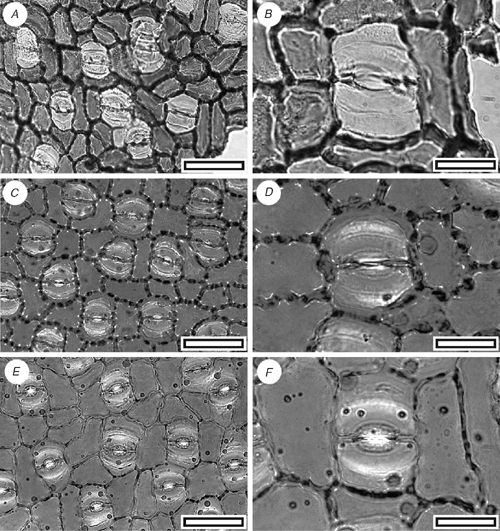
CUT-P-FBA (Fig. 15)
|
Reference specimen and locality: SL1427 (Arapito-1).
Description
Stomatal distribution over leaf surfaces unknown, evenly spread on stomatal surfaces, isolated; showing a clear trend towards alignment; brachyparacytic; development unclear; size range unimodal and small; at the same level as normal epidermal cells. Subsidiary-cell shape and size uneven (one smaller, one larger); thinner than over normal epidermal cells; unornamented. Guard-cell pair outline elongate; outlined by a well-defined anticlinal wall; length 21–30 µm; (medium); at the same level as subsidiary cells (exposed on surface); periclinal walls thinner than for normal epidermal cells; with prominent polar rods. Outer stomatal ledge almost covering width of guard cells. Stomatal pore elliptical, narrowing slightly at the poles. Cells over veins not distinguished as ‘venal’; normal epidermal cells isodiametric; walls wavy; glabrous; unbuttressed.
Identification
The general morphology, stomatal alignment and lack of trichome bases (some single-celled bases may be present) is comparable with Beauprea, although other Proteaceae genera are similar (Carpenter and Jordan 1997; pers. obs.). The identification as Beauprea is supported by the widespread occurrence of Beauprea pollen in the New Zealand Cenozoic (Pocknall and Crosbie 1988), including the Arapito Road section (Mildenhall 1978b).
Knightia sp.
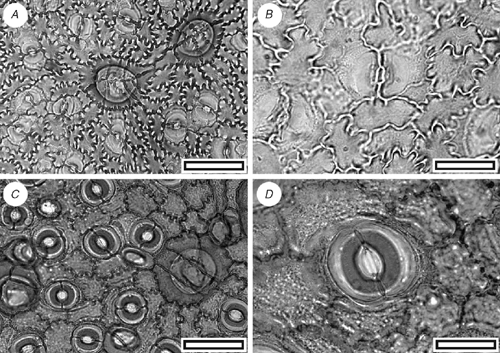
CUT-P-FBJ (Fig. 16)
|
Reference specimen and locality: SL1426 (Arapito-1).
Description
Stomatal distribution over leaf surfaces unknown, evenly spread on stomatal surfaces; stomatal complexes isolated; randomly oriented; brachyparacytic; development unclear; size range unimodal and small; at the same level as normal epidermal cells. Shape and size even; not distinct in thickness from normal epidermal cells (but all cells within areoles are much thinner than venal cells); unornamented. Guard-cell pair outline ovate; outlined by a well-defined anticlinal wall; length 15–20 µm; (small); at the same level as subsidiary cells (exposed on surface); periclinal walls the same thickness as for normal epidermal cells; with prominent T-piece thickenings at polar ends. Outer stomatal ledge almost covering width of guard cells. Stomatal pore elliptical, narrowing slightly at the poles. Epidermal-cell flanges clearly visible under TLM; cells over fine venation distinguished (elongated) as ‘venal’; normal epidermal cells highly variable from isodiametric to elongate; walls strongly sinuous; with scars of trichome bases; buttressed; unornamented. Trichomes common; scattered over venal and non-venal regions; deciduous (and therefore trichome type unknown); bases homomorphic; inserted over several modified epidermal cells; 5–10; epidermal cells under trichome base modified to form a thick, raised circular platform, on top of which sits a smooth, thick hollow collar.
Identification
The brachyparacytic stomatal complexes and multicellular trichome bases are characteristic Proteaceae features. Especially with respect to the morphology of the trichome bases and sinuous epidermal cells, the cuticle is comparable with the extant New Zealand Knightia excelsa (Pole 1998b), although the stomatal complexes of the Arapito specimens are distinctly smaller.
Myrtaceae
CUT-M-FEB (Fig. 17)
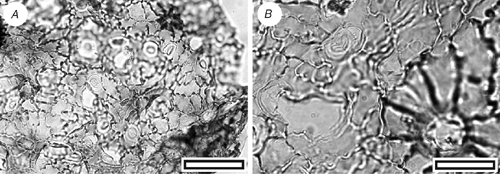
|
Reference specimen and locality: SL1448 (Arapito-1).
Description
Stomatal distribution over leaf surfaces unknown, evenly spread on stomatal surfaces; stomatal complexes isolated; randomly oriented; anisocytic; development unclear; size range unimodal and small; at the same level as normal epidermal cells. Subsidiary cells two or three; shape and size highly irregular; not distinct in thickness from normal epidermal cells; unornamented. Guard-cell pair outline circular; outlined by a well-defined anticlinal wall; length 13–18 µm; (small–medium); some concentric thickenings over guard cells; at the same level as subsidiary cells (exposed on surface); periclinal walls the same thickness asfor normal epidermal cells; little polar development between guard cells (guard cells appear as a continuous ring). Outer stomatal ledge very thin, over innermost edges of guard cells. Stomatal pore elliptical, narrowing slightly at the poles. Epidermal-cell flanges clearly visible under TLM; cells over veins not distinguished as ‘venal’; normal epidermal cells isodiametric; walls sinuous (Grade C); with scars of trichome bases; buttressed; unornamented. Trichomes abundant; scattered over venal and non-venal regions; deciduous (and therefore trichome type unknown); bases homomorphic; cantered over the junction of several epidermal cells; 6–9; similar in size to normal epidermal cell; epidermal cells around trichome base modified with thickened poral rim; epidermal cells under trichome base not clearly modified except for trichome scar (raised rim).
Identification
This cuticle morphology is essentially the same as for CUT-Z-FAH, but with the presence of common trichome bases. Metrosideros diffusa, with similar stomatal and epidermal morphology, does have trichomes but no specimens match the fossil closely in this respect. For now, it is kept distinct from CUT-Z-FAH but further specimens could indicate it is conspecific, perhaps from a different portion of the leaf.
Family indet
CUT-Z-FAH (Fig. 18A, B)
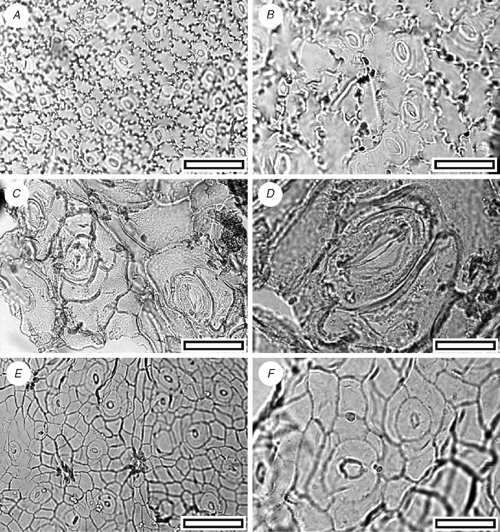
|
Reference specimen and locality: SL1450 (Arapito-1).
Description
Stomatal distribution over leaf surfaces unknown, evenly spread on stomatal surfaces; stomatal complexes isolated; randomly oriented; brachyparacytic; development unclear; size range unimodal and small; at the same level as normal epidermal cells. Shape and size uneven (one smaller, one larger); not distinct in thickness from normal epidermal cells; unornamented. Guard-cell pair outline circular; outlined by a well-defined anticlinal wall; length 10–16 µm; (small); at the same level as subsidiary cells (exposed on surface); periclinal walls the same thickness as for normal epidermal cells; little polar development between guard cells (guard cells appear as continuous ring). Outer stomatal ledge absent. Epidermal-cell flanges clearly visible under TLM; cells over veins not distinguished as ‘venal’; normal epidermal cells isodiametric; walls sinuous (Grade A); glabrous; buttressed; unornamented.
Identification
The small, often networked stomatal complexes, surrounded by sinuous–buttressed epidermal cells, closely match the New Zealand Metrosideros diffusa Forst. f. No lid cells have been found, which would confirm the identification; this may be simply because of the small size of the cuticle fragments.
CUT-Z-FDH (Fig. 18C, D)
Reference specimen and locality: SL1448 (Arapito-1).
Description
Stomatal distribution over leaf surfaces unknown; stomatal complexes possibly evenly spread on stomatal surfaces, although unclear because of specimen size; isolated; randomly oriented; morphology not clear; development where in many cases the stomatal complex has clearly been blocked out by a series of oblique, intersecting cell divisions which form three or four cells around the stomata (comparable to an anisocytic subsidiary cell arrangement); size range unimodal and small; at the same level as normal epidermal cells. Subsidiary cells three or four; shape and size highly irregular; not distinct in thickness from normal epidermal cells; unornamented. Guard-cell pair outline circular; outlined by a well-defined anticlinal wall; length 35–49 µm; (medium–large); at the same level as subsidiary cells (exposed on surface); periclinal walls the same thickness as for normal epidermal cells; with prominent T-piece thickenings at polar ends. Outer stomatal ledge a continuous ellipse, half the width of guard cells. Stomatal pore elliptical, narrowing slightly at the poles. Epidermal-cell flanges clearly visible under TLM; cells over major veins vaguely or inconsistently distinguished (elongate) as ‘venal’; normal epidermal cells elongated; walls wavy; glabrous; unbuttressed; unornamented.
Identification
The basically anisocytic stomatal structure suggests Pseudopanax.
CUT-Z-FEJ (Fig. 18E, F)
Reference specimen and locality: SL1448 (Arapito-1).
Description
Stomatal distribution over leaf surfaces unknown, in clear areoles between veins on stomatal surfaces; stomatal complexes isolated; randomly oriented; morphology not clear; development unclear; size range unimodal, but with a wide range; at the same level as normal epidermal cells. Subsidiary cells four or five; shape and size highly irregular; not distinct in thickness from normal epidermal cells; unornamented. Guard-cell pair outline circular to ovate; outlined by a well-defined anticlinal wall; length 15–28 µm; (medium); at the same level as subsidiary cells (exposed on surface); periclinal walls the same thickness as for normal epidermal cells; little polar development between guard cells (guard cells appear as continuous ring). Outer stomatal ledge very thin, over innermost edges of guard cells. Stomatal pore elliptical, narrowing slightly at the poles. Epidermal-cell flanges clearly visible under TLM; cells over major veins vaguely or inconsistently distinguished (elongate) as ‘venal’; normal epidermal cells isodiametric; walls straight; medium-dense indumentum; unbuttressed; unornamented. Trichomes common; scattered over venal and non-venal regions but slightly more common over veins; deciduous (and therefore trichome type unknown); bases homomorphic; inserted between epidermal cells; similar in size to normal epidermal cell; epidermal cells around trichome base modified with ‘frilly’ poral rim; number of foot cells five to seven.
Identification
The small, simple stomatal complexes and trichome bases suggest Cunoniaceae.
CUT-Z-FAG (Fig. 19A, B)
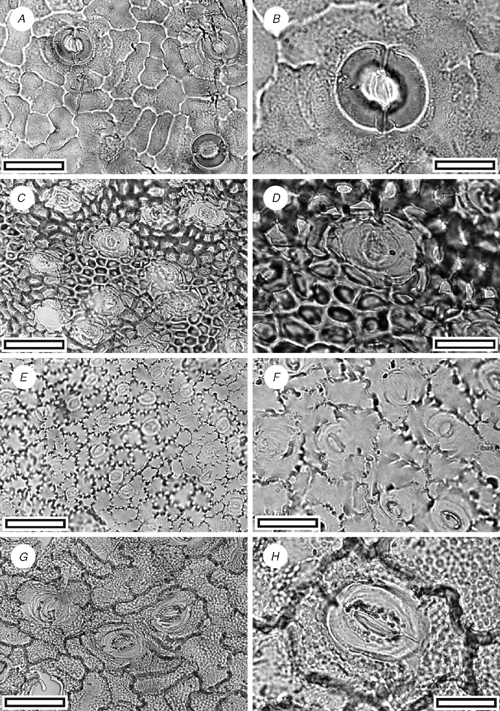
|
Reference specimen and locality: SL1448 (Arapito-1).
Description
Stomatal distribution over leaf surfaces unknown, evenly spread on stomatal surfaces; stomatal complexes isolated; randomly oriented; cyclocytic with a double-ring of subsidiary cells; development of type where all, some, or none of the subsidiary cells may have been modified by a tangential division; size range unimodal and small; at the same level as normal epidermal cells. Subsidiary cells four or five (but difficult to count under TLM); shape and size highly irregular; not distinct in thickness from normal epidermal cells; unornamented, but raised to form a broad peristomatal rim. Guard-cell pair outline circular; outlined by a well-defined anticlinal wall; length 29–32 µm; (medium); at the same level as subsidiary cells (exposed on surface); periclinal walls thicker than normal epidermal cells; with prominent polar rods. Outer stomatal ledge almost covering width of guard cells. Stomatal pore subcircular. Epidermal-cell flanges clearly visible under TLM; cells over veins not distinguished as ‘venal’; normal epidermal cells highly variable from isodiametric to elongate; walls wavy; glabrous; unbuttressed; unornamented.
Identification
The double-ring of subsidiary cells is also found in the New Zealand Griselinia lucida G.Forst., although they are much larger in the latter. The other New Zealand species, G. littoralis (Raoul.) Raoul, does not have such an obvious double-ring. A generic identity with Griselinia is possible. The double-ring and general morphology of CUT-Z-FAG is similar to at least one of the specimens (OU30891, fig. 34e–h in Pole 1996) placed in CUT-Z-022 from the Early Miocene of Foulden Hills (Pole 1996). At present, CUT-Z-FAG seems distinct on the presence of a peristomatal ring, and its broader outer stomatal ledge, but further material may show a closer relationship.
CUT-Z-FAF (Fig. 19C, D)
Reference specimen and locality: SL1434 (Arapito-1).
Description
Stomatal distribution over leaf surfaces unknown, evenly spread on stomatal surfaces; stomatal complexes isolated; randomly oriented; morphology not clear; size range unimodal and small. Subsidiary cells nine or ten; (but difficult to count under TLM); shape and size even; not distinct in thickness from normal epidermal cells (but all cells within areoles are much thinner than venal cells); unornamented. Guard-cell pair outline ovate; outer margin obscured under TLM by subsidiary cells; length 20–25 µm; (medium); periclinal walls thinner than for normal epidermal cells. Outer stomatal ledge very thin, over innermost edges of guard cells. Stomatal pore elliptical, narrowing slightly at the poles. Epidermal-cell flanges clearly visible under TLM; cells over veins not distinguished as ‘venal’; normal epidermal cells isodiametric; walls straight; unbuttressed; unornamented.
CUT-Z-FAI (Fig. 19E, F)
Reference specimen and locality: SL1440 (Arapito-1).
Description
Stomatal distribution over leaf surfaces unknown, evenly spread on stomatal surfaces; stomatal complexes isolated; randomly oriented; anisocytic; development whereby in many cases the stomatal complex clearly has been blocked out by a series of oblique, intersecting cell divisions which have resulted in three or four cells around the stomata (comparable to an anisocytic subsidiary cell arrangement); size range unimodal and small; at the same level as normal epidermal cells. Subsidiary cells three or four; shape and size highly irregular; not distinct in thickness from normal epidermal cells; distinct ‘net-like’ pattern of thickening; ornamented with a circular ridge (may be discontinuous, in which case just some ridges parallel to stomatal axis). Guard-cell pair outline elongate; not outlined by a clear anticlinal wall; length 30–32 µm; (medium); at the same level as subsidiary cells (exposed on surface); periclinal walls much thinner than for normal epidermal cells (often broken away); some wall development between guard cells, and sometimes development of T-piece thickenings at the poles. Outer stomatal ledge a continuous ellipse, half the width of guard cells. Stomatal pore elliptical, narrowing slightly at the poles. Epidermal-cell flanges clearly visible under TLM; cells over veins not distinguished as ‘venal’; normal epidermal cells elongated; walls sinuous (Grade C); glabrous; unbuttressed; unornamented. Epidermal-cell texture distinct ‘net-like’ pattern of thickening.
Identification
The basically anisocytic stomatal structure suggests Pseudopanax.
CUT-Z-FEA (Fig. 20)
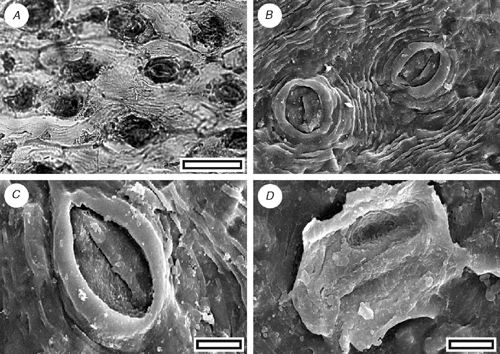
|
Reference specimen and locality: SL1134 (Tadmor-1).
Description
Stomatal distribution over leaf surfaces unknown, evenly spread on stomatal surfaces; stomatal complexes isolated, showing a clear trend towards alignment; morphology not clear; development unclear; size range unimodal and small; at the same level as normal epidermal cells. Subsidiary cells four; shape and size typically elongate parallel to stomatal axis; not distinct in thickness from normal epidermal cells; all surficial cells within the group have a granular surface; ornamented with many fine ridges parallel to stomatal axis. Guard-cell pair outline difficult to see in TLM view; outer margin obscured under TLM by surface ornamentation; length 30–38 µm; (medium); at the same level as subsidiary cells (exposed on surface); periclinal walls thicker than for normal epidermal cells. Outer stomatal ledge prominently raised and thickened, covering outer half of guard cells. Stomatal pore elliptical, narrowing slightly at the poles. Epidermal-cell flanges clearly visible under TLM; cells over veins not distinguished as ‘venal’; normal epidermal cells elongated; walls straight to slightly curved; glabrous; unbuttressed; unornamented. Trichomes not apparent.
Identification
The strong striations and prominent outer stomatal ledges suggest Quintinia; however, the scaly outer surface of the latter is absent.


