Epicuticular waxes and plant primary metabolites on the surfaces of juvenile Eucalyptus globulus and E. nitens (Myrtaceae) leaves
Martin J. Steinbauer A D , Noel W. Davies B , Cyril Gaertner C and Sylvie Derridj CA Co-operative Research Centre for Sustainable Production Forestry/CSIRO Entomology, Canberra and Department of Zoology, La Trobe University, Melbourne, Victoria 3086, Australia.
B Central Science Laboratory, University of Tasmania, Private Bag 74, Hobart, Tasmania 7001, Australia.
C Institut National de la Recherche Agronomique, Unité 1272, Physiologie de l’Insecte-Signalisation et Communication, F 78 000 Versailles, France.
D Corresponding author. Email: M.Steinbauer@latrobe.edu.au
Australian Journal of Botany 57(6) 474-485 https://doi.org/10.1071/BT09108
Submitted: 19 June 2009 Accepted: 22 September 2009 Published: 9 November 2009
Abstract
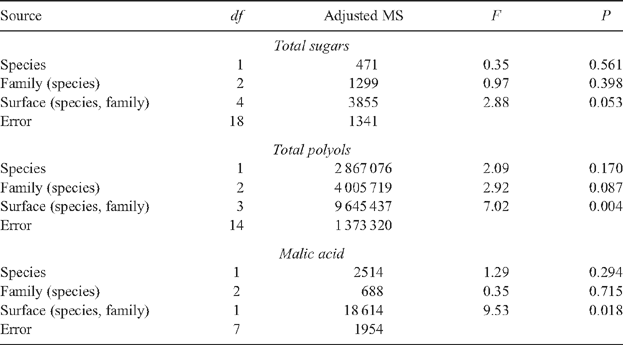
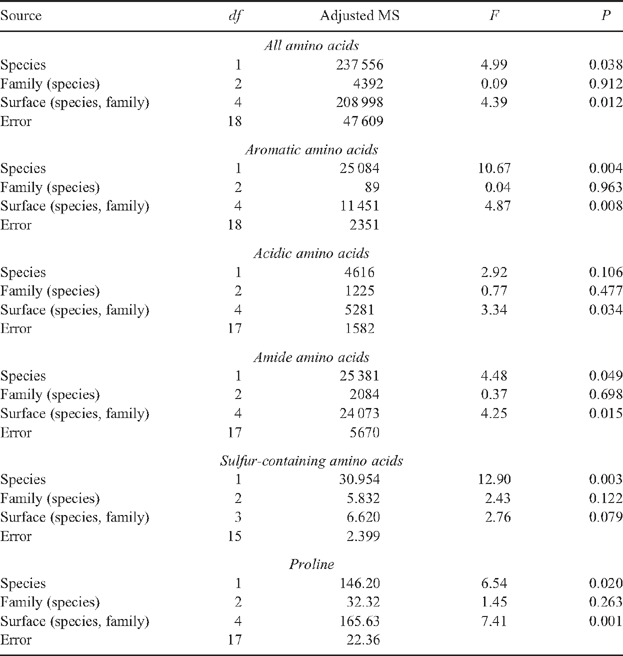
Our knowledge of the composition of the waxes on the surfaces of Eucalyptus leaves is growing but that of plant primary metabolites has been completely overlooked. The diffusion of primary metabolites above the cuticle exposes them to a variety of herbivorous taxa and has the potential to influence their responses to that plant. Juvenile leaves of two families of Eucalyptus globulus Labill. ssp. globulus and two families of E. nitens (Deane & Maiden) Maiden had 11 out of 16 of the epicuticular waxes that were detected in common. However, two phenylethyl esters (waxes) were only detected on leaves of one family of E. globulus and two benzyl esters (waxes) were not detected or were uncommon in samples from E. nitens. Wax compounds were generally found in samples from both leaf surfaces but a few were only detected in samples from particular sides. Species and families of eucalypt did not differ significantly in the concentrations of free sugars, polyols, malic acid or γ-aminobutyric acid (GABA) (all plant primary metabolites) collected from the surfaces of leaves. However, concentrations of all these metabolites were usually higher in collections from the upper surfaces of leaves. High wax abundance, especially on the lower surfaces of E. globulus leaves, is suspected to have hindered dissolution of all the primary metabolites quantified. Several free amino acids exhibited significant species-level differences in concentrations, namely the aromatic, amide and sulfur-containing amino acids as well as proline; family-level differences in amino acid concentrations were not significant. Australian and overseas evidence showing that differences in waxes and primary metabolites can be influential in plant susceptibility to herbivorous taxa is considered with respect to the threats posed by the autumn gum moth and Mycosphaerella leaf spot fungi.
Acknowledgements
Financial support for MJS to work at INRA in August–September 2003 was provided by the Australian Academy of Science under the Academy’s Scientific Visits to Europe program. The GC-MS used for the wax analyses was purchased with funds from the Australian Research Council’s Linkage Infrastructure, Equipment and Facilities scheme (reference number LE0345743). We thank Prof. Dr Caroline Müller (University of Bielefeld, Germany) for her advice on terminology and an anonymous reviewer for their constructive comments on an earlier draft of the manuscript.
Calas D,
Marion-Poll F, Steinbauer MJ
(2009) Tarsal taste sensilla of the autumn gum moth, Mnesampela privata: Morphology and electrophysiological activity. Entomologia Experimentalis et Applicata 133, 186–192.
| Crossref | GoogleScholarGoogle Scholar |

Carnegie AJ,
Johnson IG, Henson M
(2004) Variation among provenances and families of blackbutt (Eucalyptus pilularis) in early growth and susceptibility to damage from leaf spot fungi. Canadian Journal of Forest Research 34, 2314–2326.
| Crossref | GoogleScholarGoogle Scholar |

Carnegie AJ,
Keane PJ,
Ades PK, Smith IW
(1994) Variation in susceptibility of Eucalyptus globulus provenances to Mycosphaerella leaf disease. Canadian Journal of Forest Research 24, 1751–1757.
| Crossref | GoogleScholarGoogle Scholar |

Carr DJ,
Carr SGM, Lenz JR
(1985) Oriented arrays of epicuticular wax plates in Eucalyptus species. Protoplasma 124, 205–212.
| Crossref | GoogleScholarGoogle Scholar |

Chapman RF
(2003) Contact chemoreception in feeding by phytophagous insects. Annual Review of Entomology 48, 455–484.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Close DC,
Davidson NJ,
Shields CB, Wiltshire R
(2007) Reflectance and phenolics of green and glaucous leaves of Eucalyptus urnigera. Australian Journal of Botany 55, 561–567.
| Crossref | GoogleScholarGoogle Scholar |

Derridj S,
Boutin JP,
Fiala V, Soldaat LL
(1996a) Composition en métabolites primaires de la surface foliaire du poireau: étude comparative, incidence sur la sélection de la plante hôte pour pondre par un insecte. Acta Botanica Gallica 143, 125–130.

Derridj S,
Wu BR,
Stammitti L,
Garrec JP, Derrien A
(1996b) Chemicals on the leaf surface, information about the plant available to insects. Entomologia Experimentalis et Applicata 80, 197–201.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Derridj S,
Gregoire V,
Boutin JP, Fiala V
(1989) Plant growth stages in the interspecific oviposition preference of the European corn borer and relations with chemicals present on the leaf surfaces. Entomologia Experimentalis et Applicata 53, 267–276.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Dungey HS,
Potts BM,
Carnegie AJ, Ades PK
(1997) Mycosphaerella leaf disease: Genetic variation in damage to Eucalyptus nitens, Eucalyptus globulus, and their F1 hybrid. Canadian Journal of Forest Research 27, 750–759.
| Crossref | GoogleScholarGoogle Scholar |

Eigenbrode SD, Espelie KE
(1995) Effects of plant epicuticular lipids on insect herbivores. Annual Review of Entomology 40, 171–194.
| Crossref | GoogleScholarGoogle Scholar |

Fiala V,
Boutin JP,
Barry P, Derridj S
(1993) Les métabolites de surface foliaire (phylloplan): présence et rôle dans les relations plante-insecte. Acta Botanica Gallica 140, 207–216.
|
CAS |

Fiala V,
Glad C,
Martin M,
Jolivet E, Derridj S
(1990) Occurrence of soluble carbohydrates on the phylloplane of maize (Zea mays L.): variations in relation to leaf heterogeneity and position on the plant. The New Phytologist 115, 609–615.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Foster SP, Harris MO
(1992) Foliar chemicals of wheat and related grasses influencing oviposition by Hessian fly, Mayetiola destructor (Say) (Diptera, Cecidomyiidae). Journal of Chemical Ecology 18, 1965–1980.
| Crossref | GoogleScholarGoogle Scholar |

Fraenkel GS
(1959) The raison d’être of secondary plant substances. Science 129, 1466–1470.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hallam ND
(1964) Sectioning and electron microscopy of eucalypt leaf waxes. Australian Journal of Biological Sciences 17, 587–590.

Hallam ND, Chambers TC
(1970) The leaf waxes of the genus Eucalyptus L’Héritier. Australian Journal of Botany 18, 335–386.
| Crossref | GoogleScholarGoogle Scholar |

Johnson ED
(1926) A comparison of the juvenile and adult leaves of Eucalyptus globulus. The New Phytologist 25, 202–212.
| Crossref | GoogleScholarGoogle Scholar |

Jones TH,
Potts BM,
Vaillancourt RE, Davies NW
(2002) Genetic resistance of Eucalyptus globulus to autumn gum moth defoliation and the role of cuticular waxes. Canadian Journal of Forest Research 32, 1961–1969.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Lamberton JA
(1964) The occurrence of 5-hydroxy-7,4′-dimethoxy-6-methylflavone in Eucalyptus waxes. Australian Journal of Chemistry 17, 692–696.
|
CAS |

Leveau JHJ, Lindow SE
(2001) Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proceedings of the National Academy of Sciences of the United States of America 98, 3446–3453.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Lombarkia N, Derridj S
(2008) Resistance of apple trees to Cydia pomonella egg-laying due to leaf surface metabolites. Entomologia Experimentalis et Applicata 128, 57–65.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Lombarkia N, Derridj S
(2002) Incidence of apple fruit and leaf surface metabolites on Cydia pomonella oviposition. Entomologia Experimentalis et Applicata 104, 79–87.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Maher N,
Thiery D, Städler E
(2006) Oviposition by Lobesia botrana is stimulated by sugars detected by contact chemoreceptors. Physiological Entomology 31, 14–22.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Marcell LM, Beattie GA
(2002) Effect of leaf surface waxes on leaf colonization by Pantoea agglomerans and Clavibacter michiganensis. Molecular Plant-Microbe Interactions 15, 1236–1244.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Mercier J, Lindow SE
(2000) Role of leaf surface sugars in colonisation of plants by bacterial epiphytes. Applied and Environmental Microbiology 66, 369–374.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Morris BD,
Foster SP, Harris MO
(2000) Identification of 1-octacosanal and 6-methoxy-2-benzoxazolinone from wheat as ovipositional stimulants for Hessian fly, Mayetiola destructor. Journal of Chemical Ecology 26, 859–873.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Müller C, Riederer M
(2005) Plant surface properties in chemical ecology. Journal of Chemical Ecology 31, 2621–2651.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nelson EB, Hsu JST
(1994) Nutritional factors affecting responses of sporangia of Phythium ultimum to germination stimulants. Phytopathology 84, 677–683.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Östrand F,
Wallis IR,
Davies NW,
Matsuki M, Steinbauer MJ
(2008) Causes and consequences of host expansion by Mnesampela privata. Journal of Chemical Ecology 34, 153–167.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Özcan T
(2006) Total protein and amino acid compositions in the acorns of Turkish Quercus L. taxa. Genetic Resources and Crop Evolution 53, 419–429.
| Crossref | GoogleScholarGoogle Scholar |

Rapley LP,
Allen GR, Potts BM
(2004) Susceptibility of Eucalyptus globulus to Mnesampela privata defoliation in relation to a specific foliar wax compound. Chemoecology 14, 157–163.
|
CAS |

Ruan YJ,
Kotraiah V, Straney DC
(1995) Flavonoids stimulate spore germination in Fusarium solani pathogenic on legumes in a manner sensitive to inhibitors of cAMP-dependent protein kinase. Molecular Plant-Microbe Interactions 8, 929–938.
|
CAS |

Short MW, Steinbauer MJ
(2004) Floral nectar versus honeydew as food for wasp parasitoids: implications for pest management in eucalypt plantations. Australian Forestry 67, 199–203.

Soldaat LL,
Boutin JP, Derridj S
(1996) Species-specific composition of free amino acids on the leaf surface of four Senecio species. Journal of Chemical Ecology 22, 1–12.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Stammitti L,
Garrec JP, Derridj S
(1995) Permeability of isolated cuticles of Prunus laurocerasus to soluble carbohydrates. Plant Physiology and Biochemistry 33, 319–326.
|
CAS |

Steinbauer MJ,
Schiestl FP, Davies NW
(2004) Monoterpenes and epicuticular waxes help female autumn gum moth differentiate between waxy and glossy Eucalyptus and leaves of different ages. Journal of Chemical Ecology 30, 1117–1142.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Thomas DA, Barber HN
(1974) Studies on leaf characteristics of a cline of Eucalyptus urnigera from Mount Wellington, Tasmania. II. Reflection, transmission and absorption of radiation. Australian Journal of Botany 22, 701–707.
| Crossref | GoogleScholarGoogle Scholar |

Wirthensohn MG, Sedgley M
(1996) Epicuticular wax structure and regeneration on developing juvenile Eucalyptus leaves. Australian Journal of Botany 44, 691–704.
| Crossref | GoogleScholarGoogle Scholar |

Yeoh HH,
Wee YC, Watson L
(1984) Systematic variation in leaf amino-acid compositions of leguminous plants. Phytochemistry 23, 2227–2229.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

|
|


