Seedling growth rates and light requirements of subtropical rainforest trees associated with basaltic and rhyolitic soils
C. H. Lusk A D , K. M. Sendall B and P. J. Clarke CA Department of Biological Sciences, University of Waikato, Hamilton 3240, New Zealand.
B Department of Forest Resources, University of Minnesota, 1530 Cleveland Avenue N, St Paul, MN 55108, USA.
C Department of Botany, University of New England, Armidale, NSW 2350, Australia.
D Corresponding author. Email: clusk@waikato.ac.nz
Australian Journal of Botany 62(1) 48-55 https://doi.org/10.1071/BT13262
Submitted: 27 October 2013 Accepted: 19 March 2014 Published: 23 April 2014
Abstract
A trade-off between shade tolerance and growth in open conditions is widely believed to underlie the dynamics of humid forests. Little is known about how the growth versus shade tolerance trade-off interacts with other major trade-offs associated with differential adaptation to major environmental factors besides light. We asked whether the growth versus shade tolerance trade-off differed between subtropical rainforest tree assemblages native to basaltic (fertile) and rhyolitic (infertile) soils in northern New South Wales, because of the allocational costs of adaptation to low nutrient availability. Seedling relative growth rates of six basalt specialists and five rhyolite specialists were measured in a glasshouse and the minimum light requirements of each species were quantified in the field by determining the 10th percentile of juvenile tree distributions in relation to understorey light availability. A similar range of light requirements was observed in the two assemblages, and although the two fastest growing species were basalt specialists, seedling growth rates did not differ significantly between the two substrates. The overall relationship between light requirements and growth rate was weak, and there was no compelling evidence that the slope or elevation of this relationship differed between the two assemblages. Growth rates were significantly correlated, overall, with specific leaf area, and marginally with leaf area ratio. The apparent similarity of the growth versus shade tolerance trade-off in the two suites of species could reflect effects of leaf nutrient content on respiration rates; basalt specialists tended to have a smaller root mass fraction, but this may have been offset by the effects of leaf nitrogen status on respiration rates, with higher respiration rates expected on fertile basaltic soils. However, the results might also partly reflect impairment of the field performance of two basalt specialists that were heavily attacked by natural enemies.
Additional keywords: forest dynamics, light compensation point for growth, relative growth rate, shade tolerance.
Introduction
A well-known trade-off between shade tolerance and growth in open conditions is widely believed to underlie secondary succession and the dynamics of humid forests. Fast-growing pioneer trees that establish after major disturbances are eventually replaced, mainly by slower-growing species that are more tolerant of shade (e.g. Bazzaz and Pickett 1980; Shugart 1984). The trade-off between growth rate and shade tolerance is also thought to contribute to tree species coexistence in old-growth stands (Denslow 1987; Poulson and Platt 1989; Kobe 1999), where tree-fall gaps provide opportunities for regeneration of light-demanding trees (Brokaw 1985; Keddy and MacLellan 1990; Veblen 1992). This trade-off has been demonstrated in closed forests from several biomes (Hubbell and Foster 1992; Kobe et al. 1995; Lin et al. 2002; Poorter and Bongers 2006; Sánchez-Gómez et al. 2006; Lusk et al. 2013b).
Despite its documentation in many different assemblages, little is known about how the growth versus shade tolerance trade-off interacts with other major trade-offs associated with differential adaptation to major environmental factors besides light. Smith and Huston (1989) attempted an integrated explanation of succession and zonation of woody vegetation along rainfall gradients, on the basis of trade-offs between growth, shade tolerance and drought tolerance. Their framework predicted truncated successions on arid sites, where, because of the allocational costs of adaptation to drought, the dominant species should be slower growing than pioneer trees on mesic sites, and less shade-tolerant than late-successional species on mesic sites. There has been little formal testing of this framework. However, Lusk et al. (2013a) produced evidence than the relationship between shade tolerance and maximum growth rate may shift along temperature gradients in temperate rainforests, cool-temperate species being, on average, less shade-tolerant than warm-temperate species, despite having similar average growth rates. Cool-temperate species were found to develop smaller foliage areas per unit of sapwood cross-sectional area, reflecting their lower sapwood conductance and narrower vessels. A trade-off between light interception potential and frost resistance was thus hypothesised to underlie species sorting on temperature gradients.
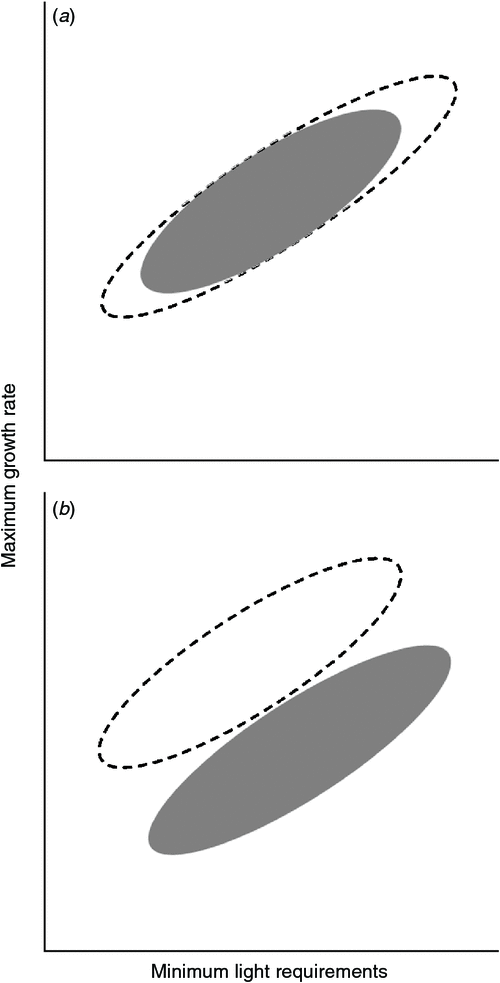
The growth versus shade tolerance trade-off might also be expected to interact with differential adaptation to edaphic gradients. Plants native to poor soils are often inherently slow-growing, reflecting selection for nutrient-conserving traits (especially slow turnover of foliage) that detract from growth rates (Lambers and Poorter 1992). A recent study showed that a temperate rainforest woody assemblage on a phosphorus-rich young alluvial soil encompassed a wider range of growth rates than that found on older, phosphorus-poor soils nearby, due to a scarcity of fast-growing trees on the latter site (Coomes et al. 2009). Because leaf area index tends to be lower on poor soils, selection for shade tolerance there may be weaker (Coomes et al. 2009). Furthermore, selection in nutrient-poor environments is likely to favour heavy allocation of carbon to roots and microbial symbionts (Paz 2003; Poorter et al. 1991; Schläpfer and Ryser 1996), presumably elevating whole-plant light compensation points as well as depressing maximum growth rates (Fig. 1b). These predictions are partially supported by evidence that tropical rainforest trees native to nutrient-poor sandstone ridges tend to have higher whole-plant compensation points (i.e. are less shade-tolerant) than congeners found on fertile alluvial soils (Baltzer and Thomas 2007); however, comparable data on maximum growth rates were not published. To our knowledge, no study has empirically compared growth versus shade tolerance trade-offs in assemblages native to soils differing significantly in nutrient availability.
|
Here we compare the light requirements and seedling growth rates of suites of subtropical rainforest trees characteristic of contrasting soil types. In northern New South Wales, rainforest occurs widely on both nutrient-rich basaltic soils and poorer rhyolitic soils (Baur 1957; Turner and Kelly 1981). The soils derived from basalt have much higher levels of total phosphorus, and although total nitrogen levels differ much less, carbon : nitrogen ratios are also much more favourable on soils developed from basalt (Table 1). Other important differences identified by Turner and Kelly (1981) include much higher exchangeable calcium in basaltic soils, and lower exchangeable aluminium. Rainforests on basalt and rhyolite in this region correspond to the ‘complex notophyll vine forest’ and ‘simple notophyll vine forest’ structural types recognised by Webb (1968). Although some species are common to both substrates, we focus here on specialists that are strongly associated with one or the other.

|
We tested two alternative hypotheses about the relationship between growth rates and light requirements in the two assemblages (Fig. 1). Coomes et al. (2009) predicted that forest assemblages on poor soils would comprise a limited range of ‘shade-tolerance strategies’, lacking both the fastest growing pioneers and the most shade-tolerant trees found on more fertile sites (Fig. 1a); the former are deemed unable to sustain their rapid tissue turnover rates in nutrient-poor environments, whereas the latter are predicted to be uncompetitive there because low leaf area indices result in a lack of deep shade. Alternatively, the relationship between light requirements and maximum growth rates could differ fundamentally between assemblages native to environments of contrasting fertility (Fig. 1b). The traits that enable plants to deal with low nutrient availability, including greater allocation of carbon to roots, should raise whole-plant compensation points as well as detracting from maximum growth rates. At a common level of shade tolerance, we might therefore expect rhyolite specialists to be slower growing than basalt specialists.
Materials and methods
Plant material
Seedlings of common species from each substrate type were obtained from a commercial nursery: six species characteristic of basalt, and five of rhyolite. We aimed to include species that represented the range of shade tolerance and growth rates present in assemblages on the two substrates (Table 2). Accordingly, we selected some species from among those that were well represented by juveniles in shaded understories (e.g. Argyrodendron trifoliolatum on basalt, Cryptocarya glaucescens on rhyolite), as well as the most common of those regenerating mainly on recently disturbed sites (e.g. Polyscias elegans on basalt, Callicoma serratifolia on rhyolite), and some that were common in environments with intermediate light (e.g. Diploglottis australis on basalt, Cryptocarya rigida on rhyolite). As relative growth rates decline during ontogeny (Evans 1972; Walters et al. 1993), we aimed to minimise initial size variation across the 10 species (Table 2).
Growth conditions
The experiment was carried out in a glasshouse at University of New England, Armidale, New South Wales (30°30′S, 151°39′E; elevation 980 m amsl). Plants were grown in 1-L pots filled with washed river sand. Nutrients were supplied in the form of 10 g Osmocote Plus (Scotts Australia, Bella Vista, NSW, Australia) slow-release fertiliser per pot. Pots were watered to field capacity twice weekly. Positions of individual pots were randomly assigned to positions on benches. Temperatures in the glasshouse ranged from 12 to 28°C, being regulated by an evaporative cooling system and under-floor heating. Light levels on the benches averaged ~15% of those measured outside the glasshouse.
Measurements
Stem growth of 9–12 seedlings per species was measured over ~250 days, from early February until late October in 2012. Stem basal diameter2 × length scales closely with whole-plant biomass (Kohyama and Hotta 1990), so changes in these dimensions of the main stem of each seedling were used as a proxy for growth of the whole plant (Baltzer and Thomas 2007). Stem volume was modelled as πr2 × length, and relative growth rates of stem volume (RGRvol) were thus estimated as:
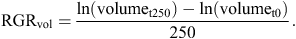
To relate growth to initial biomass distribution parameters, four to nine extra seedlings of each species were harvested at the start of this growth period and separated into leaf, stem and root fractions. Leaf area was determined using a flatbed scanner with ASSESS 2.0 image analysis software (American Phytopathological Society), enabling estimation of initial specific leaf area (SLA) and leaf area ratio (LAR); as determinants of the efficiency of light interception at whole-plant level, SLA and LAR often correlate strongly with seedling relative growth rates (e.g. Lambers and Poorter 1992; Cornelissen et al. 1996; Veneklaas and Poorter 1998). Plant material was dried at 65°C for 48 h before determining dry weights. At the end of the growth period, all remaining live seedlings were harvested. Mortality reduced the final sample sizes of Callicoma serratifolia, Flindersia schottiana and Diploglottis australis to seven each, but growth and data were eventually obtained from at least nine individuals of all other species.
Quantifying species light requirements
The 10th percentile of the distribution of each species in relation to light availability (mean daily photon flux density, PPFD10%) was used as an index of the minimum light level tolerated by each species (cf. Lusk and Reich 2000; Lusk et al. 2010). Shade-tolerant species such as Argyrodendron trifoliolatum have low PPFD10% values, whereas light-demanding species such as Polyscias elegans have high PPFD10% values (Table 2).
Sampling was carried out on transects run through both second-growth and old-growth stands, and included forest margins and tree-fall gaps.
Naturally occurring saplings with an initial height of 40–120 cm were selected randomly across as wide a range of light environments as possible, with sampling carried out along forest margins as well as within forest stands. A Nikon Coolpix 4500 digital camera (Nikon, Tokyo, Japan) and an EC-08 fisheye adaptor was used to take hemispherical photographs above each individual, with the top of the camera oriented north. Photos were taken when trees were tagged and when final growth measurements were made, and the average PPFD value was used in analyses to account for any changes in light regime over the course of the study. Photos were analysed using the Gap Light Analyzer ver. 2.0 software package (Frazer et al. 1999) to obtain the mean daily PPFD transmittance above each sapling. Cloudiness, spectral fraction and beam fraction (Frazer et al. 1999) were estimated using MODIS satellite photos to quantify the frequency of cloud cover above our main worksites on basalt (28°37′52″S, 153°20′19″E) and rhyolite (28°37′43″S, 153°21′54″E) (cf. Lusk et al. 2013b).
Statistical analysis
The statistical program SMATR ver. 2.0 (Falster et al. 2006) was used to determine the standardised major axis of the relationship between seedling growth rate and PPFD10%, and to ask whether the slope or elevation of this relationship differed between the two suites of species. The same procedure was used to examine relationships of biomass distribution parameters (SLA, LAR) with growth rates. PC-ORD (Version 6. MjM Software, Gleneden Beach, Oregon, USA) was used to perform principal components analysis, so as to determine whether multivariate analysis distinguished between species native to the two different environments. The variables included were logPPFD10%, logRGRvol, logSLA, leaf mass fraction and root mass fraction.
Results
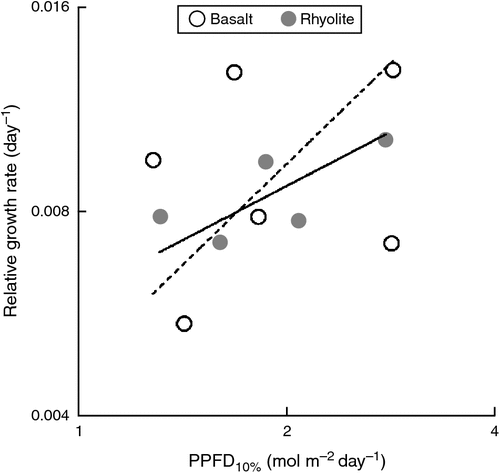
The PPFD10% data showed a similar range of light requirements in the two suites of species (Fig. 2). Although RGRvol of basalt and rhyolite specialists did not differ significantly on average (P = 0.76), there was a wider range of variation among the former (Fig. 2), which included the two fastest growing species (Polyscias elegans, Mallotus philippensis).
|
Overall, the relationship between growth rates and species light requirements (as indicated by PPFD10%) was weak (Fig. 2). The standardised major axis of this relationship did not differ significantly in slope between the two suites of species (P = 0.374), nor was there any significant difference in elevation (P = 0.8). However, the value of the test for differences in slope is questionable, in view of the weakness of the relationship among basalt specialists (Table 3).
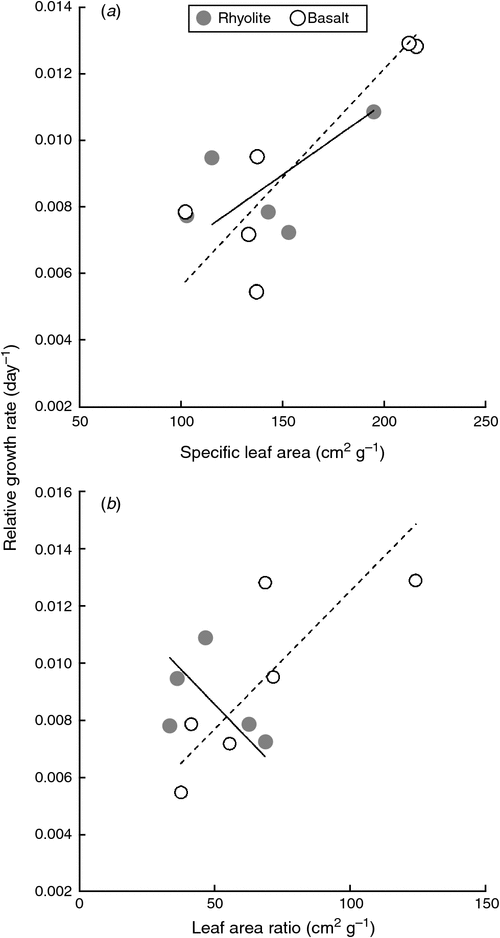
Although SLA and LAR did not differ significantly between the two suites of species (P = 0.62 and P = 0.31, respectively), the two highest values of both SLA and LAR were found in basalt specialists (Table 2). Root mass fraction tended to be higher in rhyolite specialists than in basalt specialists (mean 0.36 vs 0.31), although again, this difference was not significant (P = 0.16).
Both SLA and LAR were positively correlated overall with RGRvol, although the latter correlation was only marginally significant (r = 0.64, P = 0.034 and r = 0.57, P = 0.066, respectively). There was no compelling evidence that the standardised major axis of these two relationships differed in slope between the two assemblages (Fig. 3, P = 0.357 and P = 0.445, respectively), nor were there differences in elevation (P = 0.852 and P = 0.514, respectively). Because of weak relationships within both suites of species, neither SLA nor LAR showed clear overall relationships with PPFD10% (Table 3, Fig. 3). Root mass fraction was not correlated overall with either RGRvol or PPFD10% (P > 0.75 in both cases).
|
Principal components analysis (Fig. 4) showed the first axis of variation to be associated with biomass partitioning (leaf mass fraction, root mass fraction). This axis distinguished fairly well between the two assemblages, with rhyolite specialists mostly scoring low, and basalt specialists mostly scoring high. Axis 2 was associated with growth rate, SLA, and to a lesser extent PPFD10%.
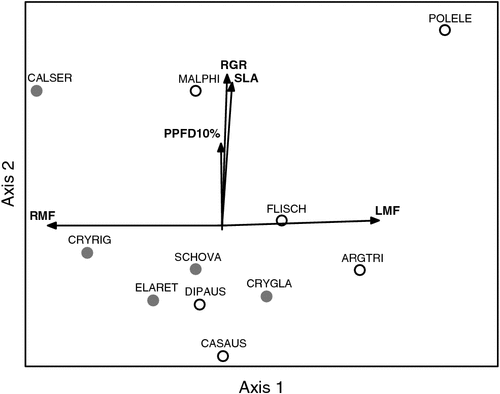
|
Discussion
At variance with our first hypothesis (Fig. 1a), basalt and rhyolite associates spanned essentially the same range of light requirements (Figs 2, 3). Our selection represents only a small fraction of typical tree species richness in Australian subtropical rainforests, especially the diverse assemblages found on basalt (Floyd 1989; Royer et al. 2009). However, we believe that our selection included the most shade-tolerant, common tree species on both substrate types, our choice being based on experience sampling in Nightcap National Park, NSW (Lusk et al. 2010; Kooyman et al. 2013; K. M. Sendall, P. B. Reich and C. H. Lusk, unpubl. data). We were unable to obtain seedlings of one of the dominant rainforest canopy species on rhyolitic soils, Ceratopetalum apetalum. However, this species is unlikely to be the most shade-tolerant, common member of the assemblage; its seedlings and saplings were relatively uncommon in the understory. We conclude that there is no compelling evidence of difference in shade tolerance between late-successional species on basaltic versus rhyolitic soils in subtropical Australia. This seems at odds with Baltzer and Thomas (2007), who reported that rainforest tree species native to fertile alluvial soils in Borneo tended to have lower compensation points (i.e. be more shade-tolerant) than congeners associated with nutrient-poor sandstone ridges nearby. However, we note that soils developed over rhyolite in Nightcap National Park often support dense rainforest ~30 m tall, so the rhyolite specialists we studied cannot be regarded as adapted to extreme low fertility. On sites with harsher edaphic conditions, where low leaf area indices mean that understory seedling growth is limited more by nutrients than by light availability, we would not expect to find highly shade-tolerant species, because in the absence of deep shade they should be uncompetitive (Coomes et al. 2009). We can say less with confidence about the other end of the shade-tolerance spectrum. Although the most light-demanding species from the two substrates had very similar PPFD10% (Fig. 2), our study omitted the widespread pioneer tree Polyscias murrayi, which is strongly associated with basaltic and other fertile soils (Floyd 1989) and known to be more light-demanding and faster growing than any of the species we did include (K. M. Sendall, P. B. Reich and C. H. Lusk, unpubl. data). Unfortunately, seedlings of P. murrayi were not available for our experiment. Therefore, it seems likely that assemblages on basalt include some species that are more light-demanding than any present in rainforest assemblages on rhyolite.
Although as predicted, the basalt specialists encompassed a wider range of growth rates than the species associated with rhyolite, growth rates of the former showed little relationship with light requirements (Fig. 2). However, seedling growth rates showed positive overall relationships with SLA and LAR, a pattern widely reported in growth analyses (e.g. Lambers and Poorter 1992; Cornelissen et al. 1996; Veneklaas and Poorter 1998). The solidity that these correlations lend to the seedling growth data suggests to us that the failure to find a clear relationship between growth rates and light requirements of basalt specialists might reflect anomalies in the field data on species light requirements. Juvenile trees of two of the basalt specialists we studied were subject to intense attacks by natural enemies during our fieldwork in Nightcap National Park; most juvenile Flindersia schottiana were heavily infested with leaf galls, and many juvenile Diploglottis australis had been damaged by stem borers that sometimes caused the death of the apex. High levels of herbivory and parasitism reduce plant growth across all light environments (Schaffer and Mason 1990; Norghauer and Newbery 2014), and so can be expected to increase the amount of light required to achieve a positive carbon balance. Our data on the light requirements of F. schottiana and D. australis may thus reflect the impact of natural enemies on their field performance and distributions; the PPFD10% values we calculated for these two species are higher than expected on the basis of their growth rates. Data published in previous comparative studies show that growth rates of these two species are moderate (Stocker 1981; Kelly et al. 2009), suggesting both are probably mid-successional trees of intermediate shade tolerance.
At variance with our second hypothesis (Fig. 1b), we found no compelling evidence that the relationship between shade tolerance and growth rate differed between basalt and rhyolite specialists. This negative result cannot be attributed solely to the putative impairment of field performance of F. schottiana and D. australis by natural enemies; growth rates and PPFD10% both overlapped considerably between the two suites of species, and the species with the lowest seedling RGR was a basalt specialist (Castanospermum australe, Table 2), which appeared healthy and vigorous under field conditions. Interestingly, our biomass partitioning data confirmed the expectation of higher root mass fraction in rhyolite specialists (Table 2, Fig. 4), which might be expected to elevate their minimum light requirements as well as reducing maximum growth rates. Yet this expectation was not met. Allocational differences might be offset by countervailing patterns in leaf respiration rates, reflecting differences in tissue nitrogen status; our own field data show that saplings growing on rhyolite have lower leaf nitrogen and respiration rates than conspecifics growing on basalt (K. M. Sendall, P. B. Reich and C. H. Lusk, unpubl. data). Data from a fertility gradient associated with a soil chronosequence in New Zealand show a less conclusive pattern (Turnbull et al. 2005). Species associated with old, infertile soils tended to have lower mass-based respiration rates than the dominant species on more fertile soil, but differences in area-based respiration rates were minimal; intraspecific variation along the chronosequence was also minimal, on both area and mass bases (Turnbull et al. 2005). It is acknowledged that tests based on our dataset of 11 species involve a high risk of false negative results; the small sample sizes limit our power to detect small, but potentially relevant, differences between the assemblages associated with basaltic and rhyolitic soils.
In conclusion, we found no clear evidence that the dominant, late-successional rainforest tree species on basaltic versus rhyolitic soils differ in shade tolerance or potential growth rates. Likewise, we found no clear evidence that the overall relationship between growth rate and shade tolerance differed substantially between assemblages from the two soil types, although the fastest growing pioneers on basalt (e.g. Polyscias murrayi) are known to outstrip any rainforest trees native to rhyolite (K. M. Sendall, P. B. Reich and C. H. Lusk, unpubl. data). Because of the small number of species involved in our study, we cannot rule out the possibility that these negative results reflect limited statistical power. Furthermore, our findings should not be extrapolated to soils of lower fertility than the rhyolitic soils in our study area, which, although relatively low in total P, have ample N levels (Table 1). Different findings may arise from studies encompassing sites where nutrient levels are low enough to significantly reduce overstorey leaf area index and hence understory light availability (cf. Coomes et al. 2009), resulting in different selective pressures.
Acknowledgements
This research was funded by ARC Discovery Project 1094606. We thank New South Wales National Parks and Wildlife Service for permission to work in Nightcap National Park.
References
Baltzer JL, Thomas SC (2007) Determinants of whole-plant light requirements in Bornean rain forest tree saplings. Journal of Ecology 95, 1208–1221.| Determinants of whole-plant light requirements in Bornean rain forest tree saplings.Crossref | GoogleScholarGoogle Scholar |
Baur G (1957) Nature and distribution of rain-forests in New South Wales. Australian Journal of Botany 5, 190–233.
| Nature and distribution of rain-forests in New South Wales.Crossref | GoogleScholarGoogle Scholar |
Bazzaz F, Pickett S (1980) Physiological ecology of tropical succession: a comparative review. Annual Review of Ecology and Systematics 11, 287–310.
| Physiological ecology of tropical succession: a comparative review.Crossref | GoogleScholarGoogle Scholar |
Brokaw NVL (1985) Gap-phase regeneration in a tropical forest. Ecology 66, 682–687.
| Gap-phase regeneration in a tropical forest.Crossref | GoogleScholarGoogle Scholar |
Coomes DA, Kunstler G, Canham CD, Wright E (2009) A greater range of shade-tolerance niches in nutrient-rich forests: an explanation for positive richness–productivity relationships? Journal of Ecology 97, 705–717.
| A greater range of shade-tolerance niches in nutrient-rich forests: an explanation for positive richness–productivity relationships?Crossref | GoogleScholarGoogle Scholar |
Cornelissen JHC, Diez PC, Hunt R (1996) Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. Journal of Ecology 84, 755–765.
| Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types.Crossref | GoogleScholarGoogle Scholar |
Denslow JS (1987) Tropical rainforest gaps and tree species diversity. Annual Review of Ecology and Systematics 18, 431–451.
| Tropical rainforest gaps and tree species diversity.Crossref | GoogleScholarGoogle Scholar |
Evans GC (1972) ‘The quantitative analysis of plant growth.’ (University of California Press: Berkeley, CA)
Falster DS, Warton DI, Wright IJ (2006) ‘SMATR: Standardised major axis tests and routines. Version 2.0.’ (Macquarie University: Sydney)
Floyd AG (1989) ‘Rainforest trees of mainland south-eastern Australia.’ (Inkata Press: Melbourne)
Frazer GW, Canham CD, Lertzman KP (1999) ‘Gap light analyzer (GLA): imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs. Users manual and program documentation, Version 2.0.’ (Simon Frazer University: Burnaby, BC, Canada; Institute of Ecosystem Studies: Millbrook, NY)
Hubbell SP, Foster RB (1992) Short-term dynamics of a neotropical forest: why ecological research matters to tropical conservation and management. Oikos 63, 48–61.
| Short-term dynamics of a neotropical forest: why ecological research matters to tropical conservation and management.Crossref | GoogleScholarGoogle Scholar |
Keddy PA, MacLellan P (1990) Centrifugal organization in forests. Oikos 59, 75–84.
| Centrifugal organization in forests.Crossref | GoogleScholarGoogle Scholar |
Kelly J, Jose S, Nichols JD, Bristow M (2009) Growth and physiological response of six Australian rainforest tree species to a light gradient. Forest Ecology and Management 257, 287–293.
| Growth and physiological response of six Australian rainforest tree species to a light gradient.Crossref | GoogleScholarGoogle Scholar |
Kobe RK (1999) Light gradient partitioning among tropical tree species through differential seedling mortality and growth. Ecology 80, 187–201.
| Light gradient partitioning among tropical tree species through differential seedling mortality and growth.Crossref | GoogleScholarGoogle Scholar |
Kobe RK, Pacala SW, Silander JA, Canham CD (1995) Juvenile tree survivorship as a component of shade tolerance. Ecological Applications 5, 517–532.
| Juvenile tree survivorship as a component of shade tolerance.Crossref | GoogleScholarGoogle Scholar |
Kohyama T, Hotta M (1990) Significance of allometry in tropical saplings. Functional Ecology 4, 515–521.
| Significance of allometry in tropical saplings.Crossref | GoogleScholarGoogle Scholar |
Kooyman RM, Zanne AE, Gallagher RV, Cornwell W, Rossetto M, O’Connor P, Parkes EA, Catterall CF, Laffan SW, Lusk CH (2013) Effects of growth form and functional traits on response of woody plants to clearing and fragmentation of subtropical rainforest. Conservation Biology 27, 1468–1477.
| Effects of growth form and functional traits on response of woody plants to clearing and fragmentation of subtropical rainforest.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3sfitFGgug%3D%3D&md5=559acdd85fae1433f4648d16fa45c014CAS |
Lambers H, Poorter H (1992) Inherent variation in growth rate between higher plants: a search for physiological causes and consequences. Advances in Ecological Research 23, 187–261.
| Inherent variation in growth rate between higher plants: a search for physiological causes and consequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXksVGiu7w%3D&md5=a56e63ef88ff03478d27a0d6cbbf1d40CAS |
Lin J, Harcombe PA, Fulton MR, Hall RW (2002) Sapling growth and survivorship as a function of light in a mesic forest of southeast Texas, USA. Oecologia 132, 428–435.
| Sapling growth and survivorship as a function of light in a mesic forest of southeast Texas, USA.Crossref | GoogleScholarGoogle Scholar |
Lusk CH, Reich PB (2000) Relationships of leaf dark respiration with light environment and tissue nitrogen content in juveniles of 11 cold-temperate tree species. Oecologia 123, 318–329.
| Relationships of leaf dark respiration with light environment and tissue nitrogen content in juveniles of 11 cold-temperate tree species.Crossref | GoogleScholarGoogle Scholar |
Lusk CH, Onoda Y, Kooyman R, Gutiérrez-Girón A (2010) Reconciling species-level versus plastic responses of evergreen leaf structure to light gradients: shade leaves punch above their weight. New Phytologist 186, 429–438.
| Reconciling species-level versus plastic responses of evergreen leaf structure to light gradients: shade leaves punch above their weight.Crossref | GoogleScholarGoogle Scholar | 20202128PubMed |
Lusk CH, Kaneko T, Grierson E, Clearwater M (2013a) Correlates of tree species sorting along a temperature gradient in New Zealand rain forests: seedling functional traits, growth and shade tolerance. Journal of Ecology 101, 1531–1541.
| Correlates of tree species sorting along a temperature gradient in New Zealand rain forests: seedling functional traits, growth and shade tolerance.Crossref | GoogleScholarGoogle Scholar |
Lusk CH, Kelly JWG, Gleason SM (2013b) Light requirements of Australian tropical vs. cool-temperate rainforest tree species show different relationships with seedling growth and functional traits. Annals of Botany 111, 479–488.
| Light requirements of Australian tropical vs. cool-temperate rainforest tree species show different relationships with seedling growth and functional traits.Crossref | GoogleScholarGoogle Scholar | 23264237PubMed |
Norghauer J, Newbery D (2014) Herbivores differentially limit the seedling growth and sapling recruitment of two dominant rain forest trees. Oecologia 174, 459–469.
Paz H (2003) Root/shoot allocation and root architecture in seedlings: variation among forest sites, microhabitats, and ecological groups. Biotropica 35, 318–332.
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87, 1733–1743.
| Leaf traits are good predictors of plant performance across 53 rain forest species.Crossref | GoogleScholarGoogle Scholar | 16922323PubMed |
Poorter H, Van der Werf A, Atkin OK, Lambers H (1991) Respiratory energy requirements of roots vary with the potential growth rate of a plant species. Physiologia Plantarum 83, 469–475.
| Respiratory energy requirements of roots vary with the potential growth rate of a plant species.Crossref | GoogleScholarGoogle Scholar |
Poulson TL, Platt WJ (1989) Gap light regimes influence canopy tree diversity. Ecology 70, 553–555.
| Gap light regimes influence canopy tree diversity.Crossref | GoogleScholarGoogle Scholar |
Royer DL, Kooyman RM, Little SA, Wilf P (2009) Ecology of leaf teeth: a multi-site analysis from an Australian subtropical rainforest. American Journal of Botany 96, 738–750.
| Ecology of leaf teeth: a multi-site analysis from an Australian subtropical rainforest.Crossref | GoogleScholarGoogle Scholar | 21628229PubMed |
Sánchez-Gómez D, Valladares F, Zavala MA (2006) Performance of seedlings of Mediterranean woody species under experimental gradients of irradiance and water availability: trade-offs and evidence for niche differentiation. New Phytologist 170, 795–806.
| Performance of seedlings of Mediterranean woody species under experimental gradients of irradiance and water availability: trade-offs and evidence for niche differentiation.Crossref | GoogleScholarGoogle Scholar | 16684239PubMed |
Schaffer B, Mason LJ (1990) Effects of scale insect herbivory and shading on net gas exchange and growth of a subtropical tree species (Guaiacum sanctum L.). Oecologia 84, 468–473.
Schläpfer B, Ryser P (1996) Leaf and root turnover of three ecologically contrasting grass species in relation to their rerformance along a productivity gradient. Oikos 75, 398–406.
| Leaf and root turnover of three ecologically contrasting grass species in relation to their rerformance along a productivity gradient.Crossref | GoogleScholarGoogle Scholar |
Shugart HH (1984) ‘A theory of forest dynamics. The ecological implications of forest succession models.’ (Springer-Verlag: Berlin)
Smith T, Huston M (1989) A theory of the spatial and temporal dynamics of plant communities. Vegetatio 83, 49–69.
| A theory of the spatial and temporal dynamics of plant communities.Crossref | GoogleScholarGoogle Scholar |
Stocker GC (1981) Regeneration of a North Queensland rain forest following felling and burning. Biotropica 13, 86–92.
| Regeneration of a North Queensland rain forest following felling and burning.Crossref | GoogleScholarGoogle Scholar |
Turnbull MH, Tissue DT, Griffin KL, Richardson SJ, Peltzer DA, Whitehead D (2005) Respiration characteristics in temperate rainforest tree species differ along a long-term soil-development chronosequence. Oecologia 143, 271–279.
| Respiration characteristics in temperate rainforest tree species differ along a long-term soil-development chronosequence.Crossref | GoogleScholarGoogle Scholar |
Turner J, Kelly J (1981) Relationships between soil nutrients and vegetation in a north coast forest, New South Wales. Australian Forest Research 11, 201–208.
Veblen TT (1992) Regeneration dynamics. In ‘Plant succession: theory and prediction’. (Eds DC Glenn-Lewin, RK Peet, TT Veblen) pp. 135–145. (Chapman and Hall: London)
Veneklaas EJ, Poorter L (1998) Growth and carbon partitioning of tropical tree seedlings in contrasting light environments. In ‘Inherent variation in plant growth. Physiological mechanisms and ecological consequences’. (Eds H Lambers, H Poorter, MMI van Vuuren) pp. 337–361. (Backhuys Publishers: Leiden, The Netherlands)
Walters MB, Kruger EL, Reich PB (1993) Relative growth-rate in relation to physiological and morphological traits for northern hardwood tree seedlings: species, light environment and ontogenic considerations. Oecologia 96, 219–231.
| Relative growth-rate in relation to physiological and morphological traits for northern hardwood tree seedlings: species, light environment and ontogenic considerations.Crossref | GoogleScholarGoogle Scholar |
Webb LJ (1968) Environmental relationships of the structural types of Australian rainforest vegetation. Ecology 49, 296–311.
| Environmental relationships of the structural types of Australian rainforest vegetation.Crossref | GoogleScholarGoogle Scholar |




