Hydrocarbon Surfactants for CO2: An Impossible Dream?
Julian Eastoe A C , Sarah Gold A and David C. Steytler BA School of Chemistry, University of Bristol, Bristol, BS8 1TS, UK.
B School of Chemical Sciences and Pharmacy, University of East Anglia, Norwich NR4 7TJ, UK.
C Corresponding author. Email: julian.eastoe@bris.ac.uk
Australian Journal of Chemistry 60(9) 630-632 https://doi.org/10.1071/CH07117
Submitted: 30 April 2007 Published: 11 September 2007
Abstract
It used to be thought that hydrocarbon ionic surfactants were incompatible with dense liquid or supercritical CO2, and fluorination of the chains was necessary to achieve any appreciable solubility. Here it is shown that branching of pendant hydrocarbon chains, especially methylation of the tips, can lead to substantial solubility enhancements in CO2 over normal linear chain hydrocarbon surfactants. In addition, increasing the number of these t-butyl-tipped groups from single-chained, through twin-tailed, to triple-chain surfactants has a profound effect on CO2 compatibility. High-pressure interfacial tension, small-angle neutron scattering, and near infrared spectroscopy have been applied to reveal the effects of surfactant structure on surface activity, aggregation, and solubility in CO2.
Acknowledgements
SG thanks the University of Bristol DTA grant for a PhD studentship. The UK research council EPSRC awarded funding through grants (EP/C523105/1, GR/L05532 and GR/L25653). CCLRC are thanked for provision of neutron scattering facilities at the Rutherford Appleton Laboratory.
[1]
J. Eastoe,
S. Gold,
D. C. Steytler,
Langmuir 2006, 22, 9832.
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |

[10]
[11]
* See Ref. [9]. In this reference it is proposed that the most effective CO2-philes should have a low FFV, which is as defined by 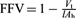 , where Vt is the physical volume of the unsolvated tail groups, l is the thickness of the interface, and Ah is the interfacial area per surfactant molecule. From a range of potential hydrocarbon surfactants for CO2 applications, AOT 4 was shown to have the most favorable FFV value.
, where Vt is the physical volume of the unsolvated tail groups, l is the thickness of the interface, and Ah is the interfacial area per surfactant molecule. From a range of potential hydrocarbon surfactants for CO2 applications, AOT 4 was shown to have the most favorable FFV value.
† The analogue AOT 3 has the same chain architecture, but is shorter than AOT 4 by one CH2 group in the linear part of the alkyl portion close to the ester linkages.
‡ One possibility is that the SC 4 signal is slightly higher owing to excess 3,5,5-trimethylhexan-1-ol, which is the precursor alcohol. It is known from careful surface tension measurements that AOT 4 and TC 4 do not suffer from this possible contamination.


