Living Radical Polymerization by the RAFT Process—A First Update
Graeme Moad A , Ezio Rizzardo A and San H. Thang AA CSIRO Molecular and Health Technologies, Bag 10, Clayton South, VIC 3169, Australia. Email: graeme.moad@csiro.au; ezio.rizzardo@csiro.au; san.thang@csiro.au

Graeme Moad obtained his B.Sc.(Hons1) in 1974 and Ph.D. in 1977 from the University of Adelaide in the field of organic free radical chemistry. Between 1977 and 1979 he undertook postdoctoral research at Pennsylvania State University in the field of biological organic chemistry. He joined CSIRO as a research scientist in 1979 and is currently a chief research scientist. Dr Moad is coauthor of the book ‘The Chemistry of Free Radical Polymerization’ which appeared as a second edition in 2006. His research interests lie in the fields of polymer design and synthesis (free radical polymerization, reactive extrusion), polymerization kinetics and mechanism, and most recently polymer nanocomposites. |

Ezio Rizzardo is a graduate of the University of New South Wales and received his Ph.D. from the University of Sydney for his studies on the photochemistry of nitro compounds. He joined CSIRO in 1976 after a postdoc at Rice University, RIMAC, and the Australian National University. His CSIRO research has focussed on developing methods for controlling free radical polymerization. For this he has received a number of awards including the RACI Australian Polymer Medal and the CSIRO Chairman's Gold Medal. Currently he is a CSIRO Fellow and a Fellow of the Australian Academy of Science. |

San H. Thang was born in Saigon, Vietnam, in 1954 and came to Australia in 1979 as a refugee. He completed his B.Sc.(Hons) degree in 1983 and Ph.D. in 1987 from Griffith University. He joined CSIRO in 1986 as a research fellow. He then moved to ICI Australia in late 1987 to undertake the challenge of industrial research. He returned to CSIRO in late 1990, and in 1995 he was co-inventor of the RAFT Process. He is currently a senior principal research scientist at CSIRO Molecular and Health Technologies where his research focusses on the interface between organic and polymer chemistry. |
Australian Journal of Chemistry 59(10) 669-692 https://doi.org/10.1071/CH06250
Submitted: 17 July 2006 Accepted: 28 September 2006 Published: 30 October 2006
Abstract
This paper provides a first update to the review of living radical polymerization achieved with thiocarbonylthio compounds (ZC(=S)SR) by a mechanism of Reversible Addition–Fragmentation chain Transfer (RAFT) published in June 2005. The time since that publication has witnessed an increased rate of publication on the topic with the appearance of well over 200 papers covering various aspects of RAFT polymerization ranging over reagent synthesis and properties, kinetics, and mechanism of polymerization, novel polymer syntheses, and diverse applications.
Introduction
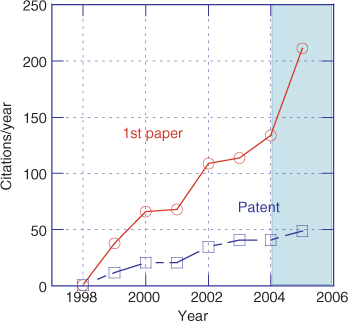
Radical polymerization is one of the most widely used processes for the commercial production of high molecular weight polymers.[1] The emergence over the last ten years of techniques for implementing living radical polymerization has provided a new set of tools for polymer chemists that allow very precise control over the polymerization process while retaining much of the versatility of conventional radical polymerization. It is no longer a formidable task to apply radical polymerization to the synthesis of blocks, stars, or other polymers of complex architecture. New materials that have the potential of revolutionizing a large part of the polymer industry continue to appear. The living radical polymerization techniques that are receiving greatest attention are nitroxide-mediated polymerization (NMP), atom-transfer radical polymerization (ATRP), and reversible addition–fragmentation chain transfer (RAFT). The increasing importance of RAFT in this context is illustrated by Fig. 1 that shows the increasing rate of citation of our first communication on RAFT with thiocarbonylthio compounds[2] and the first RAFT patent.[3] It should be noted that not all papers on RAFT polymerization cite these sources nor are all of the papers citing these documents relevant to RAFT polymerization.
|
This review is primarily intended to cover the literature on RAFT polymerization that has appeared since publication of our review of the field published in the Australian Journal of Chemistry in June 2005[4] and a review of similar scope contained within The Chemistry of Radical Polymerization.[1] We also refer to some earlier papers that were not included in that review. However, work mentioned in the previous review[4] is only mentioned again where necessary to put the more recent work in context. The last year has seen the publication of three further major reviews detailing the RAFT process.[5–7] Quiclet-Sire and Zard[8] have reviewed the application of radical addition–fragmentation processes in synthetic chemistry. Other reviews cover specific aspects of RAFT polymerization, such as computational studies related to RAFT agents and RAFT polymerization,[9] use of RAFT polymerization in heterogeneous media,[10,11] the synthesis of end functional polymers by RAFT polymerization,[12,13] the synthesis and properties of stimuli responsive block copolymers,[13] the preparation of honeycomb polymers,[14] and the control of molecular weight distributions produced by RAFT polymerization.[15] RAFT polymerization is also reviewed within works which deal more generically with radical polymerization. The process is reviewed within the chapter Living Radical Polymerization in The Chemistry of Radical Polymerization[1] and is covered in most recent reviews that, in part, relate to polymer synthesis, living polymerization, or novel architectures.[16–23] Mention should be made of the recent ACS Symposium Series volume Controlled/Living Radical Polymerization: From Synthesis to Materials in which approximately one third of the papers relate directly to RAFT polymerization.[24]
Mechanism of RAFT
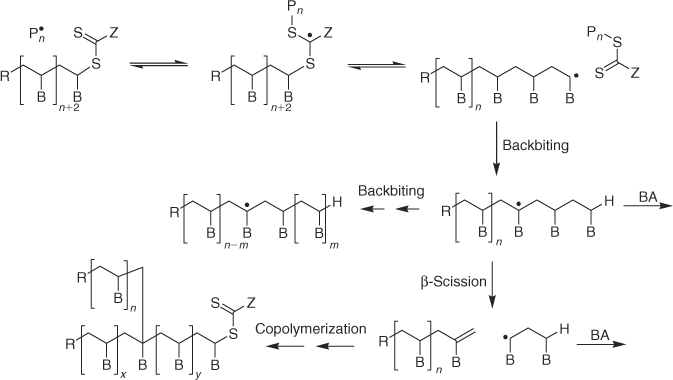
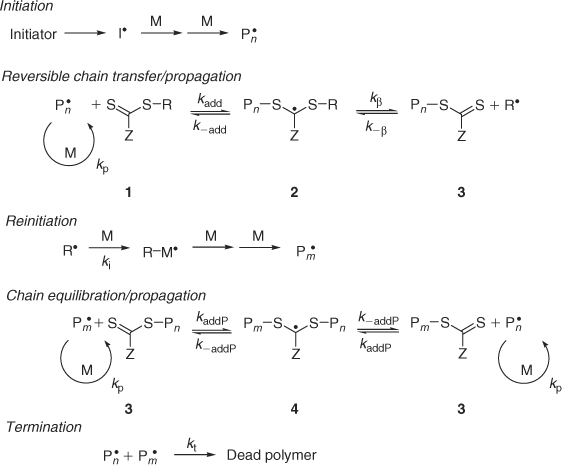
The key feature of the mechanism of RAFT polymerization with thiocarbonylthio compounds as proposed in our first communication on the subject[2] is the sequence of addition–fragmentation equilibria shown in Scheme 1. Initiation and radical–radical termination occur as in conventional radical polymerization. In the early stages of the polymerization, addition of a propagating radical (Pn•) to the thiocarbonylthio compound (RSC(Z)=S, 1) followed by fragmentation of the intermediate radical provides a polymeric thiocarbonylthio compound (PnS(Z)C=S, 3) and a new radical (R•). Reaction of this radical (R•) with monomer forms a new propagating radical (Pm•). Rapid equilibrium between the active propagating radicals (Pn• and Pm•) and the dormant polymeric thiocarbonylthio compounds 3 provides equal probability for all chains to grow and allows for the production of narrow polydispersity polymers. When the polymerization is complete (or stopped), most chains retain the thiocarbonylthio end group and can be isolated as stable materials.
|
The reactions associated with RAFT equilibria shown in Scheme 1 are in addition to those (i.e. initiation, propagation, and termination) that occur during conventional radical polymerization. In an ideal RAFT process, the RAFT agent should behave as an ideal transfer agent. Thus, as with radical polymerization with conventional chain transfer, the kinetics of polymerization should not be directly affected beyond those affects attributable to the differing molecular weights of the reacting species. Radical–radical termination is not directly suppressed by the RAFT process. Living characteristics are imparted when the molecular weight of the polymer formed is substantially lower than that which might be formed in the absence of a RAFT agent and the number of polymer molecules with RAFT agent derived ends far exceeds the number formed as a consequence of termination.
This said, RAFT polymerizations are frequently observed to stray from this ideal situation. Although the basic mechanism shown in Scheme 1 is generally not disputed, much debate continues on the kinetics of the RAFT process, the rapidity with which the various equilibria are established, and what side reactions might occur to complicate the process, the kinetics, and polymer syntheses. An IUPAC task group Towards a Holistic Mechanistic Model for RAFT Polymerizations: Dithiobenzoates as Mediating Agents was formed in 2005 under the auspices of the IUPAC Subcommittee on Modeling of Polymerization Kinetics and Processes. The first output of that working party, being a dilemma paper that summarizes the current situation, has just been published.[25]
It has been suggested at various times that the intermediates in the RAFT equilibria (2 and/or 4) might react reversibly with propagating radicals[26–28] and thus these species might be mediators of radical polymerization by a reversible termination mechanism.
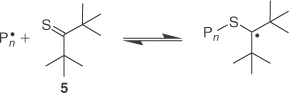
A more recent finding is that certain thioketones (e.g. 5) can act, albeit poorly, as control agents of radical polymerization.[29] The thioketones are proposed to act a spin-traps as shown in Scheme 2 and thus provide control by a reversible termination mechanism. RAFT agents (1), in particular those with poor leaving groups ‘R’, might, in principle, provide control over polymerization by similar chemistry. It is noteworthy that primary dialkyl trithiocarbonates are effectively inert in radical polymerization of styrene (with 51)[30] and BA (with 59).[31] This suggests that if or when radicals add to these trithiocarbonates, the intermediate formed undergoes no reactions other than rapid reversion to starting materials. There is no significant effect on the molecular weight distributions of the polymers formed in their presence.
|
Choice of RAFT Agents
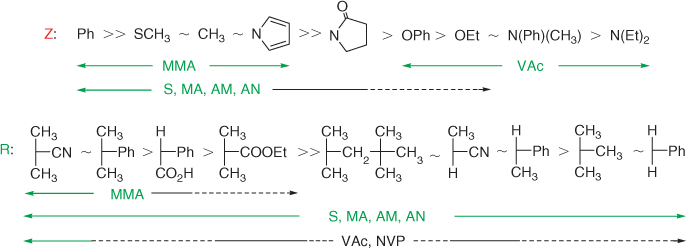
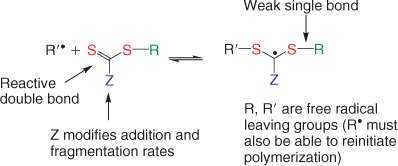
A wide variety of thiocarbonylthio RAFT agents (ZC(=S)SR 1) have been reported. A broad summary of these and the factors which influence choice of RAFT agent for a particular polymerization was presented in previous reviews.[1,4] The effectiveness of the RAFT agent depends on the monomer being polymerized and is determined by the properties of the free radical leaving group R and the group Z which can be chosen to activate or deactivate the thiocarbonyl double bond of the RAFT agent 1 and modify the stability of the intermediate radicals 2 and 4. For an efficient RAFT polymerization (Scheme 1, Fig. 2):
|
-
The initial RAFT agents 1 and the polymer RAFT agent 3 should have a reactive C=S double bond (high kadd).
-
The intermediate radicals 2 and 4 should fragment rapidly (high kβ, weak S–R bond in intermediate) and give no side reactions.
-
The intermediate 2 should partition in favour of products (kβ ≥ k–add).
-
The expelled radicals (R•) must efficiently re-initiate polymerization (ki > kp).
The properties of RAFT agents are often discussed in terms of the value of the equilibrium constants associated with radical addition to the thiocarbonylthio compound. Rates of addition are typically high (~106–108 M–1 s–1). Thus a high equilibrium constant generally implies a low fragmentation rate for the radical adduct and an increased likelihood for retardation and/or side reaction involving this species. Values of K do not, by themselves, provide sufficient information predict the ability of a RAFT agent to control polymerization.
In a given RAFT polymerization, there are at least four equilibrium constants that need to be considered.
-
KP (= kaddP/k–addP) associated with the main equilibrium.
-
K (= kadd/k–add) and Kβ (= k–β/kβ) associated with the pre-equilibrium.
-
KR (= kaddR/k–addR) associated with the reaction of the expelled radical with the initial RAFT agent (Scheme 3).
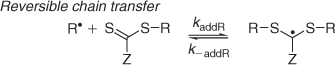
|
There may be other equilibrium constants to consider if penultimate group effects are significant (there are theoretical data[32,33] and some experimental evidence[34,35] to indicate that this is the case). There are also a further series of reactions that need to be considered that involve initiator radical derived RAFT agents. In principle, RAFT agents of differing reactivity might be derived from each radical species present.
Values of K may estimated by determining the concentrations of the radical intermediates in RAFT polymerization by EPR (or ESR) spectrometry.[36–39] Buback and co-workers[37] have reported what they term the SP-PLP-ESR-RAFT technique where the rate of build-up and decay of the intermediate following radical generation is followed by EPR spectroscopy and analyzed to provide addition and (overall) fragmentation rates. Coote and co-workers[9,32,33,40–43] have devised methods for calculating absolute values of K by applying ab initio methods. Values of K have also been estimated on the basis of simulation of the polymerization kinetics.[44–46] However, most simulations have assumed a simplified RAFT mechanism in which K is assumed independent of the radical structure. Values of K estimated on the basis of the measured concentrations of the radical intermediates are much lower than those predicted by theoretical calculations.
On the basis of computational studies on RAFT agents, Z–C(=S)SR, a RAFT agent with Z = fluorine was proposed[40,43,47] as a ‘universal’ RAFT agent able to efficiently control the polymerization of both activated (e.g. acrylates, styrene) and less-activated monomers (e.g. vinyl acetate). The full utility of this class of RAFT agent has yet to be demonstrated experimentally. It has only been tested in styrene polymerization where limited control was observed (poor correspondence between found and calculated molecular weights, slightly narrowed polydispersity).[47] The utility of dithiophosphinate esters (6; Scheme 4) as RAFT agents[48] has been questioned on the basis of the results of computational studies.[49]
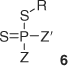
|
Matyjaszewski and Poli[50] have reported the results of density functional theory (DFT) calculations used to estimate R–S bond dissociation energies in thiocarbonylthio RAFT agents (R–S(C=S)Z) and suggest these may be used as a guide for the design of RAFT agents. Even though some parallels might be expected, it should be pointed out that it is the bond dissociation energy of R–S bond in the radical intermediate (R–S(C(•)–SR′)Z) rather than the RAFT agent (R–S(C=S)Z) that may be important in determining fragmentation rates (see Fig. 2). DFT calculations have also been applied in understanding the properties of radical adducts to phosphoryldithioacetates.[39] The suitability of DFT methods for calculations relating to RAFT polymerization has been questioned.[9,41]
The process of ‘initialization’, consumption of the initial low molecular weight RAFT agent, for styrene polymerization with cyanoisopropyl and cumyl dithiobenzoates at 70°C has been compared using in situ 1H NMR measurements.[51] The rate-determining step was found to be the rate of addition of the expelled radical (R•) to styrene which was proposed to be faster for R• = cyanoisopropyl than for R• = cumyl. Existing literature suggests that the absolute rate constants for addition to styrene by the two radicals are similar.[52,53] However, the rate constants for cyanoisopropyl radical addition to RAFT agents is substantially lower than that for cumyl.[52] Molecular orbital calculations provide support for this finding.
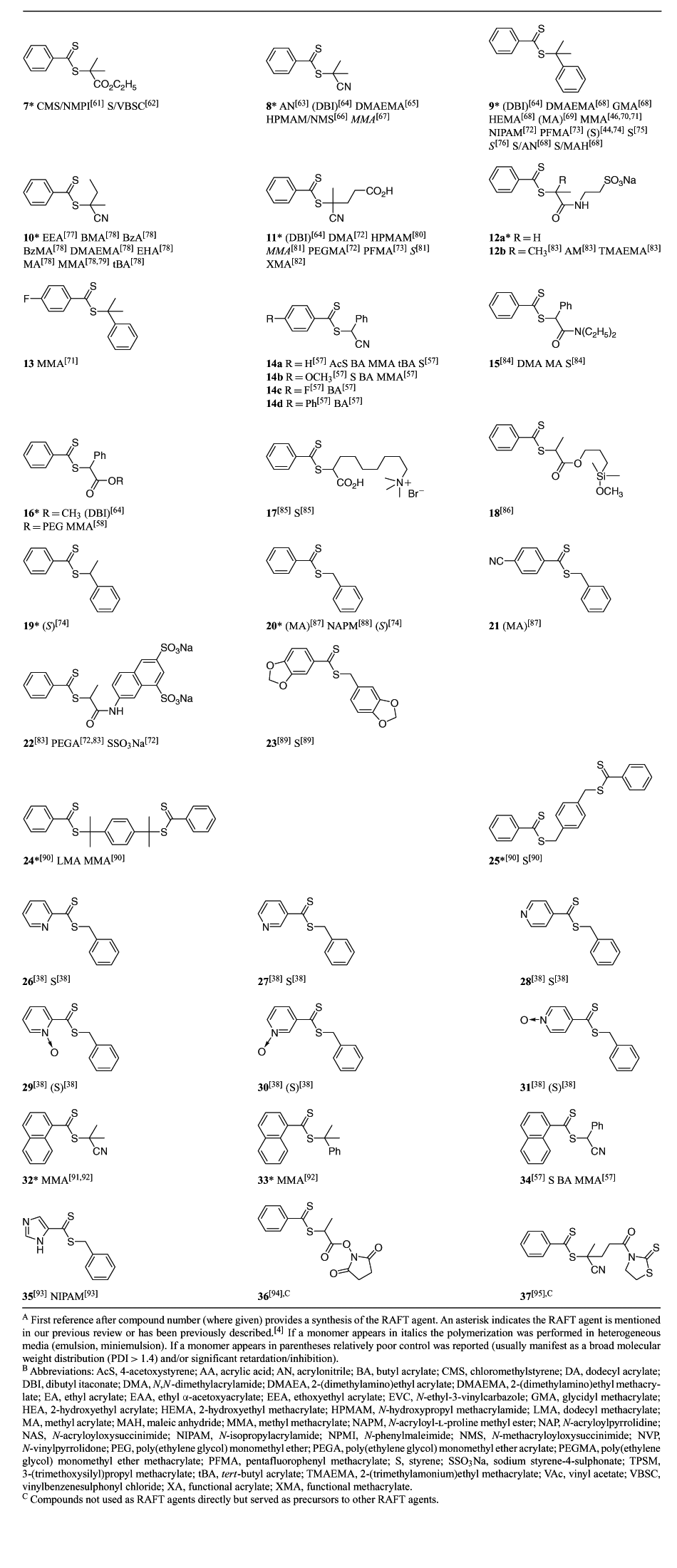
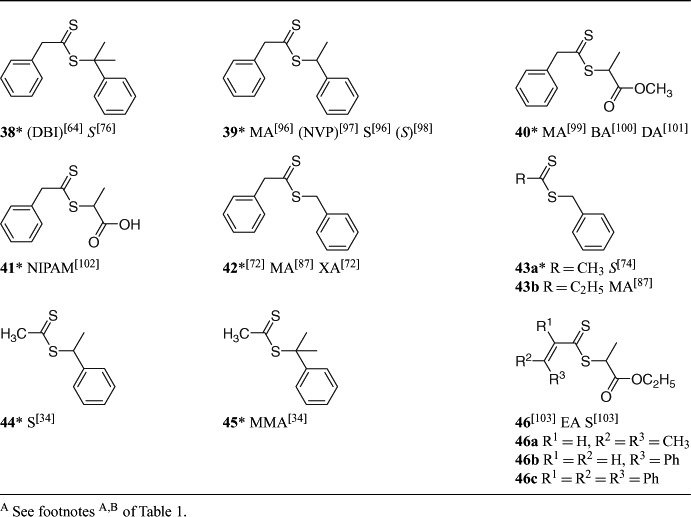
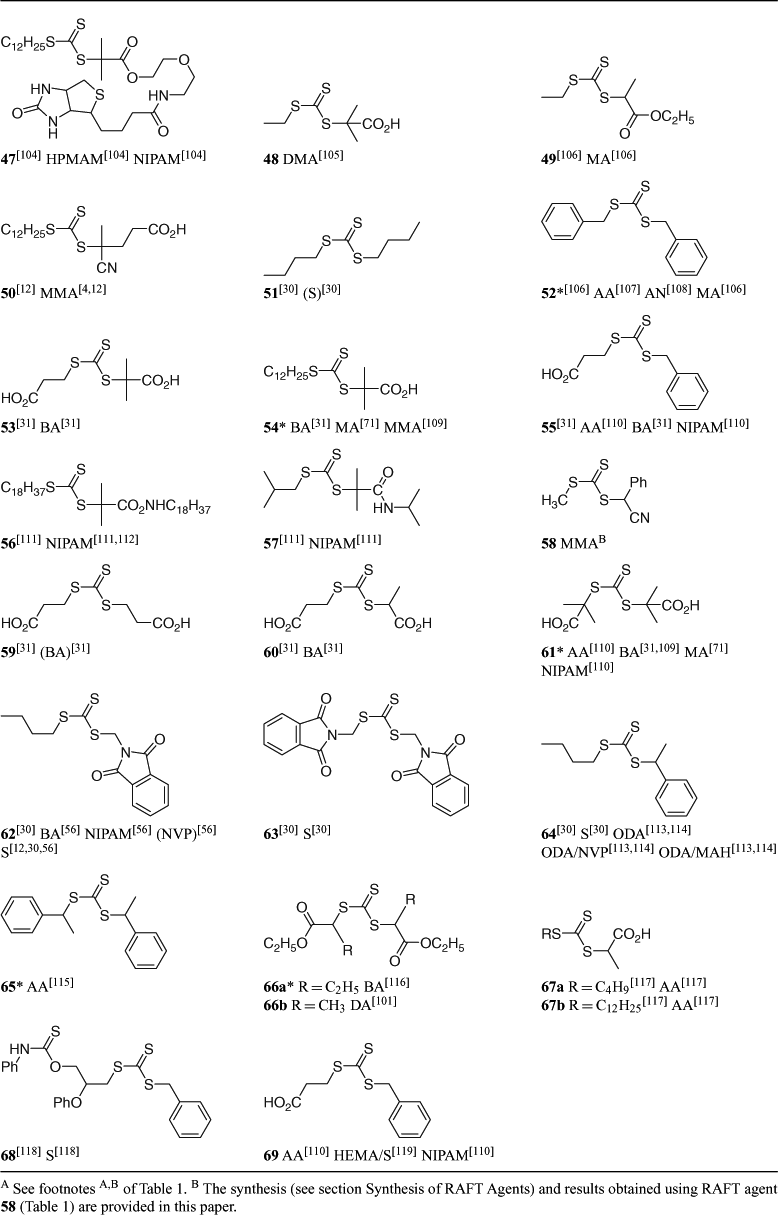
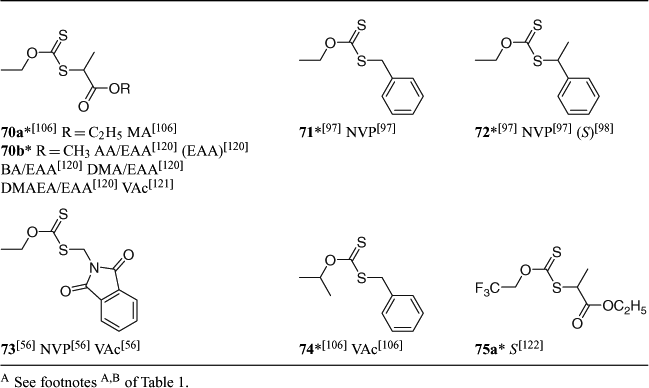
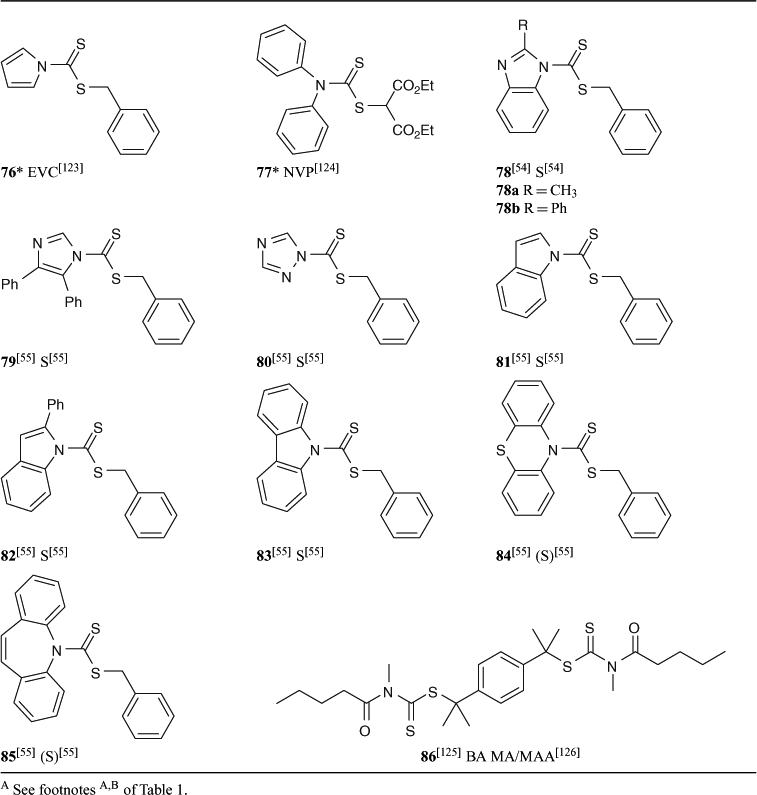
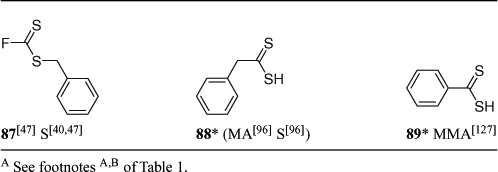
A summary of ‘new’ RAFT agents and the polymerizations in which they have been applied is included in Tables 1–6. The Tables includes some RAFT agent/monomer combinations which provide poorer molecular weight control and/or polydispersity greater than 1.4. These are indicated by the monomer being in parentheses. Often these have been investigated in order to provide an understanding of the mechanism and to allow construction of guidelines for the choice of RAFT agent.
|
|
|
|
|
|
Dithiocarbamates, where the delocalization of the nitrogen lone pair into the thiocarbonyl group is minimized by electron-withdrawing substituents or by its being part of a conjugated system (e.g. 76), are effective with styrenic and acrylic monomers. Recent work[54,55] has focussed on the relative effectiveness of a series of dithiocarbamates where the nitrogen lone pair is part of a heteroaromatic ring system (78–85) in controlling polymerization of styrene. Of these, compounds 84 and 85, where there is little delocalization of the lone pair into the aromatic system, were not effective as RAFT agents.
RAFT agents with R = phthalimidomethyl have been explored. The main instigation for this work was the synthesis of polymers with amine end-groups.[12,30,56] However, phthalimidomethyl radical is a good homolytic leaving group/initiating radical and can serve as a universal ‘R’ group in trithiocarbonate (62 and 63 with S, BA, NIPAM) or xanthate RAFT agents (73 with VAc, NVP) for monosubstituted monomers.
Secondary aromatic dithioesters with R = –CHPh(CN)[57] (14, 34) and –CHPh(CO2R) (e.g. 16)[5,58] and analogous trithiocarbonates with R = –CHPh(CN) (58) have been shown to have utility in controlling polymerization of methacrylates. Results for RAFT polymerization with trithiocarbonate 58 are presented in Table 7. Both the dithioester 14[57] and the methyl trithiocarbonate 58 (Table 7) give good molecular weight control and very narrow polydispersities. However, some retardation is evident with respect to similar polymerizations with tertiary cyanoalkyl RAFT agents, for example, methyl cyanoisopropyl trithiocarbonate,[59] under similar conditions. This may be indicative slow reinitiation by the phenylcyanomethyl radical though further study is required to establish this.
|
Fig. 3, taken from the previous review,[4] provides a general summary of how to select the appropriate RAFT agent for particular monomers. Note should be made of the dashed lines in the chart. Although some control might be achieved with these monomer RAFT agent combinations, the molecular weight distribution may be broad or they may be substantial retardation or prolonged inhibition. This proviso has been omitted in some representations of this data in the literature.
Vinylogous dithioesters, in which the thiocarbonyl and the free radical leaving group are separated by a conjugated unsaturated linkage (e.g. 90–93; Table 8), are described in the patent literature[128] but little other than passing reference has appeared in the open literature.[129] A recent paper describes the synthesis and use of 90 and 91 as RAFT agents in polymerization of EA and styrene.[103] RAFT polymerizations in the presence of 90 or 92 should provide a three-armed star.
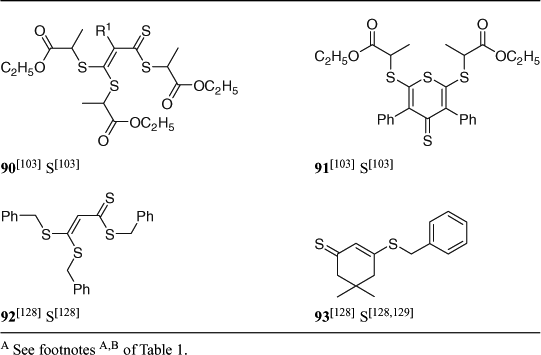
|
Synthesis of RAFT Agents
Arkema have commenced producing dibenzyl trithiocarbonate (52) on a pilot scale and have reported it as commercially available.[130] References containing syntheses of new RAFT agents by traditional methods are included in Table 1. Several papers that focus on RAFT agent synthesis are summarized in this section.
The use of thiocarbonylbisimidazole in the synthesis of RAFT agents was reported in the CSIRO patent.[128] Wood et al.[106] have now demonstrated the utility of this reagent in the synthesis of a range of trithiocarbonates, xanthates, and dithiocarbamates. Aoyagi et al.[131] have described conditions for synthesis of trithiocarbonates using minimal amounts of carbon disulfide.
A new ‘one pot’ synthesis of thiocarbonyl disulfides based on the reaction of a thiol with p-toluenesulphonyl chloride (TsCl) has appeared.[132] The proposed mechanism for the process is shown in Scheme 5. A further example of the synthesis of a tertiary trithiocarbonate (50) from the thiocarbonyl disulfide has been published.[12]
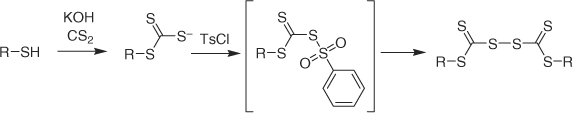
|
Two types of reagent (36[94] and 37[95]; Scheme 6) have been reported for conversion of amine functionality in polymers/surfaces to RAFT agent functionality.
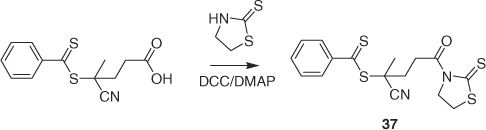
|
Attempted RAFT polymerization of MA or styrene agent with phenyldithioacetic acid meets with limited success.[96] However, the RAFT agent formed by Markovnikov addition of phenyldithioacetic acid (88) to styrene (formed by preheating a solution of the dithioacid in styrene at 70°C for 24 h) can be used directly in styrene polymerization without need for isolating the RAFT agent.[96] This strategy cannot be applied with electron deficient monomers which react with dithioacids by an anti-Markovnikov Michael-type addition.
Primary and secondary trithiocarbonates are simply made by reaction of the trithiocarbonate salt with an alkylating agent. For example, cyano(phenyl)methyl methyl trithiocarbonate (58) is synthesized as follows.[60] A solution of 2-bromo-2-phenylacetonitrile (1.1 g, 5.5 mmol) in ethyl acetate (10 mL) was added dropwise to a stirred suspension of sodium methyl trithiocarbonate (0.7 g, 5 mmol) in ethyl acetate (10 mL) and the mixture was stirred for 12 h and poured into water (20 mL). The aqueous layer was extracted with ethyl acetate, dried with MgSO4, filtered, and the solvent removed. The residue was purified by column chromatography on silica using 10% ethyl acetate in petroleum spirits to afford the title compound (0.82 g, 69%. δH 2.8 (3H, CH3), 6.2 (1H, CH), 7.2–7.6 (5H, ArH)).
The trithiocarbonate salt can be formed in situ from the thiol and carbon disulfide with triethylamine as base (e.g. in the synthesis of 62 and 64).[30]
Characterization of RAFT-Synthesized Polymers
Most papers on RAFT polymerization contain information on the characterization of RAFT-synthesized polymers and/or the RAFT process. We consider here papers where the characterization of RAFT-synthesized polymers by spectroscopic or chromatographic methods is a primary focus.
Protic end groups in RAFT-synthesized (and other) polymers can be determined by derivatization with trichloroacetyl isocyanate and 1H NMR analysis.[133] The method is effective and quantitative for hydroxy, amino, and carboxy chain ends. However, some difficulties were encountered and the method appears non-quantitative for thiol end groups. Two-dimensional chromatography techniques have been applied to characterize end-functional PMMA[109,134] and PBA[109] synthesized by RAFT polymerization.
It has been reported that oxidation of the thiocarbonylthio group by tetrahydrofuran-derived peroxides can cause problems when tetrahydrofuran is used in sample preparation for mass spectrometry.[135]
Size exclusion chromatography coupled to electrospray ionization mass spectrometry has been used to characterize RAFT-synthesized PMA.[136] The work is described below under Side Reactions in RAFT. GPC coupled with UV detection has been used to determine the butyl trithiocarbonate and phthalimidomethyl ends groups of PBA prepared with RAFT agent 62.[56] This and 13C NMR was used to estimate the extent of short- and long-chain branching in PBA as a function of conversion. Capillary zone electrophoresis has been recommended as the method of choice for characterizing RAFT-synthesized PAA oligomers.[137]
Polymerization Kinetics
Kinetic simulation is frequently used as a tool to correlate experimental data with theoretical models. New approaches to kinetic simulation of RAFT polymerization using Monte Carlo methods in solution[138] or emulsion[139] have been reported. Monteiro and co-workers have applied kinetic simulation to study various aspects of RAFT polymerization[15,140,141] including block copolymer synthesis[140] and the synthesis of polymers using bis-RAFT agents.[141] They used a method of moments to solve simplified sets of differential equations using MATLAB.
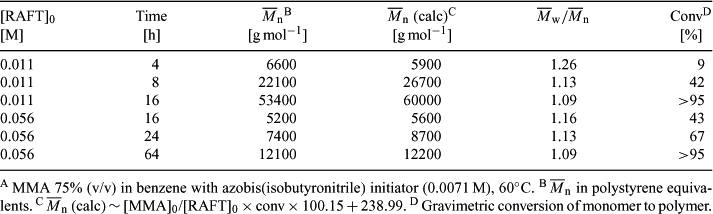
RAFT polymerization and kinetic simulation with Predici have been applied to determine chain-length dependent termination rate constants in radical polymerizations[70,99–101,121,142,143] of various monomers including MA (with 40[99,143] or 66b[143]), BA (with 40[100] or 66a[116]), DA (with 40 or 66b[101]), MMA (with 9[70]), and VAc (with 70b[121]). Most experiments[70,99,100,121] involve collecting rate of polymerization data from RAFT polymerization as a function of conversion. The quality of the data are dependent on the assessment of the importance of other chain length and conversion dependent process (the RAFT equilibria, initiator efficiency, propagation, chain transfer to monomer/polymer) and the extent to which the variations in the observed rate of polymerization with conversion can be attributed to the chain-length dependence of the termination rate constant. A more recent variant on this approach for determining chain-length dependent termination rate constants makes use of single-pulse pulsed laser polymerization (SP-PLP).[100,116]
Peklak et al.[46] have used numerical simulation to model RAFT polymerization of MMA with cumyl dithiobenzoate and study the effects of diffusion (the gel effect). Free volume theory was applied to estimate effect of diffusion on the various reaction steps including the RAFT equilibria, initiator efficiency, and bimolecular termination. A very good correlation with experimental data was obtained.
Side Reactions in RAFT Polymerization
A variety of side reactions can potentially complicate the RAFT mechanism causing retardation, byproducts and anomalies in the molecular weight distributions. Whether these occur depends on the particular RAFT agent/monomer combination and the reaction conditions. There remains some controversy surrounding the stability and possible alternate fates of the intermediate radical in RAFT polymerization. The current (mid 2006) status with respect to dithiobenzoate RAFT agents has been summarized.[25]
A detailed study of the product of RAFT polymerization of MA with both cumyl dithiobenzoate and cumyl dithiophenylacetate using coupled size exclusion chromatography–electrospray ionization mass spectrometry has been carried out with a view to detecting products which might be formed by a side reaction of the intermediate radical by radical–radical termination. As in previous studies of this nature, these were not detected.[136]
Buback and Vana[144] have proposed a new mechanism for disappearance of the product of intermediate radical termination during RAFT polymerization with dithiobenzoates. In that it does not lead to byproducts and the proposal has the potential of resolving the current impasse re the mechanism of retardation observed in these polymerizations.[25] The proposed mechanism involves the intermediate radical termination (95 or an isomer) reacting with a propagating radical to regenerate the intermediate 94 and produce 96 (identical to the normal product of radical–radical termination) as the only byproduct. While the process is thermodynamically favourable being driven by aromatization and formation of a relatively stable radical, there appears no literature precedent for the reaction of quinonoid species such as 95 with radicals to proceed as shown in Scheme 7.
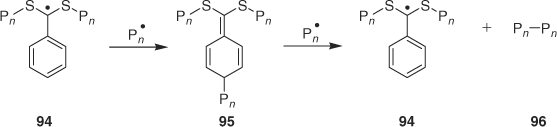
|
Another possibility is that the quinonoid species 95, if formed, reacts with radicals by hydrogen atom transfer. This process would result in formation of a new intermediate 97 to fragment to a new RAFT agent 99 and a dead polymer chain 98 (Scheme 8). Quinonoid species are well known as excellent hydrogen atom donors. The effect on kinetics would be similar to the Buback–Vana proposal[144] but would lead to the formation of a new RAFT agent 99 which might be detectable under appropriate conditions but, for the case of acrylate polymerization, may not be readily distinguished from the byproduct formed by the process mentioned in the paragraph below (Scheme 9).
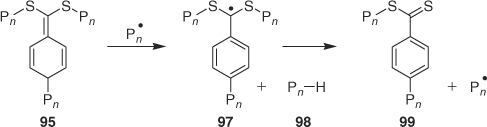
|
The above processes (Schemes 7 and 8) are only relevant to RAFT polymerizations with dithiobenzoates and closely related RAFT agents. They are not relevant to RAFT polymerizations carried out with trithiocarbonates, aliphatic dithioesters, and most other RAFT agents, many of which also give retarded polymerization.
Bimodal molecular weight distributions are often observed in RAFT polymerization of acrylate monomers when polydispersities are less than 1.2.[145] Bimodal distributions have been observed for dithiobenzoate, dithioacetate, trithiocarbonate, and dithiocarbamate RAFT agents. They are manifest as a higher molecular weight shoulder with peak molecular weight approximately twice that of the parent peak and are most pronounced for higher conversion and higher molecular weight polymers. Postma et al.[56,113] have provided evidence that the mechanism of formation is copolymerization of the macromonomer formed by the propagating radical undergoing backbiting β-scission (Scheme 9). The incidence of long chain branching by this mechanism was compared to that of short chain branching (by backbiting followed by propagation). Because β-scission competes with propagation the number of branches per molecule is greater for high monomer conversions and for higher molecular weights. Note that the proposed process does not depend on RAFT mechanism and should complicate all radical polymerizations of acrylate monomers. The process may also complicate the polymerizations of other monosubstituted monomers (e.g. VAc) but is likely to be less prevalent because other monomers generally display a reduced propensity for backbiting β-scission.
Further studies of RAFT polymerization in the presence of dithioacids (88, 89) have been reported.[96,127] These polymerizations are characterized by a prolonged inhibition period during which a RAFT agent is formed followed by normal RAFT polymerization. The efficiency of conversion of the dithioacid to the RAFT agent can be low and a variety of side reactions are proposed to occur to complicate the process.[96,127]
Reaction Conditions (Initiator, Temperature, Pressure, Solvent, Lewis Acids)
A further study of UV-initiated RAFT polymerization of MA (with 54 or 61) and MMA (with 9 or 13) with added photoinitiator has been reported.[71] The paper emphasizes the importance of selecting an irradiation wavelength, the RAFT agent, and the photoinitiator (2,4,6-trimethylbenzoyl)diphenylphosphine oxide, to avoid side reactions such as photodissociation of the RAFT agent.[71] Gamma-initiated RAFT polymerizations of MA in the presence of various dithioesters (including 20, 21, 42, 43b),[87] xanthates[146] and dithiocarbamates,[147] of MMA in the presence of 32,[91] and of NIPAM and AA in the presence of 55 or 61 in aqueous media[110] have been reported.
In the previous review we reported experiments on the influence of oxygen and degassing on the outcome of MMA polymerization.[4] Thermally initiated (no added initiator at 80°C) RAFT polymerization of MMA with 32 or 33 in the presence of oxygen provided high molecular weight polymer and remarkably good control.[92] The proposed mechanism involves formation of P(MMA-co-oxygen).
RAFT polymerization of MA in supercritical carbon dioxide with cumyl dithiobenzoate showed substantially greater retardation than when toluene was used as solvent.[69] RAFT polymerization of NVP in fluoroalcohols with xanthates (71 or 72) was carried out to achieve some stereochemical control over propagation. An increase in syndiotactic dyads from 52 to ~62% for polymerization of NVP in perfluoro-t-butanol solvent at 20°C.[97] An inhibition period was observed which might be attributed to a poor choice of ‘R’ (benzyl or 2-phenylethyl) for the xanthate RAFT agents.
RAFT polymerization is compatible with the presence of Lewis acids which can be used to influence tacticity of homopolymers and/or the tendency for alternation in copolymerization.[4] Further studies of the use of rare earth metal triflates to control the stereochemistry of polymerization of acrylamide derivatives have been reported (NIPAM,[148] amino acid derived acrylamide derivatives, N-acryloyl-l-phenylalanine methyl ester[149]). Studies on (alternating) copolymerization include styrene with MMA[150] and β-pinene with acrylonitrile.[151]
The RAFT polymerization of a cyclodextrin–styrene host–guest complex in water has been studied.[152] Molecular weights and polydispersity were controlled by the RAFT process, however, molecular weights are higher and polydispersities broader than observed for solution or bulk polymerization of styrene with the same RAFT agent. Loss of control was associated with precipitation of the polymer.
There have been further studies relating to the hydrolytic stability of RAFT agents in aqueous media.[82,83] Better results were obtained when sodium bicarbonate as opposed to carbonate was used to neutralize and solubilize the acid functional dithiobenzoate RAFT agent 11 used for control of polymerization of a glycopolymer methacrylate.[82] The effect may be related to the need to control the pH of the reaction medium particularly when using dithiobenzoate RAFT agents in aqueous media.
Arita et al.[75] have reported high temperature (up to 180°C), high pressure (1000 bar) RAFT polymerization of styrene with cumyl dithiobenzoate 9. The conditions provided improved control (attributed to a higher fragmentation rate of the RAFT intermediate) and RAFT agent decomposition was not detected (compare below).
There have been several studies on the thermal stability of RAFT agents and RAFT-synthesized polymers and the possible influence of this on the outcome of RAFT polymerization. Cumyl dithiobenzoate 9 appears substantially less stable than benzyl or phenylethyl dithiobenzoate and degrades rapidly at temperatures over 100°C.[153] The instability was attributed to reversible formation to AMS and dithiobenzoic acid. The success of high temperature polymerization (of, for example, styrene) was attributed to the fact that the RAFT agent 9 was rapidly consumed and converted to more stable polymeric RAFT agents. It was also reported that the poor control in synthesis of PMMA with dithiobenzoate RAFT agents at higher temperatures (120°C) could be attributed to the lability of the dithiobenzoate end group.[154] Other work (see later discussion on end group transformation), while confirming that thermolysis is a suitable method for end group removal, indicates that dithiobenzoate end groups of RAFT-synthesized PMMA are stable to much higher temperatures.
The effect of impurities in cumyl dithiobenzoate on RAFT polymerization of HEMA, styrene, and MA has been studied.[155] Significant retardation manifest as an inhibition period was observed with samples that had not been rigorously purified by HPLC or which had been stored for a short time (< 3 months) under refrigeration at –20°C. Samples purified by column chromatography on silica gave a significant inhibition period even when used immediately following purification. Dithiobenzoic acid was considered as a likely impurity.[155] Actual impurities in cumyl dithiobenzoate will depend on how it was synthesized and stored. We have found that cumyl dithiobenzoate purified by column chromatography on alumina with hexane as eluant and stored at 4°C for some years in the absence of light remains very effective.[156] Another potential impurity in dithiobenzoate RAFT agents is bis(thiobenzoyl)disulfide. This may form from cumyl dithiobenzoate on exposure to light and such disulfides are known to inhibit polymerization until consumed by reaction with initiator-derived radicals.[157,158]
Schubert and co-workers[77–79,119,159,160] have reported on the development and application of high-throughput techniques in developing conditions for RAFT polymerization of various (meth)acrylate and for characterization of the synthesized polymers. The methods were used to study RAFT polymerization of 2-ethoxyethyl acrylate,[77] a protected form of acrylic acid.
RAFT Polymerization in Heterogeneous Media
A large number of recent studies relate RAFT polymerization in heterogeneous media (emulsion,[67,74,81,98,117,122,139,161–166] miniemulsion,[10,76,167–175] or suspension[176]). The area has been reviewed by McCleary and Klumperman[11] and Save et al.[177]
‘Surfactant free’ emulsion polymerization making use of a hydrophilic macroRAFT agent (typically based on PAA oligomer formed with RAFT agent 67)[117,161,163,164] has been used for BA[117] and styrene.[163,164] Gilbert[164] has proposed a simple theoretical model for particle formation by self assembly during RAFT emulsion polymerization and has applied this to analyze data for styrene polymerization.
A study of the surfactant (polymerizable surfmers and classical) in RAFT miniemulsion polymerization of styrene and MMA with 11 has been carried out.[81] Bimodal molecular weight distributions were obtained when surface-active monomers were used.
For batch MMA polymerization in emulsion with cyanoisopropyl dithiobenzoate 8 higher surfactant levels and initiation rates were found to improve colloidal stability.[67] Hartmann et al.[98] have studied the effect of degassing on latex stability in RAFT emulsion polymerization of styrene with dithiophenylacetate 39 (bimodal distribution) or xanthate 72 (broad distribution but good control). They report that the use of surfactant-free polymerization media degassed through freeze–pump–thaw cycles provides stable emulsions whereas nitrogen degassing does not.
Tauer and co-workers[74,166] have examined RAFT agent concentrations in the aqueous and monomer phase and the diffusion rate of RAFT agents from the monomer to the particle phase for styrene emulsion polymerization with various dithiobenzoate (9, 19, 20) and dithioacetate (43) RAFT agents. They also provided evidence that particle entry by initiator-derived radicals, including charged species such as sulfate radical anion, can take place without monomer addition. This result appears at odds with theory that suggests that aqueous phase oligomerization (z-mer formation) occurs before particle entry. Even though RAFT agents are rapidly oxidized by sulfate radical anion this appears not to be a significant side reaction in peroxydisulfate-initiated RAFT emulsion polymerization.[74]
The preparation of liquid-filled (isooctane core) polystyrene nanocapsules by RAFT miniemulsion polymerization has been reported.[76] Although good control over polymerization was obtained with both cumyl dithiobenzoate 9 and dithiophenylacetate 38, significant retardation was observed with the dithiobenzoate RAFT agent and it was proposed this prevented development of the desired core–shell morphology.
RAFT Polymer Syntheses
Polymer syntheses by RAFT polymerization are summarized in Tables 1–6 and 8; only systems which require separate comment are mentioned here or in subsequent sections.
Several papers report on the synthesis of glycopolymers by RAFT polymerization of acrylamide,[178] vinyl ester,[179] or methacrylate[82,180,181] derivatives of glucose or lactose. Relatively broad polydispersities (
RAFT ring-opening polymerization on monomer 100 provided a polymer with anthracene units as part of the backbone (Scheme 10).[182]
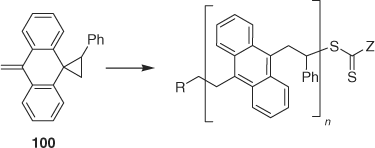
|
End-Functional Polymers and End-Group Transformations
Processes for thiocarbonylthio end group removal-transformation post RAFT polymerization have attracted significant interest.[12,30,66,183–185]
Free radical reducing agents can be used to replace the thiocarbonylthio group with hydrogen. These reducing agents comprise a free radical source and a hydrogen atom donor such as tributylstannane,[12,30] tris(trimethylsilyl)silane,[30] hypophosphite salts,[183] or isopropyl alcohol[186] and reduction takes place by a free radical chain reaction for which the propagation steps are shown in Scheme 11. An efficient hydrogen atom donor is required to limit side reactions such as reactions between the radical species, which may be manifest as the development of a bimodal molecular weight distribution. Tri-n-butylstannane is one of the most effective hydrogen atom donors but its use is complicated by the formation of byproducts which are potentially toxic and may be difficult to remove. The use of tris(trimethylsilyl)silane was explored for the reduction of butyl trithiocarbonate end groups of polystyrene.[30] However, formation of product with a bimodal molecular weight distribution indicates inefficient trapping of radicals.[30] Hypophosphite salts were demonstrated to be effective reducing agents for dithiobenzoate and trithiocarbonate (and most likely other thiocarbonylthio) end groups of acrylic and styrenic polymers and the byproducts or reduction are innocuous and readily removed.[183]
|
|
Perrier[184] reported that the thiocarbonylthio end groups could be removed from a wide range of RAFT-synthesized polymers by heating the polymer with a large excess of a radical initiator (typically AIBN) with respect to the end groups. Incomplete end group removal (~95%) reaction was observed for polystyrene with trithiocarbonate ends[30] but the process is effective for HPMAM/NMS with dithiobenzoate ends.[66]
Thermolysis provides another method for thiocarbonylthio group removal.[12,30,56,187,188] Butyl trithiocarbonate groups are cleaved from RAFT-synthesized polystyrene[12,30,187] and PBA[56,187] by heating at ~180°C in the absence of air. The dominant mechanism of end group loss depends on the particular polymer, involving concerted elimination (Chugaev process)[189,190] in the case of polystyrene and initial C–S bond homolysis in the case of PBA. Thermogravimetric analysis (TGA) of PMMA with dithiobenzoate end groups has been reported by Lima et al.,[109] Patton et al.,[90] Xu et al.,[154] and Chong et al.[188] TGA of PMMA with methyl trithiocarbonate end groups has been reported by Chong et al.[188] Loss of the PMMA dithiobenzoate end appears to involve a concerted elimination reaction. Loss of the methyl trithiocarbonate end involves C–S bond homolysis.
The thermolysis of xanthate-terminated polymers has also been reported.[56,191] For the case of both S-polystyrene and S-poly(t-butyl acrylate) O-isobutyl xanthate, the reported mechanism involves selective elimination to provide 2-butene and a polymer with a thiol end group.[191] In contrast, for the case of O-ethyl S-poly(vinyl acetate) xanthate,[56] the products are consistent with involvement of the poly(vinyl acetate) (PVAc) propagating radical and suggest a mechanism involving initial C–S bond homolysis.
The transformation of thiocarbonylthio groups to thiol end groups is often complicated by side reactions.[12,90,109,185] Bimodal molecular weight distributions are thought to arise by oxidative coupling (disulfide formation). The problem can be mitigated by reduction of the disulfide in a subsequent process (e.g. with Zn/CH3CO2H[12,192] or tris(2-carboxyethyl)-phosphine)[185]). Thus, borohydride reduction of PNIPAM with trithiocarbonate ends gave a product (with a monomodal which was treated with tris(2-carboxyethyl)phosphine to provide the same polymer with thiol ends).[185] The thiol ends were reacted with N-(1-pyrenyl)maleimide to attach a fluorescent label.
Thiols can also be produced directly by performing aminolysis in the presence of a small amount of a reducing agent (e.g. sodium bisulfite, Na2S2O4[90,109]). Thiols so formed may be transformed to sulfides by a Michael reaction. Lima et al.[109] have used this process with hydroxyethyl acrylate in forming telechelic polymers and report on the efficiency and limitations of the process.
Various functional RAFT agents used to provide end functional polymers are listed in Tables 1–6. These allow the syntheses of polymers with carboxylic acid,[12,31] hydroxyl,[109] and primary amine (via phthalimido end groups)[12,30] and other end groups.
Bipyridine (101; Scheme 12)[193] and terpyridine (102) functional dithioesters[194] have been used in RAFT polymerization and thence to form ruthenium complexes. The RAFT-synthesized polymer formed with 102 was used to make ruthenium connected polystyrene-(RuII)-PNIPAM blocks.
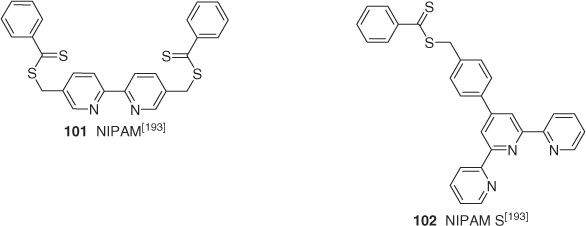
|
Chen et al.[195,196] have shown that it is possible to form new functional RAFT agents by single monomer insertion using the RAFT process. The process was used to synthesize the necessary RAFT agents for producing polymers with anthracene groups[195] or acenaphthalene[196] at the chain ends. The extent of propagation was controlled by choice of the monomer and RAFT agent concentrations and may be aided by penultimate unit effects (a first monomer addition is typically substantially faster than subsequent monomer additions).
RAFT homopolymerization of MAH results in insertion of a single MAH unit simply because the monomer has a low propensity for propagation.[197,198] This process may be used to place a MAH unit at the end of a RAFT synthesized polymer chain to create a functional macroRAFT agents.[197,198]
This single-monomer unit insertion processes have some parallel with the very early work of Zard and co-workers who described a RAFT process for synthesizing adducts of xanthates to electron-deficient monomers (e.g. N-methylmaleimide).[199]
Gradient Copolymers
It should be noted that, due to the effects of compositional drift, most copolymers prepared by RAFT polymerization fall under the description gradient copolymers.[12] Examples of gradient copolymers include a wide range of hydrophilic-hydrophobic copolymers based on long chain acrylates (e.g. octadecyl acrylate; ODA).[113,114] These copolymers include poly(ODA-grad-NVP) and poly(ODA-grad-MAH) prepared with trithiocarbonate 64[114] and which find use as dispersants in polypropylene–clay nanocomposites. Other examples are the terpolymer of styrene, MAH, and NVP[200] and various copolymers based on phosphonated monomers.[201]
A detailed study of the kinetics of RAFT copolymerization of styrene and MMA with dithioacetate RAFT agents has been reported.[34] Transfer constants (Ctr = ktr/kp, where ktr = kadd[kβ/(k–add+k–β)]; see Scheme 1) at 40°C were reported for the PMMA and polystyrene macroRAFT agents in polymerizations of MMA, styrene, and the azeotropic copolymerization of MMA and styrene (Table 9). The data indicate that in styrene polymerization, 50% conversion of the PMMA macroRAFT agent is achieved at very low monomer conversion (~0.16%), while, in MMA polymerization, the polystyrene macroRAFT agent is half consumed at a much higher conversion (~57%). The results provide further quantitative support the observation that when preparing block copolymers of methacrylates with styrene (and other monosubstituted monomers) it is best to prepare the methacrylate block first.
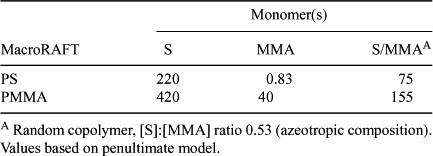
|
The results also further understanding of the observation that copolymerizations of monomers may be controlled even though homopolymerization of the same monomer may be uncontrolled or poorly controlled by particular RAFT agents.[4] Other examples of this phenomena are copolymerizations of the captodative monomer ethyl α-acetoxyacrylate (EAA) with acrylic monomers (BA, AA, DMA, DMAEA) with the xanthate RAFT agent 70[120] (EAA homopolymerization is not controlled with 70), and the above-mentioned copolymerizations of ODA and other acrylic monomers with MAH or NVP with trithiocarbonate RAFT agent 64 (MAH does not readily homopolymerize, NVP homopolymerization is not controlled with trithiocarbonates).[114]
Block Copolymers
Several approaches to polyolefin block copolymers which make use of RAFT polymerization have been reported. A synthesis of polyethylene with terminal t-butyl trithiocarbonate, dithiobenzoate or N,N-diethyldithiocarbamate groups as potential RAFT agents has been reported.[202] It should be noted that RAFT agents with R = primary alkyl would not usually be anticipated to be effective because primary alkyl radicals are poor homolytic leaving groups.
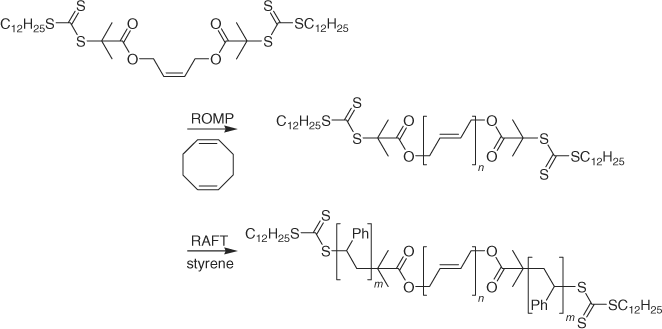
Telechelic polybutadienes bearing trithiocarbonate end groups were prepared by ring-opening metathesis polymerization (ROMP) of cyclooctadiene and subsequently used to make ABA triblocks by RAFT polymerization of styrene (Scheme 13) or t-butyl acrylate.[203]
The synthesis[125] and use[126] of dithiocarbamate RAFT agents containing blocks derived from liquid Kraton (a monohydroxy end-functional poly(ethene-co-butene)) has been described. A bis-RAFT agent (analogous to 86) was used to prepare poly(ethene-co-butene)-block-PBA-block-poly(ethene-co-butene).[126] A range of hydrophilic–hydrophobic block copolymers based on long chain acrylates (ODA) have been reported.[113,204] PODA is compatible with polypropylene.
RAFT polymerization of styrene with trithiocarbonate 103 (Scheme 14, with protected diol functionality in the ‘R’ group) was used to form a precursor from which cationic ring opening polymerization of 1,3-dioxepane was initiated.[205]
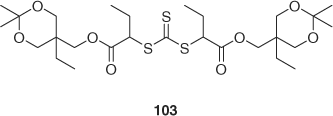
|
Polylactide-block-PDMA-block-polystyrene[206] and PEO-block-PMMA-block-polystyrene[58] were prepared from the appropriate hydroxy end-functional polymer by end-functionalizing that polymer as a RAFT agent (104 or 105 respectively; Scheme 15) and sequential RAFT polymerization.
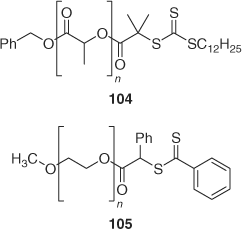
|
Other examples of block copolymers are acrylamide-based block copolymers,[105] PAN-block-PMA,[207] poly(styrene-co-AN) (SAN) block copolymers from methacrylate (DMAEMA, GMA, MMA) or poly(styrene-co-MAH) macro-RAFT agents (note that SAN and SAN block copolymers by RAFT have been reported previously[4]),[68] and PBMA-block-PDMAEMA-block-PBMA and PDMAEMA-block-PBMA-block-PDMAEMA,[208] hydrophilic–hydrophobic block copolymers (including PBA-block-PDMA, PBA-block-PNAP, PBA-block-poly(2-(methylsulfinyl)ethyl acrylate)).[72,209,210] Further examples with specific applications are mentioned in the other sections of this review.
Multiblock copolymers can be prepared from polymers containing trithiocarbonate linkages.[197,211] One method of synthesizing these involves a ring opening RAFT polymerization making use of the cyclic trithiocarbonate 106 (Scheme 16).[211] Multiblock copolymers have also been prepared from the multi-dithiocarbamate 107.[126]
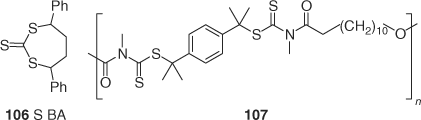
|
Star Polymers
A strategy for kinetic simulation of star formation by RAFT polymerization making use of multi-RAFT agents has been developed.[212]
Star-block copolymers with a hexa(dithiobenzoate)-functionalized ruthenium tris(bipyridine)[213] as core and di-[214] or tri-block arms[215] have been reported. Synthesis of stars with triblock arms based on acenapthalene, the coumarin derivative 108 (Scheme 17), and NIPAM provided light-harvesting systems with an energy gradient from the periphery to the core.[215]
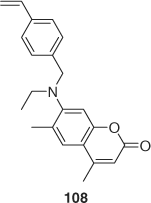
|
Dendrimer cores (16 dithiobenzoate) were synthesized and used to make star-polystyrene and star-(polystyrene-block-PMA).[216] Second or third generation polyethyleneimine dendrimers were used to prepare cores with eight or sixteen dithiobenzoate ends 109 (Scheme 18) for synthesis of star-polystyrene and star-(polystyrene-block-PMA)[217] or star-PNIPAM.[218]
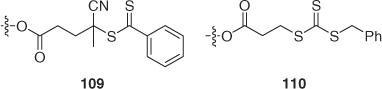
|
First, second, and third generation (three, six, and twelve arm) dendrimers functionalized with benzyl trithiocarbonate groups 110 were used to make star-polystyrene, star-PBA, star-(PBA-block-polystyrene), and star-(polystyrene-block-PBA).[219] Hyperbranched polyglycerol (functionality ~16) also functionalized as benzyl trithiocarbonate groups 110 was used to make star-PEA.[220]
A tris-trithiocarbonate RAFT agent with a thiourethane-isocyanurate core 111 (Scheme 19) was used to synthesize a three-armed star-polystyrene.[118]
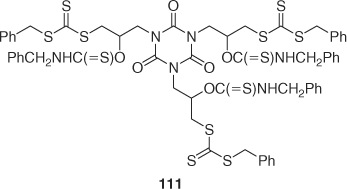
|
Microgels and Nanoparticles
In this section we consider direct formation of polymer microgels or nanoparticles in a solution polymerization process or by self-assembly and crosslinking of block copolymers. Surface functionalization of nanoparticles by RAFT polymerization is considered in the next section. Formation of particles and nanoparticles by heterogeneous polymerization (emulsion, miniemulsion polymerization) is discussed above.
The three basic processes for forming nanoparticles described in the previous review[4] will be distinguished.
-
(Co)polymerization of a di- (or higher) functional monomer to form a crosslinked core. There are two ways of adapting this process to form a star-microgel. The so-called ‘arm-first’ process for star synthesis involves synthesis of a macroRAFT agent which is then used in RAFT (co)polymerization of a multifunctional monomer. The second approach relies on the RAFT agent functionality that is retained in the crosslinked core to enable arms to be grown from the core is a subsequent RAFT polymerization step. The two approaches may be combined to synthesize mikto-arm stars. Recent examples are the preparation of stable microgels by copolymerization of divinylbenzene and 4VP[221] or of AA and ethylene glycol diacrylate[222] in the presence of polystyrene dithiobenzoate or by copolymerization of AA or AM and N,N'-methylenebisacrylamide in the presence of an O-ethyl xanthate.[223]
-
Hydrophilic–hydrophobic (or solvophilic–solvophobic) block copolymers are self-assembled to form micelles (or other supramolecular structures). A crosslinking reaction is then used to stitch together the core, shell and/or mid-block sections to form a stable structure. Again it is possible to perform a subsequent RAFT polymerization making use of retained RAFT agent functionality step to elaborate the structure. Recent examples are crosslinked micelles with a PNIPAM core produced from PNIPAM-block-PHEA or PNIPAM-block-polystyrene (cross linking with N,N′-methylenebisacrylamide)[224] and shell-crosslinked structures from poly(styrene-alt-MAH)-block-polystyrene,[225] or PEO-block-P(DMA-co-NAS)-block-PNIPAM.[226] Shell-crosslinked micelles have also been produced from RAFT-synthesized block copolymers self-assembled on gold nanoparticles.[227]
-
Self-condensing vinyl polymerization involves (co)polymerization of a monomer that also contains RAFT agent functionality. A recent example is hyperbranched PNIPAM prepared by copolymerization of NIPAM with 112 (Scheme 20)[93,228] and used in protein separation.[228]
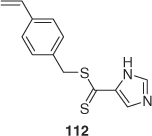
|
There have also been further studies on network formation by RAFT polymerization.[208]
Polymer Brushes, Graft Copolymers, Comb Polymers, and Surface Modification
Grafting-From Processes
Two basic approaches are used. The first involves surface modification to attach RAFT agent functionality and RAFT polymerization as a subsequent step. The second involves forming radicals on the surface (e.g. by irradiation or from attached initiator functionality) to initiate polymerization in the presence of a ‘free’ RAFT agent which becomes attached to the surface as a consequence of RAFT polymerization.
Further papers have been published on grafting to hydroxy-functional materials, for example, cellulose (e.g. cotton fabric or paper functionalized as shown in Scheme 21),[229,230] PVA (113, 114; Scheme 22).[231]
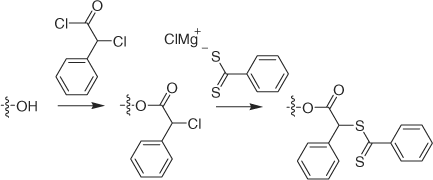
|
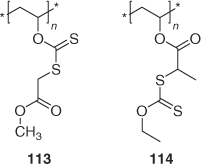
|
Functionalized multi-walled carbon nanotubes were prepared by derivatization of surface carboxy groups as dithiobenzoates (Scheme 23).[232] RAFT polymerization gave PHPMAM grafts.
|
Polystyrene–clay nanocomposites were prepared by RAFT polymerization in the presence of clay intercalated with the alkyl ammonium dithiobenzoate 17.[85]
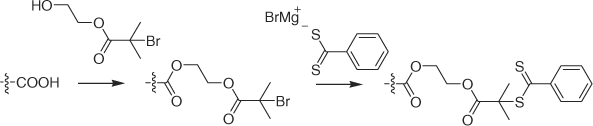
Polymerizations initiated from silica particles have been reviewed.[233] Surface modification of silica particles with silane functional RAFT agent 18 was used to attach dithiobenzoate groups which were subsequently grafted with polystyrene, PBA or PBA-block-polystyrene by RAFT polymerization.[86] Dithiobenzoate RAFT agents with tertiary ‘R’ were attached by reacting amino functional silica particles with 37.[95] These were then used to form PMMA brushes.
Porous silica particles surface were modified by immobilization of 2,2-azobis(2-cyanopentanoic acid) and grafted with MAA-EGDMA by RAFT polymerization with cumyl dithiobenzoate in the presence of l-phenylalanine anilide to leave a crosslinked molecularly imprinted film which was used as a chromatographic support.[234]
Pirri et al.[235] have described a new method for forming PDMA or a poly(DMA-block-GMA) brushes on glass or silica surfaces by RAFT polymerization. A glass microscope slide surface was derivatized with a thiol-functional organosilane, (3-mercaptopropyl)trimethoxysilane. The slide was then immersed in a N,N-dimethylformamide solution of monomer (DMA), initiator (AIBN), and RAFT agent 8 and heated to 60°C for 48 h. A similar process was applied to graft from silica beads.[235] These materials were then used to bind oligonucleotide molecules.
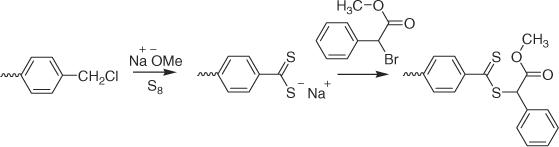
The above-mentioned processes are ‘away from’ processes where the ‘R’ is bound to the substrate. ‘Attached to’ processes where ‘Z’ is bound to the substrate have also been described. Merrifield resin and silica particle supported RAFT agents have been synthesized by sequential treatment of chloromethylphenyl functionality with sodium methoxide and elemental sulfur and methyl-α-bromophenylacetate (Scheme 24).[236]
|
A PHEMA brush was formed on a poly(tetrafluoroethylene-co-hexafluoropropylene) (FEP) film by first carrying out surface modification with an oxygen plasma to introduce peroxide functionality. This functionality was used to initiate RAFT polymerization of HEMA in the presence of cumyl dithiobenzoate to produce FEP-graft-(PHEMA).[237]
A PVDF surface was first chemically modified, to introduce hydroxyl groups. These were then esterified with 2,2-azobis(2-cyanopentanoic acid) and this functionality used to initiate RAFT polymerization of MMA, or PEGMA in the presence of cumyl dithiobenzoate. PVDF-graft-(PMMA-block-PDMAEMA) and PVDF-graft-(PPGEMA-block-PDMAEMA) were prepared.[238]
Other papers cover RAFT polymerization of AA/65 from a PVDF surface previously irradiated with an electron beam[115] and graft copolymerization from a PP surface was achieved by gamma-irradiation of a solution of styrene and 1-(2-isocyanatopropan-2-yl)-3-(propen-2-yl)benzene and 38 in the presence of a PP ‘lantern’.[239]
Several papers report the use of RAFT polymerization to prepare a backbone polymers/macroinitiator for comb polymer synthesis. Poly(N-phenyl maleimide-co-(4-chloromethylstyrene)) was prepared by RAFT copolymerization with 7 and used as a macroinitiator for ATRP of styrene (CuCl/2,2′-bipyridine catalyst) to form poly(N-phenyl maleimide-co-4-chloromethylstyrene)-comb-polystyrene.[61] Similarly poly(styrene-co-(vinylbenzenesulphonyl chloride)) prepared by RAFT copolymerization with 7 was used as a macroinitiator for ATRP of MMA (CuCl/2,2′-bipyridine catalyst).[62] Poly(styrene-co-(4-chloromethylstyrene))-block-PMA was used as a macroinitiator for cationic ring-opening polymerization of tetrahydrofuran (AgClO4 catalyst).[240]
Processes based on the use of dithiocarbamate photoiniferters need not involve RAFT. They are mentioned here because they involve the use of similar reagents and similar strategies for attachment of the thiocarbonylthio functionality. Recent reports include dithiocarbamate photoiniferter based grafting of MMA to silicon substrates,[241] and the grafting of acrylates (PEGA, TFEA) to polymer surfaces prepared by thiol-ene polymerization[242,243] in the preparation of microfluidic devices[243] and other applications.
Grafting-To Processes
Dithioesters and trithiocarbonates can act as anchoring groups for use in a grafting-to approach. The RAFT agents (benzyl dithiobenzoate 20 and dibenzyl trithiocarbonate 52) and derived RAFT-synthesized polystyrenes were shown to bind to form monolayers on gold surfaces without prior transformation of these thiocarbonylthio groups to thiols.[244]
Copolymers of N-(meth)acryloyl succinimide or pentafluorophenyl methacrylate (these monomers, NAS, NMS, and PFMA are referred to as active esters of (meth)acrylates) have been produced by RAFT polymerization and served as substrates for biofunctionalization or other grafting reactions using grafting-to processes.[66,73,245,246] Recent examples include poly(t-butyl acrylamide-block-poly(N-acryloylmorpholine-co-NAS)[245] poly(DMA-co-NAS),[246] PFMA-block-PNAM,[73] and PFMA-block-PMMA.[73]
Grafting-Through Processes
Clay nanocomposites formed by RAFT polymerization with the use of intercalants containing monomer functionality.[247] A RAFT-synthesized glycopolymer was grafted to silica particles by copolymerization of silica particles modified with 3-(trimethoxysilyl)propyl methacrylate (TPSM).[181]
PBA and PDMA grafts have been formed on poly(divinylbenzene) microspheres.[248] The process involved RAFT polymerization of the monomer in the presence of microspheres prepared by precipitation polymerization and which possess pendant double bonds. Cumyl dithiobenzoate 9 was used as RAFT agent.
Other Approaches
A PNIPAM-block-PTPSM was prepared by RAFT polymerization. This block copolymer self-assembles into micelles with PTPSM at the core and PNIPAM as a shell. A base-catalyzed sol–gel process within the PTPSM core resulted in PNIPAM-encapsulated silica nanoparticles.[249]
Supramolecular Structures
Stimuli responsive polymers and self-assembled structures have attracted significant interest. Self assembly is an integral step in the process of particle formation during emulsion polymerization using amphiphilic RAFT agents and many of the processes for forming nanoparticles and surfaces mentioned above and in the previous review.[4] The tolerance of RAFT polymerization to a wide variety of polar and protic monomers means that it is one of the most versatile for forming amphiphilic polymers.
The work of the McCormick group in designing block copolymers that contain segments that respond to changes in pH, temperature, or ionic strength has been reviewed.[13] Thermally responsive systems based on the use of polymers containing PNIPAM blocks[72,102,104,105,111,112,226,228,249–252] have been reported by many groups. Polymers with PNAPM blocks have been reported in a similar context.[88]
Honeycomb-structured, porous films formed as breath figures with pore sizes ranging from 200 nm to 7 μm have been prepared from RAFT-synthesized polymers and the dependence on polymer composition, molecular weight, and architecture has been reviewed.[14] A more general review on the formation and applications of honeycomb polymers by the breath figure approach has also been published.[253] Recent reports include honeycomb structures formed by assembly of polypyrrole-containing block copolymers[254] and comb polymers formed by grafting polystyrene from cellulose.[255] The former have been suggested as scaffolds for cell growth.[254]
Summary
The period since the publication of our last review has seen the publication of more than 200 papers on various aspects of RAFT polymerization. A change in focus from the study of the scope and mechanism of RAFT polymerization towards applications of the process is clearly evident.
Acknowledgments
We are grateful to DuPont Performance Coatings for their support of the work carried out at CSIRO Molecular and Health Technologies.
[1]
[2]
J. Chiefari,
Y. K. Chong,
F. Ercole,
J. Krstina,
J. Jeffery,
T. P. T. Le,
R. T. A. Mayadunne,
G. F. Meijs,
C. L. Moad,
G. Moad,
E. Rizzardo,
S. H. Thang,
Macromolecules 1998, 31, 5559.
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |