Synthesis of Imine and Amine-Linked Macrocycles Containing Tris-Indoles*
Hakan Kandemir A B , Ibrahim F. Sengul A C , Naresh Kumar A and David StC. Black A DA School of Chemistry, University of New South Wales (UNSW), Sydney, NSW 2052, Australia.
B Department of Chemistry, Faculty of Art and Science, Namık Kemal University, Tekirdag 59100, Turkey.
C Department of Chemistry, Gebze Technical University, PO Box 141, Kocaeli 41400, Turkey.
D Corresponding author. Email: d.black@unsw.edu.au
Australian Journal of Chemistry 70(11) 1196-1201 https://doi.org/10.1071/CH17264
Submitted: 15 May 2017 Accepted: 9 August 2017 Published: 1 September 2017
Abstract
The synthesis of 21-membered imine- and amine-linked tris-indole macrocycles starting from 7-nitroethylindoles is described. The 7-nitroethylindoles are converted to 2,2′-diindolylmethanes and the nitro groups reduced to amino. The resulting diamines undergo reactions with indole-2,7-dicarbaldehydes to form the macrocyclic imines, which can subsequently be reduced to the macrocyclic amines.
Introduction
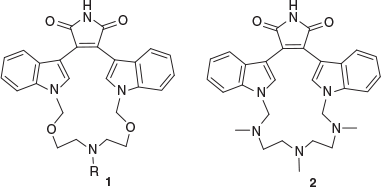
Macrocyclic compounds are important targets in the area of drug discovery due to the fact that naturally occurring examples often show biological activity. Many clinically used macrocyclic antibiotics have been reported.[1] Moreover, macrocycles have proved useful in treating disease and providing a basic preorganized scaffold that can optimally present functional binding domains. However, these compounds rarely function as classic enzyme inhibitors. Another structural advantage of macrocycles is that the ring can restrict structural flexibility and provide a degree of pre-organization.[2] Macrocycles containing indole rings provide some unique systems with importance in medicinal chemistry as they show diverse and remarkable biological activities.[3] For instance, macrocyclic bis-indolylmaleimides 1 and 2 are examples of biologically active macrocyclic compounds, which show good selectivity for inhibition of protein kinase C (PKC) and glycogen synthase kinase-3 (GSK-3) (Fig. 1).[4]
|
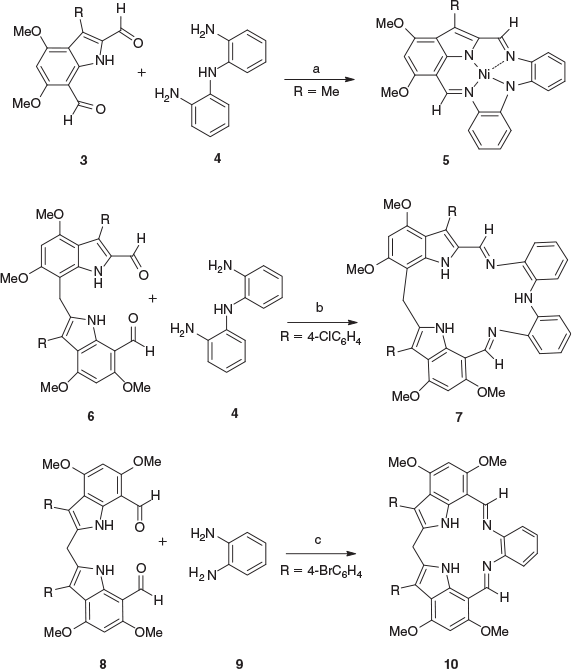
Our group has been engaged for many years both in building macrocyclic systems and in developing new chemistry of indoles. In particular, the ability to obtain indole-2,7-dicarbaldehydes through the ready formylation of activated 3-substituted-4,6-dimethoxyindoles has led to their application to the synthesis of macrocyclic imines and their metal complexes.[5] The imine bonds cannot only be generated from reactions of amines with aldehydes[5–8] but also by the direct application of the modified Vilsmeier reaction through iminium chloride chemistry.[9,10] For example, the 4,6-dimethoxy-3-methylindole-2,7-dicarbaldehyde (3) readily undergoes a metal template reaction with 2,2′-diaminodiphenylamine (4) in the presence of nickel(ii) acetate to give the macrocyclic complex 5 (Scheme 1).[5] Our group has also reported the synthesis of macrocyclic compounds which incorporate two indole moieties from reactions of 2,7′-diindolylmethane dialdehydes with diamines. For example, the dialdehyde 6 reacts with 2,2′-diaminodiphenylamine (4) to give the 18-membered macrocyclic diindolylmethane diimine 7 (Scheme 1).[11]
|
More recently, dialdehydes derived from di-(2-indolyl)arenes[12] and di-(2-indolyl)heteroarenes[13] have been shown to undergo reactions with diamines to give macrocyclic compounds containing three and four indoles. Furthermore, the synthesis of new macrocyclic systems incorporating 2,2′-diindolylmethane moieties has a special interest. Our group has explored a range of 2,2′-diindolylmethanes such as the dialdehyde 8 and shown that they can be converted into a variety of 15-membered macrocyclic systems, such as compound 10 by reaction with 1,2-diamines such as 1,2-diaminobenzene (9) (Scheme 1).[14–18] Manganese(iii) complexes derived from these macrocyclic systems can function as highly effective catalysts for alkene epoxidation. Related macrocyclic complexes derived from 2,2′-biindolyl-7,7′-dicarbaldehydes have also been prepared.[14–19]
It was therefore decided to investigate the development of imine-linked macrocyclic systems that could incorporate two indole rings in the form of a 2,2′-diindolylmethane moiety together with a further indole ring derived from an indole-2,7-dicarbaldehyde.
Results and Discussion
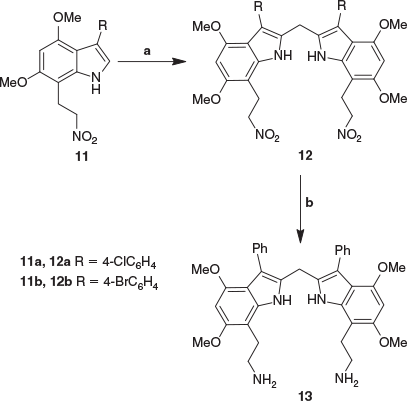
In order to construct a diamine based on the 2,2′-diindolylmethane structure, the approach was to make use of the previously reported synthesis of indole-7-tryptamines.[20] This transformation was achieved by the reaction of indole-7-carbaldehydes with nitromethane to give 7-nitrovinylindoles, which were subsequently reduced in a two-step process via the 7-nitroethylindoles to give the 7-aminoethylindoles (or 7-tryptamines). Since the synthesis of 7-nitroethylindoles 11a,b was reported,[20] they were reacted with excess formaldehyde in glacial acetic acid, following previous routes to 2,2′-diindolylmethanes.[11,12,14–16,21] Although the reaction proceeded, a complex mixture of products was obtained. However, the preparation of methylene bridged 2,2′-bis-indoles 12a,b was achieved successfully by the condensation of 7-nitroethylindoles 11a,b with an excess of formaldehyde in the presence of hydrochloric acid in anhydrous methanol (Scheme 2). The reactions were slow, taking 20 h to reach completion, and produced baseline impurities which could be removed by chromatography to give the nitroethyl compounds 12a,b as clean products.
|
The 1H NMR spectrum of the compound 12b exhibited the methylene protons attached to C7 of the indole nucleus as a triplet at 3.41 ppm, the methylene protons linking the two indole units as a singlet at 4.65 ppm, and the methylene protons adjacent to the nitro group as a triplet at 4.04 ppm. In the 13C NMR spectrum, the methylene carbon adjacent to the nitro group appeared at 74.5 ppm while the methylene carbon linking the two indole units and the methylene carbon attached to C7 of the indole nucleus appeared together at 23.1 ppm, as confirmed through HSQC and HMBC NMR experiments. This overlapping pattern of the methylene carbon linking the two indole units and the methylene carbon attached to C7 of the indole nucleus was similarly observed at 23.1 ppm in the 13C NMR spectrum of compound 12a. Finally, the high resolution mass spectra for compounds 12a and 12b revealed molecular ion peaks at 733.1825 and 820.0738 ([M + H]+), with the molecular formulae requiring 733.1832 and 820.0743 respectively.
Reduction of compounds 12a and 12b to the corresponding primary amino compounds with hydrazine hydrate and 10 % Pd/C was carried out in a mixture of ethanol and tetrahydrofuran. The mixed solvent system was employed in order to increase the solubility of the starting materials. The reaction reached completion after 8 h of heating under reflux and the resulting products were isolated. As with previous reductions of similar nitro groups, 1H NMR spectroscopy indicated that during the reduction, the halogens on the aromatic rings were also replaced by hydrogen atoms, so that both compounds 12a and 12b gave the same bis-indole diamine 13 (Scheme 2). This assignment was supported by other analytical data, with the 13C NMR spectra showing resonances at 125.5, 127.3, and 131.1 ppm corresponding to aryl carbons of the benzenoid ring and mass spectrometry showing an m/z of 605.3115, which is consistent with C37H40N4O4 requiring 605.3128. The Pd/C-catalyzed dehalogenation of aromatic halides is well documented in the literature.[22–24] Moreover, the reducing reagent hydrazine hydrate is also known to cause dehalogenation.[25]
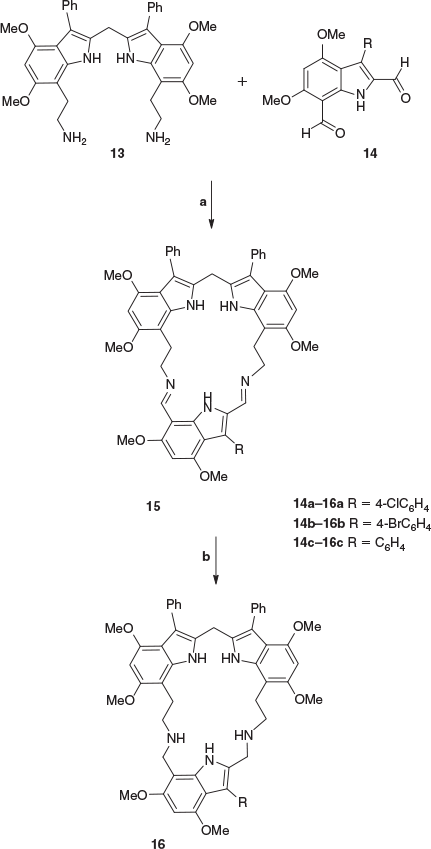
Synthesis of the unsymmetrical macrocycles 15a–c, which incorporate three indole rings, was then achieved by the tandem condensation of diamine 13 with 3-aryl-4,6-dimethoxyindole-2,7-dicarbaldehydes 14a–c in absolute ethanol. Products 15a–c precipitated from the reaction mixture and were collected by filtration in 58–71 % yields (Scheme 3). These 21-membered macrocyclic compounds were found to be stable on silica and were purified by passing through a silica plug. The high resolution mass spectra showed [M + H]+ peaks at 912.3509, 958.3022, and 878.3912, with the molecular formulae requiring 912.3528, 958.3002, and 878.3918 respectively.
|
In the 1H NMR spectrum of compound 15a, the methylene protons appeared as a singlet at 2.87 ppm and a doublet at 3.15 ppm while the imine protons appeared at 7.34 and 8.79 ppm and the methylene bridge protons appeared at 4.01 ppm. The six methoxy group singlets appeared at 3.63, 3.70, 3.77, 3.84, 3.87, and 3.92 ppm and the three indole NH proton resonances appeared at 8.40, 9.55, and 12.03 ppm.
The imine-linked macrocyclic compounds 15a–c were reduced to the corresponding amine-linked macrocyclic compounds 16a–c with sodium borohydride in a mixture of hot ethanol and tetrahydrofuran. The compounds 16a–c were found to be very polar and thus remained at the baseline of the thin-layer chromatography plate, but did not appear to have the same solubility issues in deuterated chloroform as the previous compounds, allowing for the acquisition of satisfactory analytical data.
For example, evidence for the formation of compound 16b was provided by 1H NMR data which showed the absence of the imine protons and the appearance of the methylene peaks at 4.15 and 4.24 ppm, and by 13C NMR and DEPT135 experiments indicating the appearance of seven CH2 groups at 23.0, 25.0, 25.4, 43.6, 44.2, 48.3, and 49.3 ppm. This was supported by the high resolution mass spectrum which revealed a molecular ion at 960.3338 ([M + H]+), with the molecular formula requiring 960.3336.
Given the formation of 21-membered macrocyclic diimines 15 in good yields, consideration was given to the construction of a symmetrically linked macrocycle containing four indole rings. Using a similar methodology to that already described, 7,7′- diamino-2,2′-diindolylmethane (13) was heated at reflux in ethanol with 2,2′-diindolylmethane-7,7′-dialdehyde (8), but the larger 26-membered macrocycle was not produced and the dialdehyde 8 was recovered. Use of higher boiling solvents led to reactions resulting in the formation of polymeric mixtures.
Conclusion
In conclusion, the condensation of 7-nitroethylindoles with formaldehyde led to formation of the related 2,2′-diindolylmethanes which were successfully converted into 7,7′-di(aminoethyl) derivatives. These were used for the construction of imine-bridged 21-membered macrocycles by reaction with indole-2,7-dialdehydes. Subsequent reduction of the imine groups afforded the corresponding amine-linked macrocylic systems. The macrocyclic structures 15 and 16 provide the basis for further investigation with respect to functionalization and metal complexation. Preliminary experiments indicate that metal complexation is possible but not yet selective, so that further extensive studies are required. Biological screening of these and further functionalized structures will also be of interest.
Experimental
General
Melting points were measured using a Mel-Temp melting point apparatus, and are uncorrected. Microanalyses were performed on a Carlo Erba Elemental Analyser EA 1108 at the Campbell Microanalytical Laboratory, University of Otago, New Zealand. 1H and 13C NMR spectra were obtained on a Bruker DPX300 (300 MHz) spectrometer. Mass spectra were recorded on either a Bruker FT-ICR MS (EI) or a Micromass ZQ2000 (ESI) at UNSW, or a Shimadzu LCMS QP 8000 (ESI) at the University of Otago, New Zealand. Infrared spectra were recorded with a Thermo Nicolet 370 FTIR Spectrometer using KBr discs. Ultraviolet–visible spectra were recorded using a Varian Cary 100 Scan Spectrometer. Column chromatography was carried out using Merck 230–400 mesh ASTM silica gel, while preparative thin-layer chromatography was performed using Merck silica gel 7730 60GF254.
Bis-(3-(4-Chlorophenyl)-4,6-dimethoxy-7-(2-nitroethyl)-1H-indol-2-yl)-methane (12a)
To a mixture of 7-nitroethylindole (11a) (2.27 g, 6.3 mmol) and formaldehyde solution (6 mL, 40 %) in methanol (60 mL) was added concentrated hydrochloric acid (6 mL). The mixture was heated under reflux for 20 h. The solvent was evaporated, the residue was quenched with water, and the resulting solid was filtered and purified by flash chromatography using dichloromethane as eluent to yield the title compound 12a (1.18 g, 51 %) as a cream solid. Mp 238–240°C. δH (300 MHz, DMSO-d6) 3.43, 4.67 (2t, J 14.7, 8H, CH2), 3.60 (s, 6H, OMe), 3.79 (s, 6H, OMe), 4.06 (s, 2H, CH2), 6.33 (s, 2H, H5), 7.04, 7.14 (2d, J 8.4, 8H, aryl H), 10.87 (br s, 2H, NH). δC (75 MHz, DMSO-d6) 23.1, 74.5 (CH2), 55.5, 57.0 (OMe), 89.4 (C5), 127.1, 132.5 (aryl CH), 98.6, 111.3, 112.7, 130.5, 131.5, 134.5, 136.7, 153.1, 154.0 (aryl C). vmax (KBr)/cm−1 3419, 1601, 1547, 1490, 1334, 1123, 994. λmax (THF)/nm (ϵ/cm−1 M−1) 232 (62600), 284 (28900). m/z (HRMS ESI+) 733.1825; C37H34Cl2N4O8 [M + H]+ requires 733.1832.
Bis-(3-(4-Bromophenyl)-4,6-dimethoxy-7-(2-nitroethyl)-1H-indol-2-yl)-methane (12b)
To a mixture of 7-nitroethylindole (11b) (2.13 g, 5.27 mmol) and formaldehyde solution (5 mL, 40 %) in methanol (60 mL) was added concentrated hydrochloric acid (6 mL). The mixture was heated under reflux for 20 h. The solvent was evaporated, the residue was quenched with water, and the resulting green solid was filtered and purified by flash chromatography using dichloromethane as eluent to yield the title compound 12b (1.15 g, 53 %) as a cream solid. Mp 239–241°C. (Found: C 53.48, H 4.12, N 6.77. C37H34Br2N4O8 0.1CH2Cl2 requires C 53.62, H 4.15, N 6.74 %. δH (300 MHz, DMSO-d6) 3.41, 4.65 (2t, J 14.7, 8H, CH2), 3.58 (s, 6H, OMe), 3.77 (s, 6H, OMe), 4.04 (s, 2H, CH2), 6.31 (s, 2H, H5), 6.96, 7.26 (2d, J 8.5, 8H, aryl H), 10.86 (br s, 2H, NH). δC (75 MHz, DMSO-d6) 23.1, 74.5 (CH2), 55.4, 57.0 (OMe), 89.4 (C5), 130.1, 132.9 (aryl CH), 98.5, 111.2, 112.7, 119.1, 131.4, 134.9, 136.7, 153.1, 154.0 (aryl C). vmax (KBr)/cm−1 3419, 1600, 1547, 1487, 1334, 1123, 993. λmax (THF)/nm (ϵ/cm−1 M−1) 232 (70000), 284 (33000). m/z (HRMS ESI+) 820.0738; C37H34Br2N4O8 [M]+ requires 820.0743.
2,2′-(2,2′-Methylenebis-(4,6-dimethoxy-3-phenyl-1H-indole-7,2-diyl))-diethanamine (13)
To a refluxing solution of bis-indole 12b (0.74 g, 0.9 mmol) in absolute ethanol/THF (40 mL) (3 : 1), 10 % Pd/C (0.3 g) was added under argon followed by the dropwise addition of hydrazine monohydrate (8 mL) over 15 min. The reaction mixture was heated under reflux for another 8 h. The reaction mixture was filtered through Celite and the solvent was removed under reduced pressure. The residue was quenched with water, the resulting precipitate was filtered and dried to yield the title compound 13 (0.46 g, 84 %) as a yellow-green solid. Mp 150–153°C. δH (300 MHz, CDCl3) 2.92, 2.94 (2d, J 4.2, 8H, CH2), 3.66 (s, 6H, OMe), 3.83 (s, 6H, OMe), 4.21 (s, 2H, CH2), 6.26 (s, 2H, H5), 7.22–7.37 (m, 10H, aryl H), 10.34 (br s, 2H, NH). δC (75 MHz, CDCl3) 23.1, 28.7, 41.9 (CH2), 55.4, 57.2 (OMe), 89.9 (C5), 125.5, 127.3, 131.1 (aryl CH), 103.2, 111.4, 113.6, 131.4, 135.8, 137.2, 152.3, 153.2 (aryl C). vmax (KBr)/cm−1 3272, 2931, 2834, 1601, 1519, 1495, 1453, 1332, 1210, 1123, 989. λmax (MeOH)/nm (ϵ/cm−1 M−1) 288 (17900), 290 (17600). m/z (HRMS ESI+) 605.3115; C37H40N4O4 [M + H]+ requires 605.3128.
Macrocyclic Imine 15a
A mixture of bis-indole 13 (0.33 g, 0.54 mmol) and 2,7-dicarbaldehyde 14a (0.18 g, 0.54 mmol) was heated under reflux in absolute ethanol (40 mL) overnight. The precipitate was filtered and dried. The crude product was passed through a plug of silica to yield the title compound 15a (0.34 g, 69 %) as a yellow solid. Mp 203°C (dec.). δH (300 MHz, CDCl3) 2.87 (s 2H, CH2), 3.15 (d, J 4.5, 2H, CH2), 3.63 (s, 3H, OMe), 3.67 (t, J 10.09, 2H, CH2), 3.71 (s, 3H, OMe), 3.76 (s, 3H, OMe), 3.83 (s, 3H, OMe), 3.87 (s, 3H, OMe), 3.93 (s, 3H, OMe), 4.03 (s, 2H, CH2), 6.09 (s, 1H, H5), 6.32 (s, 1H, H5′), 6.33 (s, 1H, H5″), 6.66–7.09 (m, 14H, aryl H), 7.34 (s, 1H, CH), 8.40 (br s, 1H, NH), 8.79 (s, 1H, CH), 9.55 (br s, 1H, NH), 12.03 (br s, 1H, NH). δC (75 MHz, CDCl3) 22.8, 25.2, 26.7, 61.9, 62.3 (CH2), 55.1, 55.5, 56.0, 57.2, 58.0, 60.3 (OMe), 87.7 (C5), 89.7 (C5′), 90.6 (C5″), 125.0, 125.1, 126.8, 126.9, 127.0, 130.4, 131.1, 132.2 (aryl CH), 152.0, 158.2 (CH), 100.7, 103.2, 105.9, 111.9, 112.0, 112.1, 112.5, 115.1, 120.9, 130.9, 131.2, 131.7, 135.0, 135.6, 137.1, 137.3, 138.5, 152.7, 153.0, 153.4, 158.5, 159.5 (aryl C). vmax (KBr)/cm−1 3361, 2930, 2837, 1614, 1592, 1516, 1463, 1246, 1122, 989, 699. λmax (THF)/nm (ϵ /cm−1 M−1) 232 (84100), 271 (45500), 348 (23700). m/z (HRMS ESI+) 912.3509; C55H50ClN5O6 [M + H]+ requires 912.3528.
Macrocyclic Imine 15b
A mixture of bis-indole 13 (0.16 g, 0.27 mmol) and 2,7-dicarbaldehyde 14b (0.10 g, 0.27 mmol) was heated under reflux in absolute ethanol (30 mL) overnight. The precipitate was filtered and dried. The crude product was passed through a plug of silica to yield the title compound 15b (0.15 g, 71 %) as a yellow solid. Mp 198°C (dec.). δH (300 MHz, CDCl3) 2.96 (s, 2H, CH2), 3.15 (d, J 4.7, 2H, CH2), 3.63 (s, 3H, OMe), 3.70 (s, 3H, OMe), 3.76 (s, 3H, OMe), 3.83 (s, 3H, OMe), 3.86 (s, 3H, OMe), 3.92 (s, 3H, OMe), 4.01 (s, 2H, CH2), 6.10 (s, 1H, H5), 6.31 (s, 1H, H5′), 6.32 (s, 1H, H5″), 6.67–7.23 (m, 14H, aryl H), 7.39 (s, 1H, CH), 8.54 (br s, 1H, NH), 8.80 (s, 1H, CH), 9.63 (br s, 1H, NH), 12.04 (br s, 1H, NH). δC (75 MHz, CDCl3) 22.8, 25.2, 26.8, 61.9, 62.1 (CH2), 55.1, 55.5, 56.1, 57.2, 57.9 (OMe), 87.7 (C5), 89.7 (C5′), 90.5 (C5″), 125.0, 125.1, 126.8, 126.9, 130.0, 130.4, 131.1, 132.6 (aryl CH), 151.9, 158.0 (CH), 100.7, 103.1, 105.7, 111.9, 112.3, 112.5, 115.1, 120.6, 121.0, 130.9, 131.0, 131.6, 132.2, 135.0, 135.6, 137.2, 137.3, 138.4, 152.7, 153.0, 153.4, 158.5, 159.5 (aryl C). vmax (KBr)/cm−1 3364, 2981, 2829, 1829, 1614, 1592, 1518, 1462, 1243, 1150, 1122, 991, 699. λmax (THF)/nm (ϵ/cm−1 M−1) 232 (128 500), 272 (71200), 348 (37600). m/z (HRMS ESI+) 958.3022; C55H5081BrN5O6 [M + H]+ requires 958.3002.
Macrocyclic Imine 15c
A mixture of bis-indole 13 (0.26 g, 0.42 mmol) and 2,7-dicarbaldehyde 14c (0.13 g, 0.42 mmol) was heated under reflux in absolute ethanol (30 mL) overnight. The precipitate was filtered and dried. The crude product was passed through a plug of silica to yield the title compound 15c (0.22 g, 58 %) as a yellow solid. Mp 185°C (dec.). δH (300 MHz, CDCl3) 3.08 (s, 2H, CH2), 3.17 (t, J 10.8, 2H, CH2), 3.63 (s, 3H, OMe), 3.68 (s, 6H, OMe), 3.76 (s, 3H, OMe), 3.85 (s, 3H, OMe), 3.86 (s, 6H, OMe), 3.93 (s, 3H, OMe), 4.02 (d, J 4.29, 2H, CH2), 6.12 (s, 1H, H5), 6.31 (s, 1H, H5′), 6.33 (s, 1H, H5″), 6.87–7.18 (m, 15H, aryl H), 7.52 (s, 1H, CH), 8.71 (br s, 1H, NH), 8.82 (s, 1H, CH), 9.69 (br s, 1H, NH). δC (75 MHz, CDCl3) 22.9, 25.5, 27.0, 62.0, 62.0 (CH2), 55.3, 55.6, 55.7, 56.2, 57.4, 58.0 (OMe), 87.8 (C5), 90.2 (C5′), 90.7 (C5″), 125.1, 125.2, 126.5, 126.9, 130.7, 131.1, 131.2 (aryl CH), 152.4, 158.0 (CH), 100.9, 103.6, 105.7, 112.3, 112.4, 112.7, 115.1, 122.7, 127.0, 127.0, 131.0. vmax (KBr)/cm−1 3319, 2932, 2834, 1623, 1592, 1518, 1462, 1351, 1242, 1201, 1149, 1122, 990, 699. λmax (THF)/nm (ϵ/cm−1 M−1) 293 (36900), 348 (29400). m/z (HRMS ESI+) 878.3912; C55H51N5O6 [M + H]+ requires 878.3918.
Macrocyclic Amine 16a
To a solution of cyclic indole 15a (0.10 g, 0.11 mmol) in a mixture of absolute ethanol/THF (32 mL) (1 : 3), sodium borohydride (1.00 g, 26.3 mmol) was added and the mixture was heated under reflux for 12 h. The solvent was removed under reduced pressure, and the residue was treated with water and neutralized using dilute hydrochloric acid (2 M). The resulting precipitate was filtered, dried, and recrystallized from methanol to yield the title compound 16a (0.07 g, 69 %) as a yellow-green solid. Mp 183°C (dec). δH (300 MHz, CDCl3) 2.78, 2.97 (2t, J 11.6, 4H, CH2), 3.14 (s, 4H, CH2), 3.54 (s, 2H, CH2), 3.64 (s, 3H, OMe), 3.65 (s, 3H, OMe), 3.76 (s, 3H, OMe), 3.82 (s, 3H, OMe), 3.86 (s, 3H, OMe), 3.92 (s, 3H, OMe), 4.14 (s, 2H, CH2), 4.24 (s, 2H, CH2), 6.24 (s, 1H, H5), 6.28 (s, 1H, H5′), 6.30 (s, 1H, H5″), 7.12–7.35 (m, 14H, aryl H), 9.55 (br s, 1H, NH), 10.17 (br s, 1H, NH), 10.73 (br s, 1H, NH). δC (75 MHz, CDCl3) 23.0, 25.0, 25.3, 43.5, 44.2, 48.3, 49.2 (CH2), 55.3, 55.5, 55.6, 56.8, 57.2, 57.4 (OMe), 88.9 (C5), 89.3 (C5′), 89.7 (C5″), 125.3, 125.3, 127.1, 127.1, 127.4, 131.0, 131.0, 132.2 (aryl CH), 111.4, 111.8, 112.0, 114.3, 114.8, 115.2, 130.6, 130.7, 131.7, 133.6, 135.3, 135.4, 137.2, 137.5, 137.8, 153.0, 153.0, 153.2, 153.5, 153.9 (aryl C). vmax (KBr)/cm−13314, 2931, 2835, 1600, 1493, 1334, 1203, 1147, 990. λmax (THF)/nm (ϵ/cm−1 M−1) 230 (105 500), 285 (49900). m/z (HRMS ESI+) 916.3844; C55H54ClN5O6 [M + H]+ requires 916.3841.
Macrocyclic Amine 16b
To a solution of cyclic indole 15b (0.13 g, 0.13 mmol) in a mixture of absolute ethanol/THF (32 mL) (1 : 3), sodium borohydride (1.00 g, 26.3 mmol) was added and the mixture was heated under reflux for 12 h. The solvent was removed under reduced pressure, and the residue was treated with water and neutralized using dilute hydrochloric acid (2 M). The resulting precipitate was filtered, dried, and recrystallized from methanol to yield the title compound 16b (0.08 g, 60 %) as a yellow-green solid. Mp 243°C (dec). δH (300 MHz, CDCl3) 2.80, 2.97 (2t, J 5.6, 4H, CH2), 3.14 (s, 4H, CH2), 3.54 (s, 2H, CH2), 3.64 (s, 3H, OMe), 3.65 (s, 3H, OMe), 3.77 (s, 3H, OMe), 3.82 (s, 3H, OMe), 3.89(s, 3H, OMe), 3.93 (s, 3H, OMe), 4.15 (s, 2H, CH2), 4.24 (s, 2H, CH2), 6.25 (s, 1H, H5), 6.28 (s, 1H, H5′), 6.30 (s, 1H, H5″), 7.10–7.53 (m, 14H, aryl H), 9.62 (br s, 1H, NH), 10.18 (br s, 1H, NH), 10.71 (br s, 1H, NH). δC (75 MHz, CDCl3) 23.0, 25.0, 25.4, 43.6, 44.2, 48.3, 49.3 (CH2), 50.6, 55.3, 55.5, 56.8, 57.2, 57.4 (OMe), 88.9 (C5), 89.3 (C5′), 89.7 (C5″), 125.3, 125.3, 127.1, 127.2, 130.3 132.0, 131.0, 132.5 (aryl CH), 101.4, 102.2, 111.3, 111.8, 112.0, 114.4, 114.8, 115.1, 119.9, 130.6, 130.7, 134.1, 135.3, 137.2, 137.5, 137.7, 153.0, 153.2, 153.5, 153.9 (aryl C). vmax (KBr)/cm−1 3353, 2933, 2837, 1600, 1463, 1335, 1214, 1122. λmax (THF)/nm (ϵ/cm−1 M−1) 291 (60900). m/z (HRMS ESI+) 960.3338; C55H54BrN5O6 [M + H]+ requires 960.3336.
Macrocyclic Amine 16c
To a solution of cyclic indole 15c (0.10 g, 0.11 mmol) in a mixture of absolute ethanol/THF (32 mL) (1 : 3), sodium borohydride (1.00 g, 26.3 mmol) was added and the mixture was heated under reflux for 12 h. The solvent was removed under reduced pressure, and the residue was treated with water. The resulting precipitate was filtered, dried, and recrystallized from methanol to yield the cyclic amine 16c (0.06 g, 62 %) as a yellow-green solid. Mp 159°C (dec.). δH (300 MHz, CDCl3) 2.71 (s, 2H, CH2), 2.91 (s, 2H, CH2), 3.07 (s, 4H, CH2), 3.55 (s, 3H, OMe), 3.56 (s, 3H, OMe), 3.67 (s, 3H, OMe), 3.72 (s, 3H, OMe), 3.81 (s, 3H, OMe), 3.84 (s, 3H, OMe), 4.06 (s, 2H, CH2), 4.17 (s, 2H, CH2), 6.15 (s, 1H, H5), 6.19 (s, 1H, H5′), 6.22 (s, 1H, H5″), 7.03–7.34 (m, 15H, aryl H), 9.31 (br s, 1H, NH), 10.09 (br s, 1H, NH), 10.75 (br s, 1H, NH). δC (75 MHz, CDCl3) 23.1, 25.1, 25.4, 43.4, 44.3, 48.3, 49.3 (CH2), 55.5, 55.6, 55.7, 56.9, 57.3, 57.5 (OMe), 89.1 (C5), 89.5 (C5′), 89.9 (C5″), 125.3, 125.4, 126.0, 127.1, 127.2, 127.3, 131.1, 131.2 (aryl CH), 101.6, 102.3, 111.7, 111.9, 112.1, 114.4, 114.9, 116.7, 130.7, 130.8, 130.9, 135.2, 135.5, 137.2, 137.6, 137.9, 153.1, 153.15, 153.2, 153.7, 154.0, 154.3 (aryl C). vmax (KBr)/cm−1 3305, 2931, 2834, 1601, 1518, 1495, 1451, 1333, 1203, 1147, 1124, 990, 699. λmax (THF)/nm (ϵ/cm−1 M−1) 293 (66600). m/z (HRMS ESI+) 882.4251; C55H55N5O6 [M + H]+ requires 882.4231.
Supplementary Material
1H and 13C NMR spectra of new compounds are available on the Journal’s website.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank the University of New South Wales and the Turkish Government for their financial support.
References
[1] X. Yu, D. Sun, Molecules 2013, 18, 6230.| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXpsFemurw%3D&md5=8efd978c0cda789ad4490a6bdb8c2550CAS |
[2] E. M. Driggers, S. P. Hale, J. Lee, N. K. Terrett, Nat. Rev. Drug Discov. 2008, 7, 608.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvFShsbc%3D&md5=9cd9ab208bd581e60ed494916262f1f4CAS |
[3] J. Mallinson, I. Collins, Future Med. Chem. 2012, 4, 1409.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFCht7rO&md5=5b40d9206b3bfb3b44bf0cdf534ea522CAS |
[4] H. C. Zhang, K. B. White, H. Ye, D. F. McComsey, C. K. Derian, M. F. Addo, P. Andrade-Gordon, A. J. Eckardt, B. R. Conway, L. Westover, Bioorg. Med. Chem. Lett. 2003, 13, 3049.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXms1Gmtr0%3D&md5=94618bfb6f34fd0792251a26b5a2cea3CAS |
[5] D. StC. Black, N. E. Rothnie, L. C. H. Wong, Aust. J. Chem. 1983, 36, 2407.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXhtlWgtbg%3D&md5=af094ccd53431394157849624d565e9fCAS |
[6] D. StC. Black, D. C. Craig, N. Kumar, L. C. H. Wong, J. Chem. Soc. Chem. Commun. 1985, 1172.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XhsVSqsrk%3D&md5=8cd8cbc8de8025a4e3670fb904e2e853CAS |
[7] D. StC. Black, N. Kumar, L. C. H. Wong, Synthesis 1986, 474.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXhtF2ktb8%3D&md5=4e48844f11689015b594d560a8ffa7d5CAS |
[8] P. K. Bowyer, D. StC. Black, D. C. Craig, A. D. Rae, A. C. Willis, J. Chem. Soc., Dalton Trans. 2001, 1948.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkslSktrk%3D&md5=75b93d43566774bd19c154a2221bcc5aCAS |
[9] D. StC. Black, M. C. Bowyer, A. Choy, D. C. Craig, N. Kumar, J. Chem. Soc., Perkin Trans. 1 1989, 200.
| Crossref | GoogleScholarGoogle Scholar |
[10] D. StC. Black, Adv. Nitrogen Heterocycl. 1998, 3, 85.
| Crossref | GoogleScholarGoogle Scholar |
[11] P. K. Bowyer, D. StC. Black, D. C. Craig, Tetrahedron 2005, 61, 10781.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVyntLjF&md5=d3077958ae3c7c966da73bc02ec0ae19CAS |
[12] I. F. Sengul, K. Wood, P. K. Bowyer, M. Bhadbhade, R. Chen, N. Kumar, D. StC. Black, Tetrahedron 2012, 68, 7429.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVCjtL3N&md5=bb9626e18a923dda4453c5292a0b3ca8CAS |
[13] I. F. Sengul, K. Wood, N. Kumar, D. StC. Black, Tetrahedron 2012, 68, 9050.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhtlakt7rL&md5=98e617269a54e1b1d37b7b439195c5e8CAS |
[14] D. StC. Black, Synlett 1993, 246.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXkt1Oqsbw%3D&md5=4ad81ac9d511c2229198bc1aae943ae1CAS |
[15] H. Sholihin, New Metal Complex Catalysts for Oxidation Reactions 1999, Ph.D. thesis, The University of New South Wales, Sydney, Australia.
[16] D. StC. Black, in Green Chemistry Series No 1, Collection of Lectures, Summer School on Green Chemistry, 2nd edn (Ed. P. Tundo) 2002, pp. 187–197 (INCA: Mestre, Italy).
[17] D. StC. Black, in Green Chemistry Series No 1, Collection of Lectures, Summer School on Green Chemistry, 3rd edn (Ed. P. Tundo) 2004, pp. 253–261 (INCA: Mestre, Italy).
[18] D. StC. Black, in Methods and Reagents for Green Chemistry (Eds P. Tundo, A. Perosa, F. Zecchini) 2007, pp. 219–229 (Wiley: New York, NY).
[19] D. StC. Black, N. Kumar, L. C. H. Wong, J. Chem. Soc. Chem. Commun. 1985, 1174.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XhsVSqsrY%3D&md5=294741b31ad4d1f8a813efda417b6da4CAS |
[20] H. Kandemir, I. F. Sengul, C. R. Gardner, E. L. Werry, M. L. Barron, M. Kassiou, N. Kumar, D. StC. Black, Heterocycles 2016, 93, 333.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXhtFSht7jF&md5=57797339af6e2f6b3bc61ce66a5f02d7CAS |
[21] D. StC. Black, M. C. Bowyer, N. Kumar, P. S. R. Mitchell, J. Chem. Soc. Chem. Commun. 1993, 819.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXmslWisLc%3D&md5=4cd4eed4f79cffb747e001d15c7b3b09CAS |
[22] C. Xia, J. Xu, X. Liang, Catal. Commun. 2004, 5, 383.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlvVSkurk%3D&md5=17ef58d651d1d6c0c4ddebcadfca443bCAS |
[23] H. Sajiki, A. Kume, K. Hattori, K. Hirota, Tetrahedron Lett. 2002, 43, 7247.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xns12isLg%3D&md5=d59c0512b415603183addac8bdb741c2CAS |
[24] Y. Ukisu, T. Miyadera, J. Mol. Catal. A: Chem. 1997, 125, 135.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXntlWgsLc%3D&md5=e197a16ce076c7bc76868c5759fef537CAS |
[25] A. Furst, R. C. Berlo, S. Hooton, Chem. Rev. 1965, 65, 51.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2MXjs1amtA%3D%3D&md5=fc6bc21e94dc1ac786105dca891f7f07CAS |
* David StClair Black was awarded the Australian Academy of Science David Craig Medal for 2017.


