Synthesis of Star Polymers by RAFT Polymerization as Versatile Nanoparticles for Biomedical Applications*
Jinming Hu A , Ruirui Qiao B , Michael R. Whittaker B , John F. Quinn B and Thomas P. Davis B C DA CAS Key Laboratory of Soft Matter Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui 230026, China.
B ARC Centre of Excellence in Convergent Bio-Nano Science and Technology, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville, Vic. 3052, Australia.
C Chemistry Department, University of Warwick, Coventry CV4 7AL, UK.
D Corresponding author. Email: thomas.p.davis@monash.edu
Australian Journal of Chemistry 70(11) 1161-1170 https://doi.org/10.1071/CH17391
Submitted: 11 July 2017 Accepted: 18 August 2017 Published: 13 September 2017
Abstract
The precise control of polymer chain architecture has been made possible by developments in polymer synthesis and conjugation chemistry. In particular, the synthesis of polymers in which at least three linear polymeric chains (or arms) are tethered to a central core has yielded a useful category of branched architecture, so-called star polymers. Fabrication of star polymers has traditionally been achieved using either a core-first technique or an arm-first approach. Recently, the ability to couple polymeric chain precursors onto a functionalized core via highly efficient coupling chemistry has provided a powerful new methodology for star synthesis. Star syntheses can be implemented using any of the living polymerization techniques using ionic or living radical intermediates. Consequently, there are innumerable routes to fabricate star polymers with varying chemical composition and arm numbers. In comparison with their linear counterparts, star polymers have unique characteristics such as low viscosity in solution, prolonged blood circulation, and high accumulation in tumour regions. These advantages mean that, far beyond their traditional application as rheology control agents, star polymers may also be useful in the medical and pharmaceutical sciences. In this account, we discuss recent advances made in our laboratory focused on star polymer research ranging from improvements in synthesis through to novel applications of the product materials. Specifically, we examine the core-first and arm-first preparation of stars using reversible addition–fragmentation chain transfer (RAFT) polymerization. Further, we also discuss several biomedical applications of the resulting star polymers, particularly those made by the arm-first protocol. Emphasis is given to applications in the emerging area of nanomedicine, in particular to the use of star polymers for controlled delivery of chemotherapeutic agents, protein inhibitors, signalling molecules, and siRNA. Finally, we examine possible future developments for the technology and suggest the further work required to enable clinical applications of these interesting materials.
Introduction
The synthesis of polymers with well-defined molecular architecture has been the subject of mounting interest over the past few decades. Specifically, polymers with linear, cyclic, comb, brush, hyperbranched, star, and dendritic structures have been successfully designed and synthesized.[1,2] Of these, star polymers possess a unique topology in which several linear chains (i.e. arms) are linked together at a central core. In comparison with linear polymers of similar molecular weight, star polymers have considerably lower viscosity and as such are useful as rheology control agents.[3,4] Moreover, star polymers are finding increasing application in nanomedicine, and as nanocontainers and nanoreactors.[5] Although star polymers can be prepared via cationic or anionic polymerization,[6,7] the reaction conditions required are challenging and have limited the extent to which star polymers have found widespread application. In recent years, the advent of living radical polymerization methods such as reversible addition–fragmentation chain transfer (RAFT) and atom transfer radical polymerization (ATRP) and the use of highly efficient coupling chemistry (e.g. click chemistry) has revolutionized the synthesis of polymers with diverse molecular architectures. Owing to their synthetic ease and high versatility, these techniques have provided an excellent alternative for the preparation of star polymers.[8–11]
Herein, we highlight several recent achievements in the synthesis and application of star polymers. In particular, we examine the preparation of star polymers using RAFT polymerization, exploring both core-first and arm-first synthesis. In this account, we focus primarily on work originating in our own laboratories. The advantages and disadvantages of each technique are presented, along with suggestions for achieving well-defined materials. Thereafter, we discuss several potential applications for star polymers, particularly in biomedicine. Specifically, we explore the utility of star polymers for drug and gene delivery, as magnetic resonance imaging (MRI) contrast agents or fluorescent imaging agents, and as theranostic vectors, which combine both imaging and delivery modalities. Finally, we discuss the future direction of star polymer research and the next steps required to enable widespread clinical application of these interesting materials.
The Preparation of Star Polymers via RAFT Polymerization
Star polymers are principally prepared via core-first or arm-first strategies, utilizing various polymerization or post-polymerization assembly methodologies. Herein, we focus on the fabrication of star polymers using RAFT polymerization owing to the excellent compatibility of RAFT with a variety of functional monomers. Further, in comparison with ATRP, RAFT polymerization offers a considerable advantage in that the product polymers do not require removal of metal catalyst. Nevertheless, for a discussion of star polymer preparation using other techniques, the reader is referred to several excellent review articles.[6,12,13] Recently, coupling linear polymeric chains to a multifunctional core using highly efficient coupling chemistry such as click chemistry has also been demonstrated, in particular for the synthesis of miktoarm star polymers.[14] In the paragraphs that follow, we summarize the synthesis of star polymers via RAFT polymerization using either a core-first or arm-first approach.
Core-First Approach
Core-first approaches for preparing star polymers typically require a multifunctional initiator from which the polymeric arms effectively grow. However, for RAFT polymerization, a multifunctional transfer agent (rather than an initiator) is employed. These agents include multiple thiocarbonylthio moieties that are pendant from the core. When a propagating polymer chain undergoes chain transfer with one of these thiocarbonylthio groups, formation of an arm is facilitated. Depending on the orientation of the thiocarbonylthio group, the arm either propagates outward from the core or undergoes addition and fragmentation reactions as the polymerization proceeds. The number of arms can be readily and precisely dictated by varying the number of thiocarbonylthio moieties in the RAFT agent. Obviously, a necessary precursor to this approach is the synthesis of an appropriate multifunctional RAFT agent, which introduces a degree of complexity into the overall synthesis.
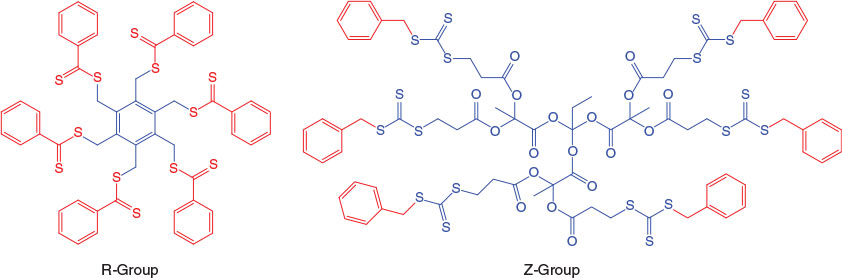
As noted above, the preparation of star polymers via a core-first methodology can be further subdivided by reference to the orientation of the thiocarbonylthio moiety in the transfer agent (see Fig. 1). These have been classified as the Z-group approach (where the thiocarbonylthio moiety remains proximal to the core and the arms undergo fragmentation and addition as the polymerization proceeds), or the R-group approach (where the core is essentially the leaving group and the chains polymerize outward from the core). The pioneering work using multivalent RAFT agents to synthesize star polymers was performed by the CSIRO group.[15] Subsequently, we have prepared a variety of star polymers using multifunctional RAFT agents based on hexakis(thiobenzoylthiomethyl)benzene,[16] β-cyclodextrin (β-CD),[17] pentaerythritol[18] or 1,1,1-tris(hydroxymethyl)propane,[18,19] (co)polymers of vinyl benzyl dithiobenzoate,[20] and dendrimers.[21,22]
Although the multifunctional transfer agents mentioned above enable the synthesis of a wide variety of polymers, these particular agents yield materials built using carbon–carbon bonds. In order to prepare degradable star polymers, stimuli-responsive bonds (e.g. disulfide bonds) must be incorporated into the structure. This can be achieved by conjugating RAFT agents that include a thiol-labile disulfide bond to a multifunctional core such that the labile bond remains proximal to the core. As a result, the arms of the star polymers can be cleft by thiol (e.g. glutathione (GSH), or dithiothreitol (DTT)), thus triggering a drop in molecular weight. This unique property may be useful for thiol-triggered release of encapsulated payloads.[23–25]
Although the core-first approach enables control over arm number and incorporation of degradable linkages, the technique does lead to some molecular weight broadening. This is true for both the Z-group and R-group approaches. In general, intermolecular (star–star) coupling is less pronounced with the Z-group approach. However, this orientation also results in thiocarbonylthio groups remaining proximal to the core, rendering the star polymers sensitive to reducing agents or amines. Moreover, at higher arm numbers and arm molecular weights, steric hindrance becomes substantial, which may impede the polymerization of bulky monomers. By contrast, the R-group technique yields star polymers with the thiocarbonylthio moiety distal from the core, which should substantially reduce steric hindrance. However, with the R-group technique, intermolecular coupling between stars is increased, leading to broader molecular weight distributions (MWDs). As such, strategies to minimize side reactions are essential to achieve high-quality star polymers. Specifically, lower arm number, reduced radical concentration, and higher concentration of RAFT agent enhance formation of well-defined star polymers for both the Z- and R-group approaches, with the particular conditions required varying between different monomer types.[26]
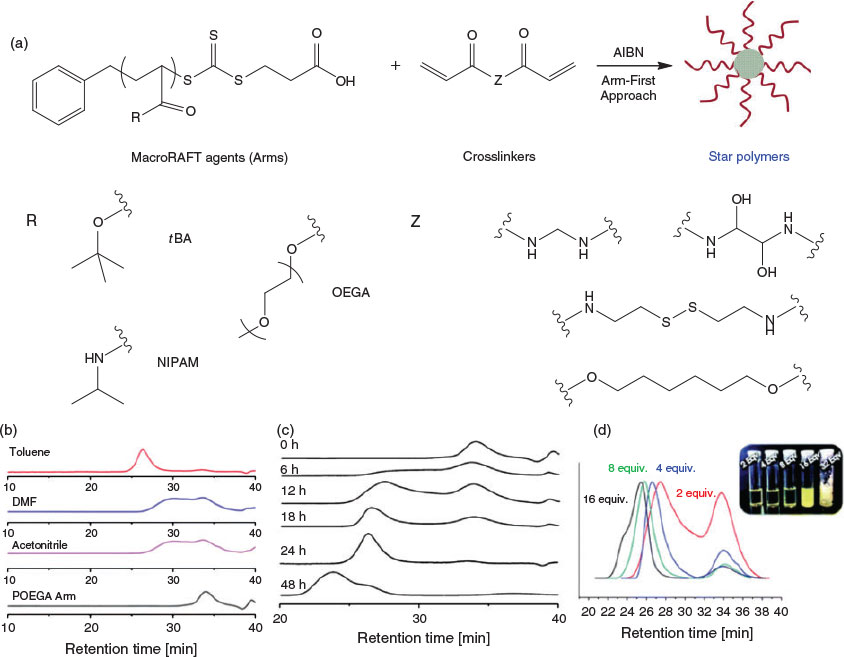
Arm-First Approach
As the name suggests, the arm-first approach requires the preparation of polymeric arms (macromolecular chain transfer agent, or ‘macroRAFT agent’) as the initial step in the star polymer synthesis. The macroRAFT agent is then chain extended with a multifunctional crosslinker, resulting in the formation of star polymers or microgels. The arm-first method abrogates the need to synthesize multifunctional chain transfer agents and therefore simplifies the synthesis. An early example of this approach in the literature involved the synthesis of polystyrene (PS) star polymers with a crosslinked poly(divinylbenzene) (PDVB) core. These materials were synthesized by chain-extending PS macroRAFT agent with divinylbenzene (DVB). The MWDs of the resulting star polymers broadened at longer polymerization times at a low crosslinker ratio (DVB/PS macroRAFT agent = 2 : 1), whereas increasing the ratio of crosslinker to macroRAFT (e.g. DVB/PS macroRAFT agent = 5 : 1) led to significant broadening of the MWD, suggesting the formation of hyperbranched structures rather than star polymers.[27] The arm number varied from 2 to 16 per star on average, depending on polymerization time and feed ratio of crosslinker to PS macroRAFT agent. Moreover, the resulting PS star polymer could be used to form macroporous films. Of course, as these materials are hydrophobic and chemically inert, they are unlikely to have significant utility for biomedical applications.
In order to prepare water-miscible and stimuli-responsive star polymers, hydrophilic poly(oligo(ethylene glycol) methyl ether methacrylate) (POEGMA) arms and acid-labile or thiol-responsive crosslinkers have been investigated. For this system, a ratio of crosslinker to macroRAFT agent of 8 : 1 led to optimal arm incorporation efficiency. Moreover, thiol groups can be generated within the star via aminolysis of the residual thiocarbonylthio moieties, furnishing new reactive sites and enabling the incorporation of various agents via Michael addition. The resulting star polymers were water soluble, and degradation could be triggered through cleavage of the crosslinkers in the presence of acid or reducing agents.[28] Additionally, hydrophilic miktoarm star polymers have been successfully prepared by a similar protocol using several different macroRAFT agents during the polymerization process.[29] These materials were shown to assemble into larger aggregates due to the incorporation of a hydrophobic portion (poly(n-butyl methacrylate)) into the miktoarm structure.
Ensuring that star polymers have narrow MWDs has been the subject of considerable attention. By screening the polymerization conditions (solvent, crosslinker, and arm molecular weight), we found that when polymerizations were conducted in a poor solvent for the crosslinker, the polydispersity index (PDI) of the resulting stars was reduced (typically <1.2) and incorporation of the arms was maximized. Conversely, polymerizations conducted in a good solvent for the crosslinker correlated with poorer incorporation of the arms and higher polydispersities (>1.5). For example, when using poly(oligo(ethylene glycol)methyl ether acrylate) (POEGA) macroRAFT (molecular mass <20 kDa) as the arm and N,N-methylenebisacrylamide or N,N-bis(acryloyl)cystamine as the crosslinker, polymerizations performed in a poor solvent for the crosslinker (toluene) afforded well-defined star polymers with very narrow MWDs, i.e. PDI < 1.2. In contrast, when using the same arm material and crosslinker with a good solvent for the crosslinker (e.g. N,N-dimethylformamide or acetonitrile), much broader MWDs were observed (Fig. 2).[30] Importantly, the MWD of the star polymers synthesized in this manner was not significantly affected by varying the arm material, providing the opportunity to prepare a wide variety of star polymers. In further work, generation of star polymers in a heterogeneous solvent was demonstrated in water by using a hydrophilic POEGMA macroRAFT agent and water-immiscible crosslinker (e.g. 1,6-hexanediol diacrylate).[31,32] Other work has shown that if the polymeric arms are able to self-assemble into micelles rather than unimers and the thiocarbonylthio moiety is embedded within the micellar core, subsequent chain extension with the crosslinker remains localized to the core, leading to low-polydispersity star polymers.[33]
|
The incorporation of functional units into the star polymer structure is important for enabling subsequent conjugation of dye or therapeutic molecules. For instance, by incorporating activated ester-based monomers (such as pentafluorophenyl acrylate (PFPA)) into the arms, it is possible to construct star polymers that can react with amine-bearing molecules. As a result, the properties (e.g. water solubility and fluorescence) of the star polymers can be easily modified.[34] Altogether, the arm-first approach represents a versatile method for preparing star polymers with narrow MWDs in which the corona (and also the core) can be readily functionalized. This paves the way for a multiplicity of possible applications.
Biomedical Applications of Star Polymers
In the emerging field of nanomedicine, water-soluble star polymers with stimuli-responsive properties are ideally suited owing to their nearly monodisperse size, stability during circulation, and ease of functionalization. In the following section, we highlight several applications of star polymers in the fields of drug and gene delivery, magnetic resonance and fluorescence imaging, and theranostics.
Star Polymers for Drug and Gene Delivery
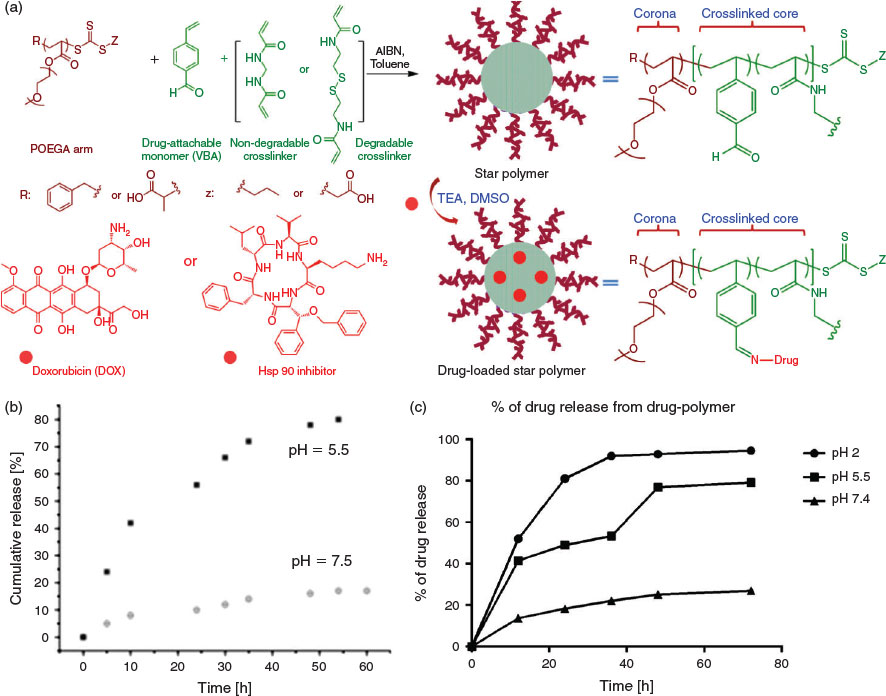
The possible application of star polymers as drug delivery vehicles has attracted considerable attention.[35] Such materials are interesting because they can potentially limit the systemic exposure of chemotherapeutic agents, thereby reducing off-target effects. To this end, star polymer comprising a POEGA corona and benzaldehyde moieties within the core have been synthesized via an arm-first protocol using POEGA macroRAFT agent.[36] The POEGA corona is included to give stability during circulation, while the benzaldehyde moieties can be used to conjugate amine-containing drugs (e.g. doxorubicin (DOX)) via the formation of imine bonds. Importantly, imine bonds are stable at neutral pH, yet labile at acidic pH. As such, the DOX remains conjugated to the star polymer during circulation (i.e. at neutral pH) but is released when the star polymers are internalized into cancer cells owing to the action of the acidic organelles (e.g. endosomes and lysosomes). Moreover, the crosslinker can be engineered to include a biodegradable linkage, providing a further functional handle on the drug release profile (Fig. 3). In addition to chemotherapeutic drugs, amine-containing protein inhibitors can also be introduced into this system using a similar protocol. As a proof-of-principle, amine-bearing heat shock protein 90 (hsp 90) inhibitor was incorporated into the system in place of DOX, with the acid-mediated release of hsp 90 inhibitor inducing cell apoptosis via a caspase 3-dependent pathway (Fig. 3).[37] These results can be attributed to the dynamic nature of the imine bonds; intact drugs and inhibitors can undergo triggered release on exposure to the acidic milieu. In addition to small-molecule chemotherapeutics and protein inhibitors, polymeric chains can also be attached to star polymer cores. Specifically, the formation of pH-sensitive bonds between aldehyde and aminooxy groups has been explored to fabricate miktoarm star polymers.[38,39]
|
Star polymers have also been used to deliver the ubiquitous cellular signalling molecule nitric oxide (NO) and so prevent biofilm formation.[40] To this end, a novel NO delivery agent was synthesized via the integration of NO donor molecules into star polymers. Specifically, amine-reactive 2-vinyl-4,4-dimethyl-5-oxazolone monomer (VDM) was introduced during the crosslinking process, and further treated with spermine and NO gas under increased pressure (e.g. 5 atm [506 kPa]) to yield N-diazeniumdiolate moieties (NONOates). The resulting NO donor-modified star polymers were shown to release NO when pH values were lower than 7.5 owing to the hydrolysis of NONOates. In vitro experiments suggested that star polymer with sustained NO release capability efficiently prevented the formation of biofilms of Pseudomonas aeruginosa, whereas neither spermine-functionalized star polymers without NO-release capability nor small-molecule-derived NO donor were able to effectively inhibit biofilm formation.
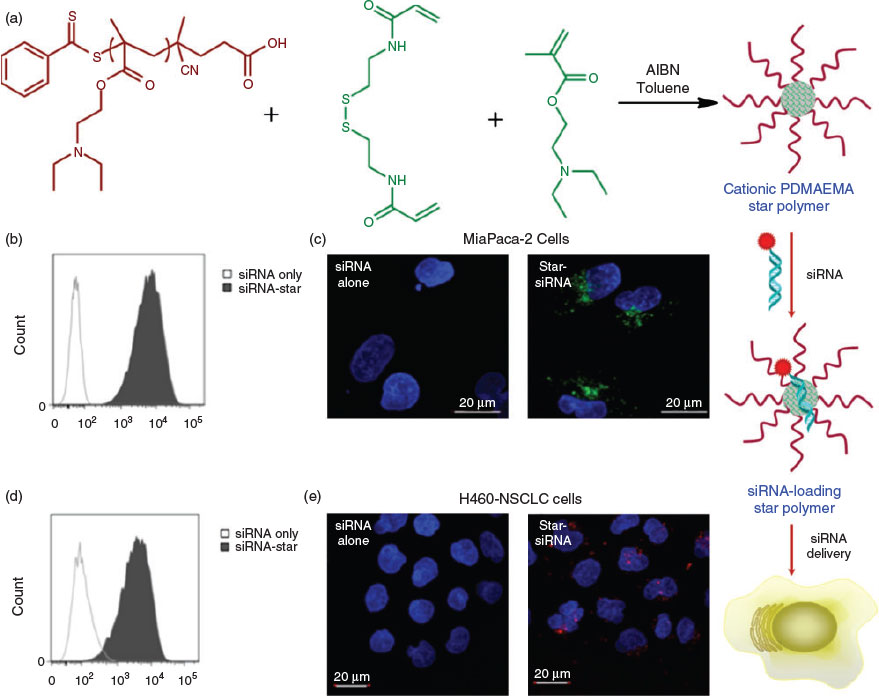
The above examples of controlled delivery using star polymers mainly involve functionalizing the core of the star, leaving the POEGA coronas intact to provide anti-fouling properties during circulation. However, the coronas can also be modified for certain applications. In recent work, the hydrophilic POEGA corona was substituted by cationic poly(2-dimethylaminoethyl methacrylate) (PDMAEMA) to facilitate intracellular gene delivery through the electrostatic interactions between negatively charged nucleic acids and cationic PDMAEMA. Using a pancreatic cell line that stably expresses high levels of firefly luciferase 2 (Luc2) gene, cationic star polymers loaded with small-interfering RNA (siRNA) where shown to reduce the expression of Luc2 mRNA by up to 50 %, with a commensurate decrease in Luc2 protein expression also observed. Further, in vivo experiments using mice injected with H460 non-small-cell lung cancer cells expressing high levels of green fluorescent protein (GFP) demonstrated that delivery of siRNA via intratumoral injection of the cationic PDMAEMA star polymers could silence GFP mRNA expression by 50 % (Fig. 4).[41] Using cationic star polymers to deliver siRNA has also been shown to have potential for treating pancreatic cancer. After systemic administration, the in vivo results indicated that the star polymer could efficiently deliver siRNA to orthotopic pancreatic tumours in mice and effectively inhibit the expression of βIII-tubulin.[42] Notably, the gene delivery capability of PDMAEMA-based cationic star polymers was not compromised by employing other synthetic protocols for the star formation, such as an ATRP arm-first approach.[43]
|
Compared with other drug and gene carriers self-assembled from amphiphilic molecules, the synthesis of star polymers is both time- and cost-effective. The ability to incorporate functional moieties and to tailor the outer corona means that star polymers can readily accommodate various chemotherapeutic agents or genes, either covalently or by electrostatic association. Moreover, star polymers exhibit good stability and are not susceptible to disassociation in the same way as self-assembled structures. Further, star polymers typically have a hydrodynamic diameter of ~20 nm, which is ideal for delivery via the enhanced permeability and retention (EPR) effect. Finally, using stimuli-responsive crosslinking agents can impart programmed degradation, thereby reducing the cytotoxicity of polymeric scaffolds.
Star Polymers for Imaging and Theranostic Applications
MRI is a powerful, non-invasive technique for the diagnosis of numerous illnesses including cancer and cardiovascular disease. During MRI, so-called contrast agents are often administered to enhance contrast and distinguish pathological regions from normal tissue. Macromolecular MRI contrast agents have several advantages over small-molecule-based agents, such as enhanced sensitivity and improved accumulation in pathogenic regions (due to the EPR effect). In this context, star polymer-based macromolecular MRI contrast agents have been prepared by conjugating small-molecule contrast agents to star polymer structures derived from hyperbranched H40 polyester, β-CD, and tetraphenylethylene cores.[44–47] These star-shaped macromolecular contrast agents have substantial benefits in terms of stability, enhanced circulation time, increased relaxivity, and potential for functional modification.
Building on our previous work on the functionalization of star polymers, we successfully prepared star polymer-based macromolecular MRI contrast agent by conjugating the gadolinium ion (Gd3+) chelating agent 1-(5-amino-3-aza-2-oxypentyl)-4,7,10-tris(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane (DO3A-tBu-NH2) to the polymer via activated ester chemistry. The macromolecular MRI contrast agent formed exhibits higher relaxivity compared with the corresponding small-molecule MRI agent. Interestingly, we found that the location of the gadolinium complex within the polymeric structure significantly affected the relaxivity. More specifically, hyperbranched structures and linear polymer labelled with gadolinium complex show similar relaxivities, both of which are superior to star polymer with the complex localized to the cores. This is presumably due to reduced accessibility of water to the star polymer cores.[48] Further studies wherein the precise location of the gadolinium complex within the star polymers is varied reveal that the star polymer having the complex at the chain ends of the hydrophilic corona has the maximal relaxivity at a low magnetic field strength (e.g. 0.47 T), whereas the star polymer with gadolinium complex randomly distributed along the hydrophilic arm has significantly compromised relaxivity under the same conditions. Moreover, the relaxivity of star polymer with the complex in the core is even worse (Fig. 5).[49] Importantly, the relaxivities of all three kinds of star polymers are profoundly affected by the magnetic field strength. Moreover, the MRI relaxivities could be further regulated by introduction of functional monomers that respond to endogenous signals of pathological regions. This can further enhance the contrast and resolution of regions of interest by selectively suppressing imaging signals in normal regions.[50,51]
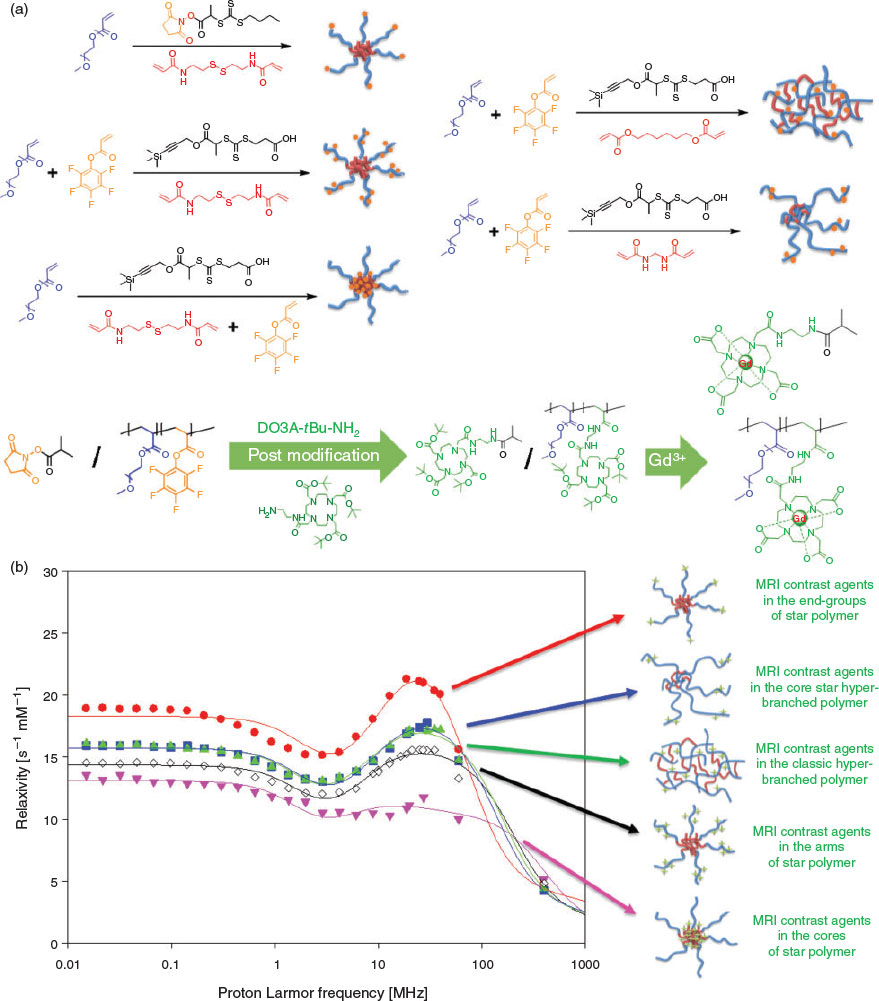
|
In addition to small-molecule MRI contrast agents, fluorophores can also be readily incorporated into star polymers, yielding fluorescent star polymers for cellular imaging.[52,53] For instance, by employing acrolein-labelled thermoresponsive poly(N-isopropylacrylamide) (PNIPAM) as the arm and aluminium tris(8-hydroxyquinoline)-bearing fluorescent crosslinker, thermoresponsive fluorescent star polymers were successfully synthesized via an arm-first approach. These materials could be further functionalized with aminooxy-group modified proteins (e.g. bovine serum albumin, BSA) by using the acrolein moieties in the corona to form dynamic imine bonds.[54] Notably, star polymers can also be used as bimodal imaging agents after incorporating both fluorophores and MRI contrast agents into the star polymer structure.[55]
The application of star polymers as nanocarriers and macromolecular imaging agents demonstrates the power and versatility of the platform. However, in most cases, star polymers have been engineered for just a single function (e.g. drug delivery or imaging). Given the variety of routes that can be used to incorporate functionality into a star polymer (i.e. via the arm monomer, crosslinker or secondary monomer in the core), there is significant potential for fabricating materials that include both an imaging modality and a therapeutic capability. These theranostic star polymers can release their payload at a precise physical location and provide instant feedback of the successful delivery. In this context, we recently developed a star polymer-based theranostic nanovector via the incorporation of both MRI contrast agent functionality and reversibly conjugated chemotherapeutic drug (DOX) (Fig. 6).[56] In brief, activated-ester monomer (PFPA) and aldehyde-functional monomer (vinyl benzaldehyde, VBA) were included in the corona and core of star polymers respectively. The PFPA and VBA monomers were subsequently modified with DO3A-tBu-NH2 chelate and DOX by exploiting the disparate reactivities of PFPA and VBA.[57] In vitro experiments demonstrated that the dual-functionalized star polymers could be readily taken up by MCF-7 breast cancer cells. Moreover, intracellular DOX release was confirmed by fluorescence lifetime imaging microscopy (FLIM) using the distinctly different fluorescence lifetimes of free DOX and conjugated DOX. Further, the star polymer-based theranostic nanovector showed significant increase in relaxivity as compared with that of small-molecule MRI contrast agent precursor (i.e. DO3A-NH2-Gd).[56]
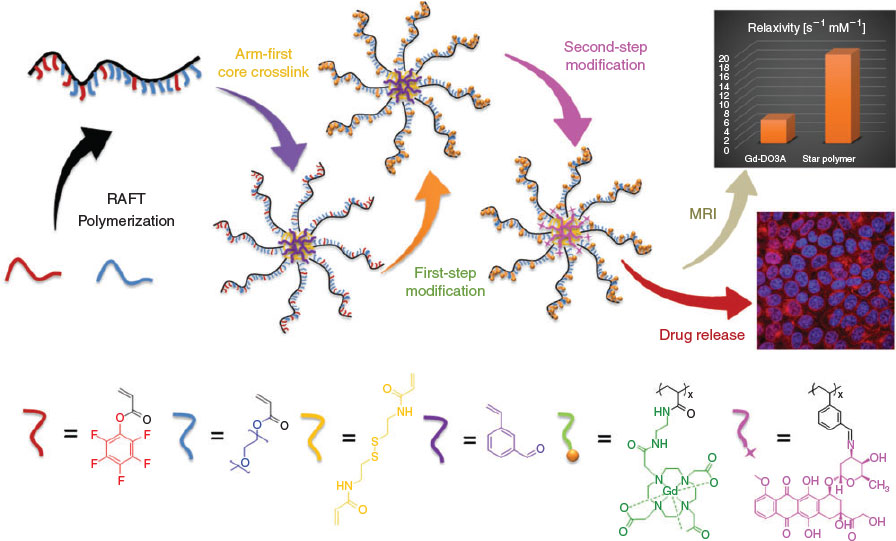
|
In Vivo Applications of Star Polymers
Despite extensive investigation of star polymers in vitro, the evaluation of their in vivo properties has been quite limited. Recently, we have started to scrutinize the in vivo performance of star polymers for drug and gene delivery applications. For example, the effect of molecular weight on the pharmacokinetics and tumour disposition of POEGA star polymers has been evaluated.[58] POEGA star polymers with varying molecular masses (49, 64, and 94 kDa) were synthesized via an arm-first approach, followed by modification with tritiated (3H) residues. The in vivo studies revealed that the POEGA star polymers were well tolerated at potential therapeutic concentrations and that the star polymer with the highest molecular mass (i.e. 94 kDa) exhibited the longest blood circulation time and tumour biodistribution. A subsequent study on the POEGA star with a molecular mass of 64 kDa suggested that it behaved similarly to PEGylated dendrimers after subcutaneous and inhaled delivery. That said, pulmonary administration of the star polymer (64 kDa) resulted in poor bioavailability (~3 %), with most of the star polymer retained in lung tissue and excreted in faeces.[59] Evidently, both the molecular weight (hydrodynamic size) and route of administration play an important role in determining the biological fate of star polymers. A further interesting study geared towards clinical application was recently carried out in which the interaction between star polymers and human blood cells was investigated. In contrast with a control star polymer bearing phenyl residues on the coronas, star polymers with thiol-reactive pyridyl disulfide (PDS) moieties possessed enhanced association with white blood cells at 37°C, particularly so with the phagocytic monocyte, granulocyte, and dendritic cell subsets.[60]
Concluding Remarks
Star polymers prepared via RAFT polymerization represent a new category of polymer nanoparticles suitable for drug and gene delivery, MRI, and theranostic applications. The use of living radical polymerization techniques to synthesize star polymers abrogates the need to use ionic polymerization, which requires more demanding synthetic conditions. Star polymers can be successfully prepared via either core-first or arm-first approaches, with the arm-first approach providing a particularly attractive route owing to its simplicity and versatility. Performing the arm-first technique in heterogenous media holds particular promise for achieving well-defined star polymers with narrow MWD. Moreover, the use of functional monomers such as PFPA or VBA in either the macroRAFT arm or core enables facile post-synthesis modification of the stars. Notably, beyond traditional RAFT polymerizations, the RAFT preparation of star synthesis is continuing to advance and recent studies have revealed that thiocarbonylthio compounds can be efficiently activated by photoirradiation or redox reactions.[61]
Importantly, star polymers have potential owing to their reasonable renal clearance and tumour accumulation.[62] As such, applications in the emerging field of nanomedicine are possible, with the integration of controlled release and imaging capability providing for novel theranostic applications. Although work to date has focussed on in vitro studies, preliminary in vivo work in our laboratory has now revealed new insights into the biodistribution and pharmacokinetic properties of star polymers.[58,59] Moreover, the specific recognition between star polymers and human blood cells has been examined as well.[60] Such investigations are a necessary next step towards ultimate clinical application of these materials. Notably, further in vivo studies have revealed a bright future for star polymers in gene therapy.[41,42]
In addition to the biomedical applications noted in the present review, star polymers can also be employed in several other fields such as membrane science, catalysis, and photonics. Given the variety of potential applications for these versatile materials, we envision that, going forward, star polymers prepared using RAFT polymerization will continue to be the subject of considerable interest.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
This work is financially supported by National Natural Science Foundation of China (NNSFC) Project no. 51673179 and the Australian Research Council Centre of Excellence in Convergent Bio-Nano Science and Technology (Project no. CE140100036). T.P.D. gratefully acknowledges the award of an Australian Laureate Fellowship.
References
[1] C. Boyer, M. H. Stenzel, T. P. Davis, J. Polym. Sci. Part A: Polym. Chem. 2011, 49, 551.| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhs1ejur%2FF&md5=9fef1c5d64cf2acf5298faa82e7576ccCAS |
[2] B. S. Sumerlin, A. P. Vogt, Macromolecules 2010, 43, 1.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVensrrK&md5=32e0009344f2087a2d399f1e794ef02cCAS |
[3] L. J. Fetters, A. D. Kiss, D. S. Pearson, G. F. Quack, F. J. Vitus, Macromolecules 1993, 26, 647.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXotFOmtQ%3D%3D&md5=f74a1e6ee9800044eae1a4683a4153a1CAS |
[4] J. M. Ren, T. G. McKenzie, Q. Fu, E. H. H. Wong, J. T. Xu, Z. S. An, S. Shanmugam, T. P. Davis, C. Boyer, G. G. Qiao, Chem. Rev. 2016, 116, 6743.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XpsFOhsrw%3D&md5=d7153d926879f76418fb7072ba4ccb8bCAS |
[5] H. F. Gao, Macromol. Rapid Commun. 2012, 33, 722.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjvVWqu7g%3D&md5=9f9da0bccf22af05887a224c0cbdb243CAS |
[6] N. Hadjichristidis, H. Iatrou, M. Pitsikalis, J. Mays, Prog. Polym. Sci. 2006, 31, 1068.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1yktrvM&md5=482ad39ccdae5ec1815bbd37946d7f28CAS |
[7] M. Morton, S. D. Gadkary, T. E. Helminiak, F. Bueche, J. Polym. Sci., Polym. Phys. Ed. 1962, 57, 471.
| 1:CAS:528:DyaF38XktFWnu7Y%3D&md5=31d0c77b64ad1c5a3286751fea16ed88CAS |
[8] A. Blencowe, J. F. Tan, T. K. Goh, G. G. Qiao, Polymer 2009, 50, 5.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksFyjsA%3D%3D&md5=4920df4a05182af206d5f7ac494e3e84CAS |
[9] H. Gao, K. Matyjaszewski, J. Am. Chem. Soc. 2007, 129, 11828.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpvVCntLY%3D&md5=e734deb835bc4485107e1bb89399813aCAS |
[10] M. R. Whittaker, M. J. Monteiro, Langmuir 2006, 22, 9746.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVKqsLbI&md5=0c8a2266bf2be54941d20b729d36cc9dCAS |
[11] Q. Zhang, L. Su, J. Collins, G. S. Chen, R. Wallis, D. A. Mitchell, D. M. Haddleton, C. R. Becer, J. Am. Chem. Soc. 2014, 136, 4325.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjtVequr8%3D&md5=370bb24fd66c33373fed47ca3e45b261CAS |
[12] D. Kuckling, A. Wycisk, J. Polym. Sci. Part A: Polym. Chem. 2013, 51, 2980.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmt1Sqs7Y%3D&md5=77a1bfdc0677d01ede6857d83d630f74CAS |
[13] J. T. Wiltshire, G. G. Qiao, Aust. J. Chem. 2007, 60, 699.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFKgur7M&md5=23e108a4fbc3fac7cad819c7f311f4d1CAS |
[14] K. Khanna, S. Varshney, A. Kakkar, Polym. Chem. 2010, 1, 1171.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtl2msLrM&md5=1f09895459542e84f53a4a69bbbe4b25CAS |
[15] T. P. Le, G. Moad, E. Rizzardo, S. H. Thang, WO 1998001478 1998.
[16] M. Stenzel-Rosenbaum, T. P. Davis, V. Chen, A. G. Fane, J. Polym. Sci. Part A: Polym. Chem. 2001, 39, 2777.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlsFKlsbo%3D&md5=bffb8c82b25fe6054f334df2022bbcbdCAS |
[17] M. H. Stenzel, T. P. Davis, J. Polym. Sci. Part A: Polym. Chem. 2002, 40, 4498.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XptlahsLc%3D&md5=a4aa255a0bb61e74ed153e0b045cc11fCAS |
[18] M. H. Stenzel, T. P. Davis, C. Barner-Kowollik, Chem. Commun. 2004, 1546.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltFWnsbY%3D&md5=c85b0f8564b5745cef187f2e0b1faeabCAS |
[19] J. Bernard, X. J. Hao, T. P. Davis, C. Barner-Kowollik, M. H. Stenzel, Biomacromolecules 2006, 7, 232.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht12rtbzP&md5=bd5d20a76c9035cf396477539ca6d383CAS |
[20] J. F. Quinn, R. P. Chaplin, T. P. Davis, J. Polym. Sci. Part A: Polym. Chem. 2002, 40, 2956.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xmt12hu7k%3D&md5=7851b436171bf91153d18c20dfe3bf4bCAS |
[21] X. J. Hao, C. Nilsson, M. Jesberger, M. H. Stenzel, E. Malmstrom, T. P. Davis, E. Östmark, C. Barner-Kowollik, J. Polym. Sci. Part A: Polym. Chem. 2004, 42, 5877.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVCjtLzL&md5=277eb26dcd3373185ad3cd2ef88c469fCAS |
[22] X. J. Hao, E. Malmstrom, T. P. Davis, M. H. Stenzel, C. Barner-Kowollik, Aust. J. Chem. 2005, 58, 483.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXltFymsb4%3D&md5=fc4007c02f394505fb0a7c9562d2692aCAS |
[23] E. Setijadi, L. Tao, J. Q. Liu, Z. F. Jia, C. Boyer, T. P. Davis, Biomacromolecules 2009, 10, 2699.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpsFGisb0%3D&md5=8e0dd81cb56296da9d061f368cf0e854CAS |
[24] J. Q. Liu, H. Y. Liu, Z. F. Jia, V. Bulmus, T. P. Davis, Chem. Commun. 2008, 6582.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVKntb3M&md5=725ddc0b38685451d705ac5eb0f57f17CAS |
[25] J. Q. Liu, L. Tao, J. T. Xu, Z. F. Jia, C. Boyer, T. P. Davis, Polymer 2009, 50, 4455.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVOju7fJ&md5=008cab048d87c9d8dfa80724086d4a05CAS |
[26] C. Barner-Kowollik, T. P. Davis, M. H. Stenzel, Aust. J. Chem. 2006, 59, 719.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFeqsr%2FP&md5=3420dafc091a3656704565a47ffa712dCAS |
[27] H. T. Lord, J. F. Quinn, S. D. Angus, M. R. Whittaker, M. H. Stenzel, T. P. Davis, J. Mater. Chem. 2003, 13, 2819.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXotlyqtLg%3D&md5=7aeb23b8a4c7921ff90d963c3020ad05CAS |
[28] J. A. Syrett, D. M. Haddleton, M. R. Whittaker, T. P. Davis, C. Boyer, Chem. Commun. 2011, 1449.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXot1Crtw%3D%3D&md5=e74f3560e42827dc8f9d108400dd1075CAS |
[29] X. H. Wei, G. Moad, B. W. Muir, E. Rizzardo, J. Rosselgong, W. T. Yang, S. H. Thang, Macromol. Rapid Commun. 2014, 35, 840.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXitFarsL0%3D&md5=bb5e5496adfca03c33dd6d0c9d4a6430CAS |
[30] J. Ferreira, J. Syrett, M. Whittaker, D. Haddleton, T. P. Davis, C. Boyer, Polym. Chem. 2011, 2, 1671.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpslKltL0%3D&md5=c03d622aad7ba82bd7b1a5809aca4938CAS |
[31] C. L. Zhang, M. Miao, X. T. Cao, Z. S. An, Polym. Chem. 2012, 3, 2656.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFansLnI&md5=9eb75c87a85ef131acefe533b2db86e4CAS |
[32] Q. Qiu, G. Y. Liu, Z. S. An, Chem. Commun. 2011, 12685.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsV2ktLjJ&md5=68a5413d0ff1c9b923f32797e68c9c66CAS |
[33] L. Zhang, K. Katapodi, T. P. Davis, C. Barner-Kowollik, M. H. Stenzel, J. Polym. Sci. Part A: Polym. Chem. 2006, 44, 2177.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjtVeltLc%3D&md5=92dac22481d1d384d142f0326a31f082CAS |
[34] C. Boyer, M. Whittaker, T. P. Davis, J. Polym. Sci. Part A: Polym. Chem. 2011, 49, 5245.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1Gqs7fI&md5=8615744919c8d67806bd79f277aed7fdCAS |
[35] G. M. Soliman, A. Sharma, D. Maysinger, A. Kakkar, Chem. Commun. 2011, 9572.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVWgs73O&md5=7319add62dc6261330b50de48dd0a114CAS |
[36] J. N. Liu, H. E. Duong, M. R. Whittaker, T. P. Davis, C. Boyer, Macromol. Rapid Commun. 2012, 33, 760.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlsFSqsLY%3D&md5=3b8b407f27d578ef3a2ce0955d24cd17CAS |
[37] S. J. Kim, D. M. Ramsey, C. Boyer, T. P. Davis, S. R. McAlpine, ACS Med. Chem. Lett. 2013, 4, 915.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFOltrzE&md5=99fb52c86d8fea9099ebb459cdbee469CAS |
[38] Z. M. Wu, H. Liang, J. A. Lu, W. L. Deng, J. Polym. Sci. Part A: Polym. Chem. 2010, 48, 3323.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotlWnsL0%3D&md5=f9303c8b50ef97cf38bf791ae61f9692CAS |
[39] Z. M. Wu, H. Liang, J. A. Lu, Macromolecules 2010, 43, 5699.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXms1Shtbc%3D&md5=501715853c2db53524da93e31cec865dCAS |
[40] H. T. T. Duong, K. Jung, S. K. Kutty, S. Agustina, N. N. M. Adnan, J. S. Basuki, N. Kumar, T. P. Davis, N. Barraud, C. Boyer, Biomacromolecules 2014, 15, 2583.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXpsVGgtbg%3D&md5=7b332dcecb7be887d34ff9f585bb47c9CAS |
[41] C. Boyer, J. Teo, P. Phillips, R. B. Erlich, S. Sagnella, G. Sharbeen, T. Dwarte, H. T. T. Duong, D. Goldstein, T. P. Davis, M. Kavallaris, J. McCarroll, Mol. Pharm. 2013, 10, 2435.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmt1yks7o%3D&md5=c28d4744cf354f44f08c60e892bb3874CAS |
[42] J. Teo, J. A. McCarroll, C. Boyer, J. Youkhana, S. M. Sagnella, H. T. T. Duong, J. Liu, G. Sharbeen, D. Goldstein, T. P. Davis, M. Kavallaris, P. A. Phillips, Biomacromolecules 2016, 17, 2337.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XpslOqsrk%3D&md5=3b63ec3904006b21ecd3e651154c70e8CAS |
[43] H. Y. Cho, A. Srinivasan, J. Hong, E. Hsu, S. G. Liu, A. Shrivats, D. Kwak, A. K. Bohaty, H.-j. Paik, J. O. Hollinger, K. Matyjaszewski, Biomacromolecules 2011, 12, 3478.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFWqt73K&md5=6adc99170a655b79bc0e610740dea9ebCAS |
[44] X. J. Li, Y. F. Qian, T. Liu, X. L. Hu, G. Y. Zhang, Y. Z. You, S. Y. Liu, Biomaterials 2011, 32, 6595.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXos1Sks7k%3D&md5=c7510acf7972ec7dde833ec16ede9ef1CAS |
[45] Y. M. Li, H. S. Yu, Y. F. Qian, J. M. Hu, S. Y. Liu, Adv. Mater. 2014, 26, 6734.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVSnurfO&md5=ff41c735f13bfeb6e288c9508d89e3d5CAS |
[46] Y. Li, Y. F. Qian, T. Liu, G. Y. Zhang, J. M. Hu, S. Y. Liu, Polym. Chem. 2014, 5, 1743.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvFGiu70%3D&md5=5c35755ab2be59c6e2b8d8da4141efd9CAS |
[47] T. Liu, X. J. Li, Y. F. Qian, X. L. Hu, S. Y. Liu, Biomaterials 2012, 33, 2521.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XntFKrtQ%3D%3D&md5=c7db597322a0bf26edb8b31e44a3a09aCAS |
[48] Y. Li, M. Beija, S. Laurent, L. V. Elst, R. N. Muller, H. T. T. Duong, A. B. Lowe, T. P. Davis, C. Boyer, Macromolecules 2012, 45, 4196.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmslKjtLc%3D&md5=8ee0a9cc141fb7ba74a92e6f123186bdCAS |
[49] Y. Li, S. Laurent, L. Esser, L. V. Elst, R. N. Muller, A. B. Lowe, C. Boyer, T. P. Davis, Polym. Chem. 2014, 5, 2592.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjslGmsb8%3D&md5=8928abd8076041d30342de901a911ec3CAS |
[50] K. W. Wang, H. Peng, K. J. Thurecht, S. Puttick, A. K. Whittaker, Polym. Chem. 2014, 5, 1760.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvFGiu7Y%3D&md5=a1489a2436af42741ca12b434f9e1912CAS |
[51] J. M. Hu, T. Liu, G. Y. Zhang, F. Jin, S. Y. Liu, Macromol. Rapid Commun. 2013, 34, 749.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslKmsLvM&md5=b4f2993fccedc7364ef76eb88c5242f0CAS |
[52] F. Cheng, E. M. Bonder, A. Doshi, F. Jakle, Polym. Chem. 2012, 3, 596.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvFalurg%3D&md5=76fcd6053704f42a9afbe45a83d31b17CAS |
[53] C. T. Adkins, E. Harth, Macromolecules 2008, 41, 3472.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlt1GhsL0%3D&md5=1c82f7cbf030529f6b72f62e69378a9aCAS |
[54] K. M. Yang, H. Liang, J. Lu, J. Mater. Chem. 2011, 21, 10390.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXosVWgtL8%3D&md5=5d1abe9075ec2d3e8eb670efde8e97c7CAS |
[55] C. T. Adkins, J. N. Dobish, C. S. Brown, B. Mayrsohn, S. K. Hamilton, F. Udoji, K. Radford, T. E. Yankeelov, J. C. Gore, E. Harth, Polym. Chem. 2012, 3, 390.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XntlehsA%3D%3D&md5=3e53ce5a3ec25a0aea8b0bf2a7765ecaCAS |
[56] Y. Li, H. T. T. Duong, S. Laurent, A. MacMillan, R. M. Whan, L. V. Elst, R. N. Muller, J. Hu, A. Lowe, C. Boyer, T. P. Davis, Adv. Healthc. Mater. 2015, 4, 148.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXivFWjuw%3D%3D&md5=f2311635b038e9c4552aa8a650b54d65CAS |
[57] Y. Li, H. T. T. Duong, M. W. Jones, J. S. Basuki, J. M. Hu, C. Boyer, T. P. Davis, ACS Macro Lett. 2013, 2, 912.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFWksLrM&md5=4cecd5c02ee21e020f40accfae0a964cCAS |
[58] S. Y. Khor, J. M. Hu, V. M. McLeod, J. F. Quinn, M. Williamson, C. J. H. Porter, M. R. Whittaker, L. M. Kaminskas, T. P. Davis, Nanomed.: Nanotechnol. Biol. Med. 2015, 11, 2099.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsFSitb3O&md5=120bf24e055facab8851ca8717cf5d80CAS |
[59] S. Y. Khor, J. M. Hu, V. M. McLeod, J. F. Quinn, C. J. H. Porter, M. R. Whittaker, L. M. Kaminskas, T. P. Davis, J. Pharm. Sci. 2016, 105, 293.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhtF2mtr%2FF&md5=b3c608484c5f3c7b3f6cf933856dcf99CAS |
[60] J. J. Glass, Y. Li, R. De Rose, A. P. R. Johnston, E. I. Czuba, S. Y. Khor, J. F. Quinn, M. R. Whittaker, T. P. Davis, S. J. Kent, ACS Appl. Mater. Interfaces 2017, 9, 12182.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXkvVyisLg%3D&md5=b3e1945b961318946e41a68e45b74a0bCAS |
[61] T. G. McKenzie, Q. Fu, M. Uchiyama, K. Satoh, J. T. Xu, C. Boyer, M. Kamigaito, G. G. Qiao, Adv. Sci. 2016, 3, 1500394.
| Crossref | GoogleScholarGoogle Scholar |
[62] M. E. Fox, F. C. Szoka, J. M. J. Frechet, Acc. Chem. Res. 2009, 42, 1141.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnslSkt7o%3D&md5=2d514a2dedcf2527ff07421c9318460aCAS |
* Thomas P. Davis was the recipient of the 2016 Batteard–Jordan Polymer Medal.