
Australian Journal of Chemistry
Volume 66 Number 3 2013
RESEARCH FRONT: Beckwith Symposium
CH13003Multi-Bond Forming Processes in Efficient Synthesis
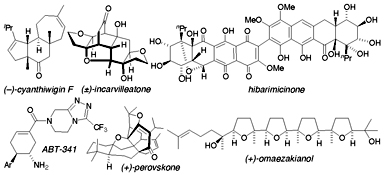
The perfect synthesis is just one step away! Complex targets are being made in fewer operations. This review classifies the strategies for short syntheses, presents landmark recent examples, and offers predictions about the future.

The papers collected for this special edition are authored by friends of Professor Athel Beckwith who attended the Beckwith Memorial Symposium held in his honour last year. This introduction sets the symposium and the papers in context of Athel’s distinguished career and contributions to chemistry.
CH125021,5-(1,7)-Biradicals and Nitrenes Formed by Ring Opening of Hetarylnitrenes
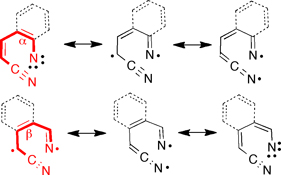
Several aromatic and heteroaromatic nitrenes and carbenes undergo photochemical and sometimes thermal ring opening. Depending on benz-annelation, the ring-opened species may have the character of either nitrenes (for α-annelation) or 1,5-(1,7-)-biradicals (for β-annelation). Both types have been observed, and they are clearly distinguished by their characteristic electron spin resonance spectra.
CH12546Production and Regulation of Levels of Amidated Peptide Hormones
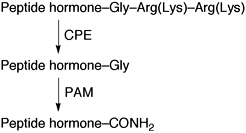
This review surveys literature concerning the regulation and inhibition of peptidylglycine α-amidating monooxygenase (PAM) and carboxypeptidase E (CPE), key enzymes involved in the formation of C-terminal amidated peptide hormones that regulate numerous physiological processes and are associated with many disease states.
CH12452Computational Evaluation of the Sulfonyl Radical as a Universal Leaving Group for RAFT Polymerisation
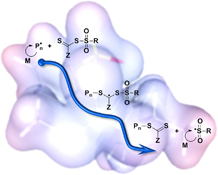
The success of the reversible addition–fragmentation chain-transfer polymerisation process depends on choosing reagents that are tailored to the monomer being polymerised. Here, we predict that sulfonyl radicals should function as universal leaving groups, capable of efficient chain transfer for a broad range of monomers. The sulfonyl end-group also offers opportunities for post-polymerisation functionalisation.
CH12480A Novel Organic Electron Donor Derived from N-Methylisatin
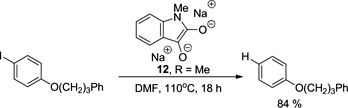
Donor 12 formed by reduction of N-methylisatin is a strong reducing agent.
CH12477Rate Coefficients for Intramolecular Homolytic Substitution of Oxyacyl Radicals at Sulfur
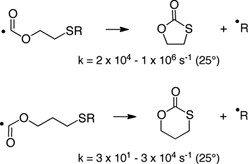
The rapid rise in the use of free radical chemistry is largely due to the plethora of rate constant data that became available over the past few decades for many classes of radicals. For oxyacyl radicals this is not the case. To address this imbalance, we now report rate constants for intramolecular homolytic substitution at sulfur.
CH12494Radical Cascade Protocol for the Synthesis of (5'S)- and (5'R)-5',8-Cyclo-2'-deoxyguanosine Derivatives
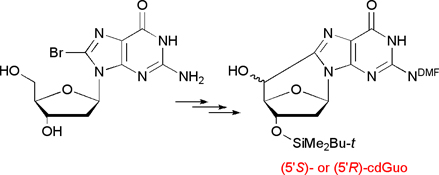
Easy access to (5′S)- or (5′R)-cdGuo building blocks for DNA modification is desirable for the full development of biochemical, biological, and biophysical studies of these lesions. The present radical cascade protocol provides facile access to these modified nucleosides.
CH12499Convenient Ambient Temperature Generation of Sulfonyl Radicals
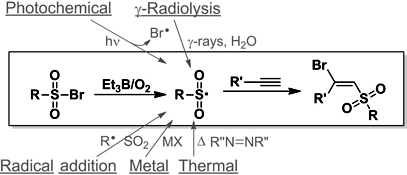
This paper presents a novel method for the efficient, ambient temperature generation of sulfonyl radicals from aryl and alkyl sulfonylbromides upon autoxidation of triethylborane (Et3B). The resultant radicals were regioselectively trapped via addition to terminal alkynes, generating a secondary vinyl radical that selectively abstracts a Br atom from RSO2Br, yielding the (E)-bromo vinylsulfones.
CH12523Kinetic Study of the Radical Azidation with Sulfonyl Azides
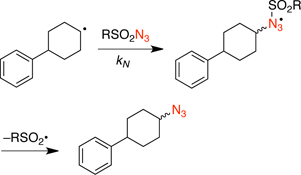
Rate constants for the addition of a secondary alkyl radical 3-pyridinesulfonyl azide and trifluoromethanesulfonyl azide were determined using bimolecular competing radical reactions.
CH12545New Elements on the Behaviour of a Bissulfinylmethyl Radical
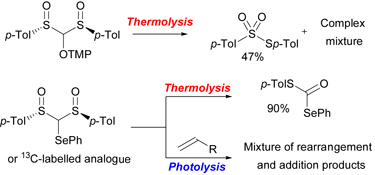
The generation of a bissulfinylmethine radical from the corresponding TEMPO and phenylselenyl bissulfoxide precursors has been studied. No univocal formation of the bissulfinyl radical has been observed in thermal or photochemical conditions. Instead, complex mixtures have been obtained, showing prominent C–S homolytic bond cleavage, which has been supported by calculations.
CH12404Synthesis of Phosphatidylcholine with Conjugated Linoleic Acid and Studies on Its Cytotoxic Activity
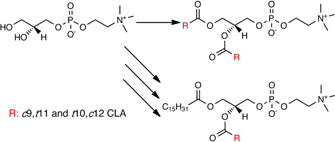
Conjugated linoleic acid (CLA) displays many physiological properties. Of particular interest is its effect on different cancer types. It can be used in the synthesis of phosphatidylcholine, which can be applicable in the targeted drug delivery of liposomes. Obtained results proved that phosphatidylcholine derivatives decreased the cytotoxic effect of CLA against healthy cell lines.
CH12181Oxyhalogen–Sulfur Chemistry: Oxidation of a Thiourea Dimer, Formamidine Disulfide, by Chlorine Dioxide
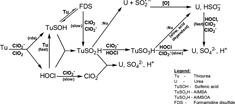
The oxidation of formamidine disulfide, FDS, the dimer of thiourea, by aqueous chlorine dioxide has been studied in highly acidic and mildly acidic media. Mass spectrometric data suggest that the oxidative pathway proceeds predominantly through the sulfinic acid while by-passing the sulfonic acid.
CH12421Dithienylbenzothiadiazole-Based Donor-Acceptor Organic Semiconductors and Effect of End Capping Groups on Organic Field Effect Transistor Performance
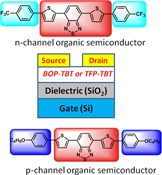
Donor-acceptor-donor based conjugated molecules using a thiophene benzothiadiazole-thiophene central core with trifluoromethyl phenyl or butoxyphenyl end capping groups were synthesised by Suzuki coupling. Both materials were tested in organic field effect transistor device geometries and show p and n-channel behaviour with respect to different end capping groups. The highest hole and electron mobilities for both materials were calculated to be 2 × 10–2 and 4 × 10–3 cm2/Vs, respectively, using top contact geometry.
CH12482Facile Fabrication of Au–F127 Nanocolloids with Different Morphologies and their Potential Bioapplications
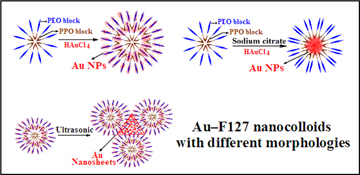
Three facile fabrication routes to prepare Au–F127 nanocolloids with different morphologies are presented and their formation mechanisms are discussed. The possible biomedical applications of the diffferent Au–F127 nanocolloids are investigated.
CH124851,3-Di(alkoxy)imidazolium-based Ionic Liquids: Improved Synthesis and Crystal Structures
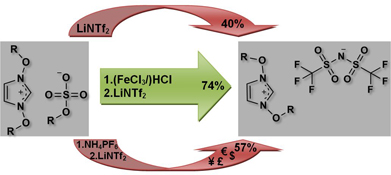
A new and convenient synthetic pathway to 1,3-di(alkoxy)imidazolium bis(trifluoromethylsulfonyl)amides and novel 1,3-di(alkoxy)imidazolium tetrachloroferrates was developed. Since an intermediate isolation step of respective hexafluorophosphates was required in previously reported preparations, they suffered from low overall yields and additional expenses. The use of FeCl3/HCl resulted in substantially improved yields and allows one-pot preparations with good scalability.



