The future of grain legumes in cropping systems
Thomas R. Sinclair A C and Vincent Vadez BA Crop Science Department, North Carolina State University, Raleigh, NC 27695, USA.
B ICRISAT (International Crops Research Institute for the Semiarid Tropics), Dryland Cereal Research Program, Patancheru 502324, Andhra Pradesh, India.
C Corresponding author. Email: trsincla@ncsu.edu
Crop and Pasture Science 63(6) 501-512 https://doi.org/10.1071/CP12128
Submitted: 3 April 2012 Accepted: 12 July 2012 Published: 14 September 2012
Abstract
Grain legume production is increasing worldwide due to their use directly as human food, feed for animals, and industrial demands. Further, grain legumes have the ability to enhance the levels of nitrogen and phosphorus in cropping systems. Considering the increasing needs for human consumption of plant products and the economic constraints of applying fertiliser on cereal crops, we envision a greater role for grain legumes in cropping systems, especially in regions where accessibility and affordability of fertiliser is an issue. However, for several reasons the role of grain legumes in cropping systems has often received less emphasis than cereals. In this review, we discuss four major issues in increasing grain legume productivity and their role in overall crop production: (i) increased symbiotic nitrogen fixation capacity, (ii) increased phosphorus recovery from the soil, (iii) overcoming grain legume yield limitations, and (iv) cropping systems to take advantage of the multi-dimensional benefits of grain legumes.
Introduction
Legumes have been cropped by humans for centuries. However, commonly legumes were included in cropping systems to provide fodder for animals and to enhance yields of subsequent grain crops. While the ancient Chinese included soybean and the Romans sometimes included pea in their crop rotations, seed harvest was usually not their prime motivation for growing these crops but rather it was the beneficial effect of improving the soil for growing cereal crops (Sinclair and Sinclair 2010). In the USA, soybean and to some extent peanut were introduced primarily as fodder crops. The potential for use of seeds of peanut, soybean, and cowpea for food and manufacturing feedstock was not recognised in the USA until the early 19th century. At that time, several uses of seeds of grain legumes in foods and industrial uses were identified. For example, George Washington Carver, who worked with comparatively primitive facilities at the Tuskegee Institute in Alabama, developed more than 100 products made from peanut seeds.
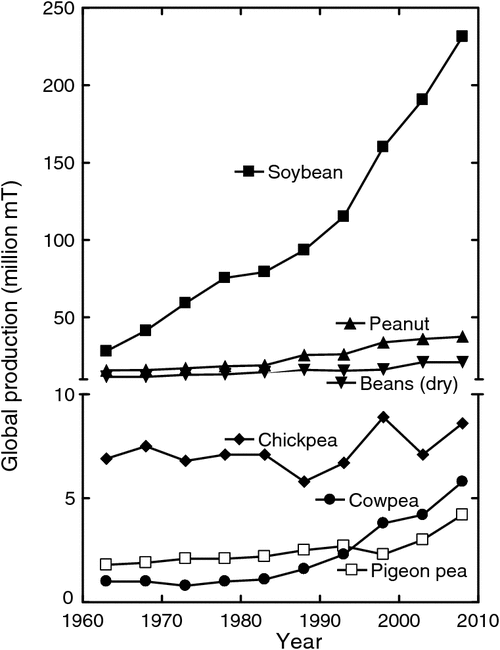
After World War II, the demand for the seeds of grain legumes, particularly soybean, began a large, steady increase (Fig. 1). There were several reasons for this increasing demand. One reason was the use of soybean seeds to supply protein for feed for animals, whose numbers increased in response to the demand for increasing consumption of meat associated with increasing affluence. A second reason was the use of the seed lipids in food products, e.g. as a cooking oil. Also, there was an increasing demand for soybean lipids in the manufacture of various products. This demand was met globally by increased production in the USA, Brazil, Argentina, and China. In addition to the increased production of soybean, in more recent times there has been a rapidly increasing demand for direct human consumption of grain legumes such as cowpea, peanut, and pigeon pea (Fig. 1). A possible further increase in demand may result from the recent suggestion that lipids from grain legumes could also be used directly as a source of biodiesel fuel.
|
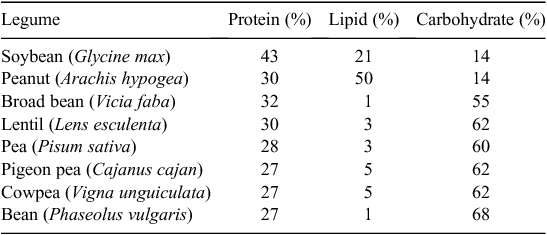
Now, legumes are a major part of many people’s diet worldwide. Direct consumption of grain legumes in many regions of the world will need to expand with human population to meet and improve their nutritional needs. The seed protein concentration of grain legumes is generally at least 25% (Table 1) and reaching as high as 50% in some genotypes of soybean. Cowpea is largely consumed across West Africa, while bean is widely consumed in the Americas and other areas worldwide. In India where much of the population is vegetarian, several legumes including pigeon pea, chickpea, mungbean, and lentils are cultivated on millions of hectares. Domestic production in India now falls short of demand and there is a net import of ~2.5–3.5 Mt pulses per year (Ali and Gupta 2012).
|
Continued increases in human population and affluence will sustain the increasing demand for grain legumes to feed animals and for direct human consumption (e.g. Ali and Gupta 2012). The expanding economies in Asia and elsewhere have already resulted in rapidly increasing demand for soybean protein to feed poultry and swine.
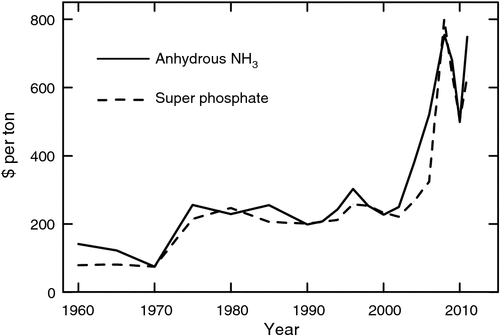
In addition to the increasing demand for grain legumes, these species bring to cropping systems the crucial capacity to decrease or eliminate the need for direct applications of some fertilisers. In many regions of the world, farmers simply do not have access to fertiliser. In regions of industrial agriculture where fertilisers are available, the skyrocketing costs of fertiliser (Fig. 2) are resulting in economic pressure on farmers to minimise fertiliser inputs. The increasing cost of energy to manufacture nitrogen (N) fertiliser has forced prices to new, higher levels. Also, the costs of mining phosphorus (P) along with an apparent dwindling of P stocks have also stimulated P prices to higher levels. Increasing costs of fossil fuels will no doubt impact directly on the availability of N and P fertilisers in future cropping systems. In fact, in some regions fertiliser is now the largest single variable cost in growing grain crops (Purdue University 2012).
|
In addition to the economic pressures to minimise fertiliser use in cropping systems, there are environmental pressures to substantially decrease the negative impact of fertiliser application for cereal crops. Nitrogen fertilisers are major sources of greenhouse gases, particularly nitrous oxide, and result in contamination of water ways and human water supplies with nitrate. Similarly, loss of P from fields can contribute to algae blooms and eutrophication of streams and lakes. The ‘dead zone’ that exists at the mouth of the Mississippi River in the Gulf of Mexico is attributable to fertiliser from crop fields in the Midwest of the USA (Burkart and James 1999; Duan et al. 2010). Current and undoubtedly future environmental regulations in many regions of the world will restrict application of fertilisers on crops.
Grain legumes bring to cropping systems a much decreased demand for fertiliser. Legumes have the capacity to meet much of their own N requirements through symbiotic N fixation so that crop growth can be potentially fully sustained without N fertiliser. Also, the potential exists to return to the approach of the ancient Chinese and Romans to use grain legumes as an important means to increase the overall N input into cropping systems. As discussed later, some grain legumes have the unique capacity to recover P from the soil that is in forms unavailable to other crops. The need to decrease P application to cropping system could be a very important attribute grain legumes might bring to cropping systems. Nevertheless, grain legumes when grown on many soils will still likely require fertilisation to provide potassium since potassium plays a key role in electric charge balancing within the rhizosphere, especially for N2-fixing legumes (Tang 1998).
In crop-livestock systems of developing countries, where cattle feed on cereal crop residues, N concentration of cereal residue is often much below the threshold needed in animal diets. Nitrogen concentrations of at least 1.0–1.2% are needed for microbial population in the rumen of livestock for an efficient feed digestion (Van Soest 1994), which are virtually never reached by cereal residues but legume residues are at or above this threshold (Sundstøl and Owen 1984). Therefore, it is possible that legume crop residues will be playing an increasing role as a source of inexpensive fodder or even key N-rich additive in cattle production.
Of course, inclusion of grain legumes in cropping systems is not without challenges. Since the plants and seeds are high in protein content, and in some species high oil content, they are attractive to diseases and insects. Also, maintaining high quality seeds stocks can be more difficult because of the comparatively low yield of parent plants and a comparatively short storage life of seeds. Farmers in developing areas in particular often cannot carry forward good quality seeds for optimum production.
Finally, certain grain legumes such as bean are often called the ‘poor man’s meat’. Consequently, outside regions where soybean and pea are major cash crops, grain legumes are still often viewed as secondary crops to facilitate the production of cereals. Grain legume production in developing countries is often ‘pushed’ to a more marginal area. For instance, the traditional chickpea production in Northern India has now almost fully shifted to the south (Gowda et al. 2009) despite the lower yield potential in these new niches. We argue in this paper, that grain legumes deserve a much more prominent role in future cropping systems in both developed and less-developed agriculture around the world.
We reviewed a decade ago key physiological traits that could be exploited for yield improvement of grain legumes (Sinclair and Vadez 2002). In view of recent research advances and expanded opportunities for legumes in cropping systems, we offer this updated review of the role of grain legumes in cropping systems. The objective of the current review is to discuss several key features of grain legumes that need to be exploited to meet the future demands for the enhanced role of grain legumes in cropping systems. These topics include (i) increased symbiotic N fixation capacity, (ii) increased P recovery from the soil, (iii) overcoming grain legume yield limitations, and (iv) the multi-dimensional benefit of legumes in cropping systems.
Symbiotic nitrogen fixation
All plants need large amounts of N because N is an essential component of all proteins and nucleic acids required for new, functioning cells. Therefore, it is not surprising that limited N availability in natural, non-legume plants systems results in restricted plant growth. Legumes ameliorated the N limitation by having evolved a capability to form a symbiosis with rhizobia and bradyrhizobia to fix atmospheric N. Symbiotic N fixation was a major evolutionary advantage because legume growth was not limited to N availability in the soil.
Nitrogen fixation symbiosis and an ability to sustain high rates of N fixation is a delicate activity for plants. Nitrogenase, which is the enzyme provided by the bacteria to fix atmospheric N, is extremely sensitive to exposure to even low concentrations of oxygen (Robson and Postgate 1980). Nitrogen fixation requires a low oxygen environment to sustain fixation so that achievement of high rates of N fixation activity requires a carefully regulated oxygen environment around nitrogenase. The regulation of oxygen concentration while maintaining high oxygen flux is a critical challenge in exploiting associative N fixation as an option to achieve high N input rates for cereal crops. The solution evolved by grain legumes is the development of specialised nodule structures that allow very controlled regulation of the oxygen atmosphere around nitrogenase to support high N fixation rates. In nodules, nitrogenase occupies the central volume of the nodule which is surrounded by a continuous cellular barrier in the inner nodule cortex that controls oxygen diffusion into the central volume (Tjepkema and Yocum 1974; Purcell and Sinclair 1993). The gas permeability of the cortex is under regulation by the plant so that oxygen concentration is maintained at a level that is not deleterious to nitrogenase, but allows high fixation rates (Minchin 1997).
Another unique feature of the nodules is that they are elegantly ‘designed’ to be responsive to the supply and removal of water and organic materials into and out the nodule. Nodules are almost exclusively dependent on water flow from the phloem flow originating in the shoots since little water is exchanged by nodules with the surrounding soils (Walsh et al. 1989). Therefore, nodule activity is very sensitive to the balance of water input from the phloem and water output to the xylem. Not only is the water input critical in sustaining the activity of the nodules, delivery of photosynthate from the leaves provides the energy resource to support the high energy process of N fixation. Xylem flow is also essential to remove the accumulating N products (Walsh et al. 1989) in the nodule to prevent feedback inhibition of N fixation activity (Serraj et al. 1999a). Without the continuous circulation of water to and from the nodules, N fixation activity cannot be sustained.
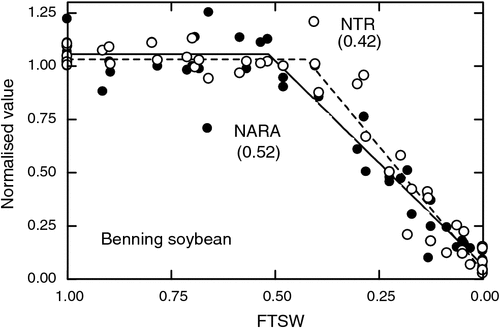
The dependence of nodule activity on water flow makes the nodules potentially very sensitive to plant water status. When turgor pressure declines in the shoots, then there is a possibility of decreased phloem flow. Even though other processes may not yet be reacting to declining water status in the shoot, the sensitivity of nodules to turgor-driven flow in the phloem can hypothetically make nodule activity especially sensitive to changes in plant water status (Serraj et al. 1999b). Nitrogen fixation tends to be one of the most sensitive processes in some grain legume plants to developing water deficit with N fixation rates often decreasing in advance of leaf gas exchange (Fig. 3). The sensitivity of N fixation to soil drying is especially pronounced in soybean and cowpea, which have capacities for high N fixation rates (Sinclair and Serraj 1995).
|
Not only is nodule activity sensitive to the balance of water influx and efflux, the fixation process is very sensitive to feedback based on the levels of N products in the nodule (Serraj et al. 1999b). While the detailed biochemical sensitivity has not been fully resolved, experimental feeding of plants with amino acids can result in dramatic decreases in fixation rate (Vadez et al. 2000). Soybean nodules are also sensitive to feeding plants with ureides, which are the compounds used by soybean and cowpea to transfer much of the N from the nodules to the shoot.
On balance, nodule activity is under tight regulation by the host plant through water supply to the nodules and N feedback regulation in the nodules. Through these regulatory processes, N fixation activity to a very large extent is under the control of the host plant. Only in extreme situation with a high preponderance of incompetent bacteria does it appear that bacteria play a major role in controlling fixation rates (Denison 2000). Unfortunately, research funds continue to be heavily focussed on ‘improving’ bacteria when the contribution of bacteria in influencing N fixation rate is likely to be very small compared with the regulation exerted by the plant. This is especially true in many cropping systems of the developing world where grain legumes are grown under abiotic limitations such as water and nutrients that would affect the whole plants to a much greater extent than the microsymbiont in the nodule.
An intriguing approach to ensure basal nodulation is the development of promiscuous host genotypes that form nodules with a range of ‘natural’ bacteria strains (Kueneman et al. 1984). The use of promiscuous soybean has led to significant N input in maize-soybean systems (Sanginga et al. 2002). It now appears in fact that promiscuous nodulation in legumes is likely more general than usually believed and host-strain specificity may be restricted to specific niches (Perret et al. 2000). In addition, even in areas where specific strains for a newly introduced legume species are lacking, as was the case when soybean cultivation began in France in the 1980s, it appears that a single inoculation is sufficient to establish the bacteria in the soil since inoculated strains remain in the soil even after long periods without cultivation of the host species (Obaton et al. 2002).
Consequently, a major shift in perspective to increase grain legume N fixation and its contribution to the N balance of the cropping system is needed. More research funds and effort need to be invested in research targeted to understanding and identifying superior host plants in their regulation of N fixation. There are large grain legume collections that have been virtually unexploited for this potential, except for soybean (Sinclair et al. 2000). One of the past difficulties for assessing genetic variability in large collections was their size and the lack of a sampling procedure to assess a representative set of germplasm. There now exists structured sets of representative germplasm from most major grain legume collections (e.g. chickpea, Upadhyaya and Ortiz 2001), which provide a gateway to explore diversity for the N fixation potential in legume germplasm collections.
Nitrogen feedback control of nitrogen fixation
To avoid the N feedback limitation on N fixation activity, it is critical that N products be expeditiously removed from nodules and sequestered in the shoot of the plant. Sequestration of the fixed N to a large extent in grain legumes is in leaf proteins. Not surprisingly, legume leaves tend to have very high N concentrations. Up to 50% of the N in soybean leaves is stored in ribulose 1,5-bisphosphate (Rubisco), which is the key photosynthetic enzyme for trapping carbon dioxide (Wittenbach et al. 1980). Rubisco is an especially effective means of storing N because this protein can be synthesised in abundance, it is innocuous in leaves, and it is stable until degraded by specific proteases (Hatayama et al. 2000). Levels of N concentrations in soybean leaves for example, can substantially exceed the concentration at which photosynthetic rates are maximised (Lugg and Sinclair 1981). A recent screening of the peanut reference collection also reveals that N levels in the field can be very large (from 1.9 to 2.8%) (Blümmel et al. 2012), which clearly opens a scope to target genotypes with high accumulation potential in the vegetative parts.
In addition to N storage in proteins of photosynthesis, some legumes have a layer of cells in leaves between the palisade and spongy mesophyll that stores glycoproteins (Klauer et al. 1996). Lansing and Franceschi (2000) examined leaves of 39 legume species and found a protein-rich, paraveinal layer in many species. No information was presented on the possibility of genetic variability within a species in the presence/absence of the paraveinal layer or in the extent of the N storage capacity of the paraveinal layer may vary among genotypes. Since the paraveinal layer is potentially an important storage structure of protein, more attention appears to be needed on the possibility of exploiting this tissue for greater N storage.
The importance of N storage in leaves in regulating N fixation rates is consistent in the high correlation (r2 = 0.83) observed in well-watered soybean between N fixation rate and leaf area (Denison et al. 1985). Greater rates of leaf area development provide more sites for the sequestration of N in enzymes of photosynthesis and higher N fixation rates. Unfortunately, many grain legumes currently tend to have slow initial development of leaf area, and flowering may occur early in the growth cycle. These development traits result in a limited capacity to store N, and N fixation rate is necessarily decreased by feedback restrictions. More rapid leaf area development and delayed flowering are options for increasing N storage and hence, N fixation activity and crop yield. In common bean, for example, climbing-type cultivars with delayed flowering and continued production of leaves after flowering commonly out-yield bush-type cultivars with early flowering (Clark and Francis 1985; Kornegay et al. 1992). Of course, delayed maturity and large leaf area can be counterproductive in environments with late-season drought or adverse temperature (Berger et al. 2012). For example, larger leaf area development can result in rapid water use under terminal drought conditions where water savings may be necessary in chickpea (Zaman-Allah et al. 2011a) and cowpea (Belko et al. 2012).
A corollary in overcoming the loss of N fixation with early flowering is to expand the period of active N fixation beyond flowering. Often grain legumes such as common bean seem to have very limited N fixation after flowering (e.g. Hungria and Neves 1987; Piha and Munns 1987) and it seems to be related to a shortage of carbohydrate supply to the nodules (Lawn and Brun 1974), but this decrease does not seem to be obligatory, at least in soybean (Denison and Sinclair 1985).
In addition to expanded N storage in leaves, another possibility is to increase N storage in stems. Stem N storage could be beneficial for eventual release of N to support growing seeds, for retention in the crop residue to enrich the soil, or for enhanced quality if used as fodder. While no screens of genotypes for storage of stem N concentrations appear to have been done in grain legumes, it may be useful to identify those genotypes that have large N stem storage capacity. Another approach for N storage is to fully exploit the capacity of some tropical legumes to produce tubers on their roots (Tropical Legumes 1979). For example, yam bean (Pachyrhizus erosus and Pachyrhizus ahipa) has been found to accumulate substantial amounts of N in their tubers (Castellanos-Ramos et al. 2009; Rodríguez-Navarro et al. 2009). A speculative research topic would be investigation of the ability of these tubers to store N either for use in seed growth by the legume crop or for providing an N source for a succeeding cereal crop.
Water-deficit impact on nitrogen fixation
As discussed previously, symbiotic N fixation of grain legumes is often quite sensitive to developing water deficits. In comparing the decrease in transpiration and N fixation rates with soil drying, it is not uncommon to have N fixation decrease in advance of leaf gas exchange as indexed by transpiration (Fig. 3). Consequently, the large need for N by the growing plant and seeds makes grain legumes especially vulnerable to water deficits. In a simulation study, Sinclair et al. (2010) found that decreasing the sensitivity of N fixation to soil drying in soybean would increase yields substantially in most soybean production areas in the USA.
Soybean has been given the most research attention of the grain legumes for genetic variation in the tolerance of N fixation to soil drying. In screens beginning with 3500 soybean plant introduction lines, Sinclair et al. (2000) identified several genotypes in which N fixation was actually more tolerant of soil drying than leaf gas exchange. Using the cultivar Jackson in which tolerance to soil drying of N fixation was equal to that of leaf gas exchange, a breeding effort resulted in the release of high-yielding germplasm with drought-resistant N fixation (Chen et al. 2007).
While Sinclair and Serraj (1995) compared the sensitivity of N fixation of nine grain legume species to soil drying and found substantial variations, their study was generally based on only a single cultivar for each species. They identified peanut as being a tolerant species, yet recently Devi et al. (2010) reported a large range in sensitivity among 17 Indian cultivars and breeding lines. The threshold fraction of transpirable soil water (FTSW) at the initiation of the decline in N fixation activity ranged from a very sensitive value of 0.59 to a tolerant value of 0.28. Genetic variation for N fixation sensitivity to water deficit in grain legumes other than soybean is largely unexplored. Breeding programs may need to be initiated to ensure high tolerance of N fixation to soil drying in genotypes released for commercial production.
Phosphorus accumulation
Like N, P is an essential element in plants and is required particularly to support energy transfer within cells. Much of the P in the plant is in inorganic form and readily reacts in the sequence of events resulting in energy transfer. Commonly the leaf cytoplasm of P-sufficient plants has concentrations in the range of 5–10 mM (Bieleski 1973). Abundant inorganic P can be stored in the vacuoles of cells in substantial excess to provide a P reserve for late-season plant growth when P in the soil solution may no longer be available. Phosphorus concentration in the cell vacuole can be as high as 25 mM (Lauer et al. 1989; Lee et al. 1990). The lack of vacuoles in young leaf cells to supply stored P causes the development of meristems to be especially inhibited by deficient P uptake (Fredeen et al. 1989; Rao et al. 1993).
Plants are well equipped to uptake P from the soil solution. There are at least two uptake pathways. The Michaelis-Menten coefficient (Km) for the high affinity P-uptake system is as low as 3 µM (Furihata et al. 1992; Schachtman et al. 1998). Bhadoria et al. (2004) found the Km for P uptake by intact peanut plants to be 10 µM. A common difficulty in recovering P from the soil is that it is not readily available to plants because P complexes in the soil with aluminum, iron and calcium. These complexes are essentially insoluble resulting in very little movement of P in the soil solution, and none of the complexes can be taken up directly by roots (Sinclair and Vadez 2002). While large amounts of P may exist in the soil, there is little evidence that the complexed P in the soil is available to cereal crops.
Grain legume species, however, have evolved mechanisms to allow recovery of P from unavailable forms. For example, when grown on soils with no available P, Ae et al. (1990) found that pigeon pea thrived for 1 month after sowing while four other crop species died from P deficiency. A similar experiment with peanut showed it survived for 2 months after sowing while three other species died (Ae and Shen 2002).
There appear to be at least three mechanisms that can be employed by grain legumes to release unavailable P in the soil for recovery by the plants. One mechanism is the exudation of organic acids from legume roots which decreases the pH in the soil surrounding the roots and releases P. Several organic acids are exuded with citrate being predominant among common bean (Shen et al. 2002), soybean and cowpea (Nwoke et al. 2008). Malate is exuded predominately by lupin, field pea, and faba bean (Nuruzzaman et al. 2005a). Chickpea was found to exude large amounts of citrate and malate (Ohwaki and Hirata 1992). Other organic acids exuded by grain legumes include oxalate, tartrate and acetate. However, the effectiveness of these organic acids in mobilising P is highly dependent on the soil and the soil environment (Hinsinger 2001; Jones et al. 2003). For example, organic acid mediated solubilisation of P by addition of citrate or oxalate varied widely among 20 contrasting soils (Jones et al. 2003). Release of organic acids may not be functional in acid soils of the Sahelian zone. It seems likely that soil characterisation will be required to select the appropriate grain legume genotype for high return of P on a particular soil.
Like many crop species, grain legumes can also release phosphatase enzymes into the soil to breakdown organic material that contains P. Lupin appears to be the most studied of the grain legumes and has been shown to employ a dual attack of exudation of organic acid and acid phosphatase (Gilbert et al. 1999). Also, genetic variation in root phosphatase activity has been demonstrated for common bean (Helal 1990).
There appears to be a third mechanism expressed by some grain legumes for recovering P from unavailable forms. Ae and Shen (2002) reported an ability of peanut and pigeon pea to recover P from unavailable forms by a contact reaction between the root surface and the insoluble P adjacent to the root. The mechanism was not resolved but they showed that a substance resides in cell walls acting directly on unavailable soil P, and it is not a pectic substance. Unfortunately, this apparently unique mechanism expressed by grain legumes has not been the subject of recent study.
A corollary with the solubilisation of P in the soil is distribution of roots through the soil volume to access insoluble P. Length and density of root hairs appear to be particularly important in contacting soil P. Root hairs disperse organic acids in the soil (Lynch 2007) and account for much of P uptake (Gahoonia and Nielsen 1998). Genetic variation has been found in root hair characteristics and have been identified in cowpea (Krasilnikoff et al. 2003), common bean (Yan et al. 2004) and soybean (Wang et al. 2004).
Since most P is in the top soil layers, a focus of improved rooting has been an increase in rooting surface in these top layers (Lynch and Brown 2001) with one approach being the identification of genotypes with root angles that encourage high rooting in the top soil layers. However, these top soil layers are also the first to dry so that there may be limited soil water in which soluble P can reside. Therefore, one of the future challenges to resolve will be to increase access to P where legumes are cultivated in low available P soil and under conditions of water limitation.
Another root alternative for scavenging soil P is the development of cluster roots, like in lupins (Neumann et al. 2000; Shane et al. 2003; Lambers et al. 2006). Cluster roots develop in response to pockets of soil P in situations of extremely low soil P availability (Lambers et al. 2006). Legumes seem to have a high potential for formation of cluster roots and their development seems to be regulated by P nutritional status (Neumann et al. 2000).
From a system perspective, the capabilities described above for legumes to acquire P from non-available pools are generally very beneficial for rotation crops such as canola, wheat or maize (e.g. Hens and Hocking 2004; Nuruzzaman et al. 2005b, Jemo et al. 2006). Faba bean appears to be one of the most promising of several legumes to express an advantage in P recovery (Nuruzzaman et al. 2005b). However, the extent of the benefit of legume P acquisition to the cropping system may be limited in some cases such as in acidic soil (Li et al. 2010).
Increasing grain legume yields
Commonly, the yields of grain legumes are substantially lower than cereals. Obviously, grain legume crops would be more attractive to farmers if yield could be increased. There are, however, several very important reasons why yields are lower than cereals and these are difficult to overcome. First, grain legumes are often assigned niches in the cropping season where duration of growth is limited due to temperature or rainfall patterns. As a consequence, these short-season crops do not have the time to acquire the resources necessary to achieve higher yields.
A second limit on grain legume yields is that their seeds are more energy dense than cereals. The higher protein content of grain legume seeds requires greater amounts of photosynthate to be used in synthesising the large amounts of protein (Sinclair and deWit 1975). Soybean and peanut are especially high in protein and lipid content, together constituting from 60 to 80% of the seed (Table 1). This fact alone limits the yields of soybean and peanut to substantially lower levels as compared with the cereals.
Maximising nitrogen fixation
Nitrogen fixation rate is highest during the vegetative growth stage when photosynthate tends to be freely available to nodules. With the initiation of grain growth, there is a shift in the supply of photosynthate away from the nodules to developing seeds (Herridge and Pate 1977). As a result, early-to-flower genotypes can have low, even negligible, N accumulation as a result of fixation (Graham and Temple 1984). Therefore, during seed growth N is retrieved from leaves and stems for transfer to the developing seeds. The loss in N from the vegetative tissues results in their senescence as a result of the ‘self-destruct’ process proposed by Sinclair and deWit (1975).
The amount of N a plant can store in vegetative tissue before seed growth is therefore crucial in determining total amounts of fixed N. As discussed previously, storage of fixed N during vegetative growth becomes a major issue. Nitrogen can be stored during vegetative development only to the extent that tissues are available to receive the fixed N. Not surprising, there is a close correlation between N storage in the vegetative tissue and crop mass. This is illustrated in Fig. 4 showing the amount of N storage in pea, wheat, and maize across a range of crop masses. These relationships are based on the empirical relationships found by Lemaire et al. (2008). While pea has the greatest storage capacity among these three species due mainly to high leaf N concentration, large plant masses still must be developed to attain high nitrogen storage levels. Often legumes are initially slow growing and do not obtain large plant masses before anthesis. Genotypes with more rapid vegetative development, particularly rapid leaf area development, are strong candidates for increasing plant N storage capacity.
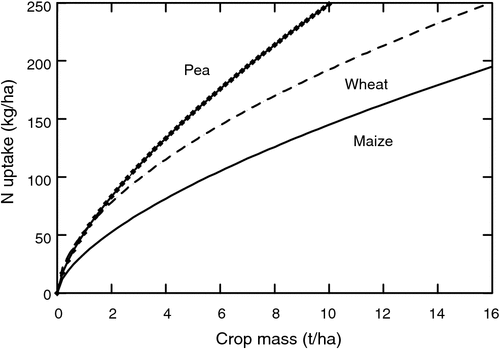
|
Minimising water-deficit limitation
In many areas, grain legumes are grown under water-limited conditions. Crops such as cowpea, pigeon pea, and chickpea are grown where soil water may be substantially limiting. Peanut is commonly grown on sandy soil with low water holding capacity. Yields are necessarily limited by the amount of water available to support growth. To quantify this relationship, the mechanistic expression of water-use efficiency derived by Tanner and Sinclair (1983) can be used. Rearranging their equation, the following expression of maximum grain yield (Ymax) is obtained.

where: W = total amount of water available for transpiration (t ha–1), HI = harvest index, k = mechanistically defined transpiration coefficient (5–6 Pa for grain legumes), VPD = mean vapour pressure deficit weighted for daily transpiration rate distribution (VPD, Pa). The weighted daily VPD is ~75% of the difference between the maximum and minimum vapour pressure (Tanner and Sinclair 1983).
In water-limited environments, a grain legume crop may not have any more than 150 mm (1500 t ha–1) of water available to it. Also, the season of growth is arid and the value of VPD may be 2.5 kPa (2500 Pa), or more. Assuming a grain legume crops with HI equal to 0.35 and a k value of 6 Pa, Ymax can be calculated as follows.

Clearly, high grain legume yields cannot be expected in such water-limited conditions. Doubling the available water to 300 mm will double yield to 3.52 t ha–1.
Aside from irrigation, a possible plant trait that could be selected for genetic modification is plant behaviour to decrease the ‘effective’ VPD. While VPD is seemingly an environmental variable, the crop could have suppressed leaf gas exchange under high VPD conditions, usually midday, so that the effective daily VPD for transpiration is shifted to a lower value. If genotypes were selected that restricted transpiration during midday, the effective VPD appropriate for Eqn 2 might be only 2.0 kPa. In this case, the maximum yield for these genotypes is increased to 1.58 t ha–1.
In fact, genotypes of several grain legumes have been identified that express a maximum transpiration rate. The soybean genotype PI 461937 was identified in soybean as having limiting leaf hydraulic conductance in the leaves resulting in a maximum transpiration rate (Sinclair et al. 2008). Devi et al. (2010) identified nine genotypes of peanut with approximately constant transpiration rates above a VPD ranging from 2.0 to 2.6 kPa. Zaman-Allah et al. (2011b) identified two peanut genotypes with reduced transpiration rates above 2.5 kPa. Figure 5 illustrates the differences in response in transpiration to increasing VPD among four peanut genotype. A search for this trait within the germplasm of all grain legumes selected for limited watered conditions is needed. In environments of intermittent drought, not only would this trait result in a lower effective VPD, but it would conserve soil water to extend the period that a crop can remain productive between rain events and avoid the consequences of severe drought.
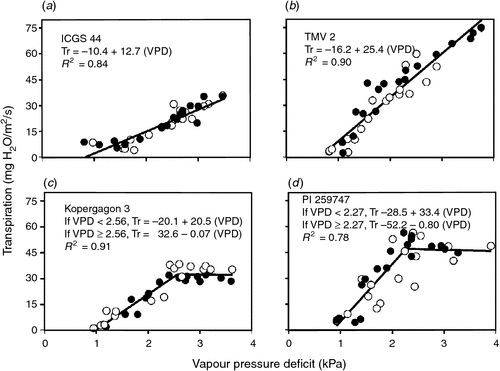
|
On drying soil, another approach to shift the effective VPD to a lower value is to initiate midday decreases in stomatal conductance earlier in the soil drying cycle. Commonly, such restrictions on transpiration begin when the fraction of FTSW is in the range of 0.25–0.40. However, there are a few reports of genetic variation within a species for the threshold value of soil water where transpiration decreases. Hufstetler et al. (2007) compared the threshold among 21 soybean genotypes grown on a sandy soil and found significant genetic differences. Devi et al. (2009) compared the threshold for the decrease in transpiration of 17 peanut genotypes grown on a silty loam soil and found a wide range of 0.22–0.71 in the FTSW threshold for the initiation of restricted water loss. Zaman-Allah et al. (2011b) also found differences in the FTSW threshold for the transpiration decline in chickpea from 0.35 to 0.63. A variation in the FTSW threshold from 0.44 to 0.68 has been found in cowpea by Belko et al. (2012). Those genotypes with a high FTSW threshold would have a lower effective VPD when soil dries, and also confer the benefit of water conservation in environments of intermittent drought.
Grain legumes in future cropping systems
Despite the many benefits that grain legumes bring to the cropping system, they are often viewed as less important than cereals and the proportion of crop land area devoted to grain legume remains relatively low in many cropping systems. Below we review some of the challenges that seem to limit more widespread adoption of grain legume production, and possible solutions to promote legumes in cropping systems.
Improving legume disease resistance
As discussed above, the tissues of grain legumes are rich in N and P, which makes them attractive for herbivory and diseases. Peanut, for example, can be devastated by foliar and viral diseases. Aschochyta blight has destroyed the entire chickpea industry in Western Australia in the late nineties (Gan et al. 2006). In other areas, this disease has forced the chickpea crop to spring sowing to avoid the spread of the disease (Abbo et al. 2003). Flower thrips or root rot diseases are primary constraints to cowpea production in the Sahel (Jackai and Daoust 1986). While cereal crops also suffer pests and diseases, chemical solutions are often employed because cereals in contrast to grain legumes in many regions of the world are grown as cash crops resulting in immediate income. Small-holder farmers cannot afford the price of fungicide or insecticide to apply to grain legumes.
An opportunity to improve pest resistance of grain legumes is to use the most recent advances of molecular techniques to exploit resistant germplasm in breeding programs by introgressing resistance genes into popular cultivars. Disease resistance is often related to the action of one or few genes that are frequently easy to move in modern breeding programs. There are also an increasing number of disease resistance genes being identified. Model legume crops and syntenic relationships between legumes may allow identification of disease resistance clusters across species, thereby accelerating the rate of disease resistance improvement (Choi et al. 2004).
The assembly of structured and representative sets of germplasm from the entire legume collection would facilitate a more thorough exploration of existing disease resistance. In addition, the molecular genetic tools now allow the exploitation of disease resistance characteristics expressed in wild relatives of cultigens. For instance, in tetraploid cultivated peanut there exists no absolute resistance to early and late leaf spot and to rust, but absolute resistance is available in the diploid wild relative of peanuts (Pande and Rao 2001). In the case of peanut, the cultivated tetraploid was isolated from its wild diploid ancestor. However, recent work has re-created a tetraploid peanut from the ‘forced’ hybridisation of diploid ancestors and duplication of chromosomes (Simpson and Starr 2001), and these synthetic tetraploids are now used to harness these resistances for the benefit of the cultigens. An example of success using this approach has been reported in breeding peanut for root nematode resistance (Chu et al. 2007). There is also similar on-going work against aschochyta blight in chickpea in Western Australia. Several breeding lines have been developed from the aschochyta-sensitive ‘Genesis’ cultivar (Khan et al. 2009). Wild chickpea germplasm has also been used to introduce disease tolerance (Singh et al. 1998; Knights et al. 2008). Increasing disease resistance is a concrete possibility that would significantly decrease the risk of cultivating legumes and would enhance their attractiveness.
Availability of good quality seeds
Poor quality seeds lead to poor crop stands and low productivity. This is especially the case for the legume production of small-holder farmers that have no access to good quality seeds. In that context, an opportunity for improvement would be the creation and promotion of farmer-based seed production units, in close relation with breeding institutions. This would allow good quality seed production and enhance farmer access, and at the same time create a new livelihood from the price premium received from seed production (Shiferaw et al. 2008).
The reasons for poor quality seeds are numerous. As discussed previously, grain legumes are often grown under marginal conditions (e.g. Gowda et al. 2009) resulting in abiotic stress causing both low yield and low seed quality. That is, low rainfall or extreme temperatures during seed growth cause losses in seed germination potential. Also, the high N and P content of grain legume seeds makes them very attractive targets for diseases and insects (Rachie and Roberts 1974). Pod-sucking insects can directly attack seeds during seed development. Post-harvest pests can be a serious problem during seed storage, for instance bruchids in cowpea seeds (Fujii et al. 1989). Another post-harvest problem for legumes with seeds high in oil content is that the lipids may not be stable and they degrade in storage resulting in loss of seed quality.
Limited adoption of grain legumes
Despite the benefits of legumes, to provide a healthy and rich source of protein and minerals while also contributing to soil fertility, the adoption of grain legumes and the intensification of legume production in cereal-legumes systems remain low. This is the case even in areas with high human populations (Kerr et al. 2007). Therefore, breeding for more productive and resilient cultivars will be of no benefit if these efforts do not include bottom-up efforts to expand adoption of grain legumes by farmers. Therefore, one of the challenges that increasing grain legume production will face in the small-holder setting of developing countries will be to understand better the socioeconomic and cultural determinants of legume production and consumption. Of course, the lack of good seed as described above is a major limit to increasing yields and expanding grain legume production. In addition, grain legumes may have a greater labour requirement (weeding, harvesting) (Snapp et al. 2002) than cereals. Since grain legumes are often cultivated by women, and become a mainstay of the diet when populations cannot afford meat, there can be a negative stigma about the value of grain legumes. To expand the cultivation of grain legumes in less developed areas, all of these socioeconomic and cultural factors need to be well understood to fully unlock the potential of legumes to improve cropping systems and the livelihoods of these farmers (Sperling et al. 1993; Snapp and Silim 2002).
Legume residue for cattle feed
In small-holder settings, which are often mixed farming systems combining crop and livestock, the main component of cattle feed is often crop residues. In India, 40% of the cattle feed is crop residue and it is predicted that this share will increase to 70% by 2020 (Roy and Singh 2008). Most of those residues come from cereals, and legumes contribute only 10% of the total. To improve rumen digestion and animal growth, it will be necessary to have crop residue that has substantially greater N concentration than the 0.6–0.8% provided by cereal residue. A recent evaluation of fodder quality in the peanut germplasm clearly showed a range of variation for fodder N concentration that could be exploited in breeding programs (Blümmel et al. 2012). More importantly, there appears to be limited or no trade-off between fodder quality traits and grain productivity. So, there is clearly an opportunity to breed both for fodder quality and pod productivity in peanut. Important topics of investigation are to fully document the quality of grain legume residue as animal fodder, and the extent of the trade-off between fodder production and seed yield. In any case, in highly populated crop-livestock systems, higher quality feed residues from grain legumes have clearly an additive role to play in the economics of the legume value chain.
Legumes in the context of cropping systems
We envision that grain legumes will have an increasing place in cropping systems in the future in both less developed and developed regions of the world. A major reason for this prediction is that grain legumes can fulfil several roles: an immediate seed crop for consumption or marketing, a means to increase soil fertility as result of its nutrient rich residues, and a key component of livestock feed. In many cropping systems, grain legumes are likely to be needed to fulfill all three roles of increased seed yield, enhanced soil fertility, and cattle feed provider. The balance between these roles will be resolved by the relative economic return from marginal increases in seed yield as compared to retention of N and P in vegetative tissue for incorporation into soils and/or for animal fodder. For situations where the price of applying fertilisers severely limits the amount of fertiliser that can be economically applied to cereal crops, it could be necessary to rely on the preceding grain legume crop to leave plant residue that will supply N and P for the succeeding crop. In view of the increasing price of fertilisers, it seems likely the role of provider of nutrients will be increasing in most cropping systems. Clearly, grain legumes will remain the backbone of farming systems in poorly endowed areas, especially for their capacity to fix N.
Consequently, a major task in the future will be the selection of species and cultivars that can be used across various cropping systems, as previously shown (Snapp et al. 1998). A major consideration will be balancing legume grain production, which offers an immediate economic return, with the accumulation of N and P in the vegetation of the grain legume crop for the benefit of subsequent cereal crops in a cropping system. The trade-off between these two roles will be dictated by the perception of risk by farmers (Lawn 1989) and the local economic conditions of fertiliser price and grain price.
Genotypes will need to be developed that offer a range of return in the use of accumulated N and P for seed growth and for retention of nutrients in the vegetative mass to be retained in the field. One important consideration in growing legumes for improving soil fertility will be the temporal dynamics of N release from legume residues. It will be useful to have legumes that release nutrients from their organic residue that coincides with the period of highest demand by the subsequent crop. For example, pigeon pea has been shown to have a relatively slow release of N from its organic matter in comparison to other legume crops (Cadisch and Giller 1997).
Conclusions for research opportunities
Major research opportunities to enhance the roles of grain legumes exist in each of the topics discussed above. Several priority areas seem apparent. Nitrogen fixation activity of grain legumes needs to be enhanced. Instead of the common focus on the bacteria component of the N fixation symbiosis, the regulation of activity by the host plant needs to be fully appreciated. A major thrust is needed to identify plant characteristics and germplasm that allow greater N fixation capacity. The ability of the host plant to store fixed N appears to be a major component of increasing N fixation input. Also, limitations of abiotic stresses, particularly water deficit, require extensive investigation.
The unique ability of some legumes to accumulate P from forms normally unavailable in many soils needs to be fully investigated. Phosphorus is expensive, and is often a limiting resource in many cropping systems. Legumes that can recover normally unavailable forms of soil P could be major assets in future cropping systems. The retention of some P, and N, in the vegetative tissue to be returned to the soil after seed harvest could be a major advance in future cropping systems.
Finally, the range of potential roles of grain legumes in cropping systems needs to be appreciated in focusing future research. There is a need for expanded research on grain legumes to maximise their productivity in terms of N and P accumulation, and increased productivity both in terms of seed production and vegetative residue returned to the cropping system. Such investigations will include assessments of the grain legume species that might be most appropriate to fit the needs of each cropping system. In a world of increasing demands for plant products, including protein and oils, at the same time of greater economic and environmental pressures on cropping systems, it is apparent that grain legumes need to become a major component of future cropping systems.
References
Abbo S, Berger J, Turner NC (2003) Evolution of cultivated chickpea: four bottlenecks limit diversity and constrain adaptation. Functional Plant Biology 30, 1081–1087.| Evolution of cultivated chickpea: four bottlenecks limit diversity and constrain adaptation.Crossref | GoogleScholarGoogle Scholar |
Ae N, Shen RF (2002) Root cell-wall properties are proposed to contribute to phosphorus (P) mobilization by groundnut and pigeonpea. Plant and Soil 245, 95–103.
| Root cell-wall properties are proposed to contribute to phosphorus (P) mobilization by groundnut and pigeonpea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnvVCitr0%3D&md5=08bc457997a5341f438937ea2859fbc6CAS |
Ae N, Arihara J, Okada K, Yoshihara T, Johansen C (1990) Phosphorus uptake by pigeon pea and its role in cropping systems of the Indian subcontinent. Science 248, 477–480.
| Phosphorus uptake by pigeon pea and its role in cropping systems of the Indian subcontinent.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXkslOitbw%3D&md5=1d5ae2eb29208b9bd86014dc13d079bdCAS |
Ali M, Gupta S (2012) Carrying capacity of Indian agriculture: pulse crops. Current Science 102, 874–881.
Belko N, Zaman MA, Diop NN, Cisse N, Ehlers JD, Ndoye O, Zombre G, Vadez V (2012) Lower soil moisture threshold for transpiration decline under water deficit correlates with lower canopy conductance and higher transpiration efficiency in drought tolerant cowpea. Functional Plant Biology 39, 306–322.
Berger JD, Buirchel BJ, Luckett DJ, Nelson MN (2012) Domestication bottlenecks limit genetic diversity and constrain adaptation in narrow-leafed lupin (Lupinus angustifolius L.). Theoretical and Applied Genetics 124, 637–652.
| Domestication bottlenecks limit genetic diversity and constrain adaptation in narrow-leafed lupin (Lupinus angustifolius L.).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC383it1Knug%3D%3D&md5=6822e62405df5555ca274340120f6b08CAS |
Bhadoria PS, El Dessougi H, Liebersbach H, Claassen N (2004) Phosphorus uptake kinetics, size of root system and growth of maize and groundnut in solution culture. Plant and Soil 262, 327–336.
| Phosphorus uptake kinetics, size of root system and growth of maize and groundnut in solution culture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmtlGgu7k%3D&md5=c7b133622eddce4a8dc69d6aaab052a2CAS |
Bieleski RL (1973) Phosphate pools, phosphate transport and phosphate availability. Annual Review of Plant Physiology 24, 225–252.
| Phosphate pools, phosphate transport and phosphate availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXltFeitbY%3D&md5=d166384e3c862fb93fdeee6d9936dfa1CAS |
Blümmel M, Ratnakumar P, Vadez V (2012) Opportunities for exploiting variations in haulm fodder traits of intermittent drought tolerant lines in a reference collection of groundnut (Arachis hypogeae L.). Field Crops Research 126, 200–206.
| Opportunities for exploiting variations in haulm fodder traits of intermittent drought tolerant lines in a reference collection of groundnut (Arachis hypogeae L.).Crossref | GoogleScholarGoogle Scholar |
Burkart MR, James DE (1999) Agricultural-nitrogen contributions to hypoxia in the Gulf of Mexico. Journal of Environmental Quality 28, 850–859.
| Agricultural-nitrogen contributions to hypoxia in the Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjt1yltL8%3D&md5=cba0311cc04cb463fd8d5a9034c75cffCAS |
Cadisch G, Giller KE (1997) ‘Driven by nature: plant residue quality and decomposition.’ (CAB International: Wallingford, UK)
Castellanos-Ramos JZ, Acosta-Gallegos JA, Orozco NR, Munoz-Ramos JJ (2009) Biological nitrogen fixation and tuber yield of yam bean in central Mexico. Agicultura Tecnica en Mexico 35, 277–283.
Chen P, Sneller CH, Purcell LC, Sinclair TR, King CA, Ishibashi T (2007) Registration of soybean germplasm lines R01-416F an R01-581F for improved yield and nitrogen fixation under drought stress. Journal of Plant Registrations 1, 166–167.
| Registration of soybean germplasm lines R01-416F an R01-581F for improved yield and nitrogen fixation under drought stress.Crossref | GoogleScholarGoogle Scholar |
Choi HK, Mun JH, Kim DJ, Zhu H, Baek JM, Mudge J, Roe B, Ellis N, Doyle J, Kiss JB, Young ND, Cook DR (2004) Estimating genome conservation between crop and model legume species. Proceedings of the National Academy of Sciences of the United States of America 101, 15 289–15 294.
| Estimating genome conservation between crop and model legume species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVKhsbzL&md5=fb3a0bded2aba5655724d8470970a56cCAS |
Chu Y, Holbrook C, Timper P, Ozias-Akins P (2007) Development of a PCR-based molecular marker to select for nematode resistance in peanut. Crop Science 47, 841–847.
| Development of a PCR-based molecular marker to select for nematode resistance in peanut.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXltlShtbg%3D&md5=229c590fa6166e255c51d0263648f6c5CAS |
Clark EA, Francis CA (1985) Bean-maize intercrops: a comparison of bush and climbing bean growth habits. Field Crops Research 10, 151–166.
| Bean-maize intercrops: a comparison of bush and climbing bean growth habits.Crossref | GoogleScholarGoogle Scholar |
Denison RF (2000) Legume sanctions and the evolution of symbiotic cooperation by rhizobia. American Naturalist 156, 567–576.
| Legume sanctions and the evolution of symbiotic cooperation by rhizobia.Crossref | GoogleScholarGoogle Scholar |
Denison RF, Sinclair TR (1985) Diurnal and seasonal variation in dinitrogen fixation (acetylene reduction) rates by field-grown soybeans. Agronomy Journal 77, 679–684.
| Diurnal and seasonal variation in dinitrogen fixation (acetylene reduction) rates by field-grown soybeans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXlslGnu7k%3D&md5=1d8e14372f1cbc3bc996d7cc9eed1539CAS |
Denison RF, Weisz PR, Sinclair TR (1985) Variability among plants in dinitrogen fixation (acetylene reduction) rates by field-grown soybean. Agronomy Journal 77, 947–950.
| Variability among plants in dinitrogen fixation (acetylene reduction) rates by field-grown soybean.Crossref | GoogleScholarGoogle Scholar |
Devi MJ, Sinclair TR, Vadez V, Krishnamurthy L (2009) Peanut genotypic variation in transpiration efficiency and decreased transpiration during progressive soil drying. Field Crops Research 114, 280–285.
| Peanut genotypic variation in transpiration efficiency and decreased transpiration during progressive soil drying.Crossref | GoogleScholarGoogle Scholar |
Devi MJ, Sinclair TR, Vadez V (2010) Genotypic variability among peanut (Arachis hypogea L.) in sensitivity of nitrogen fixation to soil drying. Plant and Soil 330, 139–148.
| Genotypic variability among peanut (Arachis hypogea L.) in sensitivity of nitrogen fixation to soil drying.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktFequ7g%3D&md5=fd01defd337c9d81438861b1a14ba5adCAS |
Duan S, Bianchi TS, Satschi PH, Armon RMW (2010) Effects of tributary inputs on nutrient export from the Mississippi and Atchafalaya Rivers to the Gulf of Mexico. Marine and Freshwater Research 61, 1029–1038.
| Effects of tributary inputs on nutrient export from the Mississippi and Atchafalaya Rivers to the Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1Snt7jF&md5=e3aee5949f6eae23c7c31f6a27d93be8CAS |
Fredeen , AL , Rao , IM , Terry , N (1989) Influence of phosphorous nutrition on growth and carbon partitioning in Glycine max. Plant Physiology 89, 225–230.
| Influence of phosphorous nutrition on growth and carbon partitioning in Glycine max.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXhtFelur0%3D&md5=62eec0f6534302a834c4a8a4f5e90755CAS |
Fujii K, Gatehouse AMR, Johnson CD, Mitchel R, Yoshida T (1989) Bruchids and legumes: economics, ecology and coevolution. In ‘Proceedings of the Second International Symposium on Bruchids and Legumes (ISBL-2)’. (Eds K Fujii, AMR Gatehouse, CD Johnson, R Mitchel, T Yoshida) pp. 303–315. (Kluwer Academic Publishers: Dordrecht, The Netherlands)
Furihata T, Suzki M, Sakurai H (1992) Kinetic characterization of two phosphate uptake systems with different affinities in suspension-cultured Catharanthus roseus protoplasts. Plant & Cell Physiology 33, 1151–1157.
Gahoonia TS, Nielsen NE (1998) Direct evidence on participation of root hairs in phosphorus (32P) uptake from soil. Plant and Soil 198, 147–152.
| Direct evidence on participation of root hairs in phosphorus (32P) uptake from soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXivFGis7g%3D&md5=31b05c1628bb1c2552cd4e48d1c951e6CAS |
Gan YT, Siddique KHM, MacLeod WJ, Jayakumar P (2006) Management options for minimizing the damage by ascochyta blight (Ascochyta rabiei) in chickpea (Cicer arietinum L.). Field Crops Research 97, 121–134.
| Management options for minimizing the damage by ascochyta blight (Ascochyta rabiei) in chickpea (Cicer arietinum L.).Crossref | GoogleScholarGoogle Scholar |
Gilbert GA, Knight JD, Vance CP, Allan DL (1999) Acid phosphatase activity in phosphorus-deficient white lupin roots. Plant, Cell & Environment 22, 801–810.
| Acid phosphatase activity in phosphorus-deficient white lupin roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlsFGnsr8%3D&md5=14e2572757afc4f8d441012c3bd9fe2fCAS |
Gowda C, Parthasarathy Rao P, Tripathi S, Gaur P, Deshmukh R (2009) Regional shift in chickpea production in India. In ‘Milestones in food legumes’. (Eds M Ali, S Kumar) pp. 21–35. (IIPR: Kanpur, India)
Graham PH, Temple SR (1984) Selection for improved nitrogen fixation in Glycine max (L.) Merr. and Phaseolus vulgaris L. Plant and Soil 82, 315–327.
| Selection for improved nitrogen fixation in Glycine max (L.) Merr. and Phaseolus vulgaris L.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXht1ykurw%3D&md5=c8d60813b8e40de29871d64966fea880CAS |
Hatayama R, Takahashi R, Ohshima M, Shibasaki B, Tokuyama T (2000) Ribulose-1,5-bisphosphate carboxylase/oxygenase from an ammonia-oxidizing bacterium, Nitrosomonas sp. K1: purification and properties. Journal of Bioscience and Bioengineering 90, 426–430.
Helal HM (1990) Varietal differences in root phosphatase activity as related to the utilization of organic phosphates. Plant and Soil 123, 161–163.
| Varietal differences in root phosphatase activity as related to the utilization of organic phosphates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXkvFKlsrg%3D&md5=de970a44e51f62ce6ac1c711146bf9ebCAS |
Hens M, Hocking P (2004) An evaluation of the phosphorus benefits from grain legumes in rotational cropping using 33P isotopic dilution. In ‘New directions for a diverse planet’. (Eds T Fischer, N Turner, J Angus, L McIntyre, M Robertson, A Borrell, D Lloyd) (The Regional Institute Ltd: Gosford, NSW) www.cropscience.org.au/icsc2004/poster/2/5/4/1190_hockingp.htm
Herridge DF, Pate JS (1977) Utilization of net photosynthate for nitrogen fixation and protein production in an annual legume. Plant Physiology 60, 759–764.
| Utilization of net photosynthate for nitrogen fixation and protein production in an annual legume.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXkslKlsw%3D%3D&md5=67eb995c2f072bd4e080bfc715ade92dCAS |
Hinsinger H (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant and Soil 237, 173–195.
| Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XovVWlsQ%3D%3D&md5=a77ca3df64a9bbec83f05e7ae8cfcc56CAS |
Hufstetler EV, Boerma HR, Carter TE, Earl HJ (2007) Genotypic variation for three physiological traits affecting drought tolerance in soybean. Crop Science 47, 25–35.
| Genotypic variation for three physiological traits affecting drought tolerance in soybean.Crossref | GoogleScholarGoogle Scholar |
Hungria M, Neves MCP (1987) Cultivar and Rhizobium strain effect on nitrogen fixation and transport in Phaseolus vulgaris L. Plant and Soil 103, 111–121.
| Cultivar and Rhizobium strain effect on nitrogen fixation and transport in Phaseolus vulgaris L.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXnt1WqsQ%3D%3D&md5=1554e0b181d0456724fef46130f214e7CAS |
Jackai LEN, Daoust RA (1986) Insect pests of cowpeas. Annual Review of Entomology 31, 95–119.
| Insect pests of cowpeas.Crossref | GoogleScholarGoogle Scholar |
Jemo M, Abaidoo RC, Nolte C, Tchienkoua M, Sanginga N, Horst WJ (2006) Phosphorus benefits from grain-legume corps to subsequent maize grown on acid soils of southern Cameroon. Plant and Soil 284, 385–397.
| Phosphorus benefits from grain-legume corps to subsequent maize grown on acid soils of southern Cameroon.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntF2isbw%3D&md5=86c608302391c0132d344f4039dd2181CAS |
Jones DL, Dennis PG, Owen AG, van Hees PAW (2003) Organic acid behavior in soils – misconceptions and knowledge gaps. Plant and Soil 248, 31–41.
| Organic acid behavior in soils – misconceptions and knowledge gaps.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtFCqsro%3D&md5=3bb095819448cf15bea58317461997e8CAS |
Kerr RB, Snapp SS, Chirwa M, Shumba L, Msachi R (2007) Participatory research on legume diversification with Malawian smallholder farmers for improved human nutrition and soil fertility. Experimental Agriculture 43, 437–453.
| Participatory research on legume diversification with Malawian smallholder farmers for improved human nutrition and soil fertility.Crossref | GoogleScholarGoogle Scholar |
Khan TN, Adhikari K, Siddique KHM, Garlinge J, Smith L, Morgan S, Boyd C (2009) Chickpea 2010 crop variety testing of germplasm developed by DAFWA/CLIMA/ICRISAT/COGGO alliance. Agribusiness Crop Updates 2010. Western Australian Agriculture Authority, Perth, Australia. pp. 9–12.
Klauer SF, Franceschi VR, Ku MSB, Zhang D (1996) Identification and localization of vegetative storage proteins in legume leaves. American Journal of Botany 83, 1–10.
| Identification and localization of vegetative storage proteins in legume leaves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xht1GisLg%3D&md5=a1553a533a95909bfa13b11649e9b1f9CAS |
Knights EJ, Southwell RJ, Schwinghamer MW, Harden S (2008) Resistance to Phytophthora medicaginis Hansen and Maxwell in wild Cicer species and its use in breeding root rot resistant chickpea (Cicer arietinum L.). Australian Journal of Agricultural Research 59, 383–387.
| Resistance to Phytophthora medicaginis Hansen and Maxwell in wild Cicer species and its use in breeding root rot resistant chickpea (Cicer arietinum L.).Crossref | GoogleScholarGoogle Scholar |
Kornegay J, White JW, Ortiz Cruz O (1992) Growth habit and gene pool effects on inheritance of yield in common bean. Euphytica 62, 171–180.
| Growth habit and gene pool effects on inheritance of yield in common bean.Crossref | GoogleScholarGoogle Scholar |
Krasilnikoff G, Gahoonia T, Nielsen NE (2003) Variation in phosphorus uptake efficiency by genotypes of cowpea (Vigna unguiculata) due to differences in root and root hair length and induced rhizosphere processes. Plant and Soil 251, 83–91.
| Variation in phosphorus uptake efficiency by genotypes of cowpea (Vigna unguiculata) due to differences in root and root hair length and induced rhizosphere processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXitlKgtLY%3D&md5=ad0b5be0276bc215a5cd57bf5dcd37f4CAS |
Kueneman EA, Root WR, Dashiell KE, Hohenberg J (1984) Breeding soybeans for the tropics capable of nodulating effectively with indigenous Rhizobium spp. Plant and Soil 82, 387–396.
| Breeding soybeans for the tropics capable of nodulating effectively with indigenous Rhizobium spp.Crossref | GoogleScholarGoogle Scholar |
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98, 693–713.
| Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits.Crossref | GoogleScholarGoogle Scholar |
Lansing AJ, Franceschi VR (2000) The paraveinal mesophyll: a specialized path for intermediary transfer of assimilates in legume leaves. Australian Journal of Plant Physiology 17, 757–767.
Lauer MJ, Blevins DH, Sierzputowska-Gracz H (1989) 31P-nuclear magnetic resonance determinations of phosphate compartmentation in leaves of reproductive soybeans (Glycine max L.) as affected by phosphate nutrition. Plant Physiology 89, 1331–1336.
| 31P-nuclear magnetic resonance determinations of phosphate compartmentation in leaves of reproductive soybeans (Glycine max L.) as affected by phosphate nutrition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXitFWmtLw%3D&md5=5bb7696af3b9f00c0af0ee619095fb6aCAS |
Lawn RJ (1989) Agronomic and physiological constraints to the productivity of tropical grain legumes and prospects for improvement. Experimental Agriculture 25, 509–528.
| Agronomic and physiological constraints to the productivity of tropical grain legumes and prospects for improvement.Crossref | GoogleScholarGoogle Scholar |
Lawn RJ, Brun WA (1974) Symbiotic nitrogen fixation in soybean: I. Effect of photosynthetic source-sink manipulations. Crop Science 14, 11–16.
| Symbiotic nitrogen fixation in soybean: I. Effect of photosynthetic source-sink manipulations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2cXkt1eis7k%3D&md5=fdfa4d3916d96085eb25f33636456351CAS |
Lee RB, Ratcliffe RG, Southon TE (1990) 31P NMR measurements of cytoplasmic and vacuolar Pi content of mature maize roots: relationships with phosphorus status and phosphate fluxes. Journal of Experimental Botany 41, 1063–1078.
| 31P NMR measurements of cytoplasmic and vacuolar Pi content of mature maize roots: relationships with phosphorus status and phosphate fluxes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXmtVyns7s%3D&md5=b3212aa0a0e1515b933918595ef9cca1CAS |
Lemaire G, Jeuffroy M-H, Gastal F (2008) Diagnosis tool for plant and crop N status in vegetative stage. Theory and practices for crop N management. European Journal of Agronomy 28, 614–624.
| Diagnosis tool for plant and crop N status in vegetative stage. Theory and practices for crop N management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXktFClt70%3D&md5=e51df4444199c09e35075c7b79c0013bCAS |
Li H, Shen J, Zhang F, Marschner P, Cawthray G, Rengel Z (2010) Phosphorus uptake and rhizosphere properties of intercropped and monocropped maize, faba bean, and white lupin in acidic soil. Biology and Fertility of Soils 46, 79–91.
| Phosphorus uptake and rhizosphere properties of intercropped and monocropped maize, faba bean, and white lupin in acidic soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktl2lsg%3D%3D&md5=d73f5037b646fc663c8f963c7cace419CAS |
Lugg DG, Sinclair TR (1981) Seasonal changes in photosynthesis of field grown soybean leaflets. 2. Relation to nitrogen content. Photosynthetica 15, 138–144.
Lynch JP (2007) Roots of the second Green Revolution. American Journal of Botany 55, 493–512.
| Roots of the second Green Revolution.Crossref | GoogleScholarGoogle Scholar |
Lynch JP, Brown KM (2001) Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant and Soil 237, 225–237.
| Topsoil foraging – an architectural adaptation of plants to low phosphorus availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XovVWltA%3D%3D&md5=d8d0e6a878c28e7632b50a5b4ad5275cCAS |
Minchin FR (1997) Regulation of oxygen diffusion in legume nodules. Soil Biology & Biochemistry 29, 881–888.
| Regulation of oxygen diffusion in legume nodules.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXks1WqsrY%3D&md5=1ae4d91ec19a92543069b0c442533f73CAS |
Neumann G, Massonneau A, Langlade N, Dinkelaker B, Hengeler C, Romheld V, Martinoia E (2000) Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Annals of Botany 85, 909–919.
| Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjs12hu7o%3D&md5=ca9eec27a5cf1fb604f55dac0992f043CAS |
Nuruzzaman M, Lambers H, Bollard MDA, Veneklaas EJ (2005a) Phosphorus uptake by grain legumes and subsequently grown wheat at different levels of residual phosphorus fertiliser. Australian Journal of Agricultural Research 56, 1041–1047.
| Phosphorus uptake by grain legumes and subsequently grown wheat at different levels of residual phosphorus fertiliser.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFChsLbE&md5=4a6366bb7db363550240fdbdc1c3777fCAS |
Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ (2005b) Phosphorus benefits of different legume crops to subsequent wheat grown in different soils of Western Australia. Plant and Soil 271, 175–187.
| Phosphorus benefits of different legume crops to subsequent wheat grown in different soils of Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXks1Ojt7g%3D&md5=3ce561cbc14aa5a035b1024a87d735bbCAS |
Nwoke OC, Diels J, Abaidoo R, Nziguheba G, Merckx R (2008) Organic acids in the rhizosphere and root characteristics of soybean (Glycine max) and cowpea (Vigna unguiculata) in relation to phosphorus uptake in poor savanna soils. African Journal of Biotechnology 7, 3620–3627.
Obaton M, Bouniols A, Piva G, Vadez V (2002) Are Bradyrhizobium japonicum stable during a long stay in soil. Plant and Soil 245, 315–326.
| Are Bradyrhizobium japonicum stable during a long stay in soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnsVGltrk%3D&md5=1a0a3020ee2779cfc371e635874371d8CAS |
Ohwaki Y, Hirata H (1992) Difference in the carboxylic acid exudation among P-starved leguminous crops in relation to carboxylic acid contents in plant tissues and phospholipid level in roots. Soil Science and Plant Nutrition 38, 235–243.
| Difference in the carboxylic acid exudation among P-starved leguminous crops in relation to carboxylic acid contents in plant tissues and phospholipid level in roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XlvFSqsbY%3D&md5=5a4904ea5651cb4beb4a8e85f455a379CAS |
Pande S, Rao N (2001) Resistance of wild Arachis species to late leaf spot and rust in greenhouse trials. Plant Disease 85, 851–855.
| Resistance of wild Arachis species to late leaf spot and rust in greenhouse trials.Crossref | GoogleScholarGoogle Scholar |
Perret X, Christian Staehelin C, Broughton WJ (2000) Molecular basis of symbiotic promiscuity. Microbiology and Molecular Biology Reviews 64, 180–201.
| Molecular basis of symbiotic promiscuity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXitFygsr0%3D&md5=ffe8f03f6676b9082388574c0b0721a3CAS |
Piha MI, Munns DN (1987) Nitrogen fixation capacity of field-grown bean compared to other grain legumes. Agronomy Journal 79, 690–696.
| Nitrogen fixation capacity of field-grown bean compared to other grain legumes.Crossref | GoogleScholarGoogle Scholar |
Purcell LC, Sinclair TR (1993) Soybean (Glycine max) nodule physical traits associated with permeability responses to oxygen. Plant Physiology 103, 149–156.
Purdue University (2012) Purdue Crop Cost & Return Guide. Purdue Extension ID-166-W. W. Lafayette, IN, USA
Rachie KO, Roberts LM (1974) Grain legumes of the lowland tropics. Advances in Agronomy 26, 1–132.
| Grain legumes of the lowland tropics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2MXktVCrurY%3D&md5=f4113054eb0fe7631d29f3b8b2658e5fCAS |
Rao IM, Fredeen AL, Terry N (1993) Influence of phosphorus limitation on photosynthesis, carbon allocation and partitioning in sugar beet and soybean grown with a short photoperiod. Plant Physiology and Biochemistry 31, 223–231.
Robson RL, Postgate JR (1980) Oxygen and hydrogen in biological nitrogen fixation. Annual Review of Microbiology 34, 183–207.
| Oxygen and hydrogen in biological nitrogen fixation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M%2FmtV2rsA%3D%3D&md5=6341ff79f5ab1ce3d406b1a2318b8fe7CAS |
Rodríguez-Navarro DN, Camacho M, Temprano F, Santamaria C, Leidi EO (2009) Assessment of nitrogen fixation potential in aphia (Pachyrhizus ahipa) and its effect on root and seed yield. Experimental Agriculture 45, 177–188.
| Assessment of nitrogen fixation potential in aphia (Pachyrhizus ahipa) and its effect on root and seed yield.Crossref | GoogleScholarGoogle Scholar |
Roy MM, Singh KA (2008) The fodder situation in rural India: future outlook. International Forestry Review 10, 217–234.
| The fodder situation in rural India: future outlook.Crossref | GoogleScholarGoogle Scholar |
Sanginga N, Okogun J, Vanlauwe B, Dashiell K (2002) The contribution of nitrogen by promiscuous soybeans to maize based cropping the moist savanna of Nigeria. Plant and Soil 241, 223–231.
| The contribution of nitrogen by promiscuous soybeans to maize based cropping the moist savanna of Nigeria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XltV2jsb4%3D&md5=be25b5024b757697f3619be0d7c9329fCAS |
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiology 116, 447–453.
| Phosphorus uptake by plants: from soil to cell.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXht1ajtbc%3D&md5=a151c2da7fb1f0f25009a053e7756fb5CAS |
Serraj R, Sinclair TR, Purcell LC (1999a) Symbiotic N2 fixation response to drought. Journal of Experimental Botany 50, 143–155.
Serraj R, Vadez V, Purcell LC, Sinclair TR (1999b) Recent advances in the physiology of drought stress effects on symbiotic N2 fixation in soybean. In ‘Highlights of nitrogen fixation research’. (Eds F Matinez, G Hernandez) pp. 49–55. (Kluwer Academic/Plenum Publications: New York)
Shane MW, De Vos M, De Roock S, Lambers H (2003) Shoot P status regulates cluster-root growth and citrate exudation in Lupinus albus grown with a divided root system. Plant, Cell & Environment 26, 265–273.
| Shoot P status regulates cluster-root growth and citrate exudation in Lupinus albus grown with a divided root system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhslOgur8%3D&md5=2deb34d1f5735f05b7a44696eede89e1CAS |
Shen H, Yan X, Zhao M, Zheng S, Wang X (2002) Exudation of organic acids in common bean as related to mobilization of aluminum- and iron-bound phosphates. Environmental and Experimental Botany 48, 1–9.
| Exudation of organic acids in common bean as related to mobilization of aluminum- and iron-bound phosphates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XksVansrs%3D&md5=75ae0ab190091c04e56f5b9cb8f87376CAS |
Shiferaw BA, Kebede TA, You L (2008) Technology adoption under seed access constraints and the economic impacts of improved pigeonpea varieties in Tanzania. Agricultural Economics 39, 309–323.
Simpson CE, Starr JL (2001) Registration of ‘COAN’ peanut. Crop Science 41, 918
| Registration of ‘COAN’ peanut.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR, deWit CT (1975) Photosynthate and nitrogen requirements for seed production by various crops. Science 189, 565–567.
| Photosynthate and nitrogen requirements for seed production by various crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2MXltlSmurk%3D&md5=84d124ce49321e78ded9e4036242a000CAS |
Sinclair TR, Serraj R (1995) Legume nitrogen fixation and drought. Nature 378, 344
| Legume nitrogen fixation and drought.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXps1anu70%3D&md5=566e34a89942c00a7176493f9f5a857aCAS |
Sinclair TR, Sinclair CJ (2010) ‘Bread, beer and the seeds of change: agriculture’s imprint on world history.’ (CAB International: Wallingford, UK)
Sinclair TR, Vadez V (2002) Physiological traits for crop yield improvement in low N and P environments. Plant and Soil 245, 1–15.
| Physiological traits for crop yield improvement in low N and P environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnvVCitro%3D&md5=87bb6b70e75af812d605a08d89e69e1bCAS |
Sinclair TR, Purcell LC, Vadez V, Serraj R, King CA, Nelson R (2000) Identification of soybean genotypes with N2 fixation tolerance to water deficits. Crop Science 40, 1803–1809.
| Identification of soybean genotypes with N2 fixation tolerance to water deficits.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR, Zwieniecki MA, Nolbrook NM (2008) Low leaf hydraulic conductance associated with drought tolerance in soybean. Physiologia Plantarum 132, 446–451.
| Low leaf hydraulic conductance associated with drought tolerance in soybean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXksFWktb4%3D&md5=33d8bb93d04b9038b64012744a783c5cCAS |
Sinclair TR, Messina CD, Beatty A, Samples M (2010) Assessment across the United States of the benefits of altered soybean drought traits. Agronomy Journal 102, 475–482.
| Assessment across the United States of the benefits of altered soybean drought traits.Crossref | GoogleScholarGoogle Scholar |
Singh KB, Robertson LD, Ocampo B (1998) Diversity for abiotic and biotic stress resistance in the wild annual Cicer species. Genetic Resources and Crop Evolution 45, 9–17.
| Diversity for abiotic and biotic stress resistance in the wild annual Cicer species.Crossref | GoogleScholarGoogle Scholar |
Snapp SS, Silim SN (2002) Farmer preferences and legume intensification for low nutrient environments. Plant and Soil 245, 181–192.
| Farmer preferences and legume intensification for low nutrient environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnvVCit78%3D&md5=2ef83a25ef34bbd38537bb73132fda06CAS |
Snapp SS, Mafongoya PL, Waddington S (1998) Organic matter technologies to improve nutrient cycling in smallholder cropping systems of Southern Africa. Agriculture, Ecosystems & Environment 71, 185–200.
| Organic matter technologies to improve nutrient cycling in smallholder cropping systems of Southern Africa.Crossref | GoogleScholarGoogle Scholar |
Snapp SS, Rohrback DD, Simtowe F, Freeman HA (2002) Sustainable soil management options for Malawi: can smallholder farmers grow more legumes? Agriculture, Ecosystems & Environment 91, 159–174.
| Sustainable soil management options for Malawi: can smallholder farmers grow more legumes?Crossref | GoogleScholarGoogle Scholar |
Sperling L, Loevinsohn ME, Ntabomvura B (1993) Rethinking the farmer’s role in plant breeding: local bean experts and onstation selection in Rwanda. Experimental Agriculture 29, 509–519.
| Rethinking the farmer’s role in plant breeding: local bean experts and onstation selection in Rwanda.Crossref | GoogleScholarGoogle Scholar |
Sundstøl F, Owen E (Eds) (1984) ‘Straw and other fibrous by-products as feed.’ (Elsevier: Amsterdam)
Tang C (1998) Factor affecting soil acidification under legumes. I. Effect of potassium supply. Plant and Soil 199, 275–282.
| Factor affecting soil acidification under legumes. I. Effect of potassium supply.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjslOns7g%3D&md5=b3c8d879ef411191e52e6585c76490e5CAS |
Tanner CB, Sinclair TR (1983) Efficient water use in crop production: Research or re-search? In ‘Limitations to efficient water use in crop’. (Eds HM Taylor, WR Jordan, TR Sinclair) pp. 1–27. (American Society of Agronomy: Madison, WI)
Tjepkema JD, Yocum CS (1974) Measurement of oxygen partial pressure within soybean nodules by oxygen microelectrode. Planta 119, 351–360.
| Measurement of oxygen partial pressure within soybean nodules by oxygen microelectrode.Crossref | GoogleScholarGoogle Scholar |
Tropical Legumes (1979) ‘Tropical legumes: resources for the future.’ (National Academy of Sciences: Washington, DC)
Upadhyaya HD, Ortiz R (2001) A mini-core subset for capturing diversity and promoting utilization of chickpea genetic resources in crop improvement. Theoretical and Applied Genetics 102, 1292–1298.
| A mini-core subset for capturing diversity and promoting utilization of chickpea genetic resources in crop improvement.Crossref | GoogleScholarGoogle Scholar |
Vadez V, Sinclair TR, Serraj R (2000) Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiologia Plantarum 110, 215–223.
| Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXnt1Cntbo%3D&md5=c02df021f9167c3b4c3fdb99a92b7564CAS |
Van Soest PJ (1994) ‘Nutritional ecology of the ruminant.’ 2nd edn. (Cornell University Press: Ithaca, NY)
Walsh KB, McCully ME, Canny MJ (1989) Vascular transport and soybean nodule function: nodule xylem is a blind alley, not a throughway. Plant, Cell & Environment 12, 395–405.
| Vascular transport and soybean nodule function: nodule xylem is a blind alley, not a throughway.Crossref | GoogleScholarGoogle Scholar |
Wang L, Liao H, Yan X, Zhuang B, Dong Y (2004) Genetic variability for root hair traits as related to phosphorus status in soybean. Plant and Soil 261, 77–84.
| Genetic variability for root hair traits as related to phosphorus status in soybean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlvVehtLY%3D&md5=0846373e87e3597cf1f29935d38ba3feCAS |
Wittenbach VA, Ackerson RC, Giaquinta RT, Hebert RR (1980) Changes in photosynthesis, ribulose biosphosphate carboxylase, proteolytic activity, and ultrastructure of soybean leaves during senescence. Crop Science 20, 225–231.
| Changes in photosynthesis, ribulose biosphosphate carboxylase, proteolytic activity, and ultrastructure of soybean leaves during senescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXksVWlu7w%3D&md5=fa44917a49d4cedae233c710d944c09dCAS |
Yan X, Liao H, Beebe SE, Blair MW, Lynch JP (2004) QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean. Plant and Soil 265, 17–29.
| QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXns12ltg%3D%3D&md5=19747913fa3527ff9e456e1881c895abCAS |
Zaman-Allah M, Jenkinson D, Vadez V (2011a) Chickpea genotypes contrasting for seed yield under terminal drought stress in the field differ for traits related to the control of water use. Functional Plant Biology 38, 270–281.
| Chickpea genotypes contrasting for seed yield under terminal drought stress in the field differ for traits related to the control of water use.Crossref | GoogleScholarGoogle Scholar |
Zaman-Allah M, Jenkinson D, Vadez V (2011b) A conservative pattern of water use, rather than deep or profuse rooting, is critical for the terminal drought tolerance of chickpea. Journal of Experimental Botany 62, 4239–4252.
| A conservative pattern of water use, rather than deep or profuse rooting, is critical for the terminal drought tolerance of chickpea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVeit7jJ&md5=8b50735efc55be793b63081206a06fbdCAS |


