Nitrogen fixation in Australian dairy systems: review and prospect
Murray UnkovichSchool of Agriculture, Food and Wine, The University of Adelaide, PMB 1, Glen Osmond, SA 5064, Australia. Email: murray.unkovich@adelaide.edu.au
Crop and Pasture Science 63(9) 787-804 https://doi.org/10.1071/CP12180
Submitted: 27 April 2012 Accepted: 7 August 2012 Published: 10 December 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Abstract
Quantitative measurement of N2 fixation has rarely been conducted in Australian dairy pastures. The available data indicate that annual N2 fixation rates in Australian dairy pastures are generally low, due to low pasture legume content. With typical legume contents of grazed pastures less than 30% of total pasture biomass production, annual N2 fixation in herbage is usually much less than 50 kg ha–1 year–1. Other factors which are likely to be able to contribute to increased N2 fixation input (rhizobia, mineral N management, soil acidity, soil water contents) will have little impact until such time as legume contents are increased. In contrast, for some hay systems, such as those using lucerne, N2 fixation input is shown to be high (200–300 kg ha–1 year–1).
While pasture clover contents remain low there is little value in study or measurement of N2 fixation, nor in complex modelling, as N2 fixation will be of little quantitative importance. However, where legume contents, and thus potential N2 fixation are increased, there is scope for investigation into potential increases in N input from this source, which is invariably linked to fertiliser application, the management of grazing and the N returns in urine and dung. These are the major influences on sward N dynamics and legume N2 fixation. The inoculant rhizobia used for white clover in Australia (TA1) is likely to be suboptimal. Isolated in Tasmania in 1953 it has been shown to be inferior in N2 fixation compared with other strains on several occasions. Root pests and diseases are likely to be prevalent and impact directly on clover root growth and perhaps nodulation.
Modelling is often used to describe the probable influence of management and/or climate on the operation of agricultural systems. Reliable modelling of N2 fixation requires capacity to integrate the effects of grazing and pasture composition on soil mineral N dynamics, the influence of this mineral N on nodulation and on suppression of N2 fixation, and environmental and management influences on soil rhizobial populations. Currently no models have demonstrated this capacity. At present, a suitably calibrated regression model is probably a good option for modelling N2 fixation in Australian dairy pastures.
Environmental benefits ensuing from increasing N2 fixation and substituting this for fertiliser N are likely to be greater off-farm (reduced GHG emissions at site of fertiliser manufacture) than on, if current fertiliser management is optimal. Nevertheless substituting fixed N for fertiliser N would have modest environmental and feed efficiency benefits.
Introduction
Nitrogen (N) in plants is the primary N source for animal and milk protein production in dairy systems. Biological dinitrogen (N2) fixation is the process whereby specialised microorganisms are able to convert N2 from the atmosphere into ammonia (NH3) via the enzyme nitrogenase. This ‘fixed’ N can then be incorporated into microbial and plant protein. This is a very important process because, along with fertiliser N (industrial N fixation), it provides the main entry point for N into agricultural systems. There are four principal forms of N2 fixation which relate to the type of N2-fixing bacteria and to the strength of their relationship with plants. Some bacteria fix N2 in a free-living state, while others fix N2 in association with plants. The associations with plants range from rather loose associations around plant roots (associative), endophytic N2-fixing bacteria residing in the vascular tissues of some grasses, and finally, highly-evolved, complex symbioses, involving morphological changes of both microbe and plant in specialised root structures (nodules). In legume symbioses the N2-fixing bacteria pass all of the fixed NH3 directly on to their plant hosts which incorporate it into plant protein. The N2-fixing symbioses with legume plants (e.g. clovers, medics, peas, beans) are the most important because they are more highly evolved and able to fix much greater amounts of N than the other associations. For example, symbiotic N2 fixation can provide for all of the N requirements of pasture legumes, while for pasture grasses the N2-fixing associations are unlikely to be able to provide more than 10% of grass N demand, even under optimal conditions.
The objective of the present review is to document the state of knowledge of N2 fixation in dairy pastures in Australia and to indicate potential areas of research which might increase the value of N2 fixation in Australian dairy systems. While the review is clearly directed at Australian field studies, the limited Australian research requires recourse to salient reviews or critical information from studies elsewhere. This review focuses primarily on perennial high-rainfall or irrigated legume pastures where much of the Australian dairy industry is located. Thorough reviews on N2 fixation in annual legume pastures can be found elsewhere (e.g. Unkovich et al. 1997; Peoples et al. 1998, 2001; Peoples and Baldock 2001) and Unkovich et al. (1998) provide a detailed study of N dynamics in grazed annual clover pastures.
Some of the more pertinent reviews on N2 fixation in grazed perennial pastures include Haynes and Williams (1993), Jarvis et al. (1995), Ledgard and Steele (1992), Ledgard (2001), Menneer et al. (2004), while the reviews of Carlsson and Huss-Danell (2003) and Cuttle et al. (2003) are also quite useful. Eckard (1998) provides salient background to the N dynamics of dairy pastures in Australia and likely responses to N fertiliser application but does not explicitly deal with N2 fixation.
Operation of the N2-fixing legume symbiosis under field conditions
Symbiotic N2 fixation is a complex process involving two organisms in a dynamic partnership subject to a range of environmental and management influences. While the physiological operation of the symbioses are generally understood (Schulze 2004; Garg and Geetanjali 2007), an ability to predict N2 fixation under field conditions requires site-specific knowledge of partner and symbiotic responses to relevant local environmental and management parameters (Russelle 2008). Interactions between grazing and competition for light with grasses exert considerable influence on the balance between grasses and legumes in pasture systems but this will not be considered in detail here. Readers are referred to Schwinning and Parsons (1996).
Mineral N depresses N2 fixation
While legumes have the capacity to fix atmospheric N2 via their symbioses with rhizobia, they are also able to take up soil mineral N like non-legume plants. Indeed they have a preference for use of soil mineral N such that the nodulation and N2 fixation processes are downregulated or turned off in the presence of significant concentrations of mineral N (see Streeter 1988). The dynamic relationship between these factors is illustrated in Fig. 1 for two annual pasture legumes grown under controlled (glasshouse) conditions. The figure highlights that (i) both nodulation and N2 fixation are downregulated by available mineral N (ii) small amounts of mineral N can stimulate growth, nodulation and N2 fixation, and (iii) there are significant differences between species in the extent of these relationships. Although it is not illustrated here, the same legume species with different rhizobia may also vary in their sensitivity to soil mineral N (Unkovich and Pate 1998).
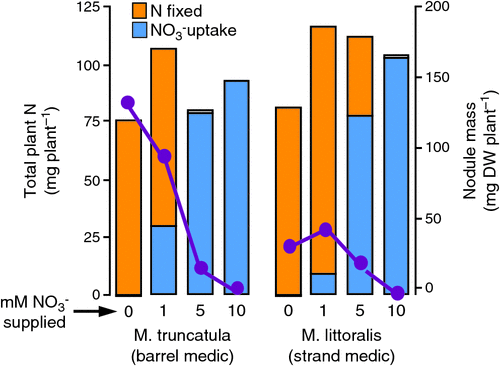
|
In the context of grazed dairy pastures, this means that returns of N in urine and dung will suppress N2 fixation if most of the resultant mineral N is not taken up by companion grasses. Similarly, application of fertiliser N to legume pastures will suppress clover N2 fixation (see e.g. Ledgard et al. 1996, 2001). Regardless of fertiliser N application, this phenomenon is most likely to occur under urine patches which may result in concentrations of readily mineralisable N equivalent to ≥1000 kg ha–1 (Haynes and Williams 1993). Such mineral N concentrations would be expected to suppress N2 fixation and nodulation for some months. Soil nitrate concentrations high enough to suppress nodulation and N2 fixation may also arise in rain-fed pastures at the end of summer and into autumn, particularly in pastures containing annual species (see Unkovich et al. 1998).
In a study in northern Victoria, Mundy (1987) used a 15N tracer to follow fertiliser uptake and N2 fixation in an irrigated white clover/ryegrass pasture following the application of 5 or 100 kg N ha–1 (Fig. 2). Pasture clover content was reduced from 40% in the 5 kg N ha–1 treatment to 20% with 100 kg N fertiliser applied. However, total mineral N uptake by clover was not reduced but fertiliser N uptake was substituted for N2 fixation, which was reduced from 74 to 45% of clover herbage N. The authors indicated that this suppression of N2 fixation continued for at least 10 weeks. These data demonstrate the dynamic interaction between soil mineral N availability, clover and grass growth, and symbiotic N2 fixation, even in the absence of grazing animals. Increased availability of soil mineral N reduces the competitive advantage of N2-fixing legumes under low soil mineral N supply, switches off legume N2 fixation and reduces pasture clover content.
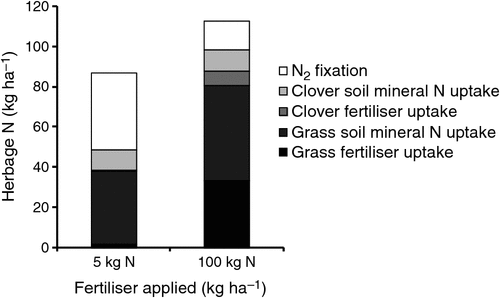
|
A second example of the effect of N fertiliser application on N2 fixation in an irrigated white clover/ryegrass dairy pasture from northern Victoria is shown in Fig. 3. Following application of 100 kg N ha–1, N2 fixation remained at ~50% of that for unfertilised pasture over the following 2 weeks.
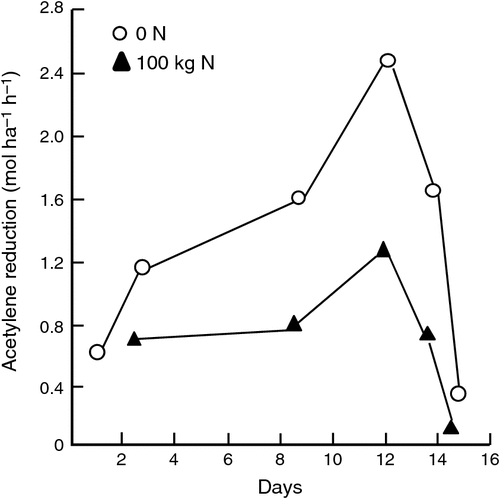
|
In a study of a rain-fed white clover pasture in western Victoria (McKenzie et al. 1998), application of 45 kg N ha–1 had no measurable impact on N2 fixation, regardless of fertiliser type (Table 1). However, in this case, differences between treatments might not be expected since prior grazing may have provided much more mineral N than the modest fertiliser application, and this effect may last many months (Menneer et al. 2004) and, furthermore, very low legume content (9%) and thus low N2 fixation (2–4 kg ha–1) would mask potential treatment effects on measured N2 fixation.
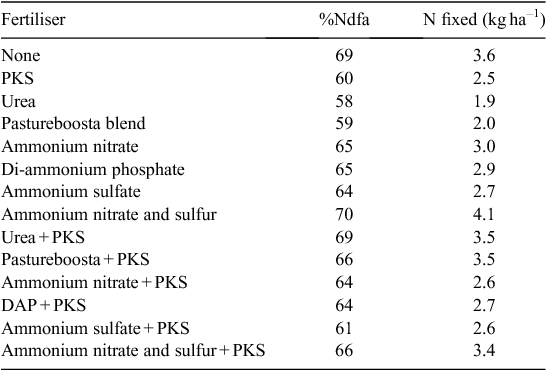
|
In a second experiment McKenzie et al. (1998) applied 0–60 kg N ha–1 to the pasture and N2 fixed ranged from 0.8–3.7 kg N ha–1. While these authors indicated a positive linear response to increasing N fertiliser N application, this seems an unlikely conclusion given the difficulties of measuring such small differences in N2 fixation at the field level (Unkovich et al. 2008). The results of these two experiments highlight the limited value in measuring N2 fixation in low clover content pastures.
Soil water limitations to N2 fixation
N2 fixation activity of legume nodules declines under high soil water contents associated with flood irrigation (Mundy et al. 1988) or water logging, possibly a consequence of the production of ethanol in nodules under anoxic conditions (Sprent and Gallacher 1976). Decreases in soil oxygen availability and subsequent declines in N2 fixation may also result from pugging or increased bulk densities under grazing (Menneer et al. 2001). Nitrogenase activity also declines at low soil water contents, and probably more so than plant growth (Davey and Simpson 1990), although it is difficult to separate these as it is often unclear whether N2 fixation activity reduction is due to reduced plant demand for N or a reduced supply of photosynthate to the nodules. Nodule activity declines with water stress, but can only recover if the water stress is moderate (Sprent 1971). Recommencement of N2 fixation after more severe stress requires regrowth of existing nodules (3–4 days, Engin and Sprent 1973), but after drought, initiation and growth of completely new nodules is required, which would take longer (5–10 days, Davey and Simpson 1990).
An example of the sensitivity of the N2-fixing nodule to soil water content is given in Fig. 4, which shows nitrogenase activity for two irrigated white clover pastures in northern Victoria. The two sites had different soil bulk densities, thus different pore space, and presumably oxygen availability, but the relative effects of soil water content were maintained. For irrigated systems there is thus a challenge to maintain soil water content within the non-limiting range to maximise N2 fixation activity.
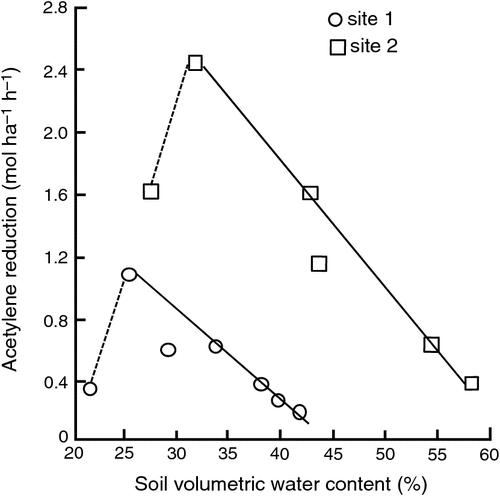
|
White clover may be more susceptible to water stress than other perennial pasture legume species and lucerne more tolerant (Kelly et al. 1989; Neal et al. 2009), Ostrowski (1972) considered white clover to be more susceptible to water than heat stress. This is a probable explanation for observed increases in pasture growth in summer in high-rainfall or irrigated (Kelly and O’Brien 1992) environments when clover contents are increased, and potential increases in N2 fixation during the warmer months of the year (see Eckard 1998, 2001). The low drought tolerance of white clover may be a significant cause of its poor persistence in many systems, even under irrigated conditions where white clover may only maintain maximal growth for 4–5 days after irrigation (Mason et al. 1987). Pasture clover content thus appears to be higher with more frequent irrigations (Dunbabin et al. 1997). Compared with other perennial legumes, lucerne may be more tolerant of water stress, producing greater biomass than five other perennial legumes when grown under deficit irrigation (Neal et al. 2009).
Temperature and N2 fixation
There is considerable inconsistency in the literature relating temperature to N2 fixation in white clover. Whitehead (1995) suggests that N2 fixation ceases below a soil temperature of 9°C, but other evidence indicates that it occurs over a wider range of temperatures (~2−40°C), and is relatively insensitive to temperature over quite a wide range (15−30°C) (Liu et al. 2010). Provided that there is adequate water available, white clover can maintain a constant N2 fixation rate over the 20–33° temperature range (Ryle et al. 1989) and thus the summer temperatures experienced in the Australian dairy regions should not be prohibitive to N2 fixation. Low temperatures may affect N2 fixation less than NO3– uptake (Hatch and Macduff 1991). While Bouchart et al. (1998) reported that N2 fixation in white clover declined more than NH4+ uptake at low temperatures (6°C), they also showed that this was due to reduced clover N demand, not to a direct effect of low temperature on N2 fixation per se. Temperatures as low as 7°C were not limiting to N2 fixation in white clover (Svenning and MacDuff 1996). In the study of three white clover pastures in western Victoria (Riffkin et al. 1997), dependence of white clover on N2 fixation did not decline during the winter months. Dart and Day (1971) found that most of the legume species studied (including red clover and lucerne) continued to fix N2 down to 2°C, and N2 fixation in lucerne was maintained up to 37°C (white clover was not included in the study). Nodulation and N2 fixation in lucerne was suggested to cease below 8°C (Bordeleau and Prévost 1994) but this is not consistent with other reports. Temperature per se is thus unlikely to have any significant direct influence on N2 fixation at the field level under Australian dairy climates, although clearly it will exert indirect influence via effects on plant development, plant water relations, and on the mineralisation of soil N.
Rhizobia and N2 fixation
The microsymbiont bacteria contained in commercial inoculants that partner the primary pasture legumes in Australian dairy systems are given in Table 2. Here it can be seen that while development of legume inoculants has continued for lucerne and annual Trifolium species, there has been no development of rhizobial inoculants for perennial Trifolium species since the initial release of TA1 in ca. 1954.

|
Inoculant rhizobia for perennial Trifolium species
The current rhizobia used in the commercial inoculant for white (Trifolium repens), red (Trifolium pratense) and strawberry (Trifolium fragiferum) clovers in Australia was isolated in Tasmania, and first tested on clovers in 1953 (Paton 1957). Initially named BA-Tas, it was renamed TA1 (Waters 1957). In combination with strain NA30 it was recommended for use as the commercial inoculant for clovers at that time (Waters 1957), primarily because it was effective on a wide range of annual and perennial Trifolium species (Paton 1957). Although TA1 was later shown to be poorly competitive with native rhizobia (Brockwell et al. 1972) on alpine soils, it had appeared to fare better in agricultural soils (Dudman and Brockwell 1968). Strain NA30 was later annexed from the culture (Brockwell and Gibson 1968) and TA1 remains the single strain in the Group B commercial inoculant for white clover available today (Pulsford and Bullard 1997). Rhizobium leguminosarum bv. trifolii strain TA1 became a benchmark organism, and studies deploying this strain of rhizobia developed into a voluminous literature internationally, but little of this relates to its field performance in N2 fixation, particularly with the varieties of white clover grown in Australia. As far as I am able to ascertain this has in fact not been examined, although it has been shown to be less effective in N2 fixation on clovers than a range of other field isolates on several occasions (see Brockwell and Gibson 1968; Riffkin et al. 1999a). Under laboratory conditions Gibson et al. (1975) found that very few field isolates could match its N2-fixing effectiveness. Meanwhile, there is a strong tendency for self selection of suitable rhizobia in the field (Baird 1955; Brockwell et al. 1972), and this may be reflected in the superior performance of some field isolates in western Victoria when compared with TA1 (Riffkin et al. 1999b). There is little doubt that significant improvements could be made with respect to the N2 fixation effectiveness of the microsymbiont used for white clover in Australia, however, while legume contents of dairy pastures remain low, there may be little benefit realised from such improvement.
Inoculant rhizobia for lucerne
Nodulation and rhizobiology of lucerne in Australia has seen more attention than white clover, probably because lucerne also has important roles outside of the dairy industry. These studies have generally shown lucerne to nodulate well and fix N with the range of rhizobia that persist in agricultural soils in Australia (Bowman et al. 1998; Ballard et al. 2003), and in this respect lucerne may be more gregarious than some other Medicago species (Ballard et al. 2003).
N2 fixation, soil acidity and salinity
The average soil pH on 44 dairy farms across the country in the survey of Gourley et al. (2010) was 5.3 (CaCl2), and 4.8 across 71 dairy farms in western Victoria (Riffkin et al. 1999a). At such low soil pH rhizobia (Richardson and Simpson 1989) and nodulation (Munns 1965b) are likely to be severely compromised. Effects may be primarily manifest through poor survival of rhizobia at low pH (Richardson and Simpson 1989; Ballard et al. 2003) and/or inhibition of legume nodulation by toxic aluminium (Unkovich et al. 1996). The white clover inoculant strain (TA1) was shown to be less persistent in acid soil than five of six other strains in a field comparison on annual clovers (Watkin et al. 2000), so this rhizobia may be relatively sensitive to low soil pH. Consistent with this, in the survey of Riffkin et al. (1999a) clover dependence on N2 fixation was negatively correlated with rhizobial numbers on light textured soils (mean pH 4.6) but not on medium textured soils (mean pH 4.9), and the amount of N2 fixed positively correlated with soil pH on light but not medium textured soils. Although lucerne has been shown to ‘select’ compatible, effective rhizobia under acid soil conditions (Ballard et al. 2003), N2 fixation will most likely be suboptimal under the typical soil pH of Australian dairy farms. While rhizobial partners more able to withstand acid soil conditions can be identified (Howieson et al. 1991), these are not a long-term solution to the problem of acid soil development which requires the addition of lime to provide improved soil chemical conditions for plant growth, legume N2 fixation (Howieson and Ballard 2004) and general soil health. Lucerne is generally considered more susceptible to problems of low soil pH than some other legume species and nodule establishment can be an issue (Munns 1965a).
Irrigation of a white clover/ryegrass pasture with saline water reduced clover growth but not grass growth (Smith et al. 1993), yet N2 fixation did not appear to be impaired at the salinities encountered (5 dS m–1). Similarly long-term applications of sewage sludge to soils under dairy pasture in NSW did not impair the operation of white clover symbioses or the effectiveness of the naturalised soil rhizobia (Munn et al. 1997).
Pests and diseases
A range of parasitic nematodes are known to infect white clover across the dairy zone, and to reduce root growth and nodulation (McLeish et al. 1997), with bacterial feeding nematodes being particularly important as pasture legume content increases (Yeates and Stirling 2008). Some pests feed directly on clover nodules (Gerard 2001) and in this case would severely compromise N2 fixation capacity. Although specific, direct effects of pests and diseases on N2 fixation have not been studied (quantified) in the field, the density of some nematode species was correlated with the amount of N2 fixed and the dependence of white clover on N2 fixation on light textured soils in a field survey in western Victoria (Riffkin et al. 1999a). This would imply that nematodes might be reducing white clover N2 fixation in this region. Where the white clover content of pastures are higher this may constitute a significant restraint on N2 fixation potential.
Quantitative estimates of N2 fixation in Australian dairy pastures
Interpreting N2 fixation data in grazed pasture systems
Before examining the available quantitative data on N2 fixation in dairy pastures it is worth considering a framework for interpretation of symbiotic N2 fixation field data. From a systems point of view the key elements are the interactive effects of soil mineral N, clover : grass ratio, and grazing pressure, on N2 fixation as shown in Fig. 5. This highlights that (1) N2 fixation generally tops up clover N demand where it cannot first be satisfied by soil mineral N supply, (2) grasses and other non-legumes are stronger competitors for mineral N than legumes and thus the mineral N demand of non-legumes tends to be met first, (3) the N returns in urine and dung from grazing animals, and fertiliser N, result in increased mineral N in the soil which tends to favour growth of grasses over legumes and to reduce N2 fixation directly, but contrary to this (4) at the lower end of the grazing spectrum, increased grazing intensity may favour the growth of clover over grass due to reduced shading of the clover, and (5) when clover content is lower it is forced to depend more on N2 fixation for its N requirement because more of the mineral N will be taken by the larger grass component.
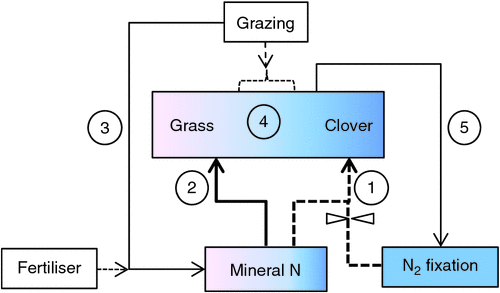
|
One must be careful when interpreting N2 fixation data, for example a clover pasture fixing 100% of its N might be considered excellent, but if the total clover production is only say 500 kg ha–1 then only the tiny amount of 12 kg N ha–1 might be fixed. Conversely, if only 20 kg N were fixed this might be quite acceptable for a pasture with a clover yield of 10 t ha–1, in which case %Ndfa would be low but total clover N might be a respectable 300 kg N ha–1. Unambiguous data on N2 fixation for pastures thus must include information on clover total N or dry matter, as well as the amount of N2 fixed, and the proportional dependence on N2 fixation (%Ndfa).
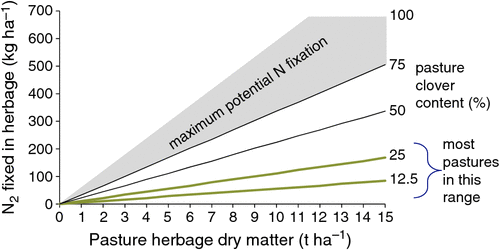
Potential (maximum) N2 fixation is established by legume total dry matter production (Fig. 6), with the realisation of this potential primarily determined by mineral N availability, soil fertility [primarily phosphorus (P)], and the abundance and competence of the microsymbiont rhizobia. The figure shows that a clover production of 14 t ha–1 could potentially sponsor up to 700 kg of N2 fixation annually.
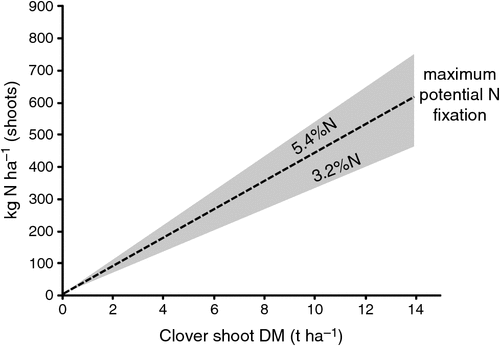
|
Problems of measurement
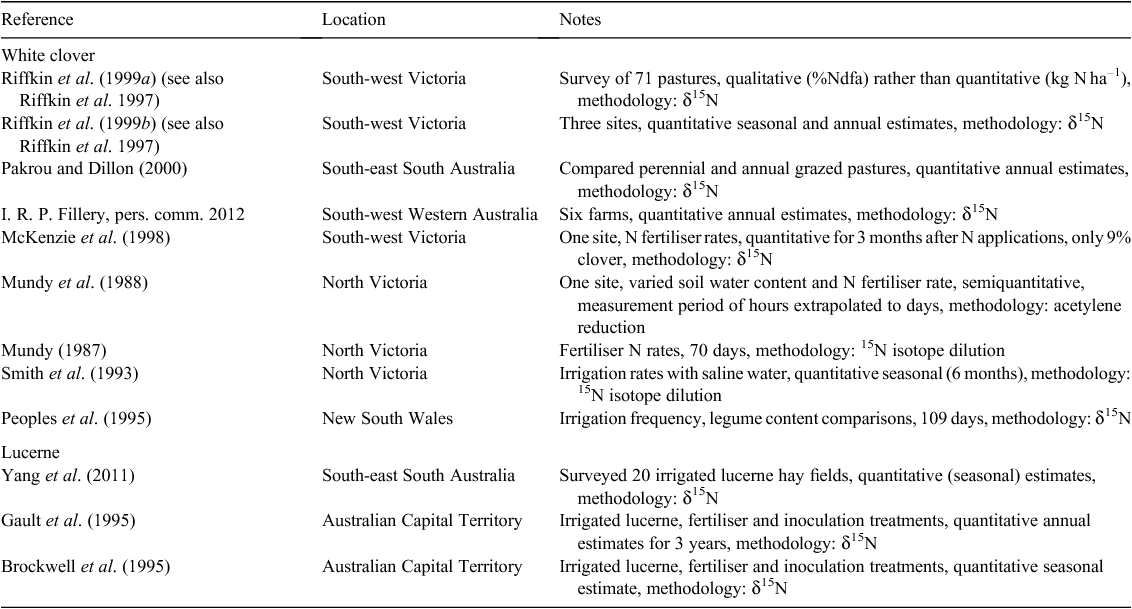
Methods for field measurement of N2 fixation have been detailed in Unkovich et al. (2008) and summarised by Peoples et al. (2009). These reports highlight that there are substantial obstacles to the reliable quantification of N2 fixation in the field and no available methodology is optimal. Those methods which use the stable isotope 15N are considered the more reliable and also give time integrated values. The natural 15N abundance (δ15N) methodology is currently the most widely deployed approach for field measurement of N2 fixation in temperate legumes. Under controlled conditions the relative activity of the N-fixing enzyme, nitrogenase, can be compared in different treatments using the acetylene reduction assay (e.g. Mundy et al. 1988), and while the assay can be applied to field samples it cannot provide reliable quantitative estimates of symbiotic N2 fixation at the field (kg ha–1 year–1) scale (Unkovich et al. 2008). Other non-isotopic techniques (N difference, N balance, regression equations) do not measure N2 fixation directly but rely on a suite of assumptions that are very often invalid, and this reduces their usefulness in many situations. Regression equations relating clover growth to the amount of N2 fixed are becoming popular (e.g. Ledgard et al. 1999; Eckard et al. 2001a; Carlsson and Huss-Danell 2003; Gourley et al. 2010) but these may not be as widely applicable as one might hope. This approach is considered in more detail in Modelling N2 fixation in dairy systems, but results of their application in Australia are not considered to constitute measurements of N2 fixation in the present review. Studies reporting quantitative field estimates of N2 fixation in Australian dairy systems are outlined in Table 3.
|
Accounting for whole-plant N
From the point of view of dairy production the N contained in legume roots that might have been input from N2 fixation may not be as important as in cropping systems (see e.g. Khan et al. 2003). However, it represents a N input to the system and as such can provide for fertility build up and N supply to companion grasses when roots senesce and the N becomes more readily available for microorganisms. This may be particularly important when studying N balances or when modelling mineral N availability in dairy pasture soils. None of the reports in Table 3 include measurement of the total N in legume roots, a task which remains an ongoing challenge (McNeill et al. 1997). In the absence of such measurement the pragmatic approach has been to apply fixed ratios of shoot : root N and multiply these by the amount of shoot N fixed to get total N2 fixation (Unkovich et al. 2010). However, in the absence of the aforementioned root N measurements (see also Wichern et al. 2008) it is difficult to have confidence in the ratios proposed. For white clover a multiplication factor of 1.7 times herbage N was proposed for estimating total clover N (herbage + stolons + roots, Jørgensen and Ledgard 1997) and this has been applied in several studies (e.g. Peoples et al. 2001; Eckard et al. 2007), however, most of the Jorgensen and Ledgard data came from pot studies where the plants were ungrazed/uncut and only grown for a few weeks. How such leaf/stolon + root N ratios might relate to field ratios for grazed perennial clover is unclear. Although they had one contrasting data point for a grazed field experiment this was not compared with the glasshouse experiments, although they were plotted on the same graph. In a field study of subterranean clover using mowing, McNeill et al. (1997) estimated below-ground plant N and came up with a similar 1.75 ratio for estimating total plant N. Unkovich et al. (2010) give a value of 2.0 for lucerne, based on a pot study. It is not clear how such multiplication factors might apply across grazing/cutting regimes, soils, water availabilities, soil fertilities or species, and thus some caution must be exercised in their use. Nevertheless application of these approximate ratios might result in a more accurate estimate of total N2 fixed than if they were not applied at all and root N was ignored.
Grazed white clover pastures
I have only been able to find 12 reports of field measurement of N2 fixation in Australian high-rainfall/irrigated perennial pastures (Table 3), although there are several other reports on rain-fed, annual or lower rainfall, perennial pastures (see Peoples and Baldock 2001).
Annual inputs
For white clover, only four of the datasets in Table 3 (Peoples et al. 1995; Riffkin et al. 1999b; Pakrou and Dillon 2000; I. R. P. Fillery, pers. comm. 2012) include annual N2 fixation estimates, the remainder of the datasets are for shorter periods of time. The work of Riffkin et al. (1999b) demonstrated at three rain-fed sites in south-west Victoria, that N2 fixation was primarily limited by the low legume (white clover) content, averaging only 8% across the three sites. Thus annual N2 fixation input in herbage was only 19–22 kg N ha–1, with the total amount (including roots) being perhaps ~1.7 times this (Jørgensen and Ledgard 1997) at 32–37 kg N ha–1 year–1. These values may be slightly under the average for the region, with an average clover content double these (19%) across 71 dairy pastures examined (Riffkin et al. 1999a), and with values of up to 50% of pasture herbage as clover recorded.
In a recent study in Western Australian dairy pastures (Table 4), similar low legume contents constrained N2 fixation to 2–87 kg ha–1 year–1 across 2 years and six farmlets (I. R. P. Fillery, pers. comm. 2012). The higher value in Farmlet 6 was for a perennial pasture whereas the other pastures contained annual legumes.

|
The most comprehensive study of the N stocks and flows in an Australian dairy pasture comes from the work of Pakrou and Dillon (2000). This study is invaluable because it used isotopic measurement of N2 fixation rather than estimation as has been used in several other N balance studies (e.g. Eckard et al. 2001a, 2007; Gourley et al. 2007). The South Australian study by Pakrou and Dillon (2000) compared a perennial, irrigated white clover/ryegrass pasture (Fig. 7) with a rain-fed, annual subterranean clover-based pasture. In contrast to the abovementioned studies, this involved the sowing of a white clover/ryegrass pasture and comparing this irrigated pasture with an adjacent, rain-fed, unrenovated annual Trifolium pasture. In the irrigated white clover pasture, legume content was just above 50%, and in the rain-fed annual pasture ~25%. Both pastures were grazed by cows, with utilisation rates around 70%.
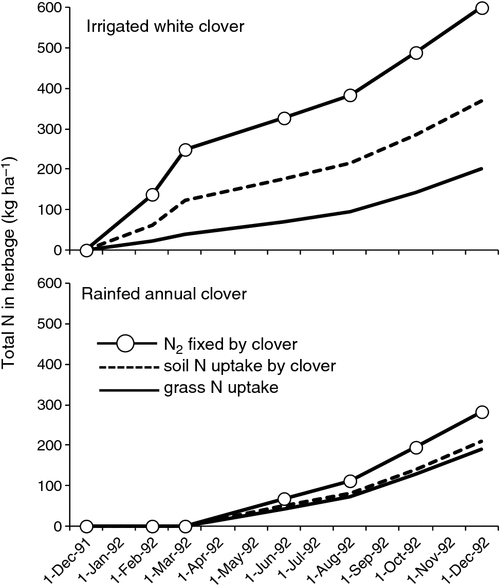
|
Over the 12-month study period the irrigated white clover pasture fixed 231 kg N ha–1 in the harvested herbage whereas the annual subterranean clover based pasture only fixed 75 kg N ha–1 (Fig. 7). The difference between the two pastures was clearly due to the increased productivity of the white clover pasture with irrigation, to the longer growing season afforded by this irrigation, and to the high clover content when compared with the annual pasture. In the annual pasture, grass N uptake dominated the accumulation of herbage N whereas in the perennial pasture clover accounted for 66% of total herbage N. In the annual pasture, soil mineral N uptake by herbage totalled 208 kg ha–1 for the growing season while N2 fixation contributed only 75 kg ha–1, clearly soil mineral N supply provided for the bulk of plant N requirements, thus limiting N2 fixation. The key element of these results is the substantial fixation of N2 when pasture productivity (17.2 t ha–1) and clover content (>50%) are high. Interestingly although productivity of the annual pasture (12.2 t ha–1) was 70% of the irrigated perennial pasture, herbage N accumulation totalled only 47% of that of the perennial pasture. Why the N concentration in herbage was lower in the annual pasture is not clear, but might relate to differential grazing management (see Unkovich et al. 1998).
The final quantitative estimate of N2 fixation in a white clover pasture is that of Peoples et al. (1995), comparing a clover-dominant (85%) with a grass-dominant (60%) pasture over 109 days, with low or high irrigation frequency. Few details of the experiment are given in the Peoples et al. (1995) review paper. Results are as one might anticipate, with greater total N accumulation in both pastures under lower soil water deficits, and greater N2 fixation with higher pasture clover content and clover N yield (Table 5).
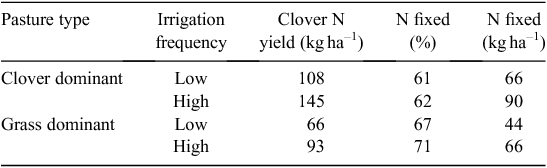
|
Based on the survey of Riffkin et al. (1999a) white clover dependence on N2 fixation in Australian dairy pastures is typically ~65%, indicating reasonable N2-fixing capacity, however, the actual amounts of N2 fixed are very much limited by low clover dry matter production as a consequence of low clover content in most pastures. Much higher rates of N2 fixation are achievable, with up to 294 kg N ha–1 year–1 being recorded for a recently sown, irrigated white clover pasture (Pakrou and Dillon 2000). In a review of perennial forage legumes in temperate/boreal environments, Carlsson and Huss-Danell (2003) report N2 fixation by white clover to be up to 545 kg N ha–1 year–1, but this did not include data on white clover from Australia. Mason et al. (1987) measured irrigated pure white clover pasture annual dry matter production of almost 23 t ha–1 in northern Victoria, which, according to Fig. 6 would provide for potential annual N2 fixation of >1000 kg N ha–1. This is higher than any value in the literature for any N2-fixing system, but nevertheless shows that the potential with this species is very high. In current dairy systems this potential is not being realised due to low pasture legume contents.
The Achilles heel: low white clover content of pastures
Similar low white clover contents of pastures were reported earlier in a survey of Australian temperate pastures (Pearson et al. 1997; Hill and Donald 1998), and also earlier in Victoria (Ward and Quigley 1992). It would thus appear that pasture clover contents, and potential N2 fixation in Australian perennial pastures has probably not improved in almost 20 years, regardless of the increased application of fertiliser N. Farmers appear reluctant to resow legumes (Ward and Quigley 1992). It may well be that for well managed, N fertilised, intensively grazed perennial ryegrass/white clover pastures, that equilibrium clover contents are around 20% resulting in the fixation of no more than ~100 kg N ha–1 year–1, similar to that observed in the UK (Parsons et al. 1991; Andrews et al. 2007) and NZ (Woodfield and Clark 2009), although Jarvis (1993) suggested that in the UK, dairy pastures were typically much lower in both clover content (<10%) and the amount of N2 fixed (10 kg ha–1 year–1). These low clover contents are likely to be suboptimal in terms of dairy production (Woodfield and Clark 2009) as well as N2 fixation and thus efforts to increase N2 fixation should be rewarded with increased milk production efficiency.
In the absence of cattle grazing and the associated deposition of high rates of urine and dung, which increase soil mineral N and most likely depress N2 fixation (Haynes and Williams 1993; Ledgard et al. 1999), dependence on N2 fixation may be higher. For example, in the irrigated pure lucerne systems of south-eastern Australia (Yang et al. 2011) lucerne dependence on N2 fixation averaged 65% and annual N2 fixation in herbage estimated to be >200 kg N ha–1.
Lucerne hay systems
While grazed lucerne pastures are used in Australian dairy systems they are of relatively minor importance compared with white clover/ryegrass pastures, but nevertheless important in the production of hay that feeds directly into the dairy system. Table 3 indicates just three studies quantifying N2 fixation of irrigated lucerne in Australia, with the only two of those (Brockwell et al. 1995; Gault et al. 1995) providing annual N2 fixation estimates being experimental sites in the ACT.
Gault et al. (1995) measured N2 fixation using δ15N natural abundance, in newly established, irrigated lucerne stands cut for hay, over a 3-year period. Experimental treatments were (1) no rhizobial inoculation and superphosphate only in the year of sowing (9 kg P ha–1), (2) rhizobial inoculation plus annual applications of superphosphate, and (3) no rhizobial inoculation, but with annual application of superphosphate and N fertiliser (33 kg N ha–1). Dry matter production and N2 fixation increased dramatically after the first year (Fig. 8), reaching 284 kg N ha year–1 for the inoculated and P fertilised treatment in the third year, although this was only marginally more than for the second year for all treatments (269–275 kg N ha–1). In the third year, the uninoculated treatment, which had not received annual applications of P fertiliser fixed much less than the other treatments. The authors estimated that total N2 fixation (including root N) over the 3-year period to exceed 1400 kg N ha–1 in the annual P fertilised treatments.
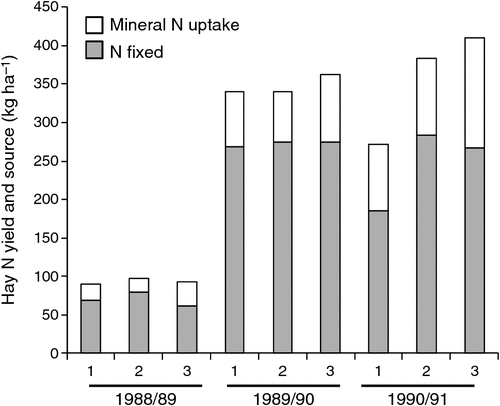
|
This study shows the potential for N2 fixation in irrigated lucerne is very high, provided that attention is paid to crop nutrition. The removal of 10–12 t ha–1 year–1 of hay exports significant quantities of nutrients, aside from N, and these would need to be replaced if growth and N2 fixation is to continue uninhibited.
The above treatments were also applied to a 4-year-old lucerne stand at the same site (Brockwell et al. 1995) and N2 fixation ranged from 83 to 97 kg N ha–1 over the 6-month period of study, giving a nominal annual rate similar to that of Gault et al. (1995). From the data of Fig. 9 it would appear that N2 fixation continues unabated at a constant rate over the warmer months where irrigation water is applied.
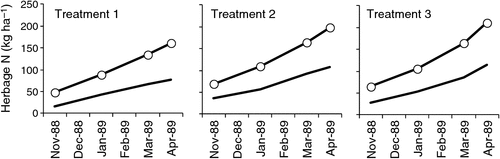
|
The final example of field measures of N2 fixation in lucerne systems comes from Yang et al. (2011) who surveyed N2 fixation in 18 irrigated lucerne hay fields in the south-east of South Australia. The estimates of N2 fixation were for standing dry matter at the time of sampling, in a system which typically has three hay cuts per year. Mean N2 fixation in standing biomass (Table 6) was 73 kg N ha–1, or 65% of lucerne herbage N. What time period these values might represent was not able to be established, but the authors considered that, on average, annual values were likely to be 3 times those observed, giving a value very similar to the annual N2 fixation indications from the studies of Brockwell et al. (1995) and Gault et al. (1995). The South Australian study also indicated that these lucerne stands continued to fix N2 many years (>25) after they were established.
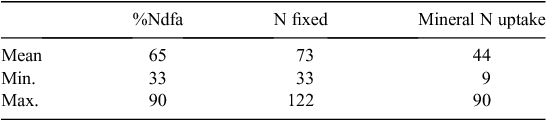
|
Together these data indicate that irrigated lucerne hay crop systems continue to fix considerable amounts of N over time. In contrast to grazed white clover systems, these hay systems export substantial quantities of N in herbage. Furthermore, they are often only grazed lightly such that the build up of soil mineral N does not occur to the extent that is seen in intensively grazed white clover pastures. In this case it is not the legume species which are driving the massive differences in N2 fixation input between lucerne and white clover, but rather the presence of the animals, and the differential management of the systems in which the legumes are utilised.
Grazing and N2 fixation
A detailed review of the impacts of grazing animals on legume N2 fixation are given in Menneer et al. (2004). The key element of grazed dairy systems is the excretion by cattle of at least 75% of the N they ingest from herbage as urine and dung (Whitehead 1995). Maximal N2 fixation is likely to come from well managed hay systems rather than grazed systems, this is because optimal clover content can be more easily managed and the urinary and dung N returns do not suppress N2 fixation. However, this does not mean that ungrazed systems will have greater N2 fixation than grazed systems. Ungrazed mixtures of clover and grass are likely to become grass dominant with shading reducing clover growth and N2 fixation (Sanford et al. 1995). In a study of an annual subterranean clover pasture grazed by sheep in Western Australia (Unkovich et al. 1998) a more heavily grazed pasture had lower grass growth and greater N2 fixation than a lightly grazed pasture. While increased grazing pressure can favour clover growth over grasses, in practise the magnitude of this generally appears quite small as the effect occurs at the lighter end of grazing intensities (Doyle et al. 2000). Increased grazing pressure usually increases the N (protein) content of clover (Unkovich et al. 1998), and indeed other pasture species (Kelly et al. 2005). The work of Pakrou and Dillon (2000) highlights the significance of the mineral N flux under grazing. Under irrigated, grazed white clover pasture, the flux of N through the soil mineral N pool was estimated to be 687 kg N ha–1, more than half of which was derived from animal returns. This study also highlights the significant role that N2 fixation can play when there is a high clover content, even in the presence of intensive grazing. Under the annual pasture, mineralisation of soil organic N was driving the available N pool, being no higher when the animals were on the pasture than when they there were absent (see Pakrou and Dillon 2000). Nevertheless excretory N returns from grazing animals showed up as the key influence on sward N dynamics and N2 fixation in these two dairy systems.
In terms of N2 fixation the key elements to note in the perennial pasture of Pakrou and Dillon (2000) are:
-
The legume (white clover) content was high (57%) because the pasture had been sown only 2 years before, this is atypical for Australian dairy pastures where legumes contents are commonly <20%,
-
Because the legume content and legume dry matter production (9.8 t ha–1) was high, N2 fixation was also high (236 kg N ha–1), excluding an additional 59 kg ha–1 (25%) estimated for clover roots,
-
After mineralisation (687 kg N ha–1), cattle intake (419 kg N ha–1) and grass mineral N uptake (389 kg N ha–1), N2 fixation was the fourth highest N flux in the system, and
-
N2 fixation was greater than the combined N losses estimated from leaching, NH3 volatilisation and denitrification (209 kg N ha–1) and thus the system appeared to be in an approximate N balance, despite there being no N fertiliser inputs.
The key elements to note for the annual, rain-fed pasture were:
-
N2 fixation was much lower than for the white clover-based pasture because (a) subterranean clover is an annual and only grows for part of the year (b) the clover content (25%) was less than half that of the perennial pasture, and (c) the annual pasture was rain-fed, not irrigated,
-
Most of the clover N was fixed (80%),
-
The system had a marginally negative N balance overall, and
-
The fixation rate of 100 kg N ha–1 year–1 in this pasture is higher than for Australian dairy pastures generally because there were no fertiliser N inputs.
Differences in N2 fixation capacity between species and cultivars
Differences between legume cultivars are unlikely to be of quantitative importance for N2 fixation input in Australian dairy systems. However, where differences in clover productivity are expressed then those cultivars with greater shoot biomass would fix more N. While this has not been examined specifically for Australian cultivars (N2 fixation has not been considered in the Australian white clover breeding program (Carol Harris, NSW DPI, pers. comm. 2012)), data on nine white clover cultivars from New Zealand (Ledgard et al. 1996) indicated that differences between cultivars in the amount of N2 fixed are related to dry matter production driven differences in clover total N accumulation, rather than to inherent differences in the N2 fixation efficiency or shoot N concentration (Fig. 10). While all three of these variables are used to calculate the amount of N fixed, it is clearly legume dry matter production which is the driving force in this dataset, and indeed in most others (Unkovich et al. 2010).
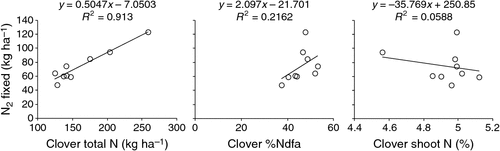
|
In an earlier study of differences in N2 fixation between white clover cultivars in New Zealand (Ledgard et al. 1990), it was concluded that there were no inherent differences in the capacity of different cultivars to fix N, and thus that N2 fixation was not a basis for substituting one for another. Generally speaking, in breeding for maximum dry matter or total N accumulation, clover breeding programs might indirectly select for maximal N2 fixation. However, this does not mean that N2 fixation is optimal nor has been selected for, because it may well be that even with the best available plant material, N2 fixation could still be limiting growth, due, for example to poorly effective rhizobia. With respect to cultivar performance in N2 fixation, in several pasture legume species it has been shown that there is a strong interaction between legume cultivar and rhizobium strain, such that optimal N2 fixation potential is achieved with specific combinations of pasture legume cultivar and rhizobial strain (see e.g. Ballard et al. 2003).
Differences in N2 fixation between species of legume will be driven as much by differential management of species/systems and environment, as by inherent differences between legume species.
Modelling N2 fixation in dairy systems
Because field measurement of biological N2 fixation is complex and expensive (Unkovich et al. 2008) modelling approaches to estimate N2 fixation hold significant attraction. The basis for model design can be either empirical (e.g. Høgh-Jensen et al. 2004; Unkovich et al. 2010) or dynamic mechanistic (e.g. Boote et al. 2008). Empirical approaches tend to correlate measured N2 fixation rates with other, more easily measured pasture properties, fit regression equations to the resulting dataset, and then apply those regressions elsewhere in time or space. Dynamic simulation models attempt to mimic the primary biological and physical processes driving plant growth (Sinclair and Seligman 1996), including N2 fixation, and usually attempt to be universally applicable upon local parameterisation. Such so called mechanistic or dynamic simulation models are usually only semi-mechanistic as they typically include some empirical approaches. Liu et al. (2010) reviewed a large number of approaches to modelling N2 fixation and the reader is referred to this thorough exposé of N2 fixation modelling, the detail of which is outside the scope of the present review.
Empirical relationships
An example of a typical empirical model for estimating N2 fixation is given in Fig. 11, which relates legume shoot dry matter production to the amount of N2 fixed. This figure is for herbage N fixed, an additional fraction can be added for fixed N possibly contained in roots.
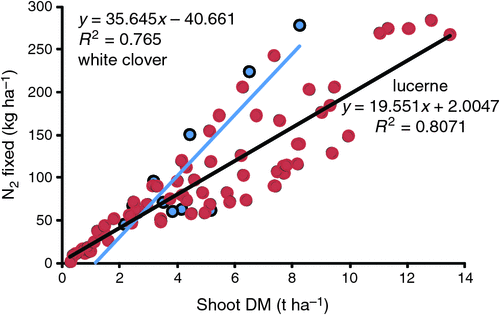
|
The pros and cons of such approaches are detailed in and Liu et al. (2010) and Unkovich et al. (2010). The primary limitation of such approaches are that, aside from the influence of dry matter production, they are naive to other possible drivers of N2 fixation, such as soil fertility, temperature, water availability, grazing intensity, non-legume pasture content, and microsymbiont performance. The net effect of such factors are of course inherent in the observed data and so have been captured for the data points presented. The problem is that once the regression is applied in another situation (time or place), these inherent effects may not apply at the application place/time.
Carlsson and Huss-Danell (2003) found significantly different regressions for grazed and mown white clover pastures, and thus the regressions are not transferable between such management regimes. Relationships which have been developed elsewhere (e.g. Ledgard et al. 1999) and applied in Australia (e.g. Eckard et al. 2001a, 2007) are thus fraught with danger, especially if applied too specifically. Such regressions have no experience beyond their derivation dataset and thus other regressions might have equal validity. For example Carlsson and Huss-Danell (2003) gave linear regressions between white clover dry matter and N fixed accounting for 91% (clover/grass) to 55% (legume monocultures) of the measured amount of N2 fixed, without accounting for N fertiliser application.
Examples such as that in Fig. 11 may approximate behaviour across regions but are unlikely to be correct at any given point and should only be applied at the scale at which the regression is derived. That is, if the data are derived for a range of treatments within a single field or farm, they could not be reliably extrapolated outside of that field or farm. Conversely, regression across a range of fields or regions might usefully be applied across such a scale, but is not likely to apply at sub field or region scale. The regressions cannot be reliably used in situations where they have no previous experience. In this way they are different to dynamic simulation models which often respond to local environmental and management influences.
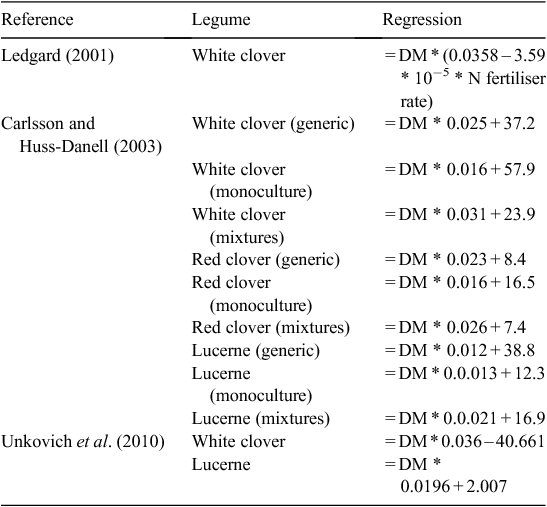
In the study of Ledgard et al. (2001) the white clover N concentration did not drop below 4.5%, whereas this was close to the average for 71 pastures investigated in Victoria (Riffkin et al. 1999a) and in the analysis of broader Australian data by Unkovich et al. (2010) the mean shoot [N] for white clover was given as 3.2%, which could account for a significant difference in the slope of the regression lines. Indeed Fig. 12 looks much like Fig. 6. Furthermore, the clover N2 fixation in the Ledgard study did not exceed 94 kg N ha–1 whereas in the Unkovich dataset the maximum was 278 kg N ha–1 and in the Carlsson dataset it exceeded 400 kg N ha–1 year–1. As much of the evidence indicates that clover content and clover dry matter production are low in Australia (≤4 t ha–1) the relevant part of Fig. 12 is near the origin. At 2 t ha–1 clover dry matter N2 fixation would range from 30 to 87 kg N ha–1 year–1 depending on which regression equation was used. Further complications arise because in some instances significant N2 fixation would be indicated with no clover dry matter (Fig. 12, Carlsson regression). This can occur with regressions when they are extended beyond their experience, or where the responses may indeed not be linear, as is likely to be the case at the lower end of the range when soil mineral N will become increasingly important.
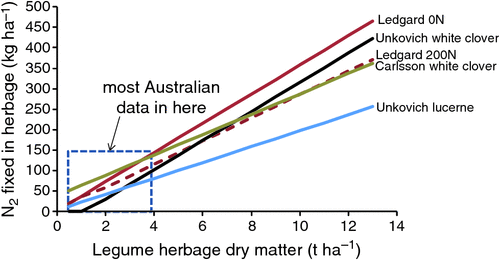
|
|
Given that a value of ~4.5% N for herbage seems typical for grazed white clover (Fig. 6 and Ledgard et al. 2001), a shoot : root N ratio of 1.7 (Jørgensen and Ledgard 1997) and the average dependence of white clover on N2 fixation in western Victoria of 65% (Riffkin et al. 1999b), this implies a total N2 fixation for current systems averaging 50 kg t–1 clover shoot dry matter, or in shoots only 29 kg t–1 herbage. While this might provide a useful rule of thumb for pastures of low (<25%) legume content from which the data have been derived, for higher legume content dairy pastures other factors may play a part in changing %Ndfa or herbage N concentration and thus alter the relationship between dry matter and N2 fixed.
Dynamic simulation models
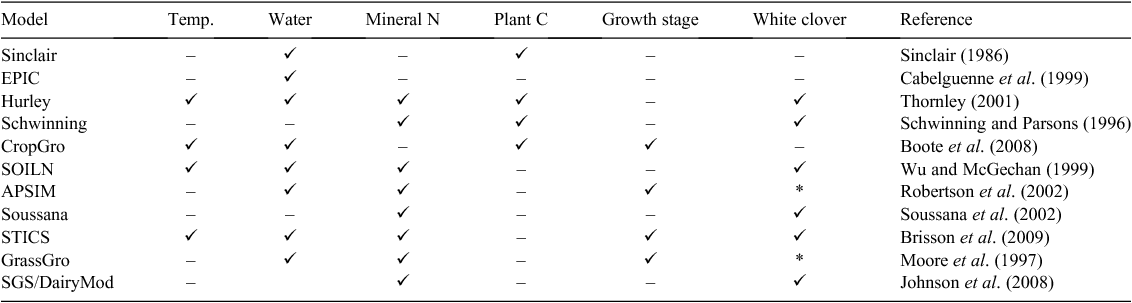
In the review of Liu et al. (2010) of nine mechanistic/process based models of N2 fixation, commonalities were the scaling of a maximum daily N2 fixation rate as a function of some combination of temperature, soil water, soil mineral N, plant carbon availability, and plant development stage. The implementation of these various factors in a range of models are shown in Table 8. Eight of the models have been used for perennial legume pasture species (white clover or lucerne).
|
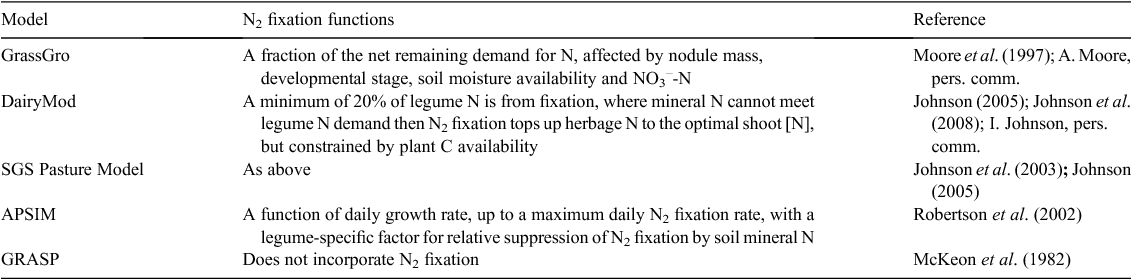
When reviewing models the first consideration is the purpose/objective of the modelling required. There are many models, either specifically for N2 fixation, or which have N2 fixation as a component, but each has been built with a different specific purpose in mind. For the present purposes it is assumed that the modelling objective is to quantify changes in legume N2 fixation in response to management and climate, rather than legume physiological responses to climate and management. Relevant pasture simulation models which have been used in Australia are given in Table 9, along with their N2 fixation simulation capacity.
|
In GrassGro the potential N2 fixation rate is calculated as the total plant N demand less N translocated from belowground reserves and N recycled from shaded leaves, multiplied by a factor for the development of nodules in early growth. This potential rate is then scaled back by low water content and high mineral N, weighted according to a nodule depth distribution (Andrew Moore, CSIRO, pers. comm.).
In the DairyMod tool, N2 fixation is linked directly to photosynthesis and a value of 6 mg C respired/mg N fixed used as a carbon cost, thus reducing growth of N2-fixing clover compared with non-fixing clover. An earlier version of the model constrained N2-dependent clover to 0.6 of the growth of mineral N-dependent clover, although I think this has now been removed. A minimum of 20% of legume N comes from N2 fixation under all conditions. Legumes are not limited for N, with N2 fixation topping herbage N up to the optimal value (Johnson 2005). Graham (2008) provides a thorough review of the DairyMod tool although does not discuss legume N2 fixation.
In APSIM (Robertson et al. 2002) N2 fixation occurs when there is insufficient mineral N to meet plant N demand, but the sensitivity with which N2 fixation is switched on in the presence of mineral N, being a cultivar specific parameter. While the model does not currently have an interaction between soil mineral N and nodulation, the N2 fixation routines are currently being revised and nodule mass will be developed into an integral part of the N2 fixation simulation routines.
While DairyMod, APSIM and GrassGro have the capacity to model N2 fixation I can find no published model output showing N2 fixation by pasture or crop legumes, or a comparison of model output with measured N2 fixation data. While the models often show good correlation of model simulated and measured dry matter production or total N, the validity of these models nevertheless remains essentially untested in terms of N2 fixation. The N2 fixation routines in both APSIM and DairyMod are currently being revised [M. Robertson (CSIRO) and I. Johnson (IMJ), pers. comms].
None of these models include any consideration of the population dynamics, effectiveness or environmental responses of the microsymbiont and thus will be unable to simulate responses of the symbiosis to management and environment in the field. Those models which ignore the microsymbiont dynamics will inevitably have limited capacity over time. There is no physiological process-based model tested for specific study of N2 fixation in Australian dairy systems. Until the N2 fixation routines in the available models have been tested against measured data they offer no more in terms of predictive N2 fixation capacity than a suitably calibrated empirical model.
Environmental costs and benefits of N2 fixation
A fair assessment of the environmental costs and benefits of legume N2 fixation in dairy systems can only be achieved with consideration of a gamut of factors impinging on the environmental balance sheet for a dairy farm. While this is beyond the scope of the present review we can briefly consider some of the issues feeding into and out of legume N in dairy farming systems. More thorough environmental analyses of dairy farming systems can be found in a recent volume (de Klein et al. 2008; Kleinman and Soder 2008; Nash and Barlow 2008) and a range of other relevant articles (Ridley et al. 2004; Andrews et al. 2007; Ledgard et al. 2009; Woodfield and Clark 2009).
Urinary N returns from dairy cattle concentrate soluble N at very high rates and provide the primary point of soluble N excess and thus the greatest opportunity for environmental impact. Generally to minimise losses of N via denitrification, leaching or NH3 volatilisation a ‘tight’ N cycle is required, necessitating the maintenance of some N limited grass to ‘mop up’ available N (Parsons et al. 1991). However, it is generally thought that a system with slightly N-deficient grass may limit feed quantity and quality and is not considered optimised in terms of animal production (Eckard 2001). This is thus not usually recommended from a milk production perspective but nevertheless could provide significant environmental benefits.
In a study in the UK, Andrews et al. (2007) considered the relative merits of (i) an unfertilised perennial ryegrass/white clover pasture (ii) a perennial ryegrass pasture receiving 200 kg N ha–1 year–1, and (iii) a perennial ryegrass only pasture supplied with 350–400 kg N ha–1 year–1. From a N cycling and NO3– leaching perspective, pastures (i) and (ii) were considered equal as the unfertilised pasture had similar N input from N2 fixation, and with a similar grazing regime the amount of N cycling through the animals was about the same. The pasture with the higher fertiliser N addition rate (iii) was considered to have a greater N footprint due to increased leaching and nitrous oxide emissions. Generally it was considered that with similar N inputs, pasture productivity and grazing intensity the environmental N footprint would be about the same; that is there may be no inherent advantage in N2 fixation per se in terms of N cycling impacts. The analysis of Andrews et al. (2007) did not include the magnitude of N2 fixed in clover roots and thus may have underestimated the difference in N inputs between treatments.
Generally then, if contrasting systems (grass versus grass/clover) are equally as productive and have the same stocking rates or animal products output, they are likely to have very similar environmental costs/benefits. This is because most of the environmental footprint from dairy systems comes from the livestock N returns not the N input per se. While substitution of fertiliser N with clover fixed N might improve the environmental balance sheet on farm, the benefit is likely to be marginal where best practise fertiliser management is already being used.
Excretal N is the primary source of nitrous oxide (N2O) emissions from dairy systems (de Klein et al. 2008; Ledgard et al. 2009). Although legume N2 fixation was previously thought to contribute directly to N2O emissions, this has been shown not to be the case (Rochette and Janzen 2005) and so the direct N2O footprint of legume fixed N2 is minimal. If one were also to include energy costs of urea fertiliser manufacture (0.73–2.14 kg CO2-e kg–1 Ledgard et al. 2011), then substituting fixed N2 for fertiliser N should have some GHG mitigation potential (Ledgard et al. 2009), but not if pasture clover contents are low. Andrews et al. (2007) considered that savings in CO2-e by substituting 200 kg N for fixed N would be negligible on a global scale but very significant on a ha–1 scale. Nitrogen fertiliser manufacture accounts for ~1% of total global CO2-e emissions. In the future if legumes with condensed tannins become available (Woodfield and Clark 2009) additional GHG benefits should accrue in terms of reduced CH4 emissions.
High land-use intensity in the dairy industry is the primary cause of environmental problems resulting from excess N (de Klein et al. 2008). While similar, well managed clover/grass and grass only pastures are likely to have the same local environmental impact, whole system or life cycle analysis suggests that overall, pastures which contain N2-fixing legumes would have a lower net environmental impact than N-fertilised pastures (Ledgard et al. 2009). While ungrazed legume-dominant hay systems would appear to have a much lower environmental impact than intensively grazed pastures as the primary animal driven mineral N fluxes would be avoided, this ignores the fact that the hay will still be fed to animals and the excretal N returned elsewhere. Although in this case it might be more effectively managed.
Managing N2 fixation in Australian dairy pastures – where to from here?
Australian dairy systems have made the inevitable drift from the exploitation of legume N in extended grazing systems to short rotational grazing of N fertilised pastures that has characterised the development of intensive, modern dairy systems elsewhere in the world. This is due to a perceived increase in system efficiency by increasing the stocking rate to utilise more of the pasture, and then supplementing the otherwise underfed cows (Lemerle et al. 1992). Such a system increases the return of urinary and dung N to pastures, further reducing legume content. It is this high intensity grazing rate that is exerting significant influences on N2 fixation by clover, through defoliation, treading and returns of urinary N which cause direct reductions in N2-fixing (nitrogenase) activity and in clover persistence. However, as legumes have several special benefits to dairy cows and to farming systems, they are likely to have a continuing, perhaps increasing role in dairy systems in the future, provided that investment is made in the appropriate areas.
The clover contents of typical dairy pastures are clearly below the optimum required for effective N2 fixation input, and perhaps below what might be optimal in terms of animal nutrition and milk production (Harris et al. 1997). Thus efforts to increase N2 fixation should be rewarded with both improved animal production efficiency, and environmental benefits. Generally lower rates of N application and moderate intensity grazing favour white clover persistence and abundance in mixed pastures (Kelly et al. 2005). Legume herbage has distinct advantages over grasses in terms of animal production and warrants inclusion in dairy pasture systems. The fact that clover is able to obtain its own N requirements from the atmosphere provides an opportunity to reduce input costs and the environmental impact of dairy agriculture.
In high-rainfall and irrigated pastures, clover contents should be able to be increased, with multiple benefits, including N2 fixation. However, under rain-fed conditions where summer droughts occur, perennial legume persistence and N2 fixation are likely to be more difficult to maintain, and occasional resowing could be required. Housed animal systems with cut and carry forage could be more reliant on legumes and N2 fixation, whereas intensively grazed pastures will inevitably have lower clover contents, higher returns of urinary and dung N (intensified through the addition of supplementary feeding when pasture supply is limited), increasing the downward pressure on legumes and N2 fixation.
Eckard et al. (2001b) points out that reduced N fertiliser use and increased dependence on legumes has now occurred in Europe, a trend which might follow here, whether this alone will be sufficient to boost pasture legume contents and N2 fixation to the required level is not clear. It is likely to also require lower stocking rates which is somewhat anachronistic to the current management paradigm in Australian dairy systems, which focuses on pasture utilisation efficiency rather than N-use efficiency. In any event if pasture legume contents are increased there will be a requirement for monitoring of legume growth and N2 fixation to ascertain whether the other factors highlighted begin to constrain N2 fixation (rhizobia effectiveness, nematodes, grazing intensity and excretal N returns). One alternative option worth exploring might be the spatial separation of clover and grass (Chapman et al. 2007; Woodfield and Clark 2009), with potential increases in N2 fixation input and scope for spatial management of fertiliser, and improved milk production. Differences in the N2-fixing potential (growth) of white clover cultivars are likely, as are differences in responses to available N (Doyle et al. 2000) but these have not been explicitly explored for Australian clover varieties.
Complex dynamic simulation models are probably not required to predict the likely outcome of changes in pasture legume content in terms of N2 fixation. This should be able to be modelled relatively simply, or with simple regression models such as that shown in Fig. 13. The DairyMod, APSIM and GrassGro models all have some capacity for N2 fixation simulation, but this is yet to be exploited. A comparison of model outputs in terms of N2 fixation against measured data are required to ascertain if the current models have anything to offer.
|
Acknowledgements
Many thanks to Ian Fillery for the provision of unpubl. data, to Andrew Moore, Ian Johnson and Michael Robertson for comment on the modelling section, and to Beverly Henry and anonymous reviewers for comment on an earlier draft. This review was conducted for Dairy Australia as part of the Dairy Moving Forward initiative.
References
Andrews M, Scholefield D, Abberton MT, McKenzie BA, Hodge S, Raven JA (2007) Use of white clover as an alternative to nitrogen fertiliser for dairy pastures in nitrate vulnerable zones in the UK: productivity, environmental impact and economic considerations. Annals of Applied Biology 151, 11–23.| Use of white clover as an alternative to nitrogen fertiliser for dairy pastures in nitrate vulnerable zones in the UK: productivity, environmental impact and economic considerations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVertrvF&md5=a72c7e3cc7eec1b32934d9fbd88af70fCAS |
Baird KJ (1955) Clover root-nodule bacteria in the New England region on NSW. Australian Journal of Agricultural Research 6, 15–26.
| Clover root-nodule bacteria in the New England region on NSW.Crossref | GoogleScholarGoogle Scholar |
Ballard RA, Shepherd BR, Charman N (2003) Nodulation and growth of pasture legumes with naturalised soil rhizobia. 3. Lucerne (Medicago sativa L.). Australian Journal of Experimental Agriculture 43, 135–140.
| Nodulation and growth of pasture legumes with naturalised soil rhizobia. 3. Lucerne (Medicago sativa L.).Crossref | GoogleScholarGoogle Scholar |
Boote K, Hoogenboom G, Jones J, Ingram K (2008) Modeling nitrogen fixation and its relationship to nitrogen uptake in the CROPGRO model. In ‘Quantifying and understanding plant nitrogen uptake for systems modeling’. (Eds L Ma, L Ahuja, V Bruulsema) pp. 13–46. (CRC Press: Florence, SC)
Bordeleau L, Prévost D (1994) Nodulation and nitrogen fixation in extreme environments. Plant and Soil 161, 115–125.
| Nodulation and nitrogen fixation in extreme environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXlt12rt7Y%3D&md5=0461b5dc0d646cc0d43fca933972eed2CAS |
Bouchart V, Macduff JH, Ourry A, Svenning MM, Gay AP, Simon JC, Boucaud J (1998) Seasonal pattern of accumulation and effects of low temperatures on storage compounds in Trifolium repens. Physiologia Plantarum 104, 65–74.
| Seasonal pattern of accumulation and effects of low temperatures on storage compounds in Trifolium repens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmvFyrtLg%3D&md5=c712911d5123c5e38391a2500152853cCAS |
Bowman A, Hebb D, Munnich D, Brockwell J (1998) Rhizobium as a factor in the re-establishment of legume-based pastures on clay soils of the wheat belt of north-western New South Wales. Australian Journal of Experimental Agriculture 38, 555–566.
| Rhizobium as a factor in the re-establishment of legume-based pastures on clay soils of the wheat belt of north-western New South Wales.Crossref | GoogleScholarGoogle Scholar |
Brisson N, Launay M, Mary B, Beaudoin N (2009) Nitrogen transformations. In ‘Conceptual basis, formalisations and parameterization of the STICS crop model’. (Eds N Brisson, M Launay, B Mary, N Beaudoin) pp. 141–165. (Quæ: Versailles Cedex)
Brockwell J, Gibson A (1968) Root-nodule bacteria for some cultivated spceis of Trifolium. Joural of The Australian Institute of Agricultural Science 34, 224–227.
Brockwell J, Bryant WG, Gault RR (1972) Ecological studies of root-nodule bacteria introduced into field environments. 3. Persistence of Rhizobium trifolii in association with white clover at high elevations. Australian Journal of Experimental Agriculture 12, 407–413.
| Ecological studies of root-nodule bacteria introduced into field environments. 3. Persistence of Rhizobium trifolii in association with white clover at high elevations.Crossref | GoogleScholarGoogle Scholar |
Brockwell J, Gault R, Peoples M, Turner G, Lilley D, Bergersen F (1995) N2 fixation in irrigated lucerne grown for hay. Soil Biology & Biochemistry 27, 589–594.
| N2 fixation in irrigated lucerne grown for hay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXlt12rtrg%3D&md5=ae689282820fb98533cb501e3f1e9efbCAS |
Cabelguenne M, Debaeke P, Bouniols A (1999) EPICphase, a version of the EPIC model simulating the effects of water and nitrogen stress on biomass and yield, taking account of developmental stages: validation on maize, sunflower, sorghum, soybean and winter wheat. Agricultural Systems 60, 175–196.
| EPICphase, a version of the EPIC model simulating the effects of water and nitrogen stress on biomass and yield, taking account of developmental stages: validation on maize, sunflower, sorghum, soybean and winter wheat.Crossref | GoogleScholarGoogle Scholar |
Carlsson G, Huss-Danell K (2003) Nitrogen fixation in perennial forage legumes in the field. Plant and Soil 253, 353–372.
| Nitrogen fixation in perennial forage legumes in the field.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlsFKqsr0%3D&md5=09aa5e349b5c3e09c40a8c92abcbb83cCAS |
Chapman D, Parsons A, Cosgrove G, Barker D, Marotti D, Venning K, Rutter S, Hill J, Thompson A (2007) Impacts of spatial patterns in pasture on animal grazing behaviour, intake and performance. Crop Science 47, 399–415.
| Impacts of spatial patterns in pasture on animal grazing behaviour, intake and performance.Crossref | GoogleScholarGoogle Scholar |
Cuttle S, Shepherd M, Goodlass G (2003) ‘A review of leguminous fertility-building crops, with particular refence to nitrogen fixation and utilisation.’ (Institute of Grassland & Environmental Research: Aberystwyth)
Dart PJ, Day JM (1971) Effects of incubation temperature and oxygen tension on nitrogenase activity of legume root nodules. Plant & Soil 35, 167–184.
| Effects of incubation temperature and oxygen tension on nitrogenase activity of legume root nodules.Crossref | GoogleScholarGoogle Scholar |
Davey AG, Simpson RJ (1990) Nitrogen fixation by subterranean clover at varying stages of nodule dehydration I. Carbohydrate status and short-term recovery of nodulated root respiration. Journal of Experimental Botany 41, 1175–1187.
| Nitrogen fixation by subterranean clover at varying stages of nodule dehydration I. Carbohydrate status and short-term recovery of nodulated root respiration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXlvVyitL4%3D&md5=21b28dabb2a1d310101110a86949dd40CAS |
de Klein C, Pinares-Patino C, Waghorn G (2008) Greenhouse gas emissions. In ‘Environmental impacts of pasture-based farming’. (Ed. R McDowell) pp. 1–33. (CAB International: Wallingford, UK)
Doyle P, Stockdale C, Lawson A, Choenm D (2000) ‘Pastures for dairy production in Victoria.’ (Department of Natural Resources and Environment: Tatura, Vic.)
Dudman WF, Brockwell J (1968) Ecological studies of root-nodule bacteria introduced into field environments. I. A survey of field performance of clover inoculants by gel immune diffusion serology. Australian Journal of Agricultural Research 19, 739–747.
| Ecological studies of root-nodule bacteria introduced into field environments. I. A survey of field performance of clover inoculants by gel immune diffusion serology.Crossref | GoogleScholarGoogle Scholar |
Dunbabin JS, Hume IH, Ireson ME (1997) Effects of irrigation frequency and transient waterlogging on the production of a perennial ryegrass/white clover pasture. Australian Journal of Experimental Agriculture 37, 165–171.
| Effects of irrigation frequency and transient waterlogging on the production of a perennial ryegrass/white clover pasture.Crossref | GoogleScholarGoogle Scholar |
Eckard R (1998) ‘A critical review of research on the nitrogen nutrition of dairy pastures in Victoria.’ (The University of Melbourne and Agriculture Victoria: Ellinbank)
Eckard R (2001) ‘Best management practices for nitrogen in intensive pasture production systems.’ (University of Melbourne: Melbourne)
Eckard R, Chapman D, White R, Smith A, Chen D, Durling P (2001a) Nitrogen balances in high rainfall, temperate dairy pastures of south eastern Australia. In ‘Proceedings of the XIX International Grasslands Congress’. São Pedro, Brazil. (Eds JA Gomide, WRS Mattos, SC Da Silva) pp. 169–170. (Fundacao Estudos Agrarios Luiz Queiroz (FEALQ), Piracicaba, Brazil)
Eckard R, McKenzie F, Lane P (2001b) The ‘Pendulum Paradigm’ – trends in nitrogen fertiliser use on temperate grass/clover pastures. In ‘Proceedings of the XVIII International Grasslands Congress’. Manitoba, Canada. (Eds JG Buchanan-Smith, LD Bailey, P McCaughey) pp. 30–33. (Extension Service, Saskatchewan Agriculture and Food: Saskatchewan, Canada)
Eckard RJ, Chapman DF, White RE (2007) Nitrogen balances in temperate perennial grass and clover dairy pastures in south-eastern Australia. Australian Journal of Agricultural Research 58, 1167–1173.
| Nitrogen balances in temperate perennial grass and clover dairy pastures in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVentrbJ&md5=15627a32b7d8740fa5419df750c57c83CAS |
Engin M, Sprent J (1973) Effects of water stress on growth and nitrogen-fixing activity of Trifolium repens. New Phytologist 72, 117–126.
| Effects of water stress on growth and nitrogen-fixing activity of Trifolium repens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXpsV2hsA%3D%3D&md5=863f34e6a71f8c7765512e54ace9d385CAS |
Garg N, Geetanjali (2007) Symbiotic nitrogen fixation in legume nodules: process and signaling. A review. Agronomy for Sustainable Development 27, 59–68.
| Symbiotic nitrogen fixation in legume nodules: process and signaling. A review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlt1Grtr8%3D&md5=6c3a9737ae84f4a28e82cd23c7d18ee2CAS |
Gault RR, Peoples MB, Turner GL, Lilley D, Brockwell J, Bergersen FJ (1995) Nitrogen fixation by irrigated lucerne during the first three years after establishment. Australian Journal of Agricultural Research 46, 1401–1425.
| Nitrogen fixation by irrigated lucerne during the first three years after establishment.Crossref | GoogleScholarGoogle Scholar |
Gerard PJ (2001) Dependence of Sitona lepidus (Coleoptera: Curculionidae) larvae on abundance of white clover Rhizobium nodules. Bulletin of Entomological Research 91, 149–152.
Gibson AH, Curnow B, Bergersen FJ, Brockwell J, Robinson C (1975) Studies of field populations of Rhizobium: effectiveness of strains of Rhizobium trifolii associated with Trifolium subterraneum L. pastures in southeastern Australia. Soil Biology & Biochemistry 7, 95–102.
| Studies of field populations of Rhizobium: effectiveness of strains of Rhizobium trifolii associated with Trifolium subterraneum L. pastures in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Gourley C, Powell J, Dougherty W, Weaver D (2007) Nutrient budgeting as an approach to improve nutrient management on Australian dairy farms. Australian Journal of Experimental Agriculture 47, 1064–1074.
| Nutrient budgeting as an approach to improve nutrient management on Australian dairy farms.Crossref | GoogleScholarGoogle Scholar |
Gourley C, Dougherty W, Aarons S, Hannah S (2010) ‘Accounting for nutrients on Australian dairy farms.’ (DPI Victoria: Melbourne)
Graham M (2008) Biophysical modelling and performance measurement. In ‘AARES 52nd Annual Conference’. Canberra. p. 25. (Australian Agricultural and Resource Economics Society: Canberra)
Harris S, Clark D, Auldist M, Waugh C, Laboyre P (1997) Optimum white clover content for dairy pastures. Proceedings of the New Zealand Grassland Association 59, 29–33.
Hatch DJ, Macduff JH (1991) Concurrent rates of N2 fixation, nitrate and ammonium uptake by white clover in response to growth at different root temperatures. Annals of Botany 67, 265–274.
Haynes RJ, Williams PH (1993) Nutrient cycling and soil fertility in the grazed pasture ecosystem. Advances in Agronomy 49, 119–199.
| Nutrient cycling and soil fertility in the grazed pasture ecosystem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXltlygs7Y%3D&md5=d67b75ea160c662c7290c715a00073f3CAS |
Hill MJ, Donald GE (1998) ‘Australian Temperate Pastures Database.’ (CD-ROM) (CSIRO: Canberra)
Høgh-Jensen H, Loges R, Jørgensen FV, Vinther FP, Jensen ES (2004) An empirical model for quantification of symbiotic nitrogen fixation in grass-clover mixtures. Agricultural Systems 82, 181–194.
| An empirical model for quantification of symbiotic nitrogen fixation in grass-clover mixtures.Crossref | GoogleScholarGoogle Scholar |
Howieson J, Ballard R (2004) Optimising the legume symbiosis in stressful and competitive environments within southern Australia – some contemporary thoughts. Soil Biology & Biochemistry 36, 1261–1273.
| Optimising the legume symbiosis in stressful and competitive environments within southern Australia – some contemporary thoughts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXls1Wmtr8%3D&md5=db4494201ddac0f88d0044de14c64994CAS |
Howieson JG, Ewing MA, Thorn CW, Revell CK (1991) Increased yield in annual species of Medicago grown in acidic soil in response to inoculation with acid tolerant Rhizobium meliloti. In ‘Plant–soil interactions at low pH’. (Ed. RJ Wright) pp. 589–595. (Kluwer Academic Publishers: Dordrecht, The Netherlands)
Jarvis S (1993) Nitrogen cycling and losses from dairy farms. Soil Use and Management 9, 99–104.
| Nitrogen cycling and losses from dairy farms.Crossref | GoogleScholarGoogle Scholar |
Jarvis SC, Scholefield D, Pain B (1995) Nitrogen cycling in grazing systems. In ‘Nitrogen fertilization and the environment’. (Ed. PE Bacon) pp. 381–419. (Marcel Dekker Inc.: New York)
Johnson I (2005) ‘Simulation models in agriculture and plant science.’ (IMJ Consultants Pty Ltd: Melbourne) Available at: www.imj.com.au/consultancy/wfsat/wfsat.html
Johnson IR, Lodge GM, White RE (2003) The Sustainable Grazing Systems Pasture Model: description, philosophy and application to the SGS National Experiment. Australian Journal of Experimental Agriculture 43, 711–728.
| The Sustainable Grazing Systems Pasture Model: description, philosophy and application to the SGS National Experiment.Crossref | GoogleScholarGoogle Scholar |
Johnson I, Chapman D, Snow V, Eckard R, Parsons A, Lambert M, Cullen B (2008) DairyMod and EcoMod: biophysical pastoral simulation models for Australia and New Zealand. Australian Journal of Experimental Agriculture 48, 621–631.
| DairyMod and EcoMod: biophysical pastoral simulation models for Australia and New Zealand.Crossref | GoogleScholarGoogle Scholar |
Jørgensen FV, Ledgard SF (1997) Contribution from stolons and roots to estimates of the total amount of N2 fixed by white clover (Trifolium repens L.). Annals of Botany 80, 641–648.
| Contribution from stolons and roots to estimates of the total amount of N2 fixed by white clover (Trifolium repens L.).Crossref | GoogleScholarGoogle Scholar |
Kelly B, O’Brien G (1992) Management of legumes for high herbage production. Proceedings of the Australian Society of Animal Production 19, 343–345.
Kelly K, Mason W, Simpson R (1989) Effects of irrigation on the productivity of white clover, red clover and lucerne in northern Victoria. In ‘5th Australian Agronomy Conference’. Perth, W. Aust. (Ed. MW Perry) p. 601. (Australian Society of Agronomy: Perth)
Kelly KB, Stockdale CR, Mason WK (2005) Effects of initial sowing rate and subsequent grazing management on the growth and clover content of irrigated white clover–perennial ryegrass swards in northern Victoria. Australian Journal of Experimental Agriculture 45, 1595–1602.
| Effects of initial sowing rate and subsequent grazing management on the growth and clover content of irrigated white clover–perennial ryegrass swards in northern Victoria.Crossref | GoogleScholarGoogle Scholar |
Khan DF, Peoples M, Schwenke G, Felton WL, Chen D, Herridge DF (2003) Effects of below-ground nitrogen on N balances of field-grown fababean, chickpea and barley. Australian Journal of Agricultural Research 54, 333–340.
| Effects of below-ground nitrogen on N balances of field-grown fababean, chickpea and barley.Crossref | GoogleScholarGoogle Scholar |
Kleinman P, Soder K (2008) Impact of hybrid dairy systems on air, soil and water quality: focus on nitrogen and phosphorus cycling. In ‘Environmental impacts of pasture-based farming’. (Ed. R McDowell) pp. 248–276. (CAB International: Wallingford, UK)
Ledgard SF (2001) Nitrogen cycling in low input legume-based agriculture, with emphasis on legume/grass pastures. Plant and Soil 228, 43–59.
| Nitrogen cycling in low input legume-based agriculture, with emphasis on legume/grass pastures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtlWkurw%3D&md5=436e29407d019354ef6ca8b7dae8b33dCAS |
Ledgard SF, Steele KW (1992) Biological nitrogen fixation in legume/grass pastures. Plant and Soil 141, 137–153.
| Biological nitrogen fixation in legume/grass pastures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38Xks1KmsLY%3D&md5=6451b3e238015018a2e73c6fe096184fCAS |
Ledgard SF, Brier GJ, Upsdell MP (1990) Effect of clover cultivar on production and nitrogen fixation in clover ryegrass swards under dairy cow grazing. New Zealand Journal of Agricultural Research 33, 243–249.
Ledgard SF, Sprosen MS, Steele KW (1996) Nitrogen fixation by nine white clover cultivars in grazed pasture, as affected by nitrogen fertilization. Plant and Soil 178, 193–203.
| Nitrogen fixation by nine white clover cultivars in grazed pasture, as affected by nitrogen fertilization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XitVahs7Y%3D&md5=2fa062e12f84f6744cc450e279508f34CAS |
Ledgard SF, Penno JW, Sprosen MS (1999) Nitrogen inputs and losses from clover/grass pastures grazed by dairy cows, as affected by nitrogen fertilizer application. The Journal of Agricultural Science 132, 215–225.
| Nitrogen inputs and losses from clover/grass pastures grazed by dairy cows, as affected by nitrogen fertilizer application.Crossref | GoogleScholarGoogle Scholar |
Ledgard SF, Sprosen MS, Penno JW, Rajendram GS (2001) Nitrogen fixation by white clover in pastures grazed by dairy cows: temporal variation and effects of nitrogen fertilization. Plant and Soil 229, 177–187.
| Nitrogen fixation by white clover in pastures grazed by dairy cows: temporal variation and effects of nitrogen fertilization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXit1aqu7o%3D&md5=d0bf77ce3176c2369cdc33f81f3143eaCAS |
Ledgard S, Schils R, Eriksen J, Luo J (2009) Environmental impacts of grazed clover/grass pastures. Irish Journal of Agricultural and Food Research 48, 209–226.
Ledgard S, Boyes M, Brentrup F (2011) ‘Life cycle assessment of local and imported fertilisers used on New Zealand farms.’ (Fertilizer and Lime Research Centre: Palmerston North)
Lemerle C, Stockdale C, Moate P, Rogers G (1992) Use of supplements with legumes. Proceedings of the Australian Society of Animal Production 19, 347–349.
Liu Y, Wu L, Baddeley JA, Watson CA (2010) Models of biological nitrogen fixation of legumes. A review. Agronomy for Sustainable Development 2, 883–905.
Mason W, Kelly K, Blaikie S, Stockdale R (1987) New directions for irrigated pastures. In ‘Proceedings of the 4th Australian Agronomy Conference: Responding to Change’. (Ed. TG Reeves) pp. 100–117. (Australian Society of Agronomy: Melbourne)
McKenzie FR, Jacobs JL, Riffkin P, Kearney G (1998) Influence of single applications of nitrogen on white clover nitrogen fixation in autumn and winter dairy pastures in south-west Victoria, Australia. African Journal of Range & Forage Science 15, 1–6.
| Influence of single applications of nitrogen on white clover nitrogen fixation in autumn and winter dairy pastures in south-west Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
McKeon G, Rickert K, Ash A, Cooksley D, Scattini W (1982) Pasture production model. Proceedings of the Australian Society for Animal Production 14, 201–204.
McLeish LJ, Berg GN, Hinch JM, Nambiar LV, Norton MR (1997) Plant parasitic nematodes in white clover and soil from white clover pastures in Australia. Australian Journal of Experimental Agriculture 37, 75–82.
| Plant parasitic nematodes in white clover and soil from white clover pastures in Australia.Crossref | GoogleScholarGoogle Scholar |
McNeill AM, Zhu C, Fillery I (1997) Use of in situ 15N-labelling to estimate the total below-ground nitrogen of pasture legumes in intact plant-soil systems. Australian Journal of Agricultural Research 48, 295–304.
| Use of in situ 15N-labelling to estimate the total below-ground nitrogen of pasture legumes in intact plant-soil systems.Crossref | GoogleScholarGoogle Scholar |
Menneer J, Ledgard S, McLay C, Silvester W (2001) What impact does dairy cow pugging have on clover N2 fixation and long-term farm production? Proceedings of the New Zealand Grassland Association 63, 63–67.
Menneer JC, Ledgard S, McLay C, Silvester W (2004) The impact of grazing animals on N2 fixation in legume-based pastures and management options for improvement. In ‘Advances in agronomy’. pp. 181–241. (Academic Press: New York)
Moore AD, Donnelly JR, Freer M (1997) GRAZPLAN: decision support systems for Australian grazing enterprises. III. Pasture growth and soil moisture submodels, and the GrassGro DSS. Agricultural Systems 55, 535–582.
| GRAZPLAN: decision support systems for Australian grazing enterprises. III. Pasture growth and soil moisture submodels, and the GrassGro DSS.Crossref | GoogleScholarGoogle Scholar |
Mundy G (1987) The nitrogen nutrition of irrigated perennial pasture during spring. In ‘Nitrogen cycling in temperate agricultural systems’. (Eds P Bacon, J Evans, R Storrier, A Taylor) pp. 185–190. (Australian Society of Soil Science: Griffiths, NSW)
Mundy GN, Jones HR, Mason WK (1988) Nitrogen fixation activity by white clover pastures during flood irrigation cycles. Australian Journal of Agricultural Research 39, 409–414.
Munn KJ, Evans J, Chalk PM, Morris SG, Whatmuff M (1997) Symbiotic effectiveness of Rhizobium trifolii and mineralisation of legume nitrogen in response to past amendment of a soil with sewage sludge. Journal of Sustainable Agriculture 11, 23–37.
| Symbiotic effectiveness of Rhizobium trifolii and mineralisation of legume nitrogen in response to past amendment of a soil with sewage sludge.Crossref | GoogleScholarGoogle Scholar |
Munns DN (1965a) Interactions of lime with nitrogen and phosphate on growth of Medicago sativa and Trifolium subterraneum. Australian Journal of Agricultural Research 16, 733–742.
| Interactions of lime with nitrogen and phosphate on growth of Medicago sativa and Trifolium subterraneum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF28XntFGr&md5=aafe4d0b10e0e980d83f0377b883f34cCAS |
Munns DN (1965b) Soil acidity and growth of a legume I. Interactions of lime with nitrogen and phosphate on growth of Medicago sativa L. and Trifolium subterraneum L. Australian Journal of Agricultural Research 16, 733–741.
| Soil acidity and growth of a legume I. Interactions of lime with nitrogen and phosphate on growth of Medicago sativa L. and Trifolium subterraneum L.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF28XntFGr&md5=aafe4d0b10e0e980d83f0377b883f34cCAS |
Nash D, Barlow K (2008) Impacts of irrigated dairying on the environment. In ‘Environmental impacts of pasture-based farming’. (Ed. R McDowell) pp. 232–248. (CAB International: Wallingford, UK)
Neal JS, Fulkerson WJ, Lawrie R, Barchia I (2009) Difference in yield and persistence among perennial forages used by the dairy industry under optimum and deficit irrigation. Crop & Pasture Science 60, 1071–1087.
| Difference in yield and persistence among perennial forages used by the dairy industry under optimum and deficit irrigation.Crossref | GoogleScholarGoogle Scholar |
Ostrowski H (1972) White clover (Trifolium repens) in sub-tropical Australia – a review. Tropical Grasslands 6, 97–106.
Pakrou N, Dillon P (2000) Key processes of the nitrogen cycle in an irrigated and a non-irrigated grazed pasture. Plant and Soil 224, 231–250.
| Key processes of the nitrogen cycle in an irrigated and a non-irrigated grazed pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXovV2hs74%3D&md5=2cb3922d80d50632a8dcf50b8713b29eCAS |
Parsons AJ, Orr RJ, Penning PD, Lockyer DR, Ryden JC (1991) Uptake, cycling and fate of nitrogen in grass-clover swards continuously grazed by sheep. Journal of Agricultural Science, Cambridge 116, 47–61.
| Uptake, cycling and fate of nitrogen in grass-clover swards continuously grazed by sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXitVKjs70%3D&md5=9e11f6bf3e293c275c09289dda0dd6efCAS |
Pate JS, Unkovich MJ (1999) Measuring symbiotic nitrogen fixation – case studies of natural and agricultural ecosystems in a Western Australian setting. In ‘Advances in physiological plant ecology’. (Eds MC Press, JD Scholes, MG Barker). (Blackwell Scientific: Oxford, UK)
Paton D (1957) Rhizobium strain trials on clovers in Tasmania 1955/56. Rhizobium Newsletter 2, 19–20.
Pearson CJ, Brown R, Collins WJ, Archer KA, Wood MS, Petersen C, Bootle B (1997) An Australian temperate pastures database. Australian Journal of Agricultural Research 48, 453–465.
| An Australian temperate pastures database.Crossref | GoogleScholarGoogle Scholar |
Peoples M, Baldock J (2001) Nitrogen dynamics of pastures: nitrogen fixation inputs, the impact of legumes on soil nitrogen to Australian farming systems. Australian Journal of Experimental Agriculture 41, 327–346.
| Nitrogen dynamics of pastures: nitrogen fixation inputs, the impact of legumes on soil nitrogen to Australian farming systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkt1Crtrw%3D&md5=0ff51c42b388da7654025c9d4c32eea8CAS |
Peoples MB, Ladha JK, Herridge DF (1995) Enhancing legume N2 fixation through plant and soil management. Plant and Soil 174, 83–101.
| Enhancing legume N2 fixation through plant and soil management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXotFals7k%3D&md5=4e26377ea7e303493bd08866e0cbb8a3CAS |
Peoples MB, Gault RR, Scammell GJ, Dear BS, Virgona J, Sandral G, Paul J, Wolfe EC, Angus JF (1998) Effect of pasture management on the contributions of fixed N to the N economy of ley-farming systems. Australian Journal of Agricultural Research 49, 459–474.
| Effect of pasture management on the contributions of fixed N to the N economy of ley-farming systems.Crossref | GoogleScholarGoogle Scholar |
Peoples MB, Bowman A, Gault R, Herridge D, McCallum M, McCormick K, Norton R, Rochester I, Scammell G, Schwenke G (2001) Factors regulating the contributions of fixed nitrogen by pasture and crop legumes to different farming systems of eastern Australia. Plant and Soil 228, 29–41.
| Factors regulating the contributions of fixed nitrogen by pasture and crop legumes to different farming systems of eastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtlWkur8%3D&md5=1d765b375b1022d4190d5a9e0f2f5ee9CAS |
Peoples M, Unkovich M, Herridge D (2009) Measuring symbiotic nitrogen fixation by legumes. In ‘Nitrogen fixation in crop production’. (Eds D Emerich, H Krishnan) pp. 125–170. (American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI)
Pulsford D, Bullard G (1997) Genetic resources research – a perspective from the root-nodule bacteria industry. Tropical Grasslands 31, 300–303.
Richardson AE, Simpson RJ (1989) Acid-tolerance and symbiotic effectiveness of Rhizobium trifolii associated with a Trifolium subterraneum L.-based pasture growing in an acid soil. Soil Biology & Biochemistry 21, 87–96.
| Acid-tolerance and symbiotic effectiveness of Rhizobium trifolii associated with a Trifolium subterraneum L.-based pasture growing in an acid soil.Crossref | GoogleScholarGoogle Scholar |
Ridley AM, Mele PM, Beverly CR (2004) Legume-based farming in Southern Australia: developing sustainable systems to meet environmental challenges. Soil Biology & Biochemistry 36, 1213–1221.
| Legume-based farming in Southern Australia: developing sustainable systems to meet environmental challenges.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXls1Wmsbg%3D&md5=0db456704b3c86609fe685d5b06edab9CAS |
Riffkin P, Quigley P, Cameron F (1997) ‘Improving the white clover feedbase by optimising nitrogen fixation.’ (Dairy Research & Development Corporation: Melbourne)
Riffkin P, Cameron F, Kearney G, Quigley P, Gault R, Peoples M, Thies J (1999a) Factors associated with biological nitrogen fixation in dairy pastures in south-western Victoria. Australian Journal of Agricultural Research 50, 261–272.
| Factors associated with biological nitrogen fixation in dairy pastures in south-western Victoria.Crossref | GoogleScholarGoogle Scholar |
Riffkin P, Quigley P, Cameron F, Peoples M, Thies J (1999b) Annual nitrogen fixation in grazed dairy pastures in south-western Victoria. Australian Journal of Agricultural Research 50, 273–281.
| Annual nitrogen fixation in grazed dairy pastures in south-western Victoria.Crossref | GoogleScholarGoogle Scholar |
Robertson M, Carberry P, Huth N, Turpin J, Probert M, Poulton P, Bell M, Wright G, Yeates S, Brinsmead R (2002) Simulation of growth and development of diverse legume species in APSIM. Australian Journal of Agricultural Research 53, 429–446.
| Simulation of growth and development of diverse legume species in APSIM.Crossref | GoogleScholarGoogle Scholar |
Rochette P, Janzen H (2005) Towards a revised coefficient for estimating N2O emissions from legumes. Nutrient Cycling in Agroecosystems 73, 171–179.
| Towards a revised coefficient for estimating N2O emissions from legumes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1agsbbJ&md5=e89f4a64d85ca7f7f1a2a10f021a4d78CAS |
Russelle M (2008) Biological dinitrogen fixation in agriculture. In ‘Nitrogen in agricultural systems’. (Eds JS Schepers, W Raun) pp. 281–360. (ASA, CSSA, SSSA: Madison, WI)
Ryle GJA, Powell CE, Timbrell MK, Gordon AJ (1989) Effect of temperature on nitrogenase activity in white clover. Journal of Experimental Botany 40, 733–739.
| Effect of temperature on nitrogenase activity in white clover.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXhvFShsw%3D%3D&md5=847c26a192223ebfe382198ce9d81d59CAS |
Sanford P, Pate JS, Unkovich MJ, Thompson AN (1995) Nitrogen fixation in grazed and ungrazed subterranean clover pasture in south-west Australia assessed by the 15N natural abundance technique. Australian Journal of Agricultural Research 46, 1427–1443.
| Nitrogen fixation in grazed and ungrazed subterranean clover pasture in south-west Australia assessed by the 15N natural abundance technique.Crossref | GoogleScholarGoogle Scholar |
Schulze J (2004) How are nitrogen fixation rates regulated in legumes? Journal of Plant Nutrition and Soil Science 167, 125–137.
| How are nitrogen fixation rates regulated in legumes?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjsFGlsbw%3D&md5=9a4fb7d7f6c28df1c8e15546774671f3CAS |
Schwinning S, Parsons AJ (1996) Analysis of the coexistence mechanisms for grasses and legumes in grazing systems. Journal of Ecology 84, 799–813.
| Analysis of the coexistence mechanisms for grasses and legumes in grazing systems.Crossref | GoogleScholarGoogle Scholar |
Sinclair T (1986) Water and nitrogen limitations in soybean grain production I. Model development. Field Crops Research 15, 125–141.
| Water and nitrogen limitations in soybean grain production I. Model development.Crossref | GoogleScholarGoogle Scholar |
Sinclair TR, Seligman NG (1996) Crop modeling: from infancy to maturity. Agronomy Journal 88, 698–704.
| Crop modeling: from infancy to maturity.Crossref | GoogleScholarGoogle Scholar |
Smith CJ, Chalk PM, Noble CL, Prendergast JB, Robertson F (1993) Nitrogen fixation in a white clover-grass pasture irrigated with saline groundwater. Irrigation Science 13, 189–194.
| Nitrogen fixation in a white clover-grass pasture irrigated with saline groundwater.Crossref | GoogleScholarGoogle Scholar |
Soussana JF, Minchin FR, Macduff JH, Raistrick N, Abberton MT, Michaelson-Yeates TPT (2002) A simple model of feedback regulation for nitrate uptake and N2 fixation in contrasting phenotypes of white clover. Annals of Botany 90, 139–147.
| A simple model of feedback regulation for nitrate uptake and N2 fixation in contrasting phenotypes of white clover.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlvFOht7w%3D&md5=0b3b06c5d5e91f4b0c72220234c09ab6CAS |
Sprent J (1971) Effects of water stress on nitrogen fixation in root nodules. Plant and Soil 35, 225–228.
| Effects of water stress on nitrogen fixation in root nodules.Crossref | GoogleScholarGoogle Scholar |
Sprent J, Gallacher A (1976) Anaerobisis in soybean root nodules under water stress. Soil Biology & Biochemistry 8, 317–320.
| Anaerobisis in soybean root nodules under water stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XltF2ltLc%3D&md5=91b936a935f10dd93049d1eb9e9b571fCAS |
Streeter J (1988) Inhibition of legume nodule formation and N2 fixation by nitrate. Critical Reviews in Plant Sciences 7, 1–23.
| Inhibition of legume nodule formation and N2 fixation by nitrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXnt1CgtQ%3D%3D&md5=a8525d2afe5dc81ae8383c17b1e6f8a0CAS |
Svenning MM, MacDuff JH (1996) Low root temperature retardation of the mineral nitrogen induced decline in N2 fixation by a northern ecotype of white clover. Annals of Botany 77, 615–621.
| Low root temperature retardation of the mineral nitrogen induced decline in N2 fixation by a northern ecotype of white clover.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xkt1Oltrw%3D&md5=5c8270f17a34792a3a6b5b39ebc39fb2CAS |
Thornley JHM (2001) Simulating grass-legume dynamics: a phenomenological submodel. Annals of Botany 88, 905–913.
| Simulating grass-legume dynamics: a phenomenological submodel.Crossref | GoogleScholarGoogle Scholar |
Unkovich MJ, Pate JS (1998) Symbiotic effectiveness and tolerance to early season nitrate availability in indigenous populations of subterranean clover rhizobia from SW Australian pastures. Soil Biology & Biochemistry 30, 1435–1443.
| Symbiotic effectiveness and tolerance to early season nitrate availability in indigenous populations of subterranean clover rhizobia from SW Australian pastures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXkslCmtr8%3D&md5=bcf8873c40ad2d062ed6c1f86db58620CAS |
Unkovich MJ, Sanford P, Pate JS (1996) Nodulation and nitrogen fixation by subterranean clover in acid soils as influenced by lime application, toxic aluminium, soil mineral N, and competition from annual ryegrass. Soil Biology & Biochemistry 28, 639–648.
| Nodulation and nitrogen fixation by subterranean clover in acid soils as influenced by lime application, toxic aluminium, soil mineral N, and competition from annual ryegrass.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XjvF2it70%3D&md5=663dd9b0761a9346ddd68fad6aff961bCAS |
Unkovich MJ, Pate JS, Sanford P (1997) Nitrogen fixation by annual legumes in Australian Mediterranean agriculture. Australian Journal of Agricultural Research 48, 267–293.
| Nitrogen fixation by annual legumes in Australian Mediterranean agriculture.Crossref | GoogleScholarGoogle Scholar |
Unkovich MJ, Sanford P, Pate JS, Hyder M (1998) Effects of grazing on plant and soil nitrogen relations of pasture-crop rotations. Australian Journal of Agricultural Research 49, 475–485.
| Effects of grazing on plant and soil nitrogen relations of pasture-crop rotations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXisFGqtrk%3D&md5=cdf35caa901fb5325bb31eff4826f92cCAS |
Unkovich M, Herridge DF, Peoples MB, Boddey RM, Cadisch G, Giller K, Alves B, Chalk PM (2008) ‘Measuring plant-associated nitrogen fixation in agricultural systems.’ ACIAR Monograph No.136. (Australian Centre for International Agricultural Research: Canberra)
Unkovich M, Baldock J, Peoples M (2010) Prospects and problems of simple linear models for estimating symbiotic N2 fixation by crop and pasture legumes. Plant and Soil 329, 75–89.
| Prospects and problems of simple linear models for estimating symbiotic N2 fixation by crop and pasture legumes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtFGluro%3D&md5=c05ee242ea3d19e2913b94d0648d1115CAS |
Ward G, Quigley P (1992) The botanical composition of high-rainfall pastures in south-west Victoria. In ‘Looking back: planning ahead. Proceedings of the 6th Australian Agronomy Conference’. (Eds K Hutchinson, P Vickery) p. 530. (Australian Society of Agronomy: Armidale)
Waters L (1957) Rhizobium strain trials at Lismore 1956. Rhizobium Newsletter 2, 19–20.
Watkin ELJ, O’Hara GW, Howieson JG, Glenn AR (2000) Identification of tolerance to soil acidity in inoculant strains of Rhizobium leguminosarum bv. trifolii. Soil Biology & Biochemistry 32, 1393–1403.
| Identification of tolerance to soil acidity in inoculant strains of Rhizobium leguminosarum bv. trifolii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmsVWgt7Y%3D&md5=463a7d725ddc815c61f44ae05b565bf9CAS |
Whitehead DC (1995) ‘Grassland nitrogen.’ (CAB International: Wallingford, UK)
Wichern F, Eberhardt E, Mayer J, Joergensen RG, Müller T (2008) Nitrogen rhizodeposition in agricultural crops: methods, estimates and future prospects. Soil Biology & Biochemistry 40, 30–48.
| Nitrogen rhizodeposition in agricultural crops: methods, estimates and future prospects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtF2iu7bJ&md5=745fcb739e46e0e7ebb0f36017de1d03CAS |
Woodfield D, Clark D (2009) Do forage legumes have a role in modern dairy farming systems? Irish Journal of Agricultural and Food Research 48, 137–147.
Wu L, McGechan MB (1999) Simulation of nitrogen uptake, fixation and leaching in a grass/white clover mixture. Grass and Forage Science 54, 30–41.
| Simulation of nitrogen uptake, fixation and leaching in a grass/white clover mixture.Crossref | GoogleScholarGoogle Scholar |
Yang H, Unkovich MJ, McNeill AM, Wang X (2011) Symbiotic N2 fixation and nitrate utilisation in irrigated lucerne (Medicago sativa) systems. Biology and Fertility of Soils 47, 377–385.
| Symbiotic N2 fixation and nitrate utilisation in irrigated lucerne (Medicago sativa) systems.Crossref | GoogleScholarGoogle Scholar |
Yeates GW, Stirling GR (2008) Regional patterns among soil nematode assemblages in Australasian pastures and effects of management practices. Australasian Plant Pathology 37, 298–307.
| Regional patterns among soil nematode assemblages in Australasian pastures and effects of management practices.Crossref | GoogleScholarGoogle Scholar |


 and lucerne
and lucerne  grown in Australia. Data from
grown in Australia. Data from 