Insights into fighting against blackleg disease of Brassica napus in Canada
Xuehua Zhang A and W. G. Dilantha Fernando A BA Department of Plant Science, University of Manitoba, 66 Dafoe Road, Winnipeg, MB, R3T 2N2, Canada.
B Corresponding author. Email: Dilantha.Fernando@umanitoba.ca
Crop and Pasture Science 69(1) 40-47 https://doi.org/10.1071/CP16401
Submitted: 25 October 2016 Accepted: 15 June 2017 Published: 15 August 2017
Journal compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Blackleg disease, caused by the ascomycete fungal pathogen Leptosphaeria maculans, is a devastating disease of canola (Brassica napus) in Australia, Canada and Europe. Although cultural strategies such as crop rotation, fungicide application, and tillage are adopted to control the disease, the most promising disease control strategy is the utilisation of resistant canola varieties. However, field populations of L. maculans display a high evolutionary potential and are able to overcome major resistance genes within a few years, making disease control relying on resistant varieties challenging. In the early 1990s, blackleg resistance gene Rlm3 was introduced into Canadian canola varieties and provided good resistance against the fungal populations until the early 2000s, when moderate to severe blackleg outbreaks were observed in some areas across western Canada. However, the breakdown of Rlm3 resistance was not reported until recently, based on studies on R genes present in Canadian canola varieties and the avirulence allele frequency in L. maculans populations in western Canada. The fact that Rlm3 was overcome by the evolution of fungal populations demands canola breeding programs in Canada to be prepared to develop canola varieties with diversified and efficient R genes. In addition, frequent monitoring of fungal populations can provide up-to-date guidance for proper resistance genes deployment. This literature review provides insights into the outbreaks and management of blackleg disease in Canada.
Additional keywords: avirulence, Leptosphaeria maculans, resistance.
Introduction
During the past three decades, the cultivation and production of canola (Brassica napus, oilseed rape) have grown rapidly and canola has become the second most important oilseed crop, after soybean, with an estimated production of 67.91 million tonnes globally in 2016 (USDA 2017). In Canada, canola is the number one cash crop, with a production of 18.4 million tonnes in 2016 (Statistics Canada 2016). Canola is mainly cultivated in the western provinces of Saskatchewan, Alberta and Manitoba, with a low to substantial amount of the crop grown in other provinces. Canola contributed $26.7 billion to the Canadian economy in 2016. Annually, Canada exports 90% of canola seeds, oil and meals to ~55 foreign markets worldwide (Canola Council of Canada 2017). In 2015, Canada exported 3.97 million tonnes of canola seeds to China, one of the most important export markets. To ensure continuous growth of the Canadian canola industry, the Canola Council of Canada established a new strategic plan, ‘Keep it Coming 2025’, to encourage an annual production of 26 million tonnes of canola by 2025.
Blackleg caused by the fungal pathogen Leptosphaeria maculans is the most severe disease of canola, causing more than $900 million economic losses per growing season worldwide (Fitt et al. 2008). In western Canada, blackleg caused up to 50% yield losses in individual fields during the 1980s, when blackleg susceptible variety, Westar was widely cultivated (Juska et al. 1997). Following the first wave of major blackleg outbreaks in the Canadian prairies, blackleg-resistant varieties were released in the early 1990s. Until the early 2000s, blackleg disease was well controlled by using resistant varieties and 4-year crop rotations (Kutcher et al. 2013). However, due to the favourable economic returns through canola, many growers adopted 2-year rotations or even grew canola in successive years across the prairies. This led to the erosion of blackleg resistance in some fields since 2002 and became widespread by 2012 (Hwang et al. 2016). This literature review summarises the outbreaks and management of blackleg disease in Canada.
Blackleg disease caused by Leptosphaeria maculans
Until 2001, strains of L. maculans were classified into two pathotypes: the highly virulent, aggressive ‘A’ group strains that cause stem cankers on canola, and the nonaggressive, weakly virulent, ‘B’ group strains that do not cause stem cankers on canola (Williams and Fitt 1999). Later, ‘A’ pathotype isolates were divided into different pathogenicity groups (PG) according to the differential B. napus varieties test, whereas ‘B’ pathotype isolates (PG1 group) were classified as another species, termed L. biglobosa (Shoemaker and Brun 2001; Kuusk et al. 2002; Chen and Fernando 2006). Leptosphaeria biglobosa species were divided into six subclades and a few of them including L. biglobosa ‘canadensis’, L. biglobosa ‘brassicae’ and L. biglobosa ‘thlaspii’ were present in Canada (Mendes-Pereira et al. 2003). To date, these two species have been found to coexist in North America, Australia and Europe, whereas only L. biglobosa has been identified in China (West and Fitt 2005; Fitt et al. 2006; Magyar et al. 2006; Karolewski et al. 2007; Brazauskiene et al. 2011; Zhang et al. 2014).
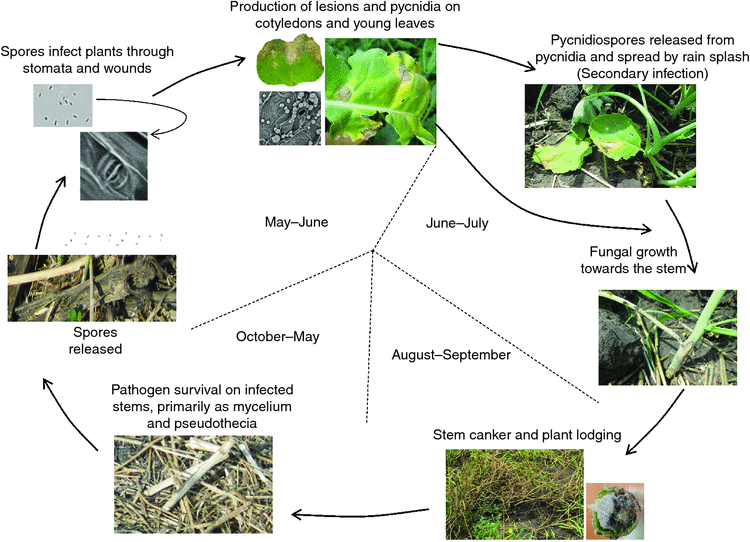
Leptosphaeria maculans has been recorded on crucifers since 1791, but the severe damage to Brassica species was only recorded in the last four decades (Rouxel and Balesdent 2005). The fungus can survive on infected stems or other parts of crop residues for several years in the form of mycelia, pycnidia and pseudothecia (West et al. 2001; Li et al. 2007b). Leptosphaeria maculans is able to attack nearly all parts of the plant, including cotyledons, leaves, stems, roots and pods, and cause leaf lesions and stem cankers (Fig. 1). Leptosphaeria maculans has both sexual and asexual stages on host plants and can either be monocyclic or polycyclic depending on the source of inoculum (Li et al. 2007a). In the case of ascospores as the primary inoculum, the disease is considered as monocyclic. However, the disease may be considered as polycyclic when pycnidiospores are the primary inoculum or as secondary inoculum (Li et al. 2007a). The period of ascospore release varies from region to region and generally coincides with the emergence of young plants (Savage et al. 2013). Ascospores are released in June in western Canada (Guo and Fernando 2005), May in Australia (Khangura et al. 2001) and late September–early October in western and central Europe (Huang et al. 2005). The epidemiology of blackleg differs between continents and regions because of differences in climate, growing season, cultivars and especially fungal populations (West et al. 2001; Fitt et al. 2006). Although the incidence of seed infection by L. maculans and L. biglobosa is relatively low, seed-borne inoculum is a major concern in transporting L. maculans into countries where L. maculans has not been identified, such as China (Fitt et al. 2006; Zhang et al. 2014; Fernando et al. 2016; Van de Wouw et al. 2016a, 2016b).
In Europe, ascospore showers are believed to be the major inoculum (Fitt et al. 2006). In Australia, the major inoculum of blackleg is ascospores, in combination with pycnidiospores (Barbetti 1976; Marcroft et al. 2004). In western Canada, pycnidiospores are the most important sources of inoculum in infection and disease development (Petrie 1995; Guo and Fernando 2005; Ghanbarnia et al. 2011; Dilmaghani et al. 2013). Ascospores are mainly dispersed by wind thus can travel long distances, whereas pycnidiospores can only travel short distances by rain-splash. Therefore, in Australia, the recommended distance between canola fields is more than 400 m as canola plants grown within 400 m are in higher risk of infection than that of more than 400 m (Marcroft et al. 2004). In western Canada, however, the recommended distance between canola fields is at least 50–100 m to reduce the impact of inoculum movement (Guo and Fernando 2005). After harvest, infected plant residues remain in the fields and supply inoculum for the following season. The life cycle of L. maculans in western Canada is illustrated in Fig. 2.
|
Blackleg disease in Canada
The first wave of severe blackleg outbreaks in the 1980s
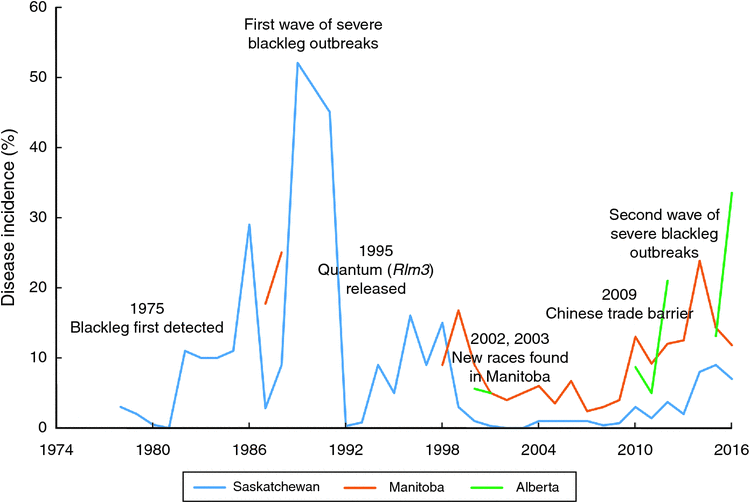
In Canada, L. maculans was first identified in Saskatchewan in 1975 (McGee and Petrie 1978), and later in Manitoba, Alberta and British Columbia (Gugel and Petrie 1992). Prior to the 1970s, only L. biglobosa was identified in Canada and blackleg was not a major concern in rapeseed production. First widespread blackleg disease was observed in 1982, when blackleg caused 6% yield losses in Saskatchewan, with the highest of 56% yield losses in some fields (Juska et al. 1997). Westar, a variety that performed much better than all previously registered varieties but very susceptible to blackleg, was widely cultivated in Canada during 1984 and the late 1980s (Juska et al. 1997). Lack of blackleg management experience combined with the incentives and pressures to achieve higher canola production, Canadian growers adopted tight rotations to grow blackleg susceptible variety Westar. This led to accumulation of infected stubbles in the field and wide spread of severe blackleg outbreaks in the Canadian prairies. In 1987, yield losses from blackleg reached 10% in Alberta. Similarly, blackleg caused 10% yield losses in Manitoba in 1988 (Juska et al. 1997). In 1989, disease incidence of blackleg reached 52% in Saskatchewan (Fig. 3).
|
Fighting against blackleg disease in the early 1990s
When the entire canola industry was threatened by severe blackleg outbreaks in the 1980s, strategies such as the application of cultural practices, development of disease-resistant varieties, and fungicide applications were adopted by growers. Westar was abandoned in 1991 and new varieties lower in yield but resistant to blackleg were released and cultivated in the early 1990s (Kutcher et al. 2010a). Since 1995, many blackleg resistant varieties such as Quantum, Q2, Hi-Q, and Conquest were released. It is now known that most of these varieties carried the same single resistance gene, Rlm3. In 1994, fungicides became available for blackleg control in Canada (Juska et al. 1997). At the same time, cultural practices such as crop rotation, deep tillage, delayed seeding, and seed testing for blackleg infestation were applied in disease control. These strategies largely contributed to the reduced disease incidence and disease severity. For example, in the early 1990s, provincial canola yield losses from blackleg declined to 1% in Alberta (Gugel and Petrie 1992).
Blackleg resistance erosion in Canada
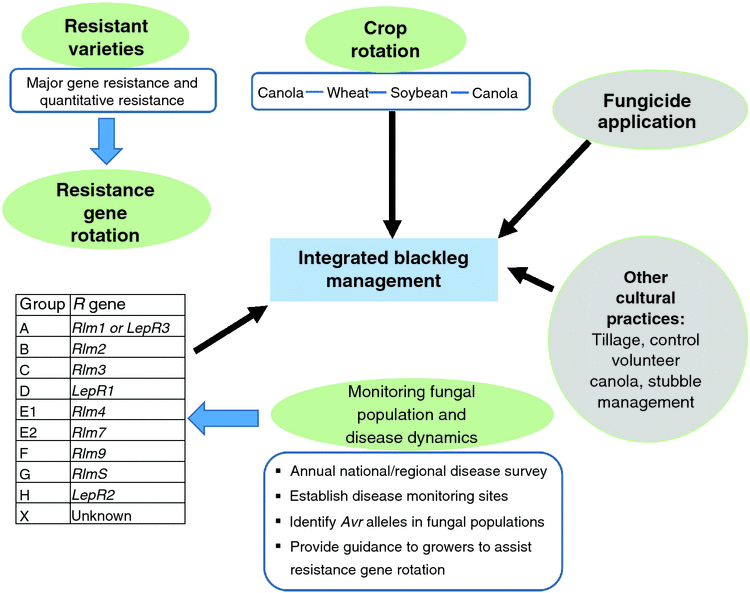
Breeding for blackleg resistance is fundamental to successful disease management (Li and McVetty 2013). In B. napus, there are two types of resistance against blackleg, qualitative resistance (R gene, major gene) mediated by single major genes and quantitative resistance (adult plant resistance) controlled by multiple genetic factors (quantitative trait loci - QTL) (Rimmer 2006; Raman et al. 2013). Although blackleg-resistant varieties have been released since the early 1990s and all commonly grown varieties and high erucic acid rapeseed have moderate to high level of blackleg resistance, the erosion of resistance in some fields was identified in 2002 and 2003, when severe infection was observed in blackleg-resistant varieties. In recent years, Canadian plant disease survey results suggested blackleg incidence is on the rise from 2010 to 2016 (Fig. 3). However, it is difficult to determine which genes have been broken down due to resistance genes and resistance types in these varieties were generally unknown until recently. A study conducted in 2012 revealed the presence of Rlm1, Rlm2, Rlm3, Rlm4, Rlm9, RlmS, LepR1 and LepR2 in Canadian canola varieties, with Rlm3 gene being predominant (Zhang et al. 2016). This study further identified Rlm1, Rlm2 and Rlm3 were the top three most frequently used R genes in Canadian B. napus varieties. In addition, the study also revealed the presence of moderate to high level adult plant resistance in more than 50% of Canadian canola varieties (Zhang et al. 2016). In the fungal populations, however, the frequency of the corresponding avirulence gene of Rlm3, AvrLm3 was very low (Liban et al. 2016; Zhang et al. 2016; W. G. Dilantha Fernando, unpubl. data). More specifically, low frequency of the AvrLm3 allele was observed in 2010 and 2011, followed by a very low frequency or lack of AvrLm3 allele in fungal populations in 2012, 2013, 2014 and 2015 (Liban et al. 2016; Zhang et al. 2016; W. G. Dilantha Fernando, unpubl. data). These findings strongly suggested the erosion of Rlm3 in western Canada.
The race shift in L. maculans populations
Prior to 2005, L. maculans isolates were classified into PG2, PG3, PG4, and PGT based on their interaction phenotypes on a few differential B. napus varieties, including Glacier (Rlm2 and Rlm3), Quinta (Rlm1 and Rlm3), and Westar (no resistance). However, a few limited PG cannot fully illustrate population variations of the pathogen. To better address population variation of L. maculans, a new term, race structure was introduced by Balesdent et al. (2005) to describe population structures of L. maculans populations. To date, at least 14 avirulence (Avr) genes have been identified in L. maculans and a few of them have been cloned (Liban et al. 2016). Knowledge on these Avr genes has largely enabled molecular and phenotypic methods for the analysis of race structures in field L. maculans populations.
The genetic diversity and complexity of the L. maculans population in western Canada has changed over time. Although varieties with moderate to high levels of blackleg resistance were cultivated in the Canadian prairie, a shift in the L. maculans populations became evident in the early 2000s. All isolates collected from Manitoba and Saskatchewan in 1991 belonged to the PG2 group (Kutcher et al. 1993). PG2 isolates remained the most common isolates found in western Canada until the year 2000, but new PGT isolates were identified in isolates collected between 1998 and 2000, and a new PG3 isolate was detected in Manitoba in 1999 (Fernando and Chen 2003; Chen and Fernando 2006; Rimmer 2006). Population structure analysis of L. maculans isolates collected between 1997 and 2005 in western Canada demonstrated high frequency of a few avirulence genes such as AvrLm4, AvrLm6 and AvrLm7 (Kutcher et al. 2010b). Dilmaghani et al. (2009) reported a high frequency of AvrLm3 and AvrLm9 in 2005 and 2006 fungal populations in western Canada. However, the frequency of AvrLm3 and AvrLm9 decreased to a very low level in 2010 and 2011 as reported by Liban et al. (2016).
Since 2012, blackleg incidence in Manitoba and Alberta were more than 10%. In Manitoba, the highest disease incidence was observed in 2014, 23.8% of plants surveyed showed stem canker. Lower level (less than 5%) of blackleg incidence was observed in Saskatchewan until 2013, but it increased since 2014. To better understand the second severe outbreak of blackleg disease, W. G. Dilantha Fernando (unpubl.) conducted a study to assess disease incidence and avirulence allele distribution of L. maculans populations in Manitoba, Canada from 2010 to 2015. Among fungal populations, high frequencies of AvrLm2, AvrLm4, AvrLm5, AvrLm6, AvrLm7, AvrLm11, and AvrLmS alleles were detected, whereas low frequencies or lack of AvrLm1, AvrLm3, AvrLm9, AvrLepR1, and AvrLepR2 alleles were observed. From 2010 to 2015, a decrease in the frequency of AvrLm1, AvrLm2, AvrLm3, AvrLm9, and AvrLepR1 alleles was identified, which indicated defeat of the corresponding R genes. A total of 180 races were identified in 964 isolates, with three major races, AvrLm-2-4-5-6-7-11, AvrLm-2-4-5-6-7-11-S, and AvrLm-1-4-5–6-7-11-(S), accounting for 24.9% of the isolates. The decrease in the frequency of these avirulence alleles could be explained by the strong selection pressure exerted by these R genes in Canadian canola varieties.
Trade barriers due to blackleg disease
Blackleg disease can cause trade barriers on canola seeds exports to major markets. Due to the potential risk of introducing blackleg disease into China, in 2009, restrictions on Canadian canola exports to China was imposed by the Chinese government, resulting in reduced canola exports to China from 3.1 million tonnes in 2009 to 1.5 million tonnes in 2010 (Canola Council of Canada). Following the trade issues triggered by blackleg in 2009, the canola industry and government of Canada have supported many research projects to achieve a science-based solution to mitigate losses and risks from blackleg disease. These research projects covered almost all aspects of the canola supply chain from host resistance (novel resistance genes, defence mechanism), fungal population genetics, and agronomic practices through to seed transportation and processing. In 2016, Canadian and Chinese governments agreed to continue their discussion on a permanent science-based solution for blackleg issues (Canola Council of Canada).
Integrated blackleg management strategies
In spite of the effectiveness of resistance genes in disease control, rapid erosion of blackleg resistance in commercial crops due to the increase in the frequency of the virulent isolates has been reported. In France, Rlm1 resistance was overcome within 5 years of release of Rlm1-carrying varieties (1996–1999) (Rouxel et al. 2003). Similarly, in Australia, ‘sylvestris’ resistance (Rlm1 and LepR3) was lost within 3 years of commercial release in the Eyre Peninsula (Sprague et al. 2006). This is not unusual as there is a typical boom and bust cycle in blackleg resistance under field conditions (Rouxel et al. 2003; Brun et al. 2010; Delourme et al. 2014). The phenomenon that a well performing variety with single major resistance gene is grown over a large area is described as the boom phase of the cycle. For example, in western Canada, a typical boom phase is the early 1990s to the 2000s, when Rlm3-carrying canola varieties were widely grown and blackleg was well controlled by genetic resistance. Extensive use of Rlm3 led to changes in the pathogen population, resulting in the increase in disease severity, or breakdown of the resistance. The bust cycle then comes when the variety was not grown in the field, and the frequency of virulent isolates decrease over time (Brun et al. 2010; Delourme et al. 2014). In western Canada, the finding that Rlm3 was overcome by the evolution of fungal populations further highlighted the high evolutionary potential of the pathogen (Zhang et al. 2016). This is not surprising as L. maculans has a mixed reproduction system, and avirulence genes are located in unstable genomic regions (McDonald and Linde 2002; Soyer et al. 2014).
Foliar fungicide applications have been proven to be of limited value to maintain canola yield (Huang et al. 2011; Liu 2014). A few studies have investigated the effect of fungicide on L. maculans and L. biglobosa, and most of these studies revealed that L. maculans was more sensitive to fungicides than L. biglobosa (Griffiths et al. 2003; Eckert et al. 2010; Huang et al. 2011). Among different L. maculans isolates, variations in sensitivity to QoI fungicides (fungicides with the action mode of action of Quinone outside inhibitor) were observed in Canada (Liu 2014). The timing of fungicide application is crucial in blackleg control as the fungicides are not able to control the disease once the pathogen has reached the stem (Steed et al. 2007; Peng et al. 2012; Liu 2014). Although foliar fungicides have been shown to reduce disease severity and increase yield in blackleg susceptible canola varieties, there is no economic benefit of using fungicide in resistant canola varieties (Bailey et al. 2000; Liu 2014). Reduction of disease with yield gain on MR or R-rated cultivars can only be achieved when there is severe erosion of resistance in a cultivar due to pathogen shifts (from Avr to avr) (Liu 2014). Although foliar fungicide products including pyraclostrobin (Headline®, BASF), propiconazole (Tilt®, Syngenta) and azoxystrobin (Quadris®, Syngenta) are available, growers in western Canada only consider in applying fungicide when the pathogen caused significant production issues (Peng et al. 2012). In Australia, azole-based fungicides were widely used in seed treatments between 2005 and 2014, foliar fungicide (prothioconazole + tebuconazole) for in-crop L. maculans control was not available until 2012. Unlike Canada, the use of a seed-dressing fungicide in Australia has been shown to gain an economic yield benefit (Marcroft and Potter 2008).
R-gene rotations and resistance groups
Marcroft et al. (2012) demonstrated that rotation of R genes can minimise disease pressure by manipulating fungal populations. Since 2012, resistance group(s) based on their R-gene complement has been assigned to all commercial canola varieties in Australia. This information is updated and released biannually to growers in the GRDC Blackleg Management Guide (Van de Wouw et al. 2016b). To understand the performance of each resistance group across canola-growing regions in Australia, disease monitoring sites have been established and assessed for blackleg disease. This allows GRDC to provide a warning to growers if high level of disease severity is observed in the resistant group. Rotations of cultivars with different components of resistance genes have become evidently effective, but it requires the identification of resistance genes in commercial canola cultivars (Marcroft et al. 2012). The combination of major gene resistance and quantitative resistance to L. maculans in canola varieties is able to provide improved durability of blackleg resistance (Brun et al. 2010; Marcroft et al. 2012; Delourme et al. 2014). Similarly, it is possible to apply an R-gene rotation strategy in the Canadian prairie to control blackleg, given the growing understanding and knowledge of host resistance in canola varieties, pathogen avirulence in L. maculans populations, and their interactions. Research scientists and the industry are interested in adopting this strategy to better control the disease, however more efforts are required to develop varieties with diversified R genes and understand Avr alleles in fungal populations. In February 2017, the Western Canada Canola and Rapeseed Recommending Committee adopted this strategy in principle, so seed companies could use a resistance group on their label. If R-gene rotation strategy is available, there is a need to develop an integrated blackleg management strategy to maximise effectiveness of the R-gene rotation strategy (Fig. 4). In this integrated strategy, crop rotation is essential to reduce fungal inoculum, whereas fungicide application and tillage could be taken into consideration in some cases, a prudent R-gene rotation strategy based on good understanding of resistant varieties and fungal population and disease dynamics is the key to successful blackleg disease management.
Conclusions and future prospects
In Canada, the Rlm3 gene has successfully protected the canola industry from blackleg disease during the early 1990s to the early 2000s. However, breakdown of Rlm3 was observed due to the high evolutionary potential and emergence of new races in the blackleg fungal populations. At a recent Western Canada Canola and Rapeseed Recommending Committee meeting in Saskatoon, Canada, a decision was made to introduce new blackleg resistance labels on varieties to introduce an R-gene rotational strategy in Canada. Based on known R genes, and assigned to groups, these labels will offer more detail on a variety’s resistance package. Such labels have successfully been used in other countries helping the growers with less breakdown of resistance in their canola varieties, and allowing the growers and the seed industry to manage the disease through genetics. The authors feel that it is a step forward in the right direction in the reduction of disease caused by L. maculans in canola/rapeseed. Deployment of canola varieties with diversified known R genes or novel resistance genes, and ideally, with the combination of quantitative resistance is of great significance for public and private breeding programs. To facilitate a proper and effective utilisation of R genes in disease control, it is important to monitor R genes in canola varieties and Avr allele frequency in field fungal populations. For the long-term, integrated disease control strategies with the efficient utilisation of resistance genes, R-gene rotation, crop rotation, and fungicide application need to be deployed.
Acknowledgements
The authors wish to thank the Canola Council of Canada, especially Mr Clint Jurke for spearheading the projects that are discussed in the manuscript and for their financial assistance through CARP. The authors also thank SaskCanola for their support through ASP/GF2 projects, and the NSERC Discovery and NSERC-CRD to W.G.D.F. to further enhance our knowledge on the canola-blackleg system.
References
Bailey KL, Johnston AM, Kutcher HR, Gossen BD, Morrall RAA (2000) Managing crop losses from foliar diseases with fungicides, rotation, and tillage in the Saskatchewan Parkland. Canadian Journal of Plant Science 80, 169–175.| Managing crop losses from foliar diseases with fungicides, rotation, and tillage in the Saskatchewan Parkland.Crossref | GoogleScholarGoogle Scholar |
Balesdent MH, Barbetti MJ, Li H, Sivasithamparam K, Gout L, Rouxel T (2005) Analysis of Leptosphaeria maculans race structure in a worldwide collection of isolates. Phytopathology 95, 1061–1071.
| Analysis of Leptosphaeria maculans race structure in a worldwide collection of isolates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVemt7jL&md5=eee2a33366e3706cfb548b921d51b926CAS |
Barbetti MJ (1976) The role of pycnidiospores of Leptosphaeria maculans in the spread of blackleg disease in rape. Australian Journal of Experimental Agriculture and Animal Husbandry 16, 911–914.
| The role of pycnidiospores of Leptosphaeria maculans in the spread of blackleg disease in rape.Crossref | GoogleScholarGoogle Scholar |
Brazauskiene I, Piliponyte A, Petraitiene E, Brazauskas G (2011) Diversity of Leptosphaeria maculans/L. biglobosa species complex and epidemiology of phoma stem canker on oilseed rape in Lithuania. Journal of Plant Pathology 93, 577–585.
Brun H, Chevre AM, Fitt BDL, Powers S, Besnard AL, Ermel M, Huteau V, Marquer B, Eber F, Renard M, Andrivon D (2010) Quantitative resistance increases the durability of qualitative resistance to Leptosphaeria maculans in Brassica napus. New Phytologist 185, 285–299.
| Quantitative resistance increases the durability of qualitative resistance to Leptosphaeria maculans in Brassica napus.Crossref | GoogleScholarGoogle Scholar |
Canola Council of Canada (2017) Industry overview. Available at: www.canolacouncil.org/markets-stats/industry-overview/ (accessed 1 March 2017).
Chen Y, Fernando WGD (2006) Prevalence of pathogenicity groups of Leptosphaeria maculans in western Canada and North Dakota, USA. Canadian Journal of Plant Pathology 28, 533–539.
| Prevalence of pathogenicity groups of Leptosphaeria maculans in western Canada and North Dakota, USA.Crossref | GoogleScholarGoogle Scholar |
Delourme R, Bousset L, Ermel M, Duffé P, Besnard AL, Marquer B, Fudal I, Linglin J, Chadœuf J, Brun H (2014) Quantitative resistance affects the speed of frequency increase but not the diversity of the virulence alleles overcoming a major resistance gene to Leptosphaeria maculans in oilseed rape. Infection, Genetics and Evolution 27, 490–499.
| Quantitative resistance affects the speed of frequency increase but not the diversity of the virulence alleles overcoming a major resistance gene to Leptosphaeria maculans in oilseed rape.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsVOkt70%3D&md5=a655a967b3d83b00df7a2049a900d424CAS |
Dilmaghani A, Balesdent MH, Didier JP, Wu C, Davey J, Barbetti MJ, Rouxel T (2009) The Leptosphaeria maculans-Leptosphaeria biglobosa species complex in the American continent. Plant Pathology 58, 1044–1058.
| The Leptosphaeria maculans-Leptosphaeria biglobosa species complex in the American continent.Crossref | GoogleScholarGoogle Scholar |
Dilmaghani A, Gout L, Moreno-Rico O, Dias JS, Coudard L, Castillo-Torres N, Balesdent MH, Rouxel T (2013) Clonal populations of Leptosphaeria maculans contaminating cabbage in Mexico. Plant Pathology 62, 520–532.
| Clonal populations of Leptosphaeria maculans contaminating cabbage in Mexico.Crossref | GoogleScholarGoogle Scholar |
Eckert MR, Rossall S, Dl B (2010) Effects of fungicides on in vitro spore germination and mycelial growth of the phytopathogens Leptosphaeria maculans and L. biglobosa (phoma stem canker of oilseed rape). Pest Management Science 66, 396–405.
Fernando WGD, Chen Y (2003) First Report on the presence of Leptosphaeria maculans pathogenicity Group-3, the causal agent of Blackleg of canola in Manitoba. Plant Disease 87, 1268
| First Report on the presence of Leptosphaeria maculans pathogenicity Group-3, the causal agent of Blackleg of canola in Manitoba.Crossref | GoogleScholarGoogle Scholar |
Fernando W, Zhang X, Amarasinghe C (2016) Detection of Leptosphaeria maculans and Leptosphaeria biglobosa causing blackleg disease in canola from Canadian canola seed lots and dockage. Plants 5, 12
| Detection of Leptosphaeria maculans and Leptosphaeria biglobosa causing blackleg disease in canola from Canadian canola seed lots and dockage.Crossref | GoogleScholarGoogle Scholar |
Fitt BDL, Brun H, Barbetti MJ, Rimmer SR (2006) World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). European Journal of Plant Pathology 114, 3–15.
| World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus).Crossref | GoogleScholarGoogle Scholar |
Fitt BDL, Hu BC, Li ZQ, Liu SY, Lange RM, Kharbanda PD, Butterworth MH, White RP (2008) Strategies to prevent spread of Leptosphaeria maculans (phoma stem canker) onto oilseed rape crops in China; costs and benefits. Plant Pathology 57, 652–664.
| Strategies to prevent spread of Leptosphaeria maculans (phoma stem canker) onto oilseed rape crops in China; costs and benefits.Crossref | GoogleScholarGoogle Scholar |
Ghanbarnia K, Dilantha Fernando WG, Crow G (2011) Comparison of disease severity and incidence at different growth stages of naturally infected canola plants under field conditions by pycnidiospores of Phoma lingam as a main source of inoculum. Canadian Journal of Plant Pathology 33, 355–363.
| Comparison of disease severity and incidence at different growth stages of naturally infected canola plants under field conditions by pycnidiospores of Phoma lingam as a main source of inoculum.Crossref | GoogleScholarGoogle Scholar |
Griffiths KM, Bacic A, Howlett BJ (2003) Sterol composition of mycelia of the plant pathogenic ascomycete Leptosphaeria maculans. Phytochemistry 62, 147–153.
| Sterol composition of mycelia of the plant pathogenic ascomycete Leptosphaeria maculans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XptlCktLw%3D&md5=8bf0e1dfe70ea3372eb67bbe09dd301fCAS |
Gugel PK, Petrie GA (1992) History, occurrence, impact and control of blackleg of rapeseed. Canadian Journal of Plant Pathology 14, 36–45.
| History, occurrence, impact and control of blackleg of rapeseed.Crossref | GoogleScholarGoogle Scholar |
Guo XW, Fernando WGD (2005) Seasonal and diurnal patterns of spore dispersal by Leptosphaeria maculans from canola stubble in relation to environmental conditions. Plant Disease 89, 97–104.
| Seasonal and diurnal patterns of spore dispersal by Leptosphaeria maculans from canola stubble in relation to environmental conditions.Crossref | GoogleScholarGoogle Scholar |
Huang YJ, Fitt BDL, Jedryczka M, Dakowska S, West JS, Gladders P, Steed JM, Li ZQ (2005) Patterns of ascospore release in relation to phoma stem canker epidemiology in England (Leptosphaeria maculans) and Poland (Leptosphaeria biglobosa). European Journal of Plant Pathology 111, 263–277.
| Patterns of ascospore release in relation to phoma stem canker epidemiology in England (Leptosphaeria maculans) and Poland (Leptosphaeria biglobosa).Crossref | GoogleScholarGoogle Scholar |
Huang YJ, Hood JR, Eckert MR, Stonard JF, Cools HJ, King GJ, Rossall S, Ashworth M, Fitt BDL (2011) Effects of fungicide on growth of Leptosphaeria maculans and L. biglobosa in relation to development of phoma stem canker on oilseed rape (Brassica napus). Plant Pathology 60, 607–620.
| Effects of fungicide on growth of Leptosphaeria maculans and L. biglobosa in relation to development of phoma stem canker on oilseed rape (Brassica napus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVGlt77P&md5=971a84c9dcd7c20af6c98a192b4ccbcbCAS |
Hwang S-F, Strelkov SE, Peng G, Ahmed H, Zhou Q, Turnbull G (2016) Blackleg (Leptosphaeria maculans) severity and yield loss in Canola in Alberta, Canada. Plants 5, 31
| Blackleg (Leptosphaeria maculans) severity and yield loss in Canola in Alberta, Canada.Crossref | GoogleScholarGoogle Scholar |
Juska A, Busch L, Tanaka K (1997) The blackleg epidemic in Canadian rapeseed as a ‘normal agricultural accident’. Ecological Applications 7, 1350–1356.
Karolewski Z, Walczak D, Kosiada T, Lewandowska D (2007) Occurrence of Leptosphaeria maculans and L biglobosa in oilseed rape leaves with different symptoms of stem canker. Phytopathologia Polonica 44, 43–50.
Khangura RK, Barbetti MJ, Salam MU, Diggle AJ (2001) Maturation of pseudothecia and ascospore discharge by blackleg fungus on canola residues in Western Australia: preliminary results from field observations. In ‘Proceedings 12th Australian Research Assembly on Brassicas Congress’. (Ed. S Marcroft) pp. 87–91. (Organising Committee of ARAB: Geelong, Vic.)
Kutcher HR, van den Berg CGJ, Rimmer SR (1993) Variation in pathogenicity of Leptosphaeria maculans on Brassica spp based on cotyledon and stem reactions. Canadian Journal of Plant Pathology 15, 253–258.
| Variation in pathogenicity of Leptosphaeria maculans on Brassica spp based on cotyledon and stem reactions.Crossref | GoogleScholarGoogle Scholar |
Kutcher HR, Yu F, Brun H (2010a) Improving blackleg disease management of Brassica napus from knowledge of genetic interactions with Leptosphaeria maculans. Canadian Journal of Plant Pathology 32, 29–34.
| Improving blackleg disease management of Brassica napus from knowledge of genetic interactions with Leptosphaeria maculans.Crossref | GoogleScholarGoogle Scholar |
Kutcher HR, Balesdent MH, Rimmer SR, Rouxel T, Chèvre M, Delourme R, Brun H (2010b) Frequency of avirulence genes in Leptosphaeria maculans in western Canada. Canadian Journal of Plant Pathology 32, 77–85.
| Frequency of avirulence genes in Leptosphaeria maculans in western Canada.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltF2mtL0%3D&md5=25b72b95f7fe7033c7de63ac6e26b0eaCAS |
Kutcher HR, Brandt SA, Smith EG, Ulrich D, Malhi SS, Johnston AM (2013) Blackleg disease of canola mitigated by resistant cultivars and four-year crop rotations in western Canada. Canadian Journal of Plant Pathology 35, 209–221.
| Blackleg disease of canola mitigated by resistant cultivars and four-year crop rotations in western Canada.Crossref | GoogleScholarGoogle Scholar |
Kuusk AK, Happstadius I, Zhou L, Steventon LA, Giese H, Dixelius C (2002) Presence of Leptosphaeria maculans group A and group B isolates in Sweden. Journal of Phytopathology 150, 349–356.
| Presence of Leptosphaeria maculans group A and group B isolates in Sweden.Crossref | GoogleScholarGoogle Scholar |
Li G, McVetty PBE (2013) Genetics and gene mapping of disease resistance in Brassica. In ‘Translational genomics for crop breeding: Vol. 1 – Biotic stress’. (Eds RK Varshney, R Tuberosa) pp. 327–344. (Wiley: Hoboken, NJ)
Li H, Kuo J, Barbetti MJ, Sivasithamparam K (2007a) Differences in the responses of stem tissues of spring-type Brassica napus cultivars with polygenic resistance and single dominant gene-based resistance to inoculation with Leptosphaeria maculans. Canadian Journal of Botany 85, 191–203.
| Differences in the responses of stem tissues of spring-type Brassica napus cultivars with polygenic resistance and single dominant gene-based resistance to inoculation with Leptosphaeria maculans.Crossref | GoogleScholarGoogle Scholar |
Li H, Sivasithamparam K, Barbetti MJ (2007b) Soilborne ascospores and pycnidiospores of Leptosphaeria maculans can contribute significantly to blackleg disease epidemiology in oilseed rape (Brassica napus) in Western Australia. Australasian Plant Pathology 36, 439–444.
| Soilborne ascospores and pycnidiospores of Leptosphaeria maculans can contribute significantly to blackleg disease epidemiology in oilseed rape (Brassica napus) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Liban SH, Cross DJ, Kutcher HR, Peng G, Fernando WGD (2016) Race structure and frequency of avirulence genes in the western Canadian Leptosphaeria maculans pathogen population, the causal agent of blackleg in brassica species. Plant Pathology 65, 1161–1169.
| Race structure and frequency of avirulence genes in the western Canadian Leptosphaeria maculans pathogen population, the causal agent of blackleg in brassica species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xht1yht7nK&md5=18674ce689f066f0fb10b986891fc9d4CAS |
Liu C (2014) Evaluation of fungicides for management of blackleg disease on canola and QoI-fungicide resistance in Leptosphaeria maculans in western Canada. Master’s Thesis, University of Manitoba, Canada.
Magyar D, Barasits T, Fischl G, Fernando WGD (2006) First report of the natural occurrence of the teleomorph of Leptosphaeria maculans on oilseed rape and airborne dispersal of ascospores in Hungary. Journal of Phytopathology 154, 428–431.
| First report of the natural occurrence of the teleomorph of Leptosphaeria maculans on oilseed rape and airborne dispersal of ascospores in Hungary.Crossref | GoogleScholarGoogle Scholar |
Marcroft SJ, Potter TD (2008) The fungicide fluquinconazole applied as seed dressing to canola reduces Leptosphaeria maculans (blackleg) severity in south-eastern Australia. Australasian Plant Pathology 37, 396–401.
| The fungicide fluquinconazole applied as seed dressing to canola reduces Leptosphaeria maculans (blackleg) severity in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntVWhtLg%3D&md5=8d40abc3c0a3624b2921f91bede949efCAS |
Marcroft SJ, Sprague SJ, Pymer SJ, Salisbury PA, Howlett BJ (2004) Crop isolation, not extended rotation length, reduces blackleg (Leptosphaeria maculans) severity of canola (Brassica napus) in south-eastern. Australian Journal of Experimental Agriculture 44, 601–606.
| Crop isolation, not extended rotation length, reduces blackleg (Leptosphaeria maculans) severity of canola (Brassica napus) in south-eastern.Crossref | GoogleScholarGoogle Scholar |
Marcroft SJ, Van de Wouw AP, Salisbury PA, Potter TD, Howlett BJ (2012) Effect of rotation of canola (Brassica napus) cultivars with different complements of blackleg resistance genes on disease severity. Plant Pathology 61, 934–944.
| Effect of rotation of canola (Brassica napus) cultivars with different complements of blackleg resistance genes on disease severity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslSkurjJ&md5=7133e66bf40c3fe505b1fe971f67496bCAS |
McDonald BA, Linde C (2002) Pathogen population genetics, evolutionary potential, and durable resistance. Annual Review of Phytopathology 40, 349–379.
| Pathogen population genetics, evolutionary potential, and durable resistance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xos1Cltbg%3D&md5=23ccd90e8bb1f92b3aa9067a51063c92CAS |
McGee D, Petrie GA (1978) Variability of Leptospaheria maculans in relation to blackleg of oilseed rape. Phytopathology 68, 625–630.
| Variability of Leptospaheria maculans in relation to blackleg of oilseed rape.Crossref | GoogleScholarGoogle Scholar |
Mendes-Pereira E, Balesdent MH, Brun H, Rouxel T (2003) Molecular phylogeny of the Leptosphaeria maculans–L. biglobosa species complex. Mycological Research 107, 1287–1304.
| Molecular phylogeny of the Leptosphaeria maculans–L. biglobosa species complex.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmvVKltA%3D%3D&md5=908b7174d2d2ef8d9f2a2e12996f7d40CAS |
Peng G, Fernando WGD, Kirkham CL, Lange R, Kutcher HR, McLaren DL, Johnson EN, Turkington TK (2012) Mitigating the risk of blackleg disease of canola using fungicide strategies. Available at: www.usask.ca/soilsncrops/conference-proceedings/previous_years/Files/2012/index.php (accessed 1 March 2017).
Petrie GA (1995) Long-term survival and sporulation of Leptosphaeria maculans (blackleg) on naturally-infected rapeseed/canola stubble in Saskatchewan. Canadian Plant Disease Survey 75, 23–34.
Raman H, Raman R, Larkan N (2013) Genetic dissection of blackleg resistance loci in rapeseed (Brassica napus L.). In ‘Plant breed from lab to fields’. (Ed. SB Andersen) pp. 85–118. (InTech: Rijeka)
Rimmer SR (2006) Resistance genes to Leptosphaeria maculans in Brassica napus. Canadian Journal of Plant Pathology 28, S288–S297.
| Resistance genes to Leptosphaeria maculans in Brassica napus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xlt1Gqtrc%3D&md5=a3b0417c852ac188d848e101599583fcCAS |
Rouxel T, Balesdent MH (2005) The stem canker (blackleg) fungus, Leptosphaeria maculans, enters the genomic era. Molecular Plant Pathology 6, 225–241.
| The stem canker (blackleg) fungus, Leptosphaeria maculans, enters the genomic era.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlvVCrt78%3D&md5=d54714fa1d5073784bf2401c81a8d70cCAS |
Rouxel T, Penaud A, Pinochet X, Brun H, Gout L (2003) A 10-year survey of populations of Leptosphaeria maculans in France indicates a rapid adaptation towards the Rlm1 resistance gene of oilseed rape. European Journal of Plant Pathology 109, 871–881.
| A 10-year survey of populations of Leptosphaeria maculans in France indicates a rapid adaptation towards the Rlm1 resistance gene of oilseed rape.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXotV2jsbg%3D&md5=3fc352d56b767bdf63155e1f793e52baCAS |
Savage D, Barbetti MJ, MacLeod WJ, Salam MU, Renton M (2013) Temporal patterns of ascospore release in Leptosphaeria maculans vary depending on geographic region and time of observation. Microbial Ecology 65, 584–592.
| Temporal patterns of ascospore release in Leptosphaeria maculans vary depending on geographic region and time of observation.Crossref | GoogleScholarGoogle Scholar |
Shoemaker RA, Brun H (2001) The teleomorph of the weakly aggressive segregate of Leptosphaeria maculans. Canadian Journal of Botany 79, 412–419.
| The teleomorph of the weakly aggressive segregate of Leptosphaeria maculans.Crossref | GoogleScholarGoogle Scholar |
Soyer JL, El Ghalid M, Glaser N, Ollivier B, Linglin J, Grandaubert J, Balesdent MH, Connolly LR, Freitag M, Rouxel T, Fudal I (2014) Epigenetic control of effector gene expression in the plant pathogenic fungus Leptosphaeria maculans. PLOS Genetics 10, e1004227
Sprague SJ, Balesdent MH, Brun H, Hayden HL, Marcroft SJ, Pinocher X, Rouxel T, Howlett BJ (2006) Major gene resistance in Brassica napus (oilseed rape) is overcome by changes in virulence of populations of Leptosphaeria maculans in France and Australia. European Journal of Plant Pathology 114, 33–40.
| Major gene resistance in Brassica napus (oilseed rape) is overcome by changes in virulence of populations of Leptosphaeria maculans in France and Australia.Crossref | GoogleScholarGoogle Scholar |
Statistics Canada (2016) ‘Production of principal field crops. November 2016.’ (Statistic Canada: Ottawa, Ontario)
Steed JM, Baierl A, Fitt BDL (2007) Relating plant and pathogen development to optimise fungicide control of phoma stem canker (Leptosphaeria maculans) on winter oilseed rape (Brassica napus). European Journal of Plant Pathology 118, 359–373.
| Relating plant and pathogen development to optimise fungicide control of phoma stem canker (Leptosphaeria maculans) on winter oilseed rape (Brassica napus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntlWrurg%3D&md5=137cb45145acc4dfa6560bc0de47765eCAS |
United States Department of Agriculture (2017) ‘World agricultural production.’ (USDA: Washington, DC)
Van de Wouw AP, Elliott VL, Ware A, Lindbeck K, Howlett BJ, Marcroft SJ (2016a) Infection of canola pods by Leptosphaeria maculans and subsequent seed contamination. European Journal of Plant Pathology 145, 687–695.
| Infection of canola pods by Leptosphaeria maculans and subsequent seed contamination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhvFKktLbO&md5=8e53e278ddf1b224a0379a20454c9591CAS |
Van de Wouw P, Marcroft SJ, Howlett BJ (2016b) Blackleg disease of canola in Australia. Crop & Pasture Science 67, 273–283.
| Blackleg disease of canola in Australia.Crossref | GoogleScholarGoogle Scholar |
West JS, Fitt BDL (2005) Population dynamics and dispersal of Leptosphaeria maculans (blackleg of canola). Australasian Plant Pathology 34, 457–461.
| Population dynamics and dispersal of Leptosphaeria maculans (blackleg of canola).Crossref | GoogleScholarGoogle Scholar |
West JS, Kharbanda PD, Barbetti MJ, Fitt BDL (2001) Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathology 50, 10–27.
| Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe.Crossref | GoogleScholarGoogle Scholar |
Williams RH, Fitt BDL (1999) Differentiating A and B groups of Leptosphaeria maculans, causal agent of stem canker (blackleg) of oilseed rape. Plant Pathology 48, 161–175.
| Differentiating A and B groups of Leptosphaeria maculans, causal agent of stem canker (blackleg) of oilseed rape.Crossref | GoogleScholarGoogle Scholar |
Zhang X, White RP, Demir E, Jedryczka M, Lange RM, Islam M, Li ZQ, Huang YJ, Hall M, Zhou G, Wang Z, Cai X, Skelsey P, Fitt BDL (2014) Leptosphaeria spp., phoma stem canker and potential spread of L. maculans on oilseed rape crops in China. Plant Pathology 63, 598–612.
| Leptosphaeria spp., phoma stem canker and potential spread of L. maculans on oilseed rape crops in China.Crossref | GoogleScholarGoogle Scholar |
Zhang X, Peng G, Kutcher HR, Balesdent M-H, Delourme R, Fernando WGD (2016) Breakdown of Rlm3 resistance in the Brassica napus–Leptosphaeria maculans pathosystem in western Canada. European Journal of Plant Pathology 145, 659–674.
| Breakdown of Rlm3 resistance in the Brassica napus–Leptosphaeria maculans pathosystem in western Canada.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhvFKks73N&md5=937372888dd3343482093375ee84ea5fCAS |