Elevated field atmospheric CO2 concentrations affect the characteristics of winter wheat (cv. Bologna) grains
Francesca Verrillo A , Franz-Werner Badeck B , Valeria Terzi B G , Fulvia Rizza B , Letizia Bernardo B , Antimo Di Maro C , Clara Fares D , Alessandro Zaldei E , Francesco Miglietta E , Anna Moschella F , Marcella Bracale A and Candida Vannini AA Dipartimento Biotecnologie e Scienze della Vita, Università degli Studi dell’Insubria, Via J.H. Dunant 3, 21100 Varese, Italy.
B CREA-GB, Research Centre for Genomics and Bioinformatics, Via San Protaso 302, Fiorenzuola d’Arda, 29017 Piacenza, Italy.
C Dipartimento di Scienze e Tecnologie Ambientali Biologiche e Farmaceutiche, Università degli Studi della Campania ‘Luigi Vanvitelli’, Via Vivaldi 43, 81100 Caserta, Italy.
D CREA-CI, Research Centre for Cereal and Industrial Crops, S.S 16 Km 675, 71121 Foggia, Italy.
E CNR-IBIMET, Istituto di Biometeorologia, Via G. Caproni, 8-50145 Firenze, Italy.
F CREA-CI, Research Centre for Cereal and Industrial Crops, Via di Corticella 133, 40128 Bologna, Italy.
G Corresponding author. Email: valeria.terzi@crea.gov.it
Crop and Pasture Science 68(8) 713-725 https://doi.org/10.1071/CP17156
Submitted: 19 April 2017 Accepted: 6 September 2017 Published: 11 October 2017
Journal Compilation © CSIRO 2017 Open Access CC BY-NC-ND
Abstract
The aim of this study was to investigate the impact of elevated concentration of carbon dioxide (CO2), as expected over coming decades, on yield and quality of winter bread wheat (Triticum aestivum L.). Plants (cv. Bologna) were grown by using the free-air CO2 enrichment (FACE) system at Fiorenzuola d’Arda under ambient (control) and elevated (570 ppm, e[CO2]) CO2 concentrations for two growing seasons. We addressed whether there would be a response of wheat grains to elevated CO2 concentration in terms of the contents of nitrogen (N), micro- and macronutrients, proteins and free amino acids. Under e[CO2], total wheat biomass and grain yield increased in both years of the study. Grain N percentage was reduced under e[CO2], but grain N yield (kg ha–1) was increased. Among macro- and micronutrients, a decrease in zinc concentration was observed. The proteome pattern was significantly different in grains grown at the two different CO2 levels, but the observed changes were highly dependent on interactions with prevailing environmental conditions. Finally, a negative trend was observed in the early germination rates of seeds from plants grown under e[CO2] compared with the controls. The results suggest that the expected increase in CO2 levels and their interactive effects with environmental variables may influence agronomic performance by increasing yield and negatively affecting quality.
Additional keywords: climate change, candidate core proteins, proteomics.
Introduction
Over the last 200 years, human activities have accelerated global changes in climate. One of the most relevant of these changes is the increase in atmospheric concentration of carbon dioxide, which, since 1750, has risen from ~280 to 400 ppm, and it is expected to reach ~550 ppm by mid-21st Century (IPCC 2013). This increase is expected to have a great impact on the growth and development of crops, particularly C3 plants such as wheat. DaMatta et al. (2010) reviewed the literature concerning the effects of CO2 increase on crop physiology, suggesting some beneficial effects such as yield increase and better water-use efficiency. On the other hand, these beneficial effects can be offset by a decrease in grain quality (Nuttall et al. 2017). In addition, when moving from greenhouse and open-top chamber experiments to the more realistic situation of the free-air CO2 enrichment (FACE) experiment, the interaction among elevated CO2 and other environmental variables was shown to introduce higher levels of complexity in plant response (DaMatta et al. 2010). Many studies have focused on the effects of elevated concentrations of CO2 on the technological and nutritional quality of wheat grains, showing that elevated CO2 can alter C and nitrogen (N) metabolism in plants, thereby altering the chemical composition of vegetative plant parts (Cotrufo et al. 1998; Loladze 2002). These conditions affect the redistribution and availability of metabolites for the developing grain, subsequently affecting both grain yield and quality (Kimball et al. 2001; Fernando et al. 2014). Some FACE experiments demonstrated only a slight decrease in grain protein levels of bread wheat under elevated CO2, whereas others pointed to consistent and statistically significant reductions in protein and micronutrient contents (Kimball et al. 2001; Wieser et al. 2008; Högy et al. 2009a; Erbs et al. 2010; Fernando et al. 2012a, 2012b, 2014; Tausz et al. 2013; Myers et al. 2014; Nuttall et al. 2017). In durum wheat, elevated CO2 decreased the quality of grains and derived pasta, the main negative effect being on gluten content (Fares et al. 2016). Nuttall et al. (2017) reviewed the current knowledge of effects of high temperature and elevated environmental CO2 on wheat-grain functional properties, and concluded that elevated CO2 concentrations generally lead to a reduction in protein percentage and a shift in grain composition, with consequent negative impact on bread-making characteristics. However, those authors emphasise that differing results have been obtained among FACE experiments because of the interaction between CO2 concentration and other environmental factors. CO2 enrichment was shown to affect even the proteome profile of wheat grains under well-watered, temperate conditions (Högy et al. 2009b) and Mediterranean rainfed conditions (Fernando et al. 2015) in FACE facilities during a single growing season.
The aim of the present work was to investigate effects of elevated CO2 on yield and grain quality of bread wheat cultivated in a Southern European environment. The winter wheat (Triticum aestivum L.) genotype Bologna has been used as model because of the prevalence of winter cereal cultivation in several warm temperate and Mediterranean areas. Bologna is representative of frost-resistant winter wheats well suited to exploit long growing seasons with autumn sowing, not only for cool winters but also in warm continental and mountain climates subject to random frost episodes in the context of the warming climate, as in the Po Valley. Using a FACE facility, we exposed wheat plants to 570 ppm CO2, which simulates the terrestrial tropospheric concentration predicted for 2050 (www.ipcc.ch/). This treatment, e[CO2], was compared with an ambient control, a[CO2]. The experiments were performed for two growing seasons to consider effects under the environmental conditions encountered during different years. Key characteristics related to grain quality were considered, including contents of N, free amino acids and minerals, and composition of seed-storage proteins. Seed proteome profiles were obtained to look for proteins that were differentially expressed between a[CO2] and e[CO2] in both seasons, and which therefore represent good candidates to be directly affected by elevated CO2 in wheat (core proteins) independent of interactions of CO2 with prevailing environmental conditions.
Materials and methods
Plant material
The superior bread-making wheat cv. Bologna was used. Bologna is currently the most cultivated variety in Italy owing to its high yield stability and excellent quality. It has a winter growth habit, characterised by a wide range of adaptability, high levels of cold and lodging resistance, and high tolerance to fusarium head blight (FHB), and is classified as ‘Frumento di Forza’ (FF, Improver Wheat) from a technological point of view. Cultivar Bologna (pedigree H89092/H89136//Soissons) has been bred by SIS, Società Italiana Sementi, San Lazzaro di Savena, BO, Italy (www.sisonweb.com/it/index.php).
Experimental setup
Wheat plants were grown within the FACE facility of the Research Centre for Genomics and Bioinformatics (CREA-GB) at Fiorenzuola d’Arda (44.927°N, 9.893°E), in the Po Valley (Italy), at elevation 60–80 m a.m.s.l. and with a warm continental climate. The field soil is alkaline (pH 8.09), with the following characteristics: total carbonate, 10.19%; inorganic C, 12.22 g kg–1; total C, 28.1 g kg–1; organic C, 15.9 g kg–1; organic matter, 2.74%; total N, 0.10%; C : N ratio 15.6; P2O5, 21.7 mg kg–1; cation exchange capacity, 6.85 cmol(+) kg–1.
Experimental units were plots 2.2 m by 1.36 m. The FACE treatment, with the e[CO2] target value set at 570 ppm, was replicated in four octagons inscribed in circles of 14 m diameter. The a[CO2] controls were replicated four times in octagons without FACE. Within the octagons, plots were replicated four times in the first growing season (2011–12; total 4 × 4 = 16 plots per treatment), and two times in the second growing season (2012–13; total 4 × 2 = 8 plots per treatment). Designations Y1E, Y1A, Y2E and Y2A refer to year (Y1, 2011–12; Y2, 2012–13) × CO2 treatment (E, e[CO2]; A, a[CO2]) combinations.
Apart from the CO2 fumigation, plants were treated according to local agronomic practices. In 2011–12, the sowing, start of fumigation and harvest dates were 19 October 2011, 16 November 2011 and 2 July 2012, respectively. FACE treatment was stopped when leaves were senescent on 14 June 2012. FACE treatment was interrupted for 20 days in February 2012 when the plots were covered with snow. Plots were fertilised with application of an N-P-K fertiliser at pre-seeding and two topdressings with ammonium nitrate for a total of 149 kg N ha–1.
In 2012–13, the sowing, start of fumigation and harvest dates were 24 October 2012, 9 November 2012 and 11 July 2013, respectively. Plots were again fertilised with application of N-P-K fertiliser at pre seeding and two topdressings with ammonium nitrate. However, the fertiliser doses were increased to a total of 234 kg N ha–1 because, in the first season, the export of N with harvested grains and straw had exceeded the amount applied as fertiliser, and the experiment was planned as non-nutrient-limited.
Climatic conditions
Air temperature, precipitation, relative humidity, and global radiation were measured and recorded at 10-min intervals with an automatic meteorological station located within the field site of the FACE experiment at Fiorenzuola.
Grain yield and nitrogen content
After harvest, aboveground biomass, grain yield, 1000-grain weight and harvest index were determined. Grain N content was determined by the Kjeldahl method, and grain crude protein content calculated as 5.7 × N. Grain CHN content was determined by TruSpec CHN elemental analyzer (LECO Corp., St. Joseph, MI, USA) following the manufacturer’s specifications. Grain N yield (kg ha–1) was calculated from grain yield and grain N content. These traits were determined for every plot.
For each growing season, samples for each treatment were obtained for further analyses from the pool of grains harvested in four a[CO2] and four e[CO2] experimental plots. For each analysis, at least three technical replicates were performed.
Mineral profiling
Wheat kernels were ground by mortar and pestle, using liquid nitrogen. To avoid metallic contaminations, a metallic grinder was not used. A pulverised sample (~200 mg) was accurately weighted inside a pressure-resistant vessel (100 mL) and digested with 3 mL 69% concentrated, ultrapure nitric acid and 9 mL 36% concentrated, ultrapure hydrochloric acid. The reactor was closed, starting the digestion procedure in a microwave oven following the program: step 1, 250 W for 2 min; step 2, 0 W for 1 min; step 3, 250 W for 2 min; step 4, 400 W for 2 min; step 5, 500 W for 5 min; step 6, 0 W for 1 min; step 7, 600 W for 5 min; step 8, oven ventilation for 6 min. After cooling the vessels to room temperature, each sample was diluted to 20 mL in a low-density polyethylene container with ultrapure water produced by a Millipore Milli-Q Advantage A10 system (Merck, Darmstadt, Germany). Ultrapure nitric acid (600 µL 69% concentrated) was added to the solution to obtain a final concentration of 2% nitric acid. The final solution obtained was analysed by inductively coupled plasma-optical emission spectroscopy (iCAP 6300; ThermoFisher Scientific, Waltham, MA, USA) for the determination of Ca, Cu, Fe, K, Mg, Mn and Zn. Determination of trace elements was performed by using external calibration.
Amino acid analyses
Wheat flour was extracted with 80% cold ethanol in the presence of nor-Leu (50 nmol) as an internal standard, homogenised with a Teflon pestle, and centrifuged at ~14 000g at 4°C. The supernatant was lyophilised, treated with 3% sulfosalicylic acid (500 µL) to precipitate any protein fraction still present, and centrifuged again (Iriti et al. 2009). Then 30 µL of this extract was directly analysed. Each sample was individually prepared and analysed in triplicate.
Aliquots of samples were directly analysed on a Biochrom 30 amino acid analyser (Biochrom, Cambridge, UK) equipped with a post-column ninhydrin derivatisation system, adapting the procedure of Moore and Stein (1963). Total free amino-acid content was obtained with a single analysis, simplifying the acquisition of raw data.
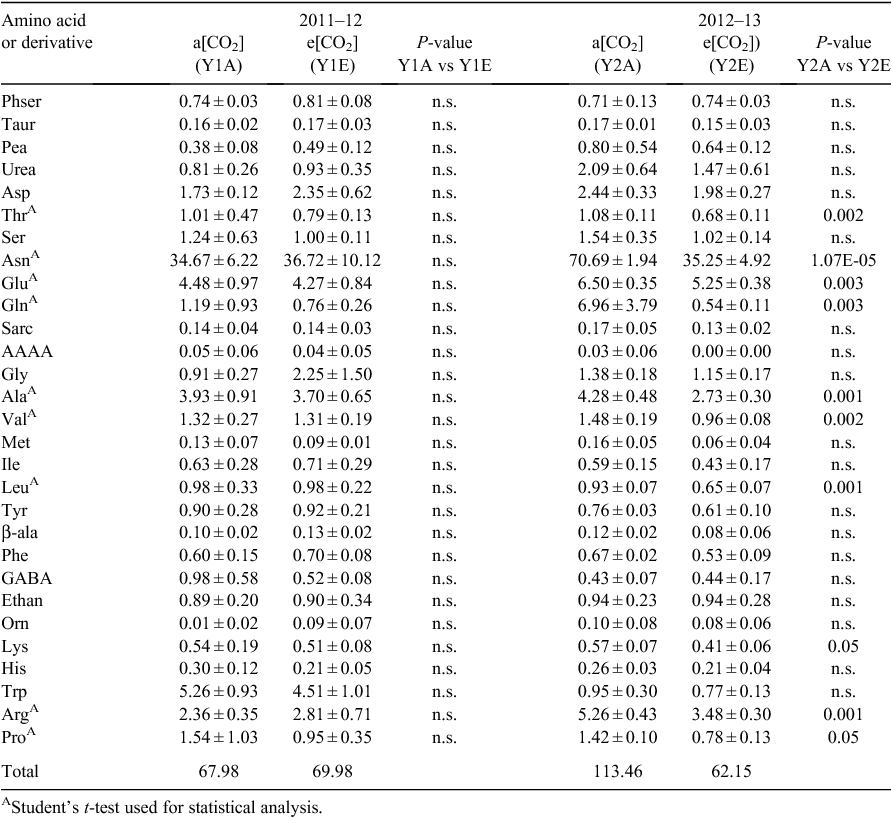
For the free amino-acid profiles, standard codes were used, along with other abbreviations, as follows: AAAA, L-α-aminoadipic acid; Ala, L-alanine; Arg, L-arginine; Asn, L-asparagine; Asp, L-aspartic acid; Ethan, ethanolamine; GABA, γ-amino-butyric acid; Gln, L-glutamine; Glu, L-glutamic acid; Gly, glycine; His, L-histidine; Ile, L-isoleucine; Leu, L-leucine; Lys, L-lysine; Met, L-methionine; Orn, L-ornithine; Pea, o-phosphoethanolamine; Phe, L-phenylalanine; Phser, o-phospho-L-serine; Pro, L-proline; Sarc, L-sarcosine; Ser, L-serine; Taur, taurine; Thr, L-threonine; Trp, L-tryptophan; Tyr, L-tyrosine; Val, L-valine; β-ala, β-alanine.
Two-dimensional gel electrophoresis (2DE) proteomic analysis
Seed protein was extracted according to Wang et al. (2006) with minor modification. Briefly, 1 g finely powdered seeds was suspended in 10 mL iced 10% w/v TCA–acetone, vortexed, filtered with Miracloth, and centrifuged at 16 000g for 10 min at 4°C. After washing once with iced 80% 0.1 m ammonium acetate in methanol and twice with 10 mL iced 80% acetone, pellets were resuspended in 5 mL ice-cold sodium dodecyl sulfate (SDS) extraction buffer (30% w/v sucrose, 0.1 m Tris-HCl pH 8, 2% w/v SDS, 2% v/v β-mercaptoethanol, 1.5% w/v polyvinylpyrrolidone, 1 mm PMSF, 1 mm cocktail antiprotease) and then extracted with an equal volume of phenol-saturated 500 mm Tris-HCl, pH 8. Proteins were precipitated overnight by adding five volumes cold 0.1 m ammonium acetate in methanol, and solubilised in rehydration sample buffer (7 m urea, 2 m thiourea, 4% (w/v) CHAPS detergent, 1% (v/v) Triton X-100, 20 mm Tris, 1% (w/v) DTT (dithiothreitol), 0.2% (w/v) ampholine 4-7). Protein concentration was estimated according to the Bradford (1976) assay. Three independent protein extractions were performed from each sample.
Total proteins (500 mg) were loaded onto an 18-cm, pH 4–7 linear gradient or pH 3–10 nonlinear gradient (NL) Immobiline DryStrip gels (IPG strips) (GE Healthcare, Uppsala, Sweden), and separated as described by Marsoni et al. (2008). The SDS-PAGE gels were visualised by the modified Colloidal Coomassie Brilliant Blue staining method (Aina et al. 2007). Each separation was repeated three times for each biological replicate to ensure the protein pattern reproducibility.
Image acquisition and spot detection
Gels were digitalised using GS-800 imaging systems (Bio-Rad, Munich, Germany), and the images (TIFF format, 300 dpi, 16 bit) were analysed with PDQuest software version 8.0.1 (Bio-Rad). Only the protein spots present in all gels for each sample were considered for the statistical analysis. Statistical analysis (Student’s t-test at P = 0.05) identified proteins that significantly increased or decreased (≥1.5-fold in relative abundance) after the different treatments with respect to the control. These spots were selected for tandem mass spectrometry (MS/MS) analysis.
In-gel digestion and mass spectrometry analysis
Selected spots were manually excised from the 2D gels, washed twice and stored in 50% ethanol at 4°C until digestion. Spot digestion was performed as previously described (Marsoni et al. 2008). The extracted tryptic fragments were analysed by MS/MS after reverse-phase separation of peptides by liquid chromatography–electrospray ionisation MS/MS (LC-ESI-MS/MS). All experiments used a Finningan LXQ linear ion-trap mass spectrometer, equipped with a Finningan Surveyor MS plus HPLC system (Thermo Electron Corp., Waltham, MA, USA).
Chromatography separations were conducted on a BioBasic C18 column (150 mm internal diameter, 150 mm length, 5 mm particle size; Thermo Electron Corp.), using a linear gradient from 5% to 75% acetonitrile, containing 0.1% formic acid, for 50 min with a flow of 2 mL min–1. Acquisitions were performed in the data-dependent MS/MS scanning mode (full MS scan range of 400–1400 m/z followed by zoom scan for the most intense ion from the MS scan and full MS/MS for the most intense ion from the zoom scan), thus enabling a dynamic exclusion window of 3 min.
Protein identification was performed by searching in the National Center for Biotechnology Information (NCBI) viridiplantae and/or EST-viridiplantae protein database using the MASCOT program (www.matrixscience.com). The following parameters were adopted for database searches: complete carbamidomethylation of cysteines, partial oxidation of methionines, peptide mass tolerance 1.2 Da, fragment mass tolerance 0.8 Da, and missed cleavage 1. For positive identification, the ion score (–10log(P)) had to be over the significance threshold level of P = 0.05. Spectra were also compared with the in situ database by SEQUEST algorithm incorporated in BIOWORKSBROWSER 3.3 software (ThermoFisher Scientific) against the Triticum subset database (31 852 entries) obtained from the NCBI-nr database (https://www.ncbi.nlm.nih.gov/) and, in case of mismatch, against the full NCBI-nr database (25 877 237 entries). The searches were carried out as described in Marsoni et al. (2008). If needed, the name of unknown proteins was annotated by protein similarity search performed by alignment analysis against the NCBI-nr database by using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Non-identified spectra were submitted to de novo analysis by PepNovo software, using default parameters (http://proteomics.ucsd.edu/Software/PepNovo). Only those PepNovo results that received a mean probability score of at least 0.5 were accepted. Peptide candidates obtained by PepNovo were edited according to MS BLAST rules, and an MS BLAST search was performed against the NCBI-nr database at: http://genetics.buh.harvard.edu/msblast. Statistical significance of hits was evaluated according to the MS BLAST scoring scheme. In order to assign a correct identification, we also take into account a minimum of two different matching peptides and correspondence of the molecular weight.
Detection of carbonylated proteins
The proteins were extracted as described above. Proteins (20 µg) were derivatised with DNPH (2,4-dinitrophenylhydrazine) as previously described with some modifications (Levine et al. 1994). Briefly, the proteins were denatured adding SDS at the final concentration of 6%. The derivatisation was performed by adding one volume of 10 mm DNPH in 2 n HCl. Only 2 n HCl was added to the negative control. After incubation for 30 min at room temperature, the mixture was neutralised by adding one volume of neutralisation solution (2 m Tris, 30% glycerol). Proteins were separated by 12% SDS-PAGE and transferred to PVP (polyvinylpyrrolidone) membrane (SERVA Electrophoresis, Heidelberg, Germany). The oxidative modified proteins were detected by using anti-DNP (dinitrophenyl-group) antibodies (Sigma-Aldrich, St. Louis, MO, USA) and visualised by a chemiluminescence detection kit (SuperSignal; Pierce Biotechnology, Rockford, IL, USA). To monitor the equal loading of samples, CCBB (colloidal Coomassie Brilliant Blue) was used to stain the proteins in a duplicate gel. Gel and immunoblot images were acquired by using GS-800 imaging systems and analysed using ImageJ software (Bio-Rad Laboratories, Hercules, CA, USA).
Germination test
Seeds were surface-sterilised with 10% sodium hypochlorite solution for 10 min and then rinsed with distilled water. Hydrated seeds were transferred in Petri dishes (100 mm diameter) containing a Whatman filter in the presence of 5 mL distilled water, pH 7.1. Ten dishes, each containing 10 seeds, were prepared for each test and incubated in the dark at 25°C. After 2, 4 and 7 days, the germinated seeds were counted. Seeds were considered germinated when root elongation was >3 mm. Tests were performed in triplicate.
Statistical analyses
Results for grain yield and N content were analysed with analysis of variance (ANOVA) as a split-plot design using R statistical software (R Foundation for Statistical Computing, Vienna: www.R-project.org/). The remaining results were presented as mean of the technical replicates ± standard deviation (s.d.). Differences between treatments for the different measured variables were tested by one-way ANOVA, followed by the Bonferroni-Holm’s post-hoc test (Daniel’s XL Toolbox version 6.60: http://xltoolbox.sourceforge.net).
Results
Climatic conditions
The climatic conditions during both growing seasons were sufficiently moist, with 487 mm precipitation over the crop growth cycle in 2011–12 and 1027 mm in 2012–13. A developing drought risk in March–April 2012 did not materialise due to rainfall in April. In both growing seasons, the ratio potential evapotranspiration : rainfall remained well below 1 (0.66 and 0.30, respectively). No marked temperature extremes were registered (FW Badeck, F Rizza, C Maré, E Mazzucotelli, L Cattivelli, A Zaldei, F Miglietta, unpubl. data), and no frost damage occurred (Badeck and Rizza 2015).
Yield parameters and seed vigour
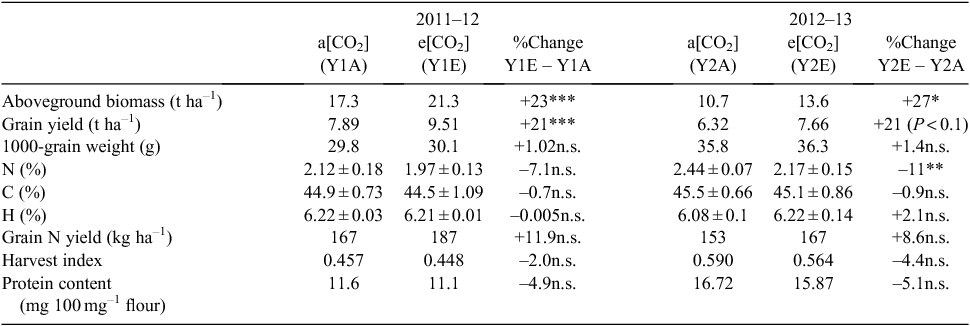
Total wheat biomass and grain yield were higher under e[CO2] than a[CO2] in both years of the study (Table 1). The biomass increase was significant in both years. Grain yield increased by 21% in the two years, significantly in 2011–12 and as a trend (P = 0.0919) in 2012–13. No significant CO2 treatment effect was found for 1000-grain weight and harvest index, which showed slight increase and decrease, respectively. Heading date did not significantly differ between CO2 treatments in either year. The grain N content (%) was reduced under e[CO2], showing a non-significant trend in the first year, and a significant effect in the second. However, a positive, although non-significant trend was evident when comparing grain N yield (kg ha–1), with an increase of 11.9% in Y1E vs Y1A and an increase of 8.6% in Y2E vs Y2A.
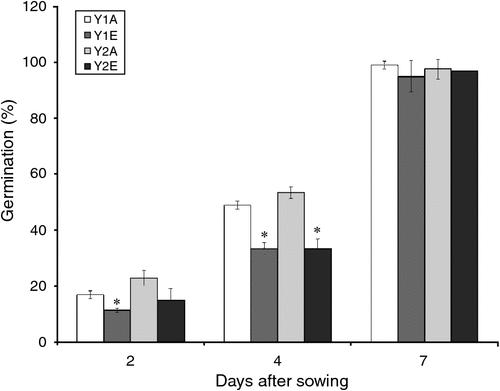
A negative trend was observed in the early germination rates of seeds from plants grown under e[CO2] compared with the control (Fig. 1).
|
Grain quality
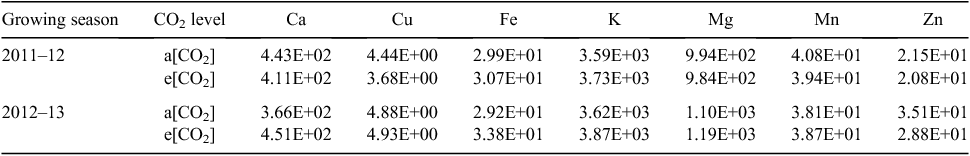
No significant differences in free amino-acid contents of individual grains were found between CO2 treatments in the first year (Table 2). In the second year, the grain content of free Ala, Arg, Asn, Glu, Gln, Leu, Lys, Pro, Thr and Val was lower under e[CO2] than a[CO2]. However, overall content of free amino acids was higher in Y2A than Y1A, and particularly the relative amounts of Asn, Arg, Asp, Gln, Glu, and Trp (Table 2), whereas Y2E and Y1E had similar amino acid contents. No significant effect of CO2 concentration or of growing season was observed on grain macro- and micronutrient contents (Table 3), except for a decrease in Zn concentration in samples grown under e[CO2] compared with the control in both years.
|
Changes in seed proteome profiles
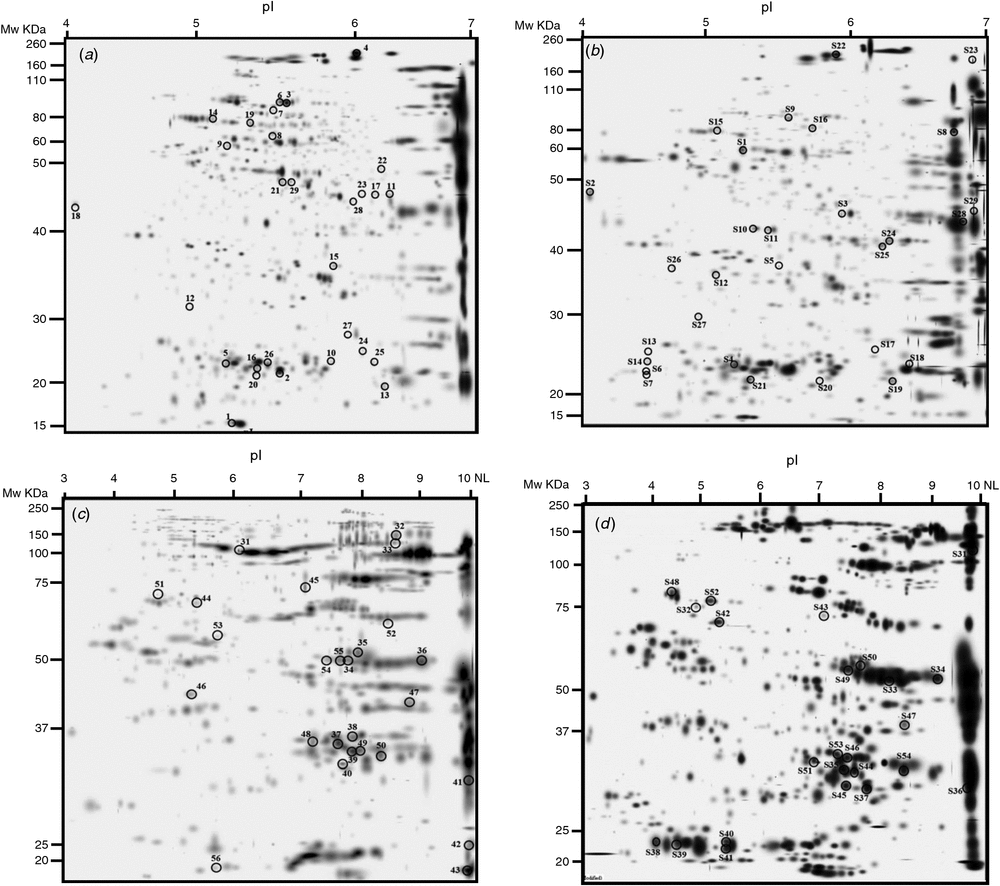
We analysed total soluble proteins by 2DE with two pH ranges: 4–7 (Fig. 2a, b) and 3–10 NL (Fig. 2c, d). In the pH 4–7 gels, we detected ~577 spots in Y1A, 566 spots in Y1E, 593 spots in Y2A and 629 spots in Y2E. The similarity of the gels was 82.6% for Y1E vs Y1A and 69.3% for Y2E vs Y2A. In the pH 3–10 NL gels, we detected 791 spots in Y1A, 622 spots in Y1E, 591 spots in Y2A and 534 spots in Y2E. The similarity of the gels was 59% for both growing seasons.
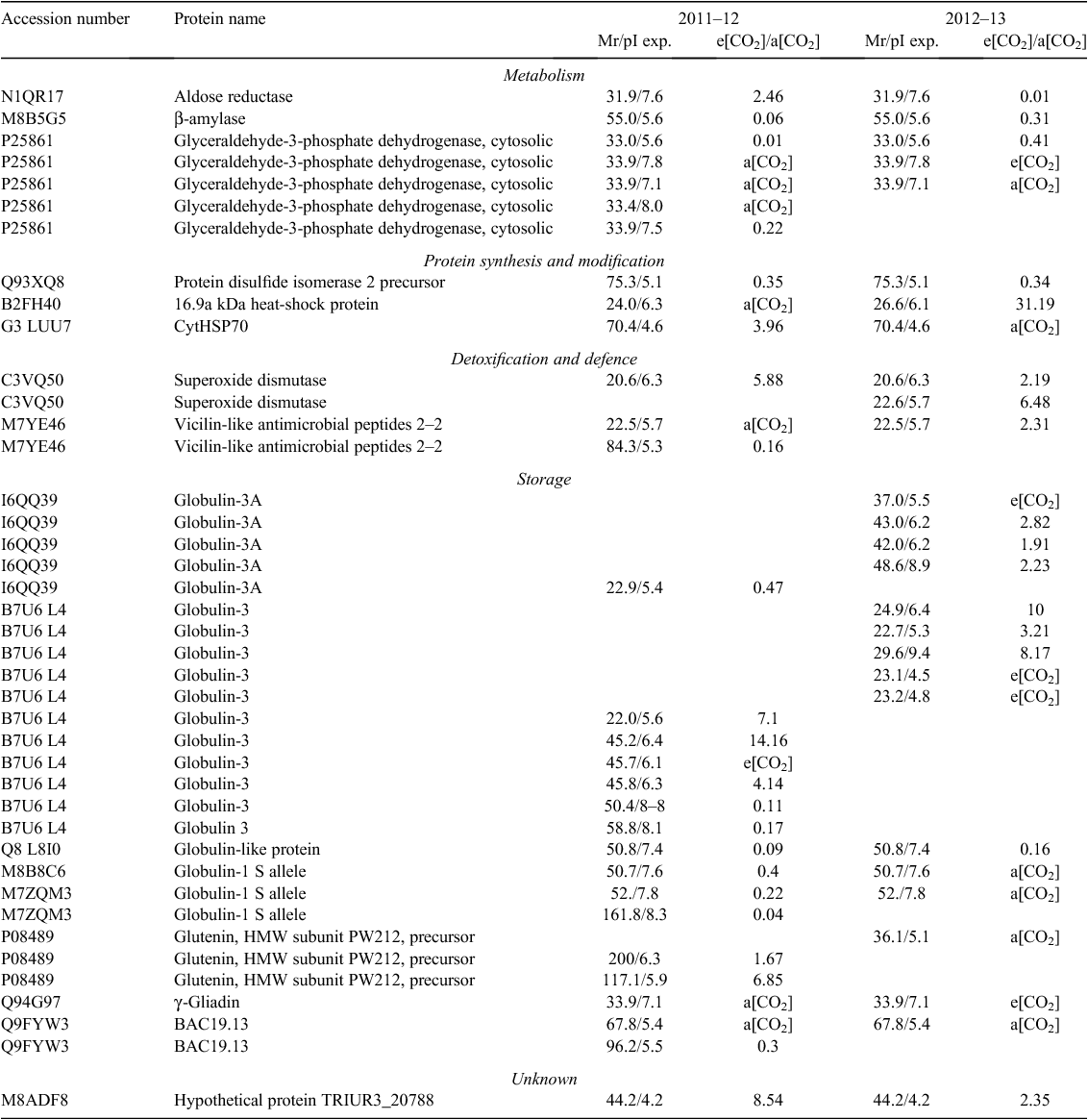
Overall proteomic analysis revealed 52 differentially expressed protein spots in samples grown under e[CO2] compared with the control in 2011–12, and 53 in 2012–13. Comparison of Y1E and Y1A samples revealed 37 protein spots that were down-accumulated (71%), whereas 15 spots were up-accumulated (29%). Comparison of Y2E and Y2A samples showed that 22 protein spots were down-accumulated (42%) and 31 up-accumulated (58%). The differentially expressed protein spots common to both years are reported in Table 4; these proteins represent good candidates to be directly affected by increased CO2 concentration in wheat (core proteins).
|
The differentially expressed proteins identified by MS/MS were classified into five groups according to their putative physiological functions: metabolism, protein synthesis and modification, detoxification and defence, storage, and unknown proteins. Approximately 35% and 30% of the differentially expressed protein spots in the 2011–12 and 2012–13 samples are metabolic proteins, and ~35% and 42% are storage proteins, respectively (Supplementary materials tables S1 and S2, available at the journal’s website).
Some protein spots corresponding to high-molecular-weight glutenin subunits (HMW-GS) accumulated during both growing seasons under e[CO2]. CO2 enrichment also resulted in the differential expression of several protein spots identified as globulin-3, globulin-3A, globulin-3C, globulin-1 S allele, and globulin-like protein. Each globulin was identified in multiple spots, with some exhibiting a lower relative molecular mass (MW) and more acidic isoelectric point (pI) than the theoretical values. Analysis of sequence coverage indicated that these spots were produced by cleavage of the N-termini of these proteins. These results are consistent with previous investigations indicating that globulin-3 family proteins can be processed at internal cleavage sites to yield polypeptides with a large range of molecular weights and pI values (Singh et al. 2001; Dupont et al. 2011; Koziol et al. 2012). Interestingly, the spots corresponding to full-length globulin were down-accumulated under e[CO2], whereas those corresponding to endo-proteolytic events accumulated or appeared in samples grown under these conditions.
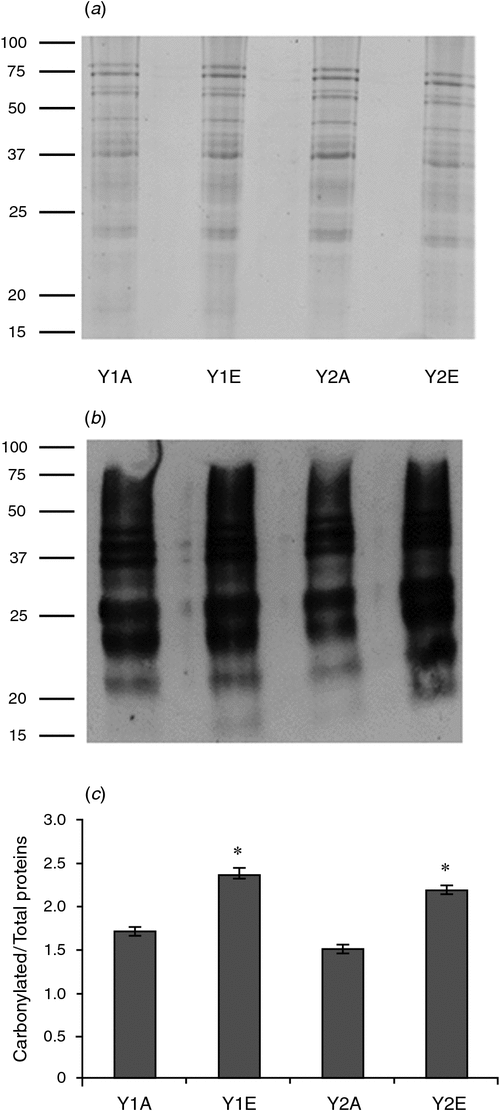
Proteome analysis revealed that one superoxide dismutase (SOD) accumulated in wheat grains grown under e[CO2] in both years. Moreover, wheat grains obtained during 2012–13 also accumulated two isoforms of an ascorbate peroxidase (APX). One-dimensional PAGE of seed protein extracts was obtained and the presence of carbonyl groups was detected by western blotting using the DNPH immunoassay. The e[CO2] treatment resulted in greater accumulation of oxidatively modified polypeptides in wheat grains during both growing seasons, as revealed by an increase in carbonylated protein levels (Fig. 3).
|
Approximately one-third of the protein spots expressed differentially between seeds grown in e[CO2] and the control are involved in metabolic processes. However, we identified different proteins across the two growing seasons, indicating that the influence of the e[CO2] treatment on seed metabolic activity is strongly influenced by additional environmental effects (Table 4). Only β-amylase and some isoforms of glyceraldehyde-3-phosphate dehydrogenase were down-accumulated in the Y1E and Y2E samples.
Discussion
The effects of climate change on our ecosystems are already severe and widespread, and ensuring food security in the face of climate change is among the most daunting challenges facing humankind. Among the actions needed to build resilience to climate change into agricultural production systems is a deeper knowledge of the impact of changed environmental conditions on the yield and quality of cereals. In this context, we have evaluated the agronomic performance and seed quality of an autumn-sown bread wheat variety cultivated in open field conditions in a FACE system, at the CO2 concentrations expected in 2050. Because interactions between elevated CO2 and other environmental conditions make it difficult to interpret the impact of increasing CO2 levels on wheat grains, we compared the responses of wheat during two growing seasons.
In the warm continental climate of the Po Valley, a significant increase in aboveground biomass was observed for cv. Bologna plants grown at elevated CO2. As reported in Table 1, the biomass increases of 23% and 27% in the first and second year, respectively, are values even higher than reported in similar FACE trials (Högy et al. 2009a). In both years, we observed a 21% yield increase at elevated CO2. This result is in agreement with the data obtained in several other FACE experiments (DaMatta et al. 2010; Arachchige et al. 2017; Nuttall et al. 2017). An average grain-yield increase of 15% in bread wheat grown at elevated CO2 in FACE experiments has been found through meta-analyses (Ainsworth and Long 2005). Increases of 10% and of 26% have been reported in German and Australian environments, respectively (Högy et al. 2010; O’Leary et al. 2015).
The first conclusion is therefore a confirmation of the ‘fertiliser’ role of elevated CO2 even in winter wheat in the Southern European environment considered here.
According to our data, the relative biomass allocation to seeds, the harvest index (HI), was quite stable (Table 1) and only 2% and 4.4% lower under e[CO2] in 2011–12 and 2012–13, respectively. Variability for this agronomic parameter has been found among durum wheat cultivars grown at different CO2 levels (Aranjuelo et al. 2013). Those authors observed that different genotypes increase or reduce their growth, and consequently their HI, under elevated CO2 as a result of different regulation of light-saturated rate of CO2 assimilation and different N assimilation. Therefore, HI has been identified as a parameter that influences responsiveness to elevated CO2 in durum wheat (Aranjuelo et al. 2013).
Total protein and N concentrations declined in seeds of cv. Bologna grown under e[CO2]. This observation is in agreement with other studies demonstrating that the most pronounced impact of elevated CO2 on nutritional and functional quality of common wheat was the significant reduction in protein concentration by 3.5–14.3% (Kimball et al. 2001; Wieser et al. 2008; Bloom et al. 2010; Fernando et al. 2012a; Broberg et al. 2017). Several hypotheses have been proposed to explain this decline: (i) excess accumulation of carbohydrates leads to reduced grain protein concentration (biomass dilution); (ii) decreased N-uptake rate under elevated CO2; (iii) elevated CO2 inhibits N assimilation (Taub and Wang 2008; Bloom et al. 2010). Högy et al. (2009a) hypothesised that the N concentration decrease is a result of the limited N supply from vegetative parts of the plant and that ‘the current rates of N fertilizer are probably inadequate to maintain existing grain quality standards’. However, increases in N fertilisation pose several problems in terms of costs and environmental consequences, and probably result in higher biomass and yield instead of promoting the distribution of N into grains (Högy and Fangmeier 2008). Nowak et al. (2004) agree that N availability has a key role in modulating the plant response to elevated CO2, even if the physiological and molecular mechanisms of this decrease are not fully understood. Recently, Fernando et al. (2017) tested the hypothesis that N types (NH4+ and NO3–) and their ratio can be associated with grain N concentration under elevated CO2. Those authors found that when equal quantities of NH4+ and NO3– are supplied to the plant under elevated CO2, post-anthesis N uptake is stimulated and the reduction in grain N concentration is avoided.
However, if we take into account the grain N yield, an increase was observed under Y1E and Y2E growth conditions. Högy et al. (2009c) found that the total protein concentration of spring wheat grown at elevated CO2 was significantly decreased, but that protein yield showed an opposite trend.
Because changes in seed C : N ratio can affect seed vigour (Hampton et al. 2013) and because we have found an increase in C : N ratio in e[CO2] seeds, germination tests were done, finding that parental CO2 environment did not have an effect on germination percentage. However, a slower germination was observed in the e[CO2] seeds. The same effect was observed by Thürig et al. (2003) for seven different graminoids, non-legume forbs and legumes species. Those authors in fact observed that the fraction of germinated seeds was not significantly different between seeds produced at ambient and elevated CO2 in those species. However, the time to germination of seeds produced under elevated CO2 was significantly shortened in two species (Sanguisorba minor, Trifolium pratense) and prolonged in Briza media. No difference was observed in the remaining five species. In the annual grass Bromus rubens, the plant population reacted to elevated CO2 by producing seeds with smaller reserves and lower initial performance (Huxman et al. 1998). Variations in C : N ratio and seed size can be responsible for the difference in seed germination, but other factors may be involved. However, in the present experiment, neither 1000-kernel weight nor grain-size distribution changed significantly under e[CO2]. The whole-proteome characterisation of cv. Bologna seeds did show the accumulation of oxidatively modified polypeptides under e[CO2]. The accumulation of APX and other defence-related proteins in spring wheat grains under elevated CO2 was also reported by Qiu et al. (2008) and Högy et al. (2009b). In plants, SOD and APX are two of the main ROS-scavenging enzymes, and constitute a highly efficient system for removing superoxide and hydrogen peroxide. The observed increase in protective antioxidative mechanisms suggests a higher oxidative level in wheat grains grown under elevated CO2. ROS can act as damaging or signalling molecules depending on the delicate equilibrium between ROS production and scavenging. If the ROS level exceeds the threshold, cells are exposed to oxidative stress. Several stressful environmental conditions (such as drought, high light levels, pathogens) induce high levels of cellular ROS, which can damage biomolecules such as lipids, proteins and DNA. Protein carbonylation, one of the most harmful, irreversible oxidative modifications of protein, involves the introduction of carbonyl groups into protein-bound amino acids, and it can disrupt protein activity thereby contributing to cell injury. Protein carbonylation is considered a hallmark of oxidative damage; we therefore performed protein carbonyl analysis to assess the extent of oxidative stress under e[CO2] (Fedorova et al. 2014). The results obtained indicate that pro-oxidant conditions and oxidative stress occur during seed development under elevated CO2. Seed performance is highly dependent on parental environmental cues, especially during seed formation and maturation (Donohue 2009). Plants of various species exhibit altered antioxidant capacity and an increased oxidative stress under elevated CO2 (Cheeseman 2006; Qiu et al. 2008; Naudts et al. 2014). This increased oxidative stress under elevated CO2 might be due to increased canopy temperatures (Kimball et al. 1999; Högy et al. 2009b). Uncontrolled ROS production might have an impact on grain germination (Bykova et al. 2011).
Future work will be necessary to understand the possible significance of elevated-CO2-induced oxidative stress and the impact of protein carbonylation on grain germination. However, we speculate that the observed decrease in the speed of germination, but not in germination rate per se, could be a consequence of carbonylation of the proteins in addition to reduced seed protein content under elevated CO2 (Hampton et al. 2013). In summary, parental CO2 environment seems responsible for variation in seed vigour, a key character for successful crop production and for the future of seed industry.
Moreover, we have compared the quality of the seeds produced in treatments e[CO2] vs a[CO2] at the two levels of free individual amino-acid content and storage-protein composition. We observed that the relative amounts of Asn, Arg, Asp, Gln, Glu and Trp were significantly higher only in the Y2A samples. Overall, our data indicate that the differences in N, protein, and free amino-acid contents in e[CO2] vs a[CO2] seeds were greater in 2012–13 than in 2011–12. The differences in biomass production and grain yield were consistent between years, suggesting that the observed effect might be due to a lower N uptake or assimilation rate under e[CO2]. Other studies report a decrease in specific amino acids such as Gln, Lys, Arg and Pro (DaMatta et al. 2010) or a general decrease in both essential and non-essential amino acids (Högy et al. 2010). In any case, the variations of several amino acids highlight a relationship among N translocation compounds–N storage and CO2 concentration (Arp et al. 1998), considering that Asp, Asn, Glu and Gln are generally regarded as the major N-translocation compounds in plants (Persson et al. 2006) and Arg is often a major N-storage compound in plants (Winter et al. 2015). Noteworthy also is the variation in Trp, a precursor of several tryptophan-derived signals such as indole-3-acetic acid (IAA or auxins), which are involved in most aspects of plant development (Pelagio-Flores et al. 2011). Finally, observed changes in Asn (4C : 2N) vs Gln (5C : 2N) retrieved suggest different efficiency in terms of C usage at different CO2 concentration (Zheng 2009).
In conclusion, elevated CO2 and other environmental variables seem to interact, giving a range of minor effects on the wheat grain composition.
The whole proteome of seeds grown under e[CO2] vs a[CO2] has also been characterised to obtain better insight into storage protein composition, directly linked to technological properties of wheat seeds. Previous results indicated that elevated CO2 induces alterations in gluten protein-subunit composition, which, in turn, affects the rheological characteristics of wheat flour. For example, the glutenin : gliadin ratio diminished in winter wheat cv. Batis grown under elevated CO2 at low N fertilisation and increased at higher N doses (Wieser et al. 2008). Moreover, a 3-year study of three bread-wheat varieties demonstrated a reduction in grain protein content and an increase in the glutenin : gliadin ratio (Panozzo et al. 2014). Our data are in disagreement with previous reports indicating that rising CO2 levels have a negative impact on HMW-GS content in wheat (Högy et al. 2009b; Fernando et al. 2015); we have observed an accumulation of some HMW-GS under e[CO2]. These findings suggest that the impact of elevated CO2 on glutenin subunit accumulation may depend not only on CO2 level but also on its interaction with the plant genotype or additional environmental variables. Glutenins and gliadins contribute ~80% to grain protein content, with albumin and globulins covering the remaining 20%. In our trials, we have observed a down-accumulation of full-length globulins and up-accumulation of those forms corresponding to endo-proteolytic events. Globulin expression is well known to be environmentally dependent in some wheat cultivars (Altenbach and Kothari 2004; Sancho et al. 2008; Laino et al. 2010; Pompa et al. 2013). This class of proteins is multifunctional, especially in seeds. In addition to their well-established role in nutrient storage, globulins may be important for plant defence against fungi and insects (Macedo et al. 1993; Dunwell et al. 2000; Donaldson et al. 2001; Lane 2002). Various peptide fragments of globulins exhibit antimicrobial activity in several plant species (Chung et al. 1997; Marcus et al.1999; Yamada et al. 1999), and they may play a role in inhibiting the growth of potential pathogens around the germinating seed. The same holds true for other antimicrobial proteins such as a vicilin protein that accumulated in the Y1E samples, and one avenin-like protein and one chitinase that accumulated in Y2E grains. The induction of such proteins cannot be explained simply by the e[CO2] treatment, but might be linked to the microbial populations that developed in the 2 years. In addition, Arachchige et al. (2017) found a higher abundance of avenin-like seed proteins and of serpin-Z1C like defence proteins in wheat genotypes under elevated CO2.
In conclusion, the e[CO2] conditions increased the grain yield and biomass production of a winter wheat (cv. Bologna) grown in a Mediterranean environment and increased its C : N ratio, whereas grain mineral content was not affected, except for a decline in Zn concentration. This Zn decrease has been found in other comparisons involving wheat grains grown at elevated CO2 (Broberg et al. 2017). Moreover, the e[CO2] treatment significantly altered the proteome pattern of cv. Bologna grains. However, the low level of overlap of differentially expressed proteins suggests that many of the changes in the grain proteome of the same cultivar are caused by exposure to elevated CO2 in conjunction with the specific environmental conditions faced during the growing season. The observed changes are therefore highly dependent on interactions with prevailing environmental conditions. This important factor should be taken into consideration when selecting genotypes with desirable resilience to the elevated CO2 conditions in the future.
Conflicts of interest
Authors declare no conflicts of interest.
Acknowledgements
Funding of the FACE experiment through the Duco project by the ‘Fondazione in rete per la ricerca agroalimentare’ within the AGER program is gratefully acknowledged. The work was partially funded by Aliqual RER project.
References
Aina R, Labra M, Fumagalli P, Vannini C, Marsoni M, Cucchi U, Bracale M, Citterio S (2007) Thiol-peptide level and proteomic changes in response to cadmium toxicity in Oryza sativa L. roots. Environmental and Experimental Botany 59, 381–392.| Thiol-peptide level and proteomic changes in response to cadmium toxicity in Oryza sativa L. roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntlCnsw%3D%3D&md5=cb835eefe5eb96b25a479244dc71d712CAS |
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165, 351–372.
| What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2.Crossref | GoogleScholarGoogle Scholar |
Altenbach S, Kothari K (2004) Transcript profiles of genes expressed in endosperm tissue are altered by high temperature during wheat grain development. Cereal Science 40, 115–126.
| Transcript profiles of genes expressed in endosperm tissue are altered by high temperature during wheat grain development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXpslWms7Y%3D&md5=64207d069527341b393864cf47a38ae5CAS |
Arachchige PMS, Ang C-S, Nicolas ME, Panozzo J, Fitzgerald G, Hirotsu N, Seneweera S (2017) Wheat (Triticum aestivum L.) grain proteome response to elevated [CO2] varies between genotypes. Journal of Cereal Science 75, 151–157.
| Wheat (Triticum aestivum L.) grain proteome response to elevated [CO2] varies between genotypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXlvFWkt7Y%3D&md5=c97ba6de7f5381f9a9a307bebae8f248CAS |
Aranjuelo I, Sanz-Sáez Á, Jauregui I, Irigoyen JJ, Araus JL, Sánchez-Díaz M, Erice G (2013) Harvest index, a parameter conditioning responsiveness of wheat plants to elevated CO2. Journal of Experimental Botany 64, 1879–1892.
| Harvest index, a parameter conditioning responsiveness of wheat plants to elevated CO2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmslSltLc%3D&md5=4a7025323a6e9bceaaac4ae1ed10ebdaCAS |
Arp WJ, Van Mierlo JEM, Berendse F, Snijders W (1998) Interactions between elevated CO2 concentration, nitrogen and water: Effects on growth and water use of six perennial plant species. Plant, Cell & Environment 21, 1–11.
| Interactions between elevated CO2 concentration, nitrogen and water: Effects on growth and water use of six perennial plant species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXitlCgsLw%3D&md5=f8bd122ea45a2788b3009c4fef309419CAS |
Badeck F-W, Rizza F (2015) A combined field/laboratory method for assessment of frost tolerance with freezing tests and chlorophyll fluorescence. Agronomy 5, 71–88.
| A combined field/laboratory method for assessment of frost tolerance with freezing tests and chlorophyll fluorescence.Crossref | GoogleScholarGoogle Scholar |
Bloom A, Burger M, Rubio-Asensio J, Cousins A (2010) Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328, 899–903.
| Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlvVeltrc%3D&md5=e3a5ba206d9116cda80f5a37846e3d35CAS |
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254.
| A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XksVehtrY%3D&md5=ec1c81995b5f1a7887c0acb71f446495CAS |
Broberg MC, Högy P, Pleijel H (2017) CO2-induced changes in wheat grain composition: Meta-analysis and response functions. Agronomy 7, 32
| CO2-induced changes in wheat grain composition: Meta-analysis and response functions.Crossref | GoogleScholarGoogle Scholar |
Bykova N, Hoehn B, Rampitsch C, Banks T, Stebbing J-A, Fan T, Knox R (2011) Redox-sensitive proteome and antioxidant strategies in wheat seed dormancy control. Proteomics 11, 865–882.
| Redox-sensitive proteome and antioxidant strategies in wheat seed dormancy control.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXitFGkur4%3D&md5=461a19d758588557bcb00ebc9c138ad4CAS |
Cheeseman J (2006) Hydrogen peroxide concentrations in leaves under natural conditions. Journal of Experimental Botany 57, 2435–2444.
| Hydrogen peroxide concentrations in leaves under natural conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnvF2lsrY%3D&md5=74a0d08d60bb014de9e92891c5f71d2bCAS |
Chung R, Neumann G, Polya G (1997) Purification and characterization of basic proteins with in vitro antifungal activity from seeds of cotton, Gossypium hirsutum. Plant Science 127, 1–16.
| Purification and characterization of basic proteins with in vitro antifungal activity from seeds of cotton, Gossypium hirsutum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXltFWjtrw%3D&md5=977f1ac22e0a542c963c2e62f5cbef75CAS |
Cotrufo MF, Ineson P, Scott A (1998) Elevated CO2 reduces the nitrogen concentration of plant tissue. Global Change Biology 4, 43–54.
| Elevated CO2 reduces the nitrogen concentration of plant tissue.Crossref | GoogleScholarGoogle Scholar |
DaMatta FM, Grandis A, Arenque BC, Buckeridge MS (2010) Impacts of climate changes on crop physiology and food quality. Food Research International 43, 1814–1823.
| Impacts of climate changes on crop physiology and food quality.Crossref | GoogleScholarGoogle Scholar |
Donaldson P, Anderson T, Lane B, Davidson A, Simmonds D (2001) Soybean plants expressing an active oligomeric oxalate oxidase from the wheat gf-2.8 (germin) gene are resistant to the oxalate-secreting pathogen Sclerotinia sclerotiorum. Physiological and Molecular Plant Pathology 59, 297–307.
| Soybean plants expressing an active oligomeric oxalate oxidase from the wheat gf-2.8 (germin) gene are resistant to the oxalate-secreting pathogen Sclerotinia sclerotiorum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xht1Gns74%3D&md5=8d8622c9758ccdc7ad1a88ddea1ea2c0CAS |
Donohue K (2009) Completing the cycle, maternal effects as the missing link in plant life histories. Philosophical Transactions of the Royal Society. B. Biological Science 364, 1059–1074.
| Completing the cycle, maternal effects as the missing link in plant life histories.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXkslGgtrY%3D&md5=fcb0f449c838f5df4784f02978c53115CAS |
Dunwell J, Khuri S, Gane P (2000) Microbial relatives of the seed storage proteins of higher plants, conservation of structure and diversification of function during evolution of the cupin superfamily. Microbiology and Molecular Biology Reviews 64, 153–179.
| Microbial relatives of the seed storage proteins of higher plants, conservation of structure and diversification of function during evolution of the cupin superfamily.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXitFygsrw%3D&md5=d141b7e364be1610aab2b51c0aab0d12CAS |
Dupont F, Vensel W, Tanaka C, Hurkman W, Altenbach S (2011) Deciphering the complexities of the wheat flour proteome using quantitative two-dimensional electrophoresis, three proteases and tandem mass spectrometry. Proteome Science 9, 10–14.
| Deciphering the complexities of the wheat flour proteome using quantitative two-dimensional electrophoresis, three proteases and tandem mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltFKktrs%3D&md5=866ab281f9939faf863fe5a7ff7f5a4eCAS |
Erbs M, Manderscheid R, Jansen G, Seddig S, Pacholski A, Weigel H (2010) Effects of free-air CO2 enrichment and nitrogen supply on grain quality parameters and elemental composition of wheat and barley grown in a crop rotation. Agriculture, Ecosystems & Environment 136, 59–68.
| Effects of free-air CO2 enrichment and nitrogen supply on grain quality parameters and elemental composition of wheat and barley grown in a crop rotation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVGrsr8%3D&md5=2ea048189fbebf4e364634f233c520cdCAS |
Fares C, Menga V, Badeck F, Rizza F, Miglietta F, Zaldei A, Codianni P, Iannucci A, Cattivelli L (2016) Increasing atmospheric CO2 modifies durum wheat grain quality and pasta cooking quality. Journal of Cereal Science 69, 245–251.
| Increasing atmospheric CO2 modifies durum wheat grain quality and pasta cooking quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XlsF2huro%3D&md5=6fc4cfaa705a543d032bc56a8d81e61fCAS |
Fedorova M, Bollineni R, Hoffmann R (2014) Protein carbonylation as a major hallmark of oxidative damage, Update of analytical strategies. Mass Spectrometry Reviews 33, 79–97.
| Protein carbonylation as a major hallmark of oxidative damage, Update of analytical strategies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXitFWnsLc%3D&md5=33b097579f86ef714ff86392e8dae613CAS |
Fernando N, Panozzo J, Tausz M, Norton R, Fitzgerald G, Seneweera S (2012a) Rising atmospheric CO2 concentration affects mineral nutrient and protein concentration of wheat grain. Food Chemistry 133, 1307–1311.
| Rising atmospheric CO2 concentration affects mineral nutrient and protein concentration of wheat grain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtF2hurk%3D&md5=961d2275c006cead3b39ad95dada6f76CAS |
Fernando N, Panozzo J, Tausz M, Norton R, Fitzgerald G, Myers S, Walker C, Stangoulis J, Seneweera S (2012b) Wheat grain quality under increasing atmospheric CO2 concentrations in a semi-arid cropping system. Journal of Cereal Science 56, 684–690.
| Wheat grain quality under increasing atmospheric CO2 concentrations in a semi-arid cropping system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1yqu7zP&md5=013ba455562ea957bdc9240d1ecaae2dCAS |
Fernando N, Panozzo J, Tausz M, Norton R, Neumann N, Fitzgerald G, Seneweera S (2014) Elevated CO2 alters grain quality of two bread wheat cultivars grown under different environmental conditions. Agriculture, Ecosystems & Environment 185, 24–33.
| Elevated CO2 alters grain quality of two bread wheat cultivars grown under different environmental conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXkslSqurs%3D&md5=ec218f01be0c351bc041251eefe8f73fCAS |
Fernando N, Panozzo J, Tausz M, Fitzgerald G, Khan A, Seneweera S (2015) Rising CO2 concentration altered wheat grain proteome and flour rheological characteristics. Food Chemistry 170, 448–454.
| Rising CO2 concentration altered wheat grain proteome and flour rheological characteristics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsFynsbfJ&md5=6de6c7459e7f835735fe66258f24fce8CAS |
Fernando N, Hirotsu N, Panozzo J, Tausz M, Norton RM, Seneweera S (2017) Lower grain nitrogen content of wheat at elevated CO2 can be improved through post-anthesis NH4 + supplement. Journal of Cereal Science 74, 79–85.
| Lower grain nitrogen content of wheat at elevated CO2 can be improved through post-anthesis NH4 + supplement.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXislygsr4%3D&md5=e2dd05851e0ba715500102c6929b9819CAS |
Hampton JG, Boelt B, Rolston MP, Chastain TG (2013) Effects of elevated CO2 and temperature on seed quality. The Journal of Agricultural Science 151, 154–162.
| Effects of elevated CO2 and temperature on seed quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjvVaqsbY%3D&md5=ebb75b150416da8dd794a91cff08735bCAS |
Högy P, Fangmeier A (2008) Effects of elevated atmospheric CO2 on grain quality of wheat. Journal of Cereal Science 48, 580–591.
| Effects of elevated atmospheric CO2 on grain quality of wheat.Crossref | GoogleScholarGoogle Scholar |
Högy P, Wieser H, Köhler P, Schwadorf K, Breuer J, Franzaring J, Muntifering R, Fangmeier A (2009a) Effects of elevated CO2 on grain yield and quality of wheat, results from a 3-year free-air CO2 enrichment experiment. Plant Biology 11, 60–69.
| Effects of elevated CO2 on grain yield and quality of wheat, results from a 3-year free-air CO2 enrichment experiment.Crossref | GoogleScholarGoogle Scholar |
Högy P, Zörb C, Langenkämper G (2009b) Atmospheric CO2 enrichment changes the wheat grain proteome. Journal of Cereal Science 50, 248–254.
| Atmospheric CO2 enrichment changes the wheat grain proteome.Crossref | GoogleScholarGoogle Scholar |
Högy P, Wieser H, Köhler P, Schwadorf K, Breuer J, Erbs M, Weber S, Fangmeier A (2009c) Does elevated atmospheric CO2 allow for sufficient wheat grain quality in the future? Journal of Applied Botany and Food Quality 82, 114–121.
Högy P, Keck M, Niehaus K, Franzaring J, Fangmeier A (2010) Effects of atmospheric CO2 enrichment on biomass, yield and low molecular weight metabolites in wheat grain. Journal of Cereal Science 52, 215–220.
| Effects of atmospheric CO2 enrichment on biomass, yield and low molecular weight metabolites in wheat grain.Crossref | GoogleScholarGoogle Scholar |
Huxman TE, Hamerlynck EP, Jordan DN, Salsman KJ, Smith SD (1998) The effects of parental CO2 environment on seed quality and subsequent seedling performance in Bromus rubens. Oecologia 114, 202–208.
| The effects of parental CO2 environment on seed quality and subsequent seedling performance in Bromus rubens.Crossref | GoogleScholarGoogle Scholar |
IPCC (2013) ‘Climate Change 2013: The physical science basis.’ Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. (Eds TF Stocker, D Qin, G-K Plattner, M Tignor, SK Allen, J Boschung, A Nauels, Y Xia, V Bex, PM Midgley) (Cambridge University Press: Cambridge, UK)
Iriti M, Di Maro A, Bernasconi S, Burlini N, Simonetti P, Picchi V, Panigada C, Gerosa G, Parente A, Faoro F (2009) Nutritional traits of bean (Phaseolus vulgaris) seeds from plants chronically exposed to ozone pollution. Journal of Agricultural and Food Chemistry 57, 201–208.
| Nutritional traits of bean (Phaseolus vulgaris) seeds from plants chronically exposed to ozone pollution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVKmt7bK&md5=45e8b66a71624622a9484fe10acf690aCAS |
Kimball B, LaMorte R, Pinter P, Wall G, Hunsaker D, Adamsen F, Leavitt S, Thompson T, Matthias A, Brooks T (1999) Free-air CO2 enrichment and soil nitrogen effects on energy balance and evapotranspiration of wheat. Water Research 35, 1179–1190.
| Free-air CO2 enrichment and soil nitrogen effects on energy balance and evapotranspiration of wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXivVCitrs%3D&md5=eca9708b029de9131e440af257d6b0b6CAS |
Kimball K, Morris C, Pinter P, Wall G, Hunsaker D, Adamsen F, LaMorte R, Leavitt S, Thompson T, Matthias A, Brooks T (2001) Elevated CO2, drought and soil nitrogen effects on wheat grain quality. New Phytologist 150, 295–303.
| Elevated CO2, drought and soil nitrogen effects on wheat grain quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjvVegsro%3D&md5=464327159c796c07dd4c9aec5c597007CAS |
Koziol A, Loit E, McNulty M, Loit A, McNulty M, MacFarlane A, Scott F, Altosaar I (2012) Seed storage proteins of the globulin family are cleaved post-translationally in wheat embryos. BMC Research Notes 5, 385–395.
| Seed storage proteins of the globulin family are cleaved post-translationally in wheat embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVKjsbfN&md5=b6f02e5ab3ff1746ad9fbf83bd5e60ceCAS |
Laino P, Shelton D, Finnie C, De Leonardis A, Mastrangelo A, Svensson B, Lafiandra D, Masci S (2010) Comparative proteome analysis of metabolic proteins from seeds of durum wheat (cv. Svevo) subjected to heat stress. Proteomics 10, 2359–2368.
| Comparative proteome analysis of metabolic proteins from seeds of durum wheat (cv. Svevo) subjected to heat stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXns1CrtLo%3D&md5=a85b666f1533931d81695c4d7c1f2138CAS |
Lane B (2002) Oxalate, germins, and higher-plant pathogens. IUBMB Life 53, 67–75.
| Oxalate, germins, and higher-plant pathogens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XktlGis7c%3D&md5=d24ff0f378bd8cdf7ae8cf17b3a9bfb2CAS |
Levine R, Williams J, Stadtman E, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods in Enzymology 233, 346–357.
| Carbonyl assays for determination of oxidatively modified proteins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmt1GgtLw%3D&md5=b41099d863d81c3049eb7dcb4f425213CAS |
Loladze I (2002) Rising atmospheric CO2 and human nutrition, toward globally imbalanced plant stoichiometry? Trends in Ecology & Evolution 17, 457–461.
| Rising atmospheric CO2 and human nutrition, toward globally imbalanced plant stoichiometry?Crossref | GoogleScholarGoogle Scholar |
Macedo M, Andrade L, Moraes R, Xavier-Filho J (1993) Vicilin variants and the resistance of cowpea (Vigna unguiculata) seeds to the cowpea weevil (Callosobruchus maculatus). Comparative Biochemistry and Physiology 105, 89–94.
Marcus J, Green J, Goulter K, Manners J (1999) A family of antimicrobial peptides is produced by processing of a 7S globulin protein in Macadamia integrifolia kernels. The Plant Journal 19, 699–710.
| A family of antimicrobial peptides is produced by processing of a 7S globulin protein in Macadamia integrifolia kernels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXntFOnsbo%3D&md5=1125ce8aa78960d82a8c737e4ed8593cCAS |
Marsoni M, Bracale M, Espen L, Prinsi B, Negri S, Vannini C (2008) Proteomic analysis of somatic embryogenesis in Vitis vinifera. Plant Cell Reports 27, 347–356.
| Proteomic analysis of somatic embryogenesis in Vitis vinifera.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXosFWjsQ%3D%3D&md5=07ef9e640b02f8fdc569603075b13165CAS |
Moore S, Stein W (1963) Chromatographic determination of amino acids by the use of automatic recording equipment. Methods in Enzymology 6, 819–831.
| Chromatographic determination of amino acids by the use of automatic recording equipment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVKrs7Y%3D&md5=9cf8a4b09e876153c3259a442dce2624CAS |
Myers S, Zanobetti A, Kloog I, Huybers P, Leakey A, Bloom A, Carlisle E, Dietterich L, Fitzgerald G, Hasegawa T, Holbrook M, Nelson R, Ottman M, Raboy V, Sakai H, Sartor K, Schwartz J, Seneweera S, Tausz M, Usui Y (2014) Increasing CO2 threatens human nutrition. Nature 510, 139–142.
| Increasing CO2 threatens human nutrition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtFygsrfE&md5=ff4c00f8bfc15c0e62e72dac0205f622CAS |
Naudts K, Van den Berge J, Farfan E, Rose P, AbdElgawad H, Ceulemans R, Janssens IA, Asard H, Nijs I (2014) Future climate alleviates stress impact on grassland productivity through altered antioxidant capacity. Environmental and Experimental Botany 99, 150–158.
| Future climate alleviates stress impact on grassland productivity through altered antioxidant capacity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXislGqsr0%3D&md5=1463a57da28e05f490e01084dc72b113CAS |
Nowak RS, Ellsworth DS, Smith SD (2004) Functional responses of plants to elevated atmospheric CO2—do photosynthetic and productivity data from FACE experiments support early predictions? New Phytologist 162, 253–280.
| Functional responses of plants to elevated atmospheric CO2—do photosynthetic and productivity data from FACE experiments support early predictions?Crossref | GoogleScholarGoogle Scholar |
Nuttall JG, O’Leary GJ, Panozzo JF, Walkea CK, Barlow KM, Fitzgerald GJ (2017) Models of grain quality in wheat—A review. Field Crops Research 202, 136–145.
| Models of grain quality in wheat—A review.Crossref | GoogleScholarGoogle Scholar |
O’Leary GJ, Christy B, Nuttall J, Huth N, Cammarano D, Stöckle C, Basso B, Shcherbak I, Fitzgerald G, Luo Q, Farre-Codina I, Palta J, Asseng S (2015) Response of wheat growth, grain yield and water use to elevated CO2 under a Free-Air CO2 Enrichment (FACE) experiment and modelling in a semi-arid environment. Global Change Biology 21, 2670–2686.
| Response of wheat growth, grain yield and water use to elevated CO2 under a Free-Air CO2 Enrichment (FACE) experiment and modelling in a semi-arid environment.Crossref | GoogleScholarGoogle Scholar |
Panozzo J, Walker CK, Partington DL, Neumann NC, Tausz M, Seneweera S, Fitzgerald GL (2014) Elevated carbon dioxide changes grain protein concentration and composition and compromises baking quality. A FACE study. Journal of Cereal Science 60, 461–470.
| Elevated carbon dioxide changes grain protein concentration and composition and compromises baking quality. A FACE study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhslyksrbO&md5=01ede2c0ef051eb6772f05cec259e194CAS |
Pelagio-Flores R, Ortíz-Castro R, Méndez-Bravo A, Macías-Rodríguez L, López-Bucio J (2011) Serotonin, a tryptophan-derived signal conserved in plants and animals, regulates root system architecture probably acting as a natural auxin inhibitor in Arabidopsis thaliana. Plant & Cell Physiology 52, 490–508.
| Serotonin, a tryptophan-derived signal conserved in plants and animals, regulates root system architecture probably acting as a natural auxin inhibitor in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXivVOnsLY%3D&md5=c97903a0dfb3f22132d690af131223ccCAS |
Persson J, Gardeström P, Näsholm T (2006) Uptake metabolism and distribution of organic and inorganic nitrogen sources by Pinus sylvestris. Journal of Experimental Botany 57, 2651–2659.
| Uptake metabolism and distribution of organic and inorganic nitrogen sources by Pinus sylvestris.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xotl2mtL4%3D&md5=ac4f6a1c27b3c0d8100c2a5b850cf43eCAS |
Pompa M, Giuliani M, Palermo C, Agriesti F, Centonze D, Flagella Z (2013) Comparative analysis of gluten proteins in three durum wheat cultivars by a proteomic approach. Journal of Agricultural and Food Chemistry 61, 2606–2617.
| Comparative analysis of gluten proteins in three durum wheat cultivars by a proteomic approach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXis1Sltb8%3D&md5=d79c09d6104be7483a6f24e4d50ff3a1CAS |
Qiu Q, Huber J, Booker F, Jain V, Leakey A, Fiscus E, Yau P, Ort D, Huber S (2008) Increased protein carbonylation in leaves of Arabidopsis and soybean in response to elevated [CO2]. Photosynthesis Research 97, 155–166.
| Increased protein carbonylation in leaves of Arabidopsis and soybean in response to elevated [CO2].Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovF2iu70%3D&md5=59d4b291dabb1296ec6b70609138641aCAS |
Sancho A, Gillabert M, Tapp H, Shewry P, Skegg P, Mills E (2008) Effect of environmental stress during grain filling on the soluble proteome of wheat (Triticum aestivum) dough liquor. Journal of Agricultural and Food Chemistry 56, 5386–5393.
| Effect of environmental stress during grain filling on the soluble proteome of wheat (Triticum aestivum) dough liquor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnt1Cltr0%3D&md5=f1a3137715bf9ba6b592e7ef72f7189eCAS |
Singh J, Blundell M, Tanner H, Skerritt J (2001) Albumin and globulin proteins of wheat flour, immunological and N-terminal sequence characterization. Journal of Cereal Science 34, 85–103.
| Albumin and globulin proteins of wheat flour, immunological and N-terminal sequence characterization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkvFOqtL4%3D&md5=012d44602da0a6001c033b4cc65b76d4CAS |
Taub D, Wang X (2008) Why are nitrogen concentrations in plant tissue lower under CO2? A critical examination of the hypotheses. Journal of Integrative Plant Biology 50, 1365–1374.
| Why are nitrogen concentrations in plant tissue lower under CO2? A critical examination of the hypotheses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVyltb7M&md5=a68a94e0ee920bf2b713c94d9fd33908CAS |
Tausz M, Tausz-Posch S, Norton G, Fitzgerald J, Nicolas M, Seneweera S (2013) Understanding crop physiology to select breeding targets and improve crop management under increasing atmospheric CO2 concentrations. Environmental and Experimental Botany 88, 71–80.
| Understanding crop physiology to select breeding targets and improve crop management under increasing atmospheric CO2 concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjslWkur4%3D&md5=d7fe4dd48b2c485a847e700ba8142791CAS |
Thürig B, Körner C, Stöcklin J (2003) Seed production and seed quality in a calcareous grassland in elevated CO2. Global Change Biology 9, 873–884.
| Seed production and seed quality in a calcareous grassland in elevated CO2.Crossref | GoogleScholarGoogle Scholar |
Wang W, Vignani R, Scali M, Cresti M (2006) Universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis 27, 2782–2786.
| Universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnslCnt7w%3D&md5=2f1be81a830f53f0b1d497f83ce74a2bCAS |
Wieser H, Manderscheid R, Erbs M, Weigel H (2008) Effects of elevated atmospheric CO2 concentrations on the quantitative protein composition of wheat grain. Agriculture and Food Chemistry 56, 6531–6535.
| Effects of elevated atmospheric CO2 concentrations on the quantitative protein composition of wheat grain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXotFGqtb8%3D&md5=d37c2f0b9a4b8b6d4b22b879898b1374CAS |
Winter G, Todd CD, Trovato M, Forlani G, Funck D (2015) Physiological implications of arginine metabolism in plants. Frontiers in Plant Science 6, 534
| Physiological implications of arginine metabolism in plants.Crossref | GoogleScholarGoogle Scholar |
Yamada K, Shimada T, Kondo M, Nishimura M, Hara-Nishimura I (1999) Multiple functional proteins are produced by cleaving Asn–Gln bonds of a single precursor by vacuolar processing enzyme. The Journal of Biological Chemistry 274, 2563–2570.
| Multiple functional proteins are produced by cleaving Asn–Gln bonds of a single precursor by vacuolar processing enzyme.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXovVKjsQ%3D%3D&md5=c03d122ffdc2b7ce272731b3d0096cbeCAS |
Zheng ZL (2009) Carbon and nitrogen nutrient balance signaling in plants. Plant Signaling & Behavior 4, 584–591.
| Carbon and nitrogen nutrient balance signaling in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFGmsbjF&md5=4ee3361c7cc479fd5eb879ad5b880fcdCAS |