First molecular identification of a begomovirus in India that is closely related to Cassava mosaic virus and causes mosaic and stunting of Jatropha curcas L.
S. K. Raj A C , S. K. Snehi A , S. Kumar A , M. S. Khan A and U. Pathre BA Plant Molecular Virology Laboratory, National Botanical Research Institute, Lucknow, 226 001, U. P., India.
B Plant Physiology Laboratory, National Botanical Research Institute, Lucknow, 226 001, U. P., India.
C Corresponding author. Email: skraj2@rediffmail.com
Australasian Plant Disease Notes 3(1) 69-72 https://doi.org/10.1071/DN08028
Submitted: 8 October 2007 Accepted: 4 May 2008 Published: 16 May 2008
Abstract
The association of a begomovirus with Jatropha mosaic disease has been found in north India. The begomovirus possessed highest identities and closest relationships with Indian and Sri Lankan cassava mosaic virus isolates.
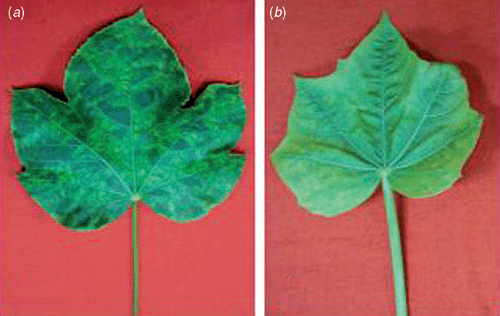
Jatropha curcas L. (of family Euphorbiaceae) is grown in India as a major commercial fuel (bio-diesel) crop. The natural occurrence of a mosaic disease was noticed on J. curcas growing in experimental plots of the National Botanical Research Institute (NBRI), Lucknow, India in the years 2006 and 2007. The disease incidence was significant (~25%). The symptoms of the disease were severe with leaf mosaic and reduced size (Fig. 1a), and stunting of whole plants (Fig. 2). With an acquisition and inoculation period of 24 h, the disease could be transmitted by whitefly (Bemisia tabaci) in a persistent manner but could not be transmitted through mechanical inoculations using leaf sap of an infected plant onto J. curcas seedlings. The whitefly-inoculated J. curcas seedlings developed systemic mosaic and chlorosis at 25–30 days post-inoculation (Fig. 1b). Therefore, infection of a whitefly-transmitted begomovirus was suspected.
|

|
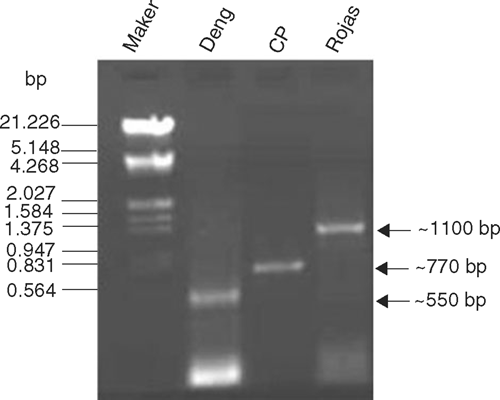
To confirm the association of a begomovirus, polymerase chain reaction (PCR) was carried out using as template total DNA extracted from naturally infected J. curcas leaf tissues. Three sets of begomovirus genus specific primers were employed: Deng A and Deng B (Deng et al. 1994), PALIv 1978 and PARIc 496 (Rojas et al. 1993), and those designed to amplify the coat protein (CP) gene of Tomato leaf curl virus (TLCV, Singh 2005). PCRs were set up in a 50 μL reaction mixture containing: template DNA (100 ng), dNTPs (10 mM each), primers (each 25 pmol), Pfu DNA polymerase (1.5 U), assay buffer (5 μL 10× Banglore Genei Pvt. Ltd) and were cycled 30 times (denaturation: 94°C for 5 min; specific annealing temperatures for 1 min according to the primers used; and extension: 72°C for 1.5 min). The annealing temperatures for Deng, Rojas and TLCV-CP primers were 52°C, 50°C and 47°C, respectively. The final extension cycle was 5 min at 72°C. PCR products were analysed by electrophoresis in 1.2% agarose gels. As expected, bands of ~550, 1100, and 770 bp were consistently amplified by Deng, Rojas and TLCV-CP primers, respectively (Fig. 3), which confirmed the association of a begomovirus with the mosaic disease of J. curcas.
|
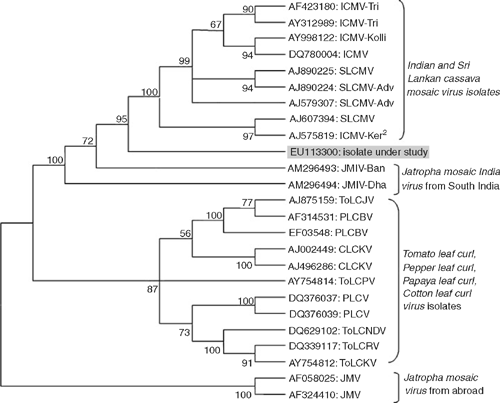
Further, the ~770 bp PCR amplicon obtained with TLCV-CP primers was cloned into pGEM-T easy vector system-1 (Promega Corporation, USA). Three clones were sequenced and the consensus data of three identical sequences was deposited in the GenBank database (Accession EU113300). Basic Local Alignment Search Tool (BLAST) analysis of the virus isolate showed 94.7% (730/771) sequence identity with Indian cassava mosaic virus sequences (AF423180, AY998122, DQ780004, AJ575819) and 94.4% (728/771) with Sri Lankan cassava mosaic virus (AJ579307, AJ607394, AJ890225, AJ890224), which are two begomovirus isolates reported from India (Saunders et al. 2002; Dutt et al. 2005). The nucleotide and amino acid sequences of the virus isolate also showed maximum 94–93% and 97–96% identities, respectively, with Indian and Sri Lankan cassava mosaic virus isolates when the Genomatix DiAlign program was used to align this new sequence with selected begomoviruses from a diverse range of host species (Table 1). Phylogenetic analysis of the virus isolate with selected begomovirus isolates using molecular evolutionary genetics analysis (MEGA) 4.0 version (Tamura et al. 2007) also revealed closest relationships with Indian and Sri Lankan cassava mosaic virus but a more distant relationship with Jatropha mosaic virus (Fig. 4). On the basis of positive PCR amplification, sequence analysis and phylogenetic relationships, the virus was identified as a begomovirus that is closely related to the isolates of Indian and Sri Lankan cassava mosaic viruses instead of Jatropha mosaic virus.
|
There are reports on occurrence of Jatropha mosaic virus on J. gossypifolia, a weed plant from Puerto Rico and Jamaica (Bird 1957; Roye et al. 2006) and natural infection of Jatropha mosaic virus on J. curcas has been reported in south India. However, the Jatropha mosaic virus infected J. curcas plants exhibited mosaic, leaf distortions and blistering (Narayana et al. 2006). Such distortions and blistering were never observed in J. curcas in the case of the virus under study. Neither Indian cassava mosaic virus nor Sri Lankan cassava mosaic virus has been reported earlier on Jatropha curcas. However, there is a report of Indian cassava mosaic virus on bittergourd (Momordica charantia) in Tamil Nadu, India (Rajinimala and Rabindran 2007). We report here the first molecular identification of a begomovirus in India that is closely related to Indian and Sri Lankan cassava mosaic virus that causes a mosaic and stunting symptoms on J. curcas.
Acknowledgement
Authors are thankful to the director, NBRI, Lucknow for facilities and Council of Scientific and Industrial Research (CSIR), New Delhi for funding under NIMITLI project.
Deng D,
McGrath PF,
Robinson DJ, Harrison BD
(1994) Detection and differentiation of whitefly-transmitted geminiviruses in plant and vector insects by the polymerase chain reaction with degenerate primers. The Annals of Applied Biology 125, 327–336.
| Crossref | GoogleScholarGoogle Scholar |

Dutt N,
Briddon RW, Dasgupta I
(2005) Identification of a second begomovirus, Sri Lankan cassava mosaic virus, causing cassava mosaic disease in India. Archives of Virology 150, 2101–2108.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Narayana DSA,
Shankarappa KS,
Govindrappa MR,
Prameela HA,
Gururaj Rao MR, Rangaswamy KT
(2006) Natural occurrence of Jatropha mosaic virus disease in India. Current Science 91, 584–586.

Rajinimala N, Rabindran R
(2007) First report of Indian cassava mosaic virus on bittergourd (Momordica charantia) in Tamil Nadu, India. Australasian Plant Disease Notes 2, 81–82.
| Crossref | GoogleScholarGoogle Scholar |

Rojas MR,
Gilbertson RL,
Russel DR, Maxwell DP
(1993) Use of degenerate primers in the polymerase chain reaction to detect whitefly transmitted geminiviruses. Plant Disease 77, 340–347.

Roye M,
Collins S, Maxwell DP
(2006) The first report of a begomovirus associated with the common weed Jatropha gossypifolia in Jamaica. Plant Pathology 55, 286.
| Crossref | GoogleScholarGoogle Scholar |

Saunders K,
Salim N,
Mali VR,
Malathi VG,
Briddon R,
Markham PG, Stanley J
(2002) Characterisation of Sri Lankan Cassava Mosaic Virus and Indian Cassava Mosaic Virus: evidence for Acquisition of a DNA B Component by a Monopartite Begomovirus. Virology 293, 63–74.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Tamura K,
Dudley J,
Nei M, Kumar S
(2007) MEGA 4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24, 1596–1599.
| Crossref | GoogleScholarGoogle Scholar | PubMed |




