Environmental levels and distribution of structural isomers of perfluoroalkyl acids after aqueous fire-fighting foam (AFFF) contamination
A. Kärrman A C , K. Elgh-Dalgren A , C. Lafossas A and T. Møskeland BA Man-Technology-Environment Research Centre, School of Science and Technology, Örebro University, SE-701 82 Örebro, Sweden.
B Det Norske Veritas (DNV), Veritasveien 1, N-1322 Høvik, Norway.
C Corresponding author. Email: anna.karrman@oru.se
Environmental Chemistry 8(4) 372-380 https://doi.org/10.1071/EN10145
Submitted: 31 December 2010 Accepted: 16 February 2011 Published: 19 August 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Environmental context. Perfluoroalkyl acids are used in many products and have spread into the environment where their persistence and potential toxicity pose a threat to humans and wildlife. The present study describes environmental contamination from usage of aqueous film forming foams, and investigates the distribution of structural isomers of perfluoroalkyl acids from a point source to the surrounding environment. Isomer patterns might be used to track contamination sources since isomer composition differs in the various products containing perfluoroalkyl acids. The environmental behaviour of these structural isomers is described, and limitations of their use to track contamination sources are identified.
Abstract. The environment (soil, water, sediment, fish, crab and mussel) around a training facility using aqueous film forming foams (AFFFs) was studied with respect to perfluorinated alkyl acids (PFAAs) and 6 : 2 fluorotelomer sulfonate (FTS) and their structural isomers. High levels of many PFAAs and 6 : 2 FTS were detected in soil, seepage water, sediment and fish liver. Structural isomers were found for sulfonates, except PFBuS, and for PFOA. Quantification using authentic standards revealed an isomer pattern of 63% linear PFOS (L-PFOS) and 80% linear PFOA (L-PFOA) in the soil at the contamination site, which indicated a source produced by electrochemical fluorination (ECF). The 6 : 2 FTS was 100% linear in all compartments thus coming from a telomerisation product. Enrichment of the linear structure of PFOS and PFOA in soil was seen with increasing distance from the training centre, and an enrichment of branched isomers for both compounds could be found in the seepage water. Sorption to sediment and accumulation in fish liver led to an enrichment of L-PFOS whereas all PFOA remained in the water body.
Additional keywords: 6 : 2 fluorotelomer sulfonates, 6 : 2 FTS, perfluorooctane sulfonate, perfluorooctanecarbocylic acid, PFOA, PFOS.
Introduction
Perfluorinated alkyl acids (PFAAs) are powerful surfactants[1] and have been used in aqueous film forming foams (AFFFs) used to extinguish fires since the 1960s.[2] AFFFs are especially efficient on fuel and solvent fires on which a film is formed that extinguishes the fire and prevents reignition of the volatile flammable liquid. It is known that non-degradable fluorinated surfactants are being used in AFFFs, such as perfluorooctane sulfonate (PFOS) and perfluorooctanecarboxylic acid (PFOA).[3,4] These compounds are under scientific and regulatory investigation since harmful properties have been observed, and PFOS is listed under Annex B in the Stockholm convention on persistent organic compounds.[5] PFOS was mainly produced by 3M Co. using a manufacturing process called electrochemical fluorination (ECF), before their voluntarily phase-out in 2002. Even though PFOS is still produced in Asia and used for specific purposes such as aviation hydraulic fluids, many countries have started to regulate PFOS and replace it when possible.[6] For example, Norwegian regulations from 2005 banned PFOS in AFFFs, textiles and preservatives as a step to phase-out perfluorinated chemicals and severely reduce emissions to the environment.[7] PFOA has historically been produced by ECF but is also commonly produced with the telomerisation method.[4] Other degradable fluorinated surfactants are also produced by telomerisation, such as perfluoroalkyl betaines that are used in DuPonts Forafac AFFF.[8] Degradation products such as 6 : 2 fluorotelomer sulfonate (6 : 2 FTS) have been found in the environment after AFFF usage.[9]
Although ECF gives mixtures of linear and branched products, telomerisation results in linear structures only and thus PFAAs produced by the two processes can be distinguished if structural isomers are separated in an analytical method.[1,10] In liquid chromatography coupled to tandem mass spectrometry (LC/MS/MS), different isomers have different signal response, for example PFOS branched isomers show a weak response in the most common transition used to quantify PFOS in humans and biota, namely m/z 499 → 99.[11] Thus using the linear isomer to quantify branched isomers using m/z 499 → 99 will give biased results. This means that authentic standards should be used in quantifying isomers.
The preservation of isomer patterns in the environment as well as uptake and elimination rates are still not completely clear. Isomeric signatures could potentially be used to differentiate contamination from direct and indirect sources (i.e. precursors) and recent contamination from historical sources. Increased knowledge of physical and biological properties of structural isomers are thus of interest. PFOA structural isomers have been studied in polar bears,[12] water[13] and biota from North America[14,15] to differentiate the source of contamination from ECF or telomerisation products. Similarly, PFOS isomers have been studied in environmental samples.[16–18] So far the studied areas or food webs have been free from a known point source and the origin of the diffuse contamination has been discussed based on the isomer pattern. Several factors besides the source composition might however influence the pattern in different environmental compartments. For example, bioaccumulation factor and water solubility may differ among structural isomers and will affect the environmental fate.
In the present study, a contaminated area was studied to enhance the understanding of behaviour and fate of structural isomers in the environment. Environmental contamination of PFAAs and 6 : 2 FTS at fire-training areas and airports has earlier been reported.[9,19–21] Presented here is a screening of PFAAs and 6 : 2 FTS in the environment around a facility for fire-fighting training at an airport in Flesland, Norway. Structural isomers, if present, were studied in soil samples from the point source (fire-training ground) and in water, sediment and biota from the surrounding environment.
Experimental
Chemicals
Ammonium acetate (>99%, p.a. for HPLC) and n-hexane (Pestanal) were purchased from Fluka (Steinheim, Germany), HPLC-grade methanol (MeOH) was from Fisher Scientific (Leicestershire, UK). Acetonitrile (AcN) and LC-MS-grade water were from Lab-scan (Sowinskiego, Poland). Ammonium hydroxide (NH4OH) 25%, sodium hydroxide (NaOH) p.a., sodium acetate p.a., hydrochloride acid (HCl) and glacial acetic acid (100%) were all purchased from E. Merck (Darmstadt, Germany). Native linear perfluorinated sulfonates (potassium perfluorobutanesulfonate (PFBS), sodium perfluorohexanesulfonate (PFHxS), sodium perfluorooctanesulfonate (PFOS) and sodium perfluorodecanesulfonate (PFDS)), and perfluorinated carboxylates (pentanoic- (PFPeA), hexanoic- (PFHxA), heptanoic- (PFHpA), octanoic- (PFOA), nonanoic- (PFNA), decanoic- (PFDA), undecanoic- (PFUnDA), dodecanoic- (PFDoDA), tridecanoic- (PFTrDA) and tetradecanoic acid (PFTeDA)) were from Wellington Laboratories (Guelph, Canada). Monomethyl- and dimethyl branched PFOS and PFOA standards (Table A1 in the Accessory publication, see http://www.publish.csiro.au/?act=view_file&file_id=EN10145_AC.pdf) were purchased from Wellington Laboratories. Labelled standards were used as internal standards (added before extraction), also from Wellington Laboratories (18O2PFHxS, 13C4PFOS, 13C2PFHxA, 13C4PFOA, 13C5PFNA, 13C2PFDA, 13C2PFUnDA). 7H-perfluoroheptanoic acid (7H-PFHpA) (98%) from ABCR (Karlsruhe, Germany) and 13C8PFOA (Wellington Laboratories) were used as recovery standards (added before injection). 1H,1H,2H,2H-perfluorooctane sulfonate (6 : 2 FTS) (purity not given by supplier) was from Interchim (Montlucon, France).
Samples
Flesland airport is located in the western part of Norway near the city of Bergen and has a fire-drill area. The area is connected to an oil separator and the seepage water is led to a lake which then flows to the sea. Soil was sampled at the training ground, seepage water from the training ground was sampled, sediment and fish liver was taken from the lake were the seepage water flows to, blue mussel and crab were taken from the sea receiving water from the lake. Fish, mussels and crabs were pooled samples from 5 fish, 30 mussels and 5 crabs respectively (Table 1). Only polypropylene bottles were used. Field blanks (empty sampling containers) were brought to each sampling location and opened, exposed to air and closed without adding any sample.

|
Extraction and clean-up
Soil, dry sediment and biota were stored at –20°C until analysis. Wet sediment and water were stored at 4°C. Soil and sediment samples were air-dried before extraction, the water content was also determined by drying for 24 h at 105°C. All biological samples were homogenised before extraction (Ultra-Turrax, IKA). From the homogenate, 1 g of sample was taken in the analytical procedure.
Internal standard (IS) was added to the dried soil or sediment, or to the homogenised biota sample, followed by addition of 0.4 mL of a 0.2-M NaOH (in methanol) solution where after the samples were left for 30 min. Extraction was performed using 4 mL of AcN, ultrasonication for 15 min and shaking for 15 min. The samples were neutralised, centrifuged and the extraction was repeated once more and the two extracts were combined. Clean-up was performed with extraction three times with n-hexane (corresponding to a volume of 2 : 1 sample extract : hexane) and with 50 mg dispersive carbon (Supelclean ENVI-Carb (20/400 mesh), Supelco Bellefonte, PA) to which 100 µL of glacial acetic acid was added. After filtration and evaporation the recovery standards (RS) 7H-PFHpA and 13C8-PFOA were added together with 2 mM ammonium acetate (aq). Blank samples (extraction blanks and field blanks) where performed in parallel with each batch of samples, and were treated in exactly the same manner as the other samples.
Water samples (200–500 mL) were filtered through glass microfibre filters (GF/B, Whatman) before extraction using Oasis WAX (6cc/150 mg, Waters Corporation, Milford, MA, USA) according to standard method ISO 25101.[22] The internal standard mixture was added before extraction. The WAX cartridges were conditioned and the water flow rate was ~1 drop per second. Sodium acetate buffer (4 mL, 0.025 M) was added and the eluate was discarded. After drying the cartridges using vacuum suction, methanol (4 mL) was added and discarded and the analytes were then eluted with 4 mL of 0.1% NH4OH/methanol solution. The eluates were collected, filtered and evaporated to suitable volume with a gentle stream of nitrogen gas. Recovery standards (13C8-PFOA, 7H-PFHpA) and ammonium acetate (aq) were added to the final extract. Extraction and field blank samples were prepared with ultra-pure laboratory produced water and were treated exactly in the same way as the samples.
Chemical analysis and quality assurance
Analysis was performed using an Acquity UPLC coupled to a Quattro Premier XE MS/MS (Waters Corporation) with an atmospheric electrospray interface operating in negative ion mode. Separation was performed on an Acquity BEH C18 2.1 × 50 mm column (100 mm for isomer analysis), 1.7 µm kept at 50°C. A chromatogram illustrating the separation of PFOS and PFOA isomers is shown in Fig. 1. An extra guard column (PFC isolator, Waters Corporation) was inserted between the pump and injector to trap contaminants originating from the LC system. Injection volume was 10 µL and the flow rate was set to 400 µL min–1 (300 µL min–1 for isomer analysis). A gradient program was employed, delivering mobile phases consisting of 2-mM ammonium acetate in methanol, and 2-mM ammonium acetate in water for quantification, and water/methanol/acetonitrile/2-mM ammonium acetate for isomer analysis. Multiple reaction monitoring was used to monitor two product ions for each compound. Quantification of structural isomers was as follows; m/z 499 → 99 was used for monomethyl substituted isomers, m/z 499 → 80 was used for dimethyl substituted isomers. For the coeluting 4,5/5,5-PFOS peak, the missing m/z 499 → 99 signal for 4,5-PFOS was used to determine the respective concentration of both isomers. The average response for 3-PFOS, 4-PFOS and 5-PFOS was used to quantify the sum of those isomers. For PFOA the m/z 413 → 369 transition was used for quantification with the exception of 5,5-PFOA for which m/z 413 → 269 was used. The average response factor of coeluting 3-PFOA, 4-PFOA and 6-PFOA was used to determine the sum of these compounds.
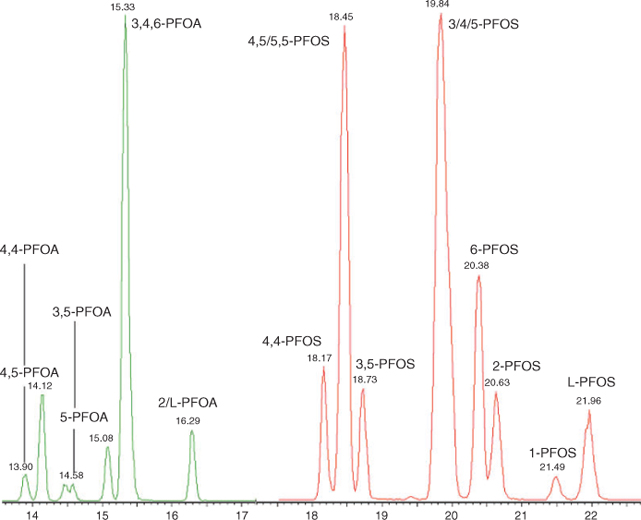
|
Concentration of the analytes in the samples was calculated using internal standard quantification. The internal standard closest in retention time was used for those compounds that did not have a corresponding labelled internal standard (PFBS, PFDS, 6 : 2 FTS, PFPeA, PFHpA, PFTrDA, PFTeDA). Samples were generally diluted five times before injection to minimise ionisation effects. Water samples were in addition diluted 100 times due to high levels and interferences. Detection and quantification for the 100× diluted samples were performed on a Xevo TQS MS/MS (Waters Corporation) with an atmospheric electrospray interface operating in negative ion mode using equivalent settings to the Quattro Premier XE but optimised for the Xevo instrument.
The limit of detection (LOD) was set to 3× the noise level. If trace levels were found in the extraction or field blanks, the LOD was set to 3× the blank signal. Two transitions were measured and the ratio between the qualifier and quantifier ions was calculated and samples with more than 50% difference were not quantified. The recoveries of the internal standards were monitored and native compounds were spiked to PFAA clean matrices (Tables A2 and A3 in the Accessory publication). Acceptable recoveries in this study were set to a range of 50–150% given the wide range of compounds analysed in different matrices. Results with less certainty were however obtained for some compounds and samples (Table A2), recoveries of carboxylates C5–C8 were on average 38–47% in soil, PFTrDA was 24% in mussel and fish liver, PFDS 44% in fish liver and PFHpA 35% in mussel. Ionisation effects due to unknown interferences were probable cause to these low recoveries. PFTeDA was not quantified in biota and PFDA could not be quantified in soil and sediment. 6 : 2 FTS was not quantified in mussel and crab due to signal enhancement and the fact that isotope dilution could not be performed without labelled internal standard. 6 : 2 FTS was detected in fish liver but not quantified for similar reasons. As for isomer specific quantification of PFOS and PFOA, only linear labelled standards are available thus making isotope dilution impossible for branched isomers. A mixture of linear and branched PFOS isomers (Fluka, Steinheim, Germany) was spiked to water and sediment confirming uniform recovery for branched and linear isomers and also indicating isomer specific preservation during the analytical method (Table A4 in the Accessory publication). Uniform recoveries and isomer specific preservation of PFOS and PFOA have been reported by others as well.[13]
Results and discussion
Environmental contamination
The environmental samples taken around the fire-training ground were clearly contaminated to a higher degree than background contamination by a mixture of PFAAs. Elevated levels of sulfonates, carboxylates and 6 : 2 FTS were seen in soil and receiving water (Tables A5–A8 in the Accessory publication). Bioaccumulation in fish liver had taken place for PFOS, PFDS and longer chain carboxylates such as PFTrDA. 6 : 2 FTS was also detected in fish liver. Mainly PFOS, PFHxS and PFTrDA were found in crab, whereas only PFTrDA was found in mussel samples.
Soil at the fire-fighting training ground contained 612 ng g–1 (dry weight, DW) 6 : 2 FTS and 273 ng g–1 DW PFOS (Table A5 in the Accessory publication). Lower levels of PFBuS, PFHxS, PFDS and carboxylates C5–C8, C11, C12 were also detected (up to 6 ng g–1 DW). Even higher levels were quantified in soil 10–20 m from the training ground (2101 ng g–1 DW 6 : 2 FTS and 1905 ng g–1 DW PFOS). The levels thereafter decreased with increasing distance. This spread indicates a movement of the chemicals not only vertically but also horizontally and suggests that the present contamination load to the site’s centre is less now than it has been historically. The velocity of the water movement at the site is not known.
The seepage water (Table A7 in the Accessory publication) contained a suite of sulfonates and carboxylates indicating a major leakage of contaminants from the training ground. Highest levels were found for 6 : 2 FTS (5110–6693 ng L–1) followed by PFOS (1427–2078 ng L–1). Carboxylates with carbon chain length C5–C8 were on average between 155 and 560 ng L–1 but also C9–C11 were detected (3.8–28 ng L–1). PFBuS was on average 97 ng L–1. Confirmation analyses with diluted extracts on Xevo TQS verified high levels in seepage water although 6 : 2 FTS was quantified at up to 25% lower concentrations in the diluted samples. The PFOS levels are however lower than in groundwater from Wurtsmith air force base (the US) that contained 4000–110 000 ng L–1 PFOS.[20]
Sediment from three locations in the lake receiving water from the training area contained PFOS between 35 and 88 ng g–1 DW, 6 : 2 FTS was on average 7 ng g–1 DW, although the second highest level was found for PFUnDA (average 15 ng g–1 DW) (Table A6 in the Accessory publication). Lower contamination was seen for the other studied compounds except for PFOA, PFNA and PFTDA, that were <LOD.
Fish liver (fresh weight, FW) from the lake contained several compounds at relatively high concentrations, average values (n = 4) were 2281 ng g–1 PFOS, 101 ng g–1 PFUnDA, 52 ng g–1 PFDS, 44 ng g–1 PFTrDA (Table A8 in the Accessory publication). PFOS and longer chain carboxylates (≥C9) therefore show bioaccumulation properties which have previously been reported in the literature.[23] 6 : 2 FTS was also found in fish liver suggesting a bioaccumulation; however the concentration could not be calculated due to signal enhancement and lack of labelled internal standard. Relatively few reports exist on 6 : 2 FTS in the scientific literature and the high levels in soil, water and detection in fish liver in the present study make 6 : 2 FTS of environmental concern.
Crab and mussel (Table A8 in the Accessory publication) were taken from where the lake flows out to the sea and only PFTrDA was found in mussel (average 0.41 ng g–1 FW). Low levels were also found in crab with PFOS having the highest concentration (average 2.3 ng g–1 FW) followed by PFTrDA (average 1.3 ng g–1 FW).
Isomer pattern
Isomer analysis using a slower chromatographic system showed more than one peak for the sulfonates PFHxS, PFOS and PFDS. PFBuS could not be properly analysed for structural isomers due to relatively short retention time and it is not clear whether they exist. Benskin et al. have shown that branched isomers are more frequent in residuals of longer carbon chains, i.e. C8 and C10.[24] In addition, PFHpS and PFOSA (not quantified) showed more than one peak. Sulfon-based perfluorinated chemicals have been produced by ECF and the major producer up until 2002 was 3M, resulting in products containing both branched and linear structures. The polyfluorinated 6 : 2 FTS showed only one single peak in the analysed samples suggesting a telomer contamination source. Branched PFOA isomers could be quantified in soil and water.
The isomer pattern of PFOS in soil was 63% linear PFOS (L-PFOS) at the training ground (0 m), assuming an ECF contamination source (Fig. 2 and Table A9 in the Accessory publication). The linear fraction is somewhat lower than expected for an ECF product typically produced by 3M although some variations in technical product composition have been reported.[25,26] The reason for this is unknown, however if PFOS precursors are used at the training centre, preferential degradation of the branched precursor structures could theoretically take place resulting in a higher fraction of branched PFOS isomers.[27] In soil the percentage of L-PFOS thereafter increased up to 85% linear at 100 m from the centre. It should be noted that this change in isomer pattern did not correlate with the total PFOS concentration change observed, for which the highest concentration was found 10 m from the centre. At 150 m, L-PFOS suddenly decreased in proportion but increased again at the next sampling point for unknown reasons (Fig. 2). Assuming that the isomer pattern at 0 m reflects the pattern in the contamination source (AFFF), horizontal spread results in enrichment of the linear structure. Thus branched structures are not sorbed to soil at the same extent as the linear, but migrate with water. 6-Monometyl branched PFOS (12.9%) and the cluster of 3/4/5-monomethyl branched PFOS (16.7%) were the highest level branched isomers at 0 m and consequently decreased in proportion with increasing distance from the centre reaching 4.6 and 9.1% at 200 m respectively. Although 6-PFOS and 1-PFOS (5% at 0 m and 1.9% at 200 m) showed a similar decrease rate, the proportion of the cluster of 3/4/5-PFOS decreased relatively more with increasing distance. Dimethyl-branched isomers were also detected in soil and 3,5-PFOS decreased with distance to the same extent as the cluster 3/4/5-PFOS (~50% decrease). The pattern of dimethyl-isomers should however be interpreted carefully due to concentrations relatively close to the detection limit. The influence of the isomer’s structure on the behaviour in soil stresses the importance to monitor isomers individually rather than as a total sum.

|
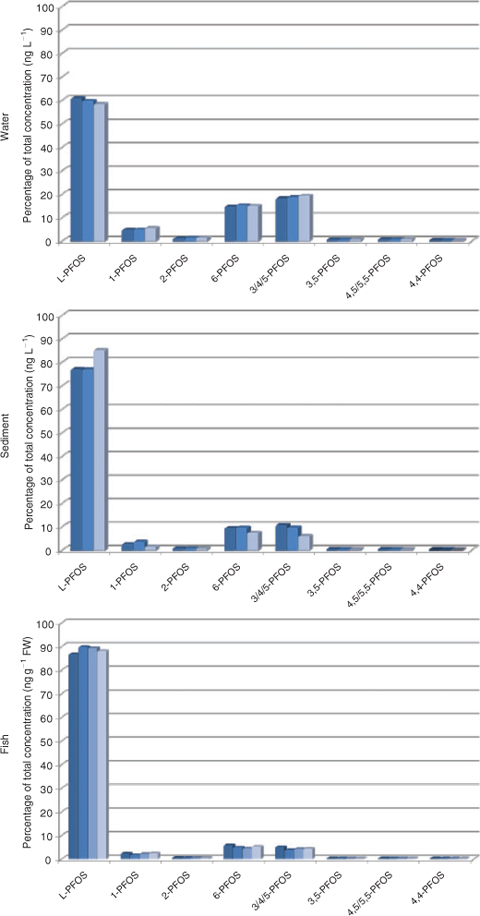
The isomer pattern thereafter changed drastically in the receiving water body, to 58–61% L-PFOS (Fig. 3 and Table A10 in the Accessory publication), thus branched structures are more water soluble, which is in agreement with the observation made in a Lake Ontario food web reported by Houde and colleagues,[16] although the branched content in the present seepage water is higher compared with Lake Ontario water (L-PFOS 43–56%). Lake Ontario water contained a mean of 5.9 ng L–1 PFOS compared with 1695 ng L–1 PFOS in the seepage water. Isomers present at the source of contamination (0 m) but not detected in soil at a distance from the source were found enriched in water. The isomer pattern in water closely resembles that of soil at 0–10 m, except for 4,5/5,5-PFOS (0.6% of total or 3.3 ng L–1 in water and 0.23% or 5.1 ng g–1 in soil). The water result together with soil characteristics indicates that branched isomers are enriched in the water-phase, but it should be noted that isomer-specific losses to surfaces during storage of water in plastic bottles were not evaluated.
|
Sediment showed an enrichment of L-PFOS with an average value of 78.5% L-PFOS (Fig. 3 and Table A10 in the Accessory publication). The most prominent isomers are the same as in water and soil, namely 3/4/5-PFOS and 6-PFOS. Dimethyl-branched isomers were also detected but at low concentrations.
In fish liver from the lake receiving contaminated water, a percentage of 87–90% L-PFOS was seen, suggesting accumulation of the linear isomer (Fig. 3 and Table A13 in the Accessory publication). Lake trout from Lake Ontario contained 88–93% L-PFOS[16] and the highest level isomers were 6-PFOS (3.8–7.1%) and 3/4/5-PFOS (2.5–4.1%). This is in accordance with the observed pattern in the present study (6-PFOS 4.3–5.7%, 3/4/5-PFOS 3.7–5.0%) despite the fact that trout from Lake Ontario had considerable lower contamination (present study 2281 ng g–1, Lake Ontario estimated at 95 ng g–1). However, the 1-PFOS was higher in the present study (1.6–2.3%) compared with Lake Ontario trout (0.9–1.1%), which might be concentration related rather than an actual difference. Crab and mussel had lower levels of PFOS and thus patterns of the less prominent branched isomers become less accurate.
PFOA found in soil (Fig. 4 and Table A11 in the Accessory publication) contained 80% linear isomer at the training centre (0 m), which suggests there is a clear contribution from a ECF source. PFOA produced by 3M using ECF has been reported to contain ~18% branched isomers,[28] which corresponds to the pattern found at the fire-fighting ground. The most prevalent isomers were 5-PFOA and the cluster of 3/4/6-PFOA. With increasing distance from the centre, the linear isomer is enriched and after 75 m, only the linear isomer was found. The PFOA concentration at 75 m is the same as at 0 m, which means that the isomer pattern of 100% L-PFOA at 75 m is not an effect of branched isomers being below the detection limit. 4,4-PFOA was also detected in soil showing a different pattern compared with other branched isomers with levels increasing from 0 to 10 m and could thereafter not be detected in remaining soil samples or in the seepage water. The proportion of linear PFOA in water was on average 74.6% (Fig. 4 and Table A12 in the Accessory publication), which is also less than in soil from the centre of the training ground and reported technical ECF PFOA, showing an enrichment of branched isomers in water. Different from that of PFOS, PFOA is not detected in any other matrix and is thus not accumulated in fish nor sorbed to sediment. PFOA isomers in water have been reported previously from Lake Ontario[14] (6–13% branched isomers) and different water bodies in Japan, the USA, China and the Netherlands[13] (13–19% branched isomers).

|
The contrary was seen for 6 : 2 FTS, which only showed one single peak thus suggesting a telomerisation source. Quantification of branched isomers of other PFAAs (PFHxS, PFHpS PFDS, PFOSA) was inhibited by the lack of standards. The other PFAAs that showed possible branched isomers were relatively low concentrated in soil. Water contained higher concentrations and the isomer pattern based on area showed a similar pattern to that of PFOS, with the tendency that higher carbon chain compounds, i.e. PFDS and PFOSA, show higher content of branched isomers.
Conclusion
The environment close to the fire-fighting training facility was highly contaminated by PFAAs and high levels of PFOS were detected despite the 2005 ban of PFOS in AFFFs. 6 : 2 FTS or its precursor(s), is a likely component in AFFFs used at the site, given the high levels found in soil and seepage water. The different environmental behaviour for branched and linear isomers as suggested here could be due to differences in soil sorption and water solubility. In this study the contamination came from AFFF-usage with clear ECF-contribution as indicated by PFOS and PFOA isomers in the soil. However, when sampling soil 100 m from the spill, the isomer-pattern wrongly indicates a much less ECF-contribution, and, in the case of PFOA, a pure telomerisation contamination. Measuring water samples might show a higher fraction of branched isomers compared with the contamination source due to the described fractionation. Using structural isomer patterns to track the source of contamination is therefore limited. Besides physicochemical properties, the time between contamination and sampling could potentially also affect the isomer pattern.
Acknowledgement
The Norwegian Climate and Pollution Agency (KLIF) is acknowledged for funding this work (from the ‘Statlig program for forurensningsovervåkning’ program). Thanks are extended to Bård Nordbø, Ingunn Correll Myhre and Jon L. Fuglestad (KLIF) for continuous feedback. Thanks also to Christian Volan (DNV), Marte Braathen (DNV), Arne Pettersen (NGI) and Bergen Havfiskerlag (local angler association) for assistance with sampling and logistics. We are also grateful for permission and assistances of site managers at Flesland airport in Bergen. Waters Corporation (Paul Silcock and Keith Worrall) is acknowledged for quality assurance work.
References
[1] E. Kissa, Fluorinated Surfactants and Repellents, 2nd edn (Ed. A. T. Hubbard) 2001 (Marcel Dekker Inc.: New York).[2] C. A. Moody, J. Field, Perfluorinated Surfactants and the environmental implications of their use in fire-fighting foams. Environ. Sci. Technol. 2000, 34, 3864.
| Perfluorinated Surfactants and the environmental implications of their use in fire-fighting foams.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlsFCksLw%3D&md5=524f2356bf4a65deb8a8cf43bbe333c1CAS |
[3] A. G. Paul, K. C. Jones, A. J. Sweetman, A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ. Sci. Technol. 2009, 43, 386.
| A first global production, emission, and environmental inventory for perfluorooctane sulfonate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVyks7%2FJ&md5=63238d1759e406591a922fb0ed3a709cCAS |
[4] K. Prevedouros, I. T. Cousins, R. C. Buck, S. H. Korzeniowski, Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32.
| Sources, fate and transport of perfluorocarboxylates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1Gru7zK&md5=14e91a9069cf17a00b47907f105e54b6CAS |
[5] The new POPs – An introduction to the nine chemicals added to the Stockholm convention by the Conference of the Parties at its fourth meeting, August 2010 2010 (Secretariat of the Stockholm Convention United Nations Environment Programme). Available at http://chm.pops.int/Programmes/New%20POPs/Publications/tabid/695/language/en-US/Default.aspx [Verified 4 April 2011].
[6] 3M phase-out plan for POSF-based products. EPA Docket 0PPT-2002-0043-0009 2002 (US Environmental Protection Agency: St Paul, MN). Available at http://www.fluoridealert.org/pesticides/pfos.fr.final.docket.0009.pdf [Verified 25 April 2011].
[7] Perfluoralkylstoffer (PFAS) og perfluoroktanylsulfonat (PFOS)-relaterte forbindelser. Handlingsplan 2005 (The Norwegian Climate and Pollution Agency (KLIF)). Available at http://www.klif.no/arbeidsomr/kjemikalier/pfos/pfas_handlingsplan.pdf [In Norwegian. Verified 4 April 2011].
[8] DuPont Forafac 1157 2010. Available at http://www2.dupont.com/Forafac/en_US/assets/downloads/Forafac11571.pdf [Verified 4 April 2011].
[9] M. Schultz, D. F. Barofsky, J. Field, Quantitative determination of fluorotelomer sulfonates in groundwater by LC MS/MS. Environ. Sci. Technol. 2004, 38, 1828.
| Quantitative determination of fluorotelomer sulfonates in groundwater by LC MS/MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXosl2jtA%3D%3D&md5=bd1c38c927aca152100c5e5bd504e473CAS |
[10] 3M, Voluntary use and exposure information profile, perfluorooctane sulfonic acid and various salt forms. US EPA Report AR226-0928 2000 (US Environmental Protection Agency).
[11] N. Riddell, G. Arsenault, J. P. Benskin, B. Chittim, J. W. Martin, A. McAlees, R. McCrindle, Branched perfluorooctane sulfonate isomer quantification and characterization in blood serum samples by HPLC/ESI-MS(/MS). Environ. Sci. Technol. 2009, 43, 7902.
| Branched perfluorooctane sulfonate isomer quantification and characterization in blood serum samples by HPLC/ESI-MS(/MS).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFCqtbbJ&md5=0d5272677575709c0b7c8d377b5d2b7bCAS |
[12] A. O. De Silva, S. A. Mabury, Isolating isomers of perfluorocarboxylates in polar bears (Ursus maritimus) from two geographical locations. Environ. Sci. Technol. 2004, 38, 6538.
| Isolating isomers of perfluorocarboxylates in polar bears (Ursus maritimus) from two geographical locations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXptF2lsr4%3D&md5=e5c534296cd8c1392f48bbc6e6c4963cCAS |
[13] J. P. Benskin, L. W. Yeung, N. Yamashita, S. Taniyasu, P. K. S. Lam, J. W. Martin, Perfluorinated acid isomer profiling in water and quantitative assessment of manufacturing source. Environ. Sci. Technol. 2010, 44, 9049.
| Perfluorinated acid isomer profiling in water and quantitative assessment of manufacturing source.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtl2jtbbK&md5=ff0ac893d479356431cf3497abbd62d0CAS |
[14] A. O. De Silva, D. C. Muir, S. A. Mabury, Distribution of perfluorocarboxylate isomers in selected samples from the North American environment. Environ. Toxicol. Chem. 2009, 28, 1801.
| Distribution of perfluorocarboxylate isomers in selected samples from the North American environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVKmt7rF&md5=838c450284307d4b965a80ff1f8a5d70CAS |
[15] V. I. Furdui, P. A. Helm, P. W. Crozier, C. Lucaciu, E. J. Reiner, C. H. Marvin, D. M. Whittle, S. A. Mabury, G. T. Tomy, Temporal trends of perfluoroalkyl compounds with isomer analysis in Lake Trout from Lake Ontario (1979–2004). Environ. Sci. Technol. 2008, 42, 4739.
| Temporal trends of perfluoroalkyl compounds with isomer analysis in Lake Trout from Lake Ontario (1979–2004).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmsFOltbc%3D&md5=954dc8b4aed94b068aae016882e8d3dcCAS |
[16] M. Houde, G. Czub, J. M. Small, S. Backus, X. Wang, M. Alaee, D. C. G. Muir, Fractionation and bioaccumulation of perfluorooctane sulfonate (PFOS) isomers in a Lake Ontario food web. Environ. Sci. Technol. 2008, 42, 9397.
| Fractionation and bioaccumulation of perfluorooctane sulfonate (PFOS) isomers in a Lake Ontario food web.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlyisbjP&md5=abca416bfa31123f22da887eb1115a5dCAS |
[17] S. Chu, R. J. Letcher, Linear and branched perfluorooctane sulfonate isomers in technical product and environmental samples by in-port derivatization-gas chromatography-mass spectrometry. Anal. Chem. 2009, 81, 4256.
| Linear and branched perfluorooctane sulfonate isomers in technical product and environmental samples by in-port derivatization-gas chromatography-mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlt1SgsLg%3D&md5=8daef32530d74be83b60760e13a3a182CAS |
[18] W. A. Gebbink, R. J. Letcher, Linear and branched perfluorooctane sulfonate isomer patterns in herring gull eggs from the colonial sites across the Laurentian Great Lakes. Environ. Sci. Technol. 2010, 44, 3739.
| Linear and branched perfluorooctane sulfonate isomer patterns in herring gull eggs from the colonial sites across the Laurentian Great Lakes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltFWgsbs%3D&md5=9fd384d471e4a4ef2c9e2a8f0aaec4d3CAS |
[19] C. A. Moody, J. A. Field, Determination of perfluorocarboxylates in groundwater impacted by fire-fighting activity. Environ. Sci. Technol. 1999, 33, 2800.
| Determination of perfluorocarboxylates in groundwater impacted by fire-fighting activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXktFektrw%3D&md5=4ce9c071f659e016bbfd0d7414b229c2CAS |
[20] C. A. Moody, G. N. Hebert, S. H. Strauss, J. Field, Occurrence and persistence of perfluorooctanesulfonate and other perfluorinated surfactants in groundwater at a fire-training area at Wurtsmith Air Force Base, Michigan, USA. J. Environ. Monit. 2003, 5, 341.
| Occurrence and persistence of perfluorooctanesulfonate and other perfluorinated surfactants in groundwater at a fire-training area at Wurtsmith Air Force Base, Michigan, USA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXitleqtrk%3D&md5=3c764fbbbd2ceeb28642c131f00bd059CAS |
[21] K. D. Oakes, J. P. Benskin, J. W. Martin, J. S. Ings, J. Y. Heinrichs, D. G. Dixon, M. R. Servos, Biomonitoring of perfluorochemicals and toxicity to the downstream fish community of Etobicoke Creek following deployment of aqueous film-forming foam. Aquat. Toxicol. 2010, 98, 120.
| Biomonitoring of perfluorochemicals and toxicity to the downstream fish community of Etobicoke Creek following deployment of aqueous film-forming foam.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmtFahtrY%3D&md5=27d62a86c977f0677a018e842e8b7f37CAS |
[22] Water quality – Determination of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) – Method for unfiltered samples using solid phase extraction and liquid chromatography/mass spectrometry ISO/DIS 25101:2009 2009 (International Organization of Standardization). Available at http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=42742 [Verified 4 April 2011].
[23] J. M. Conder, R. A. Hoke, W. De Wolf, M. H. Russell, R. C. Buck, Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ. Sci. Technol. 2008, 42, 995.
| Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXltFWmsw%3D%3D&md5=a5859b58e71eafefd64bade38f26a3c1CAS |
[24] J. P. Benskin, A. O. De Silva, J. W. Martin, Isomer profiling of perfluorinated substances as a tool for source tracking: a review of early findings and future applications. Rev. Environ. Contam. Toxicol. 2010, 208, 111.
| Isomer profiling of perfluorinated substances as a tool for source tracking: a review of early findings and future applications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlWjt77L&md5=da2f7e058f4bdd66257f7cada8195a8eCAS |
[25] G. Arsenault, B. Chittim, J. Gu, A. McAlees, R. McCrindle, V. Robertson, Separation and fluorine nuclear magnetic resonance spectroscopic (19F NMR) analysis of individual branched isomers present in technical perfluorooctanesulfonic acid (PFOS). Chemosphere 2008, 73, S53.
| Separation and fluorine nuclear magnetic resonance spectroscopic (19F NMR) analysis of individual branched isomers present in technical perfluorooctanesulfonic acid (PFOS).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVWitLzN&md5=04080835b001ccc527d0c534f2f4d371CAS |
[26] S. M. Vyas, I. Kania-Korwel, H. Lehmler, Differences in the isomer composition of perfluoroctanesulfonyl (PFOS) derivatives. J. Environ. Sci. Health A 2007, 42, 249.
| Differences in the isomer composition of perfluoroctanesulfonyl (PFOS) derivatives.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXitl2ju7o%3D&md5=a7192b5cea1ea320c5172dea8732edfdCAS |
[27] J. P. Benskin, A. Holt, J. W. Martin, Isomer-specific biotransformation rates of a perfluorooctane sulfonate (PFOS)-precursor by cytochrome P450 isozymes and human liver microsomes. Environ. Sci. Technol. 2009, 43, 8566.
| Isomer-specific biotransformation rates of a perfluorooctane sulfonate (PFOS)-precursor by cytochrome P450 isozymes and human liver microsomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht12msrvI&md5=b15120e08693e81530727cd34559df0eCAS |
[28] S. E. Loveless, C. Finlay, N. E. Everds, S. R. Frame, P. J. Gillies, J. C. O’Connor, C. R. Powley, G. L. Kennedy, Comparative responses of rats and mice exposed to linear/branched, linear, or branched ammonium perfluorooctanoate (APFO). Toxicology 2006, 220, 203.
| Comparative responses of rats and mice exposed to linear/branched, linear, or branched ammonium perfluorooctanoate (APFO).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhvVWksL8%3D&md5=ae4cd4814cda61a698afceb2da1cf15aCAS |


