Organic phosphorus in the aquatic environment
Darren S. BaldwinCSIRO Land and Water and the Murray–Darling Freshwater Research Centre, La Trobe University, PO Box 991, Wodonga, Vic. 3689, Australia. Email: darren.baldwin@csiro.au

Darren Baldwin is a biogeochemist based at the Murray–Darling Freshwater in Wodonga, Australia. His current research examines how natural and human-induced perturbations affect the movement and transformation of carbon and nutrients in aquatic ecosystems. |
Environmental Chemistry 10(6) 439-454 https://doi.org/10.1071/EN13151
Submitted: 8 August 2013 Accepted: 21 October 2013 Published: 19 December 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Environmental context. Organic phosphorus can be one of the major fractions of phosphorus in many aquatic ecosystems. This paper discusses the distribution, cycling and ecological significance of five major classes of organic P in the aquatic environment and discusses several principles to guide organic P research into the future.
Abstract. Organic phosphorus can be one of the major fractions of phosphorus in many aquatic ecosystems. Unfortunately, in many studies the ‘organic’ P fraction is operationally defined. However, there are an increasing number of studies where the organic P species have been structurally characterised – in part because of the adoption of 31P NMR spectroscopic techniques. There are five classes of organic P species that have been specifically identified in the aquatic environment – nucleic acids, other nucleotides, inositol phosphates, phospholipids and phosphonates. This paper explores the identification, quantification, biogeochemical cycling and ecological significance of these organic P compounds. Based on this analysis, the paper then identifies a number of principles which could guide the research of organic P into the future. There is an ongoing need to develop methods for quickly and accurately identifying and quantifying organic P species in the environment. The types of ecosystems in which organic P dynamics are studied needs to be expanded; flowing waters, floodplains and small wetlands are currently all under-represented in the literature. While enzymatic hydrolysis is an important transformation pathway for the breakdown of organic P, more effort needs to be directed towards studying other potential transformation pathways. Similarly effort should be directed to estimating the rates of transformations, not simply reporting on the concentrations. And finally, further work is needed in elucidating other roles of organic P in the environment other than simply a source of P to aquatic organisms.
Additional keywords: 31P NMR, analysis, eutrophication, freshwater, marine, reactive phosphorus, sediment, soil, virus.
Introduction
Phosphorus is an essential element in aquatic ecosystems. It can be the limiting nutrient controlling primary production (energy production), particularly so in many freshwater ecosystems, but also in some marine systems. High concentrations of phosphorus can contribute to excessive algal and macrophyte growth and associated water quality issues. Therefore, understanding the processes underlying P dynamics is important for the ongoing assessment and management of aquatic ecosystems.
Organic phosphorus refers to a diverse group of chemical compounds that contain both carbon and phosphorus atoms in the same molecule.[1,2] Although organic phosphorus species can account for a substantial amount of the extracellular P found in many aquatic environments, our understanding of their role in aquatic biogeochemical and ecological processes is far less advanced than our understanding of the role of inorganic forms of P – particularly the orthophosphate ion (HxPO4(3 – x)–). This may be a consequence of the often cited misconception that only orthophosphate is ecologically relevant and hence organic P is considered less important. That being said, one of the main reasons for the lack of progress in this area is the limited availability of suitable tools and techniques to easily and routinely quantify and characterise organic P in environmental samples. Although there have been a plethora of approaches to the characterisation and quantification of organic P in the aquatic environment[2–7] each approach has its limitations. Attempts to quantify and characterise organic P in aquatic ecosystems have ranged from the very simple to the technologically advanced. At its simplest dissolved ‘organic’ P has simply (and incorrectly) been defined as the difference between the total filterable P (determined by a chemical digestion step followed by a colourimetric assay) and filterable reactive P (often assumed to be orthophosphate and determined by filtration followed by colourimetric assay).[8] This is notwithstanding the fact that the most common colourimetric assay used for filterable reactive P quantification (the molybdenum blue technique) may also hydrolyse several known organic compounds[9] (although this has been questioned).[10] At the other end of the spectrum researchers have used several sophisticated (and expensive) instruments to explore organic P speciation including ultra-high field mass spectrometry,[11] X-ray absorption near edge structure (XANES) spectroscopy[12,13] (which requires a source of synchrotron radiation), and solution and solid state 31P NMR spectroscopy.[14] Of these more sophisticated approaches, based on the number of publications, solution 31P NMR spectroscopy appears to have been the most influential.
Notwithstanding the 500+ papers in the literature that deal with organic P in the aquatic environment (freshwater, estuarine, coastal and oceanic) it is difficult to develop a consensus on the importance of organic P in these environments. This is in part because many of these studies do not actually measure organic P as such, but rather report on the dynamics of operationally defined fractions. In solution, organic P is often defined as the difference between reactive P and total P[8]; in sediments it has been defined as the P remaining at the end of a sequential extraction scheme.[15] The former should more properly be referred to as non-reactive P, the latter recalcitrant P. It is only by examining specific organic P compounds, or classes of compounds, that we gain any sense of the importance and dynamics of organic P in aquatic environments. There are five classes of organic P species that have been well characterised in the aquatic environment – nucleic acids, other nucleotides, inositol phosphates, phospholipids and, to a lesser extent, phosphonates. (A sixth class of compounds that could have been included in this review, UV-sensitive humic–Fe–P compounds,[16] although interesting in themselves, are most likely Fe–orthophosphate complexes[17] and therefore not strictly organic P compounds.) This paper explores how each of these classes of compounds have been characterised and quantified in a variety of aquatic environments, explores the processes underlying their biogeochemical cycling and looks at their ecological significance. Insights gained from an exploration of the biogeochemical dynamics and ecological relevance of these compounds are then used to identify guiding principles for future research in this under-studied area.
Classes of organic P compounds in the aquatic environment
Nucleic acids (DNA and RNA)
Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are found in all living organisms. DNA encodes the genetic instructions for development and function of living organisms whereas RNA plays several roles in the coding, decoding regulation and expression of genes. In DNA and RNA the ribose sugar units of nucleotide monomers (Fig. 1) are joined to each other through the phosphate group on one sugar coordinating with the alcohol linkages on another sugar – forming a phosphodiester.
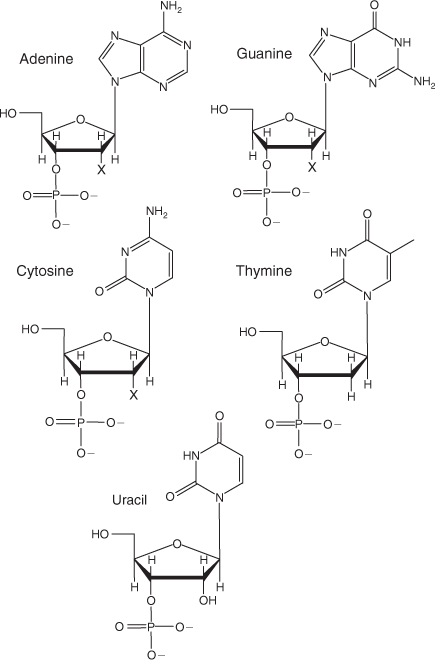
|
Identification
Many different approaches have been used to identify and quantify DNA and, to a lesser extent, RNA in aquatic samples. Some of the earliest studies that identified DNA in water samples used fluorescence assays. In this approach the DNA was isolated from other organic P by ion-exchange chromatography[18] or precipitation with cetyltrimethylammonium bromide (CTAB).[19] The DNA could then be quantified through reaction with a fluorophore (e.g. 3,5-diaminobenzoic acid or 4,6-diamidino-2-phenyl indole – DAPI) that binds to the DNA. In some instances this approach overestimated the DNA present, therefore Siuda and Chróst[20] modified the CTAB–DAPI approach by determining the fluorescence before and after treatment with the hydrolytic enzyme DNAase. Using this technique they could differentiate between free DNA and DNA that was encapsulated in viral particles or otherwise stabilised on particles. RNA has also been quantified in samples containing both DNA and RNA fluorometrically (using ethidium bromide) by comparing fluorescence of samples treated with and without RNAase.[21]
DNA and RNA have also been identified in both the water column and in sediments by 31P NMR spectroscopy. Earlier 31P NMR studies were limited in their characterisation of organic P into essentially four classes – orthophosphate, phosphomonoesters, phosphodiesters (which would include the nucleic acids) and phosphonates. Increases in the field strength of instruments coupled with techniques to minimise line broadening caused by paramagnetic ions (e.g. Fe) in the samples has allowed the unequivocal assigning of peak signals to both DNA and RNA.[22]
Quantification
Notwithstanding the methods of detection, several studies have shown that in many cases DNA, or its partially degraded derivatives, is the most common form of dissolved organic P in aquatic environments. For example, in a study of the water column in 21 lakes in Poland and Germany, Siuda et al.[23] determined that total dissolved DNA accounted for between 16 and >100 % (mean 54 %) of the total P. A subsequent study showed that between 50 and 90 % of the DNA was enzymatically hydrolysable and therefore truly dissolved DNA, not viral particles.[20] They also found that both the actual amount and relative proportion of dissolved DNA was higher in hypertrophic lakes than eutrophic lakes, which in turn was greater than for mesotrophic lakes. Similarly in a study of dissolved DNA in Tokyo Bay, Sakano and Kamatani[21] found that DNA accounted for between 12 and >100 % (mean 49 %) of the total dissolved P. In the same study the authors found that RNA composed between 0 and >100 % (mean 36 %) of the total dissolved P pool; RNA was detected in all but three samples (detection limit 1.8 µg L–1). The DNA concentration was substantially higher than RNA in 19 of their samples, the concentrations were approximately equal in eight samples and the concentration of RNA was substantially greater than DNA in six samples. Conversely Beebee[24] could identify DNA but not RNA in ‘natural freshwater samples’ – although the paper gives no details of where the samples were taken from or how many were analysed. Beebee[24] was also critical of the fluorometric determination of nucleotides (as was the case in both the Polish lake and Tokyo Bay studies). Beebee[24] suggested, but did not demonstrate, that fluorometric approaches overestimate nucleic acid concentrations, at least in the case of RNA. Only one study has used 31P NMR spectroscopy to quantify dissolved nucleic acids in aquatic systems.[25] That study showed that DNA accounted for up to 67 % of the non-reactive P in the water column in a series of Danish lakes; RNA concentrations were not reported.
DNA is also present in aquatic sediments, although not always to the same relative proportion of the total P as found for nucleic acids in the water column. For example Reitzel et al.,[26] used 31P NMR spectroscopy to determine DNA concentrations in NaOH extracts of depth profiles through the sediment of a Swedish mesotrophic lake. DNA accounted for ~2–7.5 % of the total P through the sediment core; with a maximum DNA concentration between 5 and 6 cm below the surface. It should be noted though that they pre-extracted the sediment with citrate-bicarbonate dithionite (CBD) to remove paramagnetic iron from the sample. CBD has been shown to extract organic P from sediments.[27] Conversely, Turner et al.,[28] also using 31P NMR spectroscopy but without CBD pre-treatment, showed that almost all of the organic P fraction in sediments from a constructed wetland was DNA – with some RNA hydrolysis products also present.
RNA has also been detected in NaOH extracts of aquatic sediment,[29] but its relative contribution to the total P in the sediment was not stated. It is interesting to note that a two-dimensional 1H–31P NMR correlation spectroscopic study of NaOH extracts of soil gave strong evidence that RNA is hydrolysed during the NaOH extraction procedure.[30]
Biogeochemistry
There is little available information on the biogeochemical cycling of RNA in aquatic environments; most of the available data refers specifically to DNA. The source and abundance of DNA in any given water body will depend on the relative abundance of particular groups of organisms and their mortality. Although higher organisms can contribute to the dissolved DNA pool,[29,31] algae and bacteria appear to be the main sources of DNA in both marine[32–35] and freshwater systems.[36,37] Several studies have identified viral-induced cell lysis as an important pathway for DNA release from cells. Reisser et al.[34] observed an increase in virus-like particles following the crash of an algal bloom. The increase in viral particles was closely followed by an increase in dissolved DNA. In a subsequent laboratory experiment they showed that viral-induced lysis increased both the virus numbers and amount of dissolved DNA. A similar result was observed in a laboratory experiment looking at marine bacteriophages and dissolved DNA.[35] Riemann et al.[38] estimated that ~25 % of dissolved DNA in the Baltic Sea was from viral lysis of bacterioplankton. Predation of bacteria by marine nanoflagellates,[32] protists[35] and freshwater ciliates[37] and subsequent excretion of excess DNA[32] has also been identified as another important source of extracellular DNA in aquatic ecosystems – as has ‘sloppy’ predation of phytoplankton by rotifers[39] and potentially by other organisms like protists.
The principal pathway for DNA breakdown appears to be enzymatic. In a study on the degradation of tomato plants in freshwater ecosystems, Bravo et al.[31] showed that DNA could be leached from the plants on immersion, but some of the DNA was degraded by intracellular nuclease activity before release. However, most studies attribute DNA breakdown to a suite of extracellular enzymes including phosphatase and nucleases.[40,41] Abiotic hydrolysis facilitated by mineral phases[42] is potentially an alternate pathway for the degradation of DNA in aquatic ecosystems – but it has not been demonstrated. The only abiotic degradation pathway that has been demonstrated to date is the breaking of DNA double strands induced by radiation from dissolved uranium.[43]
Sedimentation appears to be an important pathway for the loss of dissolved DNA from the water column.[29] As seston ages there is a relative increase in phosphodiesters[44] much of which is DNA.[29] Much of this is most probably bound within microbial cells growing on the settling particles,[44] but it is likely that some will be associated with the inorganic matrix. There is a large body of work suggesting that DNA can attach to clays.[45] An X-ray diffraction and molecular mechanics study has shown that DNA can form intercalated structures in layered (swelling) clays – the DNA resides between clay layers and is bound to the clay structure through phosphate bonds to clay-bound interlayer cations (e.g. Ca2+ or Mg2+). Although the bound DNA is potentially stabilised by the interaction with the clay and therefore not readily mineralised,[46] studies of DNA residence times in sediments suggest that sediment-bound DNA is not totally recalcitrant. In a study of sediment P speciation in a Swedish Lake, DNA concentrations did not reach a minimum asymptotic concentration with depth, indicating that it was still being hydrolysed.[26] Ahlgren et al.[47] estimated the half-life of DNA in sediments of the Baltic Sea to be ~8.5 years whereas Dell’Anno and Danovaro[48] estimated the residence time of DNA in deep ocean sediments was ~9.5 years compared to 40+ years for organic P in general.
Ecological significance
DNA can be an important source of P in aquatic environments especially when orthophosphate concentrations are low.[40,48] In a series of mesocosm experiments using radioactive labelled orthophosphate, adenosine triphosphate (ATP) and DNA, Løvdal et al.[49] showed that, even though orthophosphate was the preferred P substrate for bacteria and algae, both ATP and DNA were utilised in mesocosms where orthophosphate was also present. In mesocosms without added orthophosphate the turnover time for dissolved DNA decreased by a factor of 10 (from 15.6 to 1.5 h) with no liberation of free orthophosphate-indicating a tight coupling between hydrolysis and uptake. There was also a transition from uptake of P dominated by large organisms (i.e. algae) to small organisms (bacteria) when DNA and ATP was the only P source. Other studies have also recognised the importance of DNA as a P source in low orthophosphate environments. For example in the deep sea environment Dell'Anno and Danovaro[48] estimated that 47 % of the daily prokaryotic P demand was met by dissolved DNA (as well as 4 % of C demand and 7 % of prokaryotic N demand). Another environment where orthophosphate can be scarce is in Fe-rich sediments. Orthophosphate preferentially adsorbs to iron minerals compared to organic P species.[50] Therefore in Fe-rich sediments there may be substantially more dissolved organic P than orthophosphate. Shewanella is a genus of dissimilatory metal-reducing bacteria found in such environments. A laboratory study has shown that several Shewanella species were capable of using extracellular DNA not only as their sole P source but also as their sole source of both carbon and energy.[40]
Exogenous DNA plays an important role in the ecology of biofilm-forming bacteria including Shewanella sp. and Vibrio cholera. DNA is an important structural component of the extracellular polymer matrix of biofilms.[51–53] For V. cholera extracellular DNA is implicated in the development of biofilm architecture, nutrient acquisition and biofilm detachment.[53] For Shewanella sp. it is believed that prophages in the bacterial genome are important for biofilm development because of the release of extracellular DNA on cell lysis.[52]
Other nucleotides (e.g. ATP, GTP and AMP)
In addition to DNA and RNA several other nucleotides have been identified in aquatic environments (Fig. 2). They include adenosine 5′-triphosphate (5′-ATP), adenosine 5′-monophosphate (5′-AMP) and guanosine 5′-triphosphate (5′-GTP), all of which are involved in cellular energy transfer, and cyclic adenosine 3′:5′ monophosphate (cAMP) which is an intracellular messenger chemical.
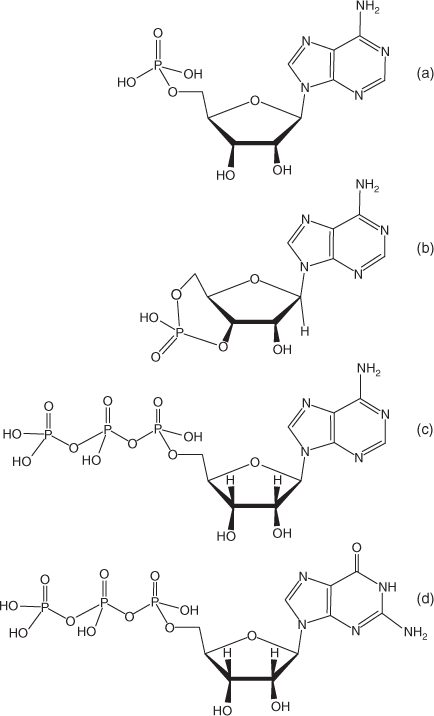
|
Identification
Some nucleotides (e.g. 5′-AMP and cAMP) appear to undergo some hydrolysis during the usual molybdenum blue colourimetric assay reaction for dissolved orthophosphate and therefore will be reported as reactive rather than organic P[9] (although this has been questioned[54]).
5′-ATP and 5′-GTP have been identified in marine systems[55] whereas cAMP has been identified in lakes.[56] These nucleotides were identified using analytical biochemical reactions. 5′-ATP and 5′-GTP were identified by the luciferin–luciferase bioluminescence reaction with pre-concentration on Mg(OH)2[55]; 5′-ATP has also been pre-concentrated using charcoal.[57] cAMP has been determined in lake waters[56] using the Gilman protein binding assay.[58] Monophosphate nucleotides including 3′-AMP, 5′-AMP, cAMP, 5′-GTP, cytidine 5′-monophosphate and uridine 5′-monophosphate have been identified, but not quantified, in lake sediments from Sweden using liquid chromatography followed by electrospray ionisation tandem mass spectroscopy.[59]
Quantification
Where they have been measured, it would appear that simple nucleotides only represent a very small proportion of the phosphate pool present in the aquatic environment, with concentrations reported in the picograms to nanograms of phosphorous per litre range. For example, ATP and GTP represented only ~0.1 % of the organic P pool in the surface water in the North Pacific Ocean.[55]
Biogeochemistry
Little is known about the biogeochemical cycling of the simple nucleotides in aquatic environments. They are obviously of biological origin and it has been suggested that peak production (and hydrolysis) occurs during algal blooms, at least in Antarctic marine waters.[57] Both 5′-ATP and 5′-AMP will adsorb to iron minerals, but to a lesser extent than orthophosphate.[50] The adsorption involves a two-step process: it has been postulated that the first (rapid) step involves surface adsorption, followed by a slower migration into the interior of the particle. Assuming that facilitated hydrolysis of the nucleotide doesn’t occur at the mineral surface,[42] migration of the nucleotide into the mineral particle could help protect the nucleotide from enzymatic hydrolysis.
Ecological significance
Although the concentration of nucleotides in the aquatic environment is low, a number studies have suggested that they may play an important role in P cycling in certain environments, particularly oligotrophic systems.[60] For example in the North Pacific subtropical Gyre, because ATP is both rapidly produced and rapidly consumed, it was estimated that the P flux through the ATP pool was up to five times faster than the general organic P pool.[55] Similarly in a mesocosm experiment Løvdal et al.[49] showed that the turnover time of ATP under P-limited conditions was only 5 min.
Cyclic AMP (and potentially 5′-ATP) may play another role in aquatic ecosystems other than a P source – i.e. quorum sensing.[61,62] Quorum sensing is a form of communication between bacterial cells based on chemical signalling. Bruns et al.[61,62] showed that there was a substantial increase in the number of both marine and freshwater bacterial species that could be cultured in the presence of cAMP, indicating that it is involved in microbial population changes. However the ecological significance of cAMP as a signalling chemical still needs to be resolved.[63]
Inositol phosphates
Inositol phosphates are a group of compounds in which a central inositol group (in one of nine potential isometric forms) is bound to between one and six phosphate groups by phosphomonoester bonds.[64] The most common of these in terrestrial soils is myo-inositol hexaphosphate (myo-IHP; Fig. 3). Myo-IHP is also called phytic acid if it is a free acid form or phytate if it is a salt – usually with alkali or alkaline earth metals. Myo-IHP is the principal storage chemical for phosphorus in plants, and is especially common in seeds.
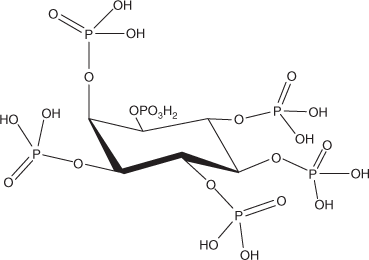
|
Identification
The analysis and distribution of inositol phosphates in aquatic environments has been the subject of several recent reviews[64,65] and therefore will only be dealt with briefly here; with particular emphasis on the more recent literature.
There have been numerous studies of inositol phosphates in soils at least dating back to the 1950s.[64] Initial studies on both freshwater and marine sediments adopted similar approaches as that used for soils. This usually involved sediment pre-extraction to remove metals and extraction of the inositol phosphates, usually into hot NaOH, followed by analysis. In a study of sediments from the Camargue wetlands in the south of France De Groot and Golterman[66] first pre-extracted the sediment with nitrilotriacetic acid-dithionite and ethylenediamine tetraacetic acid (EDTA) to remove Fe- and Ca-bound P respectively; they then extracted the phytate with hot (90 °C) 2 N NaOH and subsequently used an enzyme (phytase) to hydrolyse the phytate in the NaOH extract. It must be noted however, that phytases from some sources not only hydrolyse inositol hexaphosphates (IHPs), but can also dephosphorylate a suite of other organic phosphate species.[64] Suzumura and Kamatani[67] used a much more elaborate scheme to extract and quantify IHPs from the sediments of Tokyo Bay. They first prewashed the sediment with 1 M HCl and then extracted the phytate using hypobromite oxidation (Br2 in 3 N NaOH). The hypobromite extract was then further purified by acidification and treatment with a cation-exchange resin. Finally the IHPs were isolated by anion-exchange chromatography and identified by 31P NMR spectroscopy and gas chromatography (following esterification to acetyl derivatives). In the last decade inositol phosphates have mostly been identified in sediments using 31 P NMR spectroscopy on NaOH–EDTA sediment extracts; although there has been at least one study that used XANES.[13] Interestingly, in one study, inositol phosphates could be detected using high field mass spectroscopy, but not 31P NMR spectroscopy.[68]
Relative to sediments, there have been far fewer studies of inositol phosphates in the water column. Most have used phytase to hydrolyse phytate to orthophosphate[69–71]; although, as pointed out by several authors, phytase is not absolutely specific for inositol phosphates but can also hydrolyse other organic P esters.[64,71] Inositol phosphates have also been detected in NaOH–EDTA extracts of freeze-dried water samples using 31P NMR spectroscopy[72] although, as will be discussed later, this technique is probably not applicable for routine analysis.
Quantification
Although it has been said that large amounts of inositol phosphates are present in aquatic environments[64] the literature suggests that reality is more equivocal. De Groot and Golterman[64] estimated that inositol phosphates represented between 3 and 22.5 % (mean = 11.5 %) of the total sedimentary P in sediments of the Camarge, whereas Keller et al.,[13] using XANES spectroscopy, found that inositol phosphates accounted for 15 and 29 % of the total P in two sediment samples from a creek in the upper reaches of the Mississippi River; however much lower concentrations have been found elsewhere. For example both Turner and Newman[73] and Cheesman et al.[74] were unable to detect IHPs in a series of natural and constructed Florida wetlands. IHPs were not detected in the sediments of 6 out of 15 Danish lakes surveyed using 31P NMR spectroscopy.[75] In the lake sediments that did contain IHPs, it accounted only for between 1 and 10 % (mean = 4.4 %) of the total phosphorus pool in the top 1 cm of sediment. Similar levels of IHPs have been found in near shore coastal sediments. Suzumura and Kamatani[67] found that IHPs accounted for only 0.3–0.5 % of the total P in sediment from Tokyo Bay and inositol phosphates accounted for 0.5–4 % of the total P in a sediment core taken from a marine embayment near Helsinki.[76]
Biogeochemistry
The biogeochemistry of inositol phosphates in aquatic ecosystems remains, to some extent, enigmatic. Inositol phosphates are produced by plants. On decomposition the plant inositol phosphates are bound to, and stabilised by, soil particles.[77,78] The main pathway for inositol phosphates entering aquatic ecosystems appears to be through runoff – although this observation is based on a single study.[79] The study showed that although both the soil in the catchment and the suspended sediments in three major rivers entering Tokyo Bay had much higher IHP concentrations than the sediment within the bay, they all had similar enantiomeric signatures. Therefore the authors concluded that soil from the catchment was the principal source of IHP to the bay. Desorption of IHP from soil particles caused by increasing salinity as the soil particles enter estuaries has been proposed as a possible mechanism of mobilising IHP.[71]
Runoff from manure on pastures or from intensive agriculture has also been identified as a potential source of inositol phosphates to aquatic ecosystems. Non-ruminant vertebrates usually can’t break down inositol phosphates, so the manure of animals such as pigs and chickens tends to contain significant quantities of these compounds,[80] especially if they are fed a diet rich in inositol phosphates (e.g. one containing a large amount of grain). Similarly, seeds and grains rich in inositol phosphates are a common constituent in plant-derived fish feed used in aquaculture[81] and hence the compounds are directly added to some aquatic ecosystems.
Inositol phosphates can also be produced in situ in aquatic environments. Several species of floating macrophytes including Spirodela sp.[82] and Azolla sp.[83] have been found to contain either inositol phosphates, enzymes necessary for the synthesis of IHPs, or both. In a Spanish marsh substantially more IHP was detected in the sediments directly under stands of the emergent macrophytes Juncus subulatus and Scripus maritmus than in open water sites.[84] Subsequent analysis (based on hot NaOH extraction and subsequent hydrolysis with phytate) showed that the seeds of J. subulatus contained ~16 mg P g–1 of IHP.[84]
The major pathway for inositol phosphate degradation in aquatic ecosystems appears to be through enzymatic degradation. Phytases are a class of enzymes that are responsible for the dephosphorylation of inositol phosphates; although most can also hydrolyse other organic P species (see above). Of the four different classes of phytase, bacterial β propeller phytases appear to be the only class found in aquatic environments.[85,86] However, the extent of inositol phosphate degradation in the aquatic environment is contentious. In their study of IHP in Tokyo Bay, Suzumura and Kamatani[79] essentially presented a model where IHP is exported from the catchment attached to soil particles. Based on the measured ratio of IHP to total organic P on sediment particles there was little apparent degradation of IHP during transport down the rivers and into the estuary. Only once it reached Tokyo Bay (i.e. exposed to marine salinities) did the ratio of IHP to total organic P substantially reduce, indicating decomposition. The study by Turner and Weckstrom[76] is consistent with this model. They measured IHP (using 31P NMR spectroscopy) in a sediment core in a shallow embayment near the centre of Helsinki. Although partially open to the sea, the salinity in the embayment was only approximately one-sixth that of seawater. They found the IHP fraction in the sediments to be stable enough to suggest that it could be used as a paleo-indicator of phosphorus input to the bay. Similarly, Jørgensen et al.[75] noted the preservation of IHPs in sediments of a freshwater lake. Taken together these results suggest that exposure to a saline environment is essential for IHP degradation – possibly through release of IHP from sediment particles with increasing salinity.[71] Conversely, Hill and Cade-Menun,[87] again using 31P NMR spectroscopy, found that the IHP concentration in the sediments of drainage ditches adjacent to poultry farms in the Chesapeake catchment was substantially lower than that found in either poultry litter or cropland where the poultry litter was spread as a fertiliser. They attributed this difference to potential degradation of IHP between the farm and the drainage ditch.
Ecological significance
The ecological significance of IHP in aquatic environments remains uncertain. The Tokyo Bay study would suggest that IHP from the catchment could potentially be an important source of phosphorus to the near shore coastal environment. Indeed, in a laboratory study Suzumura and Kamatani[88] showed that under simulated marine conditions approximately half of the IHP added (at 0.75 µmol g–1 wet weight) to a sediment slurry under aerobic conditions disappeared in 60 days, whereas all the IHP disappeared in less than 40 days under anaerobic conditions. Most of the P hydrolysed from the IHP could be accounted for either in solution or adsorbed to the sediments indicating that it was available for subsequent incorporation into biomass.
The relevance of IHP to freshwater aquatic ecosystems is far less certain. Notwithstanding the title of their paper, although Golterman et al.[89] could identify IHP in aquatic sediments from a variety of wetlands from France and Spain, they did not show that this could be a source of P to the overlying water under anerobic conditions; they merely assumed it could be based on the work of Suzumura and Kamatani[88] in Tokyo Bay. It should be noted here that release of P from sediments under anaerobic conditions is a major pathway in the cycling of P in freshwater ecosystems but that conventionally it is believed to be attributable to the reductive dissolution of iron with concomitant release of orthophosphate that was adsorbed to the Fe mineral surface.[90]
It may be possible that inositol phosphates in freshwater ecosystems are more important as a carbon source than a source of P. In a laboratory study Siuda and Chróst[91] added various organic P substrates to water from Lake Constance (which had sufficient inorganic P already present) and observed the microbial dynamics. They showed that after an acclimatisation period, addition of IHP resulted in a significant increase in bacterial numbers although only a modest amount of the substrate disappeared from solution. This was compared to AMP where a significant amount of the substrate was depleted from solution, but there was little microbial growth. The authors concluded that although AMP was being utilised as a P source for the bacteria, IHP was actually being used as a carbon source.
Phospholipids
Phospholipids are a class of lipids that contain phosphate mono- or di-ester groups in the polar region of the molecule (Fig. 4). Phospholipids are one of the major components of cell membranes and have been extensively studied in environmental samples – not necessarily as a P source[92] but as a way to identify microbial community structure,[93] as well as food web dynamics.[94]
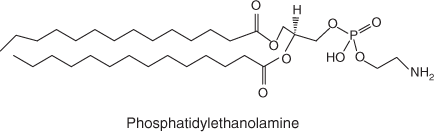
|
Identification
Methods for the identification and quantification of phospholipids in the marine environment were reviewed by Suzumura[92] and will only be briefly dealt with here. Phospholipids are extracted from either the water column or particulate matter using solvent extraction, usually with a chloroform and methanol mix. Once extracted total P in the organic phase can be determined to yield total phospholipid concentration. Alternatively the phospholipids can further be separated by a variety of chromatographic techniques including thin layer chromatography and HPLC. Typically for studies using phospholipids to characterise microbial community structures, the fatty acids are esterified with methanol before analysis by gas chromatography[93]; although rapid advances in ecogenomic characterisation of microbial communities will probably make this approach less common in the near future.[95]
31P NMR spectroscopy is not suitable for the detection of phospholipids – they are not directly extracted into NaOH which is commonly used to isolate organic P from aquatic samples. In fact there is some evidence that the NaOH extractant hydrolyses phospholipids producing glycerophosphates that are observed in the spectra.[75]
Quantification
Quantitative studies on phospholipids in aquatic ecosystems, particularly those that also quantify total P and or total organic P, are not common.[92] In one study that compared dissolved and particulate phospholipids in coastal marine environments, dissolved phospholipids represented less than 1 % of total dissolved organic P and between 3 and 13 % of total particulate P.[96] It is not surprising that the particulate fraction contained more phospholipids than the dissolved fraction. Phospholipids are not very soluble in water and would tend to be concentrated at interfaces, including on particulate matter, and, potentially, at the air–water interface. Furthermore, phospholipids are a major component of plankton which is a part of the particulate fraction. For example Oku and Kamatani[97] found that planktonic phospholipid P accounted for up to 22 % of total P in Tokyo Bay.
Biogeochemistry
Relative to other organic phosphorus compounds little is known about the biogeochemical cycling of phospholipids in aquatic ecosystems. Because they tend to concentrate at interfaces, it would be expected that the major pathway for the lateral and longitudinal movement of phospholipids through aquatic ecosystems would be as particulates – either as living cells or attached to particles. They can also be transported in stream systems in the form of aquatic foams where they are associated with humic materials.[98] The hydrolysis of phospholipids and their role as a P source in aquatic ecosystems is not well known.[92]
Ecological significance
As noted earlier, most research on phospholipids in aquatic ecosystems has focussed either on their use as a biomarkers for specific microorganisms, or their role in food webs and nutrition, both of which are outside the scope of this review. One important area where phospholipids can factor in the ecological functioning of aquatic ecosystems is as a sink for phosphorus, particularly in oligotrophic systems. For example Van Mooy et al.[99] have shown that in some marine systems phospholipid production by bacterioplankton can account for more than 20 % of all P uptake. Interestingly, when P becomes limiting several phytoplankton species will replace phospholipids with other, non P containing lipids,[100] which in turn may interfere with food web studies based on lipid composition.
Phosphonates
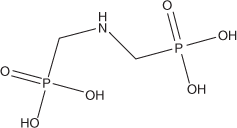
Phosphonates are a broad class of organic P compounds that contain a direct C–P bond. Natural phosphonates include a second class of phosphorus-containing lipids – the phosphonolipids.[101] Synthetic phosphonates are becoming more prevalent in the aquatic environment.[102] Of particular interest is the herbicide N-(phosphomethyl)glycine, also known as glyphosate (Fig. 5), which is the active ingredient in several commercial herbicides including Roundup. In part a result of the advent of genetically modified corn and soybean that are resistant to glyphosate, the amount of glyphosate applied to crops in the United Sates increased 8-fold between 1992 and 2007.[103]
|
Identification
It was only with the application of 31P NMR spectroscopy to explore P speciation that phosphonates were identified as potentially important fractions of dissolved and particulate P in aquatic samples.[104,105] The C–P bond in phosphonates has a specific resonance at 25 ppm in 31P NMR spectra which is substantially different from resonances associated with other P bonds to unequivocally identify phosphonates within a sample. However, little other structural information about the phosphonate structure is available from the spectra.
There are several methods available for the identification and quantification of synthetic phosphonates in aquatic samples – particularly for glyphosate. Earlier methods required pre-concentration (e.g. by ligand exchange and anion exchange), derivatisation and then separation and identification by gas chromatography–mass spectrometry.[106] More recently a technique has been described that uses direct determination, without the need for concentration or derivatisation, based on mixed-mode reversed-phase and weak anion-exchange chromatography with tandem mass spectrometry detection.[107]
Quantification
There are few studies that have quantified phosphonates (as a broad class of compounds) in aquatic samples, and in those studies that have it appears that the relative importance of phosphonates to the total P pool is quite variable. 31P NMR studies have shown that phosphonates account for between 5[108] and 25 %[109] of the dissolved organic phosphorus pool in marine samples – with most of the phosphonates associated with the high molecular weight fraction.[109] Phosphonates in marine particulate matter seems more variable. Benitez-Nielsen et al.[110] report that phosphonates account for between 3 and 18 % of the total P in sediment traps deployed in the Cariaco Basin, with the lowest values found in the deepest (1255 m) traps. Conversely, Paytan et al.[111] measured P speciation in a diverse series of particulate marine samples and could only identify phosphonates in a few samples, and then at only low concentrations. It has been reported that phosphonates account for between 6[112] and 20 %[110] of the total P in marine sediments. Phosphonates appear to be less prevalent in freshwater ecosystems compared to marine systems.[72,113–117]
Biogeochemistry
Like phospholipids, little is known about the biogeochemical cycling of phosphonates in aquatic ecosystems.[102] Although often assumed to be recalcitrant, recent molecular studies have shown that a diverse range of freshwater and marine bacteria and phytoplankton have genes associated with the production of hydrolytic enzymes capable of catalysing the breakdown of phosphonates.[118–121] Studies have also shown that phosphonates, particularly glyphosate, can be broken down by Fe-catalysed photolysis, at least in the laboratory.[122]
Ecological significance
Again, little is known on the ecological significance of phosphonates. Based on the loss of phosphonates from particulate matter with depth it has been suggested[110] that phosphonates may be an important and unrecognised source of P to aquatic environments, at least for some organisms. For example, it has been suggested that organisms like the cyanobacteria Trichodesmium sp., can outcompete other marine cyanobacteria in oligotrophic oceanic waters because they can hydrolyse phosphonates.[119] Hydrolysis of phosphonates can also be a source of bioavailable carbon for marine bacteria.[121] Interestingly, the hydrolysis of phosphonates has been implicated in the production of methane in aerobic oceanic waters.[123,124]
Synthesis
It is only by examining specific organic P compounds, or classes of compounds, that we can get any sense of the importance and dynamics of organic P in aquatic environments. But even then our knowledge is scant. For example in their review of inositol phosphates in the environment Turner et al.[64] devote ~5.5 pages to inositol phosphate dynamics in terrestrial ecosystems (mostly soils) and ~1 page to inositol phosphates in aquatic ecosystems. Similarly, in his review of inositol phosphates in aquatic environments McKelvie[65] devotes most of his paper to describing the occurrence of inositols in different aquatic environments; the section on inositol phosphate dynamics is, for the most part, speculative. Rather than a criticism of either paper, this highlights the paucity of papers that materially and unambiguously deal with inositol P dynamics (as opposed to occurrence) in aquatic environments.
Clearly organic P has the potential to affect the way aquatic ecosystems function either directly by providing a source of P (particularly where P is limiting) or indirectly through other mechanisms (see below), but further research is necessary. In this section a number of approaches are outlined that may be fruitful for ongoing research.
Ongoing need to develop methods for quickly and accurately identifying and quantifying organic P species in the environment
At its simplest organic P in the aquatic environment has been (incorrectly) defined either as the difference between reactive P and total P in the dissolved phase or the residue at the end of a sequential extraction sequence of suspended matter or sediments. These approaches are inexpensive, convenient and rapid. However, the insights gained from such an approach can only be limited. Many of the recent and most enlightening advances in our understanding of organic P dynamics in the aquatic environment have come about through the use of 31P NMR spectroscopy. The power of 31P NMR spectroscopy is that it produces information about the chemical structure of the P species present. However 31P NMR spectroscopy is not necessarily the most ideal approach for routine analysis of aquatic samples. First, the instruments are extremely expensive, costing upwards of US$500 000 to purchase. High field instruments also require ongoing maintenance – consuming both liquid helium and liquid nitrogen. Sample throughput is slow, with an acquisition time of usually between 12 and 24 h per sample depending on concentration, not counting extraction time for solid samples or evapo-concentration for aqueous samples. Finally, there are the questions of incomplete extraction (e.g. phospholipids) or degradation during the extraction phase. Notwithstanding these issues 31P NMR spectroscopy will continue to be used in the study of organic P in the environment because of its ability to assign structure, and hence origin, to several key organic P species. However lack of access to a NMR facility shouldn’t hamper on-going research into organic P species in the aquatic environment. What are needed are complementary approaches for routinely characterising and quantifying organic P species.
Ideally, techniques used to determine organic P in natural samples should:
-
Be sensitive enough to not require extraction or pre-concentration.
-
Not suffer from interferences from other compounds in the matrix (particularly organic matter or metals).
-
Offer information on the molecular structure of the organic P species present (but not necessarily the total molecular structure).
-
Have rapid sample throughput (tens of samples per day).
-
The instrumentation required for analyses should not be so expensive or specialised to the point that only a few research groups across the world have access to them; and then only on limited occasions.
None of the currently available nor emerging techniques meet all of these criteria (see Table 1).[5,6] For example, although near-edge X-ray fine structure spectroscopy (NEXAFS) essentially meets the first four criteria (at least for particulate matter) the technique requires access to a synchrotron X-ray beam line.[125]

|
In the short term HPLC possibly offers the best approach for the routine analysis of organic P in aquatic samples. Several liquid chromatographic approaches have been used to determine organic P in aquatic samples including reverse-phase, ion-pairing, ion-exchange and size exclusion chromatography.[4,126] The equipment required is not prohibitively expensive and is widely available, the technique has the potential for high sample through-put, and doesn’t need highly specialised training to use. However, the approach is not without its problems. HPLC will always require an extraction step for particulate material, or a pre-concentration step for aqueous samples (at least at current detection limits). Furthermore, most organic P compounds of interest do not adsorb in the visible or ultraviolet region and therefore analyses may require post-column derivatisation for detection and quantification. Development and commercialisation of a low-cost detector that could specifically measure total P in HPLC eluent would certainly help advance organic P research in to the future. Currently total P can be determined by directing the HPLC eluent into inductively coupled plasma–optical emission spectrophotometers or –mass spectrometers. However, these instruments are quite expensive and in the case of inductively coupled plasma–mass spectrometry, the P+ ion (30.974 Da) formed in the plasma is similar in mass to nitrogen oxide ions formed in the plasma – 15N16O+ (30.995 Da) and 14N16O1H+ (31.0581).[11,126]
Notwithstanding the techniques that are adopted, future studies on organic P in aquatic environments should not be based on the broad operationally defined definitions of organic P that have been used in the past. This will necessitate changes to several published chemical method manuals including American Public Health Association’s Standard Method.[8] At a minimum, the current component that is labelled ‘organic P’ should be renamed ‘unreactive P’. Furthermore, journal editors should discourage the use of the term ‘organic P’ unless the paper clearly shows evidence that the P is part of an organic molecule.
Expansion of the types of ecosystems studied
Most of what we know about the dynamics of organic P in aquatic environments comes from a limited number of ecosystem types. For example there is little published regarding the dynamics of the five classes of organic P species covered in this review in flowing waters (from head water streams through to large lowland rivers) or the riparian zones and floodplains associated with those water courses. Similarly although there has been some published research on organic P dynamics in natural lakes, there is also little published on man-made reservoirs (which often have substantially different hydrodynamics and physical chemistry than lakes). Furthermore, although organic P has been studied in several large near coastal wetlands and wetland complexes (e.g. the Carmague in France, Doñana marshes in Spain and the Everglades in America), there has been little research on smaller, inland wetlands like fens, bogs, water meadows or oxbow lakes – all of which are potential sites of transformation of P in the catchment.
Another related issue is that of the transferability of processes from one ecosystem type to another. For example IHP has been shown to rapidly hydrolyse under anaerobic conditions in coastal systems but that hasn’t been demonstrated for freshwater ecosystems. Another example is the role of viruses in cycling nucleic acids. This is well documented in the deep sea environment but not to the same extent in other ecosystems. Certainly viruses are an important, yet under-studied, component of many freshwater ecosystems[127,128] and there is an emerging model of the complex interaction between viruses, bacterial community structure and nutrient status, at least in lakes.[129–131]
Exploring different types of transformations
Generally speaking, most of the studies of organic P transformation in aquatic environments have centred on enzymatic hydrolysis. There are exceptions. There have been a few studies looking at adsorption and desorption of organic P on mineral surfaces[50,132] and of course the studies on the role of viruses in the cycling of P in the deep sea (see above). However there are several other potential transformative processes that have received little or no attention. Abiotic hydrolysis of model organic P compounds facilitated by mineral surfaces has been demonstrated in the laboratory[42] but not using either substrates or mineral particles from the environment. Photolysis, with or without mineral catalysis,[133] has been shown to be an important pathway for the degradation of organic compounds in aquatic environments and therefore has the potential to be involved in the degradation of organic P species.[134] As another example, change in ionic strength along salinity gradients going from freshwater to coastal waters has been inferred to be an important process in the cycling of e.g. IHPs[67,71,79] but as yet hasn’t been demonstrated experimentally. The effect of increasing ionic strength on the cycling of organic P compounds doesn’t just apply to coastal ecosystems. Many nominally freshwater ecosystems are undergoing salinisation through inappropriate land use practises, which in turn may affect biogeochemical cycling in affected ecosystems.[135]
Concentration v. flux; measuring rates of transformation
Many of the studies on organic P in aquatic environments are phenomenological. Dynamics and transformations are not directly measured but may be inferred.[110] However concentration does not necessarily equate to importance. Indeed if a chemical is limiting an active processes (e.g. primary production) then it is axiomatic that it will be in low concentrations. This is clearly demonstrated by looking at the dynamics of ATP in the deep ocean. ATP is both rapidly formed, but also rapidly hydrolysed, so that at any given time the concentration is extremely low, yet it is probably one of the key compounds involved in P cycling in that environment. Therefore, there is a pressing need to understand not just the distribution of organic P compounds in aquatic environments, but also how rapidly they are being cycled – hydrolysed, reformed or converted into new molecules that in turn can be hydrolysed.
There are several ways to assess fluxes and transformations in aquatic environments. The simplest are controlled experiments in microcosms or mesocosms that are designed to mimic components of the natural environment (e.g. core incubation experiments, sediment slurry experiments or mineral adsorption–hydrolysis experiments). These types of experiments can give important insights into the mechanisms and rates of transformations of organic P molecules under controlled systems. However, it is not always possible, or indeed sensible, to extrapolate between small-scale controlled experiments and the natural environment.[136] This in part because laboratory-scale experiments do not necessarily include synergistic or antagonistic effects that can exist in the real world. Another consideration in controlled laboratory experiments, especially those looking specifically at abiotic processes, is contamination by microbiota. Bacteria are ubiquitous and are capable of a range of transformative processes. Adoption of good laboratory practice (e.g. autoclaving material when it is appropriate to do so) coupled with inclusion of negative controls should be considered when designing such experiments.
There are several approaches that can be used to measure, or at least infer, rates of transformation in the field. The most common approach, although it is rarely labelled as such, is ‘space-for-time substitution’. In space-for-time substitution, a particular component of the ecosystem (in this case a type of organic P) is measured at sites that have undergone a perturbation at different times in the past. A common example of this approach within the biogeochemical literature is the change in concentration of a particular chemical species down the length of a sediment core (the perturbation in this case is time since burial; assuming of course that the sediment hasn’t been perturbed). Other examples include changes in chemical composition along environmental gradients (e.g. along a sediment drying gradient at the edge of a water body)[137] or changes in the composition of particulate matter with depth (settling).[111] Although useful, there are several issues with this approach. Probably the most significant is that it assumes a causal linkage between the perturbation being explored and the rate of transformation observed. An alternate approach to determining fluxes in aquatic environments is following the fate of a particular compound that has either accidently or deliberately been added to a water body (known as ‘spiralling experiments’ when carried out in flowing systems). Examples of this approach include studies that have observed the fate of glyphosate and its hydrolysis product (aminomethylphosponic acid) experimentally applied to prairie wetlands[138] and changes in organic P speciation with distance from a poultry operation.[87] However, because of the logistics in performing these types of experiments, they are often restricted to small water bodies like drains, creeks and wetlands.
In most of the examples outlined in this paper, the rates of transformation have been estimated from changes in concentration of a particular compound or, more rarely, changes in the concentration of a precursor molecule or hydrolysis product. Although valuable, our understanding of the rates and pathways of organic P transformations in aquatic ecosystems may benefit from incorporating alternate approaches into study design. One such approach would be the incorporation of stable isotope analysis to track transformation pathways. Radioactive isotopes have been used in biogeochemical research for several decades with some success; however there has been less emphasis on stable isotopes (either at natural abundance or in enriched samples). Carbon (12C/13C), nitrogen (14N/15N), oxygen (16O/18O), sulfur (32S/34S) and hydrogen (1H/2H) all have stable isotopes that have, or could be used in studying P dynamics. For example, changes in 18O to 16O ratios in phosphate have been used to determine the source of P to sediments[139] and to differentiate between biotic and abiotic cycling of P.[140] Although those studies focussed on phosphate, similar approaches could be applied to organic P.
Our understanding of organic P dynamics in aquatic ecosystems can also be expanded by embracing recent advances in ecosystem genomics; metatranscriptomics in particular has already shown promise in exploring organic P dynamics in an oligotrophic mountain lake.[141] Metatranscriptomics sequences the genes expressed within natural communities and allows an exploration of the pattern of gene expression and therefore, the pattern of transformation regulated by those genes.[142]
Investigation of other roles for organic P in aquatic environments
In an insightful discussion on microbial turnover of organic P in aquatic environments Heath[63] made the observation that most studies in this area had a focus on organic P as a source of P, particularly to fuel P-limited phytoplanktonic growth; what he termed the ‘phosphorus-limited planktonic view’. He argued that this limited our appreciation and understanding of the role organic P can play in aquatic environments. For example he argues persuasively that organic P is probably equally important as a source of carbon to aquatic bacteria. Carbon has been shown to limit microbial activity in aquatic environments[143] and therefore it is possible that bacterial exoenzyme production is as much about procuring C as it is about procuring P, often with synergistic effects. Heath also suggests there may be other roles organic P can play in the aquatic environment – e.g. the informational role of cAMP,[63] or as has been subsequently been shown, the role that DNA plays in biofilm formation. Indeed, apart from being a source or sink for P, this review clearly indicates that other ecological functions for organic P are rarely acknowledged. Therefore, although it is clear organic P can be an important source of P for aquatic organisms, other roles in the functioning of ecosystems shouldn’t be ignored.
Conclusions
Organic P represents a substantial pool of phosphorus in aquatic ecosystems and many species are not recalcitrant but undergo dynamic transformations. Hence some organic P species are important in P cycling globally. Organic P species may also play other functional roles in the ecology of aquatic ecosystems – e.g. as a source of carbon to microbiota, involved in the establishment of biofilms or as signalling chemicals for quorum sensing. However, our understanding of organic P in the aquatic environment has only advanced by abandoning operational definitions of organic P and moving towards techniques that identify specific compounds. Although there has been an increasing reliance on specific technology, particularly 31 P NMR spectroscopy, because there are other approaches that can be used, access to high end instrumentation shouldn’t be seen as an impediment to researching new aspects of organic P dynamics in aquatic ecosystems.
Acknowledgements
This work was funded under the CSIRO Water for a Healthy Country Flagship Program. The author thanks Kerry Whitworth, Grant Douglas and Rebecca Bartley for commenting on an earlier version of this paper. The author also thanks Dr Ewen Silvester (La Trobe University) for useful discussion on the use of X-ray spectroscopy in determining P speciation.
References
[1] B. L. Turner, Appendix. Organic phosphorus compounds in the environment, in Organic Phosphorus in the Environment (Eds B. L. Turner, E. Frossard, D. S. Baldwin) 2005, pp. 381–388 (CABI: Cambridge, MA).[2] A. M. Mitchell, D. S. Baldwin, Organic phosphorus in the aquatic environment: speciation, transformations and interactions with nutrient cycles, in Organic Phosphorus in the Environment (Eds B. L. Turner, E. Frossard, D. S. Baldwin) 2005, pp. 309–324 (CABI: Cambridge, MA).
[3] S. Newman, J. S. Robinson, Forms of organic phosphorus in water, soils, and sediments, in Phosophorus Biogeochemistry in Subtropical Ecosystems (Eds K. R. Reddy, G. A. O’Connor, C. L. Schelske) 1999, pp. 207–223 (Crc Press-Taylor & Francis Group: Boca Raton, FL).
[4] I. D. McKelvie, Separation, preconcentration and speciation of organic phosphorus in environmental samples, in Organic Phosphorus in the Environment (Eds B. L. Turner, E. Frossard, D. S. Baldwin) 2005, pp. 1–20 (CABI: Cambridge, MA).
[5] P. J. Worsfold, P. Monbet, A. D. Tappin, M. F. Fitzsimons, D. A. Stiles, I. D. McKelvie, Characterisation and quantification of organic phosphorus and organic nitrogen components in aquatic systems: a review. Anal. Chim. Acta 2008, 624, 37.
| Characterisation and quantification of organic phosphorus and organic nitrogen components in aquatic systems: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVWksb%2FF&md5=3d091d393733715ce553145cb1624a0fCAS | 18706309PubMed |
[6] F. Kizewski, Y. T. Liu, A. Morris, D. Hesterberg, Spectroscopic approaches for phosphorus speciation in soils and other environmental systems. J. Environ. Qual. 2011, 40, 751.
| Spectroscopic approaches for phosphorus speciation in soils and other environmental systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmt1Oqsbk%3D&md5=ce1470a95f1d66a5046e7f296f728a85CAS | 21546661PubMed |
[7] L. M. Condron, S. Newman, Revisiting the fundamentals of phosphorus fractionation of sediments and soils. J. Soils Sediments 2011, 11, 830.
| Revisiting the fundamentals of phosphorus fractionation of sediments and soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXot1ehsbk%3D&md5=6f353d86a4bbc95bcc08143d1cfcd887CAS |
[8] Standard Methods for the Examination of Water and Wastewater 2012 (American Public Health Association: Baltimore MD).
[9] D. S. Baldwin, Reactive ‘organic’ phosphorus revisited. Water Res. 1998, 32, 2265.
| Reactive ‘organic’ phosphorus revisited.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXltVeht7c%3D&md5=3b7317a5283729901235c60fc0afcfbbCAS |
[10] F. H. Denison, P. M. Haygarth, W. A. House, A. W. Bristow, The measurement of dissolved phosphorus compounds: evidence for hydrolysis during storage and implications for analytical definitions in environmental analysis. Int. J. Environ. Anal. Chem. 1998, 69, 111.
| The measurement of dissolved phosphorus compounds: evidence for hydrolysis during storage and implications for analytical definitions in environmental analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXnsV2murs%3D&md5=0ff3ada288c590316091f50eba99929bCAS |
[11] W. T. Cooper, J. M. Llewelyn, G. L. Bennett, A. C. Stenson, V. J. M. Salters, Organic phosphorus speciation in natural waters by mass spectrometry, in Organic Phosphorus in the Environment (Eds B. L. Turner, E. Frossard, D. S. Baldwin) 2005, pp. 45–74 (CABI: Cambridge, MA).
[12] A. L. Shober, D. L. Hesterberg, J. T. Sims, S. Gardner, Characterization of phosphorus species in biosolids and manures using XANES spectroscopy. J. Environ. Qual. 2006, 35, 1983.
| Characterization of phosphorus species in biosolids and manures using XANES spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1yltrvO&md5=3fc402d823d361a4b9728a4a078a827eCAS | 17071866PubMed |
[13] S. Keller, T. Q. Zhang, S. Webb, R. Brugam, K. Johnson, Z. Q. Lin, Effects of suburban land use on phosphorus fractions and speciation in the Upper Peruque Creek, Eastern Missouri. Water Environ. Res. 2008, 80, 316.
| Effects of suburban land use on phosphorus fractions and speciation in the Upper Peruque Creek, Eastern Missouri.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVSis7vJ&md5=1ef59beea0664869c85feef328b4c71eCAS | 18536482PubMed |
[14] B. J. Cade-Menun, Using phosphorus-31 nuclear magnetic resonance spectroscopy to characterize organic phosphorus in environmental samples, in Organic Phosphorus in the Environment (Eds B. L. Turner, E. Frossard, D. S. Baldwin) 2005, pp. 21–44 (CABI: Cambridge, MA).
[15] K. C. Ruttenberg, Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnol. Oceanogr. 1992, 37, 1460.
| Development of a sequential extraction method for different forms of phosphorus in marine sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXisVShsr8%3D&md5=20c0f9ab5dd487f73e3d8426eefb6192CAS |
[16] D. A. Francko, R. T. Heath, UV-sensitive complex phosphorus – association with dissolved humic material and iron in a bog lake. Limnol. Oceanogr. 1982, 27, 564.
| UV-sensitive complex phosphorus – association with dissolved humic material and iron in a bog lake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38Xks1SmsLc%3D&md5=4166e9eb7e5ba788f471e2619aa59e63CAS |
[17] P. J. Shaw, R. I. Jones, H. De Haan, The influence of humic substances on the molecular weight distributions of phosphate and iron in epilimnetic lake waters. Freshwater Biol. 2000, 45, 383.
| The influence of humic substances on the molecular weight distributions of phosphate and iron in epilimnetic lake waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkvVOksw%3D%3D&md5=7f7fb8ebeac093e38d221073ef18aaa1CAS |
[18] R. A. Minear, Characterization of naturally occuring dissolved organophosphorus compounds. Environ. Sci. Technol. 1972, 6, 431.
| Characterization of naturally occuring dissolved organophosphorus compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE38XhsFyisrw%3D&md5=e60018e21234201ab7ef06ab49c9ce88CAS |
[19] M. D. Bailiff, D. M. Karl, Dissolved and particulate DNA dynamics during a spring bloom in the Antarctic Peninsula region, 1986–87. Deep-Sea Res. A, Oceanogr. Res. Pap. 1991, 38, 1077.
| Dissolved and particulate DNA dynamics during a spring bloom in the Antarctic Peninsula region, 1986–87.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XislCnsA%3D%3D&md5=055ab33a2baec95c7f890a293d0006f2CAS |
[20] W. Siuda, R. J. Chróst, Concentration and susceptibility of dissolved DNA for enzyme degradation in lake water – some methodological remarks. Aquat. Microb. Ecol. 2000, 21, 195.
| Concentration and susceptibility of dissolved DNA for enzyme degradation in lake water – some methodological remarks.Crossref | GoogleScholarGoogle Scholar |
[21] S. Sakano, A. Kamatani, Determination of dissolved nucleic acids in seawater by the fluoresence dye ethidium bromide. Mar. Chem. 1992, 37, 239.
| Determination of dissolved nucleic acids in seawater by the fluoresence dye ethidium bromide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XisVWlsL8%3D&md5=886e31291948ce055ce29a6635e94a3eCAS |
[22] B. L. Turner, N. Mahieu, L. M. Condron, Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH–EDTA extracts. Soil Sci. Soc. Am. J. 2003, 67, 497.
| Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH–EDTA extracts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkslChsLo%3D&md5=c86a40dd5782f309bef0d2b71dde3971CAS |
[23] W. Siuda, R. J. Chróst, H. Gude, Distribution and origin of dissolved DNA in lakes of different trophic states. Aquat. Microb. Ecol. 1998, 15, 89.
| Distribution and origin of dissolved DNA in lakes of different trophic states.Crossref | GoogleScholarGoogle Scholar |
[24] T. J. C. Beebee, Identification and analysis of nucleic-acids in natural fresh-waters. Sci. Total Environ. 1993, 135, 123.
| Identification and analysis of nucleic-acids in natural fresh-waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXmsFKiur4%3D&md5=f72ad57f7b8c4d22231c85ac62dbe0efCAS |
[25] K. Reitzel, H. S. Jensen, M. Flindt, F. O. Andersen, Identification of dissolved nonreactive phosphorus in freshwater by precipitation with aluminum and subsequent 31P NMR analysis. Environ. Sci. Technol. 2009, 43, 5391.
| Identification of dissolved nonreactive phosphorus in freshwater by precipitation with aluminum and subsequent 31P NMR analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnsVWns7s%3D&md5=5936ed3e17ac31eca111ff26297fc981CAS | 19708371PubMed |
[26] K. Reitzel, J. Ahlgren, H. DeBrabandere, M. Waldeback, A. Gogoll, L. Tranvik, E. Rydin, Degradation rates of organic phosphorus in lake sediment. Biogeochem. 2007, 82, 15.
| Degradation rates of organic phosphorus in lake sediment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXislWktrw%3D&md5=b1dce6043988f20280b448df67b335a6CAS |
[27] D. S. Baldwin, The phosphorus composition of a diverse series of Australian sediments. Hydrobiol 1996, 335, 63.
| The phosphorus composition of a diverse series of Australian sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmtVarsw%3D%3D&md5=cbe87883a037621e577e26499b8d798eCAS |
[28] B. L. Turner, S. Newman, J. M. Newman, Organic phosphorus sequestration in subtropical treatment wetlands. Environ. Sci. Technol. 2006, 40, 727.
| Organic phosphorus sequestration in subtropical treatment wetlands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlCgsrvL&md5=4f18af6ea5eca3fffe3332472e6cd324CAS | 16509310PubMed |
[29] R. Shinohara, A. Imai, N. Kawasaki, K. Komatsu, A. Kohzu, S. Miura, T. Sano, T. Satou, N. Tomioka, Biogenic phosphorus compounds in sediment and suspended particles in a shallow eutrophic lake: a 31P-nuclear magnetic resonance (31P NMR) study. Environ. Sci. Technol. 2012, 46, 10572.
| Biogenic phosphorus compounds in sediment and suspended particles in a shallow eutrophic lake: a 31P-nuclear magnetic resonance (31P NMR) study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlOjt73J&md5=0a0aac7b5326d58051210f4f4f249925CAS | 22994917PubMed |
[30] J. Vestergren, A. G. Vincent, M. Jansson, P. Persson, U. Istedt, G. Grobner, R. Giesler, J. Schleucher, High-Resolution characterization of organic phosphorus in soil extracts using 2D 1H–31P NMR Correlation Spectroscopy. Environ. Sci. Technol. 2012, 46, 3950.
| High-Resolution characterization of organic phosphorus in soil extracts using 2D 1H–31P NMR Correlation Spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtlGlu74%3D&md5=8cfd3689006d544087437aede917f151CAS | 22394413PubMed |
[31] A. G. Bravo, W. Wildi, J. Pote, Kinetics of plant material mass loss and DNA release in freshwater column. Ecotoxicol. Environ. Saf. 2010, 73, 1548.
| Kinetics of plant material mass loss and DNA release in freshwater column.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1Wmu7fL&md5=c4c63fd248a756d0b1fdac32ef284e31CAS | 20570352PubMed |
[32] V. Turk, A. S. Rehnstam, E. Lundberg, A. Hagstrom, Release of bacterial DNA by marine nanoflagellates: an intermediate step in phosphorus regeneration. Appl. Environ. Microbiol. 1992, 58, 3744.
| 1:CAS:528:DyaK3sXjs1aq&md5=72f242d446166178ab0cb7acb7382facCAS | 16348813PubMed |
[33] S. C. Jiang, J. H. Paul, Viral contribution to dissolved DNA in the marine environment as determined by differential centrifugation and kingdom probing. Appl. Environ. Microbiol. 1995, 61, 317.
| 1:CAS:528:DyaK2MXivVaksL8%3D&md5=ab05b72cf5332be2b8394f22c6f88f84CAS | 16534913PubMed |
[34] W. Reisser, S. Grein, C. Krambeck, Extracellular DNA in aquatic ecosystems may in part be due to phycovirus activity. Hydrobiol 1993, 252, 199.
| Extracellular DNA in aquatic ecosystems may in part be due to phycovirus activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXktlersLk%3D&md5=8d64f5fe10e184ab810cc8f309b7c20aCAS |
[35] M. C. Alonso, V. Rodriguez, J. Rodriguez, J. J. Borrego, Role of ciliates, flagellates and bacteriophages on the mortality of marine bacteria and on dissolved-DNA concentration in laboratory experimental systems. J. Exp. Mar. Biol. Ecol. 2000, 244, 239.
| Role of ciliates, flagellates and bacteriophages on the mortality of marine bacteria and on dissolved-DNA concentration in laboratory experimental systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhsVWntrc%3D&md5=cf6d68fb8348199236d15292ccbf789bCAS |
[36] W. Siuda, H. Gude, Determination of dissolved deoxyribonucleic acid concentration in lake water. Aquat. Microb. Ecol. 1996, 11, 193.
| Determination of dissolved deoxyribonucleic acid concentration in lake water.Crossref | GoogleScholarGoogle Scholar |
[37] N. Ishii, Z. Kawabata, S. Nakano, M. G. Min, R. Takata, Microbial interactions responsible for dissolved DNA production in a hypereutrophic pond. Hydrobiol 1998, 380, 67.
| Microbial interactions responsible for dissolved DNA production in a hypereutrophic pond.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXisVWhsLo%3D&md5=21e70e52d3c257e471773243e23e2ed3CAS |
[38] L. Riemann, K. Holmfeldt, J. Titelman, Importance of viral lysis and dissolved DNA for bacterioplankton activity in a P-limited estuary, northern Baltic Sea. Microb. Ecol. 2009, 57, 286.
| Importance of viral lysis and dissolved DNA for bacterioplankton activity in a P-limited estuary, northern Baltic Sea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFelurc%3D&md5=361247eda63b48efe4ca55fdbb2797c6CAS | 18670729PubMed |
[39] J. Titelman, L. Riemann, K. Holmfeldt, T. Nilsen, Copepod feeding stimulates bacterioplankton activities in a low phosphorus system. Aquat. Biol. 2008, 2, 131.
| Copepod feeding stimulates bacterioplankton activities in a low phosphorus system.Crossref | GoogleScholarGoogle Scholar |
[40] G. E. Pinchuk, C. Ammons, D. E. Culley, S. M. W. Li, J. S. McLean, M. F. Romine, K. H. Nealson, J. K. Fredrickson, A. S. Beliaev, Utilization of DNA as a sole source of phosphorus, carbon, and energy by Shewanella spp.: ecological and physiological implications for dissimilatory metal reduction. Appl. Environ. Microbiol. 2008, 74, 1198.
| Utilization of DNA as a sole source of phosphorus, carbon, and energy by Shewanella spp.: ecological and physiological implications for dissimilatory metal reduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXit1yqsL4%3D&md5=5e1749b81a95f5aabacaa7b05e482d08CAS | 18156329PubMed |
[41] M. Heun, L. Binnenkade, M. Kreienbaum, K. M. Thormann, Functional specificity of extracellular nucleases of Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 2012, 78, 4400.
| Functional specificity of extracellular nucleases of Shewanella oneidensis MR-1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpvFKgt70%3D&md5=00e1999da5dcaf7ec82d383dc7ff37daCAS | 22492434PubMed |
[42] D. S. Baldwin, J. K. Beattie, L. M. Coleman, D. R. Jones, Phosphate ester hydrolysis facilitated by mineral phases. Environ. Sci. Technol. 1995, 29, 1706.
| Phosphate ester hydrolysis facilitated by mineral phases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXlsFersbg%3D&md5=d6f9313b4ec8e9f216b343b79c9993b8CAS | 22276899PubMed |
[43] J. D. T. Arruda-Neto, L. Nieto, H. Righi, M. A. Cotta, H. Carrer, T. E. Rodrigues, G. C. Genofre, Fragmentation of extracellular DNA by long-term exposure to radiation from uranium in aquatic environments. J. Environ. Monit. 2012, 14, 2108.
| Fragmentation of extracellular DNA by long-term exposure to radiation from uranium in aquatic environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFSitL7I&md5=15286235fc06946730c15906f5450d35CAS |
[44] K. Reitzel, J. Ahlgren, E. Rydin, S. Egemose, B. L. Turner, M. Hupfer, Diagenesis of settling seston: identity and transformations of organic phosphorus. J. Environ. Monit. 2012, 14, 1098.
| Diagenesis of settling seston: identity and transformations of organic phosphorus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XivFOlurY%3D&md5=3246031d592757682789e8ee4c71ac1cCAS | 22344567PubMed |
[45] G. W. Beall, D. S. Sowersby, R. D. Roberts, M. H. Robson, L. K. Lewis, Analysis of oligonucleotide DNA binding and sedimentation properties of montmorillonite clay using ultraviolet light spectroscopy. Biomacromolecules 2009, 10, 105.
| Analysis of oligonucleotide DNA binding and sedimentation properties of montmorillonite clay using ultraviolet light spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVOjsLzK&md5=58335be1a099d29a40427f4b445043e2CAS | 19061334PubMed |
[46] L. Celi, E. Barberis, Abiotic stabilization of organic phosphorus in the environment, in Organic Phosphorus in the Environment (Eds B. L. Turner, E. Frossard, D. S. Baldwin) 2005, pp. 113–132 (CABI: Cambridge, MA).
[47] J. Ahlgren, K. Reitzel, L. Tranvik, A. Gogoll, E. Rydin, Degradation of organic phosphorus compounds in anoxic Baltic Sea sediments: a 31P nuclear magnetic resonance study. Limnol. Oceanogr. 2006, 51, 2341.
| Degradation of organic phosphorus compounds in anoxic Baltic Sea sediments: a 31P nuclear magnetic resonance study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVOju7fI&md5=517e781d85e45b0e30b7efe11aa5ae41CAS |
[48] A. Dell'Anno, R. Danovaro, Extracellular DNA plays a key role in deep-sea ecosystem functioning. Science 2005, 309, 2179.
| Extracellular DNA plays a key role in deep-sea ecosystem functioning.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVKgtLrI&md5=34096a42cd7deb1ea9938c22131bd4b2CAS | 16195451PubMed |
[49] T. Løvdal, T. Tanaka, T. F. Thingstad, Algal–bacterial competition for phosphorus from dissolved DNA, ATP, and orthophosphate in a mesocosm experiment. Limnol. Oceanogr. 2007, 52, 1407.
| Algal–bacterial competition for phosphorus from dissolved DNA, ATP, and orthophosphate in a mesocosm experiment.Crossref | GoogleScholarGoogle Scholar |
[50] K. C. Ruttenberg, D. J. Sulak, Sorption and desorption of dissolved organic phosphorus onto iron (oxyhydr)oxides in seawater. Geochim. Cosmochim. Acta 2011, 75, 4095.
| Sorption and desorption of dissolved organic phosphorus onto iron (oxyhydr)oxides in seawater.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotleksL8%3D&md5=9504aa8272292424b9cf613d55698d51CAS |
[51] U. Böckelmann, A. Janke, R. Kuhn, T. R. Neu, J. Wecke, J. R. Lawrence, U. Szewzyk, Bacterial extracellular DNA forming a defined network-like structure. FEMS Microbiol. Lett. 2006, 262, 31.
| Bacterial extracellular DNA forming a defined network-like structure.Crossref | GoogleScholarGoogle Scholar | 16907736PubMed |
[52] J. Gödeke, K. Paul, J. Lassak, K. M. Thormann, Phage-induced lysis enhances biofilm formation in Shewanella oneidensis MR-1. ISME J. 2011, 5, 613.
| Phage-induced lysis enhances biofilm formation in Shewanella oneidensis MR-1.Crossref | GoogleScholarGoogle Scholar | 20962878PubMed |
[53] A. Seper, V. H. I. Fengler, S. Roier, H. Wolinski, S. D. Kohlwein, A. L. Bishop, A. Camilli, J. Reidl, S. Schild, Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation. Mol. Microbiol. 2011, 82, 1015.
| Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFyhu7bP&md5=496fdd7da97b23687d05e69ce1bb54c7CAS | 22032623PubMed |
[54] E. J. Monaghan, K. C. Ruttenberg, Dissolved organic phosphorus in the coastal ocean: reassessment of available methods and seasonal phosphorus profiles from the Eel River Shelf. Limnol. Oceanogr. 1999, 44, 1702.
| Dissolved organic phosphorus in the coastal ocean: reassessment of available methods and seasonal phosphorus profiles from the Eel River Shelf.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXnsVWisLs%3D&md5=32633ee24d82f80fc58c65de33b6f589CAS |
[55] K. M. Björkman, D. M. Karl, Presence of dissolved nucleotides in the North Pacific Subtropical Gyre and their role in cycling of dissolved organic phosphorus. Aquat. Microb. Ecol. 2005, 39, 193.
| Presence of dissolved nucleotides in the North Pacific Subtropical Gyre and their role in cycling of dissolved organic phosphorus.Crossref | GoogleScholarGoogle Scholar |
[56] D. A. Francko, R. G. Wetzel, The isolation of cyclic adenosine 3′–5′-monophosphate (cAMP) from lakes of different trophic status – correlation with planktonic metabolic variables. Limnol. Oceanogr. 1982, 27, 27.
| The isolation of cyclic adenosine 3′–5′-monophosphate (cAMP) from lakes of different trophic status – correlation with planktonic metabolic variables.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38Xht12hurw%3D&md5=3c8d650a85be72f2a79c7413e3da99b8CAS |
[57] M. P. Nawrocki, D. M. Karl, Dissolved ATP turnover in Bransfield Strait, Antarctica during a spring bloom. Mar. Ecol. Prog. Ser. 1989, 57, 35.
| Dissolved ATP turnover in Bransfield Strait, Antarctica during a spring bloom.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXktFWlurg%3D&md5=8743b64699b91968af4ae6646261b8edCAS |
[58] A. G. Gilman, Protein binding assays for cyclic nucleotides. Adv. Cyclic Nucleotide Res. 1972, 2, 9.
| 1:CAS:528:DyaE3sXotFygtg%3D%3D&md5=cf9ad43c47b64fc41516424cade99f55CAS | 4352300PubMed |
[59] H. De Brabandere, N. Forsgard, L. Israelsson, J. Petterson, E. Rydin, M. Waldeback, P. J. R. Sjoberg, Screening for organic phosphorus compounds in aquatic sediments by liquid chromatography coupled to ICP-AES and ESI-MS/MS. Anal. Chem. 2008, 80, 6689.
| Screening for organic phosphorus compounds in aquatic sediments by liquid chromatography coupled to ICP-AES and ESI-MS/MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXptVyrsb8%3D&md5=00bfe6f6248541fe81aedc1bc1143fb3CAS | 18665609PubMed |
[60] F. Azam, R. E. Hodson, Dissolved ATP in the sea and its utilization by marine bacteria. Nature 1977, 267, 696.
| Dissolved ATP in the sea and its utilization by marine bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXktFemtg%3D%3D&md5=c0a7d1b9be002cae848c2355dd9a72f4CAS | 876388PubMed |
[61] A. Bruns, U. Nubel, H. Cypionka, J. Overmann, Effect of signal compounds and incubation conditions on the culturability of freshwater bacterioplankton. Appl. Environ. Microbiol. 2003, 69, 1980.
| Effect of signal compounds and incubation conditions on the culturability of freshwater bacterioplankton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXivFKru7s%3D&md5=aeb5b8efa086ef7882b6be72d15018ccCAS | 12676673PubMed |
[62] A. Bruns, H. Cypionka, J. Overmann, Cyclic AMP and acyl homoserine lactones increase the cultivation efficiency of heterotrophic bacteria from the central Baltic Sea. Appl. Environ. Microbiol. 2002, 68, 3978.
| Cyclic AMP and acyl homoserine lactones increase the cultivation efficiency of heterotrophic bacteria from the central Baltic Sea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmtVejsrY%3D&md5=7013a853bcf05d0e382a84ef3ba8e87dCAS | 12147499PubMed |
[63] R. T. Heath, Microbial turnover of organic phosphorus in aquatic systems, in Organic Phosphorus in the Environment (Eds B. L. Turner, E. Frossard, D. S. Baldwin) 2005, pp. 185–204 (CABI: Cambridge, MA).
[64] B. L. Turner, M. J. Paphazy, P. M. Haygarth, I. D. McKelvie, Inositol phosphates in the environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 449.
| Inositol phosphates in the environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnsVSls7k%3D&md5=8d30c32fdff616c7fadeb3d3c9a2f944CAS | 12028785PubMed |
[65] I. D. McKelvie, Inositol phosphates in aquatic systems, in Inositol Phosphates: Linking Agriculture and the Environment (Eds B. L. Turner, A. E. Richardson, E. J. Mullaney) 2007, pp. 261–278 (CABI: Cambridge, MA).
[66] C. J. De Groot, H. L. Golterman, On the presence of organic phosphate in some Camargue sediments: evidence of the importance of phytate. Hydrobiol 1993, 252, 117.
| On the presence of organic phosphate in some Camargue sediments: evidence of the importance of phytate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXktlersb8%3D&md5=bd3d30db2004aefba9841e9b41962227CAS |
[67] M. Suzumura, A. Kamatani, Isolation and determination of inositol hexaphosphate in sediments from Tokyo Bay. Geochim. Cosmochim. Acta 1993, 57, 2197.
| Isolation and determination of inositol hexaphosphate in sediments from Tokyo Bay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXltVSisb8%3D&md5=2526a3f42f7e929238bab6b6394e2213CAS |
[68] H. El-Rifai, M. Heerboth, T. E. Gedris, S. Newman, W. Orem, W. T. Cooper, NMR and mass spectrometry of phosphorus in wetlands. Eur. J. Soil Sci. 2008, 59, 517.
| NMR and mass spectrometry of phosphorus in wetlands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvVOisrw%3D&md5=d7f719fbdccf07f6a59929dff1710f8dCAS |
[69] S. E. Herbes, H. E. Allen, K. H. Mancy, Enzymatic characterization of soluble reactive organic phosphorus in lake water. Science 1975, 187, 432.
| Enzymatic characterization of soluble reactive organic phosphorus in lake water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2MXovFyruw%3D%3D&md5=fe6f9c3b44197d89e0de809ab38f4d51CAS | 17835306PubMed |
[70] I. D. McKelvie, B. T. Hart, T. J. Cardwell, R. W. Cattrall, Use of imobilised phytase and flow injection for the determination of phosphorus species in natural waters. Anal. Chim. Acta 1995, 316, 277.
| Use of imobilised phytase and flow injection for the determination of phosphorus species in natural waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXps1WltL8%3D&md5=b7e6525498096ec8924738eae425e86eCAS |
[71] P. Monbet, I. D. McKelvie, P. J. Worsfold, Dissolved organic phosphorus speciation in the waters of the Tamar estuary (SW England). Geochim. Cosmochim. Acta 2009, 73, 1027.
| Dissolved organic phosphorus speciation in the waters of the Tamar estuary (SW England).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1arurg%3D&md5=08b9310dab4de7faf08de70beced8e0cCAS |
[72] B. J. Cade-Menun, J. A. Navaratnam, M. R. Walbridge, Characterizing dissolved and particulate phosphorus in water with 31P nuclear magnetic resonance spectroscopy. Environ. Sci. Technol. 2006, 40, 7874.
| Characterizing dissolved and particulate phosphorus in water with 31P nuclear magnetic resonance spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFCqt7zO&md5=4e46f164e001112f10c0014d7e156cccCAS | 17256541PubMed |
[73] B. L. Turner, S. Newman, Phosphorus cycling in wetland soils: the importance of phosphate diesters. J. Environ. Qual. 2005, 34, 1921.
| Phosphorus cycling in wetland soils: the importance of phosphate diesters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVygs7rK&md5=29e6a73f4a6de1989a5f133a7efde746CAS | 16151243PubMed |
[74] A. W. Cheesman, E. J. Dunne, B. L. Turner, K. R. Reddy, Soil phosphorus forms in hydrologically isolated wetlands and surrounding pasture uplands. J. Environ. Qual. 2010, 39, 1517.
| Soil phosphorus forms in hydrologically isolated wetlands and surrounding pasture uplands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXoslKmur4%3D&md5=2d6c988ae16a411f1bf8a76b3e5a1697CAS | 20830938PubMed |
[75] C. Jørgensen, H. S. Jensen, F. O. Andersen, S. Egemose, K. Reitzel, Occurrence of orthophosphate monoesters in lake sediments: significance of myo- and scyllo-inositol hexakisphosphate. J. Environ. Monit. 2011, 13, 2328.
| Occurrence of orthophosphate monoesters in lake sediments: significance of myo- and scyllo-inositol hexakisphosphate.Crossref | GoogleScholarGoogle Scholar | 21701742PubMed |
[76] B. L. Turner, K. Weckstrom, Phytate as a novel phosphorus-specific paleo-indicator in aquatic sediments. J. Paleolimnol. 2009, 42, 391.
| Phytate as a novel phosphorus-specific paleo-indicator in aquatic sediments.Crossref | GoogleScholarGoogle Scholar |
[77] L. Celi, E. Barberis, Abiotic reactions of inositol phosphates in soils, in Inositol Phosphates: Linking Agriculture and the Environment (Eds B. L. Turner, A. E. Richardson, E. J. Mullaney) 2007, pp. 207–220 (CABI: Cambridge, MA).
[78] B. L. Turner, Inositol phosphates in soil: amounts, forms and significance of the phosphorylated inositol sterioisomers, in Inositol Phosphates: Linking Agriculture and the Environment (Eds B. L. Turner, A. E. Richardson, E. J. Mullaney) 2007, pp. 186–206 (CABI: Cambridge, MA).
[79] M. Suzumura, A. Kamatani, Origin and distribution of inositol hexaphosphate in estuarine and coastal sediments. Limnol. Oceanogr. 1995, 40, 1254.
| Origin and distribution of inositol hexaphosphate in estuarine and coastal sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xot1aksQ%3D%3D&md5=ab2abd10eb489bcb66c1da42ceb42fa7CAS |
[80] C. D. Giles, B. J. Cade-Menun, J. E. Hill, The inositol phosphates in soils and manures: abundance, cycling, and measurement. Can. J. Soil Sci. 2011, 91, 397.
| The inositol phosphates in soils and manures: abundance, cycling, and measurement.Crossref | GoogleScholarGoogle Scholar |
[81] V. Kumar, A. K. Sinha, H. P. S. Makkar, G. De Boeck, K. Becker, Phytate and phytase in fish nutrition. J. Anim. Physiol. Anim. Nutr. (Berl.) 2012, 96, 335.
| Phytate and phytase in fish nutrition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xpt12jtLc%3D&md5=ef8747c590cccf2e877a3e12ee8d8dbeCAS | 21692871PubMed |
[82] C. A. Brearley, D. E. Hanke, Inositol phosphates in the duckweed Spirodela polyrhiza L. Biochem. J. 1996, 314, 215.
| 1:CAS:528:DyaK28XhtlKmtLw%3D&md5=da454a90e4836b3750ff954e6f465a82CAS | 8660286PubMed |
[83] R. Oren Benaroya, E. Zamski, E. Tel-Or, L-Myo-inositol 1-phosphate synthase in the aquatic fern Azolla filiculoides. Plant Physiol. Biochem. 2004, 42, 97.
| L-Myo-inositol 1-phosphate synthase in the aquatic fern Azolla filiculoides.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFWltrg%3D&md5=bc670d230fb45c2725b3b9d8a19ef0a0CAS |
[84] M. Reina, J. L. Espinar, L. Serrano, Sediment phosphate composition in relation to emergent macrophytes in the Donana Marshes (SW Spain). Water Res. 2006, 40, 1185.
| Sediment phosphate composition in relation to emergent macrophytes in the Donana Marshes (SW Spain).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xit1Wrtbk%3D&md5=4d6a4a6bf03e3e89b773cacb8efaea2dCAS | 16529791PubMed |
[85] C. W. Cheng, B. L. Lim, Beta-propeller phytases in the aquatic environment. Arch. Microbiol. 2006, 185, 1.
| Beta-propeller phytases in the aquatic environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhslOktbo%3D&md5=341d53df76a9ba6edfd419bd80076fcdCAS |
[86] B. L. Lim, P. Yeung, C. Cheng, J. E. Hill, Distribution and diversity of phytate-mineralizing bacteria. ISME J. 2007, 1, 321.
| Distribution and diversity of phytate-mineralizing bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXksFSnsrk%3D&md5=746f979e00ea3970536c33203beb6d21CAS | 18043643PubMed |
[87] J. E. Hill, B. J. Cade-Menun, Phosphorus-31 nuclear magnetic resonance spectroscopy transect study of poultry operations on the Delmarva Peninsula. J. Environ. Qual. 2009, 38, 130.
| Phosphorus-31 nuclear magnetic resonance spectroscopy transect study of poultry operations on the Delmarva Peninsula.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpt1Shuw%3D%3D&md5=2a28533fa0aea2298254cede4985b27fCAS | 19141802PubMed |
[88] M. Suzumura, A. Kamatani, Mineralization of inositol hexaphosphate in aerobic and anaerobic marine sediments – implications for the phosphorus cycle. Geochim. Cosmochim. Acta 1995, 59, 1021.
| Mineralization of inositol hexaphosphate in aerobic and anaerobic marine sediments – implications for the phosphorus cycle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXksFGht7k%3D&md5=36d38f8224cb3eb4ba6b9502e3941893CAS |
[89] H. Golterman, J. Paing, L. Serrano, E. Gomez, Presence of and phosphate release from polyphosphates or phytate phosphate in lake sediments. Hydrobiol. 1998, 364, 99.
| Presence of and phosphate release from polyphosphates or phytate phosphate in lake sediments.Crossref | GoogleScholarGoogle Scholar |
[90] E. E. Roden, J. W. Edmonds, Phosphate mobilization in iron-rich anaerobic sediments: microbial FeIII oxide reduction versus iron-sulfide formation. Arch. Hydrobiol. 1997, 139, 347.
| 1:CAS:528:DyaK2sXksVOksbk%3D&md5=9d86dd8769cd70bd7a50a18354086d9aCAS |
[91] W. Siuda, R. J. Chróst, Utilization of selected dissolved organic phosphorus compounds by bacteria in lake water under non-limiting orthophosphate conditions. Pol. J. Environ. Stud. 2001, 10, 475.
| 1:CAS:528:DC%2BD38XhvVKmsA%3D%3D&md5=d5726a5b04609534e30e24239e2dea47CAS |
[92] M. Suzumura, Phospholipids in marine environments: a review. Talanta 2005, 66, 422.
| Phospholipids in marine environments: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjvVClt7g%3D&md5=441b5704074f5859a616c5ca02ae5dbfCAS | 18970003PubMed |
[93] P. I. Boon, P. Virtue, P. D. Nichols, Microbial consortia in wetland sediments: A biomarker analysis of the effects of hydrological regime, vegetation and season on benthic microbes. Mar. Freshwater Res. 1996, 47, 27.
| Microbial consortia in wetland sediments: A biomarker analysis of the effects of hydrological regime, vegetation and season on benthic microbes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xis1Kru7s%3D&md5=758d1660d075a2c7e7feea449c19a2fcCAS |
[94] A. Sanseverino, M. D. Bastviken, I. Sundh, J. Pickova, A. Enrich-Prast, Methane carbon supports aquatic food webs to the fish level. PLoS ONE 2012, 7,
| Methane carbon supports aquatic food webs to the fish level.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFyrurfI&md5=68b07d676d415ced738e705959dc3ceaCAS | 22880091PubMed |
[95] J. Handelsman, Metagenomics: application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 669.
| Metagenomics: application of genomics to uncultured microorganisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjvFSgsQ%3D%3D&md5=41a13623f8d98cd3fbd7879d0ebb9d7bCAS | 15590779PubMed |
[96] M. Suzumura, E. D. Ingall, Concentrations of lipid phosphorus and its abundance in dissolved and particulate organic phosphorus in coastal seawater. Mar. Chem. 2001, 75, 141.
| Concentrations of lipid phosphorus and its abundance in dissolved and particulate organic phosphorus in coastal seawater.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjvVOqsLg%3D&md5=d234dbcc317706b818b7a4971d4985a7CAS |
[97] O. Oku, A. Kamatani, Phospholipid in plankton samples from Tokyo Bay and Sagami Bay. Nippon Suisan Gakkai Shi 1995, 61, 588.
| Phospholipid in plankton samples from Tokyo Bay and Sagami Bay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXnslyrsb0%3D&md5=0cfba021119911773d482340202b0d24CAS |
[98] M. S. Mills, E. M. Thurman, J. Ertel, K. A. Thorn, Organic geochemistry and sources of natural aquatic foams, in Humic and Fulvic Acids: Isolation, Structure, and Environmental Role (Eds J. S. Gaffney, N. A. Marley, S. B. Clark) 1996, pp. 151–192 (American Chemical Society: Washington, DC).
[99] B. A. S. Van Mooy, T. Moutin, S. Duhamel, P. Rimmelin, F. Van Wambeke, Phospholipid synthesis rates in the eastern subtropical South Pacific Ocean. Biogeosci. 2008, 5, 133.
| Phospholipid synthesis rates in the eastern subtropical South Pacific Ocean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtF2iurnF&md5=1a55337494f85246debf6d846a7a8be8CAS |
[100] B. A. S. Van Mooy, H. F. Fredricks, B. E. Pedler, S. T. Dyhrman, D. M. Karl, M. Koblizek, M. W. Lomas, T. J. Mincer, L. R. Moore, T. Moutin, M. S. Rappe, E. A. Webb, Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 2009, 458, 69.
| Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1ehtbY%3D&md5=2593ab60b49747bfd17a873567120dc6CAS |
[101] L. L. Clark, E. D. Ingall, R. Benner, Marine organic phosphorus cycling: novel insights from nuclear magnetic resonance. Am. J. Sci. 1999, 299, 724.
| Marine organic phosphorus cycling: novel insights from nuclear magnetic resonance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXls1OhsA%3D%3D&md5=7fe6ad3400d3481a7eb9ccf9aa796351CAS |
[102] B. Nowack, Environmental chemistry of phosphonates. Water Res. 2003, 37, 2533.
| Environmental chemistry of phosphonates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjsFOksrk%3D&md5=c98867f8967a4b3e906f3b3749c5e676CAS | 12753831PubMed |
[103] R. H. Coupe, S. J. Kalkhoff, P. D. Capel, C. Gregoire, Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag. Sci. 2012, 68, 16.
| Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1CnsrnI&md5=0466e54fbc410cff610c2d9fe2b0dcc8CAS | 21681915PubMed |
[104] M. A. Nanny, R. A. Minear, Characterization of soluble unreactive phosphorus using 31P nuclear magnetic resonance spectroscopy. Mar. Geol. 1997, 139, 77.
| Characterization of soluble unreactive phosphorus using 31P nuclear magnetic resonance spectroscopy.Crossref | GoogleScholarGoogle Scholar |
[105] E. D. Ingall, P. A. Schroeder, R. A. Berner, The nature of organic phosphorus in marine sediments – new insights from 31P. Geochim. Cosmochim. Acta 1990, 54, 2617.
| The nature of organic phosphorus in marine sediments – new insights from 31P.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXmtlOhtbs%3D&md5=b676e19d022da2a360e740bbda22590bCAS |
[106] E. Börjesson, L. Torstensson, New methods for determination of glyphosate and (aminomethyl)phosphonic acid in water and soil. J. Chromatogr. A 2000, 886, 207.
| New methods for determination of glyphosate and (aminomethyl)phosphonic acid in water and soil.Crossref | GoogleScholarGoogle Scholar | 10950288PubMed |
[107] C. Y. Hao, D. Morse, F. Morra, X. M. Zhao, P. Yang, B. Nunn, Direct aqueous determination of glyphosate and related compounds by liquid chromatography/tandem mass spectrometry using reversed-phase and weak anion-exchange mixed-mode column. J. Chromatogr. A 2011, 1218, 5638.
| Direct aqueous determination of glyphosate and related compounds by liquid chromatography/tandem mass spectrometry using reversed-phase and weak anion-exchange mixed-mode column.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXps1ylsL8%3D&md5=248576c6423f2025ac72ccbdcb58ea89CAS |
[108] C. L. Young, E. D. Ingall, Marine dissolved organic phosphorus composition: insights from samples recovered using combined electrodialysis/reverse osmosis. Aquat. Geochem. 2010, 16, 563.
| Marine dissolved organic phosphorus composition: insights from samples recovered using combined electrodialysis/reverse osmosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXotVOrsLg%3D&md5=e2f4cebec8c2d2cb43e3264d77f66078CAS |
[109] L. C. Kolowith, E. D. Ingall, R. Benner, Composition and cycling of marine organic phosphorus. Limnol. Oceanogr. 2001, 46, 309.
| Composition and cycling of marine organic phosphorus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjtVWgsLk%3D&md5=334970568180a8d901ea4eb2c59c4765CAS |
[110] C. R. Benitez-Nelson, L. O'Neill, L. C. Kolowith, P. Pellechia, R. Thunell, Phosphonates and particulate organic phosphorus cycling in an anoxic marine basin. Limnol. Oceanogr. 2004, 49, 1593.
| Phosphonates and particulate organic phosphorus cycling in an anoxic marine basin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXovVWqsLo%3D&md5=7dc86d8978311528fea83e2247c84b5dCAS |
[111] A. Paytan, B. J. Cade-Menun, K. McLaughlin, K. L. Faul, Selective phosphorus regeneration of sinking marine particles: evidence from 31P-NMR. Mar. Chem. 2003, 82, 55.
| Selective phosphorus regeneration of sinking marine particles: evidence from 31P-NMR.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktF2rs7k%3D&md5=f0d2b365c4a58b254174f9fa7a5ad5f8CAS | 1:CAS:528:DC%2BD3sXktF2rs7k%3D&md5=f0d2b365c4a58b254174f9fa7a5ad5f8CAS |
[112] P. Sannigrahi, E. Ingall, Polyphosphates as a source of enhanced P fluxes in marine sediments overlain by anoxic waters: evidence from 31P NMR. Geochem. Trans. 2005, 6, 52.
| Polyphosphates as a source of enhanced P fluxes in marine sediments overlain by anoxic waters: evidence from 31P NMR.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVCjs7nL&md5=5dd2a2860a4e0ca2ead69284199a63a8CAS | 1:CAS:528:DC%2BD28XhtVCjs7nL&md5=5dd2a2860a4e0ca2ead69284199a63a8CAS |
[113] L. M. Dong, Z. F. Yang, X. H. Liu, G. N. Liu, Investigation into organic phosphorus species in sediments of Baiyangdian Lake in China measured by fractionation and 31P NMR. Environ. Monit. Assess. 2012, 184, 5829.
| Investigation into organic phosphorus species in sediments of Baiyangdian Lake in China measured by fractionation and 31P NMR.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFOntbnN&md5=11248b686e2704597dc3a97d8c85c185CAS | 1:CAS:528:DC%2BC38XhtFOntbnN&md5=11248b686e2704597dc3a97d8c85c185CAS |
[114] J. Y. Liu, H. Wang, H. J. Yang, Y. J. Ma, O. C. Cai, Detection of phosphorus species in sediments of artificial landscape lakes in China by fractionation and phosphorus-31 nuclear magnetic resonance spectroscopy. Environ. Pollut. 2009, 157, 49.
| Detection of phosphorus species in sediments of artificial landscape lakes in China by fractionation and phosphorus-31 nuclear magnetic resonance spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVGgsbjP&md5=18d2f72dce9255bc127533f7c01d4460CAS | 1:CAS:528:DC%2BD1cXhsVGgsbjP&md5=18d2f72dce9255bc127533f7c01d4460CAS |
[115] R. Y. Zhang, L. Y. Wang, F. C. Wu, B. A. Song, Phosphorus speciation in the sediment profile of Lake Erhai, southwestern China: fractionation and 31P NMR. J. Environ. Sci. (China) 2013, 25, 1124.
| Phosphorus speciation in the sediment profile of Lake Erhai, southwestern China: fractionation and 31P NMR.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1ShtbbN&md5=aefa68c7de1cf299bccce04e695d3979CAS | 1:CAS:528:DC%2BC3sXhs1ShtbbN&md5=aefa68c7de1cf299bccce04e695d3979CAS |
[116] X. L. Bai, S. M. Ding, C. X. Fan, T. Liu, D. Shi, L. Zhang, Organic phosphorus species in surface sediments of a large, shallow, eutrophic lake, Lake Taihu, China. Environ. Pollut. 2009, 157, 2507.
| Organic phosphorus species in surface sediments of a large, shallow, eutrophic lake, Lake Taihu, China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntVGhtLc%3D&md5=8ca649b1390dfc81a11cbdea4f4a2bb0CAS | 1:CAS:528:DC%2BD1MXntVGhtLc%3D&md5=8ca649b1390dfc81a11cbdea4f4a2bb0CAS |
[117] A. W. Cheesman, B. L. Turner, K. R. Reddy, Soil phosphorus forms along a strong nutrient gradient in a tropical ombrotrophic wetland. Soil Sci. Soc. Am. J. 2012, 76, 1496.
| Soil phosphorus forms along a strong nutrient gradient in a tropical ombrotrophic wetland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFarsbnN&md5=f48b2c03e5190e4fca9c3dedf2c513e9CAS | 1:CAS:528:DC%2BC38XhtFarsbnN&md5=f48b2c03e5190e4fca9c3dedf2c513e9CAS |
[118] I. N. Ilikchyan, R. M. L. McKay, J. P. Zehr, S. T. Dyhrman, G. S. Bullerjahn, Detection and expression of the phosphonate transporter gene phnD in marine and freshwater picocyanobacteria. Environ. Microbiol. 2009, 11, 1314.
| Detection and expression of the phosphonate transporter gene phnD in marine and freshwater picocyanobacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmsVequrc%3D&md5=daf37d7d0e9b818e611ed863c62ba8daCAS | 1:CAS:528:DC%2BD1MXmsVequrc%3D&md5=daf37d7d0e9b818e611ed863c62ba8daCAS | 19220397PubMed |
[119] S. T. Dyhrman, P. D. Chappell, S. T. Haley, J. W. Moffett, E. D. Orchard, J. B. Waterbury, E. A. Webb, Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 2006, 439, 68.
| Phosphonate utilization by the globally important marine diazotroph Trichodesmium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1aktw%3D%3D&md5=a8641afb0eaf642b3df2ff599f4c7c5fCAS | 1:CAS:528:DC%2BD28Xht1aktw%3D%3D&md5=a8641afb0eaf642b3df2ff599f4c7c5fCAS | 16397497PubMed |
[120] H. W. Luo, H. M. Zhang, R. A. Long, R. Benner, Depth distributions of alkaline phosphatase and phosphonate utilization genes in the North Pacific Subtropical Gyre. Aquat. Microb. Ecol. 2011, 62, 61.
| Depth distributions of alkaline phosphatase and phosphonate utilization genes in the North Pacific Subtropical Gyre.Crossref | GoogleScholarGoogle Scholar |
[121] J. A. Gilbert, S. Thomas, N. A. Cooley, A. Kulakova, D. Field, T. Booth, J. W. McGrath, J. P. Quinn, I. Joint, Potential for phosphonoacetate utilization by marine bacteria in temperate coastal waters. Environ. Microbiol. 2009, 11, 111.
| Potential for phosphonoacetate utilization by marine bacteria in temperate coastal waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjt1eqtbk%3D&md5=c5ea1a50675c8fe2495f282dc9e77cf1CAS | 1:CAS:528:DC%2BD1MXjt1eqtbk%3D&md5=c5ea1a50675c8fe2495f282dc9e77cf1CAS | 18783384PubMed |
[122] Y. Chen, F. Wu, Y. Lin, N. Deng, N. Bazhin, E. Glebov, Photodegradation of glyphosate in the ferrioxalate system. J. Hazard. Mater. 2007, 148, 360.
| Photodegradation of glyphosate in the ferrioxalate system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXovFSmsLg%3D&md5=95ed1d3ab331115f23048a6c17def1a9CAS | 1:CAS:528:DC%2BD2sXovFSmsLg%3D&md5=95ed1d3ab331115f23048a6c17def1a9CAS | 17374441PubMed |
[123] D. M. Karl, L. Beversdorf, K. M. Bjorkman, M. J. Church, A. Martinez, E. F. DeLong, Aerobic production of methane in the sea. Nat. Geosci. 2008, 1, 473.
| Aerobic production of methane in the sea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvFCgsLc%3D&md5=4a5c9692a11f514531975f680874cea7CAS | 1:CAS:528:DC%2BD1cXnvFCgsLc%3D&md5=4a5c9692a11f514531975f680874cea7CAS |
[124] S. S. Kamat, H. J. Williams, L. J. Dangott, M. Chakrabarti, F. M. Raushel, The catalytic mechanism for aerobic formation of methane by bacteria. Nature 2013, 497, 132.
| The catalytic mechanism for aerobic formation of methane by bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXms1Wgt7Y%3D&md5=f930f17a84622d8f01e3716b1033242fCAS | 1:CAS:528:DC%2BC3sXms1Wgt7Y%3D&md5=f930f17a84622d8f01e3716b1033242fCAS | 23615610PubMed |
[125] J. Lehmann, D. Solomon, J. A. Brandes, H. Fleckenstein, C. Jacobsen, J. Theieme, Synchrotron-based near-edge X-ray spectroscopy of NOM in soils and sediments, in Biophysico-Chemical Processes Involving Natural Non-Living Organic Matter in Environmental Systems (Eds N. Sensei, B. Xing, P. M. Huang) 2009, pp. 723–773 (Wiley: Hoboken, NJ).
[126] M. Heerboth, Speciation of Organic Phosphorus in Soils and Surface Waters by Liquid Chromatography with High Resolution Mass Spectrometry Detection 2007, Ph.D Thesis, Florida State University, Tallahassee.
[127] R. Maranger, D. F. Bird, Viral abundance in aquatic ecosystems – a comparison between marine and fresh waters. Mar. Ecol. Prog. Ser. 1995, 121, 217.
| Viral abundance in aquatic ecosystems – a comparison between marine and fresh waters.Crossref | GoogleScholarGoogle Scholar |
[128] S. W. Wilhelm, A. R. Matteson, Freshwater and marine virioplankton: a brief overview of commonalities and differences. Freshwater Biol. 2008, 53, 1076.
| Freshwater and marine virioplankton: a brief overview of commonalities and differences.Crossref | GoogleScholarGoogle Scholar |
[129] D. Lymer, E. S. Lindstrom, K. Vrede, Variable importance of viral-induced bacterial mortality along gradients of trophic status and humic content in lakes. Freshwater Biol. 2008, 53, 1101.
| Variable importance of viral-induced bacterial mortality along gradients of trophic status and humic content in lakes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvVOhsrs%3D&md5=59fe9e56b75e167d249f4dbced900c06CAS | 1:CAS:528:DC%2BD1cXnvVOhsrs%3D&md5=59fe9e56b75e167d249f4dbced900c06CAS |
[130] D. Lymer, J. B. Logue, C. P. D. Brussaard, A. C. Baudoux, K. Vrede, E. S. Lindstrom, Temporal variation in freshwater viral and bacterial community composition. Freshwater Biol. 2008, 53, 1163.
| Temporal variation in freshwater viral and bacterial community composition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvVOhsrY%3D&md5=e898158362116741e923cdefc666b592CAS | 1:CAS:528:DC%2BD1cXnvVOhsrY%3D&md5=e898158362116741e923cdefc666b592CAS |
[131] D. Lymer, K. Vrede, Nutrient additions resulting in phage release and formation of non-nucleoid-containing bacteria. Aquat. Microb. Ecol. 2006, 43, 107.
| Nutrient additions resulting in phage release and formation of non-nucleoid-containing bacteria.Crossref | GoogleScholarGoogle Scholar |
[132] R. Olsson, R. Giesler, J. S. Loring, P. Persson, Adsorption, desorption, and surface-promoted hydrolysis of glucose-1-phosphate in aqueous goethite (α-FeOOH) suspensions. Langmuir 2010, 26, 18760.
| Adsorption, desorption, and surface-promoted hydrolysis of glucose-1-phosphate in aqueous goethite (α-FeOOH) suspensions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVGktr3E&md5=65363d47661da27516d9b4c6b2082449CAS | 1:CAS:528:DC%2BC3cXhsVGktr3E&md5=65363d47661da27516d9b4c6b2082449CAS | 21087005PubMed |
[133] J. A. Howitt, D. S. Baldwin, G. N. Rees, B. T. Hart, Photodegradation, interaction with iron oxides and bioavailability of dissolved organic matter from forested floodplain sources. Mar. Freshwater Res. 2008, 59, 780.
| Photodegradation, interaction with iron oxides and bioavailability of dissolved organic matter from forested floodplain sources.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1WnsbvK&md5=5721bd3942c59504f24880b43c551dffCAS | 1:CAS:528:DC%2BD1cXht1WnsbvK&md5=5721bd3942c59504f24880b43c551dffCAS |
[134] Z. Yiyong, UV-sensitive P compounds: release mechanism, seasonal fluctuation and inhibitory effects on alkaline phosphatase activity in a shallow Chinese freshwater lake (Donghu Lake). Hydrobiol 1996, 335, 55.
| UV-sensitive P compounds: release mechanism, seasonal fluctuation and inhibitory effects on alkaline phosphatase activity in a shallow Chinese freshwater lake (Donghu Lake).Crossref | GoogleScholarGoogle Scholar |
[135] D. S. Baldwin, G. N. Rees, A. M. Mitchell, G. Watson, J. Williams, The short-term effects of salinization on anaerobic nutrient cycling and microbial community structure in sediment from a freshwater wetland. Wetlands 2006, 26, 455.
| The short-term effects of salinization on anaerobic nutrient cycling and microbial community structure in sediment from a freshwater wetland.Crossref | GoogleScholarGoogle Scholar |
[136] G. Englund, S. D. Cooper, Scale effects and extrapolation in ecological experiments. Adv. Ecol. Res 2003, 33, 161.
| Scale effects and extrapolation in ecological experiments.Crossref | GoogleScholarGoogle Scholar |
[137] D. S. Baldwin, Effects of exposure to air and subsequent drying on the phosphate sorption characteristics of sediments from a eutrophic reservoir. Limnol. Oceanogr. 1996, 41, 1725.
| Effects of exposure to air and subsequent drying on the phosphate sorption characteristics of sediments from a eutrophic reservoir.Crossref | GoogleScholarGoogle Scholar |
[138] D. Degenhardt, D. Humphries, A. J. Cessna, P. Messing, P. H. Badiou, R. Raina, A. Farenhorst, D. J. Pennock, Dissipation of glyphosate and aminomethylphosphonic acid in water and sediment of two Canadian prairie wetlands. J. Environ. Sci. Health B 2012, 47, 631.
| Dissipation of glyphosate and aminomethylphosphonic acid in water and sediment of two Canadian prairie wetlands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xms1WktLs%3D&md5=8ce2527fb39cc8cf1856b9575bda1484CAS | 1:CAS:528:DC%2BC38Xms1WktLs%3D&md5=8ce2527fb39cc8cf1856b9575bda1484CAS | 22560025PubMed |
[139] D. P. Jaisi, R. E. Blake, Tracing sources and cycling of phosphorus in Peru Margin sediments using oxygen isotopes in authigenic and detrital phosphates. Geochim. Cosmochim. Acta 2010, 74, 3199.
| Tracing sources and cycling of phosphorus in Peru Margin sediments using oxygen isotopes in authigenic and detrital phosphates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltFKhtbw%3D&md5=b641ecfd8c27308b0fadb824a2d8fddaCAS |
[140] D. P. Jaisi, R. K. Kukkadapu, L. M. Stout, T. Varga, R. E. Blake, Biotic and abiotic pathways of phosphorus cycling in minerals and sediments: insights from oxygen isotope ratios in phosphate. Environ. Sci. Technol. 2011, 45, 6254.
| Biotic and abiotic pathways of phosphorus cycling in minerals and sediments: insights from oxygen isotope ratios in phosphate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXosVSrs7w%3D&md5=db779a7d57647b2416f46d202d17e422CAS | 21732604PubMed |
[141] M. Vila-Costa, S. Sharma, M. A. Moran, E. O. Casamayor, Diel gene expression profiles of a phosphorus limited mountain lake using metatranscriptomics. Environ. Microbiol. 2013, 15, 1190.
| Diel gene expression profiles of a phosphorus limited mountain lake using metatranscriptomics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlsFSisbs%3D&md5=aaef58c0d36b6525a75a742d098da0c0CAS | 23176588PubMed |
[142] M. A. Moran, Metatranscriptomics: eavesdropping on complex microbial communities. Microbe 2009, 4, 329.
[143] A. M. Mitchell, D. S. Baldwin, G. N. Rees, Alterations to potential phosphorus release processes from anaerobic freshwater sediments with additions of different species of labile carbon, in Phosphates in Sediments (Eds L. Serrano, H. L. Golterman) 2005, pp. 43–54 (Backhuys Publishers: Leiden, the Netherlands).
[144] J. A. Brandes, E. Ingall, D. Paterson, Characterization of minerals and organic phosphorus species in marine sediments using soft X-ray fluorescence spectromicroscopy. Mar. Chem. 2007, 103, 250.
| Characterization of minerals and organic phosphorus species in marine sediments using soft X-ray fluorescence spectromicroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpslentA%3D%3D&md5=892b1663885d642916c05ab8da5c9a8cCAS |
[145] Z. He, C. W. Honeycutt, T. Ohno, J. F. Hunt, B. J. Cade-Menun, Phosphorus features in FT-IR spectra of natural organic matter. Chin. J. Geochem 2006, 25, 259.
| Phosphorus features in FT-IR spectra of natural organic matter.Crossref | GoogleScholarGoogle Scholar |


