Recalcitrant pharmaceuticals in the aquatic environment: a comparative screening study of their occurrence, formation of phototransformation products and their in vitro toxicity
Marlies Bergheim A B F , Richard Gminski A , Bernd Spangenberg C , Malgorzata Dębiak D , Alexander Bürkle D , Volker Mersch-Sundermann A , Klaus Kümmerer A E and Reto Gieré BA University Medical Center Freiburg, Department of Environmental Health Sciences, Section of Toxicology, Breisacher Strasse 115B, D-79106 Freiburg, Germany. Email: richard.gminski@uniklinik-freiburg.de; volker.mersch-sundermann@uniklinik-freiburg.de
B Institute of Earth and Environmental Sciences, University of Freiburg, Albertstrasse 23b, D-79104 Freiburg, Germany. Email: giere@uni-freiburg.de
C University of Applied Sciences, Process Engineering and Environmental Technologies, Badstrasse 24, D-77652 Offenburg, Germany. Email: spangenberg@hs-offenburg.de
D Molecular Toxicology Group, Department of Biology, University of Konstanz, Universitätsstrasse 10, D-78457 Konstanz, Germany. Email: alexander.buerkle@uni-konstanz.de; debiakma@yahoo.com
E Present address: Leuphana University Lüneburg, Institute of Sustainable and Environmental Chemistry, Scharnhorststraße 1/C13, D-21335 Lueneburg, Germany. Email: klaus.kuemmerer@uni.leuphana.de
F Corresponding author. Email: marlies.bergheim@gmail.com
Environmental Chemistry 11(4) 431-444 https://doi.org/10.1071/EN13218
Submitted: 1 December 2013 Accepted: 9 April 2014 Published: 11 July 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Environmental context. Many pharmaceuticals on the market have not undergone detailed evaluation for potential aquatic toxicity. We found that most tested pharmaceuticals were persistent, that phototransformation products were likely to be formed as a result of UV treatment of wastewater and that some transformation products were more toxic to bacteria than their precursor pharmaceutical compound. Thus UV treatment of wastewater does not seem appropriate to completely degrade or transform micropollutants into harmless compounds.
Abstract. Data allowing for a complete environmental risk assessment of pharmaceuticals and their photoderatives in the environment are still scarce. In the present study, in vitro toxicity and both bio- and photopersistence of various pharmaceuticals (aciclovir, allopurinol, cetirizine, cimetidine, fluconazole, hydrochlorothiazide, lisinopril, phenytoin, primidone, ranitidine, sotalol, sulpiride, tramadol and valsartane) as well as their phototransformation products were evaluated in order to fill data gaps and to help prioritise them for further testing. Twelve out of the fourteen compounds investigated were found to be neither readily nor inherently biodegradable in the Organisation of Economic Cooperation and Development-biodegradability tests. The study further demonstrates that the photo-induced transformation of the pharmaceuticals was faster upon irradiation with a Hg lamp (UV light) than with a Xe lamp emitting a spectrum that mimics sunlight. Comparing the non-irradiated with the respective irradiated solutions, a higher acute and chronic toxicity against bacteria was found for the irradiated solutions of seven compounds (cetirizine, cimetidine, hydrochlorothiazide, ranitidine, sulpiride, tramadol and valsartane). No cyto- and genotoxic effects were found in human cervical (HeLa) and liver (Hep-G2) cells for any of the investigated compounds or their phototransformation products. This comparative study documents that phototransformation products can arise as a result of UV treatment of wastewater containing these pharmaceuticals. It further demonstrates that some phototransformation products may have a higher environmental risk potential than the respective parent compounds because some phototransformation products exhibited a higher bacterial toxicity.
Additional keywords: biodegradation, HeLa cells, Hep-G2 cells, irradiation, predicted environmental concentrations (PECs), UV, Vibrio fischeri.
Introduction
The increasing contamination of freshwater systems worldwide with micropollutants is a key environmental problem. Over the last decades, pharmaceuticals have become the focus of attention.[1] Numerous studies report the presence and ecotoxicity of pharmaceuticals in various environments, including influents and effluents of sewage treatment plants (STPs), surface waters, groundwater and drinking water.[2] Some pharmaceuticals are even considered to be ubiquitous.[3] However, data allowing for a complete environmental risk assessment are still rare especially in regard to long-term toxicity or additive and synergistic effects.[4]
Furthermore, it is generally accepted that the persistence, fate and effects of pharmaceuticals can only be characterised if underlying data also relate to degradation properties of these compounds. Biodegradation products can be formed through a variety of natural processes, including bacterial activity in sewage treatment plants.[5,6] Once discharged into surface waters, products of incomplete degradation can also be formed as a consequence of abiotic processes, such as photochemical and photolytic processes triggered by sunlight, and hydrolysis. All of these degradation processes can form transformation products (TPs), which may persist in the environment and for which neither environmental concentration nor ecotoxicity or human toxicity are known. A few studies have already documented the existence of such TPs or have shown that a great number of different phototransformation products (PTPs) can be formed from a single pharmaceutical compound.[7–10] However, these studies only focus on the structural characterisation of PTPs and do not report any toxicity data. Because pharmaceuticals and other chemical compounds are frequently detected in surface, ground and drinking water, new technical approaches have been tested or used to improve the quality of treated and purified sewage water, including advanced oxidation treatment processes (AOP), such as, ozonolysis, photolysis, chlorination, different filtration processes and UV irradiation.[11,12] Some of these processes have proven highly efficient in removing certain compounds, whereas other micropollutants have been shown to degrade only partially.[8,9,13,14] Thus, TPs are not only formed by natural processes (e.g. sunlight), but also by water treatment. As neither chemical properties nor environmental fate or toxicity are known, any naturally or technically formed persistent TPs are of special environmental concern. Before new technical approaches can be widely recommended as novel water-cleaning technology, the effects of TPs formed as a result of this technology on aquatic life and human beings need to be investigated.
In order to identify environmentally relevant pharmaceuticals and to evaluate their fate and effect on the aquatic environment, we investigated the bio- and photodegradability of 14 pharmaceutical compounds. For this purpose, three widely used Organisation of Economic Cooperation and Development (OECD)-standardised biodegradation tests were performed as well as photodegradation tests using a Xe or Hg lamp to respectively simulate natural sunlight and photochemical reactions within technical processes. Further information on the environmental risk was obtained by calculating predicted environmental concentrations (PECs) of the compounds in accordance with the European Medicines Evaluation Agency (EMA) guideline.[15] All photodegradation tests were monitored with regard to formation of PTPs. As a first screening assay, and to prioritise pharmaceuticals for further conventional testing, a variety of eco- and human toxicity tests were performed with the pharmaceutical parent compounds as well as with their PTPs.
Materials and methods
Chemicals
All chemicals used for this study were at least of analytical grade. The active compounds were purchased from Sigma Aldrich (Steinheim, Germany) except for aciclovir and valsartane, which were respectively purchased from Dr Ehrenstorfer (Augsburg, Germany) and USA Pharmacopeia (Rockville, MD, USA).
All solutions were prepared using ultra-pure water, obtained from a Milli-Q Millipore Reagent-Water-System (Eschborn, Germany).
Environmental relevance and PECs
In order to select environmentally relevant compounds, a variety of pharmaceutical compounds were first analysed with regard to their PECs. Compounds were selected for this experimental study only if their PECs exceeded the action limit of 0.01 µg L–1 set by the EMA.[15]
PECs were determined in accordance with the specifications of phase-I of the ‘Guideline on the environmental risk assessment of medicinal products for human use’ recommended by the EMA and as described elsewhere.[15,16] Briefly, the PEC (phase-I) is based on the maximum daily dose consumed per inhabitant (data from Germany used in this study), which is meant to be a representation of the daily and actual consumption rate in a worst-case scenario. Furthermore, a factor for market penetration is used as another first and simple approach to represent the proportion of inhabitants being treated daily with the specific active compound. The PEC is finally calculated by dividing these combined values through daily per-capita wastewater flow and by considering further dilution in surface waters.
If the calculated PEC (phase-I) exceeds the action limit of 0.01 µg L–1, the EMA recommends another, more precise PEC calculation to be conducted in accordance with the specifications of phase-II of the guideline on the environmental risk assessment.[15] Only two minor variations were made: first, the rate of adsorption to sewage sludge was derived from the adsorption measured in Zahn–Wellens test (see below) and second, the consumption rate was based on updated daily defined dose (DDD) values derived from the report released by Schwabe and Paffrath.[17]
Briefly, the PEC (phase-II) is based on a precise calculation of the consumption rate, which is based on DDD and its conversion into a quantity scale by the World Health Organization (WHO) conversion factor. To obtain a more precise PEC, this study also considered a possible loss of the active compounds through human metabolism by incorporating pharmacokinetic data (http://www.fachinfo.de/, accessed 24 May 2014).
Biodegradation tests
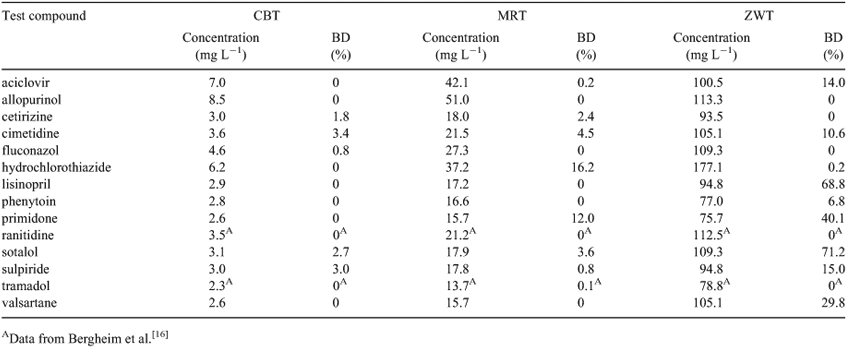
To evaluate the PTPs of the studied recalcitrant pharmaceutical compounds, as a first step, a more detailed evaluation was performed on biological degradation. For this purpose three different biodegradation tests were applied in accordance to the 1992 OECD test guidelines described in detail elsewhere.[16] The standard test period for all biodegradation experiments was 28 days, and all test series were run as duplicates. Table 1 summarises all different biodegradation test vessels that were used for the three types of biodegradation tests.

|
Closed bottle test (CBT)
The CBT (OECD 301D) is recommended by the OECD (1992) as a first test for assessing whether or not organic compounds are readily biodegradable in the aquatic environment. Accordingly, the CBT was performed with a low bacterial density, a low nutrient content, and with a low concentration of test compound (see Table 2). The amount of each test compound corresponded to a theoretical oxygen demand (ThOD) of 5 mg L–1 (without nitrification). According to the test guideline, a test compound is classified as readily biodegradable if biodegradation, expressed as the percentage of oxygen consumed in the test vessel, exceeds 60 % within a period of 10 days after oxygen consumption reached 10 %.
All test vessels were inoculated with an aliquot from the effluent of a local municipal sewage treatment plant (STP) (STP Kenzingen, Germany; 13 000 inhabitant equivalents). Two drops of inoculum were added to 1 L of medium. The process of aerobic biodegradation was monitored daily by measuring oxygen concentration in the test vessels with an optode oxygen sensor system (Fibox 3 PreSens, Regensburg, Germany), except for allopurinol, hydrochlorothiazide, ranitidine and tramadol, for which the oxygen concentration in the test vessels was measured with an oxygen electrode (Oxi 196 with EO 196-1.5, WTW Weilheim, Germany).
Manometric respirometry test (MRT)
The MRT (OECD 301F) is a second test recommended by the OECD for assessing whether or not organic compounds are readily biodegradable in the aquatic environment. Compared to the CBT and the Zahn–Wellens test (see below), it was performed with a medium bacterial density, a medium nutrient content and a medium concentration of test compound (see Table 2). The amounts of test compounds corresponded to a ThOD of 30 mg L–1. In analogy to the CBT, a test compound was classified as readily biodegradable if biodegradation, expressed as the percentage of oxygen consumed in the test vessel, exceeded 60 % within a period of 10 days after oxygen consumption reached 10 %.
All test vessels were inoculated with 40 mL of an aliquot from the effluent of the same STP as in the CBT. Aliquots of 80 mL were added to 1 L of mineral media. The process of aerobic biodegradation was measured automatically and daily by using an automatic analyser (System OxiTop OC100, WTW, Weilheim, Germany), which determines the microbial oxygen consumption by measuring CO2 production through pressure measurements.
Zahn–Wellens test (ZWT)
The ZWT (OECD 302B) is a tier-2 biodegradability test recommended by the OECD for assessing the inherent biodegradability of organic compounds, e.g. in sewage treatment. For assessment of inherent biodegradability, a high nutrient content and a high bacterial diversity are commonly used. With concentrations of the test compounds equivalent to 50 mg of dissolved organic carbon (DOC) per litre (77–177 mg L–1), the compounds were added to the test containers as the only source of carbon. A test compound is classified as inherently biodegradable if the DOC concentration was reduced by more than 70 %.
The sludge required as inoculum was obtained from the STP at Kenzingen (see CBT). The dry matter content in all test vessels was adjusted to 5.0 g L–1. The process of aerobic biodegradation was monitored at specific time intervals by measuring DOC loss in the test vessels with a total organic carbon (TOC) analyser (TOC 5000, Shimadzu GmbH, Duisburg, Germany).
DOC measurement
The progress of aerobic or anaerobic biodegradation as well as the degradation by photochemical processes or photolysis were monitored by measuring the DOC content. The latter was determined in three replicates according to European standard procedure EN 1484 by using a TOC analyser (TOC 5000, Shimadzu GmbH, Duisburg, Germany). Prior to chemical analysis, samples of the biodegradation test were filtered (cut-off 0.45 µm, Sartorius, Goettingen, Germany) in order to meet the conditions for DOC measurements, and measured continually over the course of the 28-day test period. Samples of the irradiation experiments were measured directly after irradiation for fixed time periods (2, 4, 8, 16, 32, 64 and 128 min).
High performance liquid chromatography–UV–mass spectrometry (HPLC-UV-MS) analysis
A HPLC system (Agilent Technologies, Waldbronn, Germany, HPLC 1100 series) consisting of two G1312A binary pumps, an ALS G1329A + ALS Therm G1330B sampler, a G1316A column oven (temperature set at 40 °C) and a G1322A degasser (Agilent, Germany) was used for chemical analysis. Chromatographic separation was performed on an RP-18 column (CC 70/3 NUCLEODUR 100-3 C18 ec, Macherey and Nagel, Dueren, Germany), protected by a CC 8/4 NUCLEODUR 100-5 C18 ec (Macherey and Nagel, Dueren, Germany) guard column. For elution, 0.1 % formic acid in water (HCOOH: solution A) and 100 % acetonitrile (CH3CN: solution B) were used by applying the following linear gradient: 0 min 1 % B, 20 min 45 % B, 22.3 min 55 % B, 25 min 80 % B, 26 min 1 % B, 30 min 1 % B. The sample injection volume was 20 μL, and the flow rate was set to 0.5 mL min–1. The total run time was 30 min. Test compounds at concentrations of 1, 2.5, 5, 10, 25 and 50 mg L–1 were used to establish the corresponding standard calibration curves. Quality controls at 10 mg L–1 were included in each run and were within ±20 % bias. The protonated molecule of each compound was monitored for quantification. Samples were either directly analysed or stored at –80 °C for subsequent analysis.
Quantification and detection were performed on a Bruker Daltonic Esquire 6000 plus ion trap mass spectrometer (IT-MS) equipped with a Bruker data analysis system and an atmospheric pressure electrospray ionisation (API-ESI) interface (Bruker Daltonic GmbH, Bremen, Germany). The mass spectrometer was connected to an Agilent 1100 Series LC system, and was operated in positive mode. The operating conditions of the source were: –500 V end plate, +4000 V capillary voltage, 2068.43 hPa nebuliser pressure, and 12 L min–1 dry gas flow at a dry temperature of 350 °C. The scan range was set to a mass to charge value (m/z) varying between 50 and 1000, and the scan time was 200 ms.
For UV detection, a UV/Vis detector (Agilent G1314 A) was used and absorbance maxima were measured at 210, 260, 275, 310 and 350 nm. Fluorescence was assessed with an Agilent G1321 A fluorescence detector (excitation 278 nm, emission 445 nm).
Irradiation experiments and absorbance spectra
Irradiation experiments were performed using a TXE 150 W xenon lamp and a TQ 150 W medium-pressure mercury lamp (UV-Consulting Peschl, Mainz, Germany) with stock solutions of the test compounds in ultra-pure water as described elsewhere.[16] The Hg lamp emits a low-intensity polychromatic spectrum of radiation from 200 to 600 nm, with some higher intensities at 254, 265, 302, 313, 366, 405/408, 436, 546 and 577/579 nm. According to the manufacturer, the total radiation flux (Φ) from 200 to 600 nm amounts to 47 W and the maximal intensities for whole spectral distribution were as follows: 4.0 (254), 1.4 (265), 1.8 (302), 4.3 (313), 6.4 (366), 3.2 (405/408), 4.2 (436), 5.1 (546) and 4.7 W (577/579 nm). The Xe lamp has a lower total photon flux and a continuous spectrum of radiation from 300 to 800 nm.
The lamps were inserted into an immersion tube of silica glass that was equipped with a cooling circuit to maintain the temperature of the irradiated solutions at 20 ± 2 °C. The stock solutions of 10 mg L–1 each were transferred into another vessel, which surrounded the concentrically formed lamp and the cooling circuit. These solutions were irradiated and further evaluated in terms of DOC, HPLC-UV-MS experiments as well as by the growth inhibition test. Furthermore, depending on water solubility, concentrations of up to 1 g L–1 were also irradiated as a stock solution for the other toxicity assays.
Immediately following irradiation, the absorbance spectra (Perking Elmer Instruments, Waltham, MA, USA) as well as the DOC (see DOC measurement above) were measured.
Bacterial toxicity bioassays
Growth inhibition test (EN ISO 10712: 1995)
The growth inhibition test was performed according to the EN ISO 10712 test guideline (1995) in order to investigate the effects of the irradiated and non-irradiated samples on bacterial growth (for details, see Bergheim et al.[16]). Briefly, a monoculture strain of P. putida (ATCC 50026), obtained from the German collection of microorganisms and cell cultures (DSMZ, Braunschweig, Germany), was used as inoculum. The toxicity of the test compounds was determined by comparing bacterial growth in samples from the test vessels with those of the blanks and without test compounds. This procedure was applied for the irradiated and non-irradiated (parent compounds) solutions.
Bioluminescence assay
Application on TLC plate. For further assessment of bacterial toxicity, 1 µL up to a maximal 50 µL (100 ng up to a maximal 6 mg) of the photodegradation samples (0 and 128 min) were spotted band-wise (4 mm) on a thin-layer-chromatography (TLC) plate (10 × 10 cm2) (Merck, Munich, Germany) with fluorescent dye using a LINOMAT III (Camag, Mutenz, Switzerland). For solvent and positive control, 2 µL of distilled water and 10 µL of 3,5-dichlorophenol (concentration 50 mg L–1) were spotted onto the TLC plate.
Photobacteria. To start the bacterial culture, freeze-dried luminous bacteria (LUMISmini, LCK484, Hach Lange GmbH, Düsseldorf, Germany) were pipetted into a reactivation solution provided by the manufacturer. The bacteria were cultivated for 25 to 30 h in an autoclaved medium containing the following components: NaCl, NaH2PO4·H2O, K2HPO4, MgSO4·H2O, (NH4)2HPO4, glycerine, peptone from casein and yeast extract. To increase luminescence, 1.25 mL of phosphate buffer and 0.15 mL of a 2.5 % H2O2 solution were added to the bacterial solution 15 min before starting the test. After 15 min, the TLC plate was dipped two times for 2 s into the bacteria suspension. Redundant dipping solution was wiped off gently with a wiper. After dipping the plate into the bacteria-containing solution, a clean glass plate was placed on top of the TLC plate and was then directly placed below a light-sensitive camera (ST-1603ME CCD camera with 1.56 megapixel, Santa Barbara Instrument Group, Santa Barbara, CA, USA) at a distance of 30 cm measuring the luminescence for 10 min. A video–densitometric quantification method was used to evaluate the degree of inhibition of the bacterial illumination.[18]
Cell viability assays
To measure the PTP cytotoxicity to human cells, the water-soluble tetrazolium (WST-1) assay and the neutral red (NR)-uptake assay were used. Both assays were performed on Hep-G2 cells (hepatocellular carcinoma), whereas the NR-uptake assay was also performed on HeLa cells (human cervical cancer). The cells were grown as a monolayer culture in T-75 flasks and subcultured twice per week at 37 °C in a humid atmosphere containing 5 % CO2 in air. HeLa cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, high glucose, Invitrogen, Karlsruhe, Germany) supplemented with 10 % foetal calf serum (FCS) Gold (PAA, Pasching, Austria) and 1 % penicillin–streptomycin (PAA) supplemented with 15 % FCS Gold 1 % penicillin–streptomycin. The adherent cells were detached by adding 2–4 mL of a mixture containing 0.25 % trypsin + 1 mM ethylenediaminetetraacetic acid (EDTA) (Invitrogen) and 5 mg mL–1 trypsin + 2.2 mg mL–1 EDTA (Invitrogen).
WST-1 assay
For the WST-1 experiment, 5 × 105 Hep-G2 cells were seeded in each well of a 96-well microplate and incubated for 24 h. Subsequently, the medium was replaced with fresh medium (200 µL per well) for the control sample, with medium containing the test solution (1 : 10) for the test wells, with medium and distilled water (1 : 10) for the solvent controls and with medium containing 0.01 % Triton X (Merck, Darmstadt, Germany) for the positive control. The microplate was incubated under standard culture conditions for 48 h. Cells were then washed with PBS (PAA) and a 5 vol % WST-1 solution (Roche Diagnostics, Mannheim, Germany) in a phenol red-free RPMI medium (Invitrogen) was added to each well. After 1-h incubation at 37 °C, absorbance was measured at 435 nm using a microplate reader (Tecan, Crailsheim, Germany).
NR-uptake assay
The NR-uptake assay was performed according to Repetto et al.[19] Cells were seeded and treated as described above for the WST-1 assay. HeLa cells and Hep-G2 were respectively seeded into each well at a density of 4 × 103 and 5 × 105 cells per well. After an exposure period of 48 h, cells were washed with PBS, and 200 µL of fresh medium containing 0.5 mg L–1 of NR solution (stock solution was prepared with 4 mg L–1 of NR; Sigma–Aldrich, Taufkirchen, Germany) was added into each well. After 3-h incubation at standard culture conditions, the cells were again washed twice with 100 µL of PBS, and then 200 µL of a destaining solution (ethanol 99 %, formic acid 99 %, distilled water, v/v 50 : 1 : 49) were added. After shaking the plate for 20 min at 300 rpm, absorbance was measured at 540 nm using a microplate reader (Tecan, Crailsheim, Germany).
Genotoxicity: fluorimetric detection of alkaline DNA unwinding (FADU)
Genotoxic effects of the PTPs were assessed by performing the FADU assay.[20–22] Briefly, HeLa cells were cultivated and treated as described for the cell viability assays. For the experiments, 3 × 105 cells (for 1-h exposure) and 2 × 105 cells (for 24-h exposure) were seeded into each well. Instead of a chemical positive control, X-rays (X-ray generator: CHF Müller, Hamburg, Germany, 70-keV energy) were used to determine test reliability and cell sensitivity. For this purpose, the X-ray dose was modified by variation of irradiation time at a fixed dose rate. Cells were irradiated on ice in a 96-well plate at the following condition: 3 min of irradiation with X-rays corresponded to an energy dose of 1 Gy.
Cells were trypsined, washed with PBS and re-suspended in suspension buffer (14 mM β-mercaptoethanol, 250 mM meso-inositol, 1 mM MgCl2, 10 mM sodium phosphate buffer, pH 7.4) at a final cell titre of 2 × 105 cells mL–1. Aliqots (70 μL) of the cell suspension were pipetted into a 96-well plate. The next steps were carried out by the automated FADU robot. First, 70 µL of lysis buffer (9 M urea, 10 mM NaOH, 2.5 mM 1,2-cyclohexanedinitrilotetraacetic acid, 0.1 % SDS) was added and incubated for 12 min at 0 °C. On top of the cell lysate, a pre-chilled alkaline buffer (42 % lysis buffer, 0.2 M NaOH) was overlaid and the alkaline unwinding was performed at 30 °C. After 60 min, 140 µL of a neutralisation buffer (81 M glucose, 14 mM β-mercaptoethanol) were added and incubated for 30 min at 22 °C. Samples were then mixed with Sybr-Green solution (1 : 8333 (v/v) in H2O, Invitrogen). Fluorescence was measured in a 96-well plate reader at 492 (excitation) and 520 nm (emission). All samples were measured as duplicates. DNA integrity was calculated as described elsewhere.[23]
Results
Environmental concentrations and relevance
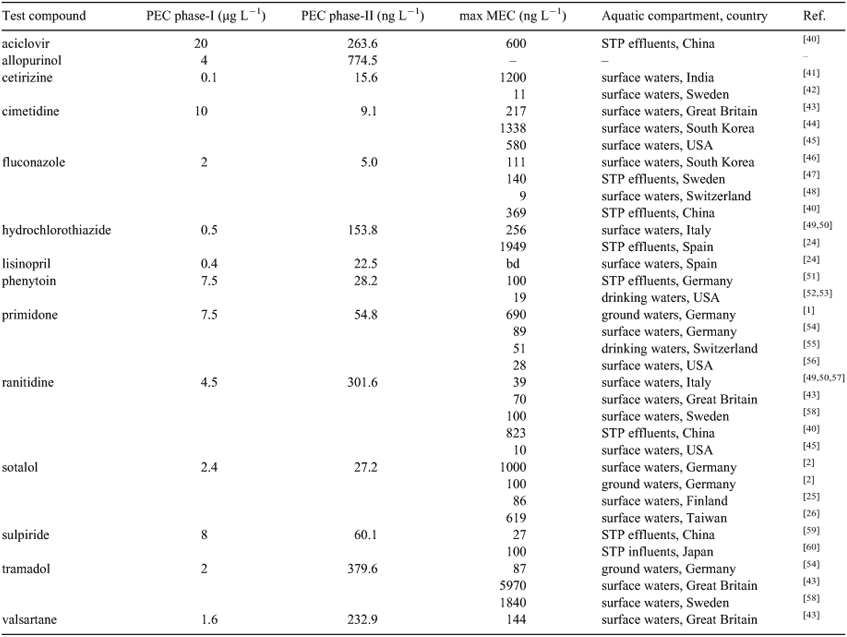
The calculated PECs (phase-I calculations) for all selected compounds exceeded the action limit of 0.01 µg L–1 (Table 3). As recommended by the EMA, a more precise exposure calculation was therefore also conducted (phase-II calculations).
The results of phase-II calculations were one to three orders of magnitude below the PECs of phase-I. The highest PEC (phase-II) was calculated for allopurinol, but there are no data in the literature on its occurrence in the aquatic environment. The lowest PEC (phase-II) was calculated for the antifungal drug fluconazole (Table 3). Most of the measured environmental concentrations (MEC) taken from the literature were one or two orders of magnitudes higher than the PEC (phase-II).
Biodegradability according to the OECD tests
All validity criteria of the OECD test guideline were met, and none of the tested compounds were toxic to the inocula. Table 2 summarises the results of the three biodegradation tests. Because biodegradability is related to the blank vessels and expressed as a percentage, the result of the biodegradability assessment can have negative values. For visual clarity these were set to zero.
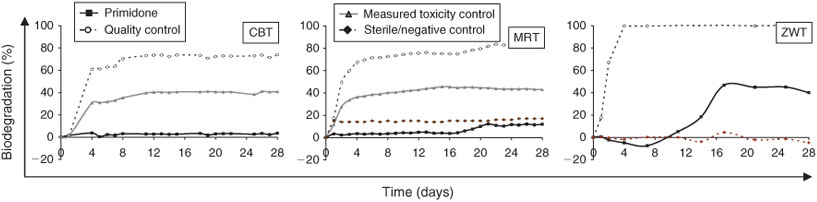
With respect to the oxygen consumption, all active compounds could be classified as not readily biodegradable in the CBT and MRT. In the ZWT and in terms of DOC loss, almost all compounds were further classified as not inherently biodegradable. The only exceptions are the two active compounds lisinopril and sotalol, for which DOC loss reached a value of 69 and 71 % at the end of the ZWT (Table 2). Moreover, a DOC loss of at least 40 % was reached for primidone after 2 weeks, but no further DOC loss occurred to the end of the test (Fig. 1).
Formation of phototransformation products (PTP)
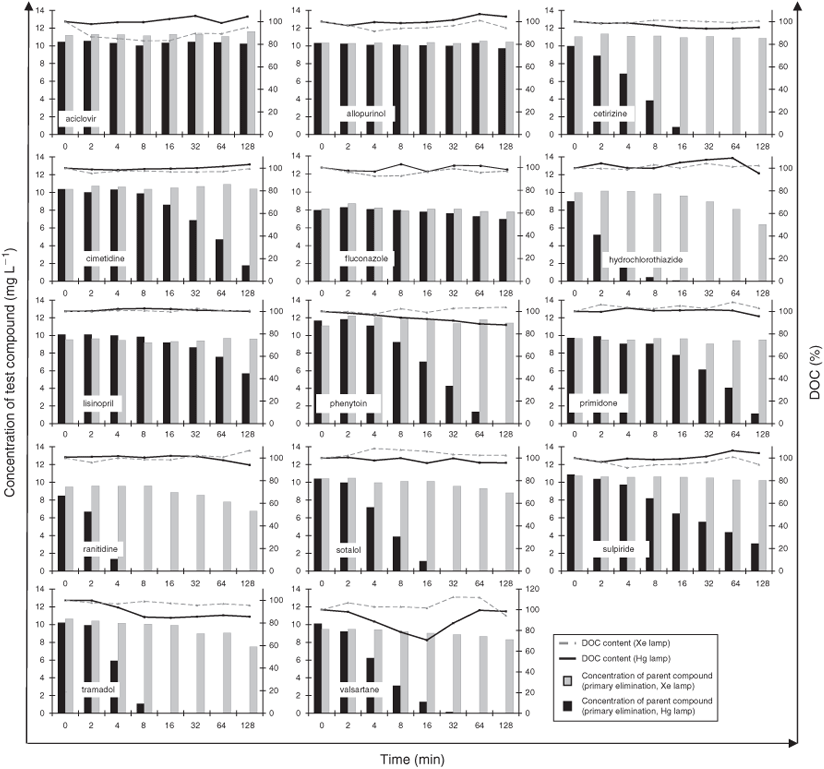
With respect to the HPLC-UV-MS analysis, deviations of all quality controls were within the limits of ±20 % for both experiments (Xe and Hg lamp). Fig. 2 shows the variations in DOC as well as the concentration of the test compounds as a function of irradiation time for all active compounds and during the irradiation experiments.
|
Over the course of the two irradiation experiments, the DOC content remained constant for all compounds tested, irrespective of the lamp used for the irradiation. Minor variations in DOC content were only observed for valsartane.
Over the course of the irradiation experiment with the Hg lamp, a constant and distinct decrease of concentration of the parent compounds was recorded for all of the tested compounds, except for aciclovir, allopurinol and fluconazole. In contrast, over the entire irradiation with the Xe lamp, a distinct decrease of the concentrations was only recorded for the three parent compounds hydrochlorothiazide, ranitidine and tramadol, and a minor decrease was additionally found for sotalol and valsartane (Fig. 2).
Fig. S1 (Supplementary material) shows the absorbance intensity of all non-irradiated and irradiated samples as a function of wavelength and during the irradiation experiments with the Hg lamp. The absorbance spectra of the solutions containing the compounds cetirizine, hydrochlorothiazide, phenytoin, ranitidine, sulpiride, tramadol and valsartane were clearly modified during irradiation. No variations in the absorbance bands were found for the rest of the tested compounds' solutions.
In contrast, when the irradiation experiments were carried out with the Xe lamp, no variations of the absorbance bands were found for any of the compounds. Only a minor decrease of the absorbance maximum of hydrochlorothiazide was found (data not presented).
The formation of PTPs was further investigated by means of HPLC-UV-MS analysis. The intensity in the extracted ion chromatogram (EIC) spectra in terms of signals and UV absorbencies were compared in each respective sample of the photodegradation experiments (data not presented). After irradiation, newly formed MS signals or newly formed UV absorbencies may both represent PTPs.
No such new signals were found in the HPLC-UV-MS analysis for aciclovir, allopurinol or fluconazole. In combination with the corresponding constant DOC values mentioned above, the constant concentration of the parent compounds as well as the absorbance spectra, which remained invariable in both quality and quantity, it becomes evident that no PTPs were formed for these three active compounds, neither in the experiments with the Hg lamp, nor in the experiments with the Xe lamp.
However, for all other parent compounds, when exposed to the Hg light source, new UV and MS signals as well as the above-mentioned constant DOC values and the distinct reduction in concentration of the parent compounds make it apparent that PTPs have been formed.
In contrast, when exposed to the Xe light source, new UV and MS signals, reductions of the concentration of the parent compounds as well as variations of the absorbance spectra are much less significant, thus the extent of formation of PTPs is less pronounced.
Toxicity against P. putida and V. fischeri
Toxicity was tested for all active compounds that were not biodegradable and for all active compounds that were transformed into PTPs during irradiation with the Hg lamp.
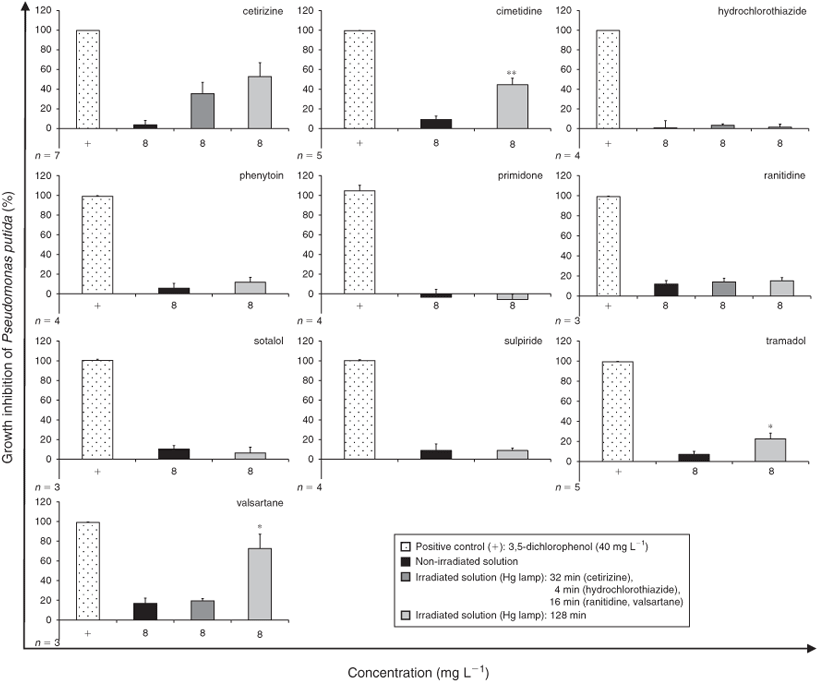
A first screen for bacterial toxicity was undertaken at a relatively high concentration of 8 mg L–1 using the widespread bacterial species P. putida. Comparing non-irradiated and irradiated samples, toxicity was significantly higher in the irradiated samples of cimetidine, tramadol and valsartane. An increase of toxicity after irradiation was also found for the antihistamine cetirizine (Fig. 3).
|
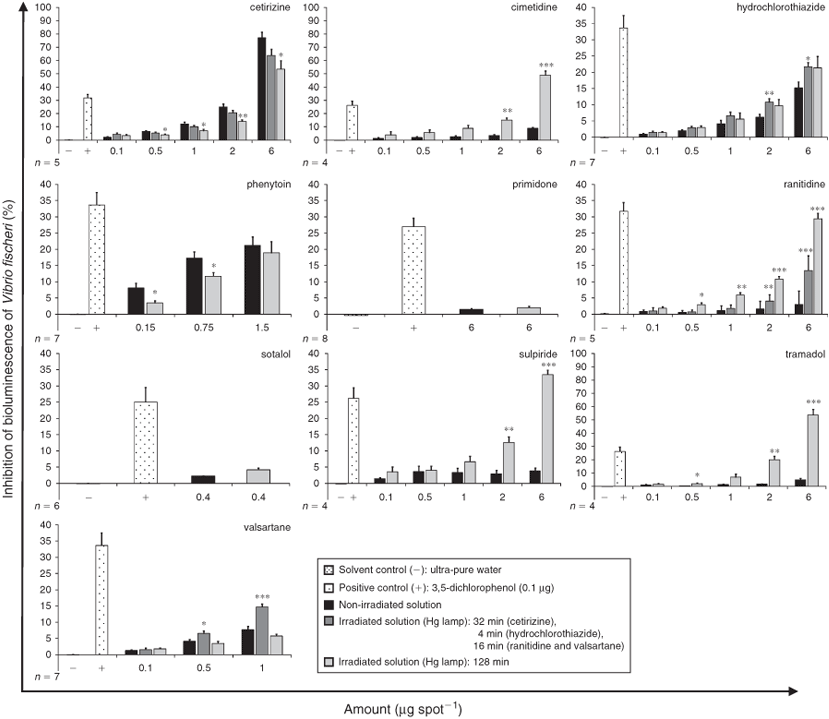
Further evaluation of bacterial toxicity was undertaken for the same non-irradiated and irradiated active compounds using V. fischeri luminescence bacteria. Results are shown in Fig. 4, where inhibition of bioluminescence is plotted as a function of concentration and irradiation exposure. Comparing non-irradiated and irradiated samples, toxicity was significantly higher for the irradiated samples of the pharmaceutical compounds hydrochlorothiazide, ranitidine, sulpiride, tramadol and valsartane. In contrast, after irradiation, toxicity was decreased for phenytoin and cetirizine. No toxicity, neither for the non-irradiated nor irradiated solutions, was found for primidone and sotalol.
Cell viability and DNA integrity of Hep-G2 and HeLa cells
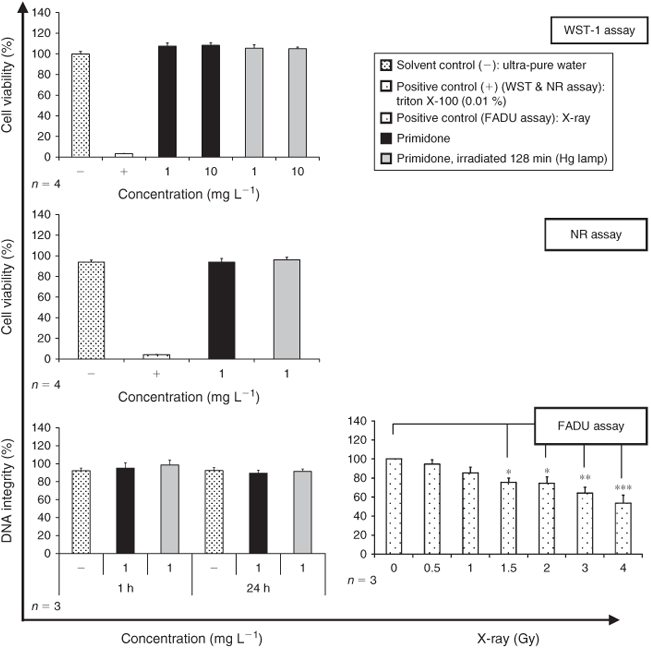
Toxicity was further investigated by means of two cell viability tests (WST-1 and NR assays) and one genotoxicity test (FADU assay) using human Hep-G2 and HeLa cells. Fig. 5 shows an exemplary result for primidone.
The cell viability and DNA integrity of Hep-G2 and HeLa cells were not affected by any of the pharmaceutical compounds tested. Moreover, toxicity was not affected after exposure to the PTPs.
Discussion
In the present study the persistence of a series of selected pharmaceuticals in the aquatic environment was evaluated with respect to biotic and abiotic transformation processes and toxicity. These tests were performed in an effort to generate data to prioritise the various substances for further testing.
Biodegradation
Biodegradation is the key degradation mechanism for organic compounds in the aquatic environment. In this study, the biodegradability of 14 pharmaceutically active compounds was investigated in detail with a test battery consisting of three widely used and standardised OECD biodegradability tests. The results demonstrate the high resistance of most of the investigated compounds towards biological degradation processes. Therefore, it can be assumed that without further treatment steps these substances may accumulate in aquatic systems. Our results are consistent with the findings of other investigations, which have documented the presence in different aquatic matrices of most of the pharmaceuticals studied here (see Table 3). Surprisingly, in the literature, the screening and detection of the parent compound allopurinol in the aquatic environment has not yet been described although our study revealed it to be neither biodegradable nor photodegradable, and even though the PEC value (775 ng L–1, phase-II) estimated here for this compound was actually the highest of all the PECs calculated.
Our investigations also show that under optimal conditions the two active compounds sotalol and lisinopril are at least inherently biodegradable. Similarly, Gros et al.[24] did not detect lisinopril in surface waters. However, despite its strong tendency to degrade biologically under optimal conditions, sotalol has already been detected in different aquatic compartments.[2,25,26] This pseudopersistence is obviously the result of high discharge rates, and our study demonstrates that an environmental risk assessment does not only have to take into account biodegradation rates but also the detection of the parent compounds of interest in the environment.
Phototransformation
The results of our irradiation experiments confirm that most pharmaceuticals undergo phototransformation to form PTPs. Only 3 (aciclovir, allopurinol and fluconazole) out of the 14 tested pharmaceutical compounds were completely resistant to any UV- or light-induced transformation.
In order to study photodegradation we carried out two different test series, in which PTPs were shown to be formed by UV light (Hg lamp) or by simulated sunlight (Xe lamp), although the latter produced only less PTPs even after 2 h of direct irradiation because of the lower total energy emitted by this lamp and the almost missing UV radiation of the spectrum emitted. High-energy radiation is needed to degrade carbon–carbon double and single bonds. Furthermore, the different pharmaceuticals have different absorption maxima in terms of wavelength and absorbance intensity, i.e. different molar extinction coefficients. This explains the different results for the Hg and the Xe lamps.
For standardisation we used ultra-pure water as recommended by Fatta-Kassinos et al.[27] Although transformation rates in natural water samples could be significantly higher – because nitrate, nitrite and organic compounds such as humic aids could act as photosensitiser in surface waters and accelerate reaction rates[28,29] – radiation will not penetrate the whole water body as underwater transmittance of sunlight is attenuated by water itself and by dissolved and suspended organic matter, which is present in natural water columns. Furthermore, the light intensity depends on altitude and season. Therefore, we decided to work with a highly standardised test system using purified water only in order to obtain basic knowledge.
Our study demonstrates that the high-energy UV light (Hg lamp) resulted in the formation of PTPs, even after a short period of irradiation. As a consequence, routine use of UV irradiation as a new technology in water purification is not appropriate, at least not as a sole technique and without any further purification steps. It can result in incomplete degradation of parent compounds, and undesirable or possibly hazardous micropollutants may be formed.
Nevertheless, photodegradation products of the compounds studied here have not yet been identified in the aquatic environment and not yet been searched for, and it remains unclear whether the PTPs determined during these laboratory-scale experiments are also formed under natural conditions in the aquatic environment. Consequently, not only must the issue of reducing the emissions of parent compounds into the aquatic environment be tackled, more work is also needed to characterise the chemical properties and toxicity of PTPs.
Toxicity
The possibility of multiple as well as long-term but unnoticed toxicological effects on aquatic life or even on human beings from exposure to PTPs is a matter of concern. We therefore not only investigated the possible formation of PTPs, but also initiated first studies on the toxicity of the observed PTPs using different in vitro screening test systems.
Two different bacterial strains were used for the bacterial toxicity tests. The bacteria V. fischeri are commonly used in monitoring studies as they have been shown to be very suitable as a biological detector.[18] Nevertheless, V. fischeri is a marine bacterium and not common in surface waters. Therefore we also performed the growth inhibition test with P. putida, which is less often used in monitoring studies, but is common in water and soil habitats.
The results of our bacterial toxicity screening assays showed irradiation to have a detoxifying effect on the two compounds phenytoin and cetirizine albeit only in the bioluminescence assay with V. fischeri. In contrast, a significant increase in toxicity was recorded for most of the investigated parent compounds after high-energetic UV irradiation (Hg lamp). For example, the toxicity of irradiated samples of tramadol and cimetidine (initial amount of each: 6 µg per spot) were shown to be significantly more toxic than the non-irradiated samples, and even more toxic than the positive control 3,5-dichlorophenol (0.1 µg per spot). Similarly, we found that the PTPs of sulpiride were more toxic than the positive control 3,5-dichlorophenol (0.1 µg per spot). To our knowledge there has been no previous study dealing with the increased toxicity of sulpiride after irradiation. Photochemical degradation was examined by Skibiński et al.[9] for the structurally related compound amisulpiride, but toxicity assessment for PTPs from amisulpiride has not been reported. For ranitidine we also found that PTPs have a higher toxicity towards V. fischeri than its parent compound, whereby the effect was almost as high as the one of the positive control (Fig. 4). This result is in accordance with studies carried out by Isidori et al.,[14] who found that PTPs are more toxic to crustaceans and rotifers than the parent compound ranitidine. Isidori et al.[14] observed that ranitidine is oxidised at the sulfide atom. Based on their results we conclude that the closely related compound cimetidine may also be oxidised at the sulfide atom, because both compounds resemble each other in terms of their thioether component. This hypothesis is further consolidated by the result of our LC-MS analysis, where a new strong signal with a corresponding [M + H]+ ion of m/z 269 was detected (Fig. S2 of the Supplementary material), which may represent the oxidised cimetidine. Latch et al.[30] also suggested that cimetidine sulfoxide represents a photolysis product.
Nevertheless, in Germany, an environmental risk from exposure to PTPs of cimetidine is probably low because of decreasing prescription rates and the low PEC value (9 ng L–1). However, cimetidine is one of the four most abundantly used pharmaceuticals in South Korea.[31] Consequently, environmental risks cannot be fully excluded, especially because of the toxicity of the irradiated parent compound cimetidine reported here.
We found the opposite results for cetirizine: toxicity was reduced after irradiation in the V. fischeri bioluminescence assay, but was increased in the P. putida growth inhibition test. However, on its own the parent compound cetirizine was very toxic to V. fischeri: at an initial amount of 6 µg per spot it was ~2.5 times more toxic than the positive control. Interestingly, all irradiated samples of the studied histamine H1- and H2-antagonists (cimetidine, cetirizine and ranitidine) revealed higher toxicity to at least one of the two bacterial strains used. Whether the class of pharmacologic agents comprised by antihistamines is generally transformed into toxic PTPs should be investigated in the future. Different outcomes of the two bacterial toxicity tests were also found for the irradiated samples of valsartane. Samples irradiated for 16 min were more toxic to V. fischeri than samples irradiated for 128 min, whereas P. putida was more susceptible to the 128-min sample. A strong new signal with a corresponding [M + H]+ ion of m/z 414 was detected in the LC-MS analysis for the 16-min sample but not for the 128-min sample. Recently, Bianchini et al.[32] clarified the chemical structure of two PTPs of valsartane with one PTP showing the same signal with a corresponding m/z of 414 ([M + H]+). However, in the study presented here we could clearly demonstrate for the first time that high-energy UV irradiation of the parent compound valsartane generates toxic PTPs.
With regard to the number of irradiated samples that have a higher bacterial toxicity than the parent compound, the growth inhibition test with P. putida seems to be less sensitive than the V. fischeri bioluminescence assay. This finding is in accordance with the fact that Pseudomonas species are common inhabitants of aquatic environments[33] and thus are able to tolerate environmental stresses.[34] Neumann et al.[35] showed that P. putida are even able to change cell morphology as a response to toxic compounds. An increase of the volume of the cell reduces the overall membrane surface on which toxic compounds can attach, and efflux pumps are more effective. Furthermore, Vodovnik et al.[36] assumed that this adaptation is a consequence of changes in the fatty acid composition of the membrane. This adaptation mechanism may be a reason why the two bacterial toxicity tests exhibited different results for the compounds hydrochlorothiazide, cetirizine, ranitidine, sulpiride and valsartane. As a consequence, we recommend more detailed ecotoxicity investigations for environmental risk assessment studies on PTPs.
Once they have entered surface waters, recalcitrant PTPs may also enter ground and drinking water. They can, however, also be formed during UV disinfection of drinking water. Expected concentrations of PTPs in drinking water are still low and so far, studies to screen for cytotoxicity or genotoxicity resulting from PTPs and tested with human cell lines are extremely rare. To our knowledge there have been no comparable previous studies on the evaluation of PTPs formed by irradiation of pharmaceutical compounds. Thus, the aim of this study was not only to assess the ecotoxicity of PTPs, but also to conduct an initial screening of the cytotoxicity and genotoxicity of the parent compounds and PTPs as a very first step in assessing their possible risk to humans. We therefore also screened for cytotoxicity in terms of lysosomal membrane integrity and mitochondrial dehydrogenases activity, and for genotoxicity with the FADU assay. To simulate realistic conditions, we performed our experiments at the lowest concentrations that could be tested (1 or 10 mg L–1). During our study, neither cytotoxicity nor genotoxicity were found for any of the investigated pharmaceutical compounds and their respective irradiated samples. However, in comparison with the results from the bacterial toxicity tests it is important to keep in mind that two completely different cells (bacteria and human cell lines) were used.
In the literature it is often assumed that PTPs bear a higher risk to humans and to aquatic life than the parent compounds. This assumption is often made without any experimental evidence. We recommend that literature dealing with the toxicity of PTPs should explicitly differentiate between the two notations ‘phototoxicity’ and ‘toxicity triggered by PTPs’. This becomes obvious when looking at the OECD’s definition of phototoxicity, which states that phototoxicity is a toxic response from a substance applied to the body, which is increased after the body’s subsequent exposure to light.[37] Thus, this definition does not describe phototoxicity as a toxic response resulting from the uptake of PTPs. The need for a clear differentiation of these terms is confirmed by the results from Han et al.,[38] who proved phototoxic hemolysis for the PTPs of hydrochlorothiazide, albeit only if exposed cells were additionally irradiated. Without further UV irradiation, the authors found that the PTPs did not induce significant hemolysis. This low toxicity of the PTPs from hydrochlorothiazide is in accordance with our results from the toxicity assessment with human cell lines. Furthermore, we recommend also using the same clear notational differentiation for the two terms ‘photogenotoxicity’ and ‘genotoxicity of PTPs’. Chételat et al.[39] proved that some fluoroquinolones induce photogenotoxic effects, whereas they did not find any genotoxic effects when cells were exposed with pre-irradiated samples, hence, with samples containing the PTPs. In the FADU assay conducted here, we also did not find any genotoxic effects of the PTPs derived from the tested fluoroquinolones. We therefore strongly recommend that a clear distinction should be made between ‘phototoxicity’ and ‘toxicity of PTPs’ to avoid false assumptions.
Furthermore, in view of the huge diversity of degradation products in the aquatic environment it seems adequate to focus first on the assessment of toxicity before extensive and costly chemical analyses are conducted. We therefore recommend toxicity screening before time-consuming structural elucidation is initiated.
Summary
-
None of the 14 active compounds tested could be classified as readily biodegradable in the closed bottle and the manometric respirometry tests.
-
Two (lisinopril and sotalol) out of fourteen investigated active compounds could be classified as inherently biodegradable in the ZWT.
-
The PECs according to phase-I of the EMA guideline exceeded the action limit of 0.01 µg L–1.
-
According to the literature all active compounds, except for allopurinol and lisinopril, have been detected in aquatic compartments.
-
During 128-min irradiation with an Hg lamp, 11 (cetririzine, cimetidine, hydrochlorothiazide, lisinopril, phenytoin, primidone, ranitidine, sotalol, sulpiride, tramadol and valsartane) out of 14 active compounds were transformed into unknown PTPs.
-
During 128-min irradiation with a Xe lamp, 3 (hydrochlorothiazide, ranitidine and tramadol) out of 14 active compounds were partly transformed into unknown PTPs.
-
Bacterial toxicity was found for the irradiated samples (Hg lamp) of seven (cetirizine, cimetidine, hydrochlorothiazide, ranitidine, sulpiride, tramadol and valsartane) active compounds in comparison to their respective non-irradiated samples (i.e. parent compounds). For two irradiated samples (cetirizine and phenytoin), bacterial toxicity to V. fischeri was reduced after the irradiation process.
-
None of the samples, non-irradiated or irradiated, were found to be cytotoxic or genotoxic to human Hep-G2 and HeLa cells.
Conclusions
-
Many pharmaceutical compounds can be very resistant to biological degradation and therefore, may accumulate in the water cycle.
-
The results show that PTPs are likely to be formed from poorly biodegradable compounds during high-energy UV-water treatment processes. Thus, in these cases water treatment with UV light is not appropriate to completely degrade or transform micropollutants into harmless compounds.
-
The PTPs forming during this process can possibly pose a higher risk to aquatic life because toxic effects to bacteria have been proven in the present study. However, we have as yet been unable to identify a risk to humans in the in vitro toxicity tests with human cell lines.
-
In view of the huge diversity of degradation products we recommend to first focus on toxicity assessment before extensive and costly analyses in terms of structural elucidation are conducted.
Supplementary material
Additional material to this study (absorbance spectra, Fig. S1); TIC, EIC and UV spectra, exemplarily shown for the photodegradation experiment with cimetidine, Fig. S2) is available from the journal online (see http://www.publish.csiro.au/?act=view_file&file_id=EN13218_AC.pdf).
Acknowledgements
Marlies Bergheim thanks the German Federal Environmental Foundation (DBU), Stiftung Viamedica (Director: Prof. Daschner), the Frankfurter Allgemeine Zeitung (FAZ) and the Vereinte Studienstiftung of the University of Freiburg for providing financial support through scholarships.
References
[1] T. Heberer, Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol. Lett. 2002, 131, 5.| Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xjt1Wju7s%3D&md5=a335a869c3b031f0332ffad3862d71a6CAS | 11988354PubMed |
[2] A Comprehensive Literature Review: Monitoring Data on the Occurrence of Pharmaceuticals in the Environment from the German Federal Environmental Agency (UBA) 2011 (German Federal Environmental Agency: Dessau-Rosßlau).
[3] R. Loos, B. M. Gawlik, G. Locoro, E. Rimaviciute, S. Contini, G. Bidoglio, EU-wide survey of polar organic persistent pollutants in European river waters. Environ. Pollut. 2009, 157, 561.
| EU-wide survey of polar organic persistent pollutants in European river waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVOisLnF&md5=04507916b89297d128f196d774fd5da5CAS | 18952330PubMed |
[4] P. Bottoni, S. Caroli, A. Caracciolo, Pharmaceuticals as priority water contaminants. Toxicol. Environ. Chem. 2010, 92, 549.
| Pharmaceuticals as priority water contaminants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjt1Wjsr0%3D&md5=308aeec013b1dfc0f2c5ed7c21b877d1CAS |
[5] J. B. Quintana, S. Weiss, T. Reemtsma, Pathway’s and metabolites of microbial degradation of selected acidic pharmaceutical and their occurrence in municipal wastewater treated by a membrane bioreactor. Water Res. 2005, 39, 2654.
| Pathway’s and metabolites of microbial degradation of selected acidic pharmaceutical and their occurrence in municipal wastewater treated by a membrane bioreactor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlvV2hsbY%3D&md5=de6e773c7cc4266f7a3fd956ad3f47e1CAS | 15979124PubMed |
[6] C. Zwiener, S. Seeger, T. Glauner, F. H. Frimmel, Metabolites from the biodegradation of pharmaceutical residues of ibuprofen in biofilm reactors and batch experiments. Anal. Bioanal. Chem. 2002, 372, 569.
| Metabolites from the biodegradation of pharmaceutical residues of ibuprofen in biofilm reactors and batch experiments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XivValu70%3D&md5=100ba268680eb9df76e8fee1475aad6dCAS | 11939633PubMed |
[7] M. Addamo, V. Augugliaro, A. Di Paola, E. Garcia-Lopez, V. Loddo, G. Marci, L. Palmisano, Removal of drugs in aqueous systems by photoassisted degradation. J. Appl. Electrochem. 2005, 35, 765.
| Removal of drugs in aqueous systems by photoassisted degradation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXkvFSqsLY%3D&md5=91bb77069ab4319d861b81626bb92734CAS |
[8] A. L. Boreen, W. A. Arnold, K. McNeill, Photochemical fate of sulfa drugs in the aquatic environment: sulfa drugs containing five-membered heterocyclic groups. Environ. Sci. Technol. 2004, 38, 3933.
| Photochemical fate of sulfa drugs in the aquatic environment: sulfa drugs containing five-membered heterocyclic groups.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXkvVGgtLk%3D&md5=0435b606b8c37af4fead3cede3587a1dCAS | 15298203PubMed |
[9] R. Skibiński, Identification of photodegradation product of amisulpride by ultra-high-pressure liquid chromatography-DAD/ESI-quadrupole time-of-flight-mass spectrometry. J. Pharmaceut. Biomed. 2011, 56, 904.
| Identification of photodegradation product of amisulpride by ultra-high-pressure liquid chromatography-DAD/ESI-quadrupole time-of-flight-mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[10] C. Sirtori, A. Aguera, W. Gernjak, S. Malato, Effect of water-matrix composition on Trimethoprim solar photodegradation kinetics and pathways. Water Res. 2010, 44, 2735.
| Effect of water-matrix composition on Trimethoprim solar photodegradation kinetics and pathways.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltFGrtbc%3D&md5=54d3a54f060cf84c830625b2f36849adCAS | 20206373PubMed |
[11] S. K. Khetan, T. J. Collins, Human pharmaceuticals in the aquatic environment: a challenge to green chemistry. Chem. Rev. 2007, 107, 2319.
| Human pharmaceuticals in the aquatic environment: a challenge to green chemistry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlvVelsrg%3D&md5=d1cd15d8d0309c6b326563f527be49f5CAS | 17530905PubMed |
[12] W. d. Püttmann, F. Keil, J. Oehlmann, U. Schulte-Oehlmann, Strategy to reduce pharmaceuticals in drinking water – technical approach. Wassertechnische Strategien zur Reduzierung der Trinkwasserbehandlung durch Arzneimittelwirkstoffe 2008, 20, 209.
[13] M. J. Gómez, C. Sirtori, M. Mezcua, A. R. Fernández-Alba, A. Agüera, Photodegradation study of three dipyrone metabolites in various water systems: identification and toxicity of their photodegradation products. Water Res. 2008, 42, 2698.
| Photodegradation study of three dipyrone metabolites in various water systems: identification and toxicity of their photodegradation products.Crossref | GoogleScholarGoogle Scholar | 18294672PubMed |
[14] M. Isidori, A. Parrella, P. Pistillo, F. Temussi, Effects of ranitidine and its photoderivatives in the aquatic environment. Environ. Int. 2009, 35, 821.
| Effects of ranitidine and its photoderivatives in the aquatic environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsl2gtL8%3D&md5=4050f142f61b93c444ab0a0cc2ec6e38CAS | 19135254PubMed |
[15] Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use 2006 (European Medical Agency: Brussels).
[16] M. Bergheim, R. Gieré, K. Kümmerer, Biodegradability and ecotoxicity of tramadol, ranitidine, and their photoderivatives in the aquatic environment. Environ. Sci. Pollut. R. 2012, 19, 72.
| Biodegradability and ecotoxicity of tramadol, ranitidine, and their photoderivatives in the aquatic environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVaitg%3D%3D&md5=0fd2e011f7098bfca3dc46698f6fce63CAS |
[17] U. Schwabe, D. Paffrath, Pharmaceutical Prescriptions in Germany 2011 2010 (Springer: Berlin).
[18] A. Seigel, A. Schroeck, R. Hauser, B. Spangenberg, Sensitive quantification of Diclofenac and Ibuprofen using thin layer chromatography coupled with a Vibrio fisheri bioluminescence Assay. J. Liq. Chromatogr. R. T. 2011, 34, 817.
| Sensitive quantification of Diclofenac and Ibuprofen using thin layer chromatography coupled with a Vibrio fisheri bioluminescence Assay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmt1ymt74%3D&md5=59a06960fcecd3e3df3c7b15fb68d4c8CAS |
[19] G. Repetto, A. del Peso, J. L. Zurita, Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125.
| Neutral red uptake assay for the estimation of cell viability/cytotoxicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXotFakurk%3D&md5=ed01af59fd0eba28eb4b45457646225eCAS | 18600217PubMed |
[20] M. Moreno-Villanueva, R. Pfeiffer, T. Sindlinger, A. Leake, M. Müller, T. B. Kirkwood, A. Bürkle, A modified and automated version of the ‘fluorimetric detection of alkaline DNA unwinding’ method to quantify formation and repair of DNA strand breaks. BMC Biotechnol. 2009, 9, 39.
| A modified and automated version of the ‘fluorimetric detection of alkaline DNA unwinding’ method to quantify formation and repair of DNA strand breaks.Crossref | GoogleScholarGoogle Scholar | 19389244PubMed |
[21] M. Moreno-Villanueva, T. Eltze, D. Dressler, J. Bernhardt, C. Hirsch, P. Wick, G. von Scheven, K. Lex, A. Bürkle, The automated FADU-assay, a potential high-throughput in vitro method for early screening of DNA breakage. ALTEX 2011, 28, 295.
| The automated FADU-assay, a potential high-throughput in vitro method for early screening of DNA breakage.Crossref | GoogleScholarGoogle Scholar | 22130482PubMed |
[22] M. Dębiak, A. Panas, D. Steinritz, K. Kehe, A. Bürkle, High-throughput analysis of DNA interstrand crosslinks in human peripheral blood mononuclear cells by automated reverse FADU assay. Toxicology 2011, 280, 53.
| High-throughput analysis of DNA interstrand crosslinks in human peripheral blood mononuclear cells by automated reverse FADU assay.Crossref | GoogleScholarGoogle Scholar | 21115096PubMed |
[23] J. Durner, M. Dębiak, A. Bürkle, R. Hickel, F.-X. Reichl, Induction of DNA strand breaks by dental composite components compared to X-ray exposure in human gingival fibroblasts. Arch. Toxicol. 2011, 85, 143.
| Induction of DNA strand breaks by dental composite components compared to X-ray exposure in human gingival fibroblasts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmtlamtLg%3D&md5=4e16bfebce9d530254daac9713f6afa3CAS | 20490463PubMed |
[24] M. Gros, M. Petrovic, D. Barcelo, Tracing pharmaceutical residues of different therapeutic classes in environmental waters by using liquid chromatography/quadrupole-linear ion trap mass spectrometry and automated library searching. Anal. Chem. 2009, 81, 898.
| Tracing pharmaceutical residues of different therapeutic classes in environmental waters by using liquid chromatography/quadrupole-linear ion trap mass spectrometry and automated library searching.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFCmtLfE&md5=4f4f34c9c36ff2c4a7cfd5b6923e59c3CAS | 19113952PubMed |
[25] N. M. Vieno, H. Harkki, T. Tuhkanen, L. Kronberg, Occurrence of pharmaceuticals in river water and their elimination a pilot-scale drinking water treatment plant. Environ. Sci. Technol. 2007, 41, 5077.
| Occurrence of pharmaceuticals in river water and their elimination a pilot-scale drinking water treatment plant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmsVajtbc%3D&md5=7f04bbb0588fe1508a3259ac2470d95cCAS | 17711226PubMed |
[26] A. Y. C. Lin, T. H. Yu, C. F. Lin, Pharmaceutical contamination in residential, industrial, and agricultural waste streams: risk to aqueous environments in Taiwan. Chemosphere 2008, 74, 131.
| Pharmaceutical contamination in residential, industrial, and agricultural waste streams: risk to aqueous environments in Taiwan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlylurnJ&md5=e938e0f50362fc7c7d64b0c281b4c41eCAS |
[27] D. Fatta-Kassinos, M. I. Vasquez, K. Kümmerer, Transformation products of pharmaceuticals in surface waters and wastewater formed during photolysis and advanced oxidation processes – degradation, elucidation of byproducts and assessment of their biological potency. Chemosphere 2011, 85, 693.
| Transformation products of pharmaceuticals in surface waters and wastewater formed during photolysis and advanced oxidation processes – degradation, elucidation of byproducts and assessment of their biological potency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVehsbvL&md5=976514a39065a5f986dd2be2f01d1e37CAS | 21835425PubMed |
[28] M. DellaGreca, M. R. Lesce, M. Isidori, S. Montanaro, L. Previtera, M. Rubino, Phototransformation of amlodipine in aqueous solution: toxicity of the drug and its photoproduct on aquatic organisms. Int. J. Photoenergy 2007, 2007, 63 459.
| Phototransformation of amlodipine in aqueous solution: toxicity of the drug and its photoproduct on aquatic organisms.Crossref | GoogleScholarGoogle Scholar |
[29] D. M. Leech, M. T. Snyder, R. G. Wetzel, Natural organic matter and sunlight accelerate the degradation of 17 beta-estradiol in water. Sci. Total Environ. 2009, 407, 2087.
| Natural organic matter and sunlight accelerate the degradation of 17 beta-estradiol in water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXit1Sjtb4%3D&md5=29f854e11dde962efb92bec93816462cCAS | 19118869PubMed |
[30] D. E. Latch, B. L. Stender, J. L. Packer, W. A. Arnold, K. McNeill, Photochemical fate of pharmaceuticals in the environment: cimetidine and ranitidine. Environ. Sci. Technol. 2003, 37, 3342.
| Photochemical fate of pharmaceuticals in the environment: cimetidine and ranitidine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkvFyjsrw%3D&md5=304286b8ee7cef88ee3b68125207df9aCAS | 12966980PubMed |
[31] Y. Kim, K. Choi, J. Jung, S. Park, P. G. Kim, J. Park, Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ. Int. 2007, 33, 370.
| Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXisVKmurg%3D&md5=5a2f6bd1e425dc225a957f7907c952f5CAS | 17223195PubMed |
[32] R. M. Bianchini, P. M. Castellano, T. S. Kaufman, Characterization of two new potential impurities of Valsartan obtained under photodegradation stress condition. J. Pharm. Biomed. Anal. 2011, 56, 16.
| Characterization of two new potential impurities of Valsartan obtained under photodegradation stress condition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsVyltL4%3D&md5=8c7cf9f151f90b6659c4de29d448e610CAS | 21592713PubMed |
[33] I. Vaz-Moreira, O. C. Nunes, C. M. Manaia, Diversity and antibiotic resistance in Pseudomonas spp. from drinking water. Sci. Total Environ. 2012, 426, 366.
| Diversity and antibiotic resistance in Pseudomonas spp. from drinking water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmvFSjtbw%3D&md5=05b790d4513c6eb9faecd581f756cf9cCAS | 22521167PubMed |
[34] N. J. Palleroni, The Pseudomonas story. Environ. Microbiol. 2010, 12, 1377.
| The Pseudomonas story.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptFOhu74%3D&md5=a326c7fa8f744101af60e7aa67f94203CAS | 20553550PubMed |
[35] G. Neumann, Y. Veeranagouda, T. B. Karegoudar, O. Sahin, I. Mausezahl, N. Kabelitz, U. Kappelmeyer, H. J. Heipieper, Cells of Pseudomonas putida and Enterobacter sp. adapt to toxic organic compounds by increasing their size. Extremophiles 2005, 9, 163.
| Cells of Pseudomonas putida and Enterobacter sp. adapt to toxic organic compounds by increasing their size.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjtlKqtbk%3D&md5=fa428ec87c77d1d49e1da32ca78b1ca9CAS | 15765202PubMed |
[36] M. Vodovnik, M. Bistan, M. Zorec, R. M. Logar, Membrane changes associated with exposure of Pseudomonas putida to selected environmental pollutants and their possible roles in toxicity. Acta Chim. Slov. 2012, 59, 83.
| 1:CAS:528:DC%2BC38Xlt1alsrY%3D&md5=34cb540b1193a05c40661ced3bd36badCAS | 24061176PubMed |
[37] OECD Guideline for testing of chemicals: In vitro 3T3 NRU phototoxicity test 2004 (Organisation of Economic Cooperation and Development: Paris).
[38] K. D. Han, K. M. Bark, E. P. Heo, J. K. Lee, J. S. Kang, T. H. Kim, Increased phototoxicity of hydrochlorothiazide by photodegradation. Photodermatol. Photo. 2000, 16, 121.
| Increased phototoxicity of hydrochlorothiazide by photodegradation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXksF2qurY%3D&md5=b04e3fa654bd4f5f41dfb8c61efa3098CAS |
[39] A. A. Chételat, S. Albertini, E. Gocke, The photomutagenicity of fluoroquinolones in tests for gene mutation, chromosomal aberration, gene conversion and DNA breakage (Comet assay). Mutagenesis 1996, 11, 497.
| The photomutagenicity of fluoroquinolones in tests for gene mutation, chromosomal aberration, gene conversion and DNA breakage (Comet assay).Crossref | GoogleScholarGoogle Scholar | 8921512PubMed |
[40] K. Yu, B. Li, T. Zhang, Direct rapid analysis of multiple PPCPs in municipal wastewater using ultrahigh performance liquid chromatography–tandem mass spectrometry without SPE pre-concentration. Anal. Chim. Acta 2012, 738, 59.
| Direct rapid analysis of multiple PPCPs in municipal wastewater using ultrahigh performance liquid chromatography–tandem mass spectrometry without SPE pre-concentration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xps1Kmt7k%3D&md5=eab08d44e56993fe8364379b1e7a5accCAS | 22790701PubMed |
[41] J. Fick, H. Soderstrom, R. H. Lindberg, C. Phan, M. Tysklind, D. G. J. Larsson, Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522.
| Contamination of surface, ground, and drinking water from pharmaceutical production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsV2lt7bI&md5=8ea48cee25d7165aab4c2d1f8ad46cebCAS | 19449981PubMed |
[42] J. Kosonen, L. Kronberg, The occurrence of antihistamines in sewage waters and in recipient rivers. Environ. Sci. Pollut. R. 2009, 16, 555.
| The occurrence of antihistamines in sewage waters and in recipient rivers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXot1Cjtr8%3D&md5=e500ed9fffe2d2a4821de90c1b77d2acCAS |
[43] B. Kasprzyk-Hordern, R. M. Dinsdale, A. J. Guwy, The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363.
| The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXps1Wkug%3D%3D&md5=52c394b51f3f956276ac17929c5f9570CAS | 19022470PubMed |
[44] K. Choi, Y. Kim, J. Park, C. K. Park, M. Kim, H. S. Kim, P. Kim, Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea. Sci. Total Environ. 2008, 405, 120.
| Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFOjurrF&md5=045bebd20245444c023eeed637b3290fCAS | 18684486PubMed |
[45] D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber, H. T. Buxton, Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202.
| Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhslOitLg%3D&md5=57329dcacf84659eb3f2f1ed1da4126aCAS | 11944670PubMed |
[46] J. W. Kim, H. S. Jang, J. G. Kim, H. Ishibashi, M. Hirano, K. Nasu, N. Ichikawa, Y. Takao, R. Shinohara, K. Arizono, Occurrence of pharmaceutical and personal care products (PPCPs. in surface water from Mankyung River, South Korea. J. Health Sci. 2009, 55, 249.
| Occurrence of pharmaceutical and personal care products (PPCPs. in surface water from Mankyung River, South Korea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXkt1yku7g%3D&md5=477c31e8b24ea49404bdd0b82429a779CAS |
[47] H. Lindberg, J. Fick, M. Tysklind, Screening of antimycotics in Swedish sewage treatment plants – waters and sludge. Water Res. 2010, 44, 649.
| Screening of antimycotics in Swedish sewage treatment plants – waters and sludge.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhvFegsLo%3D&md5=659b8373d67936c43e5c1ed330842310CAS |
[48] M. Kahle, I. J. Buerge, A. Hauser, M. D. Mueller, T. Poiger, Azole fungicides: occurrence and fate in wastewater and surface waters. Environ. Sci. Technol. 2008, 42, 7193.
| Azole fungicides: occurrence and fate in wastewater and surface waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVems77L&md5=efbbbf75bc31da2939824df48ef3f478CAS | 18939546PubMed |
[49] D. Calamari, E. Zuccato, S. Castiglioni, R. Bagnati, R. Fanelli, Strategic survey of therapeutic drugs in the rivers Po and Lambro in northern Italy. Environ. Sci. Technol. 2003, 37, 1241.
| Strategic survey of therapeutic drugs in the rivers Po and Lambro in northern Italy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtlCjur4%3D&md5=6445416a8f0e7003bd8b00236a3e3fbbCAS |
[50] E. Zuccato, S. Castiglioni, R. Fanelli, G. Reitano, R. Bagnati, C. Chiabrando, F. Pomati, C. Rossetti, D. Calamari, Pharmaceuticals in the environment in Italy: causes, occurrence, effects and control. Environ. Sci. Pollut. R. 2006, 13, 15.
| Pharmaceuticals in the environment in Italy: causes, occurrence, effects and control.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xks1GnsA%3D%3D&md5=e859b05b51712a89afde29d0eed003ffCAS |
[51] T. Heberer, Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J. Hydrol. 2002, 266, 175.
| Tracking persistent pharmaceutical residues from municipal sewage to drinking water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnvFyjur0%3D&md5=6ba934c364dfe957a1fbebd3dd7d3985CAS |
[52] M. J. Benotti, R. A. Trenholm, B. J. Vanderford, J. C. Holady, B. D. Stanford, S. A. Snyder, Pharmaceuticals and endocrine disrupting compounds in US drinking water. Environ. Sci. Technol. 2009, 43, 597.
| Pharmaceuticals and endocrine disrupting compounds in US drinking water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFSqsLjM&md5=3b863378ee80774ba89fb0fbeba08984CAS | 19244989PubMed |
[53] G. M. Bruce, R. C. Pleus, S. A. Snyder, Toxicological relevance of pharmaceuticals in drinking water. Environ. Sci. Technol. 2010, 44, 5619.
| Toxicological relevance of pharmaceuticals in drinking water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnvFGntLc%3D&md5=bf449e6faefc1836b6c4a4b84a44ec56CAS | 20575537PubMed |
[54] D. Hummel, D. Loeffler, G. Fink, T. A. Ternes, Simultaneous determination of psychoactive drugs and their metabolites in aqueous matrices by liquid chromatography mass spectrometry. Environ. Sci. Technol. 2006, 40, 7321.
| Simultaneous determination of psychoactive drugs and their metabolites in aqueous matrices by liquid chromatography mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1ChsLvK&md5=88c4161c20370719e807e91e08486df8CAS | 17180984PubMed |
[55] B. Morasch, F. Bonvin, H. Reiser, D. Grandjean, L. F. de Alencastro, C. Perazzolo, N. Chevre, T. Kohn, Occurrence and fate of micropollutants in the Vidy Bay of Lake Geneva, Switzerland. Part II: micropollutant removal between wastewater and raw drinking water. Environ. Toxicol. Chem. 2010, 2259, 1658.
[56] Y. C. Guo, S. W. Krasner, Occurrence of primidone, carbamazepine, caffeine, and precursors for n-nitrosodimethylamine in drinking water sources impacted by wastewater. J. Am. Water Resour. Assoc. 2009, 45, 58.
| Occurrence of primidone, carbamazepine, caffeine, and precursors for n-nitrosodimethylamine in drinking water sources impacted by wastewater.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjt1CgsLc%3D&md5=a3f36b270c277eb1bf5e2806b580c2e2CAS |
[57] S. Castiglioni, R. Fanelli, D. Calamari, R. Bagnati, E. Zuccato, Methodological approaches for studying pharmaceuticals in the environment by comparing predicted and measured concentrations in River Po, Italy. Regul. Toxicol. Pharmacol. 2004, 39, 25.
| Methodological approaches for studying pharmaceuticals in the environment by comparing predicted and measured concentrations in River Po, Italy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntVOguw%3D%3D&md5=278d8438c51bb05b57635ffe911848efCAS | 14746777PubMed |
[58] J. Fick, R. H. Lindberg, L. Kai, E. Brorstoem-Lundén, Results from the Swedish National Screening programme 2010 2011 (IVL Swedish Environmental Research Institute: Stockholm, Sweden).
[59] H. D. Zhou, C. Y. Wu, X. Huang, M. J. Gao, X. H. Wen, H. Tsuno, H. Tanaka, Occurrence of selected pharmaceuticals and caffeine in sewage treatment plants and receiving rivers in Beijing, China. Water Environ. Res. 2010, 82, 2239.
| Occurrence of selected pharmaceuticals and caffeine in sewage treatment plants and receiving rivers in Beijing, China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVCit7jN&md5=e0c35c35e5c3bf2ec3e1698d3ca1c039CAS |
[60] T. Okuda, Y. Kobayashi, R. Nagao, N. Yamashita, H. Tanaka, S. Tanaka, S. Fujii, C. Konishi, I. Houwa, Removal efficiency of 66 pharmaceuticals during wastewater treatment process in Japan. Water Sci. Technol. 2008, 57, 65.
| Removal efficiency of 66 pharmaceuticals during wastewater treatment process in Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXivVOrtbw%3D&md5=24f7dcd9f752b02b1d35823b8cb49b9aCAS | 18192742PubMed |