Aquatic toxicity of manufactured nanomaterials: challenges and recommendations for future toxicity testing
Aaron G. Schultz A E , David Boyle A , Danuta Chamot A , Kimberly J. Ong A , Kevin J. Wilkinson B , James C. McGeer C , Geoff Sunahara D and Greg G. Goss AA Department of Biological Sciences, University of Alberta, Edmonton, AB, T6E4W1, Canada.
B Department of Chemistry, University of Montreal, PO Box 6128, Succursale Centre-Ville Montreal, QC, H3C 3J7, Canada.
C Biology Department, Wilfrid Laurier University, Waterloo, ON, N2L 3C5, Canada.
D Aquatic and Crop Resource Development, National Research Council of Canada, 6100 Royalmount Avenue, Montreal, QC, H4P 2R2, Canada.
E Corresponding author. Email: ags2@ualberta.ca

Aaron Schultz is a Post-Doctoral Fellow in the laboratory of Prof. Greg Goss at the University of Alberta, Canada. He is a fish physiologist and aquatic toxicologist and received his B.Sc. (with 1st Class Honours) in 2006 and Ph.D. from Deakin University, Geelong, Australia, in 2010. His primary research interests focus on studying the adaptive mechanisms employed by fish and other aquatic organisms that allow them to survive in constantly changing environments and in response to anthropogenic contaminants such as nanomaterials. |

David Boyle is a Post-Doctoral Fellow in the research group of Prof. Greg Goss at the University of Alberta, Canada. He received his B.Sc. from the University of Bath, UK, in Applied Biology in 2001 and his M.Sc. in 2003 and Ph.D. in 2008 both from King's College London, UK. After an appointment as a Research Scientist at the National Institute for Nutrition and Seafood Research, Bergen, Norway, he has worked as a Post-Doctoral Research Fellow in the field of aquatic nanotoxicology, firstly at Plymouth University, UK, and since 2012, at the University of Alberta, Canada. |

Danuta Chamot is a Senior Research Associate in the laboratory of Prof. Greg Goss in the Department of Biological Sciences at the University of Alberta. She received her B.Sc. in 1988 and M.Sc. in 1990 from the University of Toronto and her Ph.D. in plant molecular biology from the University of Bern (Switzerland) in 1993. She has spent most of her career studying stress-regulated gene expression in aquatic microbes. Since joining the Goss lab, she has been closely involved in research on the physiological and toxicological effects of pollutants on fishes. |

Kimberly J. Ong is a graduate of the Biological Sciences Ph.D. program at the University of Alberta in Edmonton, Canada in 2013. She earned her B.Sc. in Marine and Freshwater Biology at the University of Guelph in 2006. Her most recent work focussed on developing and validating appropriate biological testing techniques for nanotoxicity studies, and is also interested in the effects of nanoparticles on fish development, behaviour and physiology. |

Kevin J. Wilkinson is Professor at the Université de Montréal. His research is aimed at gaining a molecular level understanding of contaminant bioavailability and mobility. Current projects are examining the nature of the physicochemical processes influencing trace metal bioaccumulation by microorganisms and determinations of the fate (dissolution, aggregation) and bioavailability (bioaccumulation, genomic and proteomic effects) of engineered nanomaterials. Wilkinson is currently an Associate Editor of Environmental Chemistry (2010–) and in the past has edited two volumes of Biophysicochemistry of Environmental Systems. He has over 100 publications, over 4000 citations to his work and an h-index of 40. He has recently established a world-class analytical laboratory specialising in the characterisation of nanomaterials: Center for the Analysis and Characterization of Engineered Nanomaterials (CACEN). |

Jim McGeer is an Associate Professor in the Biology Department and Director of the Institute for Water Science at Wilfrid Laurier University. He completed B.Sc. Agr. and M.Sc. degrees at the University of British Columbia and then a Ph.D. at the University of Dundee in 1995. He joined Laurier in 2006 following postdoctoral studies at Ben-Gurion and McMaster Universities and then a period as a federal government scientist and research manager at Mining and Mineral Sciences division of Natural Resources Canada. The McGeer lab is focussed on solutions based research directed at integrating fundamental understandings of how inorganic contaminants impinge on physiological processes in aquatic organisms and the application of this understanding in prediction models that contribute to environmental protection by reducing uncertainty. |

Geoffrey Sunahara is a Senior Research Officer at the National Resource Council of Canada (NRC). He received his B.Sc. in 1976 and M.Sc. in 1979, both from the University of Toronto, and his Ph.D. (Pharmaceutical Sciences) in 1984 from the University of British Columbia. He completed a 2-year Fogarty post-doctoral fellowship at the National Institute of Environmental Health Sciences (NIH). After working at the Nestec Research Centre (Switzerland) until 1994, he then joined NRC to develop the Applied Ecotoxicology group that focuses on the ecotoxicology of emerging contaminants including nanomaterials, biodiesel and other bioproducts. A major emphasis is made upon innovation, ecological relevance, risk assessment and modes of toxicity. |

Prof. Greg Goss is appointed in the Department of Biological Science, Faculty of Science at the University of Alberta with a cross-appointment to the School of Public Health at the University of Alberta. He is a fellow of National Institute of Nanotechnology, the Scientific Director of University of Alberta Water Initiative and Director of the Office of Environmental Nanosafety at the University of Alberta. Dr Goss works jointly with industry, governments and academia to examine the environmental toxicology of micropollutants including nanomaterials, pharmaceuticals and personal care products, hydraulic fracturing fluid and hydrocarbon contaminated fluids. |
Environmental Chemistry 11(3) 207-226 https://doi.org/10.1071/EN13221
Submitted: 9 December 2013 Accepted: 22 May 2014 Published: 20 June 2014
Environmental context. The increased use of nanomaterials in industrial and consumer products requires robust strategies to identify risks when they are released into the environment. Aquatic toxicologists are beginning to possess a clearer understanding of the chemical and physical properties of nanomaterials in solution, and which of the properties potentially affect the health of aquatic organisms. This review highlights the main challenges encountered in aquatic nanotoxicity testing, provides recommendations for overcoming these challenges, and discusses recent studies that have advanced our understanding of the toxicity of three important OECD nanomaterials, titanium dioxide, zinc oxide and silver nanomaterials.
Abstract. Aquatic nanotoxicologists and ecotoxicologists have begun to identify the unique properties of the nanomaterials (NMs) that potentially affect the health of wildlife. In this review the scientific aims are to discuss the main challenges nanotoxicologists currently face in aquatic toxicity testing, including the transformations of NMs in aquatic test media (dissolution, aggregation and small molecule interactions), and modes of NM interference (optical interference, adsorption to assay components and generation of reactive oxygen species) on common toxicity assays. Three of the major OECD (Organisation for Economic Co-operation and Development) priority materials, titanium dioxide (TiO2), zinc oxide (ZnO) and silver (Ag) NMs, studied recently by the Natural Sciences and Engineering Research Council of Canada (NSERC), National Research Council of Canada (NRC) and the Business Development Bank of Canada (BDC) Nanotechnology Initiative (NNBNI), a Canadian consortium, have been identified to cause both bulk effect, dissolution-based (i.e. free metal), or NM-specific toxicity in aquatic organisms. TiO2 NMs are most toxic to algae, with toxicity being NM size-dependent and principally associated with binding of the materials to the organism. Conversely, dissolution of Zn and Ag NMs and the subsequent release of their ionic metal counterparts appear to represent the primary mode of toxicity to aquatic organisms for these NMs. In recent years, our understanding of the toxicological properties of these specific OECD relevant materials has increased significantly. Specifically, researchers have begun to alter their experimental design to identify the different behaviour of these materials as colloids and, by introducing appropriate controls and NM characterisation, aquatic nanotoxicologists are now beginning to possess a clearer understanding of the chemical and physical properties of these materials in solution, and how these materials may interact with organisms. Arming nanotoxicologists with this understanding, combined with knowledge of the physics, chemistry and biology of these materials is essential for maintaining the accuracy of all future toxicological assessments.
Additional keywords: nanoparticles, nanotoxicology, silver, titanium dioxide, zinc oxide.
Introduction
The increasingly rapid production and release of a novel type of synthetic material collectively termed nanomaterials (NMs), necessitates their investigation as possible threats to ecosystems (see reviews by Baun et al.,[1] Handy et al.,[2] Klaine et al.,[3] Moore,[4] Scown et al.,[5] Peralta-Videa et al.,[6] Klaine et al.,[7] Bondarenko et al.[8] and Ray et al.[9]). These materials are defined as small materials that are at most 1–100 nm in one dimension and have unique properties and functions resulting from their small size.[10,11] NMs have recently been redefined by the European Commission as ‘a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50 % or more of the particles in the number size distribution, one or more external dimensions is in the size range 1–100 nm’.[12] Nanotechnology and the production and utilisation of NMs is a multibillion-dollar per annum commercial industry that is expanding rapidly. Increased production efficiencies and advances in the development of NMs have enabled an increased number of nano-enabled medical, industrial and consumer products.[13]
Currently, the most widely used NMs in consumer products are engineered metal-containing and metal oxide (MeO) NMs, including nAg, nTiO2 and nZnO. In 2013, the Woodrow Wilson database listed 1628 products containing NMs, 383 of which contained Ag NMs, 179 contained TiO2 and 36 products contained ZnO NMs (Project on Emerging Nanotechnologies, see http://www.nanotechproject.org/cpi/about/analysis/, accessed 25 October 2013). The growing interest and use of these metal-based and metal oxide NMs for future applications will likely result in greater release of these NMs and their resulting ionic counterparts into aquatic environments. Indeed, a growing number of studies have already confirmed the release of Ag NMs into wastewater from washing machines,[14] clothing,[15–17] toothpaste, shampoo and detergent,[15] whereas TiO2 NMs have been reported to be released from textiles[18] and sunscreens.[19,20]
In response to these concerns, many national agencies worldwide have established research consortia, including but not limited to Centres for Environmental Implications of Nanotechnology (CEINT, USA), NanoFATE (UK), NanoSafe (Australia), Managing Risks of Nanomaterials (MARINA, EU), NanoValid (EU) and Natural Sciences and Engineering Research Council of Canada (NSERC), National Research Council of Canada (NRC) and the Business Development Bank of Canada (BDC) Nanotechnology Initiative (Environment Canada) (NNBNI, Canada). Under the Organisation for Economic Co-operation and Development (OECD), a list of priority NMs was defined and consortia were tasked with providing environmental characterisation and toxicological data of these materials for use in risk analysis.[21] This review provides an update of the toxicological properties of key OECD materials with a focus on the findings of the NNBNI consortium (Canada). Other metal-containing and organic NMs, including but not limited to, gold NMs,[22,23] carbon nanotubes[22,24,25] and silicon-based NMs[22,26] also merit toxicological analysis, however they are beyond the scope of this review. Here we highlight some of the key advances in our understanding of aquatic nanotoxicological testing methods including NM transformations (dissolution, agglomeration and interaction with small molecules) in aqueous test media and NM interference with common toxicological assays. The characterisation requirements and controls that will allow researchers to assess both NM transformations and assay interference, and accurately interpret toxicological results are also discussed. Finally, we discuss the advances in our understanding of the toxicology of three key OECD materials: TiO2, Ag and ZnO NMs and conclude with a section on primary knowledge-gaps and key recommendations for future toxicity studies of NM.
NM testing requires advanced characterisation for proper interpretation
Accurate testing of NM toxicity to aquatic organisms is necessary to determine whether specific regulations should be mandated for NMs, however, conventional in vitro and in vivo tests employed for traditional toxicants may not be appropriate for many NMs. We discuss two major points where the absence of a thorough characterisation will result in an incomplete or incorrect interpretation of toxicological or environmental results. First, physicochemical transformations of NMs readily occur in aquatic and test media leading to uncertainty during toxicity testing, which then affects our confidence in providing accurate risk assessments. Second, the small size and unique colloidal properties of NMs can lead to unpredictable interactions with the test media and its constituents, including the organisms therein. These NM transformations and interactions are discussed in detail below, as well as recommendations for advanced characterisation and proper controls to incorporate so accurate interpretation of toxicity experiments can be made.
NM transformations in aqueous test media
Numerous abiotic factors influence the physicochemical state of NMs, both spatially and over time. Any variation in exposure conditions will create difficulties in the interpretation of toxicity experiments. In contrast to soluble, well dispersed toxicants, nanotoxicologists must understand both the novel properties of the material and the colloidal nature of the NMs. Fig. 1 identifies the key factors that should be considered when designing toxicity studies. For example, it is necessary to quantify factors such as dissolution (Fig. 1a), agglomeration and sedimentation (Fig. 1b) or the adsorption of ions and macromolecules (Fig. 1c) in order to understand bioavailability and toxicity. Improved knowledge of the catalytic activity of the NMs (generation of reactive oxygen species (ROS), bandgaps for semiconductors and quantum confinement (Fig. 1d)) will also allow the researcher to better understand the potential effect this may have on nanotoxicity, which will facilitate our ability to properly assess the risk of the NMs.
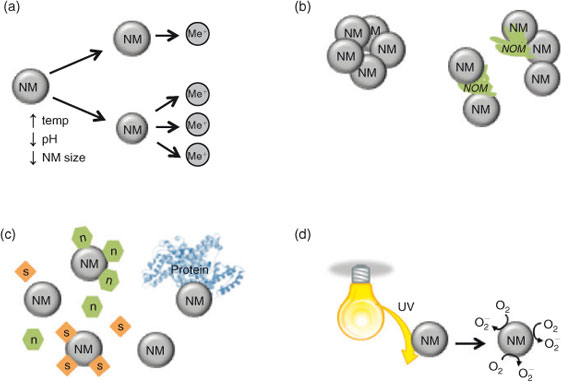
|
Dissolution of NMs
It is well known that aquatic organisms are particularly sensitive to free metals,[27] and exposure at sub-lethal concentrations can affect several processes, including development,[28] movement,[29,30] hatch rate[31–33] and ion balance.[34] A major concern associated with metal-based NMs is the dissolution and release of ionic species in aquatic matrices. For example, nominal concentration[35] and diameter will affect dissolution[36–38] whereby the relatively larger surface area to volume ratio in smaller particles results in more exposed atoms and higher dissolution rates.[36,37] The mechanism of dissolution of NMs is different than that for bulk materials, and not always fully predictable based on theoretical calculations.[36,37,39] Stable surface coatings, binding of molecules from the test media or exudates from the organism, or aggregation of NMs can block exposed NM surfaces, potentially reducing dissolution rates.[40] In contrast, elevated temperatures[41] and low pH[36,38,42] tend to increase the rate of dissolution of metals and metal oxides. Media composition will also affect dissolution whereby constituents may complex dissolved metals, compete for binding sites on an organism[42] or form a surface coating on the NMs themselves, thereby abrogating toxic effects.[43] Although most studies are performed at circumneutral pH, the potential exists for colloidal NMs that are accumulated by a cell to encounter acidic compartments such as lysosomes, which may increase dissolution inside the cell.[37,44,45] This so called ‘Trojan horse’ effect will be discussed later in this review (see section Summary of progress on OECD priority nanomaterials: TiO2, ZnO and Ag).
Monitoring free metal release from a NM is essential to distinguish metal ion effects from NM-specific effects. Carefully designed studies have shown that NMs can have effects distinct from their ionic forms, resulting in damage by different mechanisms than those reported for their dissolution (free ion) constituents.[37,46–49] Therefore, great care must be taken to determine not simply NM dissolution throughout an experiment but also trace metal speciation where relevant. Because some analytical techniques measure total dissolution (e.g. dialysis, ultracentrifugation, ultrafiltration) whereas others measure either free ions (e.g. ion selective electrode) or ionic species (e.g. diffusion in thin film gradient technique, voltammetry), data interpretation must carefully take into account methodological considerations. Furthermore, because dissolution rates can change over time as NMs either aggregate, bind to other molecules or surfaces or translocate across membranes, it is highly recommended to follow dissolution under the precise experimental conditions (e.g. medium, concentration, time, light) being used. It is no longer acceptable to solely measure dissolution of NMs in media or solutions other than those being used in the experiment.
Agglomeration of NMs
Agglomeration of NMs is dependent on both biotic and abiotic factors.[30] Agglomeration is known to reduce the available active surface of the NM, decrease the stability in the water column (i.e. increased sedimentation), and affect the aggregates’ ability to translocate over membranes or surfaces. Together, these processes act to reduce exposure and therefore hazard in the suspended test organisms, but may increase exposure and therefore hazard in benthic test organisms.[50]
According to classic Derjagiun, Landau, Verwey and Overbeek (DLVO) theory, agglomeration occurs when colloidal particles’ attractive forces (e.g. van der Waals) overwhelm repulsive forces (e.g. electrostatic).[51–53] Changes in a solution’s properties can affect the electrostatic double layer (EDL), and particle electric charge. Compression of the EDL in solutions of high ionic strength can reduce the repulsive forces of a NM (observed through a reduction in zeta potential), resulting in increased agglomeration.[36,54–56] Furthermore, if the pH of a solution is close to that of the point of zero charge of the NM, repulsive electrostatic forces are weakened and NMs will likely agglomerate.[36,55,56] Some electrolytes also affect the stability of NMs; in particular, high concentrations of divalent cations, such as Ca2+ or Mg2+ can cause increased agglomeration as a result of particle bridging, charge screening or surface complexation.[54,56–58]
Sterically stabilised NMs, such as those with capping agents, may not be as susceptible to changes in abiotic conditions as electrostatically stabilised NMs.[30,54] In aquatic matrices, NMs are known to adsorb molecules and form a ‘corona’,[59–62] a dynamic outer layer that coats the NM and can change agglomeration kinetics among other properties. Spontaneous adherence, or replacement of the coating by natural organic matter (NOM) in natural aquatic environments can produce charge and steric stabilisation of NMs.[56–58] Similarly, within a whole organism, intracellular and serum proteins can contribute to this coating around the NM.[30,63] The extent of surface coverage on the NM can change the agglomeration state. Whereas complete coverage may result in steric or electrostatic stabilisation, only partial coverage may cause increased agglomeration due to destabilisation by charge neutralisation, bridging or the interaction of oppositely charged patches of the NM.[30,36,53,64] The type and combination of proteins or organic molecules,[30] their concentration[58,65] and the incubation period[30,57] are all known to affect these interactions and must be taken into consideration when performing nanotoxicology experiments.
Monitoring the size of NM agglomerates, their sedimentation and the population of remaining monodispersed NMs throughout an experiment is essential when predicting their distribution and availability to organisms in aquatic environments.[30] A few studies have suggested that only NMs of a specific size will be taken up by cells or organisms,[66,67] whereas aggregates above a threshold size may be prevented from translocating across protective membranes, such as the embryonic fish chorion.[68] Furthermore, large agglomerates that form will likely settle, effectively concentrating the NM at the bottom of the experimental chamber. This may be particularly important in cell culture studies[69,70] and experiments where the test organism is located at the bottom of the test vessel (e.g. zebrafish embryos or benthic organisms[71,72]).
Small molecule interactions
Upon addition to aquatic media, NMs adsorb to small particles in the water column, which may be critical components for an organism’s survival. Although nanotoxicologists tend to ascribe the effects of NM exposure as a direct toxic effect, these additional interactions may simply affect organismal survival by sequestering nutrients and essential minerals, such as, hormones, growth factors, salts, proteins, nutrients and vitamins.[73] Depletion of any of these factors by their adsorption to NMs may reduce their effective concentration and indirectly limit growth and survival.[74,75] For example, Guo et al.[76] found that amino acids and vitamins are adsorbed to single-walled carbon nanotubes (SWCNTs), depleting essential nutrients that may result in decreased cell health. Ions such as calcium or phosphate have been shown to be sequestered by NMs,[75,77] potentially leading to homeostatic imbalances or deficiencies. Similarly, the inhibition of enzyme activity necessary for fish hatch has been shown to result in lethality in embryo tests.[47] It has been speculated that binding of NMs to the gills and skin[78] of fishes may lead to interactions with proteases and lysozymes that protect fish from bacterial infection.[79]
Nanomaterial interference with toxicological assays
High-throughput in vitro assays are commonly used to test the toxicity of substances on aquatic organisms. In recent years, several reports have emerged indicating that a variety of NMs can interfere with standard toxicity assays, including cellular viability tests, such as alamar blue[80–82] and neutral red[80,81,83] lactate dehydrogenase (LDH) release,[82–86] and tetrazolium dye based assays (water soluble tetrazolium, WST[80,87]; 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide, XTT[88]; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, MTT[83,89–92]). Although nanoparticle-assay interferences are becoming more regularly reported, the majority of published nanotoxicity papers still do not test for interference,[82] resulting in difficulties interpreting data. The interactions that occur between the NM and components of an assay are still not well understood, making it difficult to predict the consistency of each test. To date, the primary modes of NM-assay interference identified are: (1) direct optical interference by the NM, (2) adsorption of the NM to important assay components and (3) the generation of ROS by the NM (see Fig. 2). Each of these modes is discussed below with subsequent considerations for selecting assays of NM toxicity.
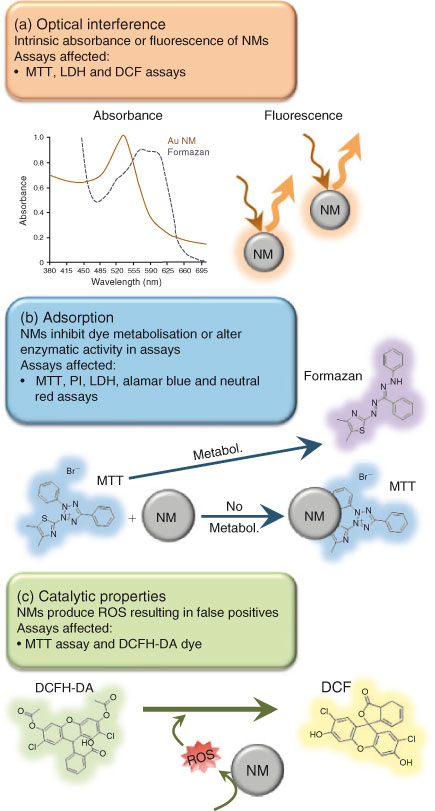
|
Optical interference
Many plate-based assays are based on an end-point spectroscopic measurement of dyes or indicators. One main mode of interference is that significant absorbance or fluorescence of the NM occurs at the wavelength at which the assay is measured. For example, Au NMs generally have a strong absorbance peak between 520 and 580 nm.[93,94] They thus have the potential to interfere with the MTT assay because the absorbance peak of the Au NM directly overlaps with the absorbance of the formazan salts in the assay (Fig. 2a). Moreover, the quantum properties of semiconductor materials can result in their absorbance or the production of interfering fluorescence spectra across UV-visible ranges.[95–99]
NMs may also interfere with the optical properties and output of indicator molecules. When a NM binds to the indicator molecule, this may lead to either a reduction in resonance frequency, and therefore intensity, or a shift in the absorbance maxima for the dye. Such an effect will lead to an apparent decrease in the absorbance or fluorescence measurement and thus an underestimation or overestimation of biological effect, depending on the assay characteristics.[87,99–103]
Adsorption to assay components
Owing to their small size and relatively high surface area to volume ratio, NMs can adsorb to the components of the assay and obstruct proper transformation of indicator molecules.[80,89] For example, the MTT assay is contingent on the production and subsequent dissolution of MTT-formazan crystals. However, adsorption to SWCNTs renders the dye insoluble, thereby affecting the final colourimetric measurement[89] (Fig. 2b). Membrane impermeable dyes such as propidium iodide (PI) are often used to identify non-viable cells with compromised membranes (e.g. LIVE/DEAD Viability Assay Kit, Life Technologies). If NMs can bind to and traffic the dyes into viable cells false positives may result,[104,105] and in other conditions, NM binding to the dye may prevent the dye from translocating and may make it appear that there is reduced toxicity.
It is well known that proteins and biomolecules can form a ‘corona’ (see reviews by Dawson et al.[61,106] and Monopoli et al.[107]) around a NM and that these interactions can affect the activity of enzymes.[108] Consequently, assays that measure enzymatic activity are likely to be affected by NMs. For example, the presence of active LDH in test media is often used as an indicator of cell death following its release into the media. As it has been shown that NMs can interact with LDH and decrease enzyme activity,[108] likely through structural deformation of the enzyme, this could result in inaccurate results regarding cell viability whereby toxicity would be masked.[84,85]
NM generation of ROS
The physicochemical properties of some NMs enable them to undergo oxidation and produce ROS that can potentially reduce dyes to their fluorescent forms,[92,100] resulting in false positive tests of toxicity. For example, incubation of carbon-based NMs with 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA) (a highly oxidisable dye that is a commonly used as a marker of oxidative stress) results in the production of fluorescent 2′,7′-dichlorodihydrofluorescein (DCF) molecules even when cells are not present (Fig. 2c).[100] Therefore, investigators should be knowledgeable about the standard potential of dyes and oxidation state[100] of the NM. If the potential for NM-mediated oxidation of the dye exists, alternative assays should be utilised to prevent assay interference and the generation of false positives.
Recommendations
Some assays appear to be more prone to error than others and therefore, validating assays with each unique formulation of the NM is essential. NM characteristics including method of synthesis, surface structure, core material, shape, etc. can modify the degree of assay interference.[91,92,104,108] Varying the concentration of other biomolecules in the assay, such as bovine serum albumin or foetal calf serum, may also influence NM interference.[84,92,108] Preliminary tests assessing a range of NM concentrations can indicate whether the assay is appropriate for the NM used. In many cases, only high concentrations of NM significantly affect an assay,[87,90] and lower concentrations may be deemed acceptable; minimisation of nanoparticles in the final product by centrifugation or filtration may limit effects. Because many spectroscopic assays are affected by the presence of NMs, the use of dye-free methods avoids the issues presented. For example, flow cytometry, manual cell counting techniques, electrical cell-based impedance protocols[109] or clonogenic assays[110] can be used to assess viability.
A significant issue is our current inability to predict the dominant mode of interference by each NM formulation on a particular assay, therefore, each assay will be differentially affected by the type and characteristics of the NM studied. To be able to validate or create new standardised tests, high-throughput assay interference testing of NMs with similar cores and different physico-chemical characteristics is needed to enhance our understanding of, and ability to, predict interference. Adsorption capacity, dissolution, optical properties and redox potential are particular NM characteristics that should be explored. Given the propensity for NMs to influence assay results, it is recommended that: (a) assays be conducted with careful control over the final concentrations of NMs, (b) multiple assays be used to corroborate results[81,85] and (c) tests for NM interference be conducted and reported for each assay and NM used.
Summary of progress on OECD priority NMs: TiO2, ZnO and Ag
There have been a substantial number of excellent, informative and extensive literature reviews published in the past few years on aquatic nanotoxicology.[8,111–114] Moreover, multiple research consortia have contributed to the extensive and rapidly growing body of knowledge in the field of nanotoxicology. In order to reduce some of the complexity and overlap with these recent reviews, we have focussed this review on the three major NM groups that were extensively covered recently by the NNBNI-Environment Canada, Canadian consortium. Relevant published results contributing to our understanding of the toxicity of each NM type are highlighted.
For each of the three OECD priority materials, evidence for both bulk effect, dissolution based (i.e. free metal) or a specific toxicity attributable to the unique properties of the NM itself have been identified. In the subsequent sections, we discuss each OECD priority material and summarise recent research indicating distinct mechanisms of toxicity that might be attributable to each material. Fig. 3 summarises each of the potential nanospecific, ionic or bulk mechanisms of toxicity relevant to metal and metal oxide NMs. It is not the intention here to perform a comprehensive review of the NM literature, but rather to draw attention to studies of note that have illustrated well the properties of the NM that are important to toxicity and which should inform future testing strategies.
Titanium dioxide NMs
Titanium dioxide (TiO2) is a metal oxide that has been used traditionally as a white pigment for paints, cosmetics, confectionaries, etc. Natural forms of TiO2 include the minerals ilmenite (FeTiO2), rutile (TiO2) and sphene (CaSiTiO5), with ilenite and rutile being the predominant forms (USEPA[115]). Engineered TiO2 is generally produced in two-phase forms or as a combination of these phases. The physico-chemical properties of nanosized anatase and rutile (including hydrophobicity and photoreactivity) are quite different than their micrometre-sized counterparts.[115] These properties can be exploited in novel or improved consumer products or industrial processes including UVA–UVB sunscreens, semiconductors (anatase bandgap energy = 3.2 eV or 385 nm), air deodorisers or wastewater treatment technology.[116,117] These new products and processes may pose a burden to the aquatic environment if TiO2 NMs are released unintentionally. The actual environmental concentrations of TiO2 NMs are not known, although model calculations offer some estimates.[64,118,119] Based on probabilistic material flow analysis of product life cycle, the Nowack group calculated the predicted environmental concentrations (PEC) for nano-TiO2 in Switzerland to be 0.021 mg L–1 in surface water, and an annual increase of 1.203 mg kg–1 year–1 for sediment and 11.2 ng kg–1 year–1 for soil.[120] In another paper, they estimated 0.015 and 0.002 mg L–1 in surface water, an annual increase of 358 and 53 mg kg–1 year–1 in sediment and an annual increase of 1.28 and 0.53 mg kg–1 year–1 in soil for Europe and the US.[121]
It is not known whether these concentrations have an ecotoxicological effect in the environment. ‘Effects’ studies of TiO2 NMs (and other NMs) have most often been conducted in the laboratory using methods that were developed to test the toxicity of soluble chemicals. Whether these methods are appropriate for non-soluble chemicals and particulates such as TiO2 NMs is controversial. For example, the use of mass-based concentrations (e.g. milligrams per litre) is problematic when comparing anatase and rutile because they possess the same molar mass (79.87 g mol–1), but they may differ in size (i.e. nanometric v. micrometric scale) or aspect ratio (spherical, needles, rods).
There is a body of knowledge describing the toxicity of nanosized and micrometre-sized TiO2 on aquatic organisms, including algae, invertebrates and fish (see reviews of Baun et al.,[1] Klaine et al.,[3] the United States Environmental Protection Agency (US EPA),[116] Farré et al.,[122] Kent,[123] Handy et al.,[2] Menard et al.[124] and Navarro et al.[125]). Although there have been exceptions reported in the literature,[126] there is broad agreement that TiO2 NMs can cause effects in aquatic organisms under certain test conditions; however, the levels of toxicity reported (most obvious where determined effect concentration (effective concentration, ECx) values have been calculated) show considerable variability between studies. For example, the average 72-h median effective concentrations (EC50) for Evonik Degussa P25 (mixed-phase: 80 % anatase and 20 % rutile) nanoparticle on algal growth calculated from three separate studies[127–129] was 28 ± 22 (s.e.) mg L–1. This value is 2-fold lower than the 96-h EC50 value of 50.1 mg L–1 for Evonik Degussa P25 on algal growth calculated by Metzler et al.[130] This variability in EC50 values for P25 is likely related to differences in test sample preparation, algae species and exposure durations, use of artificial sunlight,[127] a non-standard toxicity test medium[129] or a UV pre-illumination period.[128] Inconsistencies like these, present challenges to risk assessors as well as national and international regulators of TiO2 NMs, who seek to minimise risk, and need to be considered for future toxicity experiments.
Another source of variability can be attributed to the reporting of the research, such as the physico-chemical characterisation of TiO2 NMs before and during the ecotoxicity assays,[2] preparation of TiO2 NM test samples and experimental procedures used for the toxicity assays and the toxicity response endpoints, making comparisons between studies problematic.[131,132] Based on the principles described above (see NM transformation in aquatic test media), TiO2 NMs (primary particles) can form concentration- and time-dependent agglomerates in solution and these processes are strongly dependent on the pH and ionic strength of the test media.[56,127–130,133–135] In turn, these dynamic physicochemical processes may alter the toxic responses of organisms to TiO2 NMs. Research issues and strategies to develop a comprehensive environmental assessment for TiO2 NMs have been described elsewhere.[116] Also, the OECD has sponsored an international program (Working Party on Manufactured Nanomaterials, WPMN) to characterise the fate and ecotoxicity of TiO2 NMs for risk assessment.[136] The issue of inter-experimental variability should be better resolved after the results on the P25 from the OECD WPNM are published. Preliminary results have been described recently by Hund-Rinke and Hennecke.[137]
Despite considerable variability in reported toxicological responses of organisms to TiO2 NMs, further analyses of the literature can be performed. TiO2 NMs comprise a small group of NMs for which particle size controls (i.e. micrometre or bulk sized TiO2) have often been included in toxicity assessments. In order to identify trends and draw a consensus as to the potential for nanoscale specific effects of TiO2, the following discussion has selected some physico-chemical and toxicological data from studies with similar experimental conditions and endpoints. Within comparable studies, an additional weighting has been given to the most recent literature.
Table 1 summarises the results of eight studies that compare the toxicities of nano-sized TiO2 (i.e. primary particle diameter <100 nm) and ‘bulk’ TiO2 (i.e. primary particle diameter >100 nm) on freshwater algae, using different toxicity test methods. The studies used TiO2 samples that were obtained from various manufacturers or suppliers, and included anatase or anatase–rutile combinations, of varying purity. Test samples were prepared by homogenisation, stirring or ultrasonication, and the TiO2 materials were characterised in the test media by using a variety of analytical techniques. Only a limited number of physico-chemical characteristics of the primary and secondary particles were consistently reported in the studies.
Algal toxicity assays were based on international (International Organization for Standardization or OECD) or national (China) toxicity test guidelines, using different algal species. Effects of the TiO2 NMs on algal growth were measured using cell counts or chlorophyll fluorescence. EC50 values are expressed as milligrams per litre (nominal concentrations). In some studies, biochemical measurements of photosynthetic efficiency, algal cell damage and oxidative stress[129,135,138,139] were also provided. Detailed discussion of the effects on algal biomarkers (MTT, LDH, DCFDA, etc.) is beyond the scope of the present review. Based on the present study dataset (Table 1), there was a clear difference between the average specific surface area (SSA) of nanosized (195 m2 g–1; n = 7) and micrometre-sized TiO2 (8 m2 g–1; n = 6) particles. In contrast, EC50 values (mg L–1) for the nanosized particles (52 ± 28 (SE); n = 8) were similar to those of the micrometre-sized samples (55 ± 19; n = 6). Nonetheless, closer examination of the individual studies of the dataset provides strong and convincing evidence that a size (SSA)–effects relationship with toxicity may exist. Seven of the nine TiO2 NMs were more toxic than their micrometre-sized counterpart (used as a reference), as indicated by the elevated nano-toxicity ratio (NTR) values, where EC50 (micrometre)/EC50 (nano) is >1. Only two TiO2 NM samples yielded NTR values <1. Of these, Warheit et al.[140] used nano- and micrometre-TiO2 samples coated with alumina, and Hartmann et al.[127] reported difficulty in obtaining a reliable EC50 value (241 mg L–1, Hombikat UV100). Therefore, weight of this evidence suggests that size or SSA (nano- v. micrometre-TiO2) is involved at least in part with the toxicity of TiO2 on algal growth, and that the TiO2 surface coating can alter this cause–effect relationship. This size-related inhibition of algal growth is not caused by a ‘shading effect’, as shown by others[127,133,141] but is likely mediated by sorption of secondary TiO2 NMs to algal cell aggregates by electrostatic surface charge interactions.[130] Examination using light microscopy (phase contrast and fluorescence), scanning (SEM) and transmission electron microscopy (TEM) shows that TiO2 NM aggregates can bind directly to algae (see also Fig. 3a, step 1), as well as the algal aggregates (flocculated by algal secretion of exo-polymeric substances in an extracellular matrix).[129,130,139,142] In fact, large TiO2 NM aggregates trapped more algal cells than the bulk-TiO2 aggregates,[133] and can occur during incubation under UV-light as well as in the dark.[127,139] Interestingly, adsorption of TiO2 NMs to algae may also have novel detrimental food chain effects; Campos et al.[143] report binding of TiO2 NMs to algae and sedimentation potentially leading to food depletion in Daphnia magna, a filter feeder, and resulting effects on life history traits including reproduction.
The manner in which TiO2 NMs interact with cell surfaces is relevant to their toxicity in aquatic invertebrates, fishes and algae. It is possible that cytotoxicity can be caused by cell entry of small TiO2 NMs or agglomerates (presumably by endocytosis; Fig. 3a, step 2) associated with damage to the cell wall or membrane.[135,139] Nonetheless, recent evidence suggests that direct contact between TiO2 NMs and cell surfaces may be more important to toxicity, especially becasue the bioavailability of TiO2 NMs may be low. Lin et al.[135] studied the effects of nano-anatase on Chlorella sp. incubated in OECD media for 4 days. They reported that the 96-h EC50 (growth inhibition) of pristine anatase was 4.9 mg L–1, which increased to 47 mg L–1 in the presence of humic acid (HA). Using SEM, they observed that algal cells that were incubated with nano-anatase for 24 h became flattened and damaged when compared to control cells (no TiO2 NM added). This TiO2 NM effect was not observed when TiO2 NM-treated cells were incubated in the presence of HA, suggesting that cytotoxicity depended on the direct contact between TiO2 NM and algal cells (Fig. 3a, step 3). In more complex aquatic organisms, toxicity appeared most often to be related to binding of the NM to sensitive external epithelia, in the absence of significant tissue accumulation. Toxicity, including effects on growth and reproduction, was associated with occlusion of the gut with TiO2 NM aggregates and decreased feeding in Daphnia magna, a filter feeder.[144] Furthermore, effects observed in the internal tissues of rainbow trout exposed to 1 mg L–1 TiO2 NM for 14 days were most likely explained by a developing systemic hypoxia arising from occlusion of the gill by TiO2 NMs and resultant gill injury.[145] This hypothesis has since been given further credence by the observation that injection of TiO2 NMs into caudal vasculature, an approach that avoids the issue of bioavailability, resulted in no significant effects in internal tissues, including the kidney, the principle site of accumulation.[146,147] In spite of their apparent low bioavailability, the interaction of TiO2 NMs at external epithelia may have resulted in important ecological effects. For example, Ramsden et al.[148] report decreased fecundity in zebrafish exposed to TiO2 NMs, even though the accumulation of the NM was not observed.
It is possible that environmentally released TiO2 NMs (those residing at the top part of the water column) may be exposed to natural sunlight and UV irradiation. Current data are scant, however, elevated toxicity has generally been observed in organisms where exposure to TiO2 NMs has been coupled with UV irradiation. In zebrafish, the presence of sunlight has been shown to increase the toxicity of TiO2 NMs by several orders of magnitude.[149] Importantly, the increased sensitivity of zebrafish to photo-activated TiO2 NMs may be apparent only during later developmental stages and this may be overlooked in typical tests of 72 or 96-h duration post fertilisation. Elevated toxicity of photo-activated TiO2 NMs appears to be linked to ROS generation because different solar UV spectra induced distinct intracellular ROS in Daphnia (Fig. 3a, step 4 and 5). Studies have also shown that TiO2 NM toxicity is enhanced under simulated solar radiation (UVA) as compared to ambient laboratory lighting[150,151]; a linear correlation between ROS production and D. magna immobilisation was also observed. Similar effects have not been observed in algae; however, studies have generally pre-illuminated P25 samples to avoid the direct toxicity of UV light to algae.[128] The 72-h EC50 data indicated that UV pre-irradiation did not alter the inhibitory effect of P25 on growth (control: visible fluorescent lighting). Similar observations were reported by Hund-Rinke and Simon[141] using anatase pre-irradiated with simulated sunlight (Table 1). These consistent negative results suggest that photo-activation of TiO2 NMs is not involved in algal growth inhibition; however, there was no confirmation that P25 was photo-activated under these experimental conditions. It is also possible that concentrations of bioavailable photo-activated TiO2 NMs or their activation by-products (e.g. ROS) proximal to the algal cell wall were not sufficient to yield a measurable effect.
Although an effort was made to include results of the recent literature in order to interpret prior ecotoxicity data, interpretation of endpoint data (such as EC50 analysis) should not be done in isolation of the morphological changes of TiO2 NMs (agglomeration). Appropriate experimental design will allow for the control of these dynamic processes and reduce the inter-experimental variability that is presently an issue for risk assessors and regulators of this emerging NM.
Zinc oxide NMs
The toxicity of ZnO NMs to aquatic organisms has recently been the subject of several excellent reviews[8,112,113] that offer greater scope to the subject than is afforded here and to which we refer readers seeking a more comprehensive overview of the literature. Rather, the intention here is to highlight studies that have identified: (i) properties of the ZnO NMs that are important to toxicity in aquatic organisms; (ii) likely environmental scenarios of importance and (iii) recommended approaches for more robust toxicity testing and future avenues for investigation.
Zinc oxide is a widely used metal-oxide NM with applications in many commercial products including industrial coatings and pigments, electronics, bioremediation and personal care items. The most recent estimate from 2010 reported global production of ZnO NMs in excess of 30 000 metric tonnes, and is predicted to have since increased further.[152] The broad utility of ZnO NMs is attributable to their optical, piezo-electrical and antimicrobial properties, which are unique to, or enhanced on, the nanoscale (see Xu and Wang[153] for an excellent review of the properties of ZnO NMs). Zinc oxide NMs are wide-bandgap semiconductors with a bandgap energy of 3.3 eV at room temperature,[154] which offers potential for their use in: photovoltaic cells for solar energy harnessing,[155] the photocatalytic degradation of organic pollutants[156] and the attenuation of light in the UVA–UVB range (peak absorbance ~370 nm), which is integral to the effectiveness of ZnO NMs in sunscreens.[157] Uncoated ZnO NMs are also soluble in aqueous media (unlike TiO2 NMs, and more so than Ag NMs) releasing Zn2+ from the NM surface.[158,159] Although this offers further commercial applications of ZnO NMs, including better delivery of Zn2+ as a nutritional supplement,[160] it can also be detrimental in aquatic environments because Zn2+ is a well-characterised toxicant at higher concentrations in aquatic organisms, e.g. fish (see recent review by Hogstrand[161]). For this reason, Zn2+ levels in the environment are tightly regulated in most jurisdictions (e.g. in Canada, by the Canadian Council of Resource and Environment Ministers).[162] Accordingly, a major challenge in studies of ZnO NMs has been to discriminate between effects attributable to nanoscale specific properties of ZnO NMs, including the Trojan horse hypothesis (Fig. 3b, step 6) and effects caused through the release of Zn2+ (Fig. 3b, step 7), in order to establish if ZnO NMs present a novel risk to aquatic organisms.
Overall, ZnO NMs have been shown to be toxic to a diverse range of both fresh and marine aquatic organisms including algae, molluscs, crustaceans and fishes.[163] A key issue emerging in the literature for ZnO, as well as other metal NMs, is the importance of the physico-chemistry of the test media to the behaviour of ZnO NMs, and its influence on toxicity (see NM transformation in aquatic test media). In suspensions, uncoated ZnO NMs aggregate, sediment and solubilise, and equilibrium Zn2+ concentrations are affected by pH, particle size (especially where ZnO NM < 6 nm) and ionic strength of the media.[158,159] Toxicity in marine organisms has often been closely correlated with the concentration of released Zn2+. For example, cell growth inhibition (measured as decreased cell numbers and chlorophyll concentrations) of the marine phytoplankton Thalassiosira pseudonana exposed to ZnO NMs was closely correlated with the free Zn2+ concentration in suspension.[164] This observation may also extend to freshwater organisms[165]; the toxicity of ZnO NMs to freshwater crustaceans was closely correlated with Zn2+ release.[166] Dissolution may also underpin observations of increased toxicity on the nanoscale compared to bulk ZnO[165,167] because the rate of dissolution is closely correlated with particle surface area.[159]
An important emerging effect of ZnO NMs (and other metal oxide NMs) is in early life stage fishes. In particular, researchers from several groups have reported decreased hatching of zebrafish embryos exposed to ZnO NMs.[47,168–170] For example, Ong et al.[47] reported a decrease in hatching of zebrafish exposed to 10 and 100 mg L–1 of ZnO NMs that eventually culminated in the mortality of unhatched embryos. Interestingly, where ZnO NM exposed embryos were mechanically freed from their chorions, larvae exhibited no treatment dependent developmental deformities or significant effects on larval behaviours (including spontaneous activity) leading the authors to conclude that it was the process itself rather than embryonic development that was sensitive to the ZnO NMs.[47] The hatching process in zebrafish is initiated by the release of a protease, zebrafish hatching enzyme (ZHE1), into the embryonic fluid, and it culminates with zebrafish movements physically disrupting the ZHE1 weakened chorion.[171] Although Ong et al.[47] observed no effect on the movement of hatched larvae, ZnO NMs inhibited protease activity in isolates of chorionic fluid extracted from zebrafish embryos in vitro. This specific inhibition of ZHE1 by ZnO NMs has also been further elucidated since by Lin et al.[172] who reported the in vitro inhibition of the proteolytic activity of recombinant ZHE1 expressed in Escherichia coli.
The mechanistic basis for hatch impairment in embryos exposed to ZnO NMs has been debated in the literature, but a weight of evidence implicates Zn2+dissolution (Fig. 3b, step 8). Although NMs have been demonstrated to inhibit enzymes in vitro through a particle specific mechanism,[108] physical separation of ZnO NMs from embryos with a Zn2+ porous membrane did not ameliorate a zebrafish embryo hatch.[172] Furthermore, the use of a metal ion chelator and the doping of ZnO NMs with Fe, which decreased Zn NM dissolution, both improved hatching rates.[170] In contrast, Ong et al.[47] reported no hatch impairment in embryos exposed to Zn2+ at dissolution control concentrations. Nonetheless, dissolution analysis was performed in ultrapure water whereas dissolution will likely be greater at the higher ionic strength of the tap water used in the experiments and also likely in the high ionic strength environment of chorionic fluid where nanoscale materials have been observed.[173] Moreover, the study of Ong et al.[47] is one of very few to directly compare the toxicity of uncoated and coated NMs where they found minor effects on hatch in embryos exposed to polyacrylic acid stabilised ZnO NMs, further suggesting that Zn2+ dissolution dominates as the source of hatch impairment and therefore it is difficult to define the role of colloidal NM interaction with the hatching enzyme in Zn2+-containing NMs.[47]
Although the role of released Zn2+ towards toxicity has not been fully resolved, Zn2+ appears to have greater bioavailability than nanoscale ZnO. Whereas binding of NMs to cell membranes has been postulated to contribute to toxicity,[2] empirical evidence of ZnO NMs crossing cell membranes has been scant to date. Where the uptake of ZnO NMs has been measured directly, accumulation of Zn by aquatic organisms appears to be strongly correlated with Zn2+, including in benthic organisms where sedimentation could lead to elevated localised concentrations of ZnO NMs. Using a mass balance approach to the partitioning of 68Zn-labelled ZnO NMs in a simplified marine system with the benthic invertebrate Corophium volutator, Larner et al.[174] reported that 97 % of 68Zn was associated with the sediment with relatively little in water (2.5 %). Less than 0.5 % was accumulated in C. volutator after 10 days. Nevertheless, the partitioning of ZnO NMs to the sediment was strongly correlated with dissolution: almost identical mass balances of Zn were observed in systems where 68Zn2+ was added (as ZnCl2). Moreover, Zn accumulation in C. volutator was strongly correlated with the dissolved phase; Zn bioconcentration factors were identical for both ionic and nanoscale Zn and strongly correlated with the concentrations of aqueous phase 68Zn2+, again strongly indicating that dissolved Zn2+ was the bioavailable Zn fraction. Other studies with benthic organisms have also reported similar observations. Biodynamic modelling of Zn accumulation in the estuarine snail Peringia ulvae revealed comparable uptake and efflux rates of Zn2+ and ZnO NMs suggesting that Zn2+ was the bioavailable fraction.[175] Together these studies strongly indicated that the behaviour of ZnO NMs in the environment, even during relatively short periods (10 days in the study of Larner et al.[174]) may be little different from Zn2+.
Although these studies have undoubtedly demonstrated the important role of Zn2+ release in the toxicity of ZnO NMs, the high NM dissolution rates may potentially obscure other important facets of toxicity, including nanoscale specific properties. Nonetheless, the pre-irradiation of ZnO NMs with visible, UVA or UVB light had no observed effect on their toxicity to freshwater algae.[128] However, it should be noted that the experimental design did not include a bulk control for the comparison of phototoxicity and dissolution of ZnO NMs provided acutely toxic Zn2+ concentrations within 24 h.
Moreover, the minimal effect of shape (spheres, rods, needles) and size of the ZnO NMs on their toxicity to marine algae was attributed to the rapid dissolution (and to a lesser extent agglomeration) observed for all ZnO NMs.[176] Furthermore, at elevated concentrations of ZnO NMs (10 and 80 mg L–1) no corresponding increase in toxicity was observed over a 72-h exposure.[176] Zinc oxide NMs rapidly attain equilibrium with free Zn2+ in seawater (reportedly within 4 h in Peng et al.[176]) and thus increasing the concentration of ZnO NMs above 10 mg L–1 may not have altered the Zn2+ concentrations in solution.[176] Although this is further evidence for the principle role of Zn2+ in the toxicity of ZnO NMs, full characterisation of the dissolution behaviour is essential in order to ensure that the toxicity of ZnO NMs is not underestimated. Similar characterisation of NM behaviour is also relevant where the concentrations of the ZnO NMs are below solubility limits; comparable toxicity of Zn2+, ZnO NMs and bulk ZnO to sea urchin embryos is perhaps unsurprising because complete solubilisation of both nano and bulk ZnO will occur within 12 h.[177] To fully assess nanoscale specific effects of ZnO NMs where high rates of solubilisation are expected, investigators should seek to identify biomarkers for ZnO NMs that are responsive during the very short-term (hours) experiments.
Silver NMs
Silver is one of the most common elements manufactured into NMs.[111,178] Most of the applications for Ag NMs are focussed on the antibacterial and antifungal properties of the manufactured NM that result from the release of free silver ions (Ag+). In aquatic systems, ionic forms of Ag can induce toxicity in sensitive organisms at very low (μmol L–1) concentrations.[179,180] As a result, the threshold concentration of Ag in water quality criteria and guidelines is low relative to other metals.[181] The combination of high production volumes for Ag NMs, uncertainty about the bioavailability, uptake and stability of NM forms of metals and a low toxicity threshold for Ag+ has resulted in an elevated level of concern over the potential environmental effects (see the reviews of Fabrega et al.[111] and Reidy et al.[178]). Although there has been considerable effort towards characterising Ag NM toxicity, a comprehensive understanding is still lacking, due in part, to the complexities of the particle behaviour in aquatic media. As for other NMs, factors that have been identified as being important for Ag NM toxicity include its size, shape and surface area, its behaviour in environmental media (including agglomeration, oxidation and dissolution), as well as the influence of coating or capping agents and manufacturing impurities.[178] A mechanistic understanding of the Ag NMs is further complicated by the observation that its behaviour and toxicity depends on the geochemistry of the exposure medium and the age of the material.[113,182,183]
Research efforts towards resolving the uncertainties about the mechanisms of Ag NM toxicity have focussed on the adsorption and uptake of intact NMs (Fig. 3c, step 9), the transformation and dissolution (oxidation) of Ag particles in solution (Fig. 3c, step 10) and possible biological interactions (see the reviews of Shaw and Handy[112] and Levard et al.[184]). Some studies assign the toxic effects to Ag+ dissolution in the test media[185–188] (Fig. 3c, step 10) or within the cells (known as the ‘Trojan horse effect’,[188–191] Fig. 3c, step 11), whereas others conclude that intact NMs induce responses[189–192] (Fig. 3c, step 12). Many of these studies take a comparative approach and include treatments with both Ag NMs and Ag+ at similar concentrations in order to allow a direct comparison of concentrations that induce responses. Both acute and chronic sub-lethal toxicity have been examined. A few of the studies exploit the fact that the mechanism of Ag+ toxicity occurs by blockade of Na+ uptake as a result of Na+/K+-ATPase inhibition on respiratory surfaces in freshwater fish[179,193] (Fig. 3c, step 13) or by Ag+ biouptake over a CuI transporter in algae[188,194,195] (Fig. 3c, step 14). These works apply physiological study design elements focussed on mechanistic uptake measurements to investigate mechanisms of Ag NM uptake and toxicity.
Studies that compare Ag NM responses to those of Ag+ can be loosely grouped into two categories. The first includes studies that show responses at elevated Ag NM exposure concentrations relative to the Ag+ response concentrations. The second group includes those studies where responses occur at similar Ag+ concentrations or at relatively low concentrations, for example near water quality guidelines and criteria values. In works where Ag NM toxicity threshold concentrations are elevated and are above those for Ag+ in the same organism, authors often implicate dissolution, and release of Ag+ as a significant causal factor. For example, Hoheisel et al.,[186] working with Daphnia magna and fathead minnows exposed to uncapped Ag NMs found that EC50 and EC20 concentrations (ranging from 46 to 89 µg Ag L–1) were ~5-fold higher for NMs than for Ag+, and therefore concluded that particle dissolution was the likely cause of Ag NM toxicity. Jo et al.[196] compared a variety of solution preparation methods for uncapped Ag NMs and reported 24-h EC50 values as low as 4.2 µg Ag L–1 but that spanned three orders of magnitude. Nonetheless, dissolved Ag concentrations in solution (as determined by ultrafiltration) explained toxicity and thus they concluded that particle dissolution was responsible for the observed effects. Studies with Daphnia magna exposed to Ag NMs coated with polyvinylpyrrolidone (PVP) noted reduced Na+ influx and disruption of Na+ regulation at 500 µg Ag L–1, however this effect was also attributed to dissolution of Ag+ given that is was not seen when cysteine was added to the medium (cysteine–Ag+ complexes are not bioavailable[197]). In some of these studies, there is an indication that both NMs and Ag+ induce toxicity, and it is generally not possible to distinguish one potential cause from another. For example, although effective concentrations were relatively high (ranging from 1.3 to 10.6 mg Ag L–1), Laban et al.[198] concluded that both NMs and dissolved forms of Ag were responsible for acute toxicity (96-h LC50) in fathead minnow embryos. Similarly, Massarsky et al.[199] determined an LC50 for zebrafish embryos exposed to a polyacrylate capped Ag NM to be 1180 µg Ag L–1, whereas for Ag+, the LC50 was 70 µg Ag L–1. Nonetheless, even though the dissolution of particles was very low (0.5 %), it was not possible to exclude the presence of Ag+ as a possible contribution to the observed toxic effects. Finally, although Leclerc and Wilkinson[188] showed that when exposed to nAg, Ag biouptake by Chlamydomonas reinhardtii exceeded what was predicted based upon measured Ag+ concentrations, their measurements of darkfield–hyperspectral microscopy and expression levels of the CuI transport protein (CTR2) indicated that nAg increased Ag biouptake by locally increasing surface concentrations of Ag+. In other words, Ag biouptake was increased by oxidation of the nAg at the cell surface, but only Ag+ could be considered to enter the cells.
In the second group of studies Ag NMs are shown to induce acute or chronic sub-lethal effects at similar concentrations to Ag+. Often sensitive organisms are used and response thresholds are very low, at or near water quality guidelines and criteria values. These studies generally reach the conclusion that Ag NMs induce toxicity independently or in combination with Ag+. For example, Das et al.[200] characterised acute toxicity of carboxy-functionaliszed capped Ag NMs in Daphnia magna (48-h EC50 of 2.75 µg Ag L–1) and concluded that responses were NM specific as insufficient Ag+ was present to induce toxicity. Citrate capped Ag NMs, which had a very low dissolution rate, were shown to independently reduce Na+ uptake and inhibit Na+/K+ ATPase in juvenile rainbow trout.[46] Allen et al.[185] found that the acute toxicity (48-h LC50) of citrate coated Ag NMs and AgNO3 were similar (1.1 µg Ag L–1) in Daphnia magna and also documented accumulated NMs within Daphnia magna. Although it would seem reasonable to suggest that NMs and perhaps dissolved Ag+ were responsible for Ag NM toxicity, the authors did not reach this conclusion. Exposure of the freshwater mussel, Elliptio complanata, to Ag NMs at 0.8, 4 and 20 µg L–1 resulted in a suite of sub-lethal cellular responses, some of which were similar to Ag+ but others, for example the presence of ubiquitin in the digestive gland, were unique to the NM.[201] Responses unique to Ag NM exposure that do not occur in matched Ag+ exposures are an indirect way to distinguish between the potential toxicity of particles and that of dissolved Ag+ in solution.
Genotoxicity and cytotoxicity studies have contributed to our understanding of particle v. Ag+ responses. In these types of studies, the effects of Ag NMs vary considerably depending on NM characteristics, species and endpoints, but generally responses are reduced when compared to those provoked by Ag+ (reviewed by de Lima et al.[202] and Kim and Ryu[203]). Nonetheless, the pattern of responses can be used to demonstrate particle specific effects that are distinct from those attributed to Ag+. For example, gene expression profiles in zebrafish gills have been shown to differ for Ag NMs and AgNO3, suggesting that the biological interaction of the NMs is unique.[49] The observation of unique responses between Ag NMs and Ag+ was also found by Powers et al.[204] for developmental neurotoxicity endpoints in developing zebrafish, and by Poynton et al.[205] for gene expression profiles in Daphnia magna. In the latter study, the 48-h EC50 values for Ag NMs (citrate coated) was 1.8 µg Ag L–1 as compared to 0.04 µg Ag L–1 for AgNO3.[205] In the study by Pham et al.,[206] expression levels of the genes coding for metallothionien and glutathione were induced by exposures at 1 µg L–1 of Ag NM but not to exposures of 1.6 µg L–1 of AgNO3, indicating that NMs directly increase metal detoxification and antioxidant defence responses. Hepatic expression of stress related genes in medaka also differed between exposures to either Ag NMs or AgNO3 (both at 1 µg Ag L–1) after the first day of exposure but were similar on subsequent days.[207]
A key research question is whether intact Ag NMs induce toxic responses independently or whether they arise from Ag+ from NM dissolution.[112,178] From the data currently available it would appear that both mechanisms can occur. If particles undergo transformation and dissolution to release Ag+ to a sufficient degree then toxicity can be induced. The relatively high inherent toxicity of Ag+ means that only a small degree of dissolution need occur to produce toxicity at intermediate Ag NM concentrations (e.g. tens to hundreds of milligrams of Ag per litre). When Ag NMs are stable (Ag+ release is low) it may be possible for NM induced toxic effects to occur, perhaps at low concentrations in sensitive organisms.[46,200,208] A third scenario is that stable Ag NMs are not bioavailable and in this scenario very high concentrations may be required to induce toxicity. Greatly compounding the interpretation of the studies seeking to distinguish the effects of NMs and free ion is the perhaps counterintuitive observation that particle dissolution increases greatly at lower particle concentrations.[35,41] Such an observation again reinforces the contention that Ag NMs must be characterised under the precise experimental conditions of the exposure.
Ultimately, one of the key questions is whether environmental protection criteria and guideline values, which are measured either as total (e.g. Environment Canada) or total dissolved Ag (e.g. USA Environmental Protection Agency) are sufficiently protective.[186] In this regard, most studies with Ag NMs show effects thresholds above water quality guidelines and criteria, however there are a few that show effects at levels at or below regulatory values. Clearly, understanding these Ag NMs should be a priority. For example, the gastropod snail Physa acuta appears to be particularly sensitive to Ag NMs. In chronic exposures, reproductive impairment (egg production) occurred at and above 0.01 µg Ag L–1.[209] Stress responses and thyroid hormone signalling dynamics in the frog (Rana catesbeiana) were also disrupted by Ag NMs at very low levels (0.6 µg Ag L–1).[210] The significance of these studies and the determination of whether water quality guidelines are sufficiently protective should be of primary consideration.
Data gaps and recommendations
In this section, data gaps and recommendations have not been placed in any particular order of importance and are intended to serve as a guide to improve future experimental design, execution and data reporting. For each of the three NMs above, key recommendations, identified by the NSERC-NRC-BDC consortium are provided below in their order of importance. Each of these points and others discussed below are critical to the advancement of the field of aquatic nanotoxicology and are vital to allow future comparisons among NMs and among experimental systems. This information is crucial for regulators in order to allow the development of quantitative structure–property relationships in predictive toxicology.
Key gaps in TiO2 NM research
-
Thorough characterisation of TiO2 NM agglomeration over the duration of the toxicological study
-
Experiments performed under simulated sunlight as appropriate for the properties
-
Investigations of toxicity over longer-term exposures
-
Investigations of toxicity of NMs to marine organisms, especially phytoplankton
Key gaps in ZnO NM research
-
Experiments with numerous controls (e.g. ionic metal, bulk material and NM sizes)
-
Determinations of the relative roles of Zn2+ and ZnO NMs on biouptake
-
Determination of ZnO solubility in complex toxicological media by carefully distinguishing among Zn2+ and Zn complexes
-
Determination of the role of surface coatings on fate, bioaccumulation and bioavailability
-
Determination of the trophic transfer of ZnO NMs in aquatic ecosystems
-
Investigations of ZnO toxicity under simulated sunlight as appropriate for the properties
-
Investigations of the toxicity of ZnO NMs to marine organisms, especially phytoplankton
-
Discrimination of the bioavailability and fate of natural from anthropogenic ZnO
Key gaps in Ag NM research
-
Experiments with numerous controls (ionic metal, metal complexes)
-
Further comparisons in toxicity among Ag NMs, ionic Ag+ and other Ag complexes, such as Ag nanoclusters; mechanisms of Ag uptake
-
Bioaccumulation and trophic transfer of Ag NMs in aquatic ecosystems
-
Investigations of Ag NM transformations in aqueous systems and discover how Ag NM transformations affect bioavailability
-
Identification of the role of Ag NM size on its transformations
-
Investigations of the toxicity of NMs to marine organisms, especially phytoplankton
Early nanotoxicology studies included minimal NM characterisation limited to their pristine state, within stock solutions. It is now clear that NMs must be characterised as they are found in the aquatic test media, such as cell culture media. Interpretation of data from these early studies is, therefore, challenging and difficult to compare with many (but not all!) modern studies. Whereas there is ongoing debate regarding which NM properties require routine characterisation in nanotoxicological studies, it is recommended that NM size, agglomeration, crystal structure, composition, surface coating and solubility be considered.[211] Furthermore, the degree and type of characterisation required should be NM specific. Researchers should also attempt to characterise the NMs throughout the duration of the toxicity experiments (start, middle and end). The time course NM characterisation will provide researchers with a clear indication of any alterations in the NM properties that may have occurred during the investigation. In addition, there should be a focus on reporting important chemical components such as pH, ionic strength and the presence of nutrients (as found in cell culture media) or proteins that may alter the physiochemical properties of NM. Greater characterisation of both NMs, as well as the aquatic test media used in toxicity studies will allow more direct comparisons between studies and will enhance our understanding of the toxicity of the NMs.
Suitable controls are essential in any NM toxicity study but dependent on the specific type of NM being investigated. The importance of ionic metal controls has previously been discussed (see sections Zinc oxide NM and Silver NM); however, a bulk material (micrometre sized) control should also be considered for metal and metal oxide NM. To date, particle size controls have been limited primarily to studies assessing the aquatic toxicity of TiO2 NMs (see section Titanium dioxide NMs), with few other studies (e.g. Zn and Ag NMs) incorporating such controls. The inclusion of NM size controls will enable nano-specific effects to be more comprehensively investigated and permit more informative comparisons to be made between studies. This information will improve our understanding of NM toxicity to aquatic organisms and consequently enable risk assessors to predict more accurately the types and specific properties of NMs that incite toxicity.
In the presence of UV, some NMs possess the unique ability to undergo quantum confinement (bandgap semiconductors[91–94,212]), and catalytic activity (generation of ROS[92,100,213]), because of their small size and unique properties. These UV-mediated NM transformations have the potential to enhance the toxicity of the NMs to aquatic organisms, therefore, future studies should consider the effects of UV exposure on toxicity levels. Finally, NM toxicity may be enhanced or diminished over time, therefore, chronic toxicity involving bioaccumulation and trophic transfer of NMs should be studied to gain a greater understanding of the effects NMs may have on entire aquatic ecosystems over time.
In summary, the field of nanotoxicology has recently made great gains in our understanding of the toxicological properties for these specific OECD relevant materials. It was a great early challenge to adapt toxicological principles and techniques designed for well dispersed toxicants to the nascent field of colloid (nano) toxicology. Specifically, alteration in experimental design to identify the different behaviour of these materials as colloids, developing an ability and appreciation necessary to understand the chemical and physical properties of these materials in solution and developing and understanding how these materials may interact with organisms were essential for proper and accurate toxicological assessments. This review summarised the fundamental gains in our understanding on how to do and interpret toxicology for a few high volume NMs in commercial applications today and we have outlined some key gaps in knowledge and recommendations for study design that are essential to perform. Moreover, we will continue to be challenged by new materials and nanoformulations entering the marketplace. Training toxicologists with a firm multidisciplinary understanding of the physics, chemistry and biology of these materials is essential for accurate toxicological assessment.
References
[1] A. Baun, N. B. Hartmann, K. Grieger, K. O. Kusk, Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology 2008, 17, 387.| Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmsVKrsbw%3D&md5=b1d730df43c335581f031cec34ef2334CAS | 18425578PubMed |
[2] R. Handy, T. Henry, T. Scown, B. Johnston, C. Tyler, Manufactured nanoparticles: their uptake and effects on fish – a mechanistic analysis. Ecotoxicology 2008, 17, 396.
| Manufactured nanoparticles: their uptake and effects on fish – a mechanistic analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmsVKrsb0%3D&md5=41bcbae7e5e480c259adf68f58c83532CAS | 18408995PubMed |
[3] S. J. Klaine, P. J. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. Y. Lyon, S. Mahendra, M. J. McLaughlin, J. R. Lead, Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825.
| Nanomaterials in the environment: behavior, fate, bioavailability, and effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVersLjJ&md5=66bb5f70ddef5f791cd00b10e5afac24CAS | 19086204PubMed |
[4] M. N. Moore, Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ. Int. 2006, 32, 967.
| Do nanoparticles present ecotoxicological risks for the health of the aquatic environment?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFCqur3P&md5=7e1b6fe9c9a7d6d8c4a0cc5610a5294cCAS | 16859745PubMed |
[5] T. M. Scown, R. van Aerle, C. R. Tyler, Review: Do engineered nanoparticles pose a significant threat to the aquatic environment? Crit. Rev. Toxicol. 2010, 40, 653.
| Review: Do engineered nanoparticles pose a significant threat to the aquatic environment?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptFGrsr4%3D&md5=b8d6ae80b92dcf7d903e2d9d38c21e02CAS | 20662713PubMed |
[6] J. R. Peralta-Videa, L. Zhao, M. L. Lopez-Moreno, G. de la Rosa, J. Hong, J. L. Gardea-Torresdey, Nanomaterials and the environment: a review for the biennium 2008–2010. J. Hazard. Mater. 2011, 186, 1.
| Nanomaterials and the environment: a review for the biennium 2008–2010.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFart7o%3D&md5=f82e97c5e83e6f815923d27a7b14f149CAS | 21134718PubMed |
[7] S. J. Klaine, A. A. Koelmans, N. Horne, S. Carley, R. D. Handy, L. Kapustka, B. Nowack, F. von der Kammer, Paradigms to assess the environmental impact of manufactured nanomaterials. Environ. Toxicol. Chem. 2012, 31, 3.
| Paradigms to assess the environmental impact of manufactured nanomaterials.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1yksbfL&md5=d4cb74111fdb6a76fe2d7775f5fed067CAS | 22162122PubMed |
[8] O. Bondarenko, K. Juganson, A. Ivask, K. Kasemets, M. Mortimer, A. Kahru, Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch. Toxicol. 2013, 87, 1181.
| Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXosFyqsbo%3D&md5=663a78dabe0ea4b19ac13b7de4c420faCAS | 23728526PubMed |
[9] P. C. Ray, H. Yu, P. P. Fu, Toxicity and environmental risks of nanomaterials: challenges and future needs. J. Environ. Sci. Health – C. Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 1.
| Toxicity and environmental risks of nanomaterials: challenges and future needs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhslShtrc%3D&md5=4cf50c00be3da7090d288bed281ff3fcCAS | 19204862PubMed |
[10] M. C. Roco, Nanotechnology: convergence with modern biology and medicine. Curr. Opin. Biotechnol. 2003, 14, 337.
| Nanotechnology: convergence with modern biology and medicine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXltVSqs7c%3D&md5=0ec9abf4196ac0853548d807fec557b8CAS | 12849790PubMed |
[11] S. K. Sahoo, S. Parveen, J. J. Panda, The present and future of nanotechnology in human health care. Nanomedicine 2007, 3, 20.
| The present and future of nanotechnology in human health care.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXktFCmsrk%3D&md5=dc0d8bcb2b0899ffac3d88a2b003c771CAS | 17379166PubMed |
[12] H. Rauscher, G. Roebben, V. Amenta, A.B. Sanfeliu, L. Calzolai, H. Emons, C. Gaillard, N. Gibson, T. Linsinger, A. Mech, L. Q. Pesudo, K. Rasmussen, J. Riego Sintes, B. Sokull-Klüttgen, H. Stamm, Towards a review of the EC Recommendation for a definition of the term ‘nanomaterial’ – Part 1. Compilation of information concerning the experience with the definition. European Commission, Joint Research Centre, Report. EUR 26567 EN (Eds H. Rauscher, G. Roebben) 2014 (Publications Office of the European Union: Luxembourg).
[13] Nanomaterials state of the market Q3 2008: stealth success, broad impact. State of the market report 2008 (Research Lux Inc.: New York). Available at https://portal.luxresearchinc.com/research/document_excerpt/3735 [Verified 13 June 2014].
[14] J. Farkas, P. Christian, J. A. Gallego-Urrea, N. Roos, M. Hassellov, K. E. Tollefsen, K. V. Thomas, Uptake and effects of manufactured silver nanoparticles in rainbow trout (Oncorhynchus mykiss) gill cells. Aquat. Toxicol. 2011, 101, 117.
| Uptake and effects of manufactured silver nanoparticles in rainbow trout (Oncorhynchus mykiss) gill cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFGgsLjP&md5=1795c853bf9767108cf91df80c7363b6CAS | 20952077PubMed |
[15] T. Benn, B. Cavanagh, K. Hristovski, J. D. Posner, P. Westerhoff, The release of nanosilver from consumer products used in the home. J. Environ. Qual. 2010, 39, 1875.
| The release of nanosilver from consumer products used in the home.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVKlu7zO&md5=665579b3e77d38985564355f31a44340CAS | 21284285PubMed |
[16] T. M. Benn, P. Westerhoff, Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133.
| Nanoparticle silver released into water from commercially available sock fabrics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXktlKjsL4%3D&md5=16dae2000740ddd05f5125241df26259CAS | 18589977PubMed |
[17] L. Geranio, M. Heuberger, B. Nowack, The behavior of silver nanotextiles during washing. Environ. Sci. Technol. 2009, 43, 8113.
| The behavior of silver nanotextiles during washing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFOiu7zL&md5=546294f39bba8ea9b99ec8ba01443058CAS | 19924931PubMed |
[18] L. Windler, C. Lorenz, N. von Goetz, K. Hungerbuhler, M. Amberg, M. Heuberger, B. Nowack, Release of titanium dioxide from textiles during washing. Environ. Sci. Technol. 2012, 46, 8181.
| Release of titanium dioxide from textiles during washing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpsVGlurc%3D&md5=bad54cb7ef18652fe2aec97eccea63a3CAS | 22746197PubMed |
[19] C. Botta, J. Labille, M. Auffan, D. Borschneck, H. Miche, M. Cabie, A. Masion, J. Rose, J. Y. Bottero, TiO2-based nanoparticles released in water from commercialized sunscreens in a life-cycle perspective: structures and quantities. Environ. Pollut. 2011, 159, 1543.
| TiO2-based nanoparticles released in water from commercialized sunscreens in a life-cycle perspective: structures and quantities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltFOqtr4%3D&md5=d5ff3a5ff83d15269ef6bb915f563116CAS | 21481996PubMed |
[20] T. Poiger, H. R. Buser, M. E. Balmer, P. A. Bergqvist, M. D. Muller, Occurrence of UV filter compounds from sunscreens in surface waters: regional mass balance in two Swiss lakes. Chemosphere 2004, 55, 951.
| Occurrence of UV filter compounds from sunscreens in surface waters: regional mass balance in two Swiss lakes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXisFOntb4%3D&md5=03fa73aae518c4637f1a169d6d2346aeCAS | 15051365PubMed |
[21] Important issues on risk assessment of manufactured nanomaterials – Series on the Safety of Manufactured Nanomaterials. OECD Environment, Health and Safety Publications. ENV/JM/MONO(2012)8 2012 (Organisation for Economic Co-operation and Development: Paris, France).
[22] P. o. E. Nanotechnologies, Consumer Products Inventory, 2013. Available at http://www.nanotechproject.org/cpi [Verified 25 October 2013].
[23] K. Saha, S. S. Agasti, C. Kim, X. Li, V. M. Rotello, Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739.
| Gold nanoparticles in chemical and biological sensing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1ehtL0%3D&md5=e15c695f83badd25e2629e7e57ac5a49CAS | 22295941PubMed |
[24] Z. Liu, S. Tabakman, K. Welsher, H. Dai, Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Research. 2009, 2, 85.
| Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFSjtr%2FP&md5=e9a8f9f48c7a36dc610568e942e0e0dbCAS | 20174481PubMed |
[25] F. Zhou, D. Xing, B. Wu, S. Wu, Z. Ou, W. R. Chen, New insights of transmembranal mechanism and subcellular localization of noncovalently modified single-walled carbon nanotubes. Nano Lett. 2010, 10, 1677.
| New insights of transmembranal mechanism and subcellular localization of noncovalently modified single-walled carbon nanotubes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXkt1Gku7Y%3D&md5=2d5a6a53936872ccf7724ba69764cf08CAS | 20369892PubMed |
[26] Y.-C. Chen, X.-C. Huang, Y.-L. Luo, Y.-C. Chang, Y.-Z. Hsieh, H.-Y. Hsu, Non-metallic nanomaterials in cancer theranostics: a review of silica- and carbon-based drug delivery systems. Sci. Technol. Adv. Mater. 2013, 14, 044407.
| Non-metallic nanomaterials in cancer theranostics: a review of silica- and carbon-based drug delivery systems.Crossref | GoogleScholarGoogle Scholar |
[27] P. Campbell, Interactions between trace metals and aquatic organisms: a critique of the free-ion activity model, in Metal Speciation and Bioavailability in Aquatic Systems (Eds A Tessier, DR Turner) 1995, pp. 45–102 (Wiley: New York).
[28] B. Jezierska, K. Ługowska, M. Witeska, The effects of heavy metals on embryonic development of fish (a review). Fish Physiol. Biochem. 2009, 35, 625.
| The effects of heavy metals on embryonic development of fish (a review).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFOiur7O&md5=4f99a81c61cb71150a96e0db5b50d639CAS | 19020985PubMed |
[29] S. K. Misra, A. Dybowska, D. Berhanu, S. N. Luoma, E. Valsami-Jones, The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225.
| The complexity of nanoparticle dissolution and its importance in nanotoxicological studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFyiu7rI&md5=87340f427883184849b7a18cf9e12102CAS | 23000548PubMed |
[30] J. Fatisson, I. R. Quevedo, K. J. Wilkinson, N. Tufenkji, Physicochemical characterization of engineered nanoparticles under physiological conditions: effect of culture media components and particle surface coating. Colloids Surf. B Biointerfaces 2012, 91, 198.
| Physicochemical characterization of engineered nanoparticles under physiological conditions: effect of culture media components and particle surface coating.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1yrsbzO&md5=1841ce90344866156f1079f5c0245084CAS | 22119565PubMed |
[31] B. Jezierska, K. Lugowska, M. Witeska, The effects of heavy metals on embryonic development of fish (a review). Fish Physiol. Biochem. 2009, 35, 625.
| The effects of heavy metals on embryonic development of fish (a review).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFOiur7O&md5=4f99a81c61cb71150a96e0db5b50d639CAS | 19020985PubMed |
[32] M. Ç. Witeska, B. Jezierska, J. Chaber, The influence of cadmium on common carp embryos and larvae. Aquaculture 1995, 129, 129.
| The influence of cadmium on common carp embryos and larvae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXksFantLc%3D&md5=cf408129cd0dd2e00cf45a24d9bcfb4cCAS |
[33] G. Klein-Macphee, J. A. Cardin, W. J. Berry, Effects of silver on eggs and larvae of the winter flounder. Trans. Am. Fish. Soc. 1984, 113, 247.
| Effects of silver on eggs and larvae of the winter flounder.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXktVWqu7Y%3D&md5=d32ef0400dca26730f7b263736ff1c76CAS |
[34] C. M. Wood, C. Hogstrand, F. Galvez, R. S. Munger, The physiology of waterborne silver toxicity in freshwater rainbow trout (Oncorhynchus mykiss) 1. The effects of ionic Ag+. Aquat. Toxicol. 1996, 35, 93.
| The physiology of waterborne silver toxicity in freshwater rainbow trout (Oncorhynchus mykiss) 1. The effects of ionic Ag+.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XksF2htL4%3D&md5=727532a493095cd21f8ebe832df6a4a2CAS |
[35] M. Hadioui, S. Leclerc, K. J. Wilkinson, Multimethod quantification of Ag+ release from nanosilver. Talanta 2013, 105, 15.
| Multimethod quantification of Ag+ release from nanosilver.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmt1WjurY%3D&md5=a2cd9d269a709af1c2d216816cd619e6CAS | 23597981PubMed |
[36] S. W. Bian, I. A. Mudunkotuwa, T. Rupasinghe, V. H. Grassian, Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059.
| Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: influence of pH, ionic strength, size, and adsorption of humic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXkvVSls7w%3D&md5=37d89ce7ec956cbd16cc3c2279c783b2CAS | 21500814PubMed |
[37] R. F. Domingos, D. F. Simon, C. Hauser, K. J. Wilkinson, Bioaccumulation and effects of CdTe/CdS quantum dots on Chlamydomonas reinhardtii – nanoparticles or the free ions? Environ. Sci. Technol. 2011, 45, 7664.
| Bioaccumulation and effects of CdTe/CdS quantum dots on Chlamydomonas reinhardtii – nanoparticles or the free ions?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVyhurfI&md5=9deb034004d86b3580b8d9531dbfaa36CAS | 21842898PubMed |
[38] H. Zhang, B. Chen, J. F. Banfield, Particle size and pH effects on nanoparticle dissolution. J. Phys. Chem. C 2010, 114, 14876.
| Particle size and pH effects on nanoparticle dissolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVCksr3P&md5=f8d06b25bb32eee34840f087a5f138cbCAS |
[39] L. Tang, B. Han, K. Persson, C. Friesen, T. He, K. Sieradzki, G. Ceder, Electrochemical stability of nanometer-scale Pt particles in acidic environments. J. Am. Chem. Soc. 2010, 132, 596.
| Electrochemical stability of nanometer-scale Pt particles in acidic environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFOrtrbM&md5=1b4ec5f1b2928ce2e846755401f6e43dCAS | 20017546PubMed |
[40] V. Merdzan, R. F. Domingos, M. Monteiro, M. Hadioui, K. J. Wilkinson, The effects of different coatings on zinc oxide nanoparticles and their influence on dissolution and bioaccumulation by green alga, C. reinhardtii. Sci. Total Environ. 2014, 488–489, 316.
| The effects of different coatings on zinc oxide nanoparticles and their influence on dissolution and bioaccumulation by green alga, C. reinhardtii.Crossref | GoogleScholarGoogle Scholar | 24836387PubMed |
[41] J. Liu, R. H. Hurt, Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169.
| Ion release kinetics and particle persistence in aqueous nano-silver colloids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXit1Wqsrc%3D&md5=eda8eefb62b5e038312ad7a39ce5d935CAS | 20175529PubMed |
[42] M. Li, D. Lin, L. Zhu, Effects of water chemistry on the dissolution of ZnO nanoparticles and their toxicity to Escherichia coli. Environ. Pollut. 2013, 173, 97.
| Effects of water chemistry on the dissolution of ZnO nanoparticles and their toxicity to Escherichia coli.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVektrrI&md5=15b7f7bdc149ffb4c2f102fd7bd40442CAS | 23202638PubMed |
[43] J. Liu, D. A. Sonshine, S. Shervani, R. H. Hurt, Controlled release of biologically active silver from nanosilver surfaces. ACS Nano 2010, 4, 6903.
| Controlled release of biologically active silver from nanosilver surfaces.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlaitb3L&md5=f74a2c9fb88e9b8a96314791af98dfb3CAS | 20968290PubMed |
[44] A. M. Studer, L. K. Limbach, L. Van Duc, F. Krumeich, E. K. Athanassiou, L. C. Gerber, H. Moch, W. J. Stark, Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett. 2010, 197, 169.
| Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptVagu7o%3D&md5=a8491c019fc5252427d8f811ab64d75dCAS | 20621582PubMed |
[45] A. Shiohara, S. Prabakar, A. Faramus, C.-Y. Hsu, P.-S. Lai, P. T. Northcote, R. D. Tilley, Sized controlled synthesis, purification, and cell studies with silicon quantum dots. Nanoscale. 2011, 3, 3364.
[46] A. G. Schultz, K. J. Ong, T. MacCormack, G. Ma, J. G. C. Veinot, G. G. Goss, Silver nanoparticles inhibit sodium uptake in juvenile rainbow trout (Oncorhynchus mykiss). Environ. Sci. Technol. 2012, 46, 10295.
| 1:CAS:528:DC%2BC38XhtF2ku77P&md5=b87e5f3c65e198a7062a02838efcb6e4CAS | 22891970PubMed |
[47] K. J. Ong, X. Zhao, M. E. Thistle, T. J. Maccormack, R. J. Clark, G. Ma, Y. Martinez-Rubi, B. Simard, J. S. Loo, J. G. Veinot, G. G. Goss, Mechanistic insights into the effect of nanoparticles on zebrafish hatch. Nanotoxicology 2014, 8, 295.
| Mechanistic insights into the effect of nanoparticles on zebrafish hatch.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFemur3L&md5=06a7978833540837ae933d7d04ce8bedCAS | 23421642PubMed |
[48] J. N. Meyer, C. A. Lord, X. Y. Yang, E. A. Turner, A. R. Badireddy, S. M. Marinakos, A. Chilkoti, M. R. Wiesner, M. Auffan, Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans. Aquat. Toxicol. 2010, 100, 140.
| Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFGjs7bI&md5=40459a41abbeb56b27471610585121bdCAS | 20708279PubMed |
[49] R. J. Griffitt, K. Hyndman, N. D. Denslow, D. S. Barber, Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles. Toxicol. Sci. 2009, 107, 404.[Published online early 10 December 2008].
| Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVehtrY%3D&md5=1f5b6fce37074ccf84178dcaed08cce3CAS | 19073994PubMed |
[50] J. E. Ward, D. J. Kach, Marine aggregates facilitate ingestion of nanoparticles by suspension-feeding bivalves. Mar. Environ. Res. 2009, 68, 137.
| Marine aggregates facilitate ingestion of nanoparticles by suspension-feeding bivalves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotl2ksL8%3D&md5=98c25a841cc93e087356fc06b3d9f7bbCAS | 19525006PubMed |
[51] B. Derjaguin, L. Landau, Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 1993, 43, 30.
| Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes.Crossref | GoogleScholarGoogle Scholar |
[52] E. J. W. Verwey, J. Th. G. Overbeek, Long distance forces acting between colloidal particles. Trans. Faraday Soc. 1946, 42, B117.
| Long distance forces acting between colloidal particles.Crossref | GoogleScholarGoogle Scholar |
[53] J. Buffle, K. J. Wilkinson, S. Stoll, M. Filella, J. Zhang, A generalized description of aquatic colloidal interactions: the three-colloidal component approach. Environ. Sci. Technol. 1998, 32, 2887.
| A generalized description of aquatic colloidal interactions: the three-colloidal component approach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlsVyqt7w%3D&md5=35469604d23585c4800c0dd1924ca4fbCAS |
[54] A. M. E. Badawy, T. P. Luxton, R. G. Silva, K. G. Scheckel, M. T. Suidan, T. M. Tolaymat, Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260.
| Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions.Crossref | GoogleScholarGoogle Scholar |
[55] J. Jiang, G. Oberdörster, P. Biswas, Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77.
| Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlSksA%3D%3D&md5=1d7b8bd47a1175239c0853647affa86bCAS |
[56] R. F. Domingos, N. Tufenkji, K. J. Wilkinson, Aggregation of titanium dioxide nanoparticles: role of a fulvic acid. Environ. Sci. Technol. 2009, 43, 1282.
| Aggregation of titanium dioxide nanoparticles: role of a fulvic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotlKktA%3D%3D&md5=bb858a0eeb0445fe92775dcda539c1f6CAS | 19350891PubMed |
[57] K. L. Chen, M. Elimelech, Influence of humic acid on the aggregation kinetics of fullerene (C60) nanoparticles in monovalent and divalent electrolyte solutions. J. Colloid Interface Sci. 2007, 309, 126.
| Influence of humic acid on the aggregation kinetics of fullerene (C60) nanoparticles in monovalent and divalent electrolyte solutions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjtl2jt78%3D&md5=1e0c214b2bc39b8d345de94e91379958CAS | 17331529PubMed |
[58] Y. Zhang, Y. Chen, P. Westerhoff, J. Crittenden, Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res. 2009, 43, 4249.
| Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFWks73O&md5=65df838c58ebce11743c0085be26fa1cCAS | 19577783PubMed |
[59] T. Cedervall, I. Lynch, S. lindman, T. Berggård, E. Thulin, H. Nilsson, K. Dawson, S. Linse, Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050.
| S. lindman, T. Berggård, E. Thulin, H. Nilsson, K. Dawson, S. Linse, Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXisVWrsro%3D&md5=c875dac5258f37232c25b0c73d6df947CAS | 17267609PubMed |
[60] I. Lynch, A. Salvati, K. A. Dawson, Protein-nanoparticle interactions: what does the cell see? Nat. Nanotechnol. 2009, 4, 546.
| Protein-nanoparticle interactions: what does the cell see?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtV2ltbrP&md5=1e903930fa54405ee42996cb6567bb00CAS | 19734922PubMed |
[61] M. Lundqvist, J. Stigler, G. Elia, I. Lynch, T. Cedervall, K. A. Dawson, Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265.
| Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1SgtrvJ&md5=34a46219acd1941ab50c194a6a721abeCAS | 18809927PubMed |
[62] E. Casals, T. Pfaller, A. Duschl, G. J. Oostingh, V. Puntes, Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623.
| Time evolution of the nanoparticle protein corona.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnsFGhtbc%3D&md5=96c1714ded8ee4c756325b0ced841f9eCAS | 20553005PubMed |
[63] S. Deguchi, T. Yamazaki, S.-a. Mukai, R. Usami, K. Horikoshi, Stabilization of C60 nanoparticles by protein adsorption and its implications for toxicity studies. Chem. Res. Toxicol. 2007, 20, 854.
| Stabilization of C60 nanoparticles by protein adsorption and its implications for toxicity studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlt1Ogtbg%3D&md5=3e72fb337c825e70c9adb9b3fc04a31aCAS | 17503852PubMed |
[64] B. Nowack, J. F. Ranville, S. Diamond, J. A. Gallego-Urrea, C. Metcalfe, J. Rose, N. Horne, A. A. Koelmans, S. J. Klaine, Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ. Toxicol. Chem. 2012, 31, 50. [Published online early 9 December 2011].
[65] M. Baalousha, A. Manciulea, S. Cumberland, K. Kendall, J. R. Lead, Aggregation and surface properties of iron oxide nanoparticles: influence of pH and natural organic matter. Environ. Toxicol. Chem. 2008, 27, 1875.
| Aggregation and surface properties of iron oxide nanoparticles: influence of pH and natural organic matter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVersLjF&md5=7498511e79e4766c4437605b0d56535eCAS | 19086206PubMed |
[66] B. D. Chithrani, A. A. Ghazani, W. C. W. Chan, Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662.
| Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhvVCjsLk%3D&md5=cc0479ae85e5b160362e877e6c545d91CAS | 16608261PubMed |
[67] L. K. Limbach, Y. Li, R. N. Grass, T. J. Brunner, M. A. Hintermann, M. Muller, D. Gunther, W. J. Stark, Oxide nanoparticle uptake in human lung fibroblasts: effects of particle size, agglomeration, and diffusion at low concentrations. Environ. Sci. Technol. 2005, 39, 9370.
| Oxide nanoparticle uptake in human lung fibroblasts: effects of particle size, agglomeration, and diffusion at low concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtV2jsLjM&md5=cad96dfd3988931910df09db7067b25fCAS | 16382966PubMed |
[68] D. M. Rawson, T. Zhang, D. Kolicharan, W. L. Jonbloed, Field emission scanning electron microscopy and transmission electron microscopy studies of the chorion, plasma membrane and syncytial layers of the gastrula-stage embryo of the zebrafish Brachydanio rerio: a consideration of the structural and functional relationships with respect to cryoprotectant penetration. Aquacult. Res. 2000, 31, 325.
| Field emission scanning electron microscopy and transmission electron microscopy studies of the chorion, plasma membrane and syncytial layers of the gastrula-stage embryo of the zebrafish Brachydanio rerio: a consideration of the structural and functional relationships with respect to cryoprotectant penetration.Crossref | GoogleScholarGoogle Scholar |
[69] D. Kühnel, W. Busch, T. Meißner, A. Springer, A. Potthoff, V. Richter, M. Gelinsky, S. Scholz, K. Schirmer, Agglomeration of tungsten carbide nanoparticles in exposure medium does not prevent uptake and toxicity toward a rainbow trout gill cell line. Aquat. Toxicol. 2009, 93, 91.
| Agglomeration of tungsten carbide nanoparticles in exposure medium does not prevent uptake and toxicity toward a rainbow trout gill cell line.Crossref | GoogleScholarGoogle Scholar | 19439373PubMed |
[70] S. Laurent, C. Burtea, C. Thirifays, U. O. Häfeli, M. Mahmoudi, Crucial ignored parameters on nanotoxicology: the importance of toxicity assay modifications and ‘cell vision’. PLoS ONE 2012, 7, e29997.
| Crucial ignored parameters on nanotoxicology: the importance of toxicity assay modifications and ‘cell vision’.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht12mu74%3D&md5=ebd6af170ca4315b9fd0c211bc6c9232CAS | 22253854PubMed |
[71] W. Bai, Z. Zhang, W. Tian, X. He, Y. Ma, Y. Zhao, Z. Chai, Toxicity of zinc oxide nanoparticles to zebrafish embryo: a physicochemical study of toxicity mechanism. J. Nanopart. Res. 2010, 12, 1645. [Published online early 29 August 2009].
[72] X. Zhu, J. Wang, X. Zhang, Y. Chang, Y. Chen, The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio). Nanotechnology 2009, 20, 195103.
| The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio).Crossref | GoogleScholarGoogle Scholar | 19420631PubMed |
[73] D. Brunner, J. Frank, H. Appl, H. Schöffl, W. Pfaller, Serum-free cell culture: the serum-free media interactive online database. ALTEX 2010, 27, 53.
| 20390239PubMed |
[74] A. Casey, E. Herzog, F. M. Lyng, H. J. Byrne, G. Chambers, M. Davoren, Single walled carbon nanotubes induce indirect cytotoxicity by medium depletion in A549 lung cells. Toxicol. Lett. 2008, 179, 78.
| Single walled carbon nanotubes induce indirect cytotoxicity by medium depletion in A549 lung cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmsFKrtr4%3D&md5=d73efd1d6064f0a46665bba8c2f8b4d8CAS | 18502058PubMed |
[75] M. Horie, K. Nishio, K. Fujita, S. Endoh, A. Miyauchi, Y. Saito, H. Iwahashi, K. Yamamoto, H. Murayama, H. Nakano, N. Nanashima, E. Niki, Y. Yoshida, Protein adsorption of ultrafine metal oxide and its influence on cytotoxicity toward cultured cells. Chem. Res. Toxicol. 2009, 22, 543.
| Protein adsorption of ultrafine metal oxide and its influence on cytotoxicity toward cultured cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhvFGms7w%3D&md5=2edb3372dad626b9d4ea35566ae345d1CAS | 19216582PubMed |
[76] L. Guo, A. Von Dem Bussche, M. Buechner, A. Yan, A. B. Kane, R. H. Hurt, Adsorption of essential micronutrients by carbon nanotubes and the implications for nanotoxicity testing. Small 2008, 4, 721.
| Adsorption of essential micronutrients by carbon nanotubes and the implications for nanotoxicity testing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXosFynt7k%3D&md5=ed88389761e9751648a755588b071affCAS | 18504717PubMed |
[77] X. Zhao, B. C. Heng, S. Xiong, J. Guo, T. T.-Y. Tan, F. Y. C. Boey, K. W. Ng, J. S. C. Loo, In vitro assessment of cellular responses to rod-shaped hydroxyapatite nanoparticles of varying lengths and surface areas. Nanotoxicology 2011, 5, 182.
| In vitro assessment of cellular responses to rod-shaped hydroxyapatite nanoparticles of varying lengths and surface areas.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFCqur3K&md5=f866e507a6615fb42cb5c1684228b8e7CAS | 21609137PubMed |
[78] B. D. Johnston, T. M. Scown, J. Moger, S. A. Cumberland, M. Baalousha, K. Linge, R. van Aerle, K. Jarvis, J. R. Lead, C. R. Tyler, Bioavailability of nanoscale metal oxides TiO2, CeO2, and ZnO to fish. Environ. Sci. Technol. 2010, 44, 1144.
| Bioavailability of nanoscale metal oxides TiO2, CeO2, and ZnO to fish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht12lsA%3D%3D&md5=fec7191171ecb259ad7b0b2321864a70CAS | 20050652PubMed |
[79] A. E. Ellis, Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 2001, 25, 827.
| Innate host defense mechanisms of fish against viruses and bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmsFWkt7g%3D&md5=aaa3465b0c1538bf549a8c5b45ec75d3CAS | 11602198PubMed |
[80] A. Casey, E. Herzog, M. Davoren, F. M. Lyng, H. J. Byrne, G. Chambers, Spectroscopic analysis confirms the interactions between single walled carbon nanotubes and various dyes commonly used to assess cytotoxicity. Carbon 2007, 45, 1425.
| Spectroscopic analysis confirms the interactions between single walled carbon nanotubes and various dyes commonly used to assess cytotoxicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmtVGktrY%3D&md5=6134fbd56a2ffb4096073b524196d8f5CAS |
[81] N. A. Monteiro-Riviere, A. O. Inman, L. W. Zhang, Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol. Appl. Pharmacol. 2009, 234, 222.
| Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksVentg%3D%3D&md5=0d41a187daf720ac4f7c15480b87aa35CAS | 18983864PubMed |
[82] K. J. Ong, T. J. MacCormack, R. J. Clark, J. D. Ede, V. A. Ortega, L. C. Felix, M. K. Dang, G. Ma, H. Fenniri, J. G. Veinot, G. G. Goss, Widespread nanoparticle-assay interference: implications for nanotoxicity testing. PLoS ONE 2014, 9, e90650.
| Widespread nanoparticle-assay interference: implications for nanotoxicity testing.Crossref | GoogleScholarGoogle Scholar | 24618833PubMed |
[83] R. Guadagnini, B. Halamoda Kenzaoui, L. Cartwright, G. Pojana, Z. Magdolenova, D. Bilanicova, M. Saunders, L. Juillerat, A. Marcomini, A. Huk, M. Dusinska, L. M. Fjellsbø, F. Marano, S. Boland, Toxicity screenings of nanomaterials: challenges due to interference with assay processes and components of classic in vitro tests. Nanotoxicology 2013, in press. [Published online early 27 July 2013].
| Toxicity screenings of nanomaterials: challenges due to interference with assay processes and components of classic in vitro tests.Crossref | GoogleScholarGoogle Scholar | 24286383PubMed |
[84] G. Wang, J. Zhang, A. H. Dewilde, A. K. Pal, D. Bello, J. M. Therrien, S. J. Braunhut, K. A. Marx, Understanding and correcting for carbon nanotube interferences with a commercial LDH cytotoxicity assay. Toxicology 2012, 299, 99.
| Understanding and correcting for carbon nanotube interferences with a commercial LDH cytotoxicity assay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XosVaks7g%3D&md5=e19e1c887285fdccd759550ad432ea47CAS | 22634321PubMed |
[85] S. Bancos, D. H. Tsai, V. Hackley, J. L. Weaver, K. M. Tyner, Evaluation of viability and proliferation profiles on macrophages treated with silica nanoparticles in vitro via plate-based, flow cytometry, and coulter counter assays. ISRN Nanotechnology. 2012, 2012, 1.
| Evaluation of viability and proliferation profiles on macrophages treated with silica nanoparticles in vitro via plate-based, flow cytometry, and coulter counter assays.Crossref | GoogleScholarGoogle Scholar |
[86] X. Han, R. Gelein, N. Corson, P. Wade-Mercer, J. Jiang, P. Biswas, J. N. Finkelstein, A. Elder, G. Oberdörster, Validation of an LDH assay for assessing nanoparticle toxicity. Toxicology 2011, 287, 99.
| Validation of an LDH assay for assessing nanoparticle toxicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsFGns78%3D&md5=c9081455e9f01a9564e3c9fb04ef7a3fCAS | 21722700PubMed |
[87] C. Darolles, N. Sage, J. Armengaud, V. Malard, In vitro assessment of cobalt oxide particle toxicity: identifying and circumventing interference. Toxicol. In Vitro 2013, 27, 1699.
| In vitro assessment of cobalt oxide particle toxicity: identifying and circumventing interference.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht12gtb3E&md5=3c5612f009cc729f7bbad51a4b8495faCAS | 23624240PubMed |
[88] S. Wang, H. Yu, J. K. Wickliffe, Limitation of the MTT and XTT assays for measuring cell viability due to superoxide formation induced by nano-scale TiO2. Toxicol. In Vitro 2011, 25, 2147.
| Limitation of the MTT and XTT assays for measuring cell viability due to superoxide formation induced by nano-scale TiO2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVKmt7vM&md5=800b84700151229a49e4eacb46c22713CAS | 21798338PubMed |
[89] J. M. Wörle-Knirsch, K. Pulskamp, H. F. Krug, Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett. 2006, 6, 1261.
| Oops they did it again! Carbon nanotubes hoax scientists in viability assays.Crossref | GoogleScholarGoogle Scholar | 16771591PubMed |
[90] G. Ciofani, S. Danti, D. D’Alessandro, S. Moscato, A. Menciassi, Assessing cytotoxicity of boron nitride nanotubes: interference with the MTT assay. Biochem. Biophys. Res. Commun. 2010, 394, 405.
| Assessing cytotoxicity of boron nitride nanotubes: interference with the MTT assay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXksVentrk%3D&md5=df21ff5da546ce8216fdbaada2ee5530CAS | 20226164PubMed |
[91] T. Laaksonen, H. Santos, H. Vihola, J. Salonen, J. Riikonen, T. Heikkilä, L. Peltonen, N. Kumar, D. Y. Murzin, V.-P. Lehto, J. Hirvonen, Failure of MTT as a toxicity testing agent for mesoporous silicon microparticles. Chem. Res. Toxicol. 2007, 20, 1913.
| Failure of MTT as a toxicity testing agent for mesoporous silicon microparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1OqtLjJ&md5=f21cbc8919ecaf1aa222fec6e308b7d1CAS | 17990852PubMed |
[92] L. Belyanskaya, P. Manser, P. Spohn, A. Bruinink, P. Wick, The reliability and limits of the MTT reduction assay for carbon nanotubes–cell interaction. Carbon 2007, 45, 2643.
| The reliability and limits of the MTT reduction assay for carbon nanotubes–cell interaction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1ShtL7K&md5=d3398351c30b16b2852de1084c61f319CAS |
[93] V. Amendola, M. Meneghetti, Size evaluation of gold nanoparticles by UV-Vis spectroscopy. J. Phys. Chem. C 2009, 113, 4277.
| Size evaluation of gold nanoparticles by UV-Vis spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXit1Klsbg%3D&md5=d026c326c4044113cc013446bb3f8386CAS |
[94] W. Haiss, N. T. K. Thanh, J. Aveyard, D. G. Fernig, Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal. Chem. 2007, 79, 4215.
| Determination of size and concentration of gold nanoparticles from UV-Vis spectra.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXksFCit7s%3D&md5=36025be26592be256fdbc16dc5e36db0CAS | 17458937PubMed |
[95] P. K. Jain, X. Huang, I. H. El Sayed, M. A. El-Sayed, Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics 2007, 2, 107.
| Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1yitr3L&md5=d03ba8c3c8683d95d10a5f1afd8e3232CAS |
[96] S. Mazumder, R. Dey, M. K. Mitra, S. Mukherjee, G. C. Das, Review: biofunctionalized quantum dots in biology and medicine. J. Nanomater. 2009, 2009, 1.
| Review: biofunctionalized quantum dots in biology and medicine.Crossref | GoogleScholarGoogle Scholar |
[97] V. Wilhelmi, U. Fischer, D. van Berlo, K. Schulze-Osthoff, R. P. F. Schins, C. Albrecht, Evaluation of apoptosis induced by nanoparticles and fine particles in RAW 264.7 macrophages: facts and artefacts. Toxicol. In Vitro 2012, 26, 323.
| Evaluation of apoptosis induced by nanoparticles and fine particles in RAW 264.7 macrophages: facts and artefacts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVOlsL8%3D&md5=a7e6b65d930203bfeae7af3eda0719e9CAS | 22198050PubMed |
[98] M. A. Dobrovolskaia, J. D. Clogston, B. W. Neun, J. B. Hall, A. K. Patri, S. E. McNeil, Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett. 2008, 8, 2180.
| Method for analysis of nanoparticle hemolytic properties in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXot1aiu7s%3D&md5=2786ecb8d771d780af833484b3e68f25CAS | 18605701PubMed |
[99] A. Kroll, M. H. Pillukat, D. Hahn, J. Schnekenburger, Interference of engineered nanoparticles with in vitro toxicity assays. Arch. Toxicol. 2012, 86, 1123.
| Interference of engineered nanoparticles with in vitro toxicity assays.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsFGhsbo%3D&md5=cb41010cd0c40f97437596497c29a7f8CAS | 22407301PubMed |
[100] S. H. Doak, S. M. Griffiths, B. Manshian, N. Singh, P. M. Williams, A. P. Brown, G. J. S. Jenkins, Confounding experimental considerations in nanogenotoxicology. Mutagenesis 2009, 24, 285.
| Confounding experimental considerations in nanogenotoxicology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnvVOnu7o%3D&md5=eab8fd6445432c4aa71a50d81f2b591dCAS | 19351890PubMed |
[101] A. Panas, C. Marquardt, O. Nalcaci, H. Bockhorn, W. Baumann, H. R. Paur, S. Mülhopt, S. Diabaté, C. Weiss, Screening of different metal oxide nanoparticles reveals selective toxicity and inflammatory potential of silica nanoparticles in lung epithelial cells and macrophages. Nanotoxicology 2013, 7, 259.
| Screening of different metal oxide nanoparticles reveals selective toxicity and inflammatory potential of silica nanoparticles in lung epithelial cells and macrophages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlsF2nsrY%3D&md5=ba87fa8fdcfd9f2e5a7a403632770c98CAS | 22276741PubMed |
[102] C. A. Sabatini, R. V. Pereira, M. H. Gehlen, Fluorescence modulation of acridine and coumarin dyes by silver nanoparticles. J. Fluoresc. 2007, 17, 377.
| Fluorescence modulation of acridine and coumarin dyes by silver nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnt1Kjtr0%3D&md5=97737c08428de6ebadfe408e99fb88faCAS | 17549612PubMed |
[103] J. Tournebize, A. Sapin-Minet, G. Bartosz, P. Leroy, A. Boudier, Pitfalls of assays devoted to evaluation of oxidative stress induced by inorganic nanoparticles. Talanta 2013, 116, 753.
| Pitfalls of assays devoted to evaluation of oxidative stress induced by inorganic nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1KrurbM&md5=01839119092c4540e8fe9a765f8d2a24CAS | 24148470PubMed |
[104] A. M. Keene, R. J. Allaway, N. Sadrieh, K. M. Tyner, Gold nanoparticle trafficking of typically excluded compounds across the cell membrane in JB6 Cl 41–5a cells causes assay interference. Nanotoxicology 2011, 5, 469.
| Gold nanoparticle trafficking of typically excluded compounds across the cell membrane in JB6 Cl 41–5a cells causes assay interference.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1egurw%3D&md5=4a2179bfebc2b1feb32facba6057b5b5CAS | 21090919PubMed |
[105] S. Shukla, A. Priscilla, M. Banerjee, R. R. Bhonde, J. Ghatak, P. V. Satyam, M. Sastry, Porous gold nanospheres by controlled transmetalation reaction: a novel material for application in cell imaging. Chem. Mater. 2005, 17, 5000.
| Porous gold nanospheres by controlled transmetalation reaction: a novel material for application in cell imaging.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXps1eqsr8%3D&md5=1c0d5ae05d1de881911d7f4bf1cae91aCAS |
[106] I. Lynch, K. A. Dawson, Protein–nanoparticle interactions. Nano Today 2008, 3, 40.
| Protein–nanoparticle interactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFemsb3P&md5=8ce8d64beed66e5288d1112317716149CAS |
[107] M. P. Monopoli, D. Walczyk, A. Campbell, G. Elia, I. Lynch, F. Baldelli Bombelli, K. A. Dawson, Physical–chemical aspects of protein corona: relevance to in vitroand in vivobiological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525.
| Physical–chemical aspects of protein corona: relevance to in vitroand in vivobiological impacts of nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVOnt7w%3D&md5=84d9baed4322c44b74ebe097e665d3afCAS | 21288025PubMed |
[108] T. J. MacCormack, R. J. Clark, M. K. M. Dang, G. Ma, J. A. Kelly, J. G. C. Veinot, G. G. Goss, Inhibition of enzyme activity by nanomaterials: potential mechanisms and implications for nanotoxicity testing. Nanotoxicology 2012, 6, 514.
| Inhibition of enzyme activity by nanomaterials: potential mechanisms and implications for nanotoxicity testing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVagu7zJ&md5=b6bd5194f139c754568fa0c87bcdaf20CAS | 21639725PubMed |
[109] K. B. Male, E. Lam, J. Montes, J. H. T. Luong, Noninvasive cell-based impedance spectroscopy for real-time probing inhibitory effects of graphene derivatives. Applied Materials and Interfaces. 2012, 4, 3643.
| Noninvasive cell-based impedance spectroscopy for real-time probing inhibitory effects of graphene derivatives.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xps1GisLw%3D&md5=5a94dd58db5315ece07a7eeb58e0981dCAS | 22746697PubMed |
[110] E. Herzog, A. Casey, F. Lyng, G. Chambers, H. Byrne, M. Davoren, A new approach to the toxicity testing of carbon-based nanomaterials – the clonogenic assay. Toxicol. Lett. 2007, 174, 49.
| A new approach to the toxicity testing of carbon-based nanomaterials – the clonogenic assay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1anurbJ&md5=35e30a5deda373199d9c5271b6d262ecCAS | 17920791PubMed |
[111] J. Fabrega, S. N. Luoma, C. R. Tyler, T. S. Galloway, J. R. Lead, Silver nanoparticles: behaviour and effects in the aquatic environment. Environ. Int. 2011, 37, 517.
| Silver nanoparticles: behaviour and effects in the aquatic environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFCjtr8%3D&md5=cc52ad7aa25a0f0a1db386f09879334dCAS | 21159383PubMed |
[112] B. J. Shaw, R. D. Handy, Physiological effects of nanoparticles on fish: a comparison of nanometals versus metal ions. Environ. Int. 2011, 37, 1083.
| Physiological effects of nanoparticles on fish: a comparison of nanometals versus metal ions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsVWnurg%3D&md5=697f42e64a24a5f553af154192176b10CAS | 21474182PubMed |
[113] S. Ma, D. Lin, The biophysicochemical interactions at the interfaces between nanoparticles and aquatic organisms: adsorption and internalization. J. Environ. Monitor. 2013, 15, 145.
| 1:CAS:528:DC%2BC38XhvFWgt7vN&md5=b37f110710511832cab750b3659f4c43CAS |
[114] R. Behra, L. Sigg, M. J. Clift, F. Herzog, M. Minghetti, B. Johnston, A. Petri-Fink, B. Rothen-Rutishauser, Bioavailability of silver nanoparticles and ions: from a chemical and biochemical perspective. J. R. Soc. Interface 2013, 10, 20130396.
| Bioavailability of silver nanoparticles and ions: from a chemical and biochemical perspective.Crossref | GoogleScholarGoogle Scholar | 23883950PubMed |
[115] State of the science literature review: nano titanium dioxide environmental matters. scientific, technical, research, engineering and modeling support (STREAMS) final report, EPA-600-R-10-089 2010 (United States Environmental Protection Agency: Washington, DC).
[116] Nanomaterial case studies: nanoscale titanium dioxide in water treatment and in topical sunscreen. EPA-600-R-09-057 2010 (United States Environmental Protection Agency: Washington, DC).
[117] Nanomaterial case studies workshop: developing a comprehensive environmental assessment research strategy for nanoscale titanium dioxide. EPA-600-R‐10-042 2010 (United States Environmental Protection Agency: Washington, DC).
[118] C. O. Robichaud, A. E. Uyar, M. R. Darby, L. G. Zucker, M. R. Wiesner, Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ. Sci. Technol. 2009, 43, 4227.
| Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlvFOnu7s%3D&md5=1821f69605de3f4390228613ef5549eeCAS | 19603627PubMed |
[119] F. Gottschalk, T. Sun, B. Nowack, Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287.
| Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFSlsrfP&md5=7be267556cba6b63f2cffd78eb64df51CAS | 23856352PubMed |
[120] F. Gottschalk, T. Sonderer, R. W. Scholz, B. Nowack, Possibilities and limitations of modeling environmental exposure to engineered nanomaterials by probabilistic material flow analysis. Environ. Toxicol. Chem. 2010, 29, 1036.
| 1:CAS:528:DC%2BC3cXpslyktb0%3D&md5=03eaa77bd1800357bcdb16cc222d4c55CAS | 20821538PubMed |
[121] F. Gottschalk, T. Sonderer, R. W. Scholz, B. Nowack, Modeled environmental concentrations of engineered nanomaterials (TiO(2), ZnO, Ag, CNT, fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216.
| Modeled environmental concentrations of engineered nanomaterials (TiO(2), ZnO, Ag, CNT, fullerenes) for different regions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlyhtL%2FP&md5=a8a5454d70978de5af6ee6d145a26961CAS | 20000512PubMed |
[122] M. Farré, K. Gajda-Schrantz, L. Kantiani, D. Barcelo, Ecotoxicity and analysis of nanomaterials in the aquatic environment. Anal. Bioanal. Chem. 2009, 393, 81.
| Ecotoxicity and analysis of nanomaterials in the aquatic environment.Crossref | GoogleScholarGoogle Scholar | 18987850PubMed |
[123] K. Fent, Ecotoxicology of engineered nanoparticles, in Nanoparticles in the Water Cycle (Eds F. H. Frimmel, R. Niessner) 2010, pp. 183–205 (Springer: Heidelberg).
[124] A. Menard, D. Drobne, A. Jemec, Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ. Pollut. 2011, 159, 677.
| Ecotoxicity of nanosized TiO2. Review of in vivo data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsFWksA%3D%3D&md5=e0a07dad982fba9223bd32778e65018cCAS | 21186069PubMed |
[125] E. Navarro, A. Baun, R. Behra, N. Hartmann, J. Filser, A.-J. Miao, A. Quigg, P. Santschi, L. Sigg, Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372.
| Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmsVKrsLc%3D&md5=a1cc58f914bb667a567bab9f9a54fbd5CAS | 18461442PubMed |
[126] M. Heinlaan, A. Ivask, I. Blinova, H. C. Dubourguier, A. Kahru, Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308.
| Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXktFCmtro%3D&md5=50d90e637fdb1ab19a28fcdcd2488e74CAS | 18194809PubMed |
[127] N. B. Hartmann, F. Von der Kammer, T. Hofmann, M. Baalousha, S. Ottofuelling, A. Baun, Algal testing of titanium dioxide nanoparticles – testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology 2010, 269, 190.
| Algal testing of titanium dioxide nanoparticles – testing considerations, inhibitory effects and modification of cadmium bioavailability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjslSitbc%3D&md5=4ec6ef866a731c308edda51a0183d6d8CAS | 19686796PubMed |
[128] W. M. Lee, Y. J. An, Effects of zinc oxide and titanium dioxide nanoparticles on green algae under visible, UVA, and UVB irradiations: no evidence of enhanced algal toxicity under UV pre-irradiation. Chemosphere 2013, 91, 536.
| Effects of zinc oxide and titanium dioxide nanoparticles on green algae under visible, UVA, and UVB irradiations: no evidence of enhanced algal toxicity under UV pre-irradiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFCktr8%3D&md5=a3a866f97836a9fef84a3a8f7a63b58eCAS | 23357865PubMed |
[129] J. Wang, X. Zhang, Y. Chen, M. Sommerfeld, Q. Hu, Toxicity assessment of manufactured nanomaterials using the unicellular green alga Chlamydomonas reinhardtii. Chemosphere 2008, 73, 1121.
| Toxicity assessment of manufactured nanomaterials using the unicellular green alga Chlamydomonas reinhardtii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1SntL3F&md5=5bd9fb48ba76da1a32221e8bf6e058c9CAS | 18768203PubMed |
[130] D. M. Metzler, M. Li, A. Erdem, C. P. Huang, Responses of algae to photocatalytic nano-TiO2 particles with an emphasis on the effect of particle size. Chem. Eng. J. 2011, 170, 538.
| Responses of algae to photocatalytic nano-TiO2 particles with an emphasis on the effect of particle size.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmslahtr0%3D&md5=428c977305430f0c73d95360b628cdeaCAS |
[131] R. D. Handy, N. van den Brink, M. Chappell, M. Muhling, R. Behra, M. Dusinska, P. Simpson, J. Ahtiainen, A. N. Jha, J. Seiter, A. Bednar, A. Kennedy, T. F. Fernandes, M. Riediker, Practical considerations for conducting ecotoxicity test methods with manufactured nanomaterials: what have we learnt so far? Ecotoxicology 2012, 21, 933.
| Practical considerations for conducting ecotoxicity test methods with manufactured nanomaterials: what have we learnt so far?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlsFCjur4%3D&md5=7094b1115904429d04628a9edb95e3b2CAS | 22422174PubMed |
[132] A. Kahru, H.-C. Dubourguier, I. Blinova, A. Ivask, K. Kasemets, Biotests and biosensors for ecotoxicology of metal oxide nanoparticles. Sensors 2008, 8, 5153.
| Biotests and biosensors for ecotoxicology of metal oxide nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVekt7zI&md5=1dbab9c119fea2dce31654b0eb5ea034CAS |
[133] V. Aruoja, H. C. Dubourguier, K. Kasemets, A. Kahru, Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461.
| Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVOmsrvP&md5=d2d6eadc811ff07b228e1fc0e9a2e1ebCAS | 19038417PubMed |
[134] R. F. Domingos, C. Peyrot, K. J. Wilkinson, Aggregation of titanium dioxide nanoparticles: role of calcium and phosphate. Environ. Chem. 2010, 7, 61.
| Aggregation of titanium dioxide nanoparticles: role of calcium and phosphate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjt12jtLg%3D&md5=04eb7f231336203ef7d3953f2fb5e6e8CAS |
[135] D. Lin, J. Ji, Z. Long, K. Yang, F. Wu, The influence of dissolved and surface-bound humic acid on the toxicity of TiO2 nanoparticles to Chlorella sp. Water Res. 2012, 46, 4477.
| The influence of dissolved and surface-bound humic acid on the toxicity of TiO2 nanoparticles to Chlorella sp.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xos1GisLw%3D&md5=71abaaf52f6aada4f759d50e3a500a07CAS | 22704133PubMed |
[136] OECD Working Party on Manufactured Nanomaterials 2012 (Government of Canada). Available at http://www.ic.gc.ca/eic/site/aimb-dgami.nsf/eng/h_03496.html [Verified 19 November 2013].
[137] K. Hund-Rinke, D. Hennecke, An evaluation of studies on nano TiO2 fate and ecotoxicity for risk assessment – experiences from the OECD Sponsorship Programme, in 23rd SETAC Europe Annual Meeting, 12–16 May 2013, Glasgow, Scotland 2013, p. 515 (Society of Environmental Toxicology and Chemistry).
[138] L. Chen, L. Zhou, Y. Liu, S. Deng, H. Wu, G. Wang, Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii. Ecotoxicol. Environ. Saf. 2012, 84, 155.
| Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlSgsbrP&md5=61267384f8876869359bc318288a7181CAS | 22883605PubMed |
[139] S. Dalai, S. Pakrashi, N. Chandrasekaran, A. Mukherjee, Acute toxicity of TiO2 nanoparticles to Ceriodaphnia dubia under visible light and dark conditions in a freshwater system. PLoS ONE 2013, 8, e62970.
| Acute toxicity of TiO2 nanoparticles to Ceriodaphnia dubia under visible light and dark conditions in a freshwater system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXntlCrtLs%3D&md5=af8243a96b2855fbf27a92167cb6a52aCAS | 23658658PubMed |
[140] D. B. Warheit, R. A. Hoke, C. Finlay, E. M. Donner, K. L. Reed, C. M. Sayes, Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol. Lett. 2007, 171, 99.
| Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXotVahu7o%3D&md5=20f32c7861394285833f68b843888e9aCAS | 17566673PubMed |
[141] K. Hund-Rinke, M. Simon, Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environ. Sci. Pollut. Res. Int. 2006, 13, 225.
| Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xot1eksbc%3D&md5=eb37510095d7b9e8dfb6c0f021827c3eCAS | 16910119PubMed |
[142] I. M. Sadiq, S. Dalai, N. Chandrasekaran, A. Mukherjee, Ecotoxicity study of titania (TiO2) NPs on two microalgae species: Scenedesmus sp. and Chlorella sp. Ecotoxicol. Environ. Saf. 2011, 74, 1180.
| Ecotoxicity study of titania (TiO2) NPs on two microalgae species: Scenedesmus sp. and Chlorella sp.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsVCltro%3D&md5=5d1d62b0bf79f9b15a0865bff7574b22CAS | 21481931PubMed |
[143] B. Campos, C. Rivetti, P. Rosenkranz, J. M. Navas, C. Barata, Effects of nanoparticles of TiO2 on food depletion and life-history responses of Daphnia magna. Aquat. Toxicol. 2013, 130–131, 174.
| Effects of nanoparticles of TiO2 on food depletion and life-history responses of Daphnia magna.Crossref | GoogleScholarGoogle Scholar | 23416410PubMed |
[144] X. Zhu, Y. Chang, Y. Chen, Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere 2010, 78, 209.
| Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFGgs77K&md5=e829dcc2d10cec5f1ae9406e9fa98303CAS | 19963236PubMed |
[145] D. Boyle, G. A. Al-Bairuty, C. S. Ramsden, K. A. Sloman, T. B. Henry, R. D. Handy, Subtle alterations in swimming speed distributions of rainbow trout exposed to titanium dioxide nanoparticles are associated with gill rather than brain injury. Aquat. Toxicol. 2013, 126, 116.
| Subtle alterations in swimming speed distributions of rainbow trout exposed to titanium dioxide nanoparticles are associated with gill rather than brain injury.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvV2rsrrN&md5=4df4e651820cd3327118d54331ae32dfCAS | 23178178PubMed |
[146] D. Boyle, G. A. Al-Bairuty, T. B. Henry, R. D. Handy, Critical comparison of intravenous injection of TiO2 nanoparticles with waterborne and dietary exposures concludes minimal environmentally relevant toxicity in juvenile rainbow trout Oncorhynchus mykiss. Environ. Pollut. 2013, 182, 70.
| Critical comparison of intravenous injection of TiO2 nanoparticles with waterborne and dietary exposures concludes minimal environmentally relevant toxicity in juvenile rainbow trout Oncorhynchus mykiss.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFemsr3L&md5=8e36e9518e5235e70e6e784a57bffa2aCAS | 23896679PubMed |
[147] T. M. Scown, R. van Aerle, B. D. Johnston, S. Cumberland, J. R. Lead, R. Owen, C. R. Tyler, High doses of intravenously administered titanium dioxide nanoparticles accumulate in the kidneys of rainbow trout but with no observable impairment of renal function. Toxicol. Sci. 2009, 109, 372.
| High doses of intravenously administered titanium dioxide nanoparticles accumulate in the kidneys of rainbow trout but with no observable impairment of renal function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtlOktL4%3D&md5=5e21682afabf7b843d84b011e4d94174CAS | 19332650PubMed |
[148] C. S. Ramsden, T. B. Henry, R. D. Handy, Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquat. Toxicol. 2013, 126, 404.
| Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFClt7nE&md5=fef1247b2d249517a55eada247de6c74CAS | 23084046PubMed |
[149] O. Bar-Ilan, C. C. Chuang, D. J. Schwahn, S. Yang, S. Joshi, J. A. Pedersen, R. J. Hamers, R. E. Peterson, W. Heideman, TiO2 nanoparticle exposure and illumination during zebrafish development: mortality at parts per billion concentrations. Environ. Sci. Technol. 2013, 47, 4726.
| TiO2 nanoparticle exposure and illumination during zebrafish development: mortality at parts per billion concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXkt12mu7g%3D&md5=1ff6e1b4504cc3efcf2d977866727148CAS | 23510150PubMed |
[150] H. Ma, A. Brennan, S. A. Diamond, Photocatalytic reactive oxygen species production and phototoxicity of titanium dioxide nanoparticles are dependent on the solar ultraviolet radiation spectrum. Environ. Toxicol. Chem. 2012, 31, 2099.
| Photocatalytic reactive oxygen species production and phototoxicity of titanium dioxide nanoparticles are dependent on the solar ultraviolet radiation spectrum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1GrtL%2FK&md5=6cbe95befb1f6a69a8c108a490c3e86cCAS | 22707245PubMed |
[151] H. Ma, A. Brennan, S. A. Diamond, Phototoxicity of TiO2 nanoparticles under solar radiation to two aquatic species: Daphnia magna and Japanese medaka. Environ. Toxicol. Chem. 2012, 31, 1621.
| Phototoxicity of TiO2 nanoparticles under solar radiation to two aquatic species: Daphnia magna and Japanese medaka.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XptVOltLw%3D&md5=a8ebbeafe0290126cdd1e162fefbb2d7CAS | 22544710PubMed |
[152] A. Keller, S. McFerran, A. Lazareva, S. Suh, Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1692.
| Global life cycle releases of engineered nanomaterials.Crossref | GoogleScholarGoogle Scholar |
[153] S. Xu, Z. L. Wang, One-dimensional ZnO nanostructures: solution growth and functional properties. Nano Research. 2011, 4, 1013.
| One-dimensional ZnO nanostructures: solution growth and functional properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVGnsbfM&md5=0eac7912e06d9593daefd56a6452f0c6CAS |
[154] B. Wei, K. Zheng, Y. Ji, Y. Zhang, Z. Zhang, X. Han, Size-dependent bandgap modulation of ZnO nanowires by tensile strain. Nano Lett. 2012, 12, 4595.
| Size-dependent bandgap modulation of ZnO nanowires by tensile strain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFyqtrnI&md5=dacf9fb0b77420eb80cd06a3cec460e0CAS | 22889268PubMed |
[155] S. Ameen, M. S. Akhtar, H. S. Shin, Highly sensitive hydrazine chemical sensor fabricated by modified electrode of vertically aligned zinc oxide nanorods. Talanta 2012, 100, 377.
| Highly sensitive hydrazine chemical sensor fabricated by modified electrode of vertically aligned zinc oxide nanorods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1GqurfM&md5=ca388a9487222efc44bfd524400ae8f3CAS | 23141352PubMed |
[156] R. Barnes, R. Molina, J. Xu, P. Dobson, I. Thompson, Comparison of TiO2 and ZnO nanoparticles for photocatalytic degradation of methylene blue and the correlated inactivation of gram-positive and gram-negative bacteria. J. Nanopart. Res. 2013, 15, 1432.
| Comparison of TiO2 and ZnO nanoparticles for photocatalytic degradation of methylene blue and the correlated inactivation of gram-positive and gram-negative bacteria.Crossref | GoogleScholarGoogle Scholar |
[157] M. J. Osmond, M. J. McCall, Zinc oxide nanoparticles in modern sunscreens: an analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15.
| Zinc oxide nanoparticles in modern sunscreens: an analysis of potential exposure and hazard.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnsFOmu7k%3D&md5=5733003eec28adfd03961304c9bb59c7CAS | 20795900PubMed |
[158] R. F. Domingos, Z. Rafiei, C. E. Monteiro, M. A. K. Khan, K. J. Wilkinson, Agglomeration and dissolution of zinc oxide nanoparticles: role of pH, ionic strength and fulvic acid. Environ. Chem. 2013, 10, 306.
| Agglomeration and dissolution of zinc oxide nanoparticles: role of pH, ionic strength and fulvic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlakt7vL&md5=33a65a13816158031d29506e78a0e078CAS |
[159] C. A. David, J. Galceran, C. Rey-Castro, J. Puy, E. Companys, J. Salvador, J. Monné, R. Wallace, A. Vakourov, Dissolution kinetics and solubility of ZnO nanoparticles followed by AGNES. J. Phys. Chem. C 2012, 116, 11758.
| Dissolution kinetics and solubility of ZnO nanoparticles followed by AGNES.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xmt1ersr0%3D&md5=59346db849bb044cebc98ce9778da75cCAS |
[160] M. B. Zimmermann, F. M. Hilty, Nanocompounds of iron and zinc: their potential in nutrition. Nanoscale. 2011, 3, 2390.
| Nanocompounds of iron and zinc: their potential in nutrition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotFOlsb8%3D&md5=687c284d61d6e9a742552b0da4e9081aCAS | 21483965PubMed |
[161] C. Hogstrand, Zinc, in Homeostasis and Toxicology of Essential Metals (Eds C. M. Wood, A. P. Farrell, C. J. Brauner) 2012, pp. 135–200 (Academic Press: London).
[162] Canadian Council of Resource and Environment Ministers Canadian water quality guidelines. Prepared by the task force on water quality guideline 1987, (Government of Canada: Winnipeg).
[163] H. Ma, P. L. Williams, S. A. Diamond, Ecotoxicity of manufactured ZnO nanoparticles – a review. Environ. Pollut. 2013, 172, 76.
| Ecotoxicity of manufactured ZnO nanoparticles – a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs12iu7fK&md5=d45acff53df837b938e7ee900f66e866CAS | 22995930PubMed |
[164] A. J. Miao, X. Y. Zhang, Z. Luo, C. S. Chen, W. C. Chin, P. H. Santschi, A. Quigg, Zinc oxide-engineered nanoparticles: dissolution and toxicity to marine phytoplankton. Environ. Toxicol. Chem. 2010, 29, 2814.
| Zinc oxide-engineered nanoparticles: dissolution and toxicity to marine phytoplankton.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFags7rM&md5=9b2cbf3ea7524af2b6c865bab2bb5cedCAS | 20931607PubMed |
[165] N. M. Franklin, N. J. Rogers, S. C. Apte, G. E. Batley, G. E. Gadd, P. S. Casey, Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484.
| Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlWqs7fF&md5=e1d09d97f81f4af57461dee3fda4397cCAS | 18200883PubMed |
[166] I. Blinova, A. Ivask, M. Heinlaan, M. Mortimer, A. Kahru, Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ. Pollut. 2010, 158, 41.
| Ecotoxicity of nanoparticles of CuO and ZnO in natural water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsV2ksrzI&md5=aa120ddc17203508cb77847d9999184fCAS | 19800155PubMed |
[167] S. Manzo, M. L. Miglietta, G. Rametta, S. Buono, G. Di Francia, Toxic effects of ZnO nanoparticles towards marine algae Dunaliella tertiolecta. Sci. Total Environ. 2013, 445–446, 371.
| Toxic effects of ZnO nanoparticles towards marine algae Dunaliella tertiolecta.Crossref | GoogleScholarGoogle Scholar | 23361041PubMed |
[168] S. Lin, Y. Zhao, T. Xia, H. Meng, Z. Ji, R. Liu, S. George, S. Xiong, X. Wang, H. Zhang, S. Pokhrel, L. Madler, R. Damoiseaux, A. E. Nel, High content screening in zebrafish speeds up hazard ranking of transition metal oxide nanoparticles. ACS Nano 2011, 5, 7284.
| High content screening in zebrafish speeds up hazard ranking of transition metal oxide nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVylsb3L&md5=60caf6434e665d057af7b0de0e51f7f6CAS | 21851096PubMed |
[169] R. Liu, S. Lin, R. Rallo, Y. Zhao, R. Damoiseaux, T. Xia, S. Lin, A. Nel, Y. Cohen, Automated phenotype recognition for zebrafish embryo based in vivo high throughput toxicity screening of engineered nano-materials. PLoS ONE 2012, 7, e35014.
| Automated phenotype recognition for zebrafish embryo based in vivo high throughput toxicity screening of engineered nano-materials.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlvF2gtLw%3D&md5=2493643fca99aa329617b76d3e5bac79CAS | 22506062PubMed |
[170] T. Xia, Y. Zhao, T. Sager, S. George, S. Pokhrel, N. Li, D. Schoenfeld, H. Meng, S. Lin, X. Wang, M. Wang, Z. Ji, J. I. Zink, L. Madler, V. Castranova, A. E. Nel, Decreased dissolution of ZnO by iron doping yields nanoparticles with reduced toxicity in the rodent lung and zebrafish embryos. ACS Nano 2011, 5, 1223.
| Decreased dissolution of ZnO by iron doping yields nanoparticles with reduced toxicity in the rodent lung and zebrafish embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXosl2isw%3D%3D&md5=cc7e67811a2c078efc2888b911874df3CAS | 21250651PubMed |
[171] K. Sano, K. Inohaya, M. Kawaguchi, N. Yoshizaki, I. Iuchi, S. Yasumasu, Purification and characterization of zebrafish hatching enzyme – an evolutionary aspect of the mechanism of egg envelope digestion. FASEB J. 2008, 275, 5934.
| 1:CAS:528:DC%2BD1cXhsV2murrE&md5=28057b437605cadf48711811a1d591a2CAS |
[172] S. Lin, Y. Zhao, Z. Ji, J. Ear, C. H. Chang, H. Zhang, C. Low-Kam, K. Yamada, H. Meng, X. Wang, R. Liu, S. Pokhrel, L. Mädler, R. Damoiseaux, T. Xia, H. A. Godwin, S. Lin, A. E. Nel, Zebrafish high-throughput screening to study the impact of dissolvable metal oxide nanoparticles on the hatching enzyme, ZHE1. Small 2013, 9, 1776.
| Zebrafish high-throughput screening to study the impact of dissolvable metal oxide nanoparticles on the hatching enzyme, ZHE1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslejtLzJ&md5=91365857036ca21096de510132c6c7d9CAS | 23180726PubMed |
[173] L. C. Felix, V. A. Ortega, J. D. Ede, G. G. Goss, Physicochemical characteristics of polymer-coated metal-oxide nanoparticles and their toxicological effects on zebrafish (Danio rerio) development. Environ. Sci. Technol. 2013, 47, 6589.
| 1:CAS:528:DC%2BC3sXnsFShtbw%3D&md5=df02f8a9fc3a78cbe5def9c19d4475a7CAS | 23668311PubMed |
[174] F. Larner, Y. Dogra, A. Dybowska, J. Fabrega, B. Stolpe, L. J. Bridgestock, R. Goodhead, D. J. Weiss, J. Moger, J. R. Lead, E. Valsami-Jones, C. R. Tyler, T. S. Galloway, M. Rehkamper, Tracing bioavailability of ZnO nanoparticles using stable isotope labeling. Environ. Sci. Technol. 2012, 46, 12137.
| Tracing bioavailability of ZnO nanoparticles using stable isotope labeling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVyltb7F&md5=a3d8653c7725588a2074147f2d1c7197CAS | 23050854PubMed |
[175] F. R. Khan, A. Laycock, A. Dybowska, F. Larner, B. D. Smith, P. S. Rainbow, S. N. Luoma, M. Rehkämper, E. Valsami-Jones, Stable isotope tracer to determine uptake and efflux dynamics of ZnO Nano- and bulk particles and dissolved Zn to an estuarine snail. Environ. Sci. Technol. 2013, 47, 8532.
| Stable isotope tracer to determine uptake and efflux dynamics of ZnO Nano- and bulk particles and dissolved Zn to an estuarine snail.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVWhurzJ&md5=b8362b5b25c17114c1fea03b3e1f5cf4CAS | 23802799PubMed |
[176] X. Peng, S. Palma, N. S. Fisher, S. S. Wong, Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquat. Toxicol. 2011, 102, 186.
| Effect of morphology of ZnO nanostructures on their toxicity to marine algae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjtFert74%3D&md5=8f9958cf916b48eb25324a9649a2efffCAS | 21356181PubMed |
[177] E. A. Fairbairn, A. A. Keller, L. Madler, D. Zhou, S. Pokhrel, G. N. Cherr, Metal oxide nanomaterials in seawater: linking physicochemical characteristics with biological response in sea urchin development. J. Hazard. Mater. 2011, 192, 1565.
| Metal oxide nanomaterials in seawater: linking physicochemical characteristics with biological response in sea urchin development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVygtbzL&md5=307ccb74436d7c08406e95d068831475CAS | 21775060PubMed |
[178] B. Ç. Reidy, A. Haase, A. Luch, K. Dawson, I. Lynch, Mechanisms of silver nanoparticle release, transformation and toxicity: a critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295.
| Mechanisms of silver nanoparticle release, transformation and toxicity: a critical review of current knowledge and recommendations for future studies and applications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVSrsrrO&md5=b4358ee1e498c144a56cb5224edee27bCAS |
[179] A. Bianchini, M. Grosell, S. M. Gregory, C. M. Wood, Acute silver toxicity in aquatic animals is a function of sodium uptake rate. Environ. Sci. Technol. 2002, 36, 1763.
| Acute silver toxicity in aquatic animals is a function of sodium uptake rate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xhs1Cnsbo%3D&md5=e41ca0a276f9f405caf0f5ae4dcc25bfCAS | 11993875PubMed |
[180] C. M. Wood, R. C. Playle, C. Hogstrand, Physiology and modeling of mechanisms of silver uptake and toxicity in fish. Environ. Toxicol. Chem. 1999, 18, 71.
| Physiology and modeling of mechanisms of silver uptake and toxicity in fish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXkt1Kl&md5=14ce774fc94ef81afd6aa8da14c551c4CAS |
[181] C. M. Wood, Silver, in Homeostasis and Toxicology of Non-Essential Metals (Eds C. M. Wood, A. P. Farrell, C. J. Brauner) 2012, pp. 1–65 (Elsevier: London).
[182] A. J. Kennedy, M. A. Chappell, A. J. Bednar, A. C. Ryan, J. G. Laird, J. K. Stanley, J. A. Steevens, Impact of organic carbon on the stability and toxicity of fresh and stored silver nanoparticles. Environ. Sci. Technol. 2012, 46, 10772.
| Impact of organic carbon on the stability and toxicity of fresh and stored silver nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht12ru7jF&md5=9f10630f2b1028032a8ac1652e972137CAS | 22950762PubMed |
[183] R. I. MacCuspie, K. Rogers, M. Patra, Z. Suo, A. J. Allen, M. N. Martin, V. A. Hackley, Challenges for physical characterization of silver nanoparticles under pristine and environmentally relevant conditions. J. Environ. Monit. 2011, 13, 1212.
| Challenges for physical characterization of silver nanoparticles under pristine and environmentally relevant conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXlsFSgsL8%3D&md5=aa1b3e82f4a79b7bb008bd9a429727f3CAS | 21416095PubMed |
[184] C. Levard, E. M. Hotze, G. V. Lowry, G. E. Brown, Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ. Sci. Technol. 2012, 46, 6900.
| Environmental transformations of silver nanoparticles: impact on stability and toxicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XitlGjt7o%3D&md5=4dae6e02caa0d9ab4a861e91882a1b9dCAS | 22339502PubMed |
[185] H. J. Allen, C. A. Impellitteri, D. A. Macke, J. L. Heckman, H. C. Poynton, J. M. Lazorchak, S. Govindaswamy, D. L. Roose, M. N. Nadagouda, Effects from filtration, capping agents, and presence/absence of food on the toxicity of silver nanoparticles to Daphnia magna. Environ. Toxicol. Chem. 2010, 29, 2742.
| Effects from filtration, capping agents, and presence/absence of food on the toxicity of silver nanoparticles to Daphnia magna.Crossref | GoogleScholarGoogle Scholar | 20890913PubMed |
[186] S. M. Hoheisel, S. Diamond, D. Mount, Comparison of nanosilver and ionic silver toxicity in Daphnia magna and Pimephales promelas. Environ. Toxicol. Chem. 2012, 31, 2557.
| Comparison of nanosilver and ionic silver toxicity in Daphnia magna and Pimephales promelas.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1KhurbK&md5=155c0ab7c5941c96e176e662818d829dCAS | 22887018PubMed |
[187] A. J. Kennedy, M. S. Hull, A. J. Bednar, J. D. Goss, J. C. Gunter, J. L. Bouldin, P. J. Vikesland, J. A. Steevens, Fractionating nanosilver: importance for determining toxicity to aquatic test organisms. Environ. Sci. Technol. 2010, 44, 9571.
| Fractionating nanosilver: importance for determining toxicity to aquatic test organisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVaqsL7E&md5=6131e5965547e20e4ce07cb3bf639562CAS | 21082828PubMed |
[188] S. Leclerc, K. J. Wilkinson, Bioaccumulation of nanosilver by Chlamydomonas reinhardtii – nanoparticle or the free ion? Environ. Sci. Technol. 2014, 48, 358.
| Bioaccumulation of nanosilver by Chlamydomonas reinhardtii – nanoparticle or the free ion?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvV2mtL%2FO&md5=0a3d0d9209dad88354784c44beec993dCAS | 24320028PubMed |
[189] R. J. Griffitt, K. Hyndman, N. D. Denslow, D. S. Barber, Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles. Toxicol. Sci. 2008, 107, 404.
| Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles.Crossref | GoogleScholarGoogle Scholar | 19073994PubMed |
[190] T. Li, B. Albee, M. Alemayehu, R. Diaz, L. Ingham, S. Kamal, M. Rodriguez, S. W. Bishnoi, Comparative toxicity study of Ag, Au, and Ag-Au bimetallic nanoparticles on Daphnia magna. Anal. Bioanal. Chem. 2010, 398, 689.
| Comparative toxicity study of Ag, Au, and Ag-Au bimetallic nanoparticles on Daphnia magna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnvFOit7Y%3D&md5=ffdd41bf19eae52f89e5d28acae5e1c2CAS | 20577719PubMed |
[191] C. M. Zhao, W. X. Wang, Biokinetic uptake and efflux of silver nanoparticles in Daphnia magna. Environ. Sci. Technol. 2010, 44, 7699.
| Biokinetic uptake and efflux of silver nanoparticles in Daphnia magna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFChs77P&md5=5aa2415d6f5b3b9466f92d88d7efd165CAS | 20831153PubMed |
[192] C. M. Zhao, W. X. Wang, Size-dependent uptake of silver nanoparticles in Daphnia magna. Environ. Sci. Technol. 2012, 46, 11345.
| Size-dependent uptake of silver nanoparticles in Daphnia magna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlGjsbbP&md5=8f299de2db11ba33f61248ff05abfa21CAS | 22974052PubMed |
[193] A. Bianchini, C. M. Wood, Mechanism of acute silver toxicity in Daphnia magna. Environ. Toxicol. Chem. 2003, 22, 1361.
| Mechanism of acute silver toxicity in Daphnia magna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnsVGgs78%3D&md5=a5c75f5474a1d25d8fe0433132e9bbfdCAS | 12785595PubMed |
[194] M. Solioz, A. Odermatt, Copper and silver transport by CopB-ATPase in membrane vesicles of Enterococcus hirae. J. Biol. Chem. 1995, 270, 9217.
| Copper and silver transport by CopB-ATPase in membrane vesicles of Enterococcus hirae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXlt1ajtrw%3D&md5=5c039fe8a1ac97356eb63d952c8db090CAS | 7721839PubMed |
[195] M. D. Page, J. Kropat, P. P. Hamel, S. S. Merchant, Two Chlamydomonas CTR copper transporters with a novel cys-met motif are localized to the plasma membrane and function in copper assimilation. Plant Cell 2009, 21, 928.
| Two Chlamydomonas CTR copper transporters with a novel cys-met motif are localized to the plasma membrane and function in copper assimilation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsFylurg%3D&md5=97f55c43805ac3ed07178052a0baa815CAS | 19318609PubMed |
[196] H. J. Jo, J. W. Choi, S. H. Lee, S. W. Hong, Acute toxicity of Ag and CuO nanoparticle suspensions against Daphnia magna: the importance of their dissolved fraction varying with preparation methods. J. Hazard. Mater. 2012, 227–228, 301.
| Acute toxicity of Ag and CuO nanoparticle suspensions against Daphnia magna: the importance of their dissolved fraction varying with preparation methods.Crossref | GoogleScholarGoogle Scholar | 22682800PubMed |
[197] C. M. Zhao, W. X. Wang, Regulation of sodium and calcium in Daphnia magna exposed to silver nanoparticles. Environ. Toxicol. Chem. 2013, 32, 913.
| Regulation of sodium and calcium in Daphnia magna exposed to silver nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXkslSltb4%3D&md5=14852b98bbef3ed8dc09abf50bdd17b7CAS | 23344927PubMed |
[198] G. Laban, L. F. Nies, R. F. Turco, J. W. Bickham, M. S. Sepulveda, The effects of silver nanoparticles on fathead minnow (Pimephales promelas) embryos. Ecotoxicology 2010, 19, 185.
| The effects of silver nanoparticles on fathead minnow (Pimephales promelas) embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitlKgsQ%3D%3D&md5=714c98ed37f24779c5f49a1e88b263baCAS | 19728085PubMed |
[199] A. Massarsky, L. Dupuis, J. Taylor, S. Eisa-Beygi, L. Strek, V. L. Trudeau, T. W. Moon, Assessment of nanosilver toxicity during zebrafish (Danio rerio) development. Chemosphere 2013, 92, 59.
| Assessment of nanosilver toxicity during zebrafish (Danio rerio) development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltVGjtbY%3D&md5=ffe646d61e784bb9ffbd8e4f2ea7e3dbCAS | 23548591PubMed |
[200] P. Das, M. A. Xenopoulos, C. D. Metcalfe, Toxicity of silver and titanium dioxide nanoparticle suspensions to the aquatic invertebrate, Daphnia magna. Bull. Environ. Contam. Toxicol. 2013, 91, 76.
| Toxicity of silver and titanium dioxide nanoparticle suspensions to the aquatic invertebrate, Daphnia magna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXpvFemtb8%3D&md5=8154765b64b5366f5b606bdf947ac48bCAS | 23708262PubMed |
[201] F. Gagné, J. Auclair, P. Turcotte, C. Gagnon, Sublethal effects of silver nanoparticles and dissolved silver in freshwater mussels. J. Toxicol. Environ. Health A 2013, 76, 479.
| Sublethal effects of silver nanoparticles and dissolved silver in freshwater mussels.Crossref | GoogleScholarGoogle Scholar | 23721583PubMed |
[202] R. de Lima, A. B. Seabra, N. Durán, Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867.
| Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles.Crossref | GoogleScholarGoogle Scholar | 22696476PubMed |
[203] S. Kim, D.-Y. Ryu, Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J. Appl. Toxicol. 2013, 33, 78.
| Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues.Crossref | GoogleScholarGoogle Scholar | 22936301PubMed |
[204] C. M. Powers, T. A. Slotkin, F. J. Seidler, A. R. Badireddy, S. Padilla, Silver nanoparticles alter zebrafish development and larval behavior: distinct roles for particle size, coating and composition. Neurotoxicol. Teratol. 2011, 33, 708.
| Silver nanoparticles alter zebrafish development and larval behavior: distinct roles for particle size, coating and composition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFegsrvN&md5=c7ddb9e5f853e5f58a68ed93800518ffCAS | 21315816PubMed |
[205] H. C. Poynton, J. M. Lazorchak, C. A. Impellitteri, B. J. Blalock, K. Rogers, H. J. Allen, A. Loguinov, J. L. Heckman, S. Govindasmawy, Toxicogenomic responses of nanotoxicity in Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Environ. Sci. Technol. 2012, 46, 6288.
| Toxicogenomic responses of nanotoxicity in Daphnia magna exposed to silver nitrate and coated silver nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xmt1eqsbY%3D&md5=d1785afb9efb84090e20fe85729aea34CAS | 22545559PubMed |
[206] C. H. Pham, J. Yi, M. B. Gu, Biomarker gene response in male medaka (Oryzias latipes) chronically exposed to silver nanoparticle. Ecotoxicol. Environ. Saf. 2012, 78, 239.
| Biomarker gene response in male medaka (Oryzias latipes) chronically exposed to silver nanoparticle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XivVanurg%3D&md5=3f22aa76b070146d8917d84b08dbe94eCAS | 22154143PubMed |
[207] Y. J. Chae, C. H. Pham, J. Lee, E. Bae, J. Yi, M. B. Gu, Evaluation of the toxic impact of silver nanoparticles on Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2009, 94, 320.
| Evaluation of the toxic impact of silver nanoparticles on Japanese medaka (Oryzias latipes).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFWqt7bI&md5=87137ebcef5d277c78d54213f578bf05CAS | 19699002PubMed |
[208] C. Beer, R. Foldbjerg, Y. Hayashi, D. S. Sutherland, H. Autrup, Toxicity of silver nanoparticles – nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286.
| Toxicity of silver nanoparticles – nanoparticle or silver ion?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFWhsA%3D%3D&md5=ae5dbedd2c7f03b15167835633643b00CAS | 22101214PubMed |
[209] R. Bernot, M. Brandenburg, Freshwater snail vital rates affected by non-lethal concentrations of silver nanoparticles. Hydrobiologia 2013, 714, 25.
| Freshwater snail vital rates affected by non-lethal concentrations of silver nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVegur7L&md5=3cc522ed1ebba8c74af21bc00d63f83dCAS |
[210] A. Hinther, S. Vawda, R. C. Skirrow, N. Veldhoen, P. Collins, J. T. Cullen, G. van Aggelen, C. C. Helbing, Nanometals induce stress and alter thyroid hormone action in amphibia at or below North American water quality guidelines. Environ. Sci. Technol. 2010, 44, 8314.
| Nanometals induce stress and alter thyroid hormone action in amphibia at or below North American water quality guidelines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1Gqtr3F&md5=f2516df95796ce51b7f9990686849e64CAS | 20929207PubMed |
[211] D. B. Warheit, How meaningful are the results of nanotoxicity studies in the absence of adequate material characterization? Toxicol. Sci. 2008, 101, 183.
| How meaningful are the results of nanotoxicity studies in the absence of adequate material characterization?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmsV2ruw%3D%3D&md5=1eab0439e73eb6e3758aca11271758a3CAS | 18300382PubMed |
[212] G. P. S. Marcone, Å. C. Oliveira, G. Almeida, G. A. Umbuzeiro, W. F. Jardim, Ecotoxicity of TiO2 to Daphnia similis under irradiation. J. Hazard. Mater. 2012, 211–212, 436.
| Ecotoxicity of TiO2 to Daphnia similis under irradiation.Crossref | GoogleScholarGoogle Scholar |
[213] J. F. Reeves, S. J. Davies, N. J. F. Dodd, A. N. Jha, Hydroxyl radicals (OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat. Res. – Fund. Mol. M. 2008, 640, 113.
| Hydroxyl radicals (OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjsVSgtb8%3D&md5=b8c7e04e85af026d05b4b7ecf10eebeeCAS |
[214] J. Ji, Z. Long, D. Lin, Toxicity of oxide nanoparticles to the green algae Chlorella sp. Chem. Eng. J. 2011, 170, 525.
| Toxicity of oxide nanoparticles to the green algae Chlorella sp.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmslahtr8%3D&md5=684a49929aff3524b29ff37fc802f2ecCAS |
[215] K. Hund-Rinke, K. Schlich, A. Wenzel, TiO2 nanoparticles – relationship between dispersion preparation method and ecotoxicity in the algal growth test. Environ. Sci. Eur. 2010, 22, 517.
| TiO2 nanoparticles – relationship between dispersion preparation method and ecotoxicity in the algal growth test.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFGns7nE&md5=d57fd604f191e4f5d3b0c1b71d2543e7CAS |




