Current status and future direction for examining engineered nanoparticles in natural systems
Manuel D. Montaño A F , Gregory V. Lowry B C , Frank von der Kammer D , Julie Blue E and James F. Ranville AA Colorado School of Mines, Department of Chemistry and Geochemistry, 1012 14th Street, Golden, CO 80401, USA.
B Carnegie Mellon University, Department of Civil and Environmental Engineering, 5000 Forbes Avenue, Pittsburgh, PA 15213, USA.
C Center for Environmental Implications of Nanotechnology, 1201 Hamburg Hall, Carnegie Mellon University, 5000 Forbes Avenue, Pittsburgh, PA 15213, USA.
D University of Vienna, Department of Environmental Geosciences, Althanstrasse 14 UZAII, A-1090 Vienna, Austria.
E The Cadmus Group, Inc., 100 Fifth Avenue, Suite 100, Waltham, MA 02451-8727, USA.
F Corresponding author. Email: jranvill@mines.edu

Manuel D. Montaño is a graduate student at the Colorado School of Mines earning a Ph.D. in Applied Chemistry. His current work focuses on developing techniques and methodology for the detection and characterisation of engineered nanomaterials in environmental samples, in particular utilising single particle inductively coupled plasma–mass spectrometry (ICP-MS) for analysis of ENPs in complex matrices. His previous work has included the examination of phytoremediation of heavy metals in wetland systems affected by acid mine drainage, heteroaggregation of engineered nanomaterials with naturally occurring nanoparticles and the development of single particle ICP-MS using microsecond dwell times for the purpose of environmental analysis of engineered nanomaterials. |

Dr Gregory V. Lowry is a Professor of Environmental Engineering in the Department of Civil and Environmental Engineering at Carnegie Mellon University, Pittsburgh, PA. He is also Deputy Director of the National Science Foundation (NSF) and Environmental Protection Agency (EPA) Center for Environmental Implications of Nanotechnology (CEINT). His research and teaching focuses on environmental chemistry and nanotechnology, organic and inorganic aqueous geochemistry, and subsurface processes affecting ground water quality. Dr Lowry's professional interests include: aquatic chemistry, fate and transport of chemicals in surface and subsurface waters, soil and sediment treatment, groundwater remediation, carbon sequestration and environmental issues related to fossil energy. He has published over 90 scientific articles in leading environmental engineering and science journals and 10 related book chapters. He is an associate editor of Environmental Science: Nano (a Royal Society of Chemistry journal) and is currently editing a book on nanoscale iron particles for groundwater remediation. |

Dr Frank von der Kammer completed his Ph.D. in 2005 with highest honour at Hamburg University of Technology, in the Department of Environmental Science and Technology. He is currently senior scientist and lecturer, the Head of Nanogeosciences Division and Vice Head of the Department for Environmental Geosciences at the University of Vienna. In the past, Frank has acted as a visiting Professor at the University of Pau and at the University of Aix-Marseille, France. His research interests include environmental colloids, their dynamic behaviour and interaction with trace elements, natural nanoscale processes, nanoparticle characterisation, engineered nanoparticles in the environment and the application of field flow fractionation to characterise nanoparticles in complex samples. He has published more than 50 peer-reviewed papers within both nano research and nanoparticle characterisation. |

Dr Julie Blue is Director of Environmental Research at the Cadmus Group, Inc. She has 22 years of experience in environmental research and hydrology, with expertise in groundwater, surface water, drinking water and wastewater. She applies her technical skills in areas such as endocrine-disrupting compounds, emerging wastes and climate change. She leads Cadmus' work on the effects of climate change on water resources. Her expertise includes data analysis and mathematical modelling of contaminant transport. With an M.A. in English, an M.Sc. in Earth Sciences and a Ph.D. in Hydrology, Dr Blue has written extensively for numerous documents in the areas of source water protection, water quality and climate change and water resources. |

Dr James F. Ranville is a Professor of Geochemistry in the Department of Chemistry and Geochemistry at the Colorado School of Mines. His research interests include environmental colloids, bioavailability and toxicity of trace metals and environmental nanometrology, specifically the development and the application of inductively coupled plasma–mass spectrometry and field flow fractionation to characterise nanoparticles in complex samples. He has published more than 60 peer-reviewed papers on the topics of aqueous geochemistry, nanoparticle research, and aquatic toxicology. |
Environmental Chemistry 11(4) 351-366 https://doi.org/10.1071/EN14037
Submitted: 19 February 2014 Accepted: 7 May 2014 Published: 28 July 2014
Environmental context. The detection and characterisation of engineered nanomaterials in the environment is essential for exposure and risk assessment for this emerging class of materials. However, the ubiquitous presence of naturally occurring nanomaterials presents a unique challenge for the accurate determination of engineered nanomaterials in environmental matrices. New techniques and methodologies are being developed to overcome some of these issues by taking advantage of subtle differences in the elemental and isotopic ratios within these nanomaterials.
Abstract. The increasing manufacture and implementation of engineered nanomaterials (ENMs) will continue to lead to the release of these materials into the environment. Reliably assessing the environmental exposure risk of ENMs will depend highly on the ability to quantify and characterise these materials in environmental samples. However, performing these measurements is obstructed by the complexity of environmental sample matrices, physiochemical processes altering the state of the ENM and the high background of naturally occurring nanoparticles (NNPs), which may be similar in size, shape and composition to their engineered analogues. Current analytical techniques can be implemented to overcome some of these obstacles, but the ubiquity of NNPs presents a unique challenge requiring the exploitation of properties that discriminate engineered and natural nanomaterials. To this end, new techniques are being developed that take advantage of the nature of ENMs to discern them from naturally occurring analogues. This paper reviews the current techniques utilised in the detection and characterisation of ENMs in environmental samples as well as discusses promising new approaches to overcome the high backgrounds of NNPs. Despite their occurrence in the atmosphere and soil, this review will be limited to a discussion of aqueous-based samples containing ENMs, as this environment will serve as a principal medium for the environmental dispersion of ENMs.
Introduction
Nanotechnology is a rapidly burgeoning industry. New capabilities to control matter at scales of 1 to 100 nm are producing an enormous range of novel nanomaterials, often having properties that are unique compared to matter of a similar chemical composition but larger in size. Many of these nanomaterials are already incorporated into industrial and consumer products.[1] A search of recent patent literature indicates that the trend towards incorporation of nanomaterials into products such as computers, solid state lighting, solar cells, etc. will likely continue for decades to come.[2] Governments throughout the world, and public interests groups have called for regulation to encourage the safe deployment of these new materials. This includes an assessment of potential risks that nanomaterials may pose to human health and to the environment.
Risk assessment will require an understanding of the inherent toxicity of the nanomaterials, the properties of those materials that lead to toxicity and the potential for exposure to those materials.[3] The nanotoxicology research community is currently working to modify established toxicity testing protocols to work for nanomaterials, or in some cases establishing new testing paradigms.[4] However, toxicity and ecological effects will ultimately be dose dependent and therefore accurate risk assessment also requires an ability to predict and measure environmental concentrations of engineered nanomaterials (ENMs) so that exposures can be determined. Accurately assessing exposure potential to nanomaterials has significant challenges that have not yet been adequately addressed by the nano Environmental Health and Safety (EHS) research community. In particular, the fundamental processes affecting the fate of ENMs and their distribution in the environment have not yet been determined.[5] This stems in part from the inherent kinetic instability of nanomaterials and in part from the vast number of potential ‘environmental’ conditions that an ENM may encounter.[6] Each of these environmental conditions may transform the nanomaterial, thereby changing its toxicity potential.[7,8] As noted in several recent reports by the National Research Council, significantly more work is needed to determine the ‘critical elements of interaction’ influencing ENM fate and distribution in the environment, and to develop a reliable testing strategy and suite of tools for assessing the exposure potential through the lifecycle of the nanomaterial.[9]
Releases of ENMs into the environment may occur sporadically by accidental spills, but a significant portion of ENMs' long-term release may come from consumer product manufacture, use and disposal and from intentional nanotechnology applications such as groundwater remediation and agricultural uses.[10–12] Determining environmental concentrations will rely on rigorous detection, characterisation and quantification of these materials in environmental samples.[13–15] Accurately detecting and characterising these materials in the environment is beset by several obstacles. Their small size (1–100 nm), low expected concentrations (ng L–1), and the high background of naturally occurring nanoparticulate matter (NNPs), particularly NNPs having compositions similar to ENMs, make detection of ENMs in environmental samples very difficult. In addition, a variety of environmental processes may alter the pristine, manufactured state of the engineered nanoparticles (ENPs), requiring an understanding of how these processes will affect their quantification and characterisation.[14]
In this special collection of papers, the chemical factors that influence the fate and distribution of ENMs in the environment are explored. The ultimate goal of these types of studies is to better assess the distribution of ENMs in the environment, exposure potential and ultimately biouptake into a highly complex ecosystem. Achieving this goal will require new nanometrology instrumentation or adaptation of existing instruments to make them specific towards ENMs, and to work at the very low concentration of ENMs expected in environmental and biological media. Given the central role of metrology in exposure assessment, this first paper provides a review of the challenges for the detection and characterisation of ENMs in environmental samples. A short review of instrumentation used for ENP characterisation is included. A selection of recent work performed to differentiate between naturally occurring and ENMs will be discussed. In addition, new approaches that are currently being developed to differentiate ENMs from their naturally occurring analogues will be discussed. The research reviewed herein will focus on ENMs found in aqueous environments, as life cycle assessments (LCAs) consider aqueous and soil and sediment environments to be especially important reservoirs for released ENMs.[16,17] For brevity, atmospheric and incidental nanoparticles (those created unintentionally) have been excluded from this discussion despite being a significant source of nanomaterials in the environment. Methods for the characterisation and detection of these nanomaterials have been reviewed elsewhere.[18]
Properties of ENMs
Nanomaterials are commonly defined as materials with at least one size dimension between 1 and 100 nm.[14] In addition to nanoparticles (three nano dimensions), fibres, rods, films and plates are all common nanomaterials that are manufactured and produced for their novel properties. The upper size limit of 100 nm is arbitrary and it may be more appropriate to utilise the size at which chemical and physical properties differ from their bulk counterparts as the proper nano upper size cut-off.[19] In addition to their high specific surface area and higher proportion of surface atoms, some nanomaterials can exhibit quantum confinement and novel optical-electrical properties at the nanoscale, particularly at sizes below 20 nm. For instance, the catalytic activity of gold is found to be highly dependent on the size of the nanoparticle.[20] Quantum dots specifically can have significantly different emissions depending on nanocrystal size, and thus have a very low tolerance for changes in diameter.[21]
Although any material with a size dimension between 1 and 100 nm may be classified as a nanomaterial, only certain materials at the nanoscale will exhibit properties desirable for engineering and subsequent commercial applications. These nanomaterials can possess a varying degree of composition and complexity. Some nanomaterials are composed of a single element (i.e. carbon nanotubes (CNTs), nano-Ag), whereas others can be very complex (i.e. quantum dots (QDs) with CdSe–ZnS–polymer core shell organisation). Metallic nanomaterials have many potential uses such as heterogeneous catalysis with nano-gold, and antimicrobial applications of nanosilver in such products as textiles and plastics.[22–24] Metal oxide nanoparticles such as titanium dioxide and zinc oxide are commonly found in coatings and sunscreens for their photocatalytic properties.[25–28] Cerium dioxide nanoparticles can be found in fuel additives for their ability to produce cleaner diesel exhausts.[29,30] Semiconductor nanoparticles such as QDs have found applications in both the energy sector and in biomedical imaging and drug delivery.[31–35] Lastly, carbonaceous materials such as fullerenes and CNTs have broad application in energy products, solar cells and the strength improvement of materials.[36–38]
In addition, ENMs may possess highly engineered surface coatings, aiding in the control and utilisation of properties such as dispersability, solubility, reactivity and surface binding selectivity of the nanomaterial. Commonly used surface coatings can provide electrostatic (i.e. citrate, tannic acid) or steric (i.e. poly(vinylpyrrolidine)) stabilisation to prevent aggregation and maintain a monodisperse particle population. In addition to stabilisation, some surface coatings are applied to nanomaterials to enhance their biocompatibility and transport through biological systems. Chitosan, polypeptides, fatty acids and polyethylene glycol (PEG) are common choices.[39,40]
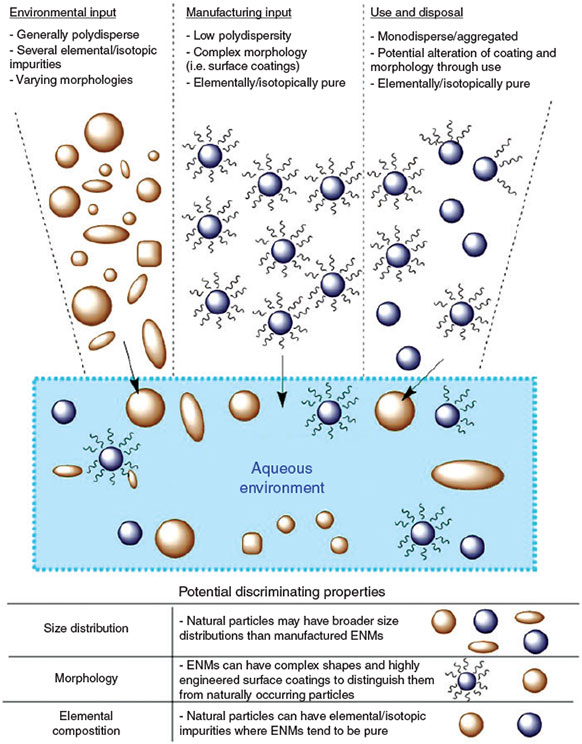
The multitude of properties and inherent complexities of these materials require robust and comprehensive analytical techniques to study them. This necessitates a multi-faceted approach for accurate characterisation. In addition, there are several environmental factors that may impede the ability to detect and characterise ENMs in the environment. The vastly greater quantity of naturally occurring nanomaterials, coupled with the multitude of environmental processes that alter the pristine nature of the ENM, will further complicate the quantification and characterisation needed for assessment of ENM risk. This is illustrated in Fig. 1, where the different inputs from environmental and anthropogenic sources will lead to a complex mixture of nanomaterials in the aqueous environment. These materials can potentially share similar or identical morphologies, compositions and properties that render most current analytical techniques inadequate for detection and characterisation. The current aim of developing methodologies and techniques is to exploit slight differences in the discriminating properties of ENMs and natural particles, which will be discussed later in this review.
|
Nanomaterials in the environment
Arguably the largest obstacle to the detection and characterisation of ENMs in environmental samples is the large proportion of naturally occurring nanomaterials and colloids. Their presence makes analyses of ENMs difficult for a variety of reasons. Because all particles scatter light to some degree, light scattering methods will be rendered useless because of their non-specific nature. Similarity in chemical composition with NNPs can obscure the concentration of ENMs obtained by elemental analysis (inductively coupled plasma mass spectrometry (ICP-MS) and optical emission spectroscopy (ICP-OES)). Particle morphologies and sizes may also be similar, making non-specific sizing methods utilising imaging (electron microscopy, particle tracking) and spectroscopy ineffective. In addition to the high background of naturally occurring nanomaterials, the environmental processes to which ENMs are subjected and subsequent transformations also make their detection and characterisation challenging.
Occurrence of natural nanomaterials
Naturally occurring nanomaterials are present in essentially all environmental samples at mass concentrations ranging from 1 to 1000 mg L–1 in surface waters and 0.01 to 80 mg L–1 in marine environments.[41] Comparatively, ENMs are expected to enter into the environment at much lower mass concentrations (ng L–1), several orders of magnitude below the concentration of natural materials.[42–44] Natural colloids have been found to follow Pareto’s power law, implying a very broad size distribution and a high degree of polydispersity.[45] Specifically, particle number concentrations increase logarithmically per decade of particle size decrease. This presumably will also be the case for natural nanoparticles over the three orders of magnitude (1–100 nm) in the defined nanoparticle size range. This is in contrast to ENPs, which are often produced to have a specific mean size with a defined upper and lower boundary. NNPs vary in size, compositions and morphology (Table 1) and can serve as interferences for detection of most engineered materials.

|
These NNPs can be formed from different pathways either through mechanical erosion and weathering (top-down synthesis) or through precipitation and biogenic pathways (bottom-up synthesis).[16,17,46,47] These materials play an important role in a multitude of environmental processes ranging from nutrient and contaminant transport to soil stability.[46,48] NNPs can further complicate the potential ENP fate and exposure by affecting the transport of these materials either by stabilising them in solutions (i.e. humic acid surface coatings) or accelerating the aggregation of these materials (i.e. NNP–ENP heteroaggregation).[49–55] NNPs interfere with the bulk chemical analysis of ENMs in environmental samples because of their similar elemental compositions. Heteroaggregation between NNPs and ENMs will also alter the effectiveness of separation-based methods such as field flow fractionation (FFF) and hydrodynamic chromatography (HDC) for characterising NNPs. Similarities in size and morphology of NNPs and ENMs greatly complicate the application of imaging techniques such as electron microscopy. Clearly the abundance of these natural materials presents a considerable, and possibly the greatest challenge to the detection and characterisation of ENMs in the environment. New methods of ENP analysis must address this challenge, as well as be sensitive to the consequences of various environmental processes acting upon and altering the pristine nature of the ENMs.
Transformation of ENMs
In their initial pristine state, ENMs are generally chemically well defined. When used for highly engineered applications (e.g. nanomedicine, photonics) there are typically monodisperse to maximise their desired function. Furthermore pristine ENMs often have specific, sometimes complex surface functionalities; in the simplest cases meant to prevent aggregation or facilitate incorporation into products. Conceivably these surface functionalities could be utilised in their detection and quantification. However, when exposed to the environment, several different chemical processes act upon these materials leaving them in an altered state that may be very different from their initial engineered or commercial form.[7] This alteration makes the detection and characterisation of these materials more difficult, and requires some knowledge of how these processes may have changed the nanomaterial, in order to identify the ENM of interest. The properties unique to ENMs as opposed to NNPs (i.e. monodispersity, well defined chemical composition, highly engineered surface coatings) are all subject to change upon entry into the environment.
Dissolution and oxidation–reduction reactions can alter the original chemical structure of the ENM. Metal and metal oxide nanomaterials, made with soft metal cations (e.g. Ag, Zn, Cu), are particularly susceptible to these reactions and may undergo dissolution or complexation with strong ligands in the environment. Silver nanoparticles for instance may oxidise rapidly to Ag+ and in reducing environments form Ag2S, or in fully oxic environments reform into halogenated insoluble precipitates (i.e. AgCl(s)).[56–58] Other nanomaterials may form an oxide shell, altering the surface composition of the material and subsequently changing its physical and chemical properties.[59–64] In addition to chemical oxidation–reduction reactions, some materials may be susceptible to photooxidation and photoreduction, which can act to change the structure and properties of the ENM. Carbonaceous nanomaterials such as CNTs and fullerenes are prone to producing carboxy and hydroxyl groups on its surface as well as generating reactive oxygen species (ROS) in the presence of sunlight.[65] The chemical alteration of the ENP, possibly accompanied by size changes, require characterisation techniques that can capture these changes and detection methods that remain sensitive to the ENPs.
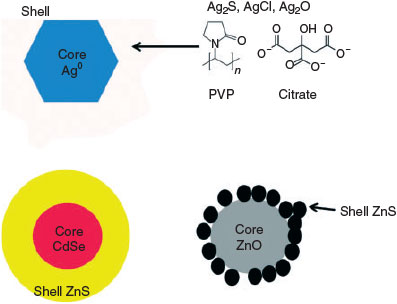
Changes to the engineered surface coatings of ENMs (Fig. 2) are expected to be commonplace in the environment. Coatings that are weakly bound to the surface to provide stabilisation are expected to be removed with relative ease in environmental samples; whereas those covalently linked to the surface of the particle may be irreversibly bound and difficult to remove.[66,67] A major pathway that may cause the loss of coatings is sunlight exposure, as sunlight-catalysed redox reactions can degrade some polymeric coatings present on ENMs.[68] The loss of engineered polymeric coatings may induce instability and facilitate (hetero-)aggregation. Conversely, polymeric-like molecules may be present in the environment and upon sorption, are capable of imparting strong electrostatic charges or steric stability to the surface of the nanomaterial preventing aggregation. Humic substances in particular have been shown to overcoat or replace the surface groups of ENMs, and impart a strong negative electrostatic charge through the numerous carboxylic acid and phenolic groups intrinsic in their molecular structure.[49,50] Other small proteins and organic molecules have been known to interact with nanomaterial surfaces resulting in changes to the dissolution, reactivity and aggregation of the ENMs.[69–71] The presence of chemically unique surface coatings could conceivably be exploited for detection through techniques such as mass spectrometry. The alteration of ENP surfaces has the effect of removing this property as a means of ENP detection and characterisation.
|
Lastly, the monodisperse nature of ENMs is not expected to persist in environmental matrices. As the surface coatings of ENMs are expected to be altered, over-coated or replaced in the environment, chemical constituents present in the environment can play a significant role in the transport and subsequently the detection and characterisation of these materials. Aggregation is generally the result of the loss of repulsive behaviour between particles, resulting in an attraction generated from van der Waals forces between particles. This can be brought on either by constriction of the electrical double layer in high ionic strength solutions, or bridging between particles by oppositely charged counter ions in solution.[72–74] Many unique ‘nano’ effects are a function of the size and surface area of the ENMs (i.e. fluorescence, surface plasmon resonance), these properties, and thus their use for ENP detection, may be lost upon aggregation. Heteroaggregation (aggregation between particles of dissimilar composition) is also expected to be a prevalent mechanism in the environment, which can further complicate the analysis of ENMs in environmental samples.[51,52,75,76] In particular, the presence of heteroaggregated ENMs leads to the need for further development of sample pretreatment methods such as chemical and mechanical dispersion and sample prefractionation by coarse filtration or centrifugation, e.g. the use of specific molecular weight cut-offs, or analytical centrifugation methods used for protein separation and characterisation.[77]
The current means of analysing ENMs in environmental samples requires a multi-faceted approach as individual analytical methods are ill-equipped to address the various obstructions that arise in the analysis of these materials. Although a great deal of work has been performed to accurately assess ENM fate and behaviour in the environment, there are still several obstacles to the application of existing nanometrology for environmental ENP quantification and characterisation, and are currently a point of emphasis in environmental research.
Current state of ENM analysis for environmental media
Owing to the intricate nature of ENMs and their subsequent alteration in environmental samples, a multifaceted approach is required for the accurate determination of these materials, as ENP detection, quantification and characterisation are all highly interrelated. An important characteristic to be determined is the size and polydispersity of the ENP. This analysis can be carried out for pristine ENMs in simple matrices, utilising a variety of techniques, yet each have their inherent drawbacks when applied to environment samples. Dynamic light scattering (DLS) is the most commonly employed high-throughput method to measure nanoparticle size in aqueous dispersions, but is less useful for the analysis of polydisperse samples because of difficulty interpreting the scattering signal. Being an ensemble technique, that is the instrument response arises from many particles, DLS is rendered essentially useless when interfering particles are present, as would generally be the case for ENMs extracted from environmental media.[49,50,78] Direct coupling to FFF and HDC at least partially overcomes the problem of polydispersity and interfering particles by providing separation of different particle sizes and presenting narrow size fractions to the DLS detector.[79,80]
An emerging light scattering technique is nanoparticle tracking analysis (NTA), tracking the Brownian motion of particles to determine diffusion coefficients and subsequently the size of the nanoparticle. The particle is first detected by light scattering, and then the distance the particle travels from its initial position within a given time interval as determined by the frame rate speed of a charge-coupled device (CCD) camera. A modified Stokes–Einstein relationship is then used to calculate the hydrodynamic diameter according to the distance travelled by the particle. Additionally, particle composition might be determined by comparing scattered light intensity from particles of the same size. The major obstacle of NTA is choosing the appropriate track length to size a statistically relevant number of particles and attain an ample particle size distribution.[81–83]
Electron microscopy (EM) techniques such as scanning (SEM) and transmission electron microscopy (TEM) are other very common analytical techniques, used in the sizing of nanomaterials. Unfortunately sample preparation for EM, as well as the imaging of the sample, requires that the sample be under vacuum, which may introduce artefacts that can alter the true environmental state of the ENM.[83,84] Some improvements have been made to preserve environmental sample integrity for EM imaging (i.e. WetSEM).[85] However, EM methods are single particle methods, and as such a size distribution is built up one particle at a time. Although this approach makes EM a low-throughput method even with automated image processing, it does offer the potential to size ENMs in the presence of interfering particles, something that DLS is incapable of. Obtaining size information by EM methods when background particles are present requires that morphological or chemical features of the ENP can still be used for identification of the ENP fraction of particles. As previously noted, environmental alteration of the ENMs may make this difficult.
An emerging sizing technique is differential centripetal sedimentation (DCS), which can provide high-resolution size information if the density of the material is known. In a common DCS analysis (disc centrifugation), the sample is injected into a spinning disc filled with liquid, in which a density gradient is generated. The sample migrates towards the outside of the disc and passes through a beam of visible light, allowing for the absorbance with time to be converted into a diameter by Stokes law (assuming a spherical geometry). Although the analysis times are dependent on the polydisperisty and density of the sample, most analyses take place on the order of only a few minutes. This rapid analysis makes it an attractive technique for the sizing of nanomaterials. However, only a few samples may be run before the fluid in the spinning disc must be drained and replaced.[86,87]
Fractionation techniques such as field flow fractionation (FFF) (e.g. flow-field flow fractionation, Fl-FFF; sedimentation-field flow fractionation, Sed-FFF) and hydrodynamic chromatography (HDC) can size nanomaterials in aqueous matrices, and for ENMs separated from soil or any solid matrix, and have the added benefit of providing size fractions for further characterisation. However they should be considered ensemble techniques as many particles are eluting from the FFF or HDC at any given time. These methods can only distinguish between natural and engineered particles if differences in chemical composition can be utilised. The most commonly used approached for this chemical identification is FFF coupled to an element-specific detector (e.g. ICP-MS). FFF and HDC are limited by extensive method development, high detection limits (dependent on detector), and non-ideal sample behaviour during separation, which may require additional sample preparation and pre-fractionation steps.[88–91] Analysis times range from tens of minutes up to an hour, making it generally faster than EM analysis but still far from being a high-throughput approach. Single particle (sp) ICP-MS, a very recently introduced technique, has the ability to size and characterise a range of metal and metal oxide nanomaterials in environmental matrices at low concentrations (ng L–1).[92–96] However, the size detection limit for this technique is dependent on the signal generated by the ablation of the nanoparticle, which may require a significant amount of ions to generate a recognisable intensity pulse. In addition, although its elemental specificity is a desirable attribute, it may be unable to differentiate between an engineered and naturally occurring nanomaterial of the same elemental composition.[81,92–95,97] Despite the limitations of FFF-ICP-MS and sp-ICP-MS, the use of these methods for ENP detection may be a significant step forward and will be elaborated upon in a subsequent discussion.
ENP surface groups and surface charge are also properties that may be important to characterise for ENMs, as they will directly influence the fate and transport of these materials in the environment. Both NMR and IR spectroscopy have been used in this respect to characterise the surface functionality of ENMs, specifically FTIR has been used to study humic acid adsorption onto silica and magnetite nanoparticles.[98–100] Yet, as previously discussed, the surface functionality of the ENM is subject to change upon exposure to the environment and may conform to the surface coatings of other naturally occurring materials in the system (i.e. humic acid coatings, biofilms). Surface charge is primarily determined through electrokinetic measurements and commonly reported as zeta potential for nanomaterials, but as an ensemble technique, the determination of surface charge for a specific nanomaterial is not possible without a prior prefractionation step that could alter the representativeness of the environmental sample.[49,50,78]
Other parameters such as particle number concentration and morphology are also very difficult to obtain in environmental samples. Particle counting techniques are obstructed by the higher number of naturally occurring particles. Aggregation of the pristine ENM may result in significant underestimates of particle number concentrations. Particle composition is subject to the many chemical reactions and processes that may severely affect the pristine or crystalline nature of the ENM, making it difficult to discern between natural and engineered analogues.
Assessing the fate of ENMs requires an ability to assess chemical composition, oxidation state, and structure. X-Ray absorption spectroscopy (XAS) is presently the only established method that allows for in situ determination of these ENM properties in environmental samples, primarily for metal and metal oxides. The advantage of XAS over other techniques is that it is non-destructive, absorption spectra can be collected directly from wet samples, including soil, sediment and tissue, and it is element specific, i.e. you collect information only on a specific element in the sample such as cerium, silver or titanium. Disadvantages are that metal concentrations of 10 to 100 mg kg–1 are required in the sample to get adequate signal. However, with fairly simple sample concentration techniques, e.g. collection of fines from specific samples, the lower end of the detection limit may be extended. XAS provides an ‘average’ speciation of the specific element in the samples and therefore does not provide ENM specific information. ENMs made from very common environmental elements such as iron or aluminium could be difficult to characterise using XAS because of the presence of high background concentrations of that element. Some recent examples of the use of XAS to assess NP fate include the ZnO and Ag NP fate in wastewater treatment plants (Ma et al.,[101] Lombi et al.[26,102]), the transformations of Ag NPs in a freshwater wetland mesocosm[26,101–103] and speciation of ZnO, CuO and TiO2 NPs in wheat and cucumber plants exposed to these nanomaterials.[104,105]
An accurate determination of environmental ENP concentration is a necessary measurement for exposure assessment. Although size is arguably the most important physical characteristic of ENMs, chemical composition is not only an important ENP characteristic, but may also serve as the best means for determining environmental concentrations. Through differences in chemical composition, ENMs might be quantified in the presence of background particles. Particle counting techniques that are non-chemical specific, such as NTA, are compromised by the higher number of naturally occurring particles. Furthermore, aggregation of the pristine ENM may result in significant underestimates of particle number concentrations.
Table 2 reviews current analytical approaches for characterising ENMs in environmental samples as a framework for determining potential future directions, namely element specific methods (i.e. sp-ICP-MS, FFF-ICPMS and XAS), for the detection, quantification and characterisation of ENMs in the environment.
New approaches
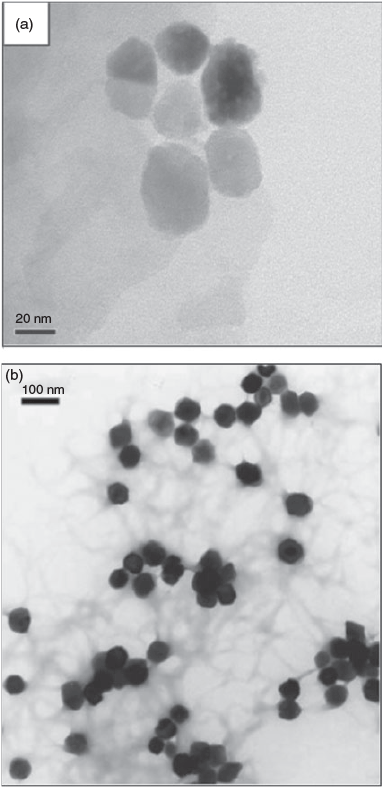
Perhaps the most direct and robust means of characterising and counting ENMs remains to be through visualisation methods, most commonly by electron microscopy. Although this is generally straightforward for simple systems, it is also obvious that visual identification is problematic for environmental samples as many naturally occurring nanomaterials share similar morphologies to commonly used ENMs. Fig. 1a, b illustrates this issue using Fe2O3 as an example. Perhaps in some unique cases highly crystalline ENMs having complex shapes might be discernable from more irregular natural materials, but as previously discussed, transformation of the ENPs may quickly alter this property.
As a result, a better discriminating property may be elemental and isotopic composition, which might be used to differentiate naturally occurring and ENMs. Although the elemental composition approach might be obvious for nanoparticulate elements that are rare (i.e. Au, Ag) it may not be possible for more commonly occurring nanoparticulate elements. The hematite example (Fig. 3a) would seem to fit this case, as both materials contain primarily iron by weight. However, natural hematite is known to contain significant amounts of impurity elements including V, Ti, Mg and Ca, among others. In contrast, hematite prepared by precipitation in the laboratory (Fig. 3b) is low in impurity elements. The following sections provide hypothetical methodologies, and discuss challenges to using elemental composition data as a means to differentiate engineered and naturally occurring nanomaterials for the purpose of quantifying and characterising ENMs in environmental samples.
|
Bulk elemental ratio approaches
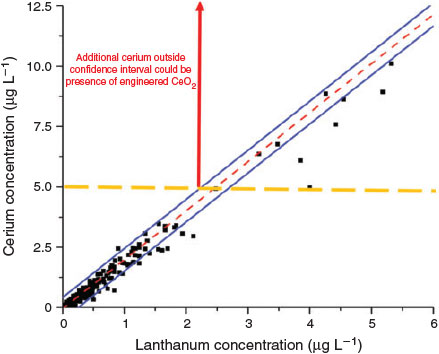
One possible method of detecting the presence of inorganic ENMs in a specific environment is to examine the elemental ratios of the nanoparticulate matter present in the system (von der Kammer et al.[106]). NNPs in aquatic systems, as well as in soils and sediments, contain several elements, in ratios that may be specific to a given geographic location, which should be a fingerprint of the natural particle population and reflect the source materials (i.e. watershed soils or aquifer materials). This can be the result of the geology of the underlying rock from which most of the particles are formed, the sources of river sediments and sediment diagenesis, dissolution, precipitation, the heteroaggregation of several different minerals or the formation of surface precipitates or coatings on the particles. Furthermore, as previously discussed, even individual mineral phases may contain minor and trace element impurities that may display ratios characteristic of a geographic region or specific field site of interest. This can directly contrast with elementally and isotopically uniform anthropogenic ENMs. Nanoparticles prepared from bottom-up syntheses are likely to be either elementally pure (e.g. metal oxides, metals) or have fixed elemental ratios (e.g. CdSe/ZnS quantum dots (QDs), Al/Ti sunscreens). In the conceptual example illustrated below (Fig. 4), a natural system will contain an assortment of natural mineral particles that contain a certain ratio of two elements, in this example cerium and lanthanum. As particle concentrations vary, either temporally or geographically, the elemental concentrations may closely co-vary. If engineered cerium dioxide (CeO2) were introduced into the system in sufficient mass, the ratio will shift towards more cerium, as lanthanum is nearly absent in these ENPs. Application of the element ratio approach requires that the elemental ratios are determined specifically for the nanoparticulate fraction of the soil or sediment, or that the ratios established on bulk samples are identical to those in the nano-range. Also, isolating the particulate fraction of the sample by filtration or centrifugation, followed by elemental analysis, will be required to improve sensitivity of the method. Whereas several techniques can provide elemental or phase data on solids (e.g. neutron activation, X-ray methods) the sensitivity and precision of ICP-MS will be needed for the concentrations of ENMs expected.
|
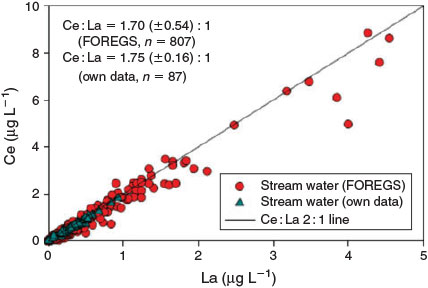
The ability to detect ENMs through perturbations in the natural ratio depends both on the amount of ENMs introduced, the magnitude of the elemental ratio in the ENP and the NNP, the variation of the ratio in the NNPs at the sampling location, the concentration of the natural nanoparticulate element and the ability of the MS to quantify the ratios with high precision. The analytical precision of the measurement on each element in the ratio will depend on several instrumental factors, including the sample processing procedures and the choice of ICP-MS (e.g. quadrupole, magnetic sector, multi-collector). The analytical methods likely contribute least to the uncertainties that limit the application. In general, high-resolution ICP-MS instruments can measure element ratios to 0.1 % (one part per thousand) accuracy. For a bulk sample analysis of element ratios, in order for an engineered CeO2 particle to be detected, it must contribute on the order of 1/1000 of the total Ce mass in a sample. If background particulate matter is in the milligrams per litre range, with Ce present as a few tenths of a percent of the particle mass, then Ce from ENMs must be present in the range of a few tens of micrograms per litre. The practical application of a bulk isotope measurement for ENP detection at realistic environmental concentrations will likely depend mostly on the natural variability of elemental ratios in the system under investigation and the concentration of background particles. Fig. 4 shows data for the correlation between La and Ce for natural waters across a wide geographic range of Europe. In principle engineered CeO2 would be detectable if it is introduced into European surface water in a quantity sufficient to shift the La/Ce ratio out of the 95 % confidence interval of the regression line. Based on the data presented in Fig. 4 concentrations on the order of 0.1–5 μg L–1 are needed, depending on the total particulate Ce concentration. Focussing on a specific geographic site, or reducing the time-scale of investigation, might reduce this value, as localised Ce/La ratios are likely to be more constant than across a widespread region. This can be illustrated even by data retrieved from sites separated by large distances, but with similar characteristics. Fig. 5 compares the Ce/La ratios for filtered surface waters (<0.45 μm) retrieved from Saliminen[107] with those locally established for small catchments draining peat bogs and wetlands in Germany, Sweden and Austria. Further data on variability of elemental ratios are needed to further evaluate the potential for success of the bulk elemental ratio approach.
|
Although bulk elemental ratios may provide a method by which to monitor the presence of ENMs, it provides minimal information on the ENM properties, and is subject to a variety of environmental factors (e.g. redox, pH) that may alter the composition of the naturally occurring mineral population in the system, particularly if particle composition is influenced by heteroaggregation. Further characterisation of the system may yield pertinent information such as size and size distribution, which combined with the elemental ratios may facilitate ENP detection.
Separation methods with elemental detection: FFF-ICP-MS
In addition to taking advantage of differences in the elemental ratios between naturally occurring and anthropogenic nanomaterials, possible differences in the size distributions of nanomaterials might be utilised to improve the element ratio approach for differentiating ENMs from NNPs. For highly engineered ENMs, in the absence of heteroaggregation with natural nanoparticles, the expected size distribution is expected to be much narrower than background nanoparticulate and colloidal matter. If an approach to isolate and measure element ratios on only the size fraction of total particulate matter that overlaps with the ENP is applied, ENP detection may be facilitated. Although serial filtration or centrifugation are possible approaches, the superior size resolution of FFF is likely to prove more successful. Additionally the direct coupling of FFF to ICP-MS provides simultaneous separation and elemental analysis, unlike sequential batch fractionation approaches. This is particularly useful as environmental processes, particularly heteroaggregation, may alter the monodisperse nature of the engineered particles, preventing the identification of a monodisperse population of ENMs. This is potentially overcome by also employing chemical or mechanical dispersion in order to examine the primary particles present in the sample.
The advantages of a front-end size fractionation coupled to element ratio measurements become apparent in the following example. Fig. 6 demonstrates a possible scenario comparing a natural system where Ti- and Fe-containing minerals are present. The fractogram shows a hypothetical clay mineral with a broad distribution from ~200 to 800 nm. When engineered titanium dioxide nanoparticles are introduced, a narrow population of additional titanium containing particles is detected in the fractogram, with no change in the iron concentrations.
In this particular scenario we assume the background iron concentration is 30 μg L–1, and titanium background concentration is ~6 μg L–1, giving a Ti/Fe ratio of 0.2 as follows (Eqn 1).
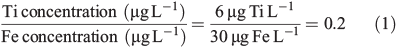
An introduction of 50 nm (25–75 nm) of titanium dioxide nanoparticles at a concentration of 60 ng Ti L–1 will change the bulk ratio of titanium to iron in the system. Averaging the concentration across the entire size range, in this example 0–1000 nm, the difference between natural and perturbed environmental ratios is minimal (Eqn 2):
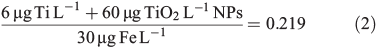
However, if we use FFF we can determine the Ti/Fe ratio at specific size ranges, allowing for the potential identification of ENMs outside the natural elemental ratio. If in fact the conditions of this example are actually met, which are: the ENMs’ elemental size distribution is narrower than the natural distribution (questionable) and elemental ratios do not display much variation across the size distribution of the natural particle size range (likely), the FFF-ICP-MS approach will be more successful than a bulk elemental ratio approach. A database of bulk elemental ratios exists,[107] which allows examination of future samples in order to observe perturbations indicative of the introduction of detectable levels of ENMs. However no such database exists for the size distribution of elemental ratios. If the size interval that is likely to be affected by ENMs displays significant variation from the bulk ratio the method will be significantly affected. Successful application might be limited to site specific studies where a suitable ‘background’ sample can be characterised for its elemental ratio size distribution and compared to a potentially affected downstream site.
The absence of heteroaggregation between ENMs and natural nanoparticles and the ability of FFF-ICP-MS to differentiate these particles could be shown on a mixture of stabilised soil nanoparticles and colloids and a 30-nm gold nanoparticle dispersion (citrate-coated gold NPs of BBI, UK). Fig. 7 shows the specific detection of the gold nanoparticles in the presence of the natural particles and also the absence of heteroaggregation because the gold ENMs are only found in their specified size region.
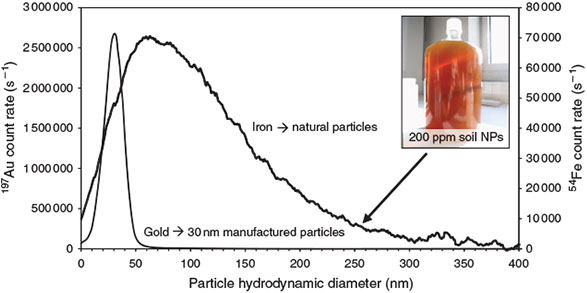
|
Time-resolved elemental analysis: microsecond-sp-ICP-MS
In recent years, sp-ICP-MS, has been used to detect and characterise engineered nanoparticles on a particle-by-particle basis in aqueous samples.[81,92,93,95,97] Utilising millisecond to microsecond dwell times, the count intensity arising from a single particle ablation event can be detected. This count intensity can then be converted into a mass using a calibration curve of dissolved standards that relate elemental mass to count intensity. From the particle mass, a diameter can also be determined assuming the appropriate geometry.
In addition to size information, determining the particle number concentration is a simple matter of counting the number of pulses and having knowledge of the sample flow rate and efficiency of the instrument’s nebuliser. Several advantages of this technique include the inherent specificity and selectivity of ICP-MS, which allows for detection and characterisation down to environmentally relevant concentrations of nanograms per litre. This technique however can be hindered by two major obstacles: a high particle number concentration and low size detection limit. High particle number concentrations may result in ‘coincidence’, where two particles are ablated and detected within the same dwell time window. This results in the apparent detection of a particle with twice the mass, as opposed to two individual particles. Additionally, smaller particles may not possess enough mass to generate a detectable signal. Single particle ICP-MS so far has been used to characterise several metallic and metal oxide nanoparticles, but is limited in its ability to only monitor for one mass at a time. Recent advances may allow for differentiating between NNPs and ENPs.
At conventional millisecond dwell times only one element can be selected by the quadrupole. Dwell times in the microsecond range allow for temporal detection of the nanoparticle as a distribution of pulse intensities, as nanoparticle events occur over the span of several hundred microseconds.[108] These pulse intensities are then summed to equate to the overall pulse intensity for a single particle. This intensity is then converted into a mass and subsequently a diameter assuming a spherical geometry. In addition to size information, the number of pulses correlates to the particle number concentration present in the sample.
At these sufficiently low settling times, where the width of the nanoparticle pulse spans several hundred milliseconds, the quadrupole can switch from one mass to the other with a short settling time. As a result, two elements can be detected within the same particle. As a result, elemental ratios can be determined on a particle-by-particle basis.[109] Naturally occurring nanomaterials may contain elemental impurities that can be detected by microsecond-sp-ICP-MS and be used to differentiate from ENMs that may have fewer elemental impurities than their natural analogues. Fig. 8 shows the analysis of river water where the minerals detected contain an elemental ratio of cerium and lanthanum. Cerium oxide particles that have been spiked into the sample will not contain a lanthanum peak, allowing for differentiation from the naturally occurring clays and minerals.

|
In addition to differentiating between particles containing different elemental ratios, ICP-MS allows for the detection of isotopic ratios. As a result, the detection and characterisation of isotopically labelled ENMs or those carrying an isotopic shift compared to natural particles is possible on a single particle basis. Some ENMs may have complex core–shell structures, with multiple elements comprising the inner and outer fractions of the particle.
Although many clay minerals in the environment will contain a mixture of elements, it is also likely that single metal oxides (e.g. CeO2, TiO2, Fe2O3) will be ubiquitously present. As with other techniques, this may require a thorough analysis of background concentrations of these particles to accurately determine the presence of ENMs. In this respect, significant deviations in the particle number concentration of these metal oxides may be a metric by which to identify the presence of anthropogenic nanomaterials.
However, to make a multi-element, high speed sp-ICP-MS analysis possible we need to reduce settling times (the time the spectrometer needs to switch to another isotope) to those much shorter than the currently encountered ones in the range on several tens of microseconds. The problem is pointed out in Fig. 9. The more often the spectrometer switches between masses, the more peak information is lost in these settling times in which no data are retrieved. This might end up in nearly total loss of the analytical information. Solutions are the decrease of settling times and the reconstruction of peaks by a convolution routine.
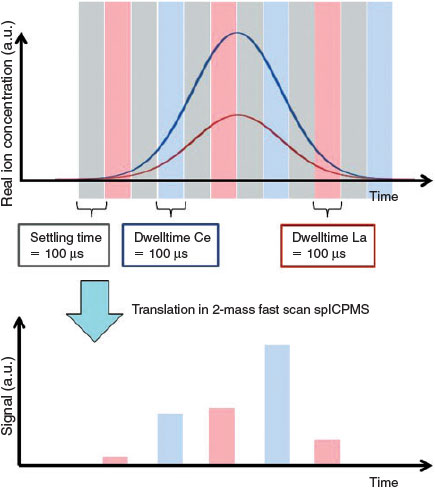
|
Conclusion
The development of accurate risk assessment models for ENMs will require the accurate determination of their fate and behaviour in environmental samples. Currently this is limited by underdeveloped methodologies that can accurately characterise these materials with sufficient specificity and sensitivity. The magnitude of naturally occurring nanomaterials, and the environmental transformations of ENMs will lead to a complex aqueous mixture of these particles requiring a multi-faceted approach necessary to accurately identify and characterise ENMs in the environment.
New highly sensitive approaches utilising differences in the elemental ratios of natural nanomaterials and likely elementally enriched ENMs may be a viable option for differentiating between these two kinds of nanomaterials. Accurate determination of elemental ratios in the background sample compared to affected sites may provide a means to identify the presence of ENMs, if the elemental ratio difference is statistically significant. This method can be improved by utilising a front-end fractionation step (i.e. field flow fractionation) to monitor changes in the elemental ratio on a size-specific basis. Lastly, improvements in single particle mass based techniques (sp-ICP-MS), may allow for the detection of elements on a particle-by-particle basis, providing a means to differentiate between complex naturally occurring nanomaterials and the more pristine ENMs. These proposed methodologies add to an ever-growing field of nanometrology. In order to develop useful life cycle assessments of ENMs for risk analysis, the accurate detection of ENMs in environmental matrices is of paramount importance.
Acknowledgements
The US Environmental Protection Agency (EPA), through its Office of Research and Development (ORD), funded the research described herein under Contract EP-C-11-039 for Scientific, Technical, Research, Engineering and Modelling Support II (STREAMS II) with the Cadmus Group. It has not been subject to EPA review and therefore does not necessarily reflect the views of EPA. No official endorsement should be inferred. This body of work was supported by: Semi-conductor Research Corporation (CSM Task Order: 425.040); EPA contract EP-C-11-039 (Cadmus Task Order Agreement 039-CSM-1); Perkin Elmer Health Sciences Inc. (PKI). The authors also acknowledge the contribution of Hamid Badiei, Samad Bazargan and Kenneth Neubauer (Perkin Elmer, Inc.) for their assistance in the collection and analysis of micro-second single particle ICP-MS data.
References
[1] M. C. Roco, C. A. Mirkin, M. C. Hersam, Nanotechnology research directions for societal needs in 2020: summary of international study. J. Nanopart. Res. 2011, 13, 897.| Nanotechnology research directions for societal needs in 2020: summary of international study.Crossref | GoogleScholarGoogle Scholar |
[2] M. E. Leitch, E. Casman, G. V. Lowry, Nanotechnology patenting trends through an environmental lens: analysis of materials and applications. J. Nanopart. Res. 2012, 14, 1283.
| Nanotechnology patenting trends through an environmental lens: analysis of materials and applications.Crossref | GoogleScholarGoogle Scholar |
[3] A. Nel, T. Xia, L. Mädler, N. Li, Toxic potential of materials at the nanolevel. Science 2006, 311, 622.
| Toxic potential of materials at the nanolevel.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XptVyrsg%3D%3D&md5=07607c278ce7f81713e9974590732786CAS | 16456071PubMed |
[4] A. Nel, T. Xia, H. Meng, X. Wang, S. Lin, Z. Ji, H. Zhang, Nanomaterial toxicity testing in the 21st century: use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2013, 46, 607.[Published online early 7 June 2012]
| 1:CAS:528:DC%2BC38Xot1Olt78%3D&md5=f0b0906b481314a2f763ddd3ecdd4180CAS | 22676423PubMed |
[5] P. Westerhoff, B. Nowack, Searching for global descriptors of engineered nanomaterial fate and transport in the environment. Acc. Chem. Res. 2013, 46, 844.[Published online early 5 September 2012]
| Searching for global descriptors of engineered nanomaterial fate and transport in the environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlSksL%2FF&md5=465f77f24ab22f48e3bc8844f500d3a5CAS | 22950943PubMed |
[6] M. R. Wiesner, J.-Y. Bottero, Environmental nanotechnology, in Applications and Impacts of Nanomaterials 2007, pp. 395–517 (McGraw Hill: New York).
[7] G. V. Lowry, K. B. Gregory, S. C. Apte, J. R. Lead, Transformations of nanomaterials in the environment. Environ. Sci. Technol. 2012, 46, 6893.
| Transformations of nanomaterials in the environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmvFajtbs%3D&md5=ffe75c0f5fd3c0eb997ff49828357fb0CAS | 22582927PubMed |
[8] C. Levard, E. M. Hotze, B. P. Colman, A. L. Dale, L. Truong, X. Y. Yang, A. J. Bone, G. E. Brown, R. L. Tanguay, R. T. Di Giulio, E. S. Bernhardt, J. N. Meyer, M. R. Wiesner, G. V. Lowry, Sulfidation of silver nanoparticles: natural antidote to their toxicity. Environ. Sci. Technol. 2013, 47, 13 440.
| A. J. Bone, G. E. Brown, R. L. Tanguay, R. T. Di Giulio, E. S. Bernhardt, J. N. Meyer, M. R. Wiesner, G. V. Lowry, Sulfidation of silver nanoparticles: natural antidote to their toxicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslWiu7zN&md5=f5e996e81c502e5ecef9c46100060a3dCAS |
[9] Committee to Develop a Research Strategy for Environmental, Health, and Safety Aspects of Engineered Nanomaterials, A research strategy for environmental, health, and safety aspects of engineered nanomaterials 2012 (National Academies Press for the National Research Council).
[10] A. Pérez-de-Luque, D. Rubiales, Nanotechnology for parasitic plant control. Pest Manag. Sci. 2009, 65, 540.
| Nanotechnology for parasitic plant control.Crossref | GoogleScholarGoogle Scholar | 19255973PubMed |
[11] M. R. Wiesner, G. V. Lowry, P. Alvarez, D. Dionysiou, P. Biswas, Assessing the risks of manufactured nanomaterials. Environ. Sci. Technol. 2006, 40, 4336.
| Assessing the risks of manufactured nanomaterials.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmvFWgsb4%3D&md5=658e5cb90b427a2f73b2955afad59c89CAS | 16903268PubMed |
[12] M. Kah, T. Hofmann, Nanopesticide research: current trends and future priorities. Environ. Int. 2014, 63, 224.
| Nanopesticide research: current trends and future priorities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitVWmur3P&md5=0d48af5ee5319da9c29ec9fc98a11cdfCAS | 24333990PubMed |
[13] S. J. Klaine, P. J. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. Y. Lyon, S. Mahendra, M. J. McLaughlin, J. R. Lead, Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825.
| Nanomaterials in the environment: behavior, fate, bioavailability, and effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVersLjJ&md5=66bb5f70ddef5f791cd00b10e5afac24CAS | 19086204PubMed |
[14] B. Nowack, T. D. Bucheli, Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5.
| Occurrence, behavior and effects of nanoparticles in the environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtF2mt7vJ&md5=af473d55f6b7c16212fec500d5b67048CAS | 17658673PubMed |
[15] M. N. Moore, Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ. Int. 2006, 32, 967.
| Do nanoparticles present ecotoxicological risks for the health of the aquatic environment?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFCqur3P&md5=7e1b6fe9c9a7d6d8c4a0cc5610a5294cCAS | 16859745PubMed |
[16] M. F. Hochella, Nanogeoscience: from origins to cutting-edge applications. Elements 2008, 4, 373.
| Nanogeoscience: from origins to cutting-edge applications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1ehtLvI&md5=d06952b73f34284821dd1a3766386d7cCAS |
[17] M. F. Hochella, S. K. Lower, P. A. Maurice, R. L. Penn, N. Sahai, D. L. Sparks, B. S. Twining, Nanominerals, mineral nanoparticles, and earth systems. Science 2008, 319, 1631.
| Nanominerals, mineral nanoparticles, and earth systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjsVamur8%3D&md5=d65e618a29744d59d6f4ac9df256c140CAS | 18356515PubMed |
[18] B. J. Majestic, G. B. Erdakos, M. Lewandowski, K. D. Oliver, R. D. Willis, T. E. Kleindienst, P. V. Bhave, A review of selected engineered nanoparticles in the atmosphere: sources, transformations, and techniques for sampling and analysis. Int. J. Occup. Environ. Health 2010, 16, 488.
| A review of selected engineered nanoparticles in the atmosphere: sources, transformations, and techniques for sampling and analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFOnsL7F&md5=a09663e8b7c364dab52e39a8ba0c3191CAS | 21222392PubMed |
[19] M. Auffan, J. Rose, J. Bottero, G. Lowry, J. Jolivet, M. Wiesner, Towards a definition of nanoparticles based on novel size-dependent properties. Nat. Nanotechnol. 2009, 4, 634.
| Towards a definition of nanoparticles based on novel size-dependent properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1aqsLrE&md5=c9f91565b01cf0e9731b5deef0b8bbc8CAS | 19809453PubMed |
[20] E. Roduner, Size matters: why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583.
| Size matters: why nanomaterials are different.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xmt1Gmu7Y%3D&md5=076ac28d5b4f345797e0c237ea81d4b0CAS | 16791330PubMed |
[21] J.-W. Luo, P. Stradins, A. Zunger, Matrix-embedded silicon quantum dots for photovoltaic applications: a theoretical study of critical factors. Energy Env. Sci. 2011, 4, 2546.
| Matrix-embedded silicon quantum dots for photovoltaic applications: a theoretical study of critical factors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFWrtrvL&md5=5f27b2267e074ecaab7d085a135ea31aCAS |
[22] T. M. Benn, P. Westerhoff, Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133.
| Nanoparticle silver released into water from commercially available sock fabrics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXktlKjsL4%3D&md5=16dae2000740ddd05f5125241df26259CAS | 18589977PubMed |
[23] S. A. Blaser, M. Scheringer, M. MacLeod, K. Hungerbühler, Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles. Sci. Total Environ. 2008, 390, 396.
| Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVOis73I&md5=69c4d3206fe10559bad96c85fdb9616dCAS | 18031795PubMed |
[24] C. N. Lok, C. M. Ho, R. Chen, Q. Y. He, W. Y. Yu, H. Sun, P. K. H. Tam, J. F. Chiu, C. M. Che, Silver nanoparticles: partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007, 12, 527.
| Silver nanoparticles: partial oxidation and antibacterial activities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkvVaitLc%3D&md5=4edecd2af31c043e8d3dcccd68d342feCAS | 17353996PubMed |
[25] H. R. Jafry, M. V. Liga, Q. Li, A. R. Barron, Simple route to enhanced photocatalytic activity of p25 titanium dioxide nanoparticles by silica addition. Environ. Sci. Technol. 2011, 45, 1563.[Published online early 31 December 2010]
| Simple route to enhanced photocatalytic activity of p25 titanium dioxide nanoparticles by silica addition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXoslek&md5=6dc46b1350e4b20698c7726687fe3895CAS | 21194213PubMed |
[26] E. Lombi, E. Donner, E. Tavakkoli, T. W. Turney, R. Naidu, B. W. Miller, K. G. Scheckel, K. Vasilev, Fate of zinc oxide nanoparticles during anaerobic digestion of wastewater and post-treatment processing of sewage sludge. Environ. Sci. Technol. 2012, 46, 9089.
| Fate of zinc oxide nanoparticles during anaerobic digestion of wastewater and post-treatment processing of sewage sludge.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVKlt7%2FK&md5=a8ff12f656878e0da34540a48c78cc9fCAS | 22816872PubMed |
[27] S. E. Cross, B. Innes, M. S. Roberts, T. Tsuzuki, T. A. Robertson, P. McCormick, Human skin penetration of sunscreen nanoparticles: in-vitro assessment of a novel micronized zinc oxide formulation. Skin Pharmacol. Physiol. 2007, 20, 148.
| Human skin penetration of sunscreen nanoparticles: in-vitro assessment of a novel micronized zinc oxide formulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXltl2gt7k%3D&md5=2fe7a4d856c4aed506a187b86bc260f5CAS | 17230054PubMed |
[28] M. J. Osmond, M. J. Mccall, Zinc oxide nanoparticles in modern sunscreens: an analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15.
| Zinc oxide nanoparticles in modern sunscreens: an analysis of potential exposure and hazard.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnsFOmu7k%3D&md5=5733003eec28adfd03961304c9bb59c7CAS | 20795900PubMed |
[29] B. Park, K. Donaldson, R. Duffin, L. Tran, F. Kelly, I. Mudway, J.-P. Morin, R. Guest, P. Jenkinson, Z. Samaras, Hazard and risk assessment of a nanoparticulate cerium oxide-based diesel fuel additive – a case study. Inhal. Toxicol. 2008, 20, 547.
| Hazard and risk assessment of a nanoparticulate cerium oxide-based diesel fuel additive – a case study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXltFyns7k%3D&md5=5418bcff2b1611630754713195e78fedCAS | 18444008PubMed |
[30] V. Sajith, C. Sobhan, G. Peterson, Experimental investigations on the effects of cerium oxide nanoparticle fuel additives on biodiesel. Adv. Mech. Eng. 2010, 2010, 581407.
| Experimental investigations on the effects of cerium oxide nanoparticle fuel additives on biodiesel.Crossref | GoogleScholarGoogle Scholar |
[31] P. V. Kamat, Quantum dot solar cells. Semiconductor nanocrystals as light harvesters. J. Phys. Chem. C 2008, 112, 18 737.
| Quantum dot solar cells. Semiconductor nanocrystals as light harvesters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1Kiu77K&md5=80deb151ad0fd8c29a9d9e6afef4caceCAS |
[32] I. Robel, V. Subramanian, M. Kuno, P. V. Kamat, Quantum dot solar cells. Harvesting light energy with CdSe nanocrystals molecularly linked to mesoscopic TiO2 films. J. Am. Chem. Soc. 2006, 128, 2385.
| Quantum dot solar cells. Harvesting light energy with CdSe nanocrystals molecularly linked to mesoscopic TiO2 films.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XoslOruw%3D%3D&md5=458b97e2980766bbb09abd0dba387b0cCAS | 16478194PubMed |
[33] X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir, S. Weiss, Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538.
| Quantum dots for live cells, in vivo imaging, and diagnostics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmslOhtw%3D%3D&md5=4c1621bb5b0ca75c827a6e05f6ec8037CAS | 15681376PubMed |
[34] J. K. Jaiswal, H. Mattoussi, J. M. Mauro, S. M. Simon, Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 2002, 21, 47.
| Long-term multiple color imaging of live cells using quantum dot bioconjugates.Crossref | GoogleScholarGoogle Scholar | 12459736PubMed |
[35] I. L. Medintz, H. T. Uyeda, E. R. Goldman, H. Mattoussi, Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435.
| Quantum dot bioconjugates for imaging, labelling and sensing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXks1Cit7k%3D&md5=e1b8a1c9d7c30c37f797e3a7070dda8fCAS | 15928695PubMed |
[36] R. H. Baughman, A. A. Zakhidov, W. A. de Heer, Carbon nanotubes – the route toward applications. Science 2002, 297, 787.
| Carbon nanotubes – the route toward applications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlvVyhsrw%3D&md5=d64b6624fe6f22ecb7aea763fa15692cCAS | 12161643PubMed |
[37] P. W. Blom, V. D. Mihailetchi, L. J. A. Koster, D. E. Markov, Device physics of polymer: fullerene bulk heterojunction solar cells. Adv. Mater. 2007, 19, 1551.
| Device physics of polymer: fullerene bulk heterojunction solar cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnsVagsL4%3D&md5=9337353b3f2068423b9fe4b67bad364dCAS |
[38] B. C. Thompson, J. M. Fréchet, Polymer–fullerene composite solar cells. Angew. Chem. Int. Ed. 2008, 47, 58.
| Polymer–fullerene composite solar cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlOrtQ%3D%3D&md5=36fb650aafce261627d19e2694241a6eCAS |
[39] A. K. Gupta, M. Gupta, Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995.
| Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXisFWr&md5=e8c2a5d123bd2bf02cf613d5f9a2e0bfCAS | 15626447PubMed |
[40] A. R. Petosa, D. P. Jaisi, I. R. Quevedo, M. Elimelech, N. Tufenkji, Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions. Environ. Sci. Technol. 2010, 44, 6532.
| Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpvVSgt7g%3D&md5=bba98cf747b566481721a2b580709409CAS | 20687602PubMed |
[41] J. Buffle, H. P. van Leeuwin, Foreword, in Environmental Particles, vol. 1 (Ed. J. Buffle, H. P. van Leeuwin) 1993 (Lewis Publishers: Chelsea, MI, USA).
[42] F. Gottschalk, T. Sonderer, R. W. Scholz, B. Nowack, Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216.
| Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlyhtL%2FP&md5=a8a5454d70978de5af6ee6d145a26961CAS | 20000512PubMed |
[43] A. A. Keller, A. Lazareva, Predicted releases of engineered nanomaterials: from global to regional to local. Environ. Sci. Technol. Lett 2014, 1, 65.[Published online early 14 October 2013]
| Predicted releases of engineered nanomaterials: from global to regional to local.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1Sqs73O&md5=6b08907e245ec9a758a6d5e5a5ab31f7CAS |
[44] A. A. Keller, S. McFerran, A. Lazareva, S. Suh, Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1692.
| Global life cycle releases of engineered nanomaterials.Crossref | GoogleScholarGoogle Scholar |
[45] J. Buffle, G. G. Leppard, Characterization of aquatic colloids and macromolecules. 1. Structure and behavior of colloidal material. Environ. Sci. Technol. 1995, 29, 2169.
| Characterization of aquatic colloids and macromolecules. 1. Structure and behavior of colloidal material.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXntFKksrs%3D&md5=1ad0a10d15bf6de9a399777fddbb1378CAS | 22280252PubMed |
[46] B. K. G. Theng, G. Yuan, Nanoparticles in the soil environment. Elements 2008, 4, 395.
| Nanoparticles in the soil environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1ehtLvL&md5=ec52b1b234bac2b44f2979c7a6489e48CAS |
[47] J. F. Banfield, H. Zhang, Nanoparticles in the environment. Rev. Mineral. Geochem. 2001, 44, 1.
| Nanoparticles in the environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XitlSntrw%3D&md5=698178a0200627168ba0b3c03e8b798aCAS |
[48] M. Taha, O. Taha, Influence of nano-material on the expansive and shrinkage soil behavior. J. Nanopart. Res. 2012, 14, 1190.
| Influence of nano-material on the expansive and shrinkage soil behavior.Crossref | GoogleScholarGoogle Scholar |
[49] S. Diegoli, A. L. Manciulea, S. Begum, I. P. Jones, J. R. Lead, J. A. Preece, Interaction between manufactured gold nanoparticles and naturally occurring organic macromolecules. Sci. Total Environ. 2008, 402, 51.
| Interaction between manufactured gold nanoparticles and naturally occurring organic macromolecules.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnt1Kqu7s%3D&md5=31a7a5255c4b927ecbd5000fe0114b44CAS | 18534664PubMed |
[50] D. P. Stankus, S. E. Lohse, J. E. Hutchison, J. A. Nason, Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents. Environ. Sci. Technol. 2011, 45, 3238.[Published online early 16 December 2010]
| Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFOqtr%2FF&md5=014ab5a691d217b2dc68d683d4946eedCAS | 21162562PubMed |
[51] P. D. Yates, G. V. Franks, S. Biggs, G. J. Jameson, Heteroaggregation with nanoparticles: effect of particle size ratio on optimum particle dose. Colloids Surf. A Physicochem. Eng. Asp. 2005, 255, 85.
| Heteroaggregation with nanoparticles: effect of particle size ratio on optimum particle dose.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhsVGms7s%3D&md5=2cf2be9c6f73983e2efa88d23efc3018CAS |
[52] P. D. Yates, G. V. Franks, G. J. Jameson, Orthokinetic heteroaggregation with nanoparticles: effect of particle size ratio on aggregate properties. Colloids Surf. A Physicochem. Eng. Asp. 2008, 326, 83.
| Orthokinetic heteroaggregation with nanoparticles: effect of particle size ratio on aggregate properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXps1elsb4%3D&md5=d88cdc4c53f19a16254196c21565c6faCAS |
[53] P. Garcia-Perez, C. Pagnoux, F. Rossignol, J.-F. Baumard, Heterocoagulation between sio2 nanoparticles and al2o3 submicronparticles; influence of the background electrolyte. Colloids Surf. A Physicochem. Eng. Asp. 2006, 281, 58.
| Heterocoagulation between sio2 nanoparticles and al2o3 submicronparticles; influence of the background electrolyte.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XkvFCkt70%3D&md5=40373e41df48e659a6ac2d60bbbf28a2CAS |
[54] S. M. Louie, R. D. Tilton, G. V. Lowry, Effects of molecular weight distribution and chemical properties of natural organic matter on gold nanoparticle aggregation. Environ. Sci. Technol. 2013, 47, 4245.
| Effects of molecular weight distribution and chemical properties of natural organic matter on gold nanoparticle aggregation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltFCjtb8%3D&md5=15bb87cd98587338b45001fe740f319eCAS | 23550560PubMed |
[55] E. M. Hotze, T. Phenrat, G. V. Lowry, Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909.
| Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVKlu7zI&md5=920b07f4a5df0e8b01da1337ad4a5016CAS | 21284288PubMed |
[56] C. Levard, E. M. Hotze, G. V. Lowry, G. E. Brown, Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ. Sci. Technol. 2012, 46, 6900.
| Environmental transformations of silver nanoparticles: impact on stability and toxicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XitlGjt7o%3D&md5=4dae6e02caa0d9ab4a861e91882a1b9dCAS | 22339502PubMed |
[57] C. Levard, S. Mitra, T. Yang, A. D. Jew, A. R. Badireddy, G. V. Lowry, G. E. Brown, Effect of chloride on the dissolution rate of silver nanoparticles and toxicity to E. coli. Environ. Sci. Technol. 2013, 47, 5738.
| Effect of chloride on the dissolution rate of silver nanoparticles and toxicity to E. coli.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmvF2js70%3D&md5=360a91aa4aabb8a8673bba0553a874dcCAS | 23641814PubMed |
[58] C. Levard, B. C. Reinsch, F. M. Michel, C. Oumahi, G. V. Lowry, G. E. Brown, Sulfidation processes of pvp-coated silver nanoparticles in aqueous solution: impact on dissolution rate. Environ. Sci. Technol. 2011, 45, 5260.
| Sulfidation processes of pvp-coated silver nanoparticles in aqueous solution: impact on dissolution rate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtlCkuro%3D&md5=78a71476ddabcc48bd33cb3b00909833CAS | 21598969PubMed |
[59] J. G. Darab, A. B. Amonette, D. S. D. Burke, R. D. Orr, S. M. Ponder, B. Schrick, T. E. Mallouk, W. W. Lukens, D. L. Caulder, D. K. Shuh, Removal of pertechnetate from simulated nuclear waste streams using supported zerovalent iron. Chem. Mater. 2007, 19, 5703.
| Removal of pertechnetate from simulated nuclear waste streams using supported zerovalent iron.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtF2jtbzK&md5=e8875e2b5073688faad7f3590b1f11efCAS |
[60] B. C. Reinsch, B. Forsberg, R. L. Penn, C. S. Kim, G. V. Lowry, Chemical transformations during aging of zerovalent iron nanoparticles in the presence of common groundwater dissolved constituents. Environ. Sci. Technol. 2010, 44, 3455.
| Chemical transformations during aging of zerovalent iron nanoparticles in the presence of common groundwater dissolved constituents.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXksVKlu7w%3D&md5=b649df551525855029193c697c576f41CAS | 20380376PubMed |
[61] M. Baalousha, P. Le Coustumer, I. Jones, J. R. Lead, Characterisation of structural and surface speciation of representative commercially available cerium oxide nanoparticles. Environ. Chem. 2010, 7, 377.
| Characterisation of structural and surface speciation of representative commercially available cerium oxide nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht12jsbzI&md5=0b1b642f638cb0089792dcbf0106a067CAS |
[62] Y. Liu, S. A. Majetich, R. D. Tilton, D. S. Sholl, G. V. Lowry, TCE dechlorination rates, pathways, and efficiency of nanoscale iron particles with different properties. Environ. Sci. Technol. 2005, 39, 1338.
| TCE dechlorination rates, pathways, and efficiency of nanoscale iron particles with different properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXovVeg&md5=afa3877f2a3c037e97d11d12aa7aa639CAS | 15787375PubMed |
[63] A. M. Derfus, W. C. W. Chan, S. N. Bhatia, Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11.
| Probing the cytotoxicity of semiconductor quantum dots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXps1SmtLo%3D&md5=a8f51446a4efc2513e69e5d4df82468dCAS |
[64] C. Noubactep, S. Caré, R. Crane, Nanoscale metallic iron for environmental remediation: prospects and limitations. Water Air Soil Pollut. 2012, 223, 1363.
| Nanoscale metallic iron for environmental remediation: prospects and limitations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xis1OnsLk%3D&md5=3e636f46d38b4c9699bcc00309694f89CAS | 22389536PubMed |
[65] W. C. Hou, C. T. Jafvert, Photochemical transformation of aqueous C60 clusters in sunlight. Environ. Sci. Technol. 2009, 43, 362.
| Photochemical transformation of aqueous C60 clusters in sunlight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFaisrnE&md5=27363eb320ead3ff07f947d92c6b266fCAS | 19238965PubMed |
[66] H.-J. Kim, T. Phenrat, R. D. Tilton, G. V. Lowry, Fe0 nanoparticles remain mobile in porous media after aging due to slow desorption of polymeric surface modifiers. Environ. Sci. Technol. 2009, 43, 3824.
| Fe0 nanoparticles remain mobile in porous media after aging due to slow desorption of polymeric surface modifiers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXkslOksrs%3D&md5=697a14a5e2c88fb0832d5790cac36510CAS | 19544894PubMed |
[67] M. P. Monopoli, D. Walczyk, A. Campbell, G. Elia, I. Lynch, F. Baldelli Bombelli, K. A. Dawson, Physical–chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525.
| Physical–chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVOnt7w%3D&md5=84d9baed4322c44b74ebe097e665d3afCAS | 21288025PubMed |
[68] Y. Cheng, L. Yin, S. Lin, M. Wiesner, E. Bernhardt, J. Liu, Toxicity reduction of polymer-stabilized silver nanoparticles by sunlight. J. Phys. Chem. C 2011, 115, 4425.
| Toxicity reduction of polymer-stabilized silver nanoparticles by sunlight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXis1akt7c%3D&md5=32dd03e52a97b8e8247f733db99b86adCAS |
[69] N. Fauconnier, J. Pons, J. Roger, A. Bee, Thiolation of maghemite nanoparticles by dimercaptosuccinic acid. J. Colloid Interface Sci. 1997, 194, 427.
| Thiolation of maghemite nanoparticles by dimercaptosuccinic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXnsVOmtbw%3D&md5=ec76abdf3913b841c5175ba2e3c27ee9CAS | 9398425PubMed |
[70] J. Lee, N. M. Donahue, Secondary organic aerosol coating of synthetic metal-oxide nanoparticles. Environ. Sci. Technol. 2011, 45, 4689.
| Secondary organic aerosol coating of synthetic metal-oxide nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXlsVeqs7s%3D&md5=bbb2b933f8a6fd0ad7573ebd7094fb72CAS | 21534558PubMed |
[71] T. Cedervall, I. Lynch, S. Lindman, T. Berggård, E. Thulin, H. Nilsson, K. A. Dawson, S. Linse, Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050.
| Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXisVWrsro%3D&md5=c875dac5258f37232c25b0c73d6df947CAS | 17267609PubMed |
[72] D. C. Sobeck, M. J. Higgins, Examination of three theories for mechanisms of cation-induced bioflocculation. Water Res. 2002, 36, 527.
| Examination of three theories for mechanisms of cation-induced bioflocculation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXovFCnt74%3D&md5=6f7ecdc1681858ea26a3a37ad051a794CAS | 11827315PubMed |
[73] N. Maximova, O. Dahl, Environmental implications of aggregation phenomena: current understanding. Curr. Opin. Colloid Interface Sci. 2006, 11, 246.
| Environmental implications of aggregation phenomena: current understanding.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1WltLfJ&md5=b4340dcc4bdfa51d8d21ad1e57249981CAS |
[74] M. Noh, T. Kim, H. Lee, C.-K. Kim, S.-W. Joo, K. Lee, Fluorescence quenching caused by aggregation of water-soluble CdSe quantum dots. Colloids Surf. A Physicochem. Eng. Asp. 2010, 359, 39.
| Fluorescence quenching caused by aggregation of water-soluble CdSe quantum dots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXivV2ju74%3D&md5=cf900162943a13e9f133a66ae8de5768CAS |
[75] M. Cerbelaud, A. Videcoq, P. Abelard, C. Pagnoux, F. Rossignol, R. Ferrando, Heteroaggregation between Al2O3 submicrometer particles and SiO2 nanoparticles: experiment and simulation. Langmuir 2008, 24, 3001.
| Heteroaggregation between Al2O3 submicrometer particles and SiO2 nanoparticles: experiment and simulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisFyit7g%3D&md5=d074c2342592d26190191b5601ff305eCAS | 18312002PubMed |
[76] M. Baalousha, A. Manciulea, S. Cumberland, K. Kendall, J. R. Lead, Aggregation and surface properties of iron oxide nanoparticles: influence of ph and natural organic matter. Environ. Toxicol. Chem. 2008, 27, 1875.
| Aggregation and surface properties of iron oxide nanoparticles: influence of ph and natural organic matter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVersLjF&md5=7498511e79e4766c4437605b0d56535eCAS | 19086206PubMed |
[77] J. Lebowitz, M. S. Lewis, P. Schuck, Modern analytical ultracentrifugation in protein science: a tutorial review. Protein Sci. 2002, 11, 2067.
| Modern analytical ultracentrifugation in protein science: a tutorial review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmslCjurg%3D&md5=29af2ee70030af82975dff65959c3575CAS | 12192063PubMed |
[78] D. Zhou, A. I. Abdel-Fattah, A. A. Keller, Clay particles destabilize engineered nanoparticles in aqueous environments. Environ. Sci. Technol. 2012, 46, 7520.
| Clay particles destabilize engineered nanoparticles in aqueous environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XovFartLc%3D&md5=680f74b56d6469de2d8b53372f676aa3CAS | 22721423PubMed |
[79] F. von der Kammer, S. Legros, T. Hofmann, E. H. Larsen, K. Loeschner, Separation and characterization of nanoparticles in complex food and environmental samples by field-flow fractionation. TRAC – Trends Analyt. Chem. 2011, 30, 425.
| Separation and characterization of nanoparticles in complex food and environmental samples by field-flow fractionation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsFals78%3D&md5=c8f3c6b484fbcf4fd394ded56c980211CAS |
[80] C. W. Isaacson, D. Bouchard, Asymmetric flow field flow fractionation of aqueous C60 nanoparticles with size determination by dynamic light scattering and quantification by liquid chromatography atmospheric pressure photo-ionization mass spectrometry. J. Chromatogr. A 2010, 1217, 1506.
| Asymmetric flow field flow fractionation of aqueous C60 nanoparticles with size determination by dynamic light scattering and quantification by liquid chromatography atmospheric pressure photo-ionization mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhslWmsb0%3D&md5=f3d172adfe7f6c86a23e5eb0b8f2f212CAS | 20070969PubMed |
[81] H. E. Pace, N. J. Rogers, C. Jarolimek, V. A. Coleman, E. P. Gray, C. P. Higgins, J. F. Ranville, Single particle inductively coupled plasma-mass spectrometry: a performance evaluation and method comparison in the determination of nanoparticle size. Environ. Sci. Technol. 2012, 46, 12 272.
| Single particle inductively coupled plasma-mass spectrometry: a performance evaluation and method comparison in the determination of nanoparticle size.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpvFehu7g%3D&md5=2c959666a930cc02c7505c4a3ade3decCAS |
[82] R. D. Boyd, S. K. Pichaimuthu, A. Cuenat, New approach to inter-technique comparisons for nanoparticle size measurements; using atomic force microscopy, nanoparticle tracking analysis and dynamic light scattering. Colloids Surf. A Physicochem. Eng. Asp. 2011, 387, 35.
| New approach to inter-technique comparisons for nanoparticle size measurements; using atomic force microscopy, nanoparticle tracking analysis and dynamic light scattering.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFansb%2FF&md5=62a4567be16b7e546cb01026ee71b66bCAS |
[83] J. Farkas, H. Peter, P. Christian, J. A. Gallego Urrea, M. Hassellöv, J. Tuoriniemi, S. Gustafsson, E. Olsson, K. Hylland, K. V. Thomas, Characterization of the effluent from a nanosilver producing washing machine. Environ. Int. 2011, 37, 1057.
| Characterization of the effluent from a nanosilver producing washing machine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsVWnurw%3D&md5=158e4099940f073c32204426d4030ca5CAS | 21470683PubMed |
[84] N. Akaighe, R. I. MacCuspie, D. A. Navarro, D. S. Aga, S. Banerjee, M. Sohn, V. K. Sharma, Humic acid-induced silver nanoparticle formation under environmentally relevant conditions. Environ. Sci. Technol. 2011, 45, 3895.
| Humic acid-induced silver nanoparticle formation under environmentally relevant conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXktFSrtbg%3D&md5=baec7aaa4e234739dc716a69b34731dcCAS | 21456573PubMed |
[85] K. Tiede, S. P. Tear, H. David, A. B. A. Boxall, Imaging of engineered nanoparticles and their aggregates under fully liquid conditions in environmental matrices. Water Res. 2009, 43, 3335.
| Imaging of engineered nanoparticles and their aggregates under fully liquid conditions in environmental matrices.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnslGqsrg%3D&md5=f0b4aa3310d3d89fb14cda556dbe202bCAS | 19501872PubMed |
[86] M. Nadler, T. Mahrholz, U. Riedel, C. Schilde, A. Kwade, Preparation of colloidal carbon nanotube dispersions and their characterisation using a disc centrifuge. Carbon 2008, 46, 1384.
| Preparation of colloidal carbon nanotube dispersions and their characterisation using a disc centrifuge.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpvFyht7c%3D&md5=06dc9a89e43e522ff46979e7178a6c67CAS |
[87] A. Neumann, W. Hoyer, M. Wolff, U. Reichl, A. Pfitzner, B. Roth, New method for density determination of nanoparticles using a CPS disc centrifuge. Colloids Surf. B Biointerfaces 2013, 104, 27.
| New method for density determination of nanoparticles using a CPS disc centrifuge.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjtFSqsL0%3D&md5=ffc2c7b50594aacf60c0f2e641928910CAS | 23298584PubMed |
[88] A. R. Poda, A. J. Bednar, A. J. Kennedy, A. Harmon, M. Hull, D. M. Mitrano, J. F. Ranville, J. Steevens, Characterization of silver nanoparticles using flow-field flow fractionation interfaced to inductively coupled plasma mass spectrometry. J. Chromatogr. A 2011, 1218, 4219.
| Characterization of silver nanoparticles using flow-field flow fractionation interfaced to inductively coupled plasma mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnslKgurY%3D&md5=d6cd8a0b645494d4ee347a445a1557a5CAS | 21247580PubMed |
[89] E. K. Lesher, J. F. Ranville, B. D. Honeyman, Analysis of ph dependent uranium(VI) sorption to nanoparticulate hematite by flow field-flow fractionation – inductively coupled plasma mass spectrometry. Environ. Sci. Technol. 2009, 43, 5403.
| Analysis of ph dependent uranium(VI) sorption to nanoparticulate hematite by flow field-flow fractionation – inductively coupled plasma mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnt1Sjtb8%3D&md5=281183837dc8f4cf03e6fdaa713c4dbaCAS | 19708373PubMed |
[90] M. Baalousha, B. Stolpe, J. R. Lead, Flow field-flow fractionation for the analysis and characterization of natural colloids and manufactured nanoparticles in environmental systems: a critical review. J. Chromatogr. A 2011, 1218, 4078.
| Flow field-flow fractionation for the analysis and characterization of natural colloids and manufactured nanoparticles in environmental systems: a critical review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnslKgtb0%3D&md5=180bc54d1c6821e40997d95296f54049CAS | 21621214PubMed |
[91] E. P. Gray, T. A. Bruton, C. P. Higgins, R. U. Halden, P. Westerhoff, J. F. Ranville, Analysis of gold nanoparticle mixtures: a comparison of hydrodynamic chromatography (HDC) and asymmetrical flow field-flow fractionation (AF4) coupled to ICP-MS. J. Anal. At. Spectrom. 2012, 27, 1532.
| Analysis of gold nanoparticle mixtures: a comparison of hydrodynamic chromatography (HDC) and asymmetrical flow field-flow fractionation (AF4) coupled to ICP-MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFOnu77O&md5=8d8f2ca8e84ec08d5b7d9d4b0dfff658CAS |
[92] R. B. Reed, D. G. Goodwin, K. L. Marsh, S. S. Capracotta, C. P. Higgins, D. H. Fairbrother, J. F. Ranville, Detection of single walled carbon nanotubes by monitoring embedded metals. Environ. Sci. Proc. Impacts 2013, 15, 204. [Published online early 7 December 2012]
| Detection of single walled carbon nanotubes by monitoring embedded metals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvFWgt7rN&md5=cfe1d3d64e856b2d1d8d2b36f9815aa5CAS |
[93] R. B. Reed, C. P. Higgins, P. Westerhoff, S. Tadjiki, J. F. Ranville, Overcoming challenges in analysis of polydisperse metal-containing nanoparticles by single particle inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2012, 27, 1093.
| Overcoming challenges in analysis of polydisperse metal-containing nanoparticles by single particle inductively coupled plasma mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XosFSksrk%3D&md5=ee7a7e1d7322123b3822463f88f0b02fCAS |
[94] D. M. Mitrano, E. K. Lesher, A. Bednar, J. Monserud, C. P. Higgins, J. F. Ranville, Detecting nanoparticulate silver using single-particle inductively coupled plasma–mass spectrometry. Environ. Toxicol. Chem. 2012, 31, 115.
| Detecting nanoparticulate silver using single-particle inductively coupled plasma–mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1yktr7E&md5=9234fb3e71b09355b5b281b35d380b3dCAS | 22012920PubMed |
[95] F. Laborda, J. Jimenez-Lamana, E. Bolea, J. R. Castillo, Selective identification, characterization and determination of dissolved silver(I) and silver nanoparticles based on single particle detection by inductively coupled plasma mass spectrometry. J. Anal. Atom. Spectrom. 2011, 26, 1362.
| Selective identification, characterization and determination of dissolved silver(I) and silver nanoparticles based on single particle detection by inductively coupled plasma mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnvVaqtro%3D&md5=573f705c12ca8eb90989c17d60dc11d3CAS |
[96] E. P. Gray, J. G. Coleman, A. J. Bednar, A. J. Kennedy, J. F. Ranville, C. P. Higgins, Extraction and analysis of silver and gold nanoparticles from biological tissues using single particle inductively coupled plasma mass spectrometry. Environ. Sci. Technol. 2013, 47, 14 315.
| Extraction and analysis of silver and gold nanoparticles from biological tissues using single particle inductively coupled plasma mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslKks7%2FO&md5=1f1decc939e7733264458b1ab0d4c851CAS |
[97] H. E. Pace, N. J. Rogers, C. Jarolimek, V. A. Coleman, C. P. Higgins, J. F. Ranville, Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 9361.
| Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVCrsLvO&md5=d3106fbeaa0633f88e64814967a64db4CAS | 22074486PubMed |
[98] J.-f. Liu, Z.-s. Zhao, G.-b. Jiang, Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949.
| Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpvVSisLw%3D&md5=55c28ff3759c6413a25dfb29dc527080CAS | 18853814PubMed |
[99] Q. Chen, D. Yin, S. Zhu, X. Hu, Adsorption of cadmium(II) on humic acid coated titanium dioxide. J. Colloid Interface Sci. 2012, 367, 241.
| Adsorption of cadmium(II) on humic acid coated titanium dioxide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsF2ltb7E&md5=dece56939e2e42a16d1d151954327169CAS | 22047914PubMed |
[100] L. Liang, L. Luo, S. Zhang, Adsorption and desorption of humic and fulvic acids on SiO2 particles at nano- and micro-scales. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 126.
| Adsorption and desorption of humic and fulvic acids on SiO2 particles at nano- and micro-scales.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotlKht7o%3D&md5=5556129a60200a2b22a3c186b833b58eCAS |
[101] R. Ma, C. Levard, F. M. Michel, G. E. Brown, G. V. Lowry, Sulfidation mechanism for zinc oxide nanoparticles and the effect of sulfidation on their solubility. Environ. Sci. Technol. 2013, 47, 2527.
| Sulfidation mechanism for zinc oxide nanoparticles and the effect of sulfidation on their solubility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXivFaluro%3D&md5=a6561168a9d878dff40dc15ce258a2bfCAS | 23425191PubMed |
[102] E. Lombi, E. Donner, S. Taheri, E. Tavakkoli, Å. K. Jämting, S. McClure, R. Naidu, B. W. Miller, K. G. Scheckel, K. Vasilev, Transformation of four silver/silver chloride nanoparticles during anaerobic treatment of wastewater and post-processing of sewage sludge. Environ. Pollut. 2013, 176, 193.
| Transformation of four silver/silver chloride nanoparticles during anaerobic treatment of wastewater and post-processing of sewage sludge.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXktFaitbw%3D&md5=b6cc5b33e4c262f5c9fb227482dcae62CAS | 23434771PubMed |
[103] G. V. Lowry, B. P. Espinasse, A. R. Badireddy, C. J. Richardson, B. C. Reinsch, L. D. Bryant, A. J. Bone, A. Deonarine, S. Chae, M. Therezien, B. P. Colman, H. Hsu-Kim, E. S. Bernhardt, C. W. Matson, M. R. Wiesner, Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland. Environ. Sci. Technol. 2012, 46, 7027.
| Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XkvVyqtbk%3D&md5=0fc37e2708edc8dea008fa6870a70b80CAS | 22463850PubMed |
[104] C. O. Dimkpa, J. E. McLean, D. E. Latta, E. Manangón, D. W. Britt, W. P. Johnson, M. I. Boyanov, A. J. Anderson, CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1125.
| CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat.Crossref | GoogleScholarGoogle Scholar |
[105] A. D. Servin, H. Castillo-Michel, J. A. Hernandez-Viezcas, B. C. Diaz, J. R. Peralta-Videa, J. L. Gardea-Torresdey, Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of tio2 nanoparticles in cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 2012, 46, 7637.
| Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of tio2 nanoparticles in cucumber (Cucumis sativus) plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XovVyhurg%3D&md5=68cceacb9677378508072d0315111ff4CAS | 22715806PubMed |
[106] F. von der Kammer, P. L. Ferguson, P. A. Holden, A. Masion, K. R. Rogers, S. J. Klaine, A. A. Koelmans, N. Horne, J. M. Unrine, Analysis of engineered nanomterials in complex matrices (environment and biota): fgeneral considerations and conceptual case studies. Environ. Toxicol. Chem. 2012, 31, 32.
| Analysis of engineered nanomterials in complex matrices (environment and biota): fgeneral considerations and conceptual case studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1yksbfF&md5=2c389671872187661fdedaf142521bfcCAS | 22021021PubMed |
[107] R. Saliminen, Geochemical Atlas of Europe Part 1 – Background information, methodology and maps 2005. Available at http://www.gtk.fi/publ/foregsatlas/ [Verified 20 December 2013].
[108] J. W. Olesik, P. J. Gray, Considerations for measurement of individual nanoparticles or microparticles by ICP-MS: determination of the number of particles and the analyte mass in each particle. J. Anal. At. Spectrom. 2012, 27, 1143.
| Considerations for measurement of individual nanoparticles or microparticles by ICP-MS: determination of the number of particles and the analyte mass in each particle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XosFSksrc%3D&md5=4e98ca7d7d5f97b73f224cbeff529c77CAS |
[109] M. D. Montaño, H. R. Badiei, S. Bazargan, J. F. Ranville, Improvements in the detection and characterization of engineered nanoparticles using spICP-MS with microsecond dwell times. Environ. Sci.: Nano. 2014, 1, 338.
| Improvements in the detection and characterization of engineered nanoparticles using spICP-MS with microsecond dwell times.Crossref | GoogleScholarGoogle Scholar |
[110] T. J. Shaw, R. Raiswell, C. R. Hexel, H. P. Vu, W. S. Moore, R. Dudgeon, K. L. Smith, Input, composition, and potential impact of terrigenous material from free-drifting icebergs in the Weddell Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 1376.
| Input, composition, and potential impact of terrigenous material from free-drifting icebergs in the Weddell Sea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXlsVWktrk%3D&md5=514d05f90e0c1cc2eafdd6e1049ca92eCAS |
[111] K. L. Chen, S. E. Mylon, M. Elimelech, Aggregation kinetics of alginate-coated hematite nanoparticles in monovalent and divalent electrolytes. Environ. Sci. Technol. 2006, 40, 1516.
| Aggregation kinetics of alginate-coated hematite nanoparticles in monovalent and divalent electrolytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XpsVGhtA%3D%3D&md5=5f12483b2a98850e9af82756e512445eCAS | 16568765PubMed |
[112] E. Neubauer, F. von der Kammer, T. Hofmann, Using FlowFFF and HPSEC to determine trace metal–colloid associations in wetland runoff. Water Res. 2013, 47, 2757.
| Using FlowFFF and HPSEC to determine trace metal–colloid associations in wetland runoff.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXksFajtbk%3D&md5=dacaa1b8b60ea2a9d44ccd917fa8caf8CAS | 23528782PubMed |
[113] E. Neubauer, S. J. Köhler, F. von der Kammer, H. Laudon, T. Hofmann, Effect of pH and stream order on iron and arsenic speciation in boreal catchments. Environ. Sci. Technol. 2013, 47, 7120.
| Effect of pH and stream order on iron and arsenic speciation in boreal catchments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnvFSlu7k%3D&md5=50fd588ac7128f5a95244ee117765b93CAS | 23692297PubMed |
[114] E. Neubauer, F. von der Kammer, K.-H. Knorr, S. Peiffer, M. Reichert, T. Hofmann, Colloid-associated export of arsenic in stream water during changing hydrological conditions. Chem. Geo. 2013, 352, 81.
| Colloid-associated export of arsenic in stream water during changing hydrological conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFOrs7rE&md5=185ea9c45a86459257c52ab823bcebf5CAS |