Biosynthesis of arsenolipids by the cyanobacterium Synechocystis sp. PCC 6803
Xi-Mei Xue A B , Georg Raber C , Simon Foster D , Song-Can Chen B E , Kevin A. Francesconi C F and Yong-Guan Zhu A E FA Key Laboratory of Urban Environment and Health, Institute of Urban Environment, Chinese Academy of Sciences, 1799 Jimei Road, Jimei District, Xiamen 361021, China.
B University of Chinese Academy of Sciences, 19A Yuquan Road, Shijingshan District, Beijing 100049, China.
C Institute of Chemistry, University of Graz, Universitaetsplatz 1, A-8010 Graz, Austria.
D Institute for Applied Ecology, University of Canberra, Bruce, ACT 2601, Australia.
E State Key Laboratory of Urban and Regional Ecology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, 18 Shuangqing Road, Haidian District, Beijing 100085, China.
F Corresponding authors. Email: ygzhu@iue.ac.cn; kevin.francesconi@uni-graz.at
Environmental Chemistry 11(5) 506-513 https://doi.org/10.1071/EN14069
Submitted: 1 April 2014 Accepted: 19 May 2014 Published: 16 September 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Environmental context. Arsenic biotransformation processes play a key role in the cycling of arsenic in aquatic systems. We show that a freshwater cyanobacterium can convert inorganic arsenic into arsenolipids, and the conversion efficiency depends on the arsenic concentration. The role of these novel arsenic compounds remains to be elucidated.
Abstract. Although methylated arsenic and arsenosugars have been verified in various freshwater organisms, lipid-soluble arsenic compounds have not been identified. Here, we report investigations with the model organism cyanobacterium Synechocystis sp. PCC 6803 wild type and ΔarsM (arsenic(III) S-adenosylmethionine methyltransferase) mutant strain, which lacks the enzymes for arsenic methylation cultured in various concentrations of arsenate (AsV). Although Synechocystis accumulated higher arsenic concentrations at the higher exposure levels, the bioaccumulation factor decreased with increasing AsV. The accumulated arsenic in the cells was partitioned into water-soluble and lipid-soluble fractions; lipid-soluble arsenic was found in Synechocystis wild type cells (3–35 % of the total depending on the level of arsenic exposure), but was not detected in Synechocystis ΔarsM mutant strain showing that ArsM was required for arsenolipid biosynthesis. The arsenolipids present in Synechocystis sp. PCC 6803 were analysed by high performance liquid chromatography–inductively coupled plasma–mass spectrometry, high performance liquid chromatography–electrospray mass spectrometry, and high resolution tandem mass spectrometry. The two major arsenolipids were characterised as arsenosugar phospholipids based on their assigned molecular formulas C47H88O14AsP and C47H90O14AsP, and tandem mass spectrometric data demonstrated the presence of the phosphate arsenosugar and acylated glycerol groups.
Additional keyword: arsenic.
Introduction
Arsenic is a widely distributed toxicant in the environment. Naturally, arsenic is found in soils and waters mainly in inorganic forms in a +3 and +5 oxidation state, arsenite (AsIII) and AsV. As a chemically active element, arsenic can affect physiological and biochemical processes of living organisms. Organisms have developed specific metabolic pathways to detoxify arsenic in the environment. Many freshwater organisms can reduce AsV to AsIII, which is then pumped out by membrane proteins, thereby decreasing the arsenic content in cells. Marine organisms tend to use biomethylation as an arsenic detoxification mechanism leading to an accumulation of organic arsenic compounds. The most abundant and common arsenic compounds are arsenobetaine, the major arsenical in marine animals, and arsenosugars, which are dominant in marine algae.[1] Arsenobetaine and arsenosugars have also been identified in freshwater[2–4] and terrestrial organisms,[5] but generally only at low concentrations.
Over the last few years, interest has turned to the investigation of arsenic-containing lipids (arsenolipids) and the possible role they might play in the transformation and detoxification of arsenic. Although the presence of arsenic in lipid fractions of fish (cod liver oil and herring oil) was first reported in 1968,[6] the structures of these lipids have mostly been elucidated only over the last 6 years. Thus, arsenic-containing fatty acids were identified in cod liver oil[7] and capelin fish meal,[8] and the closely related arsenic-containing hydrocarbons were found in capelin oil,[9] cod liver[10] and sashimi tuna.[11] A third class of arsenolipid, namely arsenosugar phospholipid, has been identified in marine algae.[12–14] Although arsenolipids are now established as common arsenic metabolites in marine animals and algae, their presence in freshwater organisms has not been demonstrated, and knowledge concerning the biological relevance and biosynthesis mechanism of the arsenolipids is still limited.
A recent report[4] that the cyanobacterium Synechocystis sp. PCC 6803 is capable of producing arsenosugars has encouraged us to explore arsenic metabolism and genetic manipulation in this organism. Synechocystis sp. PCC 6803 is an oxygenic photo-autotrophic unicellular cyanobacterium, the entire genome of which has been sequenced.[15,16] Synechocystis sp. PCC 6803 is widely used as a model organism to study molecular mechanisms of biological metabolism because of the ease with which it can be genetically modified.[17] In a preliminary study, we found that ArsM was required for arsenosugar biosynthesis in Synechocystis sp. PCC 6803 (X. M. Xue, J. Ye, G. Li, H. Gao, C. Rensing, Q. Q. Chi, K. A. Francesconi, Y. G. Zhu, unpubl. data). Here, we investigate whether Synechocystis sp. PCC 6803 can produce arsenosugar arsenolipids and to identify if ArsM is involved in arsenolipid biosynthesis.
Materials and methods
Cyanobacteria culture and harvesting
Axenic cultures of Synechocystis wild type (WT) and Synechocystis ΔarsM were grown in 250-mL Erlenmeyer flasks containing 100 mL of BG-11 medium[18] at 30 °C with shaking at 96 rpm under a 12–12 light cycle (40 μmol photons m–2 s–1) for 3 weeks. BG-11 medium was adjusted to pH 7.6 with 25 % HNO3 (Merck, Darmstadt, Germany) after adding 0.1–100 μL of 100 mM Na2HAsO4 (BZL, Beijing, China); each treatment was cultured in triplicate. For all the Synechocystis ΔarsM cultures, at least 50 μg mL–1 of the antibiotic kanamycin (Solarbio, Beijing, China) was added to the culture medium. Cells were grown to stationary phase, and cultured cells were diluted with fresh sterile medium to give an optical density at 730 nm (OD730 nm) of 0.1. After 3 weeks, ~50 mL of the cyanobacteria was harvested by centrifuging at 3220g and room temperature 25 °C for 10 min, and the cells were transferred to 15-mL polypropylene tubes with screw-caps after being washed three times with BG11 medium. The cells were broken by subjecting them to ten cycles of freezing in liquid N2 and thawing at 37 °C, and then freeze-dried. For the cells sent to Graz for characterisation of the arsenolipids, Synechocystis WT and Synechocystis ΔarsM were cultured in 2-L Erlenmeyer flasks containing 500 mL of BG-11 medium and 1 μM AsV at 30 °C.
Fractionation of arsenic in Synechocystis sp. PCC 6803
The lyophilised cyanobacteria cells were mixed with 10 mL of CHCl3/CH3OH (Fisher Scientific, Fair Lawn, NJ, USA) (2 : 1, v/v), vortexed for 1 min to assist mixing, and placed on a rolling incubator (Kylin-Bell laboratory Instruments Co., Ltd, Haimen, Jiangsu, China) overnight. The separation of the water-soluble and lipid-soluble arsenic was carried out as described by Thomson et al.[19] Samples were then centrifuged for 10 min at 3220g and room temperature 25 °C to separate residue and supernatant, and the supernatants were pipetted into 50-mL polypropylene vials. The residue was extracted again and centrifuged, and the second supernatant was combined with the first. In addition, the lipid-soluble arsenic was separated into polar arsenolipids and non-polar arsenolipids by adding 4 mL of hexane and 3 mL of CH3OH/H2O (9 : 1, v/v) according to the previously described method.[11]
Determination of total arsenic by inductively coupled plasma–mass spectrometry (ICP-MS) – Xiamen
Total arsenic concentrations were determined using an Agilent 7500cx ICPMS (Agilent Technologies, Santa Clara, CA, USA) operated in collision cell mode. The masses measured were 75As, 72Ge and 103Rh, with the last two masses serving as a check for signal stability. To monitor instrument drift, a standard solution of 10-µg L–1 arsenic was analysed every 20 samples, and the results were accepted only if the value of this ‘drift standard’ remained within 20 % of the mean value over the course of the analyses. The analytical method was validated through analyses of GBW10025 (spiral algae) supplied by the National Institute of Metrology, P. R. China (certified [total As] = 0.22 ± 0.03 mg As kg–1), found [total As] = 0.24 ± 0.06 mg As kg–1 (n = 4).
Arsenic speciation analysis – Xiamen
High performance liquid chromatography (HPLC)/ICPMS measurements were performed using an Agilent 7500cx ICPMS for element-selective detection coupled with an Agilent 1200 HPLC (Agilent Technologies, Santa Clara, CA, USA). Separation was achieved on a Hamilton PRP-X100 anion-exchange column (4.1 × 250 mm, 10 µm) with matching guard column (Hamilton Co., Reno, NV, USA). The mobile phase containing 15 mM NH4H2PO4 (Alfa Aesar, Ward Hill, MA, USA) at pH 5.6 was pumped through the column at 1.5 mL min–1. The injection volume was 100 µL and the column temperature was 40 °C. The ICPMS was tuned to monitor m/z 75 (arsenic); at the same time, m/z 77 and 82 (selenium) were monitored to verify that ArCl interferences were not present. Arsenic species were identified by retention time matching with standard arsenicals and by spiking experiments. In addition, m/z 72 (germanium) and m/z 103 (rhodium) were used as internal standards; a standard solution of 10 μg As L–1 as dimethylarsinic acid (DMAV, Accustandard, New Haven, CT, USA) was used as a drift standard introduced at a frequency of one injection every twenty samples. The various species were quantified by external calibration against DMAV.
Determination of polar arsenic species in Synechocystis cells – Graz
Extraction of polar arsenic species
Approximately 10 mg of freeze-dried cells was weighed with a precision of 0.01 mg into 1.5-mL Eppendorf tubes, and an aqueous solution (1 mL) of 10-mM malonic acid (>98 %) from Fluka (Buchs, Switzerland), adjusted to pH 5.6 with aqueous ammonia (25 %, p.a., Fluka), was added. Samples were extracted in an ultrasonic bath for 15 min followed by overnight shaking. The extracts were centrifuged for 15 min at 8900g and 4 °C. The supernatant was used directly for HPLC/ICPMS analysis. To a separate aliquot of the extracts (200 µL), 20 µL of hydrogen peroxide (30 %, p.a.) from Carl Roth GmbH (Karlsruhe, Germany) was added in order to oxidise AsIII to AsV, and thio-As species to oxo-As species.
Extraction of less polar arsenic species
Approximately 50 mg of freeze-dried cells were weighed (to a precision of 0.1 mg) directly into a centrifuge tube (10 mL, polypropylene), 5 mL of a mixture of CH3OH/H2O (9 : 1, v/v) was added, and the tubes were rotated on a rotary wheel overnight. A portion of the mixture was syringe filtered (0.2 µm) into a HPLC vial before analysis by HPLC/ICPMS. These extraction conditions are suitable for extracting many organoarsenic species, including arsenosugars, but the highly polar AsV is poorly extracted.
Ion-exchange HPLC/ICPMS analysis of polar arsenic species
Anion-exchange HPLC was performed at 40 °C with a PRP-X100 column (4.1 × 150 mm; 5-µm particle size; Hamilton Co., Reno, NV, USA) and a mobile phase of malonic acid (5 or 10 mM at pH 5.6, adjusted with aqueous ammonia) under gradient elution conditions: 0–3 min, 5 mM; 3–9 min, 10 mM; then 9–12 min, 5 mM to re-equilibrate the column before the next injection. The flow rate was 1 mL min–1, the injection volume was 20 µL. Cation-exchange HPLC was performed at 30 °C with an Ionospher 5C column (3 × 100 mm, Agilent, Waldbronn, Germany) and a mobile phase of 10-mM aqueous pyridine (Merck) adjusted to pH 2.5 with formic acid. The flow rate was 1 mL min–1; the injection volume was 20 µL. Arsenic species were identified by retention time matching and spiking experiments with standard arsenic species as previously reported.[20] Quantification was performed by external calibration against DMAV.
Determination of arsenolipids in Synechocystis cells
Extraction of arsenolipids
A portion (50 mg, weighed to a precision of 0.1 mg) of the freeze-dried cells was weighed into a centrifuge tube and extracted with a mixture of CHCl3/CH3OH (1 : 2, v/v) overnight on a rotary wheel. The mixture was centrifuged; 4.5 mL of the supernatant was transferred to a clean tube containing 0.5 mL of a 1 % aqueous NaHCO3 solution (Fluka) and the mixture was gently shaken. A portion of the CHCl3 layer was applied to a small column of silica (conditioned with 1 % formic acid in acetone/CHCl3, 1 : 1, v/v) packed into a Pasteur pipette. The silica column was washed with CH3OH (5 mL) and then CH3OH containing 1 % ammonia whereupon the arsenolipids were eluted. This arsenolipid fraction was evaporated to dryness, and the residue was re-dissolved in CH3OH (200 µL) before HPLC/ICPMS–electrospray ionisation mass spectrometry (ESIMS) (Agilent Technologies, Waldbronn, Germany) analysis.
HPLC/ICPMS and HPLC/ESIMS analysis of arsenolipids
Separations were performed with an Agilent Zorbax C8 column (150 × 4.1 mm, 5 µm, Agilent SA, Basel, Switzerland) and a mobile phase run at the following gradient elution conditions: eluent A, 0.1 % formic acid in water; eluent B, 0.1 % formic acid in CH3OH; gradient, 0–15 min 5–95 % B, 15–32 min constant 95 % B, 32 min 5 % B, 32–40 min constant 5 % B; flow, 1.0 mL min–1; injection volume, 5 µL. For ICPMS detection, the HPLC was carried out with an Agilent 1100 HPLC system connected to an Agilent 7500ce ICPMS (both from Agilent Technologies, Waldbronn, Germany). To prevent carbon deposition on the interface cones, an optional gas (1 % oxygen in argon) was introduced. The nominal mass of the molecules containing arsenic were recorded by splitting the effluent flow from the HPLC column and simultaneously determining arsenic by ICPMS and protonated molecular ions by electrospray single quadrupole MS, as previously described.[13]
For electrospray high-resolution MS measurements, the mobile phase was delivered by a Dionex Ultimate 3000 system (Thermo Scientific, Bremen, Germany). Full spectra and tandem mass spectra were obtained with a Q-Exactive hybrid quadrupole-Orbitrap mass spectrometer (Thermo Scientific); source, HESI2, positive ion mode; spray voltage, 3.2 kV; capillary temperature, 320 °C; sheath gas, 75 au (arbitrary units); aux gas, 20 au; temperature, 500 °C; settings for full scan m/z 150–2000; resolution, 70 000 (full width at half maximum height, FWHM); automatic gain control (AGC) target, 3 × 106; maximum inject time, 200 ms. Data Dependent MS2: full scan m/z 100–1100; resolution 70 000 (FWHM); AGC target 3 × 106; maximum inject time, 100 ms; the five most intense peaks were selected; dynamic exclusion time 5 s. Tandem mass spectrometry (MSMS) settings: isolation window 1 Da; normalised collision energy, 30 au; resolution 17 500 FWHM; AGC target, 1 × 105; maximum inject time, 50 ms. The simulated spectra were obtained with Xcalibur software (Thermo Scientific).
Results
Arsenic speciation in the medium
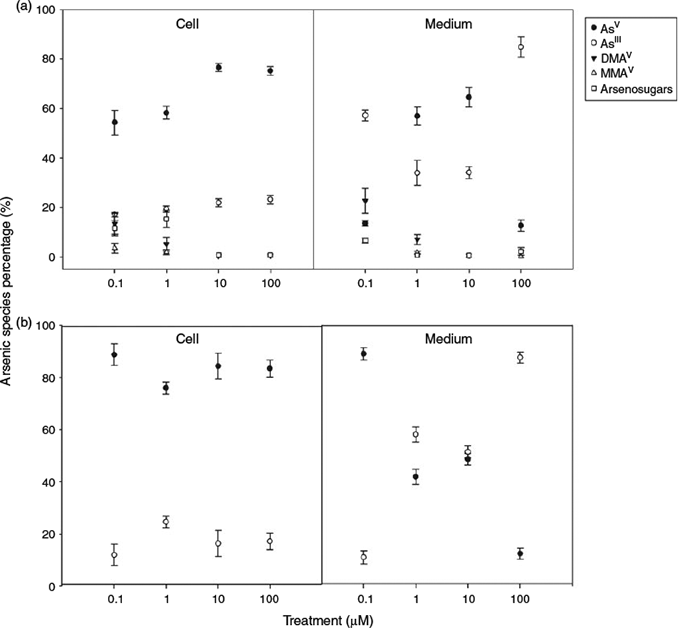
Arsenic speciation in the culture medium was investigated after the cyanobacteria were cultured for 3 weeks (Fig. 1). In Synechocystis ΔarsM medium, only inorganic As was detected for all treatments, with AsIII being the predominant species (>50 %) in the medium initially containing AsV at concentrations of 1, 10 or 100 µM. For the medium from the Synechocystis WT cultures, however, organoarsenicals (methylarsonic acid (MA) + DMA + arsenosugars) were significant at the 0.1 µM AsV exposure constituting 34 % of the total arsenic (Fig. 1). At the high exposures, AsIII was the dominant species, and organoarsenicals were either not detected or present at only trace levels.
Arsenic accumulation in cells
The arsenic concentrations in Synechocystis WT and Synechocystis ΔarsM exposed to various AsV concentrations are summarised in Table S1 of the Supplementary material. Both Synechocystis types showed a strong capability to accumulate arsenic from the medium, although Synechocystis ΔarsM cells accumulated slightly less arsenic than did Synechocystis WT cells. Increasing arsenic concentration in the medium led to greater arsenic accumulation in the cells – as arsenic exposure increased from 0.1 to 100 µM AsV, the total arsenic concentration in Synechocystis WT cells increased from 31.3 to 1293 mg kg–1 whereas the total arsenic accumulated by Synechocystis ΔarsM cells increased from 10.1 to 1067 mg kg–1. However, relative concentration factors[21] (expressed as milligrams of As per kilogram of dry mass of cells per exposure concentration in milligrams per kilogram) decreased markedly at higher arsenic exposures. After exposure to AsV for 3 weeks, the concentration factors were 1505 for Synechocystis WT and 486 for Synechocystis ΔarsM in the 0.1 µM AsV treatment, whereas for the 100 µM AsV treatment, the concentration factors were only 62 and 51 for Synechocystis WT and Synechocystis ΔarsM.
Fractionation of arsenic in Synechocystis
The total arsenic of the cyanobacteria cells was fractionated into water-soluble, lipid-soluble, and residue arsenic by extracting and partitioning between chloroform and water. Only the Synechocystis WT produced significant amounts of lipid-soluble arsenic, and the relative proportions decreased with increasing arsenic exposure (Table S1). For example, at a low exposure of 0.1 µM AsV, ~35 % of the total arsenic was lipid-soluble whereas at high exposure of 100 µM AsV, only ~3 % was lipid-soluble. Arsenolipids constituted 27 % of the total arsenic in Synechocystis WT cells treated with 1 µM AsV; the percentage of arsenolipids reduced to 6 % when the exposure level was 10 µM AsV. These data are consistent with the arsenolipid biosynthesis pathway being overloaded at higher AsV exposures. The lipid-soluble arsenic compounds were further fractionated into non-polar and polar arsenolipids by partitioning between hexane and methanol. No arsenic was found in the hexane layer thereby demonstrating that Synechocystis sp. PCC 6803 was not able to produce non-polar arsenolipids.
AsV dominates in water-soluble arsenic of cells
Arsenic biotransformation in Synechocystis sp. PCC 6803 cells exposed to different AsV concentrations was investigated (Fig. 1). For Synechocystis ΔarsM cells cultured under all treatments, only AsIII and AsV were found in the cells. In contrast, the cells from Synechocystis WT showed significant amounts of organoarsenicals, particularly at the low AsV exposures. The concentrations of arsenic species in the cells over the range of exposures were 54–77 % AsV, 17–23 % AsIII, 0.4–13 % DMA, 0.4–3.5 % MA, and 0.7–15 % arsenosugars. Organoarsenic compounds accounted for 28.5 % of the total As species for 0.1 µM AsV treatment, whereas, the proportion of organoarsenicals was reduced to 1.5 % for the higher AsV treatments (≥10 µM).
In subsequent replicate experiments, Synechocystis WT and Synechocystis ΔarsM, were individually cultured in quadruplicate. The simple methylated arsenicals MA and DMA, and three arsenosugars (glycerol, phosphate and sulfonate; see Supplementary material, Fig. S1 for structures) were produced only by Synechocystis WT. Synechocystis ΔarsM did not produce any detectable organoarsenic species (<10 µg As kg–1). The amounts of organoarsenicals produced by Synechocystis WT expressed as micrograms of As per kilogram (mean ± s.d., n = 4) of dry cells were: MA (155 ± 28); DMA (215 ± 91); glycerol arsenosugar (54 ± 17); phosphate arsenosugar (675 ± 246) and sulfonate arsenosugar (389 ± 144). The sulfate arsenosugar was present in Synechocystis WT, but only at trace, non-quantifiable levels (<20 µg As kg–1). The amounts of the organoarsenic compounds in Synechocystis, expressed as a percentage of the AsV content, were: MA (0.8 %), DMA (1.2 %), glycerol arsenosugar (0.25 %), phosphate arsenosugar (3.5 %), and sulfonate arsenosugar (2.0 %). These values are highly dependent on the extraction method, and refer to the relative amounts of arsenicals extracted into water. For example, when CH3OH/H2O (9 : 1, v/v) was used as extractant, the organoarsenic species in the extract predominated over the AsV owing to the poor methanol solubility of AsV.
Synechocystis sp. PCC 6803 produces arsenosugar phospholipids
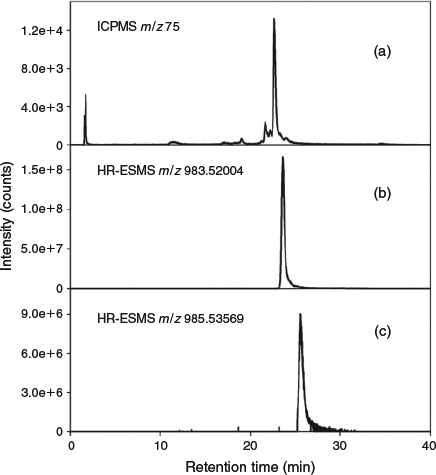
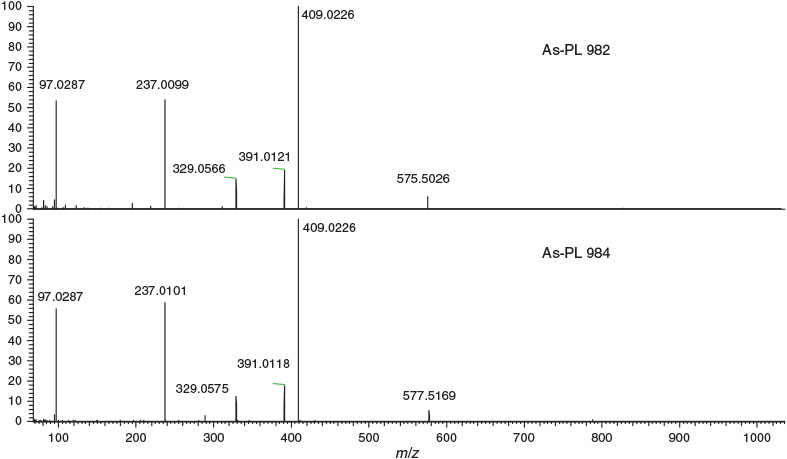
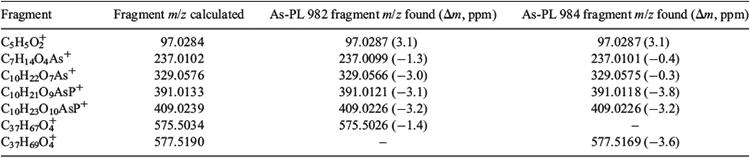
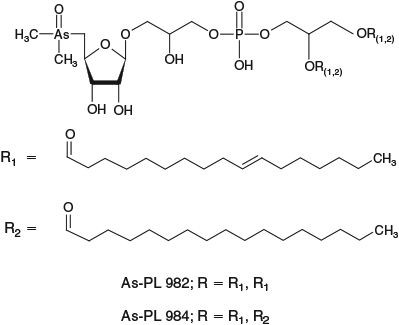
For identification of arsenic compounds in the lipid fraction of Synechocystis WT, a clean-up procedure with silica was invoked. HPLC/ICPMS analysis of the post-silica lipid fraction from Synechocystis WT showed the presence of a major arsenolipid and several minor ones (Fig. 2a); this chromatographic behaviour was similar to that found previously for arsenosugar phospholipids.[13] Investigation by HPLC/ESIMS revealed that the major arsenic compound seen by HPLC/ICPMS had [M + H]+ 983, and a minor compound had [M + H]+ 985 (Fig. 2a, b). Accurate mass measurements suggested the molecular formulas C47H88O14AsP (Anal. Calc. for [M + H]+ 983.5200; found: 983.5182, Δm = –1.8 ppm) and C47H90O14AsP (Anal. Calc. for [M + H]+ 985.5357; found: 985.5324, Δm = –3.3 ppm). Moreover, the isotope patterns from these ions matched exactly the simulated patterns for these molecular formulas (see Supplementary material, Figs S2, S3). MSMS performed on these two ions identified the fragments corresponding to the deacylated phosphate arsenosugar and the acylated glycerol group (Fig. 3, Table 1). Collectively, these data strongly support the assignment of the two structures as shown in Fig. 4.
|
|
|
|
Discussion
Arsenic in the model organism cyanobacterium Synechocystis sp. PCC 6803 was distributed between water- and lipid-soluble components. The majority of the extractable arsenic in cells was AsV for all treatments with smaller amounts of AsIII, DMAV, MAV, and arsenosugars being identified in wild type cells. Compared to other studies[4,22] with cultures of Synechocystis sp. PCC 6803, our results showed less DMAV or arsenosugars when cells were exposed to 100 μM AsV. These differences may be due to sample extraction methods. Yin et al.[22] prepared samples using 1 % HNO3 and microwave treatments, in which the original arsenic species were chemically degraded to DMAV.[23] Miyashita et al.[4] extracted samples by sonication with water. Arsenosugars may be degraded to DMAV during sonication.[2] Miyashita et al.[4] cultured Synechocystis sp. PCC 6803 for 24 h, and found that the level of phosphate arsenosugar was low and fluctuated throughout the experiment. This variability might be because phosphate arsenosugar can be derived, at least in part, from the degradation of arsenosugar phospholipids. In that regard, arsenosugar phospholipids might be the actual target arsenic compounds synthesised by algae.[13]
Reduction and efflux of arsenic are of considerable significance in arsenic detoxification by microorganisms.[24] The results from our study illustrated the capacity of the cyanobacterium Synechocystis sp. PCC 6803 to perform arsenic uptake, reduction and excretion. Synechocystis sp. PCC 6803 has a robust ability to accumulate arsenic, but a low ability to synthesise organoarsenicals (Table 1). The ability of Synechocystis to accumulate arsenic decreased with increasing arsenic concentration in the medium. For example, the bio-concentration factor decreased from 1505 to 62 when arsenic exposure increased from 0.1 to 100 μM (Table 1) for Synechocystis WT. Similar results have also been found for the green alga Chlorella vulgaris,[25,26] the marine cyanobacterium Phormidium sp.[27] and the freshwater fish, Tilapia mossambica.[28] In contrast, the uptake of arsenic by the two marine algae Fucus spiralis and Ascophyllum nodosum remained constant at external concentrations of AsV exceeding 1000 μg kg–1.[29]
Synechocystis WT was able to change the species of arsenic in the medium through absorbing AsV and subsequently excreting arsenic in a reduced form (at higher AsV exposure) or in organic forms (at low AsV exposure). For Synechocystis ΔarsM, which lacks the ability to methylate arsenic, the majority of the arsenic in the medium was AsIII. Microbial uptake of AsV can be mediated by the phosphate transport system owing to the chemical similarity of arsenate to phosphate.[30,31] The cyanobacterium took up AsV after exposure to various concentrations of AsV, and the arsenic was subsequently reduced to AsIII by ArsC.[32,33] AsIII is more toxic than AsV for it can impair physiological function by binding thiol groups in proteins.[24] To counteract the deleterious effects of AsIII, Synechocystis sp. PCC 6803 evolved with three resistance strategies, including AsIII re-oxidation,[22] arsenic methylation into less toxic species and active extrusion of AsIII from the cells by ArsB, an AsIII carrier. Previous studies[34–36] have shown that organisms could alleviate AsIII toxicity by extruding AsIII into their external environment. In this study, more AsIII was observed in the medium than in cells when the cyanobacterium was exposed to AsV, indicating that AsIII efflux might be the most important step among the three arsenic detoxification strategies.
The organoarsenicals occur in a wide range of marine and freshwater environments where they play a key role in arsenic cycling. Marine algae could transform AsV into organoarsenic compounds (MA, DMA, arsenosugars and arsenolipids) in seawater low in phosphate. The levels of arsenic in seawater normally range between 0.5 and 2 μg kg–1,[37] values comparable to phosphate levels in low phosphate waters. Low concentrations of phosphate were found to be a primary factor regulating the transformation of AsV by algae.[38] Possibly, marine algae may utilise arsenic in place of P to form membrane lipids.[13] Freshwater cyanobacteria synthesise lower quantities of organoarsenicals than do marine algae, perhaps because of the higher levels of phosphate in freshwater. Our study showed that the biosynthesis of organoarsenicals is not the primary detoxification pathway in Synechocystis sp. PCC 6803 at high AsV exposure. In the process of evolution, freshwater cyanobacteria retained several arsenic transformation pathways of marine algae, but may ultimately utilise arsenic oxidation, reduction and extrusion as the major detoxification pathways to save energy.
Although Synechocystis matched marine algae by being able to biosynthesise arsenosugar phospholipids, it differed in that the major arsenolipid was not the palmitic acid derivative found in marine algae.[12–14] Rather, the major compound contained saturated and unsaturated 17-carbon groups on the glycerol moiety. More data are required to determine if these differences have some chemotaxonomic significance.
In summary, our study showed that when exposed to AsV the model organism Synechocystis sp. PCC 6803 produces arsenosugar phospholipids, and that ArsM is a required enzyme in the biosynthesis of these lipids. We hope that our investigation with Synechocystis sp. PCC 6803 will stimulate more studies on the biosynthesis of arsenolipids, leading to an understanding of the possible function of these unusual lipids in biological systems.
Acknowledgements
The authors research is supported by the National Natural Science Foundation of China (31070101), the Ministry of Science and Technology of China (2011DFB91710), and the Austrian Science Fund (FWF) project number 23761-N17. The authors thank NAWI Graz and the Styrian Government for supporting the Graz Central Laboratory–Metabolomics. Dr Kenneth Jensen gave valuable advice on the interpretation of mass spectrometric data.
References
[1] K. A. Francesconi, Current perspectives in arsenic environmental and biological research. Environ. Chem. 2005, 2, 141.| Current perspectives in arsenic environmental and biological research.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVCjsrbF&md5=636a54db8909387d076195f7f77418b6CAS |
[2] S. Miyashita, S. Fujiwara, M. Tsuzuki, T. Kaise, Rapid biotransformation of arsenate into oxo-arsenosugars by a freshwater unicellular green alga, Chlamydomonas reinhardtii. Biosci. Biotechnol. Biochem. 2011, 75, 522.
| Rapid biotransformation of arsenate into oxo-arsenosugars by a freshwater unicellular green alga, Chlamydomonas reinhardtii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltF2lsL8%3D&md5=0a43e84cb7b1bbc4aaad93448d1e83efCAS | 21389618PubMed |
[3] S. Miyashita, M. Shimoya, Y. Kamidate, T. Kuroiwa, O. Shikino, S. Fujiwara, K. A. Francesconi, T. Kaise, Rapid determination of arsenic species in freshwater organisms from the arsenic-rich Hayakawa River in Japan using HPLC-ICP-MS. Chemosphere 2009, 75, 1065.
| Rapid determination of arsenic species in freshwater organisms from the arsenic-rich Hayakawa River in Japan using HPLC-ICP-MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltFWltLk%3D&md5=58cb051006e75cd1a9b226558f144598CAS | 19203781PubMed |
[4] S. Miyashita, S. Fujiwara, M. Tsuzuki, T. Kaise, Cyanobacteria produce arsenosugars. Environ. Chem. 2012, 9, 474.
| Cyanobacteria produce arsenosugars.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslSmtrrF&md5=440bbf9f9d60bd4122d8586dcf2fe168CAS |
[5] A. Geiszinger, W. Goessler, D. Kuehnelt, K. A. Francesconi, W. Kosmus, Determination of arsenic compounds in earthworms. Environ. Sci. Technol. 1998, 32, 2238.
| Determination of arsenic compounds in earthworms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXktVyiu7c%3D&md5=dd1cba55b06dbc7d6f2da2c2e8e07dd1CAS |
[6] G. Lunde, Analysis of arsenic in marine oils by neutron activation. Evidence of arseno organic compounds. J. Am. Oil Chem. Soc. 1968, 45, 331.
| Analysis of arsenic in marine oils by neutron activation. Evidence of arseno organic compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1cXktV2isrk%3D&md5=a447755f62f6f38b722cf2e1eece95a8CAS | 5655522PubMed |
[7] A. Rumpler, S. Edmonds, M. Katsu, K. B. Jensen, W. Goessler, G. Raber, H. Gunnlaugsdottir, K. A. Francesconi, Arsenic-containing long-chain fatty acids in cod-liver oil: a result of biosynthetic infidelity? Angew. Chem. Int. Ed. 2008, 47, 2665.
| Arsenic-containing long-chain fatty acids in cod-liver oil: a result of biosynthetic infidelity?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkvFejsL4%3D&md5=88e2bccbeae33966b484906813471103CAS |
[8] K. O. Amayo, A. Petursdottir, C. Newcombe, H. Gunnlaugsdottir, A. Raab, E. M. Krupp, J. Feldmann, Identification and quantification of arsenolipids using reversed-phase HPLC coupled simultaneously to high-resolution ICPMS and high-resolution electrospray MS without species-specific standards. Anal. Chem. 2011, 83, 3589.
| Identification and quantification of arsenolipids using reversed-phase HPLC coupled simultaneously to high-resolution ICPMS and high-resolution electrospray MS without species-specific standards.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFaktL4%3D&md5=87d42103f36c3d43af7866b8d006b1d0CAS | 21446761PubMed |
[9] G. Raber, S. Khoomrung, M. S. Taleshi, J. S. Edmonds, K. A. Francesconi, Identification of arsenolipids with GC/MS. Talanta 2009, 78, 1215.
| Identification of arsenolipids with GC/MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjtVSnsL8%3D&md5=65a56bf5ae1ed0244eb0f098aac7bb5bCAS | 19269497PubMed |
[10] U. Arroyo-Abad, J. Mattusch, S. Mothes, M. Möder, R. Wennrich, M. P. Elizalde-González, F. M. Matysik, Detection of arsenic-containing hydrocarbons in canned cod liver tissue. Talanta 2010, 82, 38.
| Detection of arsenic-containing hydrocarbons in canned cod liver tissue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXntVyktbo%3D&md5=a9eb877c83b76e27a5bb4b9978103ee4CAS | 20685432PubMed |
[11] M. S. Taleshi, J. S. Edmonds, W. Goessler, M. J. Ruiz-Chancho, G. Raber, K. B. Jensen, K. A. Francesconi, Arsenic-containing lipids are natural constituents of sashimi tuna. Environ. Sci. Technol. 2010, 44, 1478.
| Arsenic-containing lipids are natural constituents of sashimi tuna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptlymtg%3D%3D&md5=865e377839f32f31da0e188a0fd5eb9aCAS | 20099809PubMed |
[12] M. Morita, Y. Shibata, Isolation and identification of arseno-lipid from a brown alga, Undaria pinnatifida (Wakame). Chemosphere 1988, 17, 1147.
| Isolation and identification of arseno-lipid from a brown alga, Undaria pinnatifida (Wakame).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXkvVOlt78%3D&md5=268978f4517d2f3f0bf97a06e7ee7aebCAS |
[13] S. García-Salgado, G. Raber, R. Raml, C. Magnes, K. A. Francesconi, Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgae. Environ. Chem. 2012, 9, 63.
| Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgae.Crossref | GoogleScholarGoogle Scholar |
[14] A. Raab, C. Newcombe, D. Pitton, R. Ebel, J. Feldmann, Comprehensive analysis of lipophilic arsenic species in a brown alga (Saccharina latissima). Anal. Chem. 2013, 85, 2817.
| Comprehensive analysis of lipophilic arsenic species in a brown alga (Saccharina latissima).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXit1ehsr0%3D&md5=0716fece190df0381e234715ffd3a833CAS | 23394220PubMed |
[15] T. Kaneko, A. Tanaka, S. Sato, H. Kotani, T. Sazuka, N. Miyajima, M. Sugiura, S. Tabata, Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. I. Sequence features in the 1 Mb region from map positions 64 % to 92 % of the genome. DNA Res. 1995, 2, 153.
| Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. I. Sequence features in the 1 Mb region from map positions 64 % to 92 % of the genome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXptVCrt7o%3D&md5=8e469423f8c448bae5b9f90d72e9493cCAS | 8590279PubMed |
[16] T. Kaneko, S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Suquira, S. Sadamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, S. Tabata, Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996, 3, 109.
| Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xmtl2qsLc%3D&md5=23b24fff87389e795878d3914f5758c7CAS | 8905231PubMed |
[17] J. G. Williams, Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 1988, 167, 766.
| Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXhvVKqs7g%3D&md5=9d6688b8a949fa3e967263dbf33f43a2CAS |
[18] R. Rippka, J. Deruelles, J. B. Waterbury, M. Herdman, R. Y. Stanier, Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1.
| Generic assignments, strain histories and properties of pure cultures of cyanobacteria.Crossref | GoogleScholarGoogle Scholar |
[19] D. Thomson, W. Maher, S. Foster, Arsenic and selected elements in intertidal and estuarine marine algae, southeast coast, NSW, Australia. Appl. Organomet. Chem. 2007, 21, 396.
| Arsenic and selected elements in intertidal and estuarine marine algae, southeast coast, NSW, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXms1yit7s%3D&md5=71e7b70107e310574ae0c95fa0523870CAS |
[20] J. Navratilova, G. Raber, S. J. Fisher, K. A. Francesconi, Arsenic cycling in marine systems: degradation of arsenosugars to arsenate in decomposing algae, and preliminary evidence for the formation of recalcitrant arsenic. Environ. Chem. 2011, 8, 44.
| Arsenic cycling in marine systems: degradation of arsenosugars to arsenate in decomposing algae, and preliminary evidence for the formation of recalcitrant arsenic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjs1GlsLc%3D&md5=fc9c0433f676675f071cfc450fa09bebCAS |
[21] A. Geiszinger, W. Goessler, S. N. Pedersen, K. A. Francesconi, Arsenic biotransformation by the brown macroalga Fucus serratus. Environ. Toxicol. Chem. 2001, 20, 2255.
| Arsenic biotransformation by the brown macroalga Fucus serratus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XitlWitQ%3D%3D&md5=e6682d92059a06f67289d901751e1434CAS | 11596758PubMed |
[22] X. X. Yin, L. H. Wang, R. Bai, H. Huang, G. X. Sun, Accumulation and transformation of arsenic in the blue-green alga Synechocystis sp. PCC 6803. Water Air Soil Pollut. 2012, 223, 1183.
| Accumulation and transformation of arsenic in the blue-green alga Synechocystis sp. PCC 6803.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xis1Onsrg%3D&md5=8bac986faadffcc3da5cf5eba51ebcf9CAS |
[23] B. G. Gamble, J. A. Shoemaker, X. Wei, C. A. Schwegel, J. T. Creed, An investigation of the chemical stability of arsenosugars in simulated gastric juice and acidic environments using IC–ICP-MS and IC-ESI-MS/MS. Analyst 2002, 127, 781.
| An investigation of the chemical stability of arsenosugars in simulated gastric juice and acidic environments using IC–ICP-MS and IC-ESI-MS/MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XktVegsb8%3D&md5=5952bcdb431c23b90a0fc4359f98cafcCAS |
[24] R. Mukhopadhyay, B. P. Rosen, L. T. Phung, S. Silver, Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol. Rev. 2002, 26, 311.
| Microbial arsenic: from geocycles to genes and enzymes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmtVamsrg%3D&md5=922fd923fc3fabaf5827e0ad74c8b8e8CAS | 12165430PubMed |
[25] S. Maeda, S. Nakashima, T. Takeshita, S. Higashi, Bioaccumulation of arsenic by freshwater algae and the application to the removal of inorganic arsenic from an aqueous phase. Part II. By Chlorella vulgaris isolated from arsenic-polluted environment. Sep. Sci. Technol. 1985, 20, 153.
| Bioaccumulation of arsenic by freshwater algae and the application to the removal of inorganic arsenic from an aqueous phase. Part II. By Chlorella vulgaris isolated from arsenic-polluted environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXitFKmsrw%3D&md5=3e8db7e003d7a8cf051c83b31df349faCAS |
[26] L. A. Murray, A. Raab, I. L. Marr, J. Feldmann, Biotransformation of arsenate to arsenosugars by Chlorella vulgaris. Appl. Organomet. Chem. 2003, 17, 669.
| Biotransformation of arsenate to arsenosugars by Chlorella vulgaris.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmvFOrtL4%3D&md5=31a86bf4c3f1d252ed5150109fa509cdCAS |
[27] S. Maeda, S. Fujita, A. Ohki, I. Yoshifuku, S. Higashi, T. Takeshita, Arsenic accumulation by arsenic-tolerant freshwater blue-green alga (Phormidium sp.). Appl. Organomet. Chem. 1988, 2, 353.
| Arsenic accumulation by arsenic-tolerant freshwater blue-green alga (Phormidium sp.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXktlCn&md5=c4cb0f25efcf1e5e3f8ba675ad73e83eCAS |
[28] A. Suhendrayatna, A. Ohki, T. Nakajima, S. Maeda, Studies on the accumulation and transformation of arsenic in freshwater organisms II. Accumulation and transformation of arsenic compounds by Tilapia mossambica. Chemosphere 2002, 46, 325.
| Studies on the accumulation and transformation of arsenic in freshwater organisms II. Accumulation and transformation of arsenic compounds by Tilapia mossambica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXpt1ahsLs%3D&md5=033d14f4c10d7d184b04ba1ae6c39a13CAS |
[29] D. W. Klumpp, Accumulation of arsenic from water and food by Littorina littoralis and Nucella lapillus. Mar. Biol. 1980, 58, 265.
| Accumulation of arsenic from water and food by Littorina littoralis and Nucella lapillus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXkt1aiuw%3D%3D&md5=c6b986c9e0d6d53eba2282a63ec100d1CAS |
[30] D. S. Tawfik, R. E. Viola, Arsenate replacing phosphate – alternative life chemistries and ion promiscuity. Biochemistry 2011, 50, 1128.
| Arsenate replacing phosphate – alternative life chemistries and ion promiscuity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlSmt78%3D&md5=99eb9fa4c3bb56dace71ee807b8eca4eCAS | 21214261PubMed |
[31] A. A. Meharg, M. R. Macnair, Suppression of the high affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J. Exp. Bot. 1992, 43, 519.
| Suppression of the high affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XisFSis7Y%3D&md5=ba36585b0724f08998760c9d73271179CAS |
[32] L. López-Maury, A. M. Sánchez-Riego, J. C. Reyes, F. J. Florencio, The glutathione/glutaredoxin system is essential for arsenate reduction in Synechocystis sp. strain PCC 6803. J. Bacteriol. 2009, 191, 3534.
| The glutathione/glutaredoxin system is essential for arsenate reduction in Synechocystis sp. strain PCC 6803.Crossref | GoogleScholarGoogle Scholar | 19304854PubMed |
[33] L. López-Maury, F. J. Florencio, J. C. Reyes, Arsenic sensing and resistance system in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2003, 185, 5363.
| Arsenic sensing and resistance system in the cyanobacterium Synechocystis sp. strain PCC 6803.Crossref | GoogleScholarGoogle Scholar | 12949088PubMed |
[34] B. P. Rosen, M. G. Borbolla, A plasmid-encoded arsenite pump produces arsenite resistance in Escherichia coli. Biochem. Biophys. Res. Commun. 1984, 124, 760.
| A plasmid-encoded arsenite pump produces arsenite resistance in Escherichia coli.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhvVCqsw%3D%3D&md5=e0f16a8b5dd36056ade662152d38a4c9CAS | 6391481PubMed |
[35] H. Bhattacharjee, B. P. Rosen, Arsenic metabolism in prokaryotic and eukaryotic microbes, in Molecular Microbiology of Heavy Metals (Eds D. Nies, S. Silver) 2007, pp. 371–406 (Springer: New York).
[36] X. Y. Xu, S. P. McGrath, F. J. Zhao, Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol. 2007, 176, 590.
| Rapid reduction of arsenate in the medium mediated by plant roots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVSisbbO&md5=4f61fd0b2a2c63ba0a5d119cc808a573CAS | 17692074PubMed |
[37] M. O. Andreae, Distribution and speciation of arsenic in natural waters and some marine algae. Deep-Sea Res. 1978, 25, 391.
| Distribution and speciation of arsenic in natural waters and some marine algae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXks1Cqsb4%3D&md5=1d4f356c4b1a7717e90f56e4886440fcCAS |
[38] A. A. Benson, R. E. Summons, Arsenic accumulation in Great Barrier Reef invertebrates. Science 1981, 211, 482.
| Arsenic accumulation in Great Barrier Reef invertebrates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXpslCjug%3D%3D&md5=96afdcc70b2c21136e57e6c9317aa873CAS | 7455685PubMed |