Effect of episodic rainfall on aqueous metal mobility from historical mine sites
Magaly Valencia-Avellan A E , Rebecca Slack B , Anthony Stockdale C and Robert John George Mortimer DA water@leeds, School of Geography, University of Leeds, Leeds LS2 9JZ, UK.
B The Royal Horticultural Society, Harlow Carr, Crag Lane, Beckwithshaw, Harrogate, North Yorkshire, HG3 1QB, UK.
C School of Earth and Environment, University of Leeds, Leeds LS2 9JZ, UK.
D School of Animal, Rural and Environmental Sciences, Nottingham Trent University, Brackenhurst Campus, Southwell, Nottinghamshire, NG25 0QF, UK.
E Corresponding author. Email: magyvalencia80@gmail.com
Environmental Chemistry 14(8) 469-475 https://doi.org/10.1071/EN17133
Submitted: 23 July 2017 Accepted: 14 November 2017 Published: 21 March 2018
Journal compilation © CSIRO 2018 Open Access CC BY-NC-ND
Environmental context. Episodic extreme rainfall events may affect metal dynamics in rivers flowing within historical metal mining areas. This study provides an analysis of the water chemistry and geochemical processes associated with mobilisation of metals during episodic rainfall events. Findings could be used to assess the environmental quality of streams draining spoil waste areas with similar geochemical conditions, and thereby be used to guide future management strategies.
Abstract. The increasing frequency and magnitude of episodic rainfall events may affect historical metal mining areas by remobilisation and deposition of metal-rich sediments and enhancing metal-rich run off, impacting river water quality. This study assesses the effects of episodic rainfall in a Carboniferous headwater catchment contaminated by historical Pb and Zn mining. Comprehensive hourly water chemistry measurements combined with modelling using PHREEQC, WHAM/Model VII and WHAM-FTOX were used in this assessment. For the episodic event, we measured flow increases from a baseline of 0.05 to 2.12 m3 s−1 at peak flow. Changes in metal concentration were most marked for ephemeral tributary, with Pb increasing from a baseline concentration of 55 μg L−1 to a peak of 576 μg L−1. Behaviour for Pb showed great affinity to form organic complexes or bind to colloidal Al and Fe oxides, whereas for Zn and the tributary flowing subsurface a more complex behaviour was observed. For example, the dissolution of secondary metal carbonate minerals (e.g. smithsonite (ZnCO3)) is likely constrained by higher concentrations of carbonate and bicarbonate derived from increased bedrock weathering under flow conditions induced by episodic rainfall. The abundance of secondary mineral sources and circumneutral pH present during episodic rainfall are important factors controlling the mobilisation of Pb and Zn. Furthermore, episodic rainfall events could enhance metal toxicity but there are aggravating and mitigating factors that depend on site-specific chemical changes. Overall, this study highlighted the complexity of metal mobility and toxicity during these events.
Changes in climate affect the hydrological cycle (either from natural variability or anthropogenically induced changes). Extreme events like droughts and floods may have significant impacts on the quantity and quality of water bodies, with direct or indirect effects on ecosystems.[1–3] In river systems, headwater streams, ephemeral ponds and ditches are most sensitive to climatic variations because severe alterations in temperature and precipitation could affect evapotranspiration, flow, soil moisture and groundwater recharge.[4–6] Floods can be effective agents of contaminant dispersal by triggering primary pollution or remobilisation of deposited material.[7] Primary pulses are produced by major rainstorms after periods of extended drought, when soluble salts concentrated on the surface of mine wastes and spoils are quickly dissolved and flushed into receiving surface waters.[8] Runoff from watersheds draining metal-mining areas is considered an acute problem as exposed tailings produce metal-rich overflow that is often redistributed downstream.[9,10] Once in sediments, metals can remain in floodplains for decades to centuries until their remobilisation by erosion, creating a long-lived contamination problem.[11]
For the UK, there is good evidence that the frequency of longer (5–10 days) extreme rainfall events is increasing (e.g. Fowler and Kilsby[12] and reference therein); this is supported by modelling predictions of record regional winter rainfalls in future years.[13] Additionally, recent years have seen new 24- and 48-h rainfall records being established for the British Isles.[14,15] In terms of shorter timescale and higher-intensity episodic events, there is some evidence of increasing frequency;[16] however, there is an urgent need for more detailed meta-analyses. Anecdotally, there is evidence to suggest an increase in such events, such as water flowing from a cliff in the UK Yorkshire Dales for ‘the first time in living memory’.[17]
In the UK, the floods of autumn 2000 provided clear evidence of the potential effects of extreme weather events on diffuse pollution in formerly mined river catchments.[18,19] The excessive flux of metals generated by the legacy of metal mining has significantly increased the levels of metal pollution in many catchments.[20] Consequently, these catchments represent a challenge in achieving ‘good ecological and chemical status’ as required by the European Union Water Framework Directive (WFD) or other national legislation. In addition to climate-influenced factors, the dispersal of metals depends on the dynamics of each catchment.[21] For example, where rivers flow over limestone bedrock, the pH is buffered, exerting controls over mineral solubility, metal transport and bioavailability.[22,23] However, rainfall (e.g. acid rain) may produce shifts in the pH, affecting the buffering capacity of the river chemistry, allowing desorption of metals from sediments or soils.[24] Mitigating the impact of metals on water quality requires knowledge of the biogeochemistry of metal in solid and solution phases, as well as a local understanding of major sources of pollutant metals.[2,25–28]
The limited primary data about episodic high rainfall limits our understanding of its effect on metal dynamics. Thus, the present study seeks to evaluate the effects of extreme episodic rainfall on Pb and Zn dynamics at differing points within a catchment. Results are compared with those obtained from a previous comprehensive annual analysis of monthly surveys under non-episodic conditions.[22,23] From this assessment, the mobilisation and potential toxicity of dissolved metals under flow conditions derived from episodic rainfall will provide important information regarding key rainfall-induced processes in the behaviour of pollutant metals, supporting future risk mitigation strategies in similar catchments.
This study focusses on the Hebden Beck catchment in northern England, where the underlying geology consists of a succession of sandstone and mudstone (Millstone Grit) and carboniferous limestone (Fig. S1, available as Supplementary material to this paper). This headwater stream is affected by metal contamination derived from historical lead mining, where galena (PbS), sphalerite (ZnS) and barite (BaSO4) were the profitable minerals. The catchment chemistry has previously been characterised[22] and assessed with respect to water quality and ecotoxicology under seasonal conditions.[23] Three sampling stations were selected for the present study: an ephemeral tributary (ET) located in the most upstream zone, draining an area of mine spoil wastes; a perennial tributary (PT) of an underground mine channel located in middle of the stream; and a site located downstream on the main channel (MC) 2.27 km from the River Wharfe confluence and adjacent to a flow gauging station (Table S1, available as Supplementary material). Higher metal concentrations in upstream reaches may tend to be diluted by inputs from non-affected tributaries lower down the catchment; however, inputs from mine adits will also have an effect, depending on their chemical characteristics.
UK Meteorological Office daily rainfall data[29] were obtained for Pateley Bridge Ravens Nest (54°04′01.2″N 1°46′01.2″W) in order to present local seasonal drought and rainfall events for the month of August 2016 (>12 mm during high-rainfall days) (Fig. S2). The sampling campaign began at 1230 hours on 19 August and ended after a maximum of 96 h at 1250 hours on 23 August 2016. Automated water samplers (model 6712, Teledyne-ISCO, Lincoln, Nebraska, USA) were set to collect at 1- or 2-h intervals in each site. Subsamples from each time interval were filtered in the field using syringe filters (0.45 µm, polyethersulfone – hydrophilic, Sartorious) for metals (Pb, Ba, Cd, Sr, As, Zn, Cu, Co, Ni, Fe, Mn, Al), and major ions (Ca2+, Mg2+, Cl−, NO3− SO42−) and for dissolved organic and inorganic carbon (DIC and DOC) (0.45-µm nylon–polypropylene, Avonchem). Sample handling and in situ water quality measurements (temperature and pH) followed previously used methods described in Valencia-Avellan et al.[22] (detailed in the Supplementary material). Hourly rainfall data from Grimwith reservoir (code: 62046; 54°04′16.4″N 1°54′47.7″W; 3 km east of Hebden Beck) and flow data from a gauging station in the main river channel (code: F1960; 54°04′27.8″N 1°57′48.5″W) were obtained from the UK Environment Agency for the dates 18 to 23 August (Fig. S3). Several flow stages were identified; low-flow (LF), base-flow (BF), peak-flow (PF) and post peak-flow (PPF). Full details are in the Supplementary material. In ET, the first four measurements were under stagnant conditions, with the sampler positioned in a small pool near the confluence with the main channel. In PT and MC, data were collected from well-mixed areas with continuous flow. For assessing the influence of flow in the sampling sites, flow measurements from MC were used for ET and PT. Metal analysis was conducted via Inductively coupled plasma mass spectrometry (Thermo Fisher iCAPQc) using Certified Reference Material (SLRS-5, National Research Council, Canada) as a quality control and with specific limits of detection (Pb: 0.47 μg L−1, Ba: 1.45 μg L−1, Cd: 0.03 μg L−1, Sr: 1.09 μg L−1, As: 0.02 μg L−1, Zn: 2.28 μg L−1, Cu: 0.05 μg L−1, Co: 0.014 μg L−1, Ni: 0.06 μg L−1, Fe: 1.43 μg L−1, Mn: 0.15 μg L−1, Al: 1.77 μg L−1). In addition, two replicates per site and four field blanks were taken for procedural quality control. Activity of metals and solubility of relevant mineral phases were calculated from saturation of mineral forms through dissolution reactions using PHREEQC (version 3)[30] and the WATEQ4F database[31] where site-specific chemical data and major physicochemical parameters were considered as input data. Changes in DOC concentrations during episodic events may influence the concentration of metal–organic complexes; therefore, to investigate chemical speciation, we applied the Windermere Humic Aqueous Model (WHAM/Model VII).[32] Both this model and PHREEQC are used independently to evaluate different aspects of the water chemistry. In addition, toxicity of metal mixtures including protons (H+) and metals (Al3+, Zn2+, Pb2+ and Cu2+) was estimated using WHAM-FTOX.[33] Procedures for WHAM/Model VII and WHAM-FTOX were followed as described in Valencia-Avellan et al.,[23] detailed in the Supplementary material.
Results indicated that the catchment hydrology responded rapidly to rainfall. Flow levels started to increase within 2 h after the first period of rain (5 mm h−1), indicating that runoff processes are likely occurring at the surface (overland flow) and subsurface (interflow) (Fig. S3). This rapid response has been reported when rainfall exceeds the infiltration capacity of the soil, especially in peat soils where the water infiltration is low.[34]
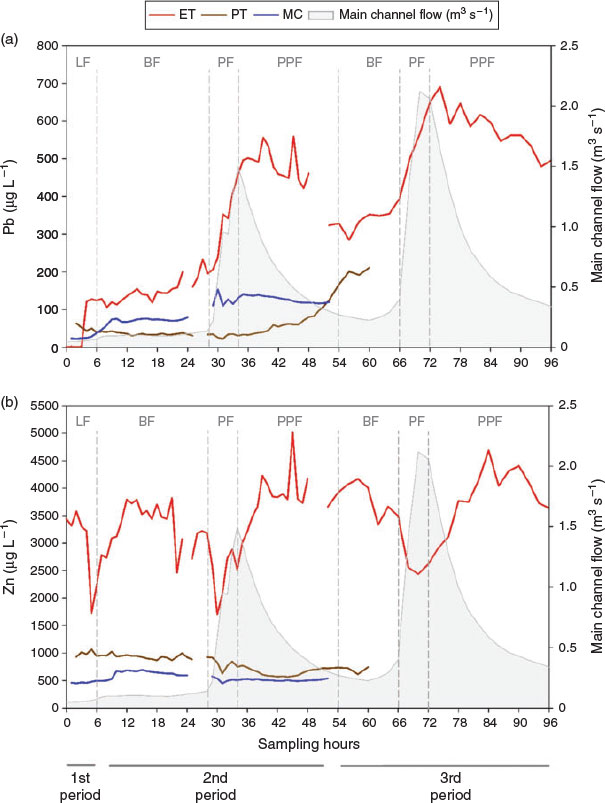
Contrasting responses were identified for Pb and Zn under increasing flow conditions (Fig. S4). Concentrations of Pb are directly influenced by flow variations, showing a peak concentration of 690.3 μg L−1, corresponding with peak flow in ET. Contrary to ET, a slow rise in concentrations was evident in PT, with maximum values (211.7 μg L−1) reached at PPF. The delayed response to episodic rainfall in PT could be due to the subsurface runoff (interflow) percolating through mine channels.[35] Peak Pb concentrations in MC (153.7 μg L−1) showed similar responses to ET but were lower in magnitude (Fig. 1a). Zinc concentrations showed an inverse relationship with episodic rainfall and flow variations (Fig. 1b), although concentrations in ET showed mixed patterns. For instance, in ET at PF, concentrations immediately decreased (55 %) but increased at PPF (194 %). This can be associated with large masses of mine wastes exposed to water producing high concentrations of dissolved Zn.[36] Less marked dilutions (47 and 35 %) were identified in sites PT and MC, perhaps due to discrete rainwater inflow in PT, and MC having the lowest concentrations of the three sites. Maximum Zn concentrations were measured in ET (5017 μg L−1) at PPF, followed by lower concentrations in PT (1069 μg L−1) at low and base flow, and in MC (694 μg L−1) at BF. The observed trends suggest that metal dilution is occurring during episodic rainfall as metal concentrations reported by Valencia-Avellan et al. [22] from a non-impacted site and MC reflected lower concentrations than ET. In addition, results revealed greater relative increases in concentrations of dissolved Pb than dissolved Zn. Furthermore, kinetics factors are likely to influence metal behaviour, and this will be related to specific mineral dissolution kinetics and other water chemistry variables such as DIC, which will directly affect the saturation indices of the minerals and will be site-specific.
|
Runoff did not alter the typical circumneutral conditions of the catchment (pH 7–8.1). Thus, geochemical signatures of each tributary are controlling their major ion chemistries (e.g. SO42−, DIC and DOC) (Table S2).[22,37] Correlation analysis showed diverse relationships at different flow conditions. Generally, stronger positive correlations were present in ET between Pb, Zn and SO42−, likewise in PT, where positive correlations were identified mainly between Zn, DIC and SO42−, whereas in MC, Pb showed strong correlations with DOC (Table S3).
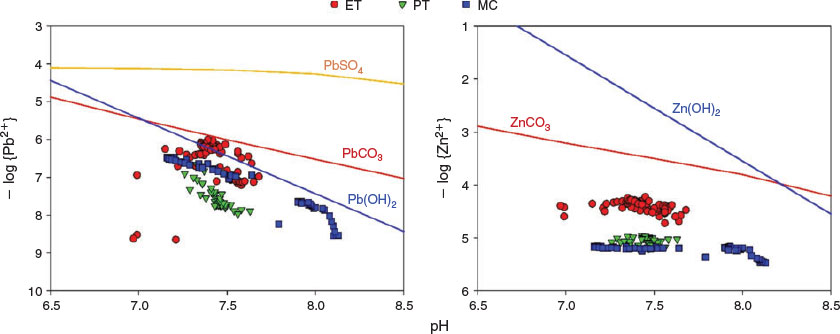
Runoff has an effect on weathering processes such as erosion of spoil heaps, size sorting of spoil particles and promoting mineral dissolution through exposure to undersaturated solution. Our results suggest that Pb and Zn concentrations are regulated by the presence and dissolution kinetics of cerussite (PbCO3) and smithsonite (ZnCO3), and dilution by water not contacting soluble minerals. Fig. 2 shows closer saturation of Pb than Zn, particularly in ET[22] and MC. This may be due to the slower dissolution kinetics of the smithsonite versus cerussite.[38] Previous work in the catchment from Valencia-Avellan et al.[22] indicated that sites with longer residence times (e.g. source pond) showed free ion concentrations closer to the theoretical saturation levels. Results agreed with prior studies[22,23,39] and emphasise the importance of smithsonite and cerussite in controlling metal mobility and transport.[40]
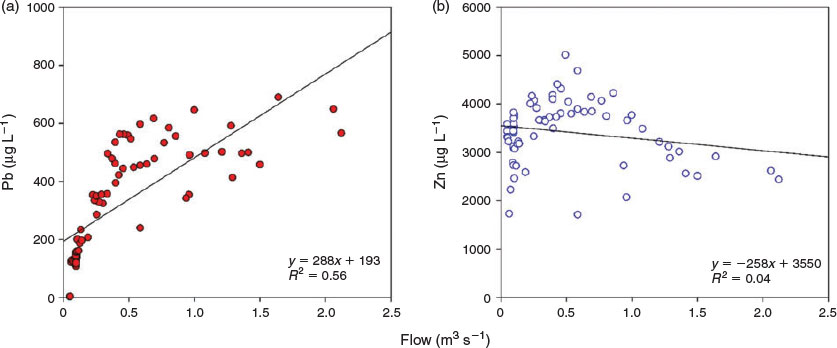
The ET data highlight the significance of extensive open spoil as a metal source during episodic flow. Relationship between flow and Pb concentrations appears initially to be linear but is less clear at flows >0.5 m3 s−1. For Zn concentrations, the behaviour was different, potentially owing to kinetic factors (Fig. 3). This suggests a bimodal response of metals to flow, possible related to the exponential fall of DIC concentrations with increasing flow in ET and MC (Fig. S5), influencing the relative saturation of the secondary minerals, principally for cerussite. Further evidence is given in Fig. S6a as the saturation index (SI) of cerussite increased with flow. Figs S6b and S7 show that the SI of smithsonite decreased when flow increased. The behaviour of smithsonite in PT agreed with studies from Pokrovsky et al.[38] regarding geochemical processes under circumneutral conditions, indicating that high flow may increase the concentrations of carbonate and bicarbonate ions, which act as inhibitors of smithsonite dissolution, reducing the equilibrium activity for Zn (Fig. S5 and Fig. 2). Although this behaviour is observed for PT potentially owing to its subsurface nature, the other sites (ET and MC) presented a reduction in the DIC with flow, coupled with an associated decrease in the SIs of smithsonite (Figs S5 and S6b). Carrol et al.[41] also stated the relevance of carbonate minerals in the sorption of metals as they function as long-term sinks, competing with other reactive minerals such as iron oxyhydroxides. Previous study of metal speciation in this catchment has identified that Pb is strongly associated with both particulate and colloidal Fe and Al oxides, whereas Zn is present mainly as inorganic complexes.[22,23] Thus, during high flood periods, the resuspension of sediments may increase the total concentrations of Pb (from particulate forms, and thus potentially available for the dissolved phase), whereas this will be a minor potential source of Zn.[42,43]
|
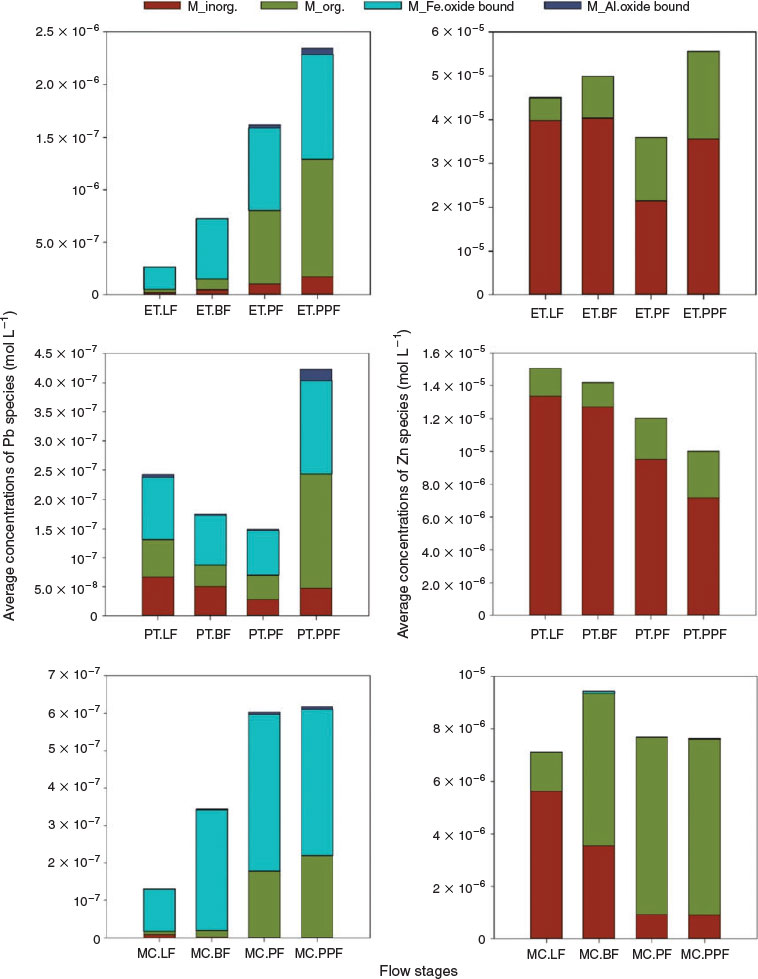
At all sites, the highest concentrations of Pb–organic complexes and oxide-bound Pb were calculated to occur at PPF. This could be explained by increased concentrations of DOC, and colloidal Fe and Al oxides (as predicted by WHAM/Model VII) at peak and post peak flow (Table S2). Nordstrom et al.[44] indicated that sorption isotherms of metals onto freshly formed Fe and Al oxides at circumneutral pH occurred in the following sequence: Pb > Cu > Zn. This low affinity of Zn for surface sorption to oxide minerals is predicted here from the abundance of Zn inorganic complexes (Fig. 4). In addition, estimations in the absence and presence of oxides precipitated show similar concentrations for Zn complexes (inorganic and organic) whereas Pb has a large tendency to be bound to DOC and Al and Fe oxides (Fig. S8) and agreeing with speciation of Zn and Pb reported by Valencia-Avellan et al.[23]
The impacts of climatic events on contaminant transport and water quality are complex because of localised effects. Current results are consistent with previous research on the transport and pollution of Pb occurring through particulate material, enhanced by episodic rainfall.[45] Likewise Gozzard et al.[46] reported the attenuation effect on Zn pollution during peak rainfall. Comparison of metal mobilisation under episodic rainfall conditions revealed that local conditions such as the abundance of secondary mineral sources and circumneutral pH are key factors controlling the mobilisation of Pb and Zn, whereas flow variations could be an enhancing factor, particularly for increasing the concentration of dissolved Pb. Thus, these results provide insight into other catchments where streams drain mine spoil under similar conditions.
The potential adverse effects of episodic-related chemistry changes in macroinvertebrate species was estimated using WHAM-FTOX, a model that assumes (based on evidence) that humic acid can be used as a proxy for organism metal binding. The model relates this metal binding to toxicity with parameterised values for the toxic potency of each metal and is based on field macroinvertebrate species data.[33] We considered two previously fitted conditions for calculating total toxicity function values (Total_FTOX): (i) Total_FTOX ≤ 2.33, no toxic effects occur; and (ii) Total_FTOX > 2.33, toxicity reflects a risk of diminished macroinvertebrate species diversity, until no species are predicted to be present at a value of 5.20. Under flow conditions induced by episodic rainfall, toxicity function was calculated in ET showing values from ~3.2 to ~3.6 that would reflect a reduction in species diversity. Lower toxicity function values predicted in PT (≤2.1) and MC (≤1.7) suggest no toxic effects from dissolved metals (Figs S9, S10, S11). For ET, calculations during episodic flow showed that short-term fluctuations in metal concentrations are slightly reflected in changes to the predicted acute toxicity to aquatic organisms. This will be due to several factors that have contrasting effects on the FTOX value, including those that would be expected to reduce the value, such as increases in DOC and a decrease in Zn concentrations, and those that may increase the value, such as higher Pb, lower Ca and Fe (competing ion) concentrations and lower concentrations of other ligands (DIC and SO42−) (Table S2). Future work would be better focussed on sites with predicted ecologically harmful levels of metal and perennial tributaries from spoil runoff areas, including a better understanding of climate variability during seasonal as well as episodic flow conditions.
In conclusion, episodic rainfall events are not altering the circumneutral conditions of the catchment. Concentrations of Pb showed a greater relative response to flow changes than Zn. The effect of surface and subsurface flow in the transport of metal–organic complexes and the dissolution of metal carbonate minerals will likely regulate the mobilisation of Pb and Zn. Metal toxicity can be influenced by site-specific chemical interactions occurring during episodic events.
Supplementary material
Supplementary material and data in support of this paper can be found on the journal’s website.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research was funded by the National Secretariat for Higher Education, Sciences, Technology and Innovation of Ecuador (SENESCYT in Spanish) through a PhD scholarship granted to Magaly Valencia-Avellan in June 2013 (grant no. 82-ARG5–2013). We thank the UK Environmental Agency for providing rainfall data and flow records. Richard Grayson (School of Geography, University of Leeds) is greatly thanked for lending the automatic samplers and the training for setting the sampling program. We also thank the anonymous reviewers for their helpful comments.
References
[1] T. Hrdinka, O. Novický, E. Hanslík, M. Rieder, Possible impacts of floods and droughts on water quality J. Hydro-Environment Res. 2012, 6, 145.| Possible impacts of floods and droughts on water qualityCrossref | GoogleScholarGoogle Scholar |
[2] J.-H. Park, E. Inam, M. H. Abdullah, D. Agustiyani, L. Duan, T. T. Hoang, K.-W. Kim, S. D. Kim, M. H. Nguyen, T. Pekthong, V. Sao, A. Sarjiya, S. Savathvong, S. Sthiannopkao, J. K. Syers, W. Wirojanagud, Implications of rainfall variability for seasonality and climate-induced risks concerning surface water quality in east Asia J. Hydrol. 2011, 400, 323.
| Implications of rainfall variability for seasonality and climate-induced risks concerning surface water quality in east AsiaCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjs1Sgsb0%3D&md5=ffc2ba8836180b828c2229d53c8df9e2CAS |
[3] S. A. Foulds, P. A. Brewer, M. G. Macklin, W. Haresign, R. E. Betson, S. M. E. Rassner, Flood-related contamination in catchments affected by historical metal mining: an unexpected and emerging hazard of climate change Sci. Total Environ. 2014, 476–477, 165.
| Flood-related contamination in catchments affected by historical metal mining: an unexpected and emerging hazard of climate changeCrossref | GoogleScholarGoogle Scholar |
[4] P. Whitehead, R. Wilby, R. Battarbee, M. Kernan, A. J. Wade, A review of the potential impacts of climate change on surface water quality Hydrol. Sci. J. 2009, 54, 101.
| A review of the potential impacts of climate change on surface water qualityCrossref | GoogleScholarGoogle Scholar |
[5] M. A. Ayers, D. M. Wolock, G. J. McCabe, L. E. Hay, G. D. Tasker, Sensitivity of Water Resources in the Delaware River Basin to Climate Variability and Change 1994 (US Government Printing Office: Reston, Virginia).
[6] P. J. Chapman, B. Reynolds, H. S. Wheater, Hydrochemical changes along stormflow pathways in a small moorland headwater catchment in mid-Wales, UK J. Hydrol. 1993, 151, 241.
| Hydrochemical changes along stormflow pathways in a small moorland headwater catchment in mid-Wales, UKCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXjtVKksLs%3D&md5=0aaad086ba9072537c278ac4a0c1a755CAS |
[7] J. R. Miller, P. J. Lechler, M. Desilets, The role of geomorphic processes in the transport and fate of mercury in the Carson River Basin, west-central Nevada Environ. Geol. 1998, 33, 249.
| The role of geomorphic processes in the transport and fate of mercury in the Carson River Basin, west-central NevadaCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhvVKksrc%3D&md5=4ebd0be7ea5865f3389390f0960f7356CAS |
[8] D. K. Nordstrom, Hydrogeochemical processes governing the origin, transport and fate of major and trace elements from mine wastes and mineralized rock to surface waters Appl. Geochem. 2011, 26, 1777.
| Hydrogeochemical processes governing the origin, transport and fate of major and trace elements from mine wastes and mineralized rock to surface watersCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVamu7nF&md5=5756e58a5ed81ece55e0706452300fe9CAS |
[9] C. F. Myers, J. Meek, S. Tuller, A. Weinberg, Non-point sources of water pollution J. Soil Water Conserv. 1985, 40, 14.
[10] U. Förstner, G. T. Wittmann, Metal Pollution in the Aquatic Environment. 1981 (Springer Verlag: Berlin).
[11] D. Ciszewski, T. M. Grygar, A review of flood-related storage and remobilization of heavy metal pollutants in river systems Water Air Soil Pollut. 2016, 227, 239.
| A review of flood-related storage and remobilization of heavy metal pollutants in river systemsCrossref | GoogleScholarGoogle Scholar |
[12] H. Fowler, C. Kilsby, A regional frequency analysis of United Kingdom extreme rainfall from 1961 to 2000 Int. J. Climatol. 2003, 23, 1313.
| A regional frequency analysis of United Kingdom extreme rainfall from 1961 to 2000Crossref | GoogleScholarGoogle Scholar |
[13] V. Thompson, N. J. Dunstone, A. A. Scaife, D. M. Smith, J. M. Slingo, S. Brown, S. E. Belcher, High risk of unprecedented UK rainfall in the current climate Nat. Commun. 2017, 8, 107.
[14] S. Burt, M. McCarthy, M. Kendon, J. Hannaford, Cumbrian floods, 5/6 December 2015 Weather 2016, 71, 36.
| Cumbrian floods, 5/6 December 2015Crossref | GoogleScholarGoogle Scholar |
[15] P. Eden, How the Heaviest Rainfall Happened 2009. Available at http://news.bbc.co.uk/1/hi/magazine/8376031.stm [Verified 20 October 2017].
[16] A. J. Robson, Evidence for trends in UK flooding Philos. Trans. R. Soc. A 2002, 360, 1327.
| Evidence for trends in UK floodingCrossref | GoogleScholarGoogle Scholar |
[17] British Broadcasting Corporation (BBC) Storm Desmond: Malham Cove Waterfall Flows Again Amid Heavy Rain 2015. Available at http://www.bbc.co.uk/news/uk-england-york-north-yorkshire-35026529 [Verified 20 October 2017].
[18] I. A. Dennis, M. G. Macklin, T. J. Coulthard, P. A. Brewer, The impact of the October–November 2000 floods on contaminant metal dispersal in the River Swale catchment, North Yorkshire, UK Hydrol. Processes 2003, 17, 1641.
| The impact of the October–November 2000 floods on contaminant metal dispersal in the River Swale catchment, North Yorkshire, UKCrossref | GoogleScholarGoogle Scholar |
[19] M. Macklin, P. Brewer, K. Hudson-Edwards, G. Bird, T. Coulthard, I. Dennis, P. Lechler, J. Miller, J. Turner, A geomorphological approach to the management of rivers contaminated by metal mining Geomorphology 2006, 79, 423.
| A geomorphological approach to the management of rivers contaminated by metal miningCrossref | GoogleScholarGoogle Scholar |
[20] W. M. Mayes, H. A. B. Potter, A. P. Jarvis, Inventory of aquatic contaminant flux arising from historical metal mining in England and Wales Sci. Total Environ. 2010, 408, 3576.
| Inventory of aquatic contaminant flux arising from historical metal mining in England and WalesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnvVemsL0%3D&md5=b6452a306cb62c68fdc1244bfa103e6cCAS |
[21] J. R. Miller, The role of fluvial geomorphic processes in the dispersal of heavy metals from mine sites J. Geochem. Explor. 1997, 58, 101.
| The role of fluvial geomorphic processes in the dispersal of heavy metals from mine sitesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjvVWjsbc%3D&md5=b1a89854c8b84803174a6ad6f0f6d7e1CAS |
[22] M. Valencia-Avellan, R. Slack, A. Stockdale, R. J. G. Mortimer, Understanding the mobilisation of metal pollution associated with historical mining in a carboniferous upland catchment Environ. Sci. Process. Impacts 2017, 19, 1061.
| Understanding the mobilisation of metal pollution associated with historical mining in a carboniferous upland catchmentCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXhtF2rsrbI&md5=f1bb93c3412c24bd172ef03bf2d309f9CAS |
[23] M. Valencia-Avellan, R. Slack, A. Stockdale, R. J. G. Mortimer, Evaluating water quality and ecotoxicology assessment techniques using data from a lead and zinc effected upland limestone catchment Water Res. 2018, 128, 49.
| Evaluating water quality and ecotoxicology assessment techniques using data from a lead and zinc effected upland limestone catchmentCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXhslWqsrvJ&md5=96819ccdd842dc0d0846e714f3d459e0CAS |
[24] W. Salomons, Environmental impact of metals derived from mining activities: processes, predictions, prevention J. Geochem. Explor. 1995, 52, 5.
| Environmental impact of metals derived from mining activities: processes, predictions, preventionCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXkvFKisLc%3D&md5=4569c45f5cf95b9cbf094130eec59870CAS |
[25] J. H. Lambing, D. A. Nimick, T. E. Cleasby, Short-term variation of trace-element concentrations during base flow and rainfall runoff in small basins, August 1999 U.S. Geol. Surv. Prof. Pap. 2004, 1652, 267.
[26] L. Wirt, K. J. Leib, D. J. Bove, M. A. Mast, J. B. Evans, G. P. Meeker, Determination of Chemical-Constituent Loads During Base-Flow and Storm-Runoff Conditions Near Historical Mines in Prospect Gulch, Upper Animas River Watershed, South-western Colorado 1999 (US Department of the Interior, US Geological Survey: Colorado, US).
[27] P. Sandén, S. Karlsson, A. Düker, A. Ledin, L. Lundman, Variations in hydrochemistry, trace metal concentration and transport during a rain storm event in a small catchment J. Geochem. Explor. 1997, 58, 145.
| Variations in hydrochemistry, trace metal concentration and transport during a rain storm event in a small catchmentCrossref | GoogleScholarGoogle Scholar |
[28] J. M. Nieto, A. M. Sarmiento, M. Olías, C. R. Canovas, I. Riba, J. Kalman, T. A. Delvalls, Acid mine drainage pollution in the Tinto and Odiel rivers (Iberian Pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva Estuary Environ. Int. 2007, 33, 445.
| Acid mine drainage pollution in the Tinto and Odiel rivers (Iberian Pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva EstuaryCrossref | GoogleScholarGoogle Scholar |
[29] UK Meteorological Office. Pateley Bridge weather forecast. Available at http://www.metoffice.gov.uk/public/weather/forecast/gcwg8jffz [Verified 1 July 2016].
[30] D. L. Parkhurst, C. A. J. Appelo, in Water Resources Investigations Report 1999 (US Geological Survey: Earth Science Information Center, Open-File Reports Section) Available at http://pubs.er.usgs.gov/publication/wri994259 [Verified 15 July 2016].
[31] J. W. Ball, D. K. Nordstrom, User’s Manual for WATEQ4F, with Revised Thermodynamic Data Base and Test Cases for Calculating Speciation of Major, Trace, and Redox Elements in Natural Waters. Open-File Report 91–183. 1991 (US Geological Survey: California, US).
[32] E. Tipping, S. Lofts, J. Sonke, Humic Ion-Binding Model VII: a revised parameterisation of cation-binding by humic substances Environ. Chem. 2011, 8, 225.
| Humic Ion-Binding Model VII: a revised parameterisation of cation-binding by humic substancesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptVWrsL0%3D&md5=2424e2ce2d3e3ab7a6de47c3b4b825e9CAS |
[33] A. Stockdale, E. Tipping, S. Lofts, S. J. Ormerod, W. H. Clements, R. Blust, Toxicity of proton–metal mixtures in the field: linking stream macroinvertebrate species diversity to chemical speciation and bioavailability Aquat. Toxicol. 2010, 100, 112.
| Toxicity of proton–metal mixtures in the field: linking stream macroinvertebrate species diversity to chemical speciation and bioavailabilityCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFGjurbM&md5=7390c61d83822f3c95b82e856d60eaadCAS |
[34] J. Holden, T. Burt, Infiltration, runoff and sediment production in blanket peat catchments: implications of field rainfall simulation experiments Hydrol. Processes 2002, 16, 2537.
| Infiltration, runoff and sediment production in blanket peat catchments: implications of field rainfall simulation experimentsCrossref | GoogleScholarGoogle Scholar |
[35] K.-P. Seiler, J. R. Gat, Groundwater Recharge from Run-off, Infiltration and Percolation. 2007 (Springer Science & Business Media: Dordrecht, The Netherlands).
[36] K. Hudson-Edwards, Sources, mineralogy, chemistry and fate of heavy metal-bearing particles in mining-affected river systems Mineral. Mag. 2003, 67, 205.
| Sources, mineralogy, chemistry and fate of heavy metal-bearing particles in mining-affected river systemsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjslansb4%3D&md5=89f23c30cb9ff6154930c5ab1f497afdCAS |
[37] A. Jones, M. Rogerson, G. Greenway, H. A. B. Potter, W. M. Mayes, Mine water geochemistry and metal flux in a major historic Pb–Zn–F orefield, the Yorkshire Pennines, UK Environ. Sci. Pollut. Res. Int. 2013, 20, 7570.
| Mine water geochemistry and metal flux in a major historic Pb–Zn–F orefield, the Yorkshire Pennines, UKCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1eltbvP&md5=ec91b0de73aec25787264f15781a07e1CAS |
[38] O. Pokrovsky, J. Schott, Surface chemistry and dissolution kinetics of divalent metal carbonates Environ. Sci. Technol. 2002, 36, 426.
| Surface chemistry and dissolution kinetics of divalent metal carbonatesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhsVWhsg%3D%3D&md5=93703f8a085c130106439bd58ab77574CAS |
[39] C. Tame, K. A. Hudson-Edwards, H. A. B. Potter, Weathering of Zinc-(Zn)-bearing mine wastes in a neutral mine drainage setting, Gunnerside Gill, Yorkshire Procedia Earth Planet. Sci. 2017, 17, 284.
| Weathering of Zinc-(Zn)-bearing mine wastes in a neutral mine drainage setting, Gunnerside Gill, YorkshireCrossref | GoogleScholarGoogle Scholar |
[40] J. M. Hammarstrom, K. S. Smith, Geochemical and mineralogic characterization of solids and their effects on waters in metal-mining environments, in Progress on Geoenvironmental Models for Selected Mineral Deposit Types (Eds R. R. Seal II, N. K. Foley) 2002, pp. 8–54 (US Geological Survey: Reston, VA).
[41] S. A. Carroll, P. A. O’Day, M. Piechowski, Rock–water interactions controlling zinc, cadmium, and lead concentrations in surface waters and sediments, US Tri-State Mining District. 2. Geochemical interpretation Environ. Sci. Technol. 1998, 32, 956.
| Rock–water interactions controlling zinc, cadmium, and lead concentrations in surface waters and sediments, US Tri-State Mining District. 2. Geochemical interpretationCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXht1Oisbo%3D&md5=257f68e0c2b944c864fed9d0315ddd7dCAS |
[42] J. F. Elder, Metal Biogeochemistry in Surface-Water Systems; A Review of Principles and Concepts. Circular 1013. 1988 (US Geological Survey: Denver, CO).
[43] S. Nagorski, T. McKinnon, J. Moore, Seasonal and storm-scale variations in heavy metal concentrations of two mining-contaminated streams, Montana, USA J. Phys. IV 2003, 107, 909.
| 1:CAS:528:DC%2BD3sXlsFCrtbs%3D&md5=d41ea8aed4576dff453969a32aa4eb19CAS |
[44] D. K. Nordstrom, R. Fernández Rubio, Efflorescent salts and their effects on water quality and mine plugging Mine Water Environ. 1999, II, 543.
[45] C. Neal, A. J. Robson, H. A. Jeffery, M. L. Harrow, M. Neal, C. J. Smith, H. P. Jarvie, Trace element inter-relationships for the Humber rivers: inferences for hydrological and chemical controls Sci. Total Environ. 1997, 194-195, 321.
| Trace element inter-relationships for the Humber rivers: inferences for hydrological and chemical controlsCrossref | GoogleScholarGoogle Scholar |
[46] E. Gozzard, W. M. Mayes, H. A. B. Potter, A. P. Jarvis, Seasonal and spatial variation of diffuse (non-point) source zinc pollution in a historically metal mined river catchment, UK Environ. Pollut. 2011, 159, 3113.
| Seasonal and spatial variation of diffuse (non-point) source zinc pollution in a historically metal mined river catchment, UKCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFehtLnL&md5=92f623919b8f0889b24264a9c13ebbe0CAS |