Phloem hydrostatic pressure relates to solute loading rate: a direct test of the Münch hypothesis
Nick Gould A E , Michael R. Thorpe B , Olga Koroleva C and Peter E. H. Minchin DA Department of Biology, University of Waikato, Private Bag 3105, Hamilton, New Zealand.
B Chemistry Department, Brookhaven National Laboratory, Upton, NY 11973, USA.
C John Innes Centre, Colney Lane, Norwich, UK.
D ICG-III Phytosphaere, Forschungszentrum Jülich, D-52425, Jülich, Germany.
E Corresponding author. Current address: HortResearch, Ruakura Research Centre, Private Bag 3123, Hamilton, New Zealand. Email: ngould@hortresearch.co.nz
Functional Plant Biology 32(11) 1019-1026 https://doi.org/10.1071/FP05036
Submitted: 17 February 2005 Accepted: 3 August 2005 Published: 28 October 2005
Abstract
According to the Münch hypothesis, a flow of solution through the sieve tubes is driven by a hydrostatic pressure difference between the source (or collection) phloem and the sink (or release) phloem. A high hydrostatic pressure is maintained in the collection phloem by the active uptake of sugar and other solutes, with a concomitant inflow of water. A lower pressure is maintained in the release phloem through solute unloading. In this work we directly test the role of solute uptake in creating the hydrostatic pressure associated with phloem flow. Solute loading into the phloem of mature leaves of barley and sow thistle was reduced by replacing the air supply with nitrogen gas. Hydrostatic pressure in adjacent sieve elements was measured with a sieve-element pressure probe, a cell pressure probe glued to the exuding stylet of aphids that had been feeding from the phloem. Sieve element sap was sampled by aphid stylectomy; sap osmotic pressure was determined by picolitre osmometry and its sugar concentration by enzyme-linked fluorescence assays. Samples were taken with a time resolution of ~2–3 min. In accordance with Münch’s proposal a drop in osmotic and hydrostatic pressure in the source phloem following treatment of the source leaf with N2 was observed. A decrease in sugar concentration was the major contributor to the change in osmotic pressure. By observing these variables at a time resolution of minutes we have direct observation of the predictions of Münch.
Keywords: anoxia, aphid stylectomy, Münch hypothesis, phloem loading, phloem pressure probe.
Introduction
According to the Münch hypothesis solution flow through the phloem occurs down a pressure gradient from the source (e.g. photosynthetic tissue) to sinks (e.g. fruits, seeds, root / shoot meristem). Hydrostatic pressure is generated at the source, in the collection phloem, through the uptake of solutes into the sieve element / companion cell complex (SE / CC). The major solutes within the phloem are sugars (most commonly sucrose), amino nitrogen compounds and potassium (Smith and Milburn 1980; Lalonde et al. 2003). In many species, sugars, amino acids and potassium appear to be actively loaded into the SE / CC from the apoplasm against a concentration gradient, via membrane-bound transporters, driven by H+ ATPase (Patrick et al. 2001). Solute uptake creates a water potential gradient between the apoplasm and SE / CC, down which water flows into the SE / CC, generating the hydrostatic pressure gradient between the source and sink phloem that drives solution flows. Thus in the Münch hypothesis the loading of solutes into the phloem is central to the mechanism driving the transport of these solutes. This theory was used to explain changes in phloem flow dynamics observed with perturbations in phloem loading (Thorpe et al. 1979; Thorpe and Minchin 1987, 1988; Minchin et al. 2002).
This work sets out to examine the much quoted but never directly tested hypothesis that the hydrostatic pressure required to drive solution through the phloem is generated by the loading of solutes into the collection phloem. Barley leaves (Hordeum vulgare; and all other C3 species so far tested) treated with replacement of air by nitrogen showed a reduced 11C export fraction, indicating reduced phloem loading (Thorpe et al. 1979; Grodzinski et al.1984; Thorpe and Minchin 1987, 1988; Minchin et al. 2002). In addition the nitrogen treatment also resulted in an increased 11C pathway transit time, a phenomenon explained by a reduced axial flow, as a result of a lower source-to-sink pressure gradient, brought about by the reduction in solute loading. Although Münch offered this hypothesis of pressure flow over 70 years ago (Münch 1930), very few studies have directly measured the phloem hydrostatic pressure (Hammel 1968; Wright and Fisher 1983; Gould et al. 2004a). By directly measuring the sieve tube hydrostatic pressure and photoassimilate transport within the translocation pathway, our aim is to directly test Münch’s theory of osmotically generated pressure-driven solution flow, by modifying solute loading and observing the coupled changes in sieve element osmotic and hydrostatic pressure. Solute loading was perturbed by an anoxic treatment on the source leaf, using N2 gas to ensure a completely anoxic environment within the leaf. Although phloem loading rate in barley (and all C3 plants tested to date) has been shown to be reduced in oxygen free air (nitrogen and carbon dioxide), in the light, oxygen-free air does not affect phloem loading in some species, due to the release of oxygen from photosynthesis (Thorpe and Minchin 1987).
Barley and sow thistle (Sonchus oleraceus) were chosen as our model species because they are easily infested by aphids (Rhopalosidium padi L. and Macrosiphum rosae L., respectively) with stylets that exude readily, giving direct access to the sieve tubes. The phloem pressure probe has been successfully used on both species (Gould et al. 2004a, b), and both plants have the C3 photosynthetic pathway for which phloem loading has shown to be reduced under anoxic conditions (Thorpe et al. 1979; Thorpe and Minchin 1987).
Materials and methods
Plant growth conditions
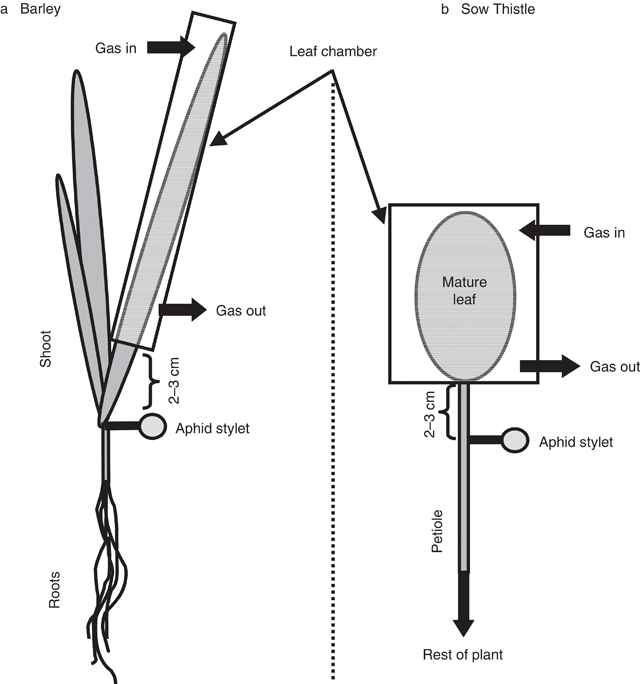
The experiments were carried out on 6-week-old sow thistle (Sonchus oleraceus L.) and 14-d-old barley (Hordeum vulgare L.) plants. Barley seeds were germinated in the dark on paper towels moistened with distilled water in Petri dishes. After 3 d, seedlings were placed on plastic mesh over an aerated solution of 0.5 mm CaCl2. After a further 3 d, the seedlings were transferred to 1-L pots containing aerated nutrient solution: 2 mm KNO3, 4 mm Ca(NO3)2, 1 mm NH4H2PO4, 1 mm (NH4)2HPO4, 1 mm MgSO4, 0.015 mm NaFeEDTA, 0.05 mm KCl, 0.002 mm MnSO4, 0.002 mm CuSO4, 0.0025 mm (NH4)6Mo7O24, 0.25 mm H3BO3, and then grown at a temperature of 22°C, relative humidity 50%, and irradiance of 800 μmol m–2 s–1 with a photoperiod of 16 h. The plants were used when they were ~2 weeks old, when the plant consisted of two mature leaves plus a third, which was still growing. Some 2–6 h before experimentation, a mature leaf was placed inside a clear perspex tubular leaf chamber (160 mm long, 10 mm diameter) and moist air was pumped into the chamber (Fig. 1a). Following at least 2 h acclimation, the air supply was replaced by N2 for 20 min before the air supply was returned.
|
The sow thistle seeds were germinated and grown individually, in 75-mm pots filled with potting compost, at a temperature of 22 / 18°C, 50% relative humidity, irradiance of 800 μmol m–2 s–1 and a photoperiod of 16 h. Plants were watered once every 2 d with tap water. The plants were used when they were ~6 weeks old during flowering, when growth had ceased in all leaves. Approximately 2–6 h before experimentation, a mature leaf was placed inside a leaf chamber (60 × 60 × 15 mm) constructed from a square Petri dish and moist air was pumped into the chamber (Fig. 1b). Following acclimation, the air supply was replaced by N2 for 20 min before the air supply was returned. To ensure that CO2 starvation did not have an effect on leaf water potential (and thus alter the water relations between the phloem and apoplast) leaf water potential was measured with a pressure bomb in both barley and sow thistle following 20 min of treatment with either N2, air or N2 gas plus 330 μmol mol–1 CO2. The results (not shown) showed no difference in leaf water potential between the three treatments.
Sieve element sap sampling and hydrostatic pressure measurements
Sieve element sap was collected from both plant species by aphid stylectomy (Fisher and Frame 1984). In barley plants, sap was collected from a stylet on the lamina of the mature leaf 2–3 cm below the leaf chamber. Aphids were held in a clip cage, stylets were cut with a radio-microcautery unit (Zapper, thorpes@xtra.co.nz), and lanolin was extruded onto the barley leaf to form a dam enclosing a freely exuding stylet. The dam was filled with water-saturated paraffin oil. In sow thistle, sap was collected from the petiole 2–3 cm from the leaf chamber. Approximately 10 aphids were caged onto the petiole with a modified critical-point drying capsule sold for electron microscopy (ProSciTech, Kelso, Qld; 1 cm3 volume). The aphid stylets were cut and, once a stylet was freely exuding, the chamber was flooded with paraffin oil. Sap samples were collected at intervals with a glass micropipette that had been filled with paraffin oil. The micropipettes were constructed from glass capillaries (1 mm O.D.; WPI Inc., Sarasota, FL) and a horizontal tip-puller (Model P-87, Sutter Instruments Co. Novato, CA). The tips of the micropipettes were broken to a diameter of ~1–5 μm.
To measure the hydrostatic pressure of a sieve element we used a method developed from that used by Wright and Fisher (1983); a glass micropipette was placed over an exuding excised stylet and sealed to the stylet with ethylcyanoacrylate adhesive (Selleys PTY limited, Padstow, NSW). The other end of the micropipette was connected via high pressure HPLC tubing to the pressure probe which consisted of a 3-MPa (100 mV) pressure transducer (RS Components Ltd, Auckland, N.Z.), a 50-μL syringe (SGE Australia Pty, Ltd, Ringwood, Vic.) driven by a stepping motor, and a valve for venting the system. The valve was left open during the gluing stage to allow sieve element sap to exude into the pressure probe. The whole system was filled with silicone oil (Dow Corning 200 / 10 cs fluid; BDH, Poole, UK) and was able to hold pressure above 3 MPa. To ensure a good bond between adhesive, micropipette and stylet, a small volume of air was left in the micropipette tip during the gluing stage. This air bubble usually dissolved at relatively low pressures (< 0.5 MPa). Once the micropipette had been sealed onto the stylet and sap was exuding into the glass micropipette, the pressure probe venting valve was closed. The pressure within the pressure probe was then increased, by injecting oil into the system from the syringe, until sap flow from the sieve element stopped. The pressure within the pressure probe then matched that within the sieve element. To demonstrate that flow had not stopped because of blockage, pressure within the system was reduced slightly after each measurement to check that sap flowed into the micropipette again. In a steady-state system (i.e. before N2 treatment) it was possible to make measurements at 1–2-min intervals. At non-steady-state, such as that experienced in this experiment after the N2 treatment, it can take longer to match the sieve element pressure; hence the time between measurements can increase to over 4 min. In addition, extra time between measurements can result when changes in treatment have to be administered (i.e. turning the N2 treatment on and off).
Owing to difficulties in providing an adequate seal onto the waxy surface of the barley leaf, the barley sieve tube hydrostatic pressure measurements were carried out on the roots, ~2–3 cm from the root base whilst the entire barley shoot was contained within the leaf chamber.
Sap analyses
The osmotic pressure of sieve element sap was measured using a freezing-point depression picolitre osmometer (University of Wales, Bangor, UK: a.d.tomos@bangor.ac.uk), following the method described by Malone et al. (1989). Sap sucrose concentration of the 2-nL samples was measured by enzyme-linked fluorescence assay (Jones et al. 1977).
Results
Sieve tube hydrostatic pressure
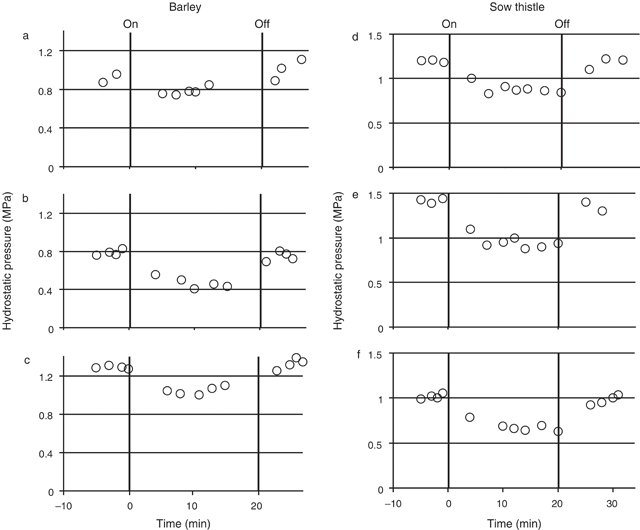
Transient hydrostatic pressure measurements were made in the sieve tubes of both the root of mature barley (five individual plants) and the petioles of mature sow thistle leaves (five individual plants) before, during and after the treatment of the source tissue with N2. For the barley the hydrostatic pressure before treatment with N2 ranged between individuals from 0.8 (± 0.007, se, n = 4) to 1.4 (± 0.059, se, n = 2) MPa. Following treatment of the source leaf with N2 gas, hydrostatic pressure decreased in all five plants (Fig. 2a–c; three plants showing typical values for all plants). The overall reduction in pressure was ~0.2–0.7 MPa (or ~14–45%). The pressure remained at the lower level for the 20-min treatment period. Removal of the N2 treatment resulted in an increase in hydrostatic pressure back to pre-treatment levels.
|
In sow thistle the mean hydrostatic pressure before treatment with N2 ranged from 1.0 (± 0.007, se, n = 4) to 1.5 (± 0.04, se, n = 3) MPa between individual plants. Following treatment of the source leaf with N2 gas, hydrostatic pressure decreased in all five plants (Fig. 2d–f; three plants showing typical values for all plants). The overall reduction in pressure was ~0.15–0.5 MPa (or ~13–43%). As in barley, the reduced sieve element pressure lasted until the N2 treatment was removed. Following removal of the treatment, the pressure returned to pre-treatment levels.
Barley: sieve element sap osmotic pressure and sucrose concentration
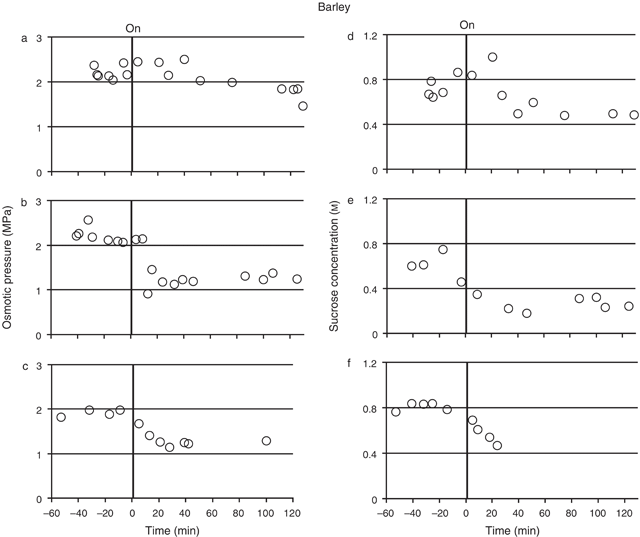
Both osmotic pressure and sucrose concentration were measured for the sieve element sap from mature barley leaves, sampled both before and during the treatment of the source leaf with N2 (Fig. 3). Transient measurements of sap osmotic pressure measurements were made in four plants (Fig. 3a–c; three plants showing typical values for all plants). Prior to the N2 treatment the osmotic pressure remained stable in each individual plant, the values for each of the four plants ranging from 1.90 (± 0.04, se, n = 4) to 2.60 (± 0.05, se, n = 7) MPa. Following treatment of the source leaf with N2 gas, sap osmotic pressure decreased, but not at the same rate in all four plants. In one plant (Fig. 3a) the reduction in osmotic pressure took over 60 min to occur, while in another the reduction started almost immediately (Fig. 3c). In the other two plants the reduction started between 0 and 30 min (Fig. 3b, for example). The overall reduction in sap osmotic pressure following N2 treatment was ~0.7–1.0 MPa (or ~30–45%).
|
Sucrose concentration was measured in three plants (Fig. 3d–f), using the same sap samples that were collected for the osmotic pressure measurements. The mean sap sucrose concentration for each of the plants, before N2 treatment, ranged from 0.60 m (± 0.06, se, n = 4) to 0.81 m (± 0.02, se, n = 5) across the plants. Following the N2 treatment, sap sucrose concentration decreased in all three plants, following a similar pattern to that of the osmotic pressure in the sap. For example, the lag in the osmotic pressure decrease observed in Fig. 3a in response to the N2 treatment was also observed in the sucrose concentration of the same sap (Fig. 3d). Likewise, the immediate response in osmotic pressure to the treatment observed in Fig. 3c was also observed in the sucrose concentration of the same sap (Fig. 3f). The overall reduction in sap sucrose concentration following N2 treatment was ~0.25–0.35 m (or 30–58%).
Sow thistle: sieve element sap osmotic pressure and sucrose concentration
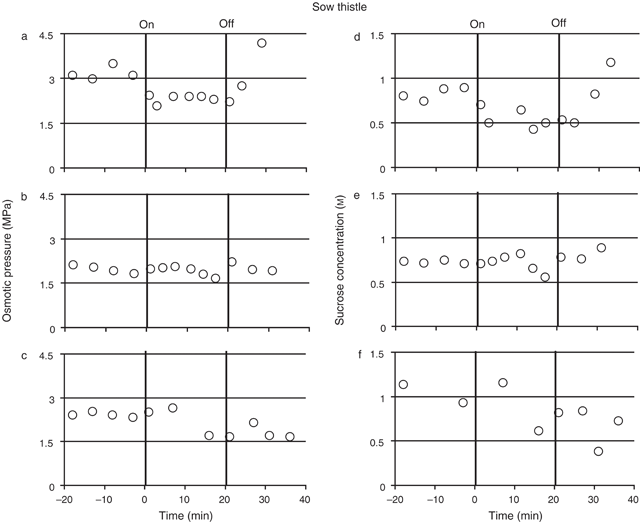
Osmotic pressure and sucrose concentrations of sieve element sap from the petioles of mature sow thistle leaves were measured in four plants before, during and after treating the source leaf with N2 (Fig. 4; measurements for three plants showing values typical for all plants). The mean osmotic pressures for each plant before the N2 treatment ranged from 2.0 MPa (± 0.07, se, n = 4) to 3.0 MPa (± 0.17, se, n = 5). Following the N2 treatment the osmotic pressure of the sap decreased by ~0.6– 0.7 MPa (or 20–30%) in all plants. The reduction occurred in less than 20 min after the start of treatment in all plants but the time varied from plant to plant (Fig. 4a–c for example). Within 5 min of the removal of the N2 treatment the osmotic pressure returned to pre treatment levels in three of the four plants. In the fourth plant the osmotic pressure remained at a lower level than the pre-treatment values for at least 16 min following the removal of the treatment (Fig. 4c).
|
The mean sucrose concentration for each plant before the N2 treatment ranged from 0.65 m (± 0.01, n = 5) to 1.40 m (± 0.1, n = 2). As observed with the barley, the sucrose concentration followed a similar pattern to the osmotic pressure, decreasing when the N2 treatment was begun and increasing following removal of the treatment. Following initiation of the N2 treatment sap sucrose concentration dropped by ~0.2–0.4 m (or 25–44%), although the time taken for the response to occur varied in all four plants (Fig. 4d–f for example). Following the removal of the N2 treatment the sucrose concentration increased in three of the four plants but remained at a decreased level in a fourth plant thus following the pattern of osmotic pressure in this plant (Fig. 4c, f).
Discussion
We set out to unequivocally test one of the fundamental requirements of the Münch hypothesis, that solute loading into the collection phloem is required to generate the hydrostatic pressure necessary to drive solution flow through the phloem.
An anoxia treatment placed upon previously photosynthesising leaves of C3 plants has earlier been shown to reduce solute loading of recently fixed photosynthate into the SE / CC (Thorpe et al. 1979; Thorpe and Minchin 1987) and increase transit time along the translocation pathway (Minchin et al. 2002). In this work, N2 gas was used to create a completely anoxic environment in the source leaves of both barley and sow thistle, thereby reducing the active loading of solutes into the phloem. Sieve tube hydrostatic pressure was then monitored to check whether or not changes in pressure could account for the previously observed changes in transit time.
Sieve tube hydrostatic pressure was measured 2–3 cm downstream of the treated tissue in both the barley and sow thistle. In the sow thistle this was the same area on the plant as the sap collected for solute analyses. In the barley, the waxy cuticle on the shoot made it difficult to seal the glass capillary to the plant so the phloem pressure probe was attached to the top of one of the roots ~2–3 cm below the shoot and the complete shoot was placed in a perspex chamber and treated with anoxia. Sap for solute analyses in barley was collected from the base of one mature leaf, which was enclosed in a leaf chamber. The comparison of sieve tube hydrostatic pressure measured in root with solute concentration in the leaf is a compromise, yet the results for the barley show similar trends in hydrostatic pressure, osmotic pressure and sucrose concentration to those measured in the same location in sow thistle.
The sow thistle had a pre-treatment hydrostatic pressure (~1.0–1.5 MPa) that was higher than in the barley (~0.8–1.4 MPa), consistent with the higher sap osmotic pressure and sucrose concentration. Pre-treatment, sap osmotic pressure of barley was ~2–2.5 MPa and sucrose concentration was ~0.6–0.8 m. In sow thistle, the pre-treatment sap osmotic pressure was ~2–3.5 MPa and sucrose concentration was ~0.65–1.4 m. Any differences in the sieve tube sucrose concentrations were probably related to the photosynthetic capacity of the load leaf upstream of the sampling point.
Following previous studies (for example Smith and Milburn 1980; Gould et al. 2004a) sucrose was probably the dominant osmoticum within the phloem of these species.
Anoxia treatment of a source leaf has been shown to reduce loading into the collection phloem of that leaf (Thorpe et al. 1979; Thorpe and Minchin 1987). The general downward trend in both osmotic pressure and sucrose concentration in both species following anoxia treatment (Figs 3, 4) is consistent with this reduced loading of the collection phloem. Different responses were observed between plants regarding the time after treatment before sap osmotic pressure and sap sucrose concentration changed. In barley the drop in osmotic pressure following anoxia treatment took less than 14 min (for example Fig. 3b, c) except one plant, which took over 60 min (Fig. 3a). In sow thistle the response time ranged from almost immediately (Fig. 3a) to over 12 min (Fig. 3b). The osmotic pressure was measured for the same sap collected for sucrose concentration analyses, and closely tracked the changes of sucrose for each individual plant. The ranges in response times of both sap osmotic pressure and sucrose concentration to the anoxia treatment may be attributed to variation in the capability of the tissue downstream of the treated areas to load solutes into the sieve tube (buffering; Minchin et al. 2002) and in sieve tube connectivity with other source and sink tissue.
Within 5 min of treating the source tissue with anoxia the hydrostatic pressure was reduced in both species. The percentage drop in hydrostatic pressure was similar in both species (~15–45%), which is also similar to the drop observed in the sap osmotic pressure and sucrose concentration in these plants. Following removal of the anoxia treatment, the hydrostatic pressure returned to pre-treated levels in both species (Fig. 2), as also observed for sap osmotic pressure and sucrose concentration in three of the four sow thistle plants (Fig. 4a, b, d, e for example; this phenomenon was not tested in barley) and can be explained by renewed loading in the source leaf. The fourth sow thistle plant did not recover either its sap osmotic pressure or sucrose concentration within 16 min of removing the treatment (Fig. 4c, f). The reason for this lack of recovery is unclear; it may be due to the gradual stoppage of flow within the sampled sieve tube. Such events have been shown to be associated with declines in osmotic pressure such as that shown in Fig. 4c (J. Pritchard pers. comm.). In the barley, the anoxia treatment continued until the end of the experiment, so nothing can be said about recovery in these plants.
Following the application of an anoxia treatment to a source leaf Minchin et al. (2002) found that transport of recently fixed 11C continued downstream of the anoxic region, but the speed of transport was reduced. This phenomenon can be explained by our results which show that sieve tube hydrostatic pressure is reduced downstream of an anoxic region, thus, reducing the hydrostatic pressure gradient required to drive solution from source to sink. Solution flow to the sink tissue does not stop completely because there is loading of solutes into the phloem along the transport pathway (buffering). The buffering capacity of the surrounding tissue is likely to dictate the regulation of hydrostatic pressure during source leaf anoxia (Minchin et al. 1984; Gould et al. 2004a; Thorpe et al. 2005) and will depend upon the type, condition and connectivity of the tissue downstream of the treated leaf. The buffering of the phloem solutes ensures that short-term changes in solute supply do not limit sink development, and short-term changes in sink requirements are not detected by the sources (Thorpe et al. 2005).
Conclusion
Using direct transient measurements of sieve tube hydrostatic pressure, we have confirmed Münch’s theory that hydrostatic pressure in the collection phloem is created and maintained through the continuing uptake of solutes into the phloem. The regulation of this pressure substantiates the interpretation that was given to account for variation in carbon flows that were associated with anoxic treatments of source tissue in previous experiments.
Acknowledgments
This work was funded by the Marsden Fund (Royal Society, NZ). In addition thanks go to Professors Roy Daniels and Warwick Silvester at the University of Waikato for providing a laboratory space and support in carrying out this work.
Fisher DB, Frame JM
(1984) A guide to the use of the exuding-stylet technique in phloem physiology. Planta 161, 385–393.
| Crossref | GoogleScholarGoogle Scholar |

Gould N,
Minchin PEH, Thorpe MR
(2004a) Direct measurements of sieve element hydrostatic pressure reveal strong regulation of sieve element hydrostatic pressure after pathway blockage. Functional Plant Biology 31, 987–993.
| Crossref | GoogleScholarGoogle Scholar |

Gould N,
Thorpe MR,
Minchin PEH,
Pritchard J, White PJ
(2004b) Solute import to elongating root cells of barley as a pressure driven bulk flow. Functional Plant Biology 31, 391–397.
| Crossref | GoogleScholarGoogle Scholar |

Grodzinski B,
Jahnke S,
Thompson RG, Sybesma C
(1984) The effect of leaf anoxia on translocation profiles of 11C and 13N labeled assimilates in petioles of Helianthus annuus L. and Lupinus albus L. Advances in Photosynthesis Research 4, 279–282.

Hammel HT
(1968) Measurement of turgor pressure and its gradient in the phloem of oak. Plant Physiology 43, 1042–1048.

Jones MGK,
Outlaw WH, Lowry OH
(1977) Enzymic assay of 10−7 to 10−14 moles of sucrose in plant tissues. Plant Physiology 60, 379–383.

Lalonde S,
Tegeder M,
Throne-Holst M,
Frommer WB, Patrick JW
(2003) Phloem loading and unloading of sugars and amino acids. Plant, Cell & Environment 26, 37–56.
| Crossref | GoogleScholarGoogle Scholar |

Malone M,
Leigh RA, Tomos AD
(1989) Extraction and analysis of sap from individual wheat leaf cells: the effect of sampling speed on the osmotic pressure of extracted sap. Plant, Cell & Environment 12, 919–926.

Minchin PEH,
Ryan KG, Thorpe MR
(1984) Further evidence of apoplastic unloading into the stem of bean: identification of the phloem buffering pool. Journal of Experimental Botany 35, 1744–1753.

Minchin PEH,
Thorpe MR,
Farrar JF, Koroleva OA
(2002) Source–sink coupling in young barley plants and control of phloem loading. Journal of Experimental Botany 53, 1671–1676.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Münch, E (1930).
Patrick JW,
Zhang W,
Tyerman SD,
Offler CE, Walker NA
(2001) Role of membrane transport in phloem translocation of assimilates and water. Australian Journal of Plant Physiology 28, 695–707.

Smith JAC, Milburn JA
(1980) Osmoregulation and the control of phloem-sap composition in Ricinus communis L. Annals of Botany 58, 577–588.

Thorpe MR, Minchin PEH
(1987) Effects of anoxia on phloem loading in C3 and C4 species. Journal of Experimental Botany 38, 221–232.

Thorpe MR, Minchin PEH
(1988) Phloem loading and transport of endogenously and exogenously labelled photo-assimilate in bean, beet, maize and cucurbit. Journal of Experimental Botany 39, 1709–1721.

Thorpe MR,
Minchin PEH, Dye EA
(1979) Oxygen effects on phloem loading. Plant Science Letters 15, 345–350.
| Crossref | GoogleScholarGoogle Scholar |

Thorpe, MR ,
Minchin, PEH ,
Gould, N ,
and
McQueen, J (2005). The stem apoplast: a potential communication channel in plant growth regulation. In ‘Vascular transport in plants’. pp. 201–220. (Elsevier / AP co-imprint: Oxford)
Wright JP, Fisher DB
(1983) Estimation of the volumetric elastic modulus and membrane hydraulic conductivity of willow sieve tubes. Plant Physiology 73, 1042–1047.



